Abstract
Activation of type 1 angiotensin II (AT1) receptors in the kidney promotes blood pressure elevation and target organ damage, but whether renal AT1 receptors influence the level of hypertension by stimulating sodium retention or by raising systemic vascular resistance has not been established. In the current studies, we used a kidney cross-transplantation strategy to determine whether increased sodium reabsorption by AT1 receptors in the kidney mediates the chronic hypertensive response to angiotensin II. We found this to be true. In addition, we also identified a second, nontrivial component of blood pressure elevation induced by activation of renal AT1 receptors that is sodium-independent. As the kidney has the capacity to limit the transmission of elevated systemic blood pressure into the renal microcirculation, prior studies struggled to clearly discriminate the relative contributions of blood pressure elevation vs. activation of AT1 receptors to hypertensive kidney injury. In our model, we found that rapid surges in blood pressure, which may overcome the kidney's capacity to prevent perturbations in renal hemodynamics, correlate closely with kidney damage in hypertension. Moreover, maximal kidney injury in hypertension may require activation of a pool of nonrenal, systemic AT1 receptors. These studies provide insight into precise mechanisms through which AT1 receptor blockade influences the progression of hypertensive kidney disease.
Keywords: kidney diseases
activation of the renin-angiotensin system (RAS) leads to blood pressure elevation and target organ damage (6, 14). The critical role of angiotensin II (ANG II) in the pathogenesis of hypertension and its complications has been shown in clinical trials wherein blockade of type 1 angiotensin (AT1) receptors dramatically lowers blood pressure, slows the progression of chronic kidney disease (CKD), and ameliorates cardiac hypertrophy (4, 11, 20). In these trials and in animal models, protection from end-organ injury during AT1 receptor inhibition correlates closely with blood pressure reduction (3, 17).
We recently explored the contribution to hypertension of AT1 receptors in the kidney vs. other tissues by exploiting a kidney cross-transplantation model (8). In this model, kidneys were transplanted between genetically matched wild-type (WT) mice and mice lacking the dominant murine AT1 receptor isoform, AT1A. With this approach, groups of mice lacking AT1A receptors only in the kidney (Kidney KO) or only in nonrenal systemic tissues (Systemic KO) were generated along with total WT and AT1A knockout (Total KO) transplant controls. During chronic ANG II infusion in these transplanted animals, blood pressure rose dramatically and similarly in the WT and Systemic KO groups, both of which express AT1A receptors in the kidney, but not in the Kidney KO and Total KO groups, both of which lack AT1A receptors in the kidney. Moreover, we performed salt balance studies showing that early in the course of ANG II-induced hypertension the WT and Systemic KO groups excreted less sodium than the Kidney KO and Total KO groups.
These studies demonstrated that activation of AT1 receptors in the kidney mediates the chronic hypertensive effects of ANG II possibly through promoting renal sodium reabsorption (8). However, the contribution of salt retention to blood pressure elevation and kidney injury in this model has not been directly explored, and the possibility remains that activation of AT1 receptors in the kidney could increase blood pressure through a salt-independent mechanism. The goal of the current experiments was therefore to discriminate the salt-dependent and -independent contributions of AT1 receptor activation in the kidney to blood pressure elevation and to characterize the roles of blood pressure elevation and regional AT1 receptor activation in the progression of kidney disease using this cross-transplant model.
MATERIALS AND METHODS
Animals.
(129 × C57BL/6)F1 mice lacking AT1A receptors for ANG II were generated as previously described (19). All mice were maintained in the animal facility of the Durham Veterans Affairs Medical Center under local and National Institutes of Health guidelines. All animal studies were approved by the Institutional Animal Care and Use Committee, Durham Veterans Affairs Medical Center (Durham, NC) and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. These studies used 2- to 4-mo-old male mice.
Mouse kidney transplantation.
