Abstract
We expanded our region-based model of water and solute exchanges in the rat outer medulla to incorporate the transport of nitric oxide (NO) and superoxide (O2−) and to examine the impact of NO-O2− interactions on medullary thick ascending limb (mTAL) NaCl reabsorption and oxygen (O2) consumption, under both physiological and pathological conditions. Our results suggest that NaCl transport and the concentrating capacity of the outer medulla are substantially modulated by basal levels of NO and O2−. Moreover, the effect of each solute on NaCl reabsorption cannot be considered in isolation, given the feedback loops resulting from three-way interactions between O2, NO, and O2−. Notwithstanding vasoactive effects, our model predicts that in the absence of O2−-mediated stimulation of NaCl active transport, the outer medullary concentrating capacity (evaluated as the collecting duct fluid osmolality at the outer-inner medullary junction) would be ∼40% lower. Conversely, without NO-induced inhibition of NaCl active transport, the outer medullary concentrating capacity would increase by ∼70%, but only if that anaerobic metabolism can provide up to half the maximal energy requirements of the outer medulla. The model suggests that in addition to scavenging NO, O2− modulates NO levels indirectly via its stimulation of mTAL metabolism, leading to reduction of O2 as a substrate for NO. When O2− levels are raised 10-fold, as in hypertensive animals, mTAL NaCl reabsorption is significantly enhanced, even as the inefficient use of O2 exacerbates hypoxia in the outer medulla. Conversely, an increase in tubular and vascular flows is predicted to substantially reduce mTAL NaCl reabsorption. In conclusion, our model suggests that the complex interactions between NO, O2−, and O2 significantly impact the O2 balance and NaCl reabsorption in the outer medulla.
Keywords: concentrating ability, kidney, oxygen balance, reactive oxygen species, sodium reabsorption
nitric oxide (NO) and superoxide (O2−) exert opposite effects in the renal medulla, and changes in the balance between the two significantly impact renal function. Whereas NO inhibits tubular NaCl reabsorption and enhances medullary blood flow by dilating blood vessels, O2− stimulates NaCl reabsorption across the medullary thick ascending limb (mTAL) and acts to reduce medullary blood flow by mechanisms that remain to be fully elucidated (13).
Under normal conditions, O2− levels in the body are kept low due to O2− scavenging by NO and superoxide dismutase (SOD). As suggested by several studies (reviewed in Ref. 36), an imbalance between NO and O2− in the kidney significantly alters renal hemodynamics and excretory function and may contribute to the development of salt-sensitive hypertension.
An imbalance between NO and O2− may also affect medullary oxygenation. Renal hypoxia is exacerbated during hypertension, and tempol reduces renal tissue hypoxia in spontaneously hypertensive rats (32, 58). Oxidative stress and subsequent reduced NO bioavailability may result in an excessive use of O2 to maintain the sodium balance (i.e., a decrease in TNa/Qo2, the ratio of transported sodium to oxygen consumption) during hypertension (58).
The objective of the current theoretical study was to investigate how shifts in the balance between NO and O2− affect medullary sodium reabsorption and oxygen availability. We developed a mathematical model of NO and O2− transport in the rat outer medulla to examine the impact of NO-O2− interactions on mTAL sodium transport and O2 consumption, under both physiological and pathological conditions.
MODEL DESCRIPTION
Our representation of the rat outer medulla is that of the region-based approach developed by Layton and Layton (31). The model represents the loops of Henle, the collecting duct (CD) system, the vasa recta, and red blood cells (RBCs). The descending limbs, ascending limbs, and CDs are represented by rigid tubules that are oriented along the corticomedullary axis, which extends from x = 0 at the corticomedullary boundary to x = L at the outer-inner medullary (OM-IM) boundary (Fig. 1A). The model separates blood flow in vasa recta into two compartments, plasma and RBCs, divided by RBC membranes. The vascular plasma and RBC compartments are also represented by rigid tubules along the corticomedullary axis. Besides tubules and vasa recta, the model considers two other sets of compartments: one consists of the RBCs within the capillaries (which we refer to as “capillary RBCs”). Capillary flow is assumed to be perpendicular to the medullary axis; thus the capillary RBC compartment is represented by rigid tubules, extending radially (i.e., perpendicular to the medullary axis) across each medullary level. We assume that the highly fenestrated nature of the capillaries results in rapid equilibration of their plasma content with local interstitium. The other set of compartments represents the combination of interstitial spaces, interstitial cells, and capillary plasma flow, and is simply referred to as the “interstitium.”
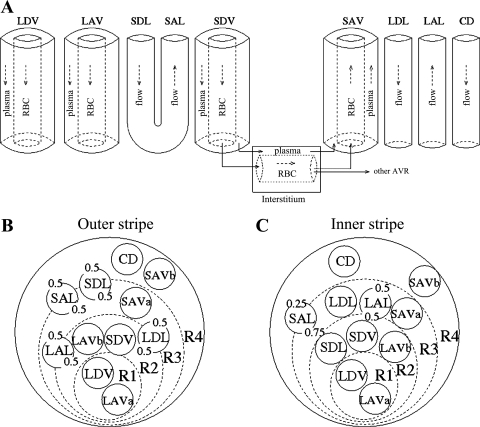
Fig. 1.
Schematic representation of tubules and vasa recta in the rat outer medulla (OM). Four concentric regions (R1–R4) are distinguished; the innermost (R1) contains the central vascular bundle. Note that R1–R4 have coincident centers; the display here is intended to minimize the figure area. A: tubules and vessels along the corticomedullary axis. B: cross section of the outer stripe. C: cross section of the inner stripe. LDV, long descending vas rectum; SDV, short descending vas rectum; LAVa and LVAb, 2 populations of LDV; SAVa and SAVb, 2 populations of SDV; LDL, long descending limb of Henle's loop; SDL, short descending limb; LAL, long ascending limb; SAL, short ascending limb; CD: collecting duct. The decimal numbers represent the relative weight of interaction between a type of vessel or tubule and a given region (i.e., the parameter κi,R in Eqs. 9–10).
The interstitium is divided into four concentric regions, which are used to represent the highly specific structural organization of the rat OM (Fig. 1, B and C): an innermost region containing the central vascular bundle (R1), where all the long descending vasa recta (i.e., DVR that reach into the inner medulla) and a third of the long ascending vasa recta (i.e., AVR that reach into the inner medulla) are sequestered; a peripheral region of the vascular bundle (R2), where the short DVR (i.e., DVR that turn within the OM) and the remaining long AVR reside; a region neighboring the vascular bundle (R3), which contains most medullary thick ascending limbs (mTALs), both long and short, and some short AVR; and the region most distant from the vascular bundle (R4), where CDs and the remaining short AVR are located. Descending limbs that reach into the inner medulla are located in R2 and R3 in the outer stripe (OS) and move toward the CDs in the inner stripe (IS). Conversely, the short descending limbs (i.e., those that turn within the OM) straddle R3 and R4 in the OS and move toward the bundle periphery (R2) in the IS.
The model yields tubular and vascular fluid flows as well as the concentration of eight species, i.e., Na+, urea, O2, deoxy-hemoglobin (Hb), oxy-hemoglobin (HbO2), nitrosyl-heme (HbNO), NO, and O2−, in each type of tubule and vessel, and in the interstitium. These variables are determined based on conservation equations and appropriate boundary conditions. Detailed equations for fluid flows and the concentrations of the first five species (Na+, urea, O2, Hb, HbO2) can be found in our previous studies (7, 8). This section focuses on the new features of our model: the transport of NO and O2−, as well as their effect on O2 metabolism and Na+ reabsorption across mTALs.
Conservation Equations
Tubules.
Steady-state conservation equations are used to predict the volumetric flow rate (Fi, V) and the concentration of solute k (Ci, k) in a tubule of type i
| (1) |
| (2) |
where x is the position along the OM, ranging from 0 at the corticomedullary junction to L at the OM-IM boundary; Ji, V and Ji, k denote the transmural flux of water and solute k into tubule i; Ailum and Aiepi, respectively, designate the cross-sectional area of the lumen of i (i.e., based on its inner diameter) and that of the surrounding epithelium. Ωi, klumand Ωi, kepi are the volumetric consumption rate of solute k in the lumen of tubule i and that of the surrounding epithelium, and Gi, kepi is the epithelial volumetric generation rate of k. Since the model does not explicitly account for tubular and vascular walls, which are represented instead as single barriers, we assume that a fixed fraction θ of the net amount of solute k that is generated in epithelia or endothelia diffuses toward the lumen, and the remainder (1 − θ) diffuses toward the interstitium. For NO and O2−, the fraction θ is taken as one-half everywhere.
Vasa recta.
As previously noted, plasma and RBCs are treated as two separate compartments. Fi, Vpl and Fi, Vrbc denote the plasma and RBC water flow rate in vessel i, respectively, so that the total water flow rate in vessel i is Fi, V = Fi,Vpl + Fi,Vrbc. Similarly, Ci, kpl and Ci, krbc denote the respective plasma and RBC concentration of solute k in vessel i. Water conservation in the plasma and RBC compartments of blood vessel i can be expressed as
| (3) |
| (4) |
where Ji, Vpl is the net transmural flux of water into plasma (i.e., from the interstitium and RBCs) and Ji, Vrbc that into RBCs. Note that Ji, Vpl = Ji, Vint − Ji, Vrbc, where Ji, Vint designates the transmural flux from interstitium to plasma.
Similarly, solute conservation in the plasma and RBC compartments of blood vessel i is expressed as
| (5) |
| (6) |
where Ji, kpl and Ji, krbc are the net transmural flux of solute k entering plasma and RBCs; Ωi, krbc, Ωi, kpl, and Ωi, kendo, respectively, denote the volumetric consumption rate of solute k in RBCs, plasma, and surrounding endothelium, and Gi, kendo is the endothelial volumetric generation rate of k. Airbc, Aipl, and Aiendo, respectively, designate the cross-sectional area of the RBC compartment, the plasma compartment, and the surrounding endothelium. The cross-sectional area of the RBC compartment is calculated as
| (7) |
Interstitium.
Water conservation equations in the interstitium of each region yield water flows into ascending vasa recta and can be found in our previous study (8). The conservation equation for solute k in region R yields the interstitial concentration of k, and is written as
| (8) |
where PR, R′, k is the permeability of the boundary between regions R and R′ to solute k, ni denotes the number of tubules or vessels of type i, ωSDV represents the fraction of short descending vasa recta (SDV) reaching to a given medullary level, QR, R′ is the capillary flow from region R to R′, QAVR is the total fluid accumulation carried away by AVR, and ARint is the area occupied by interstitial cells in region R.
