Abstract
Introduction: We are interested in the metabolic response to ingested macronutrients and the interaction between macronutrients in meals. Recently, we have determined the insulin and glucose response to ingestion of lard, olive oil, or safflower oil, fat sources varying in fatty acid composition and carbohydrate (CHO), in the form of potato.
Objective: Our aim was to determine the effect of these dietary fats ingested alone or with potato on glucagon, glucagon-like peptide-1 (GLP-1) (7-37 and 7-36 amide), and total and acyl-ghrelin concentrations.
Methods: Healthy subjects ingested 25 g fat (lard, olive oil, or safflower oil), 50 g CHO (potato), 25 g fat with 50 g CHO, or water only. Glucagon, GLP-1 (7-37 and 7-36 amide), and total and acyl-ghrelin responses were determined over 4 h.
Results: All fats when ingested alone increased glucagon. Glucagon increases were dramatically attenuated when fats were ingested with the potato. GLP-1 increased after all meals, but was greatest when fats were ingested alone. The fat-stimulated increase was completely negated when fats were ingested with potato. Both acyl and total ghrelin decreased when only fats were ingested, as expected. When potato was ingested with any of the fats, the fat-induced decrease in acyl-ghrelin response also was essentially negated. Paradoxically, ghrelin increased when potato alone was ingested.
Conclusions: The current data indicate that the glucagon, GLP-1 and ghrelin responses to ingested fats, varying in fatty acid composition, are significantly affected by co-ingestion of CHO. Overall, the interaction between ingested foods in general is likely to be complex.
GLP-1 and ghrelin responses to ingested fats, varying in fatty acid composition, are significantly affected by co-ingestion of carbohydrate.
Our laboratory is interested in the metabolic effect of macronutrients when ingested individually and in combinations. Recently, we have determined that when lard, olive oil, or safflower oil, fats considered to be high in saturated, monounsaturated, and polyunsaturated fatty acids, respectively, are ingested with potato as a source of carbohydrate, the quantitative glucose and insulin area responses were the same as when potato is ingested alone (1). However, the increase in both was delayed, reduced, and prolonged when potato was ingested with any of the fats. The ingestion of each of the three fats with potato decreased the nonesterified fatty acids concentration, and the decrease correlated with the change in insulin concentration, as expected. All three fats when ingested alone did modestly increase the insulin concentration when compared with ingestion of water alone. The C-peptide data confirmed that this represented a stimulation of insulin secretion. When lard, olive oil, or safflower oil was ingested with the potato, there was an accelerated rise in triacylglycerol concentration.
In addition to the determination of the above (1), we determined the effect of these dietary fats ingested alone or with potato on the glucagon, glucagon-like peptide-1 (GLP-1) (7-37 and 7-36 amide), and total and acyl-ghrelin concentrations. These are hormones known to respond to food intake. The results form the basis for the present report.
We consider such studies to be important because they help to elucidate the physiological effects of various dietary fats and how they interact with other macronutrients. Current data strongly emphasize that these nutrients should be considered not solely as a source of energy but also as regulators of fuel homeostasis.
Subjects and Methods
Nine healthy subjects (four females and five males) received lard as a test meal, 12 healthy subjects (six females and six males) received olive oil as a test meal, and 11 healthy subjects (six females and five males) received safflower oil as a test meal. All subjects gave informed consent before participating in the study. The study was approved by the Minneapolis Department of Veterans Affairs Medical Center and the University of Minnesota Committee on Human Subjects. The mean age of the subjects was 25 yr (range, 18–38); the mean body mass index was 23 kg/m2 (range, 19–29), with mean lean body mass of 54 kg (range, 37–67). Lean body mass was determined using a portable body impedance analyzer (RJL Systems, Clinton Township, MI). All participants had normal thyroid, liver, kidney function, lipid profiles, and glycated hemoglobin. Subjects were studied in a Special Diagnostic and Treatment Unit, which is similar to a clinical research center. Subjects were instructed to eat a light meal the evening before the test and to fast after 2000 h. After an overnight fast of 12 h, an indwelling catheter was inserted into an antecubital or forearm vein and kept patent with iv saline. Subjects ingested a test meal consisting of lard, olive oil, or safflower oil with or without mashed potatoes and mashed potatoes alone. On a separate occasion, they received water only. Each subject completed all four arms of the study. Generally, the 4-d study was conducted over a 2- to 3-wk period.