Vascularized kidney transplants were performed in mice as previously described (9). Briefly, animals were anesthetized with isoflurane, and the donor kidney, ureter, and bladder were harvested en bloc, including the renal artery with a small aortic cuff and the renal vein with a small vena caval cuff. These vascular cuffs were anastomosed to the recipient abdominal aorta and vena cava, respectively, below the level of the native renal vessels. Donor and recipient bladders were attached dome to dome. The right native kidney was removed at the time of transplant, and the left native kidney was removed through a flank incision 1–3 days later such that all kidney function in the animal was provided by the lone, transplanted kidney. The adrenal glands and their respective blood supplies were preserved intact. In the WT group, WT mice were transplanted with a WT kidney. In the Systemic KO group, Agtr1a−/− mice were transplanted with a WT kidney. In the Kidney KO group, WT mice were transplanted with an Agtr1a−/− kidney. In the Total KO group, Agtr1a−/− mice were transplanted with an Agtr1a−/− kidney.
Model of angiotensin II-induced hypertension.
One week following the kidney transplant procedure, mice from the kidney cross-transplant groups (n ≥ 6 mice per group) underwent implantation of a pressure-sensing catheter (TA11PA-C10, Transoma Medical) via the left common carotid artery as previously described (9). Allowing 7 days for reestablishment of diurnal blood pressure variation, baseline blood pressure measurements were recorded for 3 days continuously by radiotelemetry (Transoma) in conscious unrestrained animals. Then, an osmotic mini-pump (Alzet model 2004, DURECT) was implanted to infuse ANG II (1,000 ng·kg−1·min−1; Sigma) continuously for 28 days as previously described (8). All groups received normal chow (0.4% NaCl). A separate, additional group of Systemic KO mice was fed a low-salt diet (<0.01% NaCl) during the ANG II infusion period. Blood pressure measurements continued for 3 wk of ANG II infusion as previously described (8). The blood pressures of the mice fed normal chow during ANG II infusion were originally reported in a previous publication (8). On day 25, the mice were placed in metabolic cages, and urine was collected for 24 h. Urinary concentrations of albumin and aldosterone were measured in individual samples using specific ELISAs for mouse albumin (Exocell) and aldosterone (Cayman Chemical) as previously described (9, 13). Creatinine concentrations were measured with a picric acid-based method using a kit (Exocell). Albumin excretion is expressed as micrograms per milligram of creatinine. After 28 days of ANG II infusion, blood was collected via terminal cardiac puncture, and serum potassium levels were quantitated by using an IL943 Automatic Flame photometer per the manufacturer's instructions (Instrumentation Laboratory).
Aldosterone blockade.
In follow-up experiments to assess the contribution of aldosterone to kidney damage in our model, separate sets of WT (129 × C57BL/6)F1 mice (n ≥ 8 per group) underwent unilateral nephrectomy to reproduce the single-kidney element of our cross-transplant model. However, during the surgery to implant the ANG II osmotic mini-pump, these mice were also implanted subcutaneously with a pellet containing placebo (#SC-111) or spironolactone (27.8 mg·kg−1·day−1, #SM-161, Innovative Research of America), for release during the ANG II infusion period. This dose exceeds that known to block actions of aldosterone in mouse models (22).
Histopathologic analysis.
Following 28 days of ANG II infusion, kidneys and hearts were harvested, weighed, and fixed in formalin, sectioned, and stained with Masson trichrome. All of the kidneys were examined by a pathologist (P.R.) without knowledge of the genotypes or treatment groups. The pathological abnormalities in the kidney were graded based on the presence and severity of component abnormalities including glomerulosclerosis, mesangial expansion, chronic interstitial inflammation, tubular atrophy or casts, fibrosis, and vascular injury. Grading for each component was performed using a semiquantitative scale as previously described (25, 26) where 0 was no abnormality and where 1, 2, 3, and 4 represented mild, moderate, moderately severe, and severe abnormalities, respectively. The total injury score for each kidney was a summation of these component injury scores.
Statistical methods.