The first term in Eq. 8 represents the diffusion of solute into region R from adjacent regions R′. The second term is the sum of solute fluxes from tubules and long vasa recta into R. The third and fourth terms denote the composite solute fluxes at level x from all SDV and short ascending vasa recta (SAV), respectively, that are present in region R and that reach to medullary level y > x. The first term in the first pair of square brackets represents the solute flux from SDV terminating at level y = x into region R. The next term in that first pair of square brackets is the solute flux from capillary RBCs into R. The term CR′, kQR′, R − CR, k(QAVR + QR, R′) represents the net amount of solute that is carried by flow at the local concentration into AVR or into an adjoining region R′. The next two terms involving (1 − θ) represent the fraction of net solute produced by tubular epithelial and vascular endothelial cells that is released into the interstitium (see Eqs. 2 and 5). The next-to-last term denotes the net amount of solute k produced by capillary endothelium in region R (see below), and the last term denotes the consumption rate of solute k by interstitial cells in region R.
Transmural Fluxes
Tubules.
The transmural fluxes of water and solute k into tubule i are calculated as
| (9) |
| (10) |
where ri is the inner radius of the tubule, di is the product of the partial molar volume of water and the osmotic water permeability of i, σk is the reflection coefficient for solute k (taken to be 1 for all solutes), ϕk is the osmotic coefficient for solute k, and CR, k is the concentration of solute k in region R. Pi, k denotes the permeability of i to solute k, and Ψi, kactive the rate of active transport of solute k. As illustrated in Fig. 1, in some cases, fractions of a tubule or vas rectum i are distributed between two concentric regions, and κi, R is the fraction of i that is in contact with region R. The spatial dependence of the variables in the flux equations has been omitted for simplicity.
Vasa recta.
The flux of water flowing from the interstitium into DVR plasma is given by
| (11) |
The flux of water from plasma into RBCs is expressed as
| (12) |
where Δπh and Δπp are the oncotic pressures due to hemoglobin in RBCs, and to albumin and other proteins in plasma; N is Avogadro's number, kB the Boltzmann constant, and T is the absolute temperature (the product N kBT equals 19.3 mmHg/mM).
The transmural flux of solute k flowing from the interstitium into DVR plasma, and that from plasma to RBCs, are given by
| (13) |
| (14) |
Note that Ji, kpl = Ji, kint − Ji, krbc.
NO Generation and Consumption
NO is generated in the vascular endothelium and tubular epithelium, and its synthesis rate depends on O2 availability. The O2 dependence of Gi, NO is modeled using a Michaelis-Menten relationship
| (15) |
where the Michaelis-Menten constant KO2NO (taken as 38 mmHg, based on Ref. 60) is the oxygen concentration at half the maximal rate of NO production in cell layer i, itself denoted by Gi, NOmax. The vasa recta-to-tubule GNOmax ratios derive from published measurements (61), and the maximal rate of NO synthesis in vasa recta is chosen to yield interstitial NO concentrations of ∼100 nM, as determined experimentally (64).
As in our previous models of NO transport, the NO consumption reactions considered here are the auto-oxidation of NO (rate V1), the scavenging of NO by superoxide (rate V2), the irreversible reaction of NO with HbO2 to form methemoglobin (rate V3), and the reversible reaction of NO with Hb to form HbNO (rate V4)
| (16) |
| (17) |
| (18) |
| (19) |
The reaction between NO and soluble guanylate cyclase is much slower than these four reactions and is therefore neglected. The total volumetric consumption rate of NO in compartment i is then equal to
| (20) |
As described above, our model does not explicitly represent endothelial and epithelial cell barriers. As shown in Eqs. 2 and 5, we account for NO synthesis (or consumption) in these cellular layers via “source” (or “sink”) fluxes into the vascular or tubular lumen and into the interstitium. Given that solute concentrations in endothelia and epithelia are not explicitly determined, the fraction of NO consumption in these layers that is attributed to plasma or tubular lumen (Eqs. 2 and 5) is calculated based on plasma or luminal concentrations, whereas the fraction that is attributed to the interstitium (Eq. 8) is calculated based on interstitial concentrations.
Hypoxia-Induced NO Release
Even though oxygen is a precursor in the synthesis of NO, hypoxia has been shown to raise medullary NO levels in anesthetized rats (22). Several mechanisms have been proposed to explain how hypoxia may induce NO release. Stamler and colleagues (50) have postulated that S-nitrosohemoglobin (SNOHb) constitutes a pool of available NO, which can be transferred to the vessel wall upon deoxygenation. According to another hypothesis, RBC nitrite is reduced by deoxy-hemoglobin to form NO, and low Po2 stimulates NO release via this route (14). Some of the key steps in these processes remain to be fully elucidated. In a previous model (16), we used the approach developed by Chen et al. (9, 10) to model the SNOHb and RBC nitrite pathways, which suggested that neither significantly affects medullary NO concentrations. Thus, in the current model, we account for hypoxia-induced stimulation of NO release in the OM in a simple manner: we assume that as O2 availability decreases, the RBC permeability to NO (PNOrbc) also decreases, thereby preserving more NO in kidney tissue. Specifically, PNOrbc is taken to vary linearly with Po2
| (21) |
where the basal RBC permeability to NO (PNOrbc, basal) is taken as 0.1 cm/s (16). The parameters of Eq. 21 are chosen so that our previous model of NO transport (16) predicts a 14% increase in medullary NO levels (at the mid-IS in R3–R4) when medullary Po2 decreases from Pb = 28 mmHg to Pa = 12 mmHg, as observed experimentally when indomethacin is administered to anesthetized rats (23).
O2− Generation and Consumption
The rate of epithelial and endothelial O2− synthesis also depends on O2 availability. As we previously described (16), the effects of medullary hypoxia on O2− synthesis remain poorly understood. Given that some studies suggest that low Po2 stimulates O2− production (34), whereas others report an inhibitory effect (12), we consider two different hypotheses. Case A assumes that low Po2 inhibits O2− synthesis, and the oxygen dependence of the O2− generation rate is then modeled using a Michaelis-Menten relationship
| (22a) |
where Gi, O2−basal is fixed, and KO2O2− is taken as 15.4 mmHg (12). Case B assumes that low Po2 increases O2− production by 50% relative to well-oxygenated conditions (34), so that the rate of O2− synthesis under basal conditions is given by
| (22b) |
The GO2−basal ratios between vasa recta and the different types of tubules are based upon experimental measurements (34), and the basal rate of O2− synthesis in vasa recta is chosen so that predicted values of interstitial O2− are on the order of 1 nM, as discussed below.
The O2− consumption reactions considered here are the scavenging reactions with NO and with superoxide dismutase (SOD). The rate V5 of the latter reaction is calculated as
| (23) |
The total volumetric consumption rate of O2− in compartment i is given by
| (24) |
As with NO, the fraction of O2− consumed in endothelial or epithelial cells that is attributed to plasma or tubular lumen (Eqs. 2 and 5) is calculated based on plasma or luminal concentrations, whereas the fraction attributed to the interstitium (Eq. 8) is calculated based on interstitial concentrations.
To the best of our knowledge, absolute concentrations of O2− in the medulla have not been reported. We therefore use measurements of its downstream product H2O2, the medullary interstitial concentration of which is ∼100 nM (55), to estimate medullary O2− levels. We assume that at steady state, the volumetric generation rate of H2O2 is approximately counterbalanced by its consumption rate by catalase (neglecting diffusion to/from other compartments and other reactions), that is
| (25) |
The catalase content of the rat liver was estimated as 13 nmol/g wet wt liver (49), that is, ∼13 μM. Based upon a kidney-to-liver catalase activity ratio of 0.4 (56), we estimate the renal concentration of catalase to be on the order of 5 μM. Assuming that 1) the intracellular concentration of SOD is 10 μM (18), 2) kcat equals 3.4 × 107 M−1·s−1 (42), and 3) kSOD, O2− equals 1.6 × 109 M−1·s−1 (3), Eq. 25 suggests that interstitial O2− concentrations are ∼1 nM.
Capillary Endothelial Sources of NO and O2−
Recall that the model represents capillaries that traverse radially across the OM cross sections. Since there are very little quantitative data on the medullary capillary network, we assume that capillary plasma is well mixed with the local interstitium. The capillaries thus essentially carry red blood cells. To account for NO and O2− synthesis by the capillary endothelium, we assume that the latter releases NO and O2− directly into the interstitium (which includes capillary plasma). The maximal volumetric generation rate of NO and O2−, as well as the endothelial thickness, are taken to be the same in vasa recta and in capillaries. We also assume that the capillary luminal diameter is 8 μm. Thus the total cross-sectional area of capillary endothelium in a given region R at a given level x along the OM is given by
| (26) |
where ncap is the number of capillaries per bundle, (−dωSDV/dx) is the rate at which SDV break up into capillaries (see Eq. 43 below), rcap is the capillary radius, and (rR − rR − 1) is the distance between the perimeters of regions R and R − 1.
HbNO Generation and Consumption
HbNO is the product of the reversible reaction between Hb and NO (Eq. 19), and it is sequestered in RBCs. The RBC concentration of HbNO in vessel i is calculated using Eq. 6, with
| (27) |
Active and Basal O2 Consumption
We distinguish between “active” O2 consumption (that is, O2 consumption for active Na+ transport), and “basal” O2 consumption (that is, for the basal metabolism of interstitial, endothelial, and epithelial cells). In mTALs, sodium is actively reabsorbed at the basolateral membrane by Na+-K+-ATPase pumps; given the pump stoichiometry, the number of Na+ moles actively reabsorbed per mole of O2 consumed is taken to be 18 under maximal efficiency. We assume that below a critical Po2 value (denoted Pc), anaerobic metabolism provides a fraction of the energy needed to actively reabsorb Na+. The volumetric rate of active O2 consumption in mTAL epithelia (ΩmTAL, O2active) is calculated as
| (28) |
where ΨmTAL, Naactive denotes the mTAL active Na+ transport rate, and Θ(PmTAL, O2) is the fraction of that transport rate that is supported by aerobic respiration, given by
| (29) |
The meaning of the parameter a is discussed below.