Boiled, peeled white potatoes (250 g raw weight) were given in an amount to equal to 50 g starch (2). They were cooked 10–15 min in a microwave oven before being served. The amount of fat consumed either alone or mixed with potatoes was 25 g. The oils were ingested at room temperature. Lard was melted to a liquid state, which then was ingested. The meals were given in a random order at 0815 h. Each fat, or water only, was ingested within 1 min. When the meal contained potato with or without fat, the meals were ingested within approximately 5 min. Patients were allowed to consume water and coffee ad libitum. Some of the subjects drank coffee after the test meal, others did not, but this was randomly distributed. If they drank coffee, they drank coffee during all four components of the study. Although the amount of water was not quantified, when questioned, the subjects indicated that similar amounts of water/coffee were consumed for all three test meals. Baseline blood samples were obtained at 0745, 0800, and 0815 h. The test meal was ingested at 0815 h. Blood was collected every 15 min after the beginning of each meal for the first 2 h, then every 30 min for the third and fourth hours.
Glucagon and active and total ghrelin were determined by RIA using kits purchased from Linco Research (purchased by Millipore, Billerica, MA). The biologically active forms of GLP-1 [GLP-1 (7-36 amide) and GLP-1 (7-37)] were quantified using an ELISA kit from Linco Research (purchased by Millipore). For the determination of GLP-1, the blood was collected in ice-cold Vacutainer EDTA-plasma tubes to which an appropriate amount of dipeptidyl-peptidase-4 inhibitor had already been added. The blood was mixed with dipeptidyl-peptidase-4 inhibitor less than 30 sec after collection. It then was immediately returned to an ice bath and centrifuged.
For all data, the net integrated 240-min area responses, using the fasting values as baseline, were calculated using a computer program based on the trapezoid rule. Statistics were determined using repeated measures ANOVA with Prism 4 software for the Macintosh (GraphPad, La Jolla, CA). This was followed by Student’s t test for paired variates with the Microsoft Office Excel 2007 program (Microsoft Corporation, Richmond, WA) for PC. A P value of <0.05 was the criterion for significance. Data are presented as means ± sem.
Results
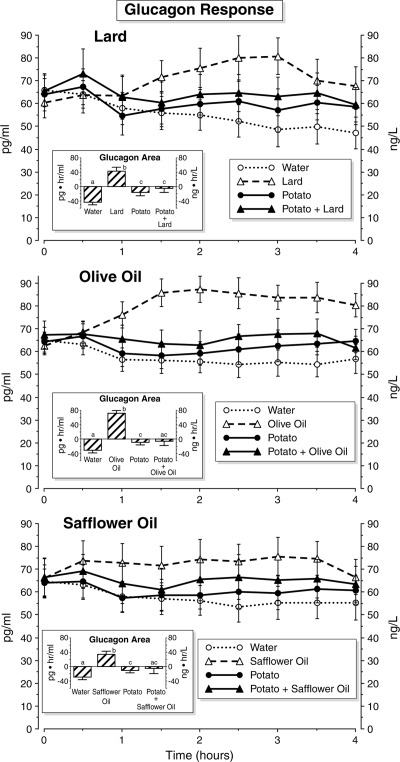
Glucagon response (Fig. 1)
Figure 1.
Time course of serum glucagon concentrations. Time points are means ± sem. Insets, Net integrated areas under the curve after test meal ingestion. Bars with different letters indicate that data are statistically significantly different (P < 0.05).