The volumetric rate of basal O2 consumption in the epithelium of tubule i, or the endothelium of vessel i, is calculated as
| (30) |
The maximal volumetric rate of O2 consumption (Ωmax, O2basal) is assumed to be the same in each compartment and is taken as 10 μM/s (8). To account for the inhibitory effects of NO on mitochondrial utilization, the Michaelis-Menten constant (KM, iO2) is taken to vary according to the local NO concentration, i.e.
| (31) |
where KM,no NOO2 is the Michaelis-Menten constant in the absence of NO, and CNOinhib is the NO concentration that doubles KM, iO2; they are respectively taken as 1 mmHg and 27 nM (3).
Active Na+ Reabsorption Across mTAL
The mTAL active Na+ transport rate is generally characterized assuming sodium-dependent Michaelis-Menten kinetics
| (32) |
where Vmax, Na (in mol Na+·m−2·s−1) is the maximal rate of Na+ transport, and KM, Na is the Michaelis-Menten constant. The metabolic requirements for this active process are high, and Na+ transport may become partly limited by insufficient O2 availability below the critical Po2 value. In addition, Na+ reabsorption across mTALs is inhibited by NO and stimulated by O2−. As a simplified approach, the effects of oxygen availability, as well as those of NO and O2−, on ΨmTAL, Naactive are incorporated separately as follows
| (33) |
where Vmax, Nao is a constant. We assume that below Pc, which is taken as 5 mmHg (7), anaerobic metabolism supplies a portion of the energy needed to actively transport Na+ across mTALs. More specifically, we assume that in the complete absence of O2, anaerobic metabolism produces enough ATP to sustain an active Na+ transport rate that is a fraction a (where 0 ≤ a ≤ 1) of the maximum rate when O2 supply is abundant. With this hypothesis
| (34) |
where αO2 is the O2 solubility coefficient, taken as 1.34 μM/mmHg. In the base case, a = 0.5. A value of 0 means that there is no anaerobic metabolism, and a value of 1 means that anaerobic metabolism can fully sustain the maximal mTAL Na+ active transport rate in the absence of O2.
Quantitative data regarding the effects of NO and O2− on NaCl transport across mTALs are very limited. The experiments demonstrating that NO inhibits, and O2− stimulates, NaCl reabsorption were performed in vitro, where some factors (such as the levels of interacting species) were not controlled. By necessity, the way in which we account for these effects is greatly simplified, and the corresponding parameters are ascribed values that are widely uncertain. To account for the inhibitory effect of NO on ΨmTAL, Naactive, we assume that Vmax, Na decreases with increasing NO concentration according to
| (35) |
Ortiz et al. (48) reported that 10 μM spermine NONOate (or SPM, an NO donor) inhibits mTAL Cl− reabsorption by 46%. At a concentration of 10 μM, SPM is expected to result in a bath concentration of 50–60 nM NO (51). Using these data, the constant β is estimated as 46.9 nM.
It has been shown that endogenously produced O2− stimulates mTAL NaCl transport independently of NO. In the absence of l-arginine, the O2− scavenger tempol (50 μM) was found to decrease mTAL Cl− reabsorption by ∼30% after 20-min incubation (46). Since the first-order rate constant for the dismutation of O2− by tempol is 6.5 × 104 M−1·s−1 (29), the concentration of O2− after a 20-min equilibration with tempol should be vanishingly small, and we assume that reducing the mTAL concentration of O2− from a reference value (CmTAL, O2−o) to zero decreases NaCl active transport by 30%. Specifically, we assume that Vmax, Na increases with increasing O2− concentration according to
| (36) |
The reference values (CmTAL, O2−o) are chosen so that h ∼ 1 in the basal configuration. Based upon preliminary simulations, CmTAL, O2−o is taken as 20 pM in case A and 350 pM in case B. The constant VmTAL, Nao was previously estimated as 25.9 nmol/(cm2·s) in the inner stripe, and 10.5 in the outer stripe, in a model that did not account for NO and O2− effects (31). To obtain a two- to threefold increase in the osmolality of the CD fluid between the corticomedullary junction and the boundary between the outer and inner medulla, those VmTAL, Nao values are multiplied by 2.2.
Permeability to NO and O2−
Aquaporin-1 (AQP1) water channels have been shown to transport NO (21). As previously described (16), we use the empirical correlation obtained by Herrera et al. (21) to estimate the permeability of tubule or vessel i (Pi, NO) to NO, given the basal RBC permeability to NO (PNOrbc, basal)
| (37) |
where Pi, f is the water permeability of tubule or vessel i. In the vessels and tubules that do not express AQP1 (i.e., AVR, ascending limbs, and CDs) Pi, f is taken as zero in Eq. 37.
Even though the lipid bilayers are almost impermeable to O2− (19), chloride channels have been shown to mediate O2− transport in endothelial cell plasma membranes (43). In the absence of more specific data, we assumed that the permeability of OM tubules and vessels to O2− is 5 × 10−4 cm/s.
The effective permeability to solute k (k = NO, O2−) of the boundary separating regions R and R′ is estimated as
| (38) |
where AF, R, R′ is the fraction of the R-R′ interface available for interstitial diffusion, Dk is the diffusivity of solute k in dilute solution, and dR, R′ is the distance between the midpoints of regions R and R′. The parameter γ is a diffusion resistance which accounts for the hindering effects of macromolecules and cells in the interstitium. We assume that γ equals 5 for both NO and O2−, based upon measured membrane-to-dilute solution NO diffusivity ratios (15). The factor τ represents the effect of tortuosity on the diffusion path length around tubules and vessels and is taken as π/2 (31). The diffusivity of NO and O2− in dilute solution is taken as 3,300 and 2,800 μm2/s, respectively (6, 38).
Boundary Conditions
The boundary conditions for the flows of water, Na+, urea, O2, Hb, and HbO2 were described previously (8). The concentrations of NO and O2− are specified at the corticomedullary junction in descending vessels and tubules (Table 1). They must also be prescribed in long ascending limbs (LAL) and long ascending vasa recta (LAV) at the boundary between the inner and outer medulla (i.e., at x = L). To do so, we assume that NO and O2− molar flow rates at x = L are equal in long ascending and descending limbs, and in long ascending and descending vasa recta. Thus the concentration of solute k (k = NO and O2−) in LAL fluid and LAV plasma at x = L is obtained by solving the following equations.
Table 1.
Vessel and tubule dimensions
| Vessel or Tubule | Inner Diameter, μm | Endothelial/Epithelial Layer Thickness, μm | Permeability to Water, μm/s |
|---|---|---|---|
| Long and short descending vasa recta | 11 | 1.0 | 1,257 |
| Long and short ascending vasa recta | 28 → 10 | 1.0 | * |
| Long descending limbs | 80 in OS | 1.1 | 3,570 in OS |
| 21 → 16 in IS | 2,295 in IS | ||
| Short descending limbs | 21 → 16 | 1.1 | 3,570 in OS |
| 3,257 in IS | |||
| Long ascending limbs | 20 | 8.0 | 0 |
| Short ascending limbs | 21 → 10 | 8.0 | 0 |
| Collecting ducts | 31 → 22 | 9.0 | 463 |
Diameters and water permeabilities are taken from Ref. 31, thicknesses from Ref. 8. IS, inner stripe. The arrow indicates that the parameter value decreases linearly along the x-axis (from x = 0 to x = L, unless indicated otherwise). The large diameter of the long descending limbs in the outer stripe (OS) represents the tortuosity of the proximal straight tubules.
Ascending vasa recta (AVR) water permeability is not given because AVR water fluxes are computed based on fluid accumulation into a region.
Long ascending limbs.
| (39) |
Long vasa recta.
| (40a) |
| (40b) |
We also assume that the RBC-to-plasma concentration ratio for solute k (k = NO and O2−) is the same in long ascending and descending vasa recta at x = L
| (41) |
The RBC concentration of HbNO is specified as 1 μM in descending vasa recta at the corticomedullary junction. This estimate is in the midrange of reported values (24, 30). At the boundary between the outer and inner medulla, the molar flow rate of HbNO leaving LDV is taken to be equal to that entering LAV
| (42a) |
| (42b) |
Parameter Values
The dimensions of tubules and vessels are given in Table 1, those of interstitial regions in Table 2. Shown in Table 3 are NO and O2− generation rates, permeabilities, and inlet boundary conditions. Kinetic parameters are listed in Table 4. The fraction of short vasa recta present at level x is given by
| (43) |
where LOS is the outer-stripe length (0.6 mm), and L is the total OM length (2.0 mm).
Table 2.
Region parameters
| R1 | R2 | R3 | R4 | |
|---|---|---|---|---|
| Region radii, μm | 36.7–42.4 | 225–121 | 348–191 | 388–226 |
| Interstitial areas, ×10−6 cm2 | 2.41–2.26 | 87.8–16.2 | 126–137 | 53.2–91.6 |
| Fractional area available for diffusion, AF, R, R+l | 0.0560–0.0398 | 0.0560–0.120 | 0.0560–0.199 | |
| Area of capillary endothelial compartment, ×10−6 cm2 | 0.0424–0.666 | 0.435–2.47 | 0.284–2.20 | 0.0924–1.10 |
Table 3.
NO and O2− transport parameters
| Descending Vasa Recta | Ascending Vasa Recta | Descending Limbs | Ascending Limbs | Collecting Ducts | |
|---|---|---|---|---|---|
| Maximal epithelial/endothelial NO generation rate, Gi,NOmax, μM·s−1 | 76.6 | 72.2* | 12.3 | 0.985 | 0.415 |
| Epithelial/endothelial O2− generation rate, Gi,O2−basal, μM·s−1 | 21.0 | 19.8* | 15.2 | 3.2 | 1.8 |
| NO permeability†, cm/s | 0.496 | 0.0122 | 0.896 → 1.387‡ | 0.0122 | 0.0122 |
| O2− permeability†, cm/s | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.0005 |
| NO concentration at inlet, nM | RBC: 0.1 | N.A. | 100 | NA | 100 |
| Plasma: 100 | |||||
| O2− concentration at inlet, nM | RBC: 0.1 | N.A. | 0.02 | NA | 0.02 |
| Plasma: 0.1 |
NO, nitric oxide; O2−, superoxide; NA, not applicable.
The AVR rate is obtained by multiplying the DVR rate by (1 − ffen), where ffen is the fraction of the AVR surface that is fenestrated (taken as 0.057, based on Ref. 35).
Permeability values correspond to the vascular or tubular wall. The red blood cell (RBC) permeability to NO is taken as 0.1 cm/s in the base case.
Depending on the descending limb portion (note that the NO permeability scales with water permeability).
Table 4.