The plasma glucagon concentration decreased slowly when only water was ingested. This decrease was less pronounced after potato alone and any potato + fat combination meal. Lard, olive oil, and safflower oil ingested independently increased the glucagon concentration. The increase was greatest after olive oil ingestion.
The net integrated glucagon area response after ingestion of water was negative. The glucagon area response after ingestion of potato was significantly less negative in all the three arms of the study when compared with the area after water ingestion. The same was true for the glucagon area response after potato + lard ingestion. The glucagon area after ingested potato was not different when compared with potato + lard. The glucagon area responses after potato + olive oil and potato + safflower oil, although slightly negative, were not different when compared with the potato alone or water. After ingestion of lard, olive oil, or safflower oil, the glucagon area response was positive and significantly higher when compared with the glucagon area after water, potato, or potato + any fat. The area response after olive oil was considerably higher than that after lard or safflower oil ingestion.
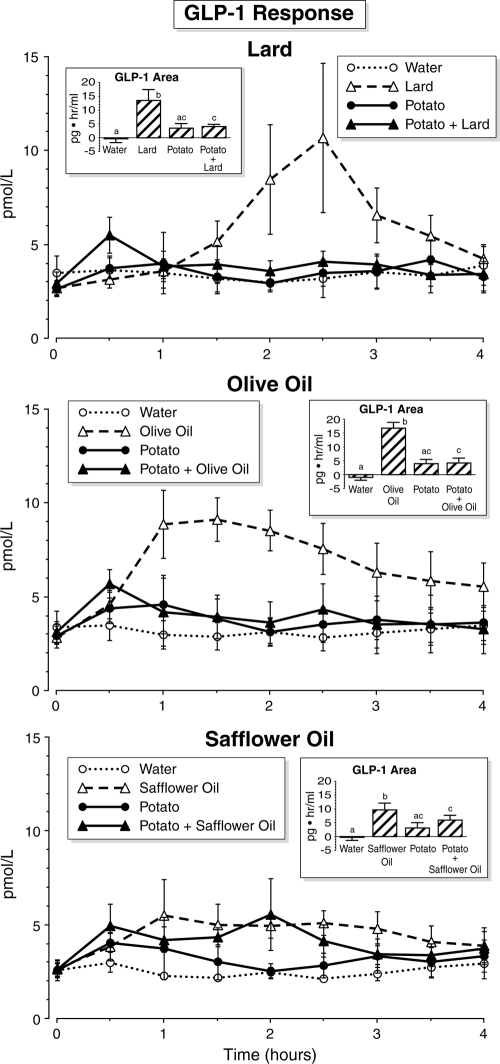
GLP-1 response (Fig. 2)
Figure 2.
Time course of serum GLP-1 concentrations. Time points are means ± sem. Insets, Net integrated areas under the curve after test meal ingestion. Bars with different letters indicate that data are statistically significantly different (P < 0.05).
The plasma GLP-1 concentration remained stable after ingestion of water only. After ingestion of lard, olive oil, or safflower oil alone, the GLP-1 concentration increased and remained elevated for the duration of the study. When lard, olive oil, or safflower oil was ingested with potato, there was only a small increase in GLP-1. That is, the increase was blunted when potato was added to the fats. The plasma GLP-1 concentration increased slightly in a biphasic response after potato ingestion.
Potato ingestion resulted in an increase in GLP-1 area, but this was not statistically significant when compared with water. The GLP-1 net area responses after ingestion of lard, olive oil, and safflower oil were significantly higher when compared with the net area response after ingestion of the respective water, potato, or potato + fat meals. Interestingly, the area response after olive oil ingestion was greater than after lard or safflower oil ingestion. The net area responses after potato and potato + lard, olive oil, or safflower oil were positive and not different. All were higher when compared with the area response after water ingestion, but this was only statistically significant when any of the fats were present.
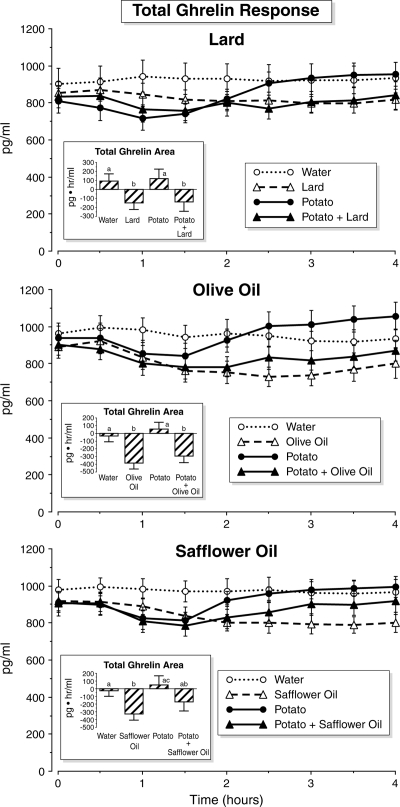
Total ghrelin response (Fig. 3)
Figure 3.
Time course of serum total ghrelin concentrations. Time points are means ± sem. Insets, Net integrated areas under the curve after test meal ingestion. Bars with different letters indicate that data are statistically significantly different (P < 0.05).
The total ghrelin concentration did not change significantly when only water was ingested. After the ingestion of potato alone, the total ghrelin decreased to a nadir at 1–1.5 h, returned to the fasting value at 2 h, and continued to increase, remaining elevated at the termination of the study. After lard or safflower oil ingestion, the total ghrelin decreased, reached a nadir at 3.5 h, and remained below baseline at the end of the study. After olive oil ingestion, the total ghrelin concentration decreased in a similar fashion, reaching a nadir at 2.5 h and remaining below the fasting level at the termination of the study. When potato was ingested with lard, olive oil, and safflower oil, respectively, there was only a small and transient initial decrease in total ghrelin concentration.
The total ghrelin net area response was slightly positive after potato ingestion, but this was not statistically significantly different from the net area response after water ingestion. The area responses after lard and potato + lard were negative. This was statistically significantly less than the area response after water or potato ingestion. A similar response was obtained with olive oil. The area response after safflower oil ingestion was negative and also statistically significantly different when compared with the area response after water or potato ingestion. However, when potato was ingested with safflower oil, the total ghrelin area response was not statistically significantly different when compared with the net areas after water or potato ingestion. Potato + lard, potato + olive oil, and potato + safflower oil ingestion resulted in a similar decrease in total ghrelin area when compared with only lard, olive oil, and safflower oil ingestion, respectively.
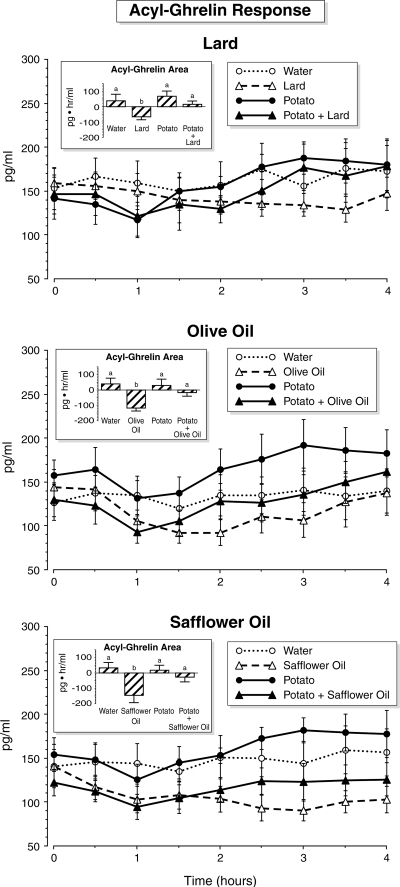
Acyl-ghrelin response (Fig. 4)
Figure 4.