Reaction kinetic parameters
| Parameter Definition | Parameter Value |
|---|---|
| NO-O2 reaction rate constant kO2, M−2·s−1 | 6.3 × 106 |
| NO-O2− reaction rate constant kNO-Sup, M−1·s−1 | 6.7 × 109 |
| NO-HbO2 reaction rate constant koxy, M−1·s−1 | 2.5 × 107 |
| NO-Hb reaction rate constants kdeoxy, M−1·s−1 and krev, s−1 | 2.5 × 107 and 10−4 |
| O2−-SOD reaction rate constant kSOD, M−1·s−1 | 1.6 × 109 |
| SOD concentration, μM | 5 |
HbO2, oxyhemoglobin.
Numerical Methods
The steady-state differential equations are discretized to form a system of nonlinear algebraic equations. A spatial discretization of 200 grid points along the medullary axis and 50 grid points in the radial direction is used. The system of coupled, nonlinear conservation equations is solved using MINPACK, a numerical package implemented in FORTRAN for solving nonlinear equations with a modification of Powell's hybrid algorithm, in an iterative approach described in a previous study (8).
RESULTS
Base-Case Description
In baseline simulations, the RBC permeability to NO is kept constant, and the O2− generation rate is taken to vary in parallel with Po2 (case A). In other words, the base case does not incorporate hypoxia-induced increases in NO release and in O2− generation. These effects are examined further below.
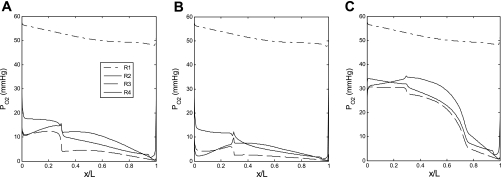
Po2 profiles are similar to those described in our recent studies (7). Briefly, our model predicts that the OM architecture results in significant Po2 gradients both in the axial and the radial directions (Fig. 2A). The segregation of long DVR at the center of the vascular bundles (in region R1) preserves enough O2 for delivery to the inner medulla (IM), while the high metabolic requirements of mTALs in the peripheral regions (R2–R4) significantly deplete O2 therein.
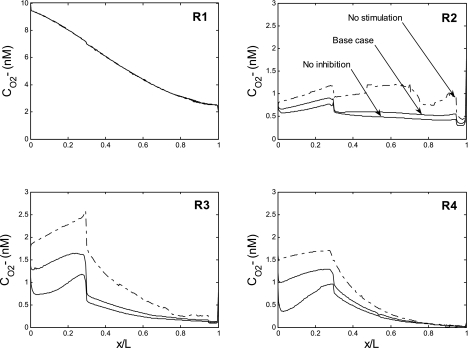
Fig. 2.
Oxygen tension (Po2) profiles in the interstitium of the 4 regions (R1–R4) in the base case (A), assuming that nitric oxide (NO) does not inhibit medullary thick ascending limb (mTAL) NaCl reabsorption (B), and assuming that O2− does not stimulate mTAL NaCl reabsorption (C). x/L denotes the ratio of the axial coordinate to total length of outer medulla. In all cases, the red blood cell (RBC) permeability to NO (PNOrbc) is kept constant, and the O2− generation rate (GO2−) is taken to vary in parallel with Po2.
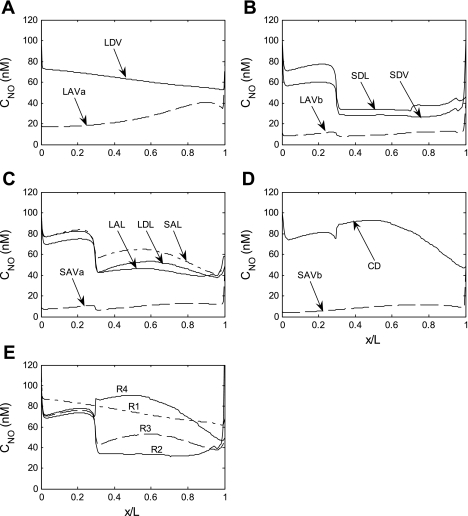
NO concentration (CNO) profiles are shown in Fig. 3. As described in our previous model of transport in cross sections of the rat OM (16), the large radial Po2 gradients in turn generate substantial radial NO and O2− concentration gradients, since the synthesis of both solutes is O2 dependent. Vasa recta endothelial cells are by far the largest source of NO, but RBCs constitute a sink for NO because hemoglobin scavenges NO at a very rapid rate (∼100 times faster than O2− does), and the RBC permeability to NO is relatively high. Thus, without the rate-limiting effects of O2 on NO generation, CNO would be lowest in the vascular bundle core, that is, the region with the highest density of RBCs. When those rate-limiting effects are taken into account, CNO is predicted be significantly higher in R1 (the region with the highest Po2) than in R2 and R3 (the regions with the poorest O2 availability) in the inner stripe (Fig. 3E).
Fig. 3.
Base case NO concentration (CNO) in tubules, vasa recta, and interstitia. A–D: regions R1, R2, R3, and R4, respectively; tubules are assigned to the region with which they are in contact for 50% or more of their inner stripe length. E: CNO profiles in the interstitium of the 4 regions.
In the outer stripe (OS) of the OM, several classes of tubules straddle two peripheral regions (see Fig. 1B), leading to significant mixing between R2, R3, and R4. Thus interstitial CNO is very similar in those three regions. It is slightly higher in R4, a region with relatively high Po2 levels and few vessels (i.e., few NO sinks). In the inner stripe (IS), the external regions are more segregated, leading to sharper CNO differences between regions.
The sharp rise in interstitial CNO in R4 at the OS-IS junction is caused by tubule migration. As illustrated in Fig. 1, the short descending limbs are near CDs in the OS (i.e., they straddle R3 and R4), but move to the immediate periphery of the vascular bundle (i.e., to R2) in the IS. These short (and thin) descending limbs generate less NO than the other tubular and vascular structures in R4. Thus, in the OS, NO diffuses from the R4 interstitium into their lumen, and when this flux abruptly ceases at the OS-IS junction, interstitial CNO increases sharply in R4. If the position of these short descending limbs were to be maintained constant in the OM, there would be no such sharp increase (results not shown). In the deep IS, R4 Po2 drops significantly, and so does the interstitial CNO in R4.
In R2 and R3, by contrast, interstitial CNO drops suddenly at the OS-IS junction. Indeed, due to an increase in the maximal rate of Na+ active transport across mTALs at the junction, Po2 levels fall significantly in R2 and R3 (see Fig. 2A), thereby greatly reducing NO generation in those regions. In addition, long descending limbs are taken to be very large in the OS (to account for the tortuosity of proximal tubules), and thus constitute a significant source of NO therein; their diameter decreases sharply at the OS-IS junction, which further reduces NO generation in R2–R3. Even though Po2 is higher in R2 (where there are fewer mTALs), interstitial CNO is higher in R3 than in R2 mainly because of the short ascending limbs: the latter tubules straddle R3 and R4 and receive a substantial amount of NO from R4, which then diffuses from the short ascending limb lumen in the R3 interstitium. Note that along the corticomedullary axis, and within each stripe, CNO generally rises and falls with Po2.
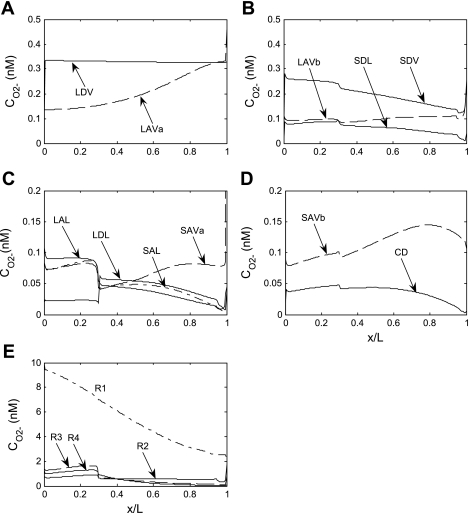
Superoxide concentration (CO2−) profiles are illustrated in Fig. 4. There is as yet no evidence that AQP1 is permeable to O2−, and vascular and tubular permeabilities to O2− are taken to be significantly lower than those to NO. Thus transmembrane CO2− gradients are predicted to be significant, and in a given region, CO2− is significantly higher in the interstitium than in tubular lumen or plasma. In the base case, the O2− generation rate is assumed to decrease with decreasing Po2, and close examination of the curves reveals that interstitial CO2− profiles closely track Po2 profiles. Note that CO2− is more elevated in R1 than in the peripheral regions not only because Po2 is higher in the vascular bundle core but also because the model postulates that there is much less water accumulation, and therefore less dilution, therein. The sharp decrease in interstitial CO2− at the OS-IS junction in the interbundle region stems from the sudden diminution in the long descending limb diameter in R2 and R3, as well as the migration of short descending limbs out of R4 (Fig. 4).
Fig. 4.
Base case O2− concentration (CO2−) in tubules, vasa recta, and interstitia. A–D: regions R1, R2, R3, and R4, respectively. E: CO2− profiles in the interstitium of the 4 regions. Note the different y-axis scales.
One measure of the concentrating capacity of the OM is the osmolality of the tubular fluid in the CD at the OM-IM junction (denoted osmCDL hereafter). Under basal conditions, the latter equals 787 mosmol/kgH2O (Table 5).
Table 5.
Effects of NO and O2− on the concentrating capacity of the OM
| osmCDL, mosmol/kgH2O |
|||
|---|---|---|---|
| Case A | Case B | Case C | |
| Base case | 787 | 878 | 729 |
| Without the NO/O2− reaction | 770 | 714 | 708 |
| Without NO-mediated inhibition of mTAL active transport | 1,333 | 1,398 | 1,328 |
| Without O2−-mediated stimulation of mTAL active transport | 493 | 584 | 453 |
osmCDL, osmolality of the collecting duct (CD) tubular fluid at the boundary between the outer (OM) and inner medulla; case A, O2− synthesis is taken to decrease with decreasing Po2 (Eq. 22a), and the RBC permeability to NO (PNOrbc) is taken to remain constant; case B, O2− synthesis is taken to increase with decreasing Po2 (Eq. 22b), and PNOrbc is taken to remain constant; case C, O2− synthesis is taken to decrease with decreasing Po2 (Eq. 22a), and PNOrbc is taken to vary with Po2 (Eq. 21); mTAL, medullary thick ascending limb.
Effects of Hypoxia
As described above, the mechanisms by which hypoxia modulates medullary NO and O2− levels remain uncertain. To incorporate the effects of hypoxia on CNO in a simple manner, we assumed that the RBC permeability to NO (PNOrbc), and thus the strength of the RBC sink, decrease with decreasing Po2 (Eq. 21). With this hypothesis, interstitial CNO is predicted to rise significantly relative to the base case in the peripheral, O2-starved regions (R2–R4), and to decrease in the central, O2-rich region (R1), as displayed in Fig. 5. Higher NO levels in mTALs result in greater inhibition of Na+ transport, and thus a diminished concentrating capacity; osmCDL, is predicted to be 729 mosmol/kgH2O in this case.