Time course of serum acyl-ghrelin concentrations. Time points are means ± sem. Insets, Net integrated areas under the curve after test meal ingestion. Bars with different letters indicate that data are statistically significantly different (P < 0.05).
When only water was ingested, the acyl-ghrelin concentration remained stable. After lard, olive oil, or safflower oil ingestion, the acyl-ghrelin decreased and remained below the baseline value at the end of the study. After potato ingestion, the acyl-ghrelin decreased and reached a nadir at 1 h, which was followed by a rebound to above the fasting value. The response to potato + lard, potato + olive oil, or potato + safflower oil was similar to that of potato alone, except the rebound was delayed and smaller.
The net acyl-ghrelin area response was positive after water or potato, and the areas were essentially identical. The acyl-ghrelin area responses were negative after ingestion of lard, olive oil, and safflower oil and were statistically significantly lower when compared with the area after water and potato + lard, olive oil, or safflower oil. When potato was ingested with any of the dietary fats, the decrease in acyl-ghrelin response induced by the ingested fat alone was essentially negated. The net acyl-ghrelin areas after potato + lard, potato + olive oil, or potato + safflower oil were not different when compared with potato alone or water.
Discussion
In the first publication from this study, we have reported for the first time that lard, olive oil, and safflower oil ingested alone all increase the insulin concentration when compared with ingestion of water alone, although the increase is modest (1). The C-peptide data confirmed that this represented a stimulation of insulin secretion. The mechanism is totally unknown. The observation that the plasma glucose did not change is compatible with a fatty acid-induced insulin resistance, or the increase may not have been sufficient to affect glucose metabolism.
In the same study, we now report that the ingestion of lard, olive oil, or safflower oil also greatly stimulated an increase in GLP-1. Thus, the fat-stimulated insulin response could be mediated, at least in part, by a rise in the GLP-1 concentration. However, if a rise in GLP-1 is mechanistically important, the fact that, quantitatively and dynamically, the responses of GLP-1 and insulin were different suggests that if GLP-1 is playing a role, it is a minor role, and only a modest increase in GLP-1 concentration is sufficient to stimulate insulin secretion.
It has been reported that ingestion of fats or glucose independently stimulates a rise in GLP-1 (3,4). It also has been reported that an increase in the plasma glucose concentration amplifies the GLP-1 stimulatory effect on insulin secretion (5). Thus, we would have expected the ingestion of potato with lard, olive oil, or safflower oil to have an additive effect in stimulating a rise in GLP-1 concentration. This was not the case. The addition of potato essentially extinguished the GLP-1 response to fat, i.e. instead of an additive effect the rise in GLP-1 was diminished. Interestingly, Vollmer et al. (6) have reported that the GLP-1 response to a mixed meal was attenuated when the plasma glucose was clamped at approximately 160 mg/dl using a glucose infusion protocol. The authors speculated that the attenuation could be explained by a hyperglycemic inhibition of gastric emptying, and this is the reason the GLP-1 response to meals is impaired in people with type 2 diabetes. Our data suggest that an elevated glucose also could have impaired the GLP-1 response to the ingested fat. This is an issue that needs to be investigated further.
A rise in glucagon concentration occurs with ingestion of proteins, fructose, and galactose (7,8,9,10). All are gluconeogenic substrates. The present data indicate that orally administered dietary fats also result in an increase in glucagon concentration. In a previous study, we also demonstrated progressively larger glucagon area responses with increasing amounts of butter when ingested with potato in individuals with type 2 diabetes (11). Thus, all ingested macronutrient constituents studied to date raise the plasma glucagon, with the exception of glucose. Ingested glucose lowers the glucagon concentration.