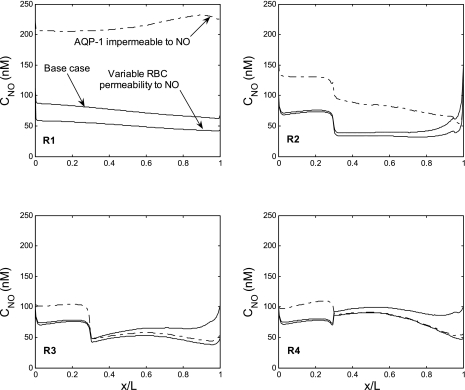
Fig. 5.
CNO profiles in the interstitium of the 4 concentric regions (R1–R4). In the base case (solid curves), PNOrbc remains constant, and aquaporin-1 (AQP1) is permeable to NO. In the second case (dotted curves), PNOrbc is taken to increase with decreasing Po2. In the third case (dash-dotted curves), AQP1 is taken to be impermeable to NO.
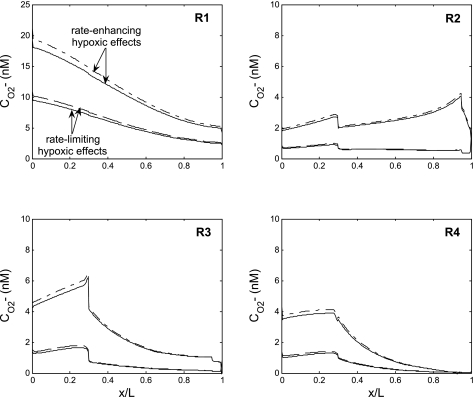
As to the effects of hypoxia on O2−, if we assume that a low Po2 enhances rather than limits O2− generation (as described by Eq. 22b), the predicted CO2− is then two to three times higher than in the base case (Fig. 6). Since the volumetric generation rate of O2− is highest in vasa recta and descending limbs, interstitial CO2− varies in proportion to the fractional area occupied by vasa recta and descending limbs within each region, as previously described (16). In the OS, this fractional area is highest in R1 and lowest in R2, and so is CO2−. In the deep IS, the relative area occupied by vasa recta and descending limbs decreases monotonically from R1 to R4, and so does interstitial CO2−. The CD fluid osmolality at the OM-IM junction is predicted to be 878 mosmol/kgH2O in this case.
Fig. 6.
CO2− profiles in the interstitium of the 4 concentric regions (R1–R4), assuming a fixed PNOrbc. The solid and dashed curves, respectively, depict CO2− with and without O2− scavenging by NO, assuming that the O2− generation rate (GO2−) decreases with decreasing Po2. The dotted and dash-dotted curves, respectively, depict CO2− with and without O2− scavenging by NO, assuming that GO2− is enhanced rather than limited by low medullary Po2. CO2− increases moderately in the absence of the NO-O2− reaction because SOD is the main O2− scavenger.
Direct NO-O2− Interactions
To assess the impact of direct NO-O2− interactions, we then set the NO-O2− reaction rate to zero. Simulations were performed for three cases: GO2− and PNOrbc were taken to be fixed or to vary with Po2. In all three scenarios, predicted O2− concentrations rose by 10% at most (Fig. 6), because the rate of O2− consumption by SOD is significantly faster than that by NO.
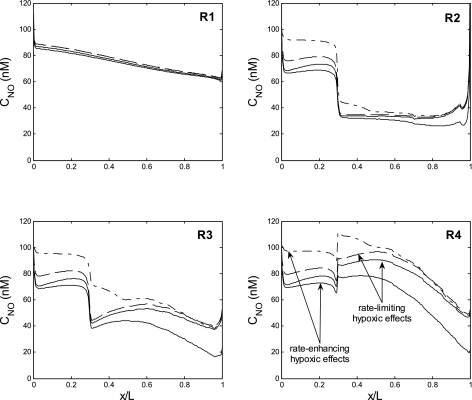
The extent to which CNO is modulated by the NO-O2− reaction depends on O2− levels, as displayed in Fig. 7. If GO2− and CO2− are low to start with (as in the base case), eliminating the NO-O2− reaction raises CNO by 2–10 nM, and osmCDL drops slightly, from 787 to 770 mosmol/kgH2O (Table 5). If CO2− is two to three times higher (as when GO2− is enhanced by low medullary Po2), eliminating the NO-O2− reaction raises CNO by up to 30 nM in R3–R4, i.e., those regions where the major NO scavenger, hemoglobin, is the least predominant. In that case, osmCDL is predicted to decrease by 20%, from 878 to 714 mosmol/kgH2O (Table 5).
Fig. 7.
CNO profiles in the interstitium of the 4 regions, assuming a fixed PNOrbc. The solid and dashed curves, respectively, depict CNO with and without NO consumption by O2−, assuming that GO2− decreases with decreasing Po2. In the absence of the NO-O2− reaction, CNO increases by <10 mM relative to the base case. The dotted and dash-dotted curves, respectively, depict CNO with and without NO consumption by O2−, assuming that GO2− is elevated under hypoxic conditions. Under that assumption, O2− scavenging of NO is predicted to significantly impact NO levels in the peripheral regions. (Note that the dashed and dash-dotted lines cannot be distinguished in R1.)
NO-Mediated Inhibition of mTAL Sodium Reabsorption
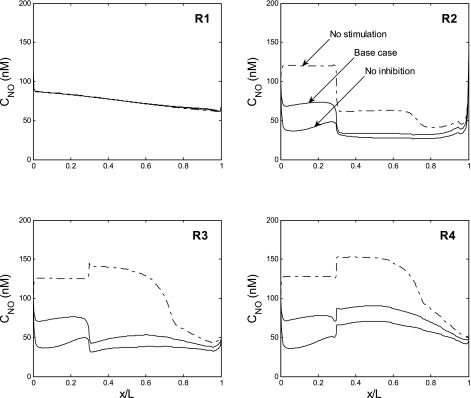
To determine the extent to which NO-induced inhibition of mTAL NaCl reabsorption affects the concentrating capacity of the OM and its oxygenation, we performed simulations in which NO effects on NaCl transport were abolished. That is, we set g(CmTAL, NO) = 1 in Eq. 33. In the absence of NO-mediated inhibition, the rates of NaCl reabsorption and O2 consumption are predicted to both rise markedly relative to the base case. In R2 and R3, Po2 drops below the critical pressure throughout most of the IS (Fig. 2B). The rate of NaCl active transport can nevertheless increase substantially because of anaerobic metabolism. The model predicts that the concentrating capacity of the OM rises by 70%, that is, osmCDL increases from 787 to 1,333 mosmol/kgH2O (Table 5). In the vascular bundle core (R1), Po2 remains relatively unchanged and oxygen delivery to the IM is preserved.
Since NO generation decreases with decreasing Po2, CNO is then significantly lower in R2–R4, relative to the base case (Fig. 8). Under these conditions, CNO remains higher in R1 than in R2–R4 along the entire medullary axis.
Fig. 8.
CNO profiles in the interstitium of the 4 regions, in the base case (solid curves), assuming that NO does not inhibit mTAL NaCl reabsorption (dotted curves), and assuming that O2− does not stimulate mTAL NaCl reabsorption (dash-dotted curves). Profiles were obtained assuming that PNOrbc is constant and that GO2− varies in parallel with Po2.
Similar trends are obtained if we assume that the RBC permeability to NO decreases with decreasing Po2, as described by Eq. 21. Eliminating NO-mediated inhibition of mTAL transport raises osmCDL by 80% in that case (Table 5). Even though the Po2 drop in R2–R4 reduces RBC removal of NO therein, the concomitant decrease in NO generation predominates and CNO is also predicted to decrease in the peripheral regions under this assumption (results not shown).
Given that NO scavenges O2−, an isolated reduction in CNO should raise CO2−. However, assuming that O2− generation decreases with decreasing Po2, the reduction in O2− consumption is accompanied by a greater reduction in O2− production, and CO2− is predicted to decrease in R2–R4 when NO-mediated inhibition of mTAL transport is eliminated (Fig. 9). The decrease is more pronounced in the OS (20–50%) than in the IS (10–20%) because Po2 drops more sharply in the upper OM. Conversely, if we were to assume fixed O2− generation rates, CO2− would increase by ∼5% in the peripheral regions in the absence of NO effects on NaCl reabsorption, given the concomitant reduction in the O2− consumption rate (results not shown).
Fig. 9.
CO2− profiles in the interstitium of the 4 regions, in the base case (solid curves), assuming that NO does not inhibit mTAL NaCl reabsorption (dotted curves), and assuming that O2− does not stimulate mTAL NaCl reabsorption (dash-dotted curves). Profiles were obtained assuming that PNOrbc is constant and that GO2− varies in parallel with Po2.
O2−-Mediated Activation of mTAL Sodium Reabsorption
As opposed to NO, O2− stimulates NaCl reabsorption across the mTAL. In the next set of simulations, we removed these O2−-induced effects to assess their importance. That is, we set h(CmTAL, NO) = 0.70 in Eq. 33.
In the absence of O2−-mediated stimulation of active transport, the rate of O2 consumption diminishes, interbundle Po2 levels increase, and so does NO production in R2–R4. Thus NO-induced inhibition of mTAL active transport rises in turn, thereby increasing Po2 levels further and exerting a positive feedback loop. Nevertheless, the limited O2 supply to the interbundle region halts the Po2 and NO increase therein. Given this positive feedback loop, the concentrating capacity of the OM is predicted to be significantly lower relative to the base case: osmCDL is then equal to 493 (vs. 787) mosmol/kgH2O (Table 5).
Without O2−-mediated stimulation of NaCl reabsorption, Po2 is predicted to hover above 20 mmHg in all regions all the way down to the mid-IS (Fig. 2C). With or without hypoxia-mediated effects on NO release, interstitial CNO is then predicted to remain higher in R3–R4 than in R1 throughout most of the medulla (Fig. 8). Indeed, as noted above, without the rate-limiting effects of O2 on NO generation rates, CNO is predicted to be the lowest in the vascular bundle core, where the relative density of Hb-carrying blood vessels is the highest.
When O2− effects on NaCl transport are eliminated, the Po2 elevation translates into an increase in O2− generation, assuming that O2− generation increases in parallel with Po2, so that interstitial CO2− rises by ∼50% in R2–R4 (Fig. 9). However, if O2− generation is taken to be fixed, the increase in O2− consumption due to higher NO levels causes CO2− to drop by ∼5% in the interbundle region (results not shown).
Contribution of Anaerobic Metabolism
In all the preceding simulations, we assumed that glycolysis provides a substantial fraction of the energy needed to actively reabsorb NaCl across mTALs when Po2 drops below the critical pressure. How would our predictions differ in the absence of anaerobic metabolism (i.e., if the parameter a in Eqs. 28 and 29 were equal to 0 instead of 0.5)? The active transport rate would then be considerably limited by the hypoxic conditions that prevail in the renal medulla, and all else being equal, the concentrating capacity of the OM would greatly diminish.
Specifically, assuming that a = 0, osmCDL drops to 621 mosmol/kgH2O under basal conditions (vs. 787 with a = 0.5). Abolishing NO-mediated inhibition of mTAL transport has a small impact on NaCl reabsorption, because the supply of O2 in the interbundle region is not sufficient to support significantly greater metabolic needs by itself. Thus, with a = 0, osmCDL only increases by 5% (to 655 mosmol/kgH2O) when NO-mediated inhibition of mTAL transport is eliminated. In contrast, in the absence of O2−-mediated stimulation of NaCl reabsorption, Po2 reaches comparable levels with and without anaerobic metabolism, NO and O2− concentration profiles are similar, and so is osmCDL (464 mosmol/kgH2O if a = 0, vs. 493 if a = 0.5).
AQP1-Mediated NO Transport
The base case assumes that AQP1 transports NO, as observed experimentally (21). Nonetheless, there is some controversy as to whether AQP1 is indeed permeable to small gases such as CO2, NH3, and NO (57). We performed simulations in which AQP1 was taken to be impermeable to NO: the NO permeability of the vessels and tubules that express AQP1 in the OM, namely, DVR and descending limbs, was set to 0.0122 cm/s, equal to that of other vessels and tubules (Table 3). As displayed in Fig. 5, in the absence of NO transport via AQP1, interstitial NO concentrations are predicted to increase significantly relative to the base case. Elevations are most pronounced in the core (R1) and immediate periphery (R2) of the vascular bundle, where all DVR, which represent the largest volumetric source of NO, are located. Interstitial CNO increases because as the resistance to NO diffusion from endothelium to plasma increases, a smaller fraction of NO makes its way into RBCs, and more NO is preserved elsewhere. The subsequent decrease in mTAL NaCl reabsorption reduces osmCDL from 787 to 736 mosmol/kgH2O (Table 6).
Table 6.
Effects of luminal flow and O2− synthesis on the concentrating capacity of the OM
| osmCDL, mosmol/kgH2O | |
|---|---|
| Base Case | 787 |
| Without anaerobic metabolism | 621 |
| Without anaerobic metabolism and no mediated inhibition of mTAL active transport | 655 |
| Without anaerobic metabolism and no mediated stimulation of mTAL active transport | 464 |
| Without facilitated NO transport via AQP1 | 736 |
| With a 20% increase in inlet water flows | 648 |
| With a 20% increase in inlet water flows and in GNO | 575 |
| With a 20% increase in inlet water flows, in GNO and in GO2− | 591 |
| With a 10-fold increase in GO2− | 1,073 |
| With a 10-fold increase in GO2− and a 25% decrease in GNO | 1,175 |
| With a 10-fold increase in GO2−and a 50% decrease in GNO | 1,297 |
| With a 10-fold increase in GO2−and a 50% decrease in TNa/Qo2 | 935 |
| With a 10-fold increase in GO2−, a 50% decrease in TNa/Qo2, and a 25% decrease in GNO | 1,020 |
| With a 10-fold increase in GO2−, a 50% decrease in TNa/Qo2, and a 50% decrease in GNO | 1,124 |
AQP1, aquaporin-1; GNO and GO2− are the volumetric rates of NO and O2− synthesis, respectively; TNa/Qo2, ratio of transported sodium to oxygen consumption. In all these simulations, GO2− is taken to decrease with decreasing Po2, and PNOrbc is fixed.
Flow-Induced Endothelial Nitric Oxide Synthase Activation
Studies have shown that increased luminal flow activates endothelial nitric oxide synthase (eNOS) and enhances NO production in DVR and TALs (47, 62). Luminal flow also stimulates O2− production in TALs (26). Flow-induced effects on O2− are partly inhibited by NO via a nonscavenging mechanism, as discussed below. To determine whether eNOS and NADPH oxidase activation by luminal flow plays an important role in the regulation of OM Na+ reabsorption, we performed simulations in which inlet volume flows (i.e., in DVR, descending limbs, and CD at x = 0) and maximal NO and O2− synthesis rates were simultaneously increased by 20%. Vasa recta express both eNOS and neuronal NOS (nNOS) (40), but in the absence of specific data on the distribution of these enzymes, we raised NO (and O2−) volumetric generation rates by 20% everywhere.
We first examined the isolated effects of increasing inlet volume flows on the OM concentrating mechanism. An increase in medullary perfusion augments O2 availability and thereby stimulates NaCl reabsorption, but this effect is more than counterbalanced by two opposite forces: a Po2-induced increase in NO synthesis, which acts to inhibit NaCl active transport, and higher loads, which mean that larger fluid flows must be concentrated. Thus the OM concentrating capacity is predicted to decrease relative to the base case. As shown in Table 6, an isolated 20% increase in inlet volume flows is calculated to lower osmCDL by 18%, from 787 to 648 mosmol/kgH2O.
When inlet volume flows and maximal NO synthesis rates (i.e., Gi, NOmax in Eq. 15) are both increased by 20%, CNO rises further, and osmCDL is predicted to drop even more, to 575 mosmol/kgH2O (Table 6). When inlet volume flows, NO synthesis rates, and O2− synthesis rates (i.e., Gi, O2−basal in Eq. 22a) are all increased by 20%, O2− exerts greater compensating effects on mTAL transport, and osmCDL climbs slightly, to 591 mosmol/kgH2O (Table 6). Together, these results suggest that an increase in tubular and vascular flows substantially reduces the OM axial osmolality gradient.
Hypertensive Conditions
Medullary infusions of the SOD inhibitor DETC have been shown to induce hypertension in rats: DETC induced an eightfold increase in interstitial CO2−, with a subsequent 40% decrease in medullary blood flow and a nearly 20-mmHg increase in blood pressure (37). To dissect some of the underlying mechanisms, we raised maximal O2− generation rates in the OM by a factor of 10. As expected, the higher levels of O2− stimulate NaCl reabsorption and O2 consumption, thereby reducing Po2 and CNO. In the interbundle region, Po2 is predicted to drop by ∼5–10 mmHg at the mid-OS and ∼2 mmHg at the mid-IS.
Per se, a 10-fold increase in O2− concentration raises osmCDL by ∼300 mosmol/kgH2O, i.e., by 35% (Table 6). The increase is limited because 1) the effects of O2− on NaCl reabsorption across the mTAL are described using a saturable expression (Eq. 33) and 2) NO still exerts significant inhibitory effects on this NaCl transport pathway.
The 35% increase in osmCDL is predicted assuming that the number of Na+ moles actively reabsorbed per mole of O2 consumed (i.e., the mTAL TNa/Qo2 ratio) remains fixed at 18. There is some evidence, however, that NO and/or reactive oxygen species (ROS) modulate the amount of O2 consumed per Na+ ion transported, as discussed below. However, the explicit impact of NO and ROS on TNa/Qo2 in the mTAL has not been measured, to the best of our knowledge. If we assume that this ratio is halved (based on data from spontaneously hypertensive rats in Ref. 59) when O2− concentrations increase 10-fold, our model then predicts a smaller (20%) increase in osmCDL (Table 6).
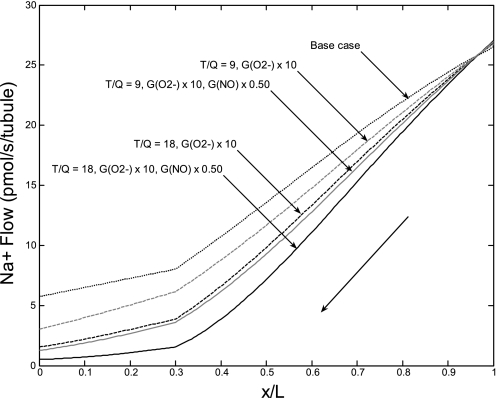
Some models of hypertension, such as the Dahl salt-sensitive rat, are also characterized by a decrease in NO production (39). In the absence of specific data, we simulated graded reductions (25 and 50%) in NO synthesis in parallel with the 10-fold increase in O2− levels. As expected, the greater the reduction in GNO, the faster NaCl transport across mTALs, and the higher the concentrating capacity of the OM (Table 6). The impact of the GO2− increase, GNO decrease, and TNa/Qo2 variations on NaCl reabsorption along the long ascending limb (LAL) is illustrated in Fig. 10. The Na+ flow at the LAL inlet (at x = L) is very similar in all cases, ∼27 pmol/s (per tubule). In the base case, it decreases to 5.8 pmol/s at the corticomedullary junction (x = 0). If the O2− synthesis rate is multiplied by 10 and Na+ reabsorption thereby stimulated, it decreases much more rapidly. If TNa/Qo2 remains equal to 18, the LAL Na+ flow decreases to 1.6 and 0.5 pmol/s at x = 0, assuming no change and a 50% decrease in GNO, respectively. If TNa/Qo2 drops to 9, it decreases slightly less, to 3.0 and 1.3 pmol/s, respectively.
Fig. 10.
Na+ flow (in pmol·s−1·tubule−1) along the LAL. The thick arrow indicates the direction of the flow, from the OM-inner medullary (IM) boundary (x/L = 1) to the corticomedullary junction (x/L = 0). The black dotted line represents the base case. In all other cases, GO2− is multiplied 10-fold to mimic hypertensive conditions. The ratio of transported sodium to oxygen consumption (TNa/Qo2) is taken as 18 (black curves) or 9 (grey curves), and the NO synthesis rate (GNO) is either maintained constant (dashed lines) or halved (solid lines). NaCl reabsorption is maximal when the Na+ flow at x = 0 is the lowest.