In the present study, the ingestion of potato alone also modestly increased the glucagon concentration when compared with the water control. Most likely, this was due to the small amount of protein present in potato (8). The addition of potato to lard, olive oil, or safflower oil abrogated the glucagon response observed after the ingestion of fat alone. This can be explained by a potato-induced increase in circulating glucose and insulin concentrations because both are reported inhibitors of glucagon release (12).
Whether GLP-1 directly inhibits glucagon secretion is uncertain (13,14). The current data strongly suggest that GLP-1 does not inhibit glucagon secretion. When any of the fats was ingested, both GLP-1 and glucagon increased. It also is of interest that the insulin, GLP-1, and glucagon concentrations were increased, but the glucose concentration remained entirely stable. As we have pointed out previously, the role of glucagon in regulating the glucose concentration remains elusive, except in the presence of hypoglycemia (15).
In the present study, both acyl and total ghrelin were suppressed for the duration of the study when lard, olive oil, or safflower oil was ingested. Ingestion of potato or potato plus lard, olive oil, or safflower oil resulted in only a small transient decrease, followed by a rebound increase in both. In contrast, in a study by Foster-Schubert et al. (16), lipid ingestion suppressed total and acyl-ghrelin less effectively than did carbohydrate ingestion. However, the test meals consisted of isocaloric beverages of mixed macronutrient composition with 80% of the calories derived from carbohydrate or lipids. Similar to results of our study, the total and acyl-ghrelin rebound was most pronounced after carbohydrate ingestion.
It has been reported that the ghrelin concentration is suppressed by insulin (17,18,19). In the present study, the insulin concentration cannot explain the biphasic acyl ghrelin response to potato or potato + any fat. In addition, the ghrelin concentration remained suppressed after ingestion of each fat, although the insulin concentration was always much lower after any fat ingestion than after potato or potato + fat ingestion. Thus, it is difficult to explain the current data based on a quantitative regulation of ghrelin by insulin. A certain amount of insulin may be necessary to maintain the mechanism by which ingested nutrients regulate ghrelin secretion. However, a rise in insulin during the meal is not necessary. Our data are compatible with such a concept. The rebound increase in ghrelin when potato or potato plus fat was ingested still remains to be explained.
Considerable evidence implicates ghrelin suppression by ingested nutrients in postprandial satiation (20). In our study, the satiety data (1) did not correlate with the ghrelin data. The ghrelin suppression after ingestion of fat or potato + fat meals vs. ingestion of potato alone was not associated with a decrease of the desire to eat, degree of hunger or proposed food intake, or with an increase of fullness sensation.
Summary
There is a complex interaction between ingested fats and carbohydrates in regard to the regulation of glucagon, GLP-1, and ghrelin as well as for insulin. We have determined that dietary fats ingested alone stimulate a rise in glucagon and GLP-1 concentration. However, the glucagon and GLP-1 increases were dramatically attenuated when the fats were ingested with the potato. The ghrelin concentration was decreased in response to fat ingested alone. Ingested potato only modestly and transiently decreased the ghrelin concentrations. The interaction of fats with potato did not change significantly the ghrelin response to ingested fat alone.
Acknowledgments
The authors thank the subjects for participating in the study; the staff of the Special Diagnostic and Treatment Unit, the staff of the Clinical Chemistry Laboratory, and the staff of the Nuclear Medicine Department for their assistance; Heidi Hoover, R.D., M.S., for help with food preparation; and Linda Hartich, M.T., for technical advice and laboratory assistance. We thank Dr. Michael Kuzkowski for advice on statistical analysis of the data.
Footnotes
This work was supported by Merit Review funds from the Department of Veterans Affairs and a grant from the National Pork Board.
F.Q.N. and M.C.G. designed the study. A.R. collected the data. All three authors analyzed the data and contributed to the writing of the manuscript.
Disclosure Summary: There is no conflict of interest.
First Published Online May 5, 2010
Abbreviation: GLP-1, Glucagon-like peptide-1.