DISCUSSION
Feedback Mechanisms
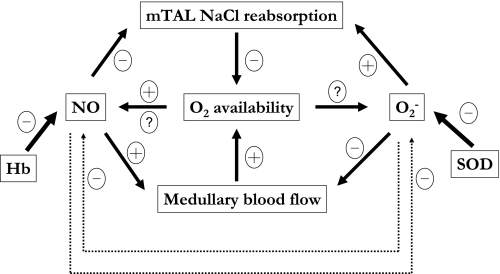
Our results suggest that NaCl reabsorption across mTALs and the concentrating capacity of the OM are substantially modulated by NO and O2−. Moreover, the effect of each solute on NaCl transport in the OM cannot be considered in isolation, given the feedback loops resulting from the reciprocal interactions between O2, NO, and O2−, which are summarized in Fig. 11.
Fig. 11.
Feedback mechanisms involving NO, O2−, and O2. NO synthesis is O2 dependent, but RBC trapping of NO may also decrease under hypoxic conditions, via uncertain mechanisms. NO is scavenged very rapidly by hemoglobin (Hb), and to a smaller extent by O2−. The relationship between O2− synthesis and Po2 remains unclear. Superoxide dismutase (SOD) consumes O2− at a faster rate than NO does. As a vasodilator, NO enhances medullary perfusion and O2 supply, whereas O2− exerts opposite effects. NO inhibits NaCl reabsorption by the thick ascending limb and therefore O2 consumption, whereas O2− stimulates both. Not shown on the diagram are the many other paracrine agents (such as endothelins and prostaglandins) that also act on vascular contraction and tubular NaCl reabsorption in the renal medulla.
The net production rate of NO and O2− is oxygen dependent. Reciprocally, NO and O2− affect oxygen availability in two ways: by modulating active transport across mTAL cells and therefore O2 consumption, and by regulating vessel contraction and therefore O2 supply. Our current model does not take into consideration vessel diameter changes, i.e., vasoactive effects are not accounted for in this study. In the absence of such vasoactive effects, our model predicts that O2−-mediated stimulation of NaCl reabsorption raises the OM concentrating capacity (evaluated as the tubular fluid osmolality in collecting ducts at the OM-IM boundary) by >50% under basal conditions. Note that the numerical values in this study are necessarily approximations, given the significant uncertainty associated with several key parameters (see above). Conversely, NO-induced inhibition of NaCl reabsorption is predicted to decrease the OM concentrating capacity by >50% (Table 5). Our prediction that active transport of NaCl would substantially increase in the absence of NO is predicated on the hypothesis that anaerobic metabolism can supply a significant fraction of the energetic requirements of mTALs. Without glycolysis, NaCl transport would only increase by 5% in the absence of NO (Table 6), because there isn't enough O2 in the interbundle region to support per se significantly greater metabolic needs.
If NO didn't inhibit NaCl active transport across mTALs, faster transport rates and therefore enhanced O2 consumption would reduce Po2, which in turn would lower NO production. The resulting decrease in CNO should then lead to vasoconstriction, which would further reduce Po2 by limiting O2 supply. However, several mechanisms would then put a break on this positive feedback loop. Hypoxic-induced NO release should partly counteract the reduction in NOS-mediated NO synthesis, as discussed above. Moreover, the medullary microcirculation is controlled by many signaling molecules. In particular, adenosine and prostaglandins, like NO, act both as saliuretic agents and as paracrine vasodilators; such agents would most likely exert mitigating effects so as to preserve medullary perfusion and raise medullary Po2. Similarly, activation of KATP channels due to reduction of intracellular ATP might hyperpolarize vasa recta pericytes to favor vasodilatation (5).
Conversely, without O2−-mediated stimulation of sodium reabsorption and O2 consumption, elevation of Po2 would favor a rise in CNO and vasodilation, tending to enhance O2 supply. In this case, however, active transport by the mTAL would also tend to increase, since it is partly limited by O2 availability under basal conditions. Thus a rise in mTAL O2 consumption would be favored, tending to offset the increase in O2 supply. Finally, it seems plausible that other paracrine agents such as endothelins or vasoconstrictor prostaglandins might be released to limit such NO-dependent vasodilation. Given the complex nature of events that balance O2 supply and consumption, we formulated the current model to facilitate prediction of the net effect of such interactions.
Impact of NO on O2− Bioavailability
Our model predicts that NO scavenging reduces O2− levels by ∼10% in the renal medulla. As recently observed by Hong and Garvin (25), flow-induced enhancement of O2− production in the TAL is reduced in the presence of NO, but this effect cannot be attributed to scavenging only. Approximately 70% of the inhibitory effect of NO on net O2− production appears to be mediated via the cGMP/PKG pathway (25). These novel findings suggest that NO and O2− may interact in more complex ways than previously thought. It is not presently known under which conditions, and precisely how, the cGMP/PKG pathway leads to inhibition of net O2− production. In the absence of data, we did not incorporate this pathway in our model.
Impact of O2− on NO Bioavailability
The impact of basal O2− levels on NO bioavailability in the OM remains to be fully ascertained, in part because there have been until now no direct measurements of medullary O2− levels. In a recent mathematical model of NO-O2− interactions in OM cross sections (17), we showed that if O2− is present in subnanomolar concentrations, it affects NO to a small extent only. In the current study, we assumed that O2− is present at higher levels (1–10 nM), based on measured H2O2 concentrations. Thus CNO increased significantly when the NO-O2− reaction rate was set to zero (Fig. 7). Moreover, when the effects of O2− on mTAL reabsorption were abolished, CNO increased even more in the interbundle region (Fig. 8). In other words, the present model indicates that O2− modulates NO levels both directly and indirectly: O2− may indirectly reduce NO generation via its stimulation of mTAL metabolic requirements, leading to reduction of O2 as a substrate for NO formation in the OM. It is likely, however, that hypoxia-induced NO release acts to compensate for the decrease in NO synthesis. Our current model suggests that hypoxia-mediated effects would not suffice to fully counteract the GNO decrease, but complete elucidation of the mechanisms by which hypoxia raises NO levels is needed to draw definitive conclusions.
Experimental observations regarding the effects of basal O2− on NO bioavailability are conflicting. Cowley and colleagues (11) observed that renal medullary interstitial infusion of the SOD mimetic tempol in anesthetized rats raised medullary blood flow and sodium excretion; these effects were partly counteracted by the dismutation of superoxide into H2O2, but were not affected by pretreatment with a NOS inhibitor (63). These results, combined with the observation that tempol by itself does not affect the basal tone of microperfused descending vasa recta (4), suggest that basal O2− has little direct impact on medullary NO levels. In contrast, several ex vivo studies have found that tempol enhances the release and diffusion of NO from mTALs (41, 45). However, these studies were performed in the absence of RBCs (i.e., of hemoglobin), and with a disrupted vascular endothelium (which, particularly when stimulated by shear, constitutes the main source of NO in vivo); under such conditions, O2− would have had a disproportionate impact on NO levels.
In contrast, studies consistently indicate that O2− significantly reduces NO bioavailability under oxidative stress conditions. In spontaneously hypertensive rats, or in rats infused with ANG II, tempol markedly affects arterial blood pressure, renal blood flow, glomerular filtration rate, and/or sodium excretion (28, 44, 54). Pretreatment with NG-nitro-l-arginine methyl ester (l-NAME) eliminates the antihypertensive effects of tempol (53), implying that, in these hypertensive animals, O2− is present at sufficient levels to significantly scavenge NO.
Hypertensive Conditions
Our model predicts that shifting the NO-O2− balance in favor of superoxide substantially enhances NaCl reabsorption across mTALs (Fig. 10) and further depletes oxygen in the OM, as experiments have suggested. Our simulations suggest that a 10-fold increase in the rate of O2− synthesis raises the concentrating capacity of the OM by ∼35%, and reduces Po2 in the peripheral regions by 5–10 mmHg in the OS and 1–3 mmHg in the IS, assuming that the mTAL TNa/Qo2 ratio remains constant. In fact, the number of Na+ moles reabsorbed per mole of O2 consumed is reported to be lower in hypertensive subjects (59). As discussed by Welch (58), possible explanations for the lower TNa/Qo2 involve 1) oxidative stress-mediated changes in the Na+ reabsorption profile, such that more Na+ is reabsorbed downstream of the proximal tubule, at a higher energy cost; 2) back-leak of Na+ in the tubular lumen; and 3) reduced efficiency of mitochondria in producing ATP in the presence of lower NO concentrations; note that NO modulates the respiration rate by inhibiting cytochrome oxidase in competition with O2 (2). The extent to which oxidative stress reduces TNa/Qo2 specifically in the mTAL remains to be ascertained. A twofold reduction would partly counteract the stimulating effects of O2− on NaCl reabsorption across the mTAL (Fig. 10), but would further reduce Po2 by a few millimeters mercury outside the vascular bundle. These results suggest that the inefficient use of O2 in hypertensive models slows down active transport while exacerbating hypoxia in the OM.
Tubular and Vascular Flow Increases
We examined the net effect of increased flow on NaCl transport in the OM, given that luminal flow stimulates O2− production, via PKC-mediated activation of NADPH oxidase (26), but also induces NOS activation in DVR and TALs. Shear stress enhances the intrinsic activity of NOS at least partly by stimulating its translocation to the membrane (47). We previously showed that per se, increases in vascular and tubular flows lower the OM concentrating capacity: despite greater O2 availability, the higher loads make it harder to concentrate the tubular fluid (7). Our earlier study, which did not include the transport of NO and O2−, predicted that a 25% increase in volume flows lowers osmCDL by 10%. The current model suggests that increasing volume flows may in fact reduce the osmolality gradient even more, because NO-mediated inhibition of mTAL transport intensifies. The flow-induced increase in O2− synthesis, if comparable to that in NO synthesis, is not sufficient to offset these effects. Hence, in the absence of other counteracting mechanisms, a 20% increase in flow rates that is accompanied by a 20% increase in the production of NO and O2− is predicted to lower osmCDL by ∼25% (Table 6).
Model Validation, Comparison, and Limitations
There are very few experimental data with which our model predictions can be compared and validated. Predicted NO concentrations in the OM fall within the range of measured values, which extend from 60–100 nM (27, 64) to 800 nM (52), but there have been no direct measurements of O2− levels in the OM, to the best of our knowledge. We compared the predicted and measured reduction in Po2 induced by the NOS inhibitor l-NAME. Li et al. (33) used BOLD MRI to detect changes in renal medullary oxygenation. In Wistar-Kyoto rats, the parameter R2* (which is inversely proportional to Po2) increased by ∼40% following administration of l-NAME. Similarly, we found that abolishing NO generation reduced interstitial Po2 in the interbundle region by 30–50%, depending on position (results not shown).