References
- Radulescu A, Hassan Y, Gannon MC, Nuttall FQ 2009 The degree of saturation of fatty acids in dietary fats does not affect the metabolic response to ingested carbohydrate. J Am Coll Nutr 28:286–295 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture 1984 Composition of foods raw, processed, prepared. Agriculture Handbook No. 8-11. Vegetables and vegetable products. Washington, DC: U.S. Government Printing Office [Google Scholar]
- Herrmann C, Göke R, Richter G, Fehmann HC, Arnold R, Göke B 1995 Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 56:117–126 [DOI] [PubMed] [Google Scholar]
- Beysen C, Karpe F, Fielding BA, Clark A, Levy JC, Frayn KN 2002 Interaction between specific fatty acids, GLP-1 and insulin secretion in humans. Diabetologia 45:1533–1541 [DOI] [PubMed] [Google Scholar]
- Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF 1992 Insulinotropic action of glucagon like peptide-I-(7–37) in diabetic and nondiabetic subjects. Diabetes Care 15:270–276 [DOI] [PubMed] [Google Scholar]
- Vollmer K, Gardiwal H, Menge BA, Goetze O, Deacon CF, Schmidt WE, Holst JJ, Meier JJ 2009 Hyperglycemia acutely lowers the postprandial excursions of glucagon-like peptide-1 and gastric inhibitory polypeptide in humans. J Clin Endocrinol Metab 94:1379–1385 [DOI] [PubMed] [Google Scholar]
- Krezowski PA, Nuttall FQ, Gannon MC, Bartosh NH 1986 The effect of protein ingestion on the metabolic response to oral glucose in normal individuals. Am J Clin Nutr 44:847–856 [DOI] [PubMed] [Google Scholar]
- Westphal SA, Gannon MC, Nuttall FQ 1990 The metabolic response to glucose ingested with various amounts of protein. Am J Clin Nutr 52:267–272 [DOI] [PubMed] [Google Scholar]
- Nuttall FQ, Khan MA, Gannon MC 2000 Peripheral glucose appearance rate following fructose ingestion in normal subjects. Metabolism 49:1565–1571 [DOI] [PubMed] [Google Scholar]
- Gannon MC, Khan MA, Nuttall FQ 2001 Glucose appearance rate after the ingestion of galactose. Metabolism 50:93–98 [DOI] [PubMed] [Google Scholar]
- Gannon MC, Ercan N, Westphal SA, Nuttall FQ 1993 Effect of added fat on the plasma glucose and insulin response to ingested potato in individuals with NIDDM. Diabetes Care 16:874–880 [DOI] [PubMed] [Google Scholar]
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB 2005 β-Cell secretary products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54:1808–1815 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Deacon CF, Schmidt WE, Holst JJ, Nauck MA 2007 Suppression of glucagon secretion is lower after oral glucose administration than during intravenous glucose administration in human subjects. Diabetologia 50:806–813 [DOI] [PubMed] [Google Scholar]
- Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ 2008 Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 57:678–687 [DOI] [PubMed] [Google Scholar]
- Nuttall FQ, Ngo A, Gannon MC 2008 Regulation of hepatic glucose production and the role of gluconeogenesis in humans: is the rate of gluconeogenesis constant? Diabetes Metab Res Rev 24:438–458 [DOI] [PubMed] [Google Scholar]
- Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE 2008 Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab 93:1971–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS 2003 The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab 284:E313–E316 [DOI] [PubMed] [Google Scholar]
- Möhlig M, Spranger J, Otto B, Ristow M, Tschöp M, Pfeiffer AF 2002 Euglycemic hyperinsulinemia, but not lipid infusion, decreases circulating ghrelin levels in humans. J Endocrinol Invest 25:RC36–RC38 [DOI] [PubMed] [Google Scholar]
- Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, Boyadjian R 2002 Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab 87:3997–4000 [DOI] [PubMed] [Google Scholar]
- Cummings DE 2006 Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav 89:71–84 [DOI] [PubMed] [Google Scholar]