The current model differs from our previous model of NO and O2− transport in the OM (16, 17) in several important respects. The latter was restricted to medullary cross sections, focused on the transport of three solutes only (NO, O2−, and ONOO−), and assumed fixed Po2 profiles. In particular, it did not consider the effects of NO and O2− on sodium reabsorption and O2 consumption in the OM, and therefore failed to capture important reciprocal interactions. In addition, basal O2− concentrations were previously taken to be subnanomolar, based on measured O2− synthesis rates in aortic endothelial cells. In this study, basal O2− concentrations are taken to be ∼10 times higher, based on experimental determinations of medullary H2O2.
Some of the significant limitations of our study were discussed above. In the absence of quantitative data, several model parameters are necessarily uncertain, particularly those related to NO- and O2−-mediated effects on NaCl reabsorption in the mTAL. In addition, our steady-state model does not account for vasomotion and hormone-induced changes in vessel diameter. Finally, we did not examine the effects of ONOO− (the product of the NO-O2− reaction) and H2O2 (the product of the SOD-O2− reaction) on medullary function. ONOO− is thought to exert potent cytotoxic effects at high concentrations and to induce vascular relaxation at lower (nM) concentrations, but the role of ONOO− in regulating kidney function remains to be investigated (36). Similarly, the far-reaching vascular effects of H2O2 have yet to be fully characterized in vivo (1).
In vivo, solute reabsorption and medullary blood flow are controlled by many endocrine and paracrine factors. This study aimed to yield a better understanding of the interactions between NO and O2− and their combined effects on tubular and vascular function in the OM. Given the complex nature of events that balance O2 supply and consumption and modulate NaCl transport, we formulated the current model to facilitate prediction of the net effect of such interactions.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK053775 to A. Edwards.
ACKNOWLEDGMENTS
The authors thank Dr. J. Chen for help with computations, and Dr. T. L. Pallone for helpful discussions.
REFERENCES
- 1. Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med 231: 237–251, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta 1504: 46–57, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Buerk D, Lamkin-Kennard K, Jaron D. Modeling the influence of superoxide dismutase on superoxide and nitric oxide interactions, including reversible inhibition of oxygen consumption. Free Radic Biol Med 34: 1488–1503, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Cao C, Edwards A, Sendeski M, Lee-Kwon W, Cui L, Cai CY, Patzak A, Pallone TL. Intrinsic nitric oxide and superoxide production regulates descending vasa recta contraction. Am J Physiol Renal Physiol 299: F1056–F1064, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao C, Lee-Kwon W, Silldorff EP, Pallone TL. KATP channel conductance of descending vasa recta pericytes. Am J Physiol Renal Physiol 289: F1235–F1245, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chen B, Deen WM. Analysis of the effects of cell spacing and liquid depth on nitric oxide and its oxidation products in cell cultures. Chem Res Toxicol 14: 135–147, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Edwards A, Layton AT. Effects of pH and medullary blood flow on oxygen transport and sodium reabsorption in the rat outer medulla. Am J Physiol Renal Physiol 298: F1369–F1383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen J, Layton AT, Edwards A. A mathematical model of oxygen transport in the rat outer medulla. I. Model formulation and baseline results. Am J Physiol Renal Physiol 297: F517–F536, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen K, Piknova B, Pittman RN, Schechter AN, Popel AS. Nitric oxide from nitrite reduction by hemoglobin in the plasma and erythrocytes. Nitric Oxide 18: 47–60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen K, Pittman RN, Popel AS. Vascular smooth muscle NO exposure from intraerythrocytic SNOHb: a mathematical model. Antioxid Redox Signal 9: 1097–1110, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Chen YF, Cowley AW, Jr, Zou AP. Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol Regul Integr Comp Physiol 285: R827–R833, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Gill PS, Welch WJ. Oxygen availability limits renal NADPH-dependent superoxide production. Am J Physiol Renal Physiol 289: F749–F753, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 52: 777–786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 107: 566–574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Denicola A, Souza JM, Radi R, Lissi E. Nitric oxide diffusion in membranes determined by fluorescence quenching. Arch Biochem Biophys 328: 208–212, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Edwards A, Layton AT. Nitric oxide and superoxide transport in a cross section of the rat outer medulla. I. Effects of low medullary oxygen tension. Am J Physiol Renal Physiol 299: F616–F633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards A, Layton AT. Nitric oxide and superoxide transport in a cross section of the rat outer medulla. II. Reciprocal interactions and tubulovascular cross talk. Am J Physiol Renal Physiol 299: F634–F647, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol 4: 161–177, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Gus'kova RA, Ivanov II, Kol'tover VK, Akhobadze VV, Rubin AB. Permeability of bilayer lipid membranes for superoxide (O2−) radicals. Biochim Biophys Acta 778: 579–585, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Hawkins BJ, Madesh M, Kirkpatrick CJ, Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell 18: 2002–2012, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herrera M, Hong NJ, Garvin JL. Aquaporin-1 transports NO across cell membranes. Hypertension 48: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Heyman SN, Goldfard M, Darmon D, Brezis M. Tissue oxygenation modified nitric oxide bioavailability. Microcirculation 6: 199–203, 1999 [PubMed] [Google Scholar]
- 23. Heyman SN, Karmeli F, Rachmilewitz D, Haj-Yehia A, Brezis M. Intrarenal nitric oxide monitoring with a Clark-type electrode: potential pitfalls. Kidney Int 51: 1619–1623, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Hobbs AJ, Gladwin MT, Patel RP, Williams DLH, Butler AR. Haemoglobin: NO transporter, NO inactivator, or NOne of the above? Trends Pharmacol Sci 23: 406–411, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Hong NJ, Garvin JL. Nitric oxide reduces flow-induced superoxide production via cGMP-dependent protein kinase in thick ascending limbs. Am J Physiol Renal Physiol 296: F1061–F1066, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hong NJ, Silva GB, Garvin JL. PKC-α mediates flow-stimulated superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 298: F885–F891, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kakoki M, Kim HS, Arendshorst WJ, Mattson DL. l-Arginine uptake affects nitric oxide production and blood flow in the renal medulla. Am J Physiol Regul Integr Comp Physiol 287: R1478–R1485, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Kopkan L, Castillo A, Navar LG, Majid DSA. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol 290: F80–F86, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci USA 89: 5537–5541, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kumura E, Yoshimine T, Takaoka M, Hayakawa T, Shiga T, Kosaka H. Hypothermia suppresses nitric oxide elevation during reperfusion after focal cerebral ischemia in rats. Neurosci Lett 220: 45–48, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 289: F1346–F1366, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Li LP, Li BSY, Storey P, Fogelson L, Li W, Prasad P. Effect of free radical scavenger (tempol) on intrarenal oxygenation in hypertensive rats as evaluated by BOLD MRI. J Magn Reson Imaging 21: 245–248, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li L, Storey P, Kim D, Li W, Prasad P. Kidneys in hypertensive rats show reduced response to nitric oxide synthase inhibition as evaluated by BOLD MRI. J Magn Reson Imaging 17: 671–675, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002 [DOI] [PubMed] [Google Scholar]
- 35. MacPhee PJ. Fluid uptake by the renal medullary vasa recta: an estimate based on a quantitative analysis of the distribution of fenestrae in the vasa recta of young Sprague-Dawley rats. Exp Physiol 83: 23–34, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Majid DS, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol 34: 946–952, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW., Jr Increased renal medullary oxidative stress produces hypertension. Hypertension 39: 667–672, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Malinski T, Taha Z, Grunfeld S, Patton S, Kapturczak M, Tomboulian P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensors. Biochem Biophys Res Commun 193: 1076–1082, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Manning RD, Jr, Hu L, Tan DY, Meng S. Role of abnormal nitric oxide systems in salt-sensitive hypertension. Am J Hypertens 14: 68S–73S, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Mattson DL, Wu F. Nitric oxide synthase activity and isoforms in rat renal vasculature. Hypertension 35: 337–341, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Mori T, Cowley AW., Jr Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension 42: 588–593, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Mueller S, Riedel HD, Stremmel W. Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal Biochem 245: 55–60, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Mumbengegwi DR, Li Q, Li C, Bear CE, Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol 28: 3700–3712, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, Abe Y. Systemic and regional hemodynamic responses to tempol in angiotensin II-infused hypertensive rats. Hypertension 37: 77–83, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Ortiz PA, Garvin JL. Interaction of O2− and NO in the thick ascending limb. Hypertension 39: 591–596, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am J Physiol Renal Physiol 287: F274–F280, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Oshino N, Chance B, Sies H, Bücher T. The role of H2O2 generation in perfused rat liver and the reaction of catalase compound I and hydrogen donors. Arch Biochem Biophys 154: 117–131, 1973 [DOI] [PubMed] [Google Scholar]
- 50. Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci USA 102: 2531–2536, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol 276: F159–F163, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Salom MG, Arregui B, Carbonell LF, Ruiz F, Gonzalez-Mora JL, Fenoy FJ. Renal ischemia induces an increase in nitric oxide levels from tissue stores. Am J Physiol Regul Integr Comp Physiol 289: R1459–R1466, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 32: 59–64, 1998 [DOI] [PubMed] [Google Scholar]
- 54. Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-iso prostaglandin F2α. Hypertension 33: 424–428, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Taylor NE, Cowley AW., Jr Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 289: R1573–R1579, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Thayer WS. Adriamycin stimulated superoxide formation in submitochondrial particles. Chem-Biol Interact 19: 265–278, 1977 [DOI] [PubMed] [Google Scholar]
- 57. Verkman AS. Does aquaporin-1 pass gas? An opposing view. J Physiol 542: 31, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Welch WJ. Intrarenal oxygen and hypertension. Clin Exp Pharmacol Physiol 33: 1002–1005, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Nephron Po2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int 59: 230–237, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Whorton AR, Simonds DB, Piantadosi CA. Regulation of nitric oxide synthesis by oxygen in vascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 272: L1161–L1166, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol Renal Physiol 276: F874–F881, 1999 [DOI] [PubMed] [Google Scholar]
- 62. Zhang Z, Pallone TL. Response of descending vasa recta to luminal pressure. Am J Physiol Renal Physiol 287: F535–F542, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Zou AP, Li N, Cowley AW., Jr Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001 [DOI] [PubMed] [Google Scholar]
- 64. Zou AP, Wu F, Cowley AW., Jr Protective effect of angiotensin II-induced increase in nitric oxide in the renal medullary circulation. Hypertension 31: 271–276, 1998 [DOI] [PubMed] [Google Scholar]