The values for each parameter within a group were expressed as means ± SE. For comparisons between groups with normally distributed data, statistical significance was assessed using ANOVA followed by unpaired t-test. For comparisons between groups with nonnormally distributed data, the Mann-Whitney U-test was employed. For comparisons within groups, normally distributed variables were analyzed by a paired t-test, whereas nonnormally distributed variables were analyzed by the Wilcoxon signed rank test.
RESULTS
Role of sodium retention in blood pressure elevation induced by activation of renal AT1 receptors.
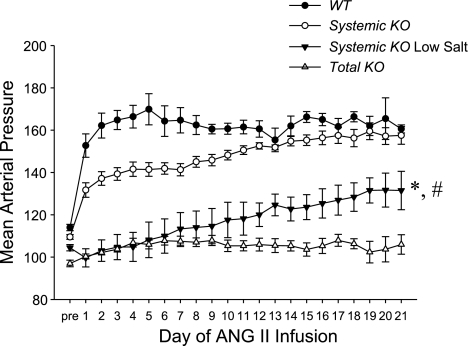
In our previous work with the kidney cross-transplant model (8), the WT and Systemic KO mice that express AT1A receptors in the kidney had marked blood pressure elevation during chronic ANG II infusion compared with the Kidney KO and Total KO mice that lack AT1A receptors in the kidney. Moreover, during the first week of ANG II infusion the WT and Systemic KO mice had reduced salt excretion compared with the other groups, suggesting that enhanced sodium reabsorption in the nephron due to activation of renal AT1 receptors was a prominent driver of hypertension in our model. Therefore, to directly test the role of sodium retention in this model, we fed a normal-salt (0.4%) or a low-salt diet (<0.01% NaCl) to groups of Systemic KO mice throughout the ANG II infusion period and monitored blood pressure continuously by radiotelemetry (Fig. 1). Mean arterial pressures in the low-salt Systemic KO group were lower than in the normal-salt Systemic KO group on every single day of ANG II infusion (P < 0.01 for each) such that the blood pressures averaged over the entire ANG II infusion period were markedly reduced in the low-salt Systemic KOs (114 ± 7 mmHg) compared with the normal-salt Systemic KOs (148 ± 2 mmHg; P < 0.0001). These data suggest that salt retention due to activation of AT1 receptors in the kidney is indeed responsible for much of the blood pressure increase in the Systemic KO group. Moreover, up through day 11 of ANG II infusion, the blood pressures in the low-salt Systemic KO group were not significantly different from those of the Total KO group fed a normal diet, suggesting that up to this point, increased salt reabsorption due to activation of AT1 receptors in the kidney mediates all of the blood pressure elevation in the Systemic KO group.
Fig. 1.
Low-salt feeding blunts the chronic hypertensive response of Systemic knockout (KO) mice to angiotensin II (ANG II). Mean arterial blood pressures (MAP) measured by radiotelemetry in the experimental groups at baseline (“pre”) and during 3 wk of ANG II infusion; n ≥ 6 mice per group. *P < 0.01 for Systemic KO low-salt vs. Systemic KO normal-salt for days 1–21; P < 0.04 for Systemic KO low-salt vs. Total KO for days 12–21 except day 17 (P = 0.07). #P = 0.01 for Systemic KO low-salt day 12 vs. day 21. WT, wild-type.
Sodium-independent effects of renal AT1 receptors on blood pressure elevation.
Despite the prominent blood pressure-lowering effects of the low-salt diet in the Systemic KO group, the daily average blood pressures in the low-salt Systemic KO group did progressively increase during the first 2 wk of ANG II infusion such that on each day subsequent to day 11 of ANG II infusion, mean arterial pressures in this group were significantly higher than in the Total KO group (P < 0.04 for each). Accordingly, during week 3 of ANG II infusion, blood pressures in the low-salt Systemic KO group (128 ± 7 mmHg) were significantly higher than in the Total KO group (104 ± 3 mmHg; P < 0.006). These data highlight a salt-independent effect on blood pressure of chronic renal AT1 receptor activation.
Reduction in blood pressure on low-salt diet protects from ANG II-induced cardiac hypertrophy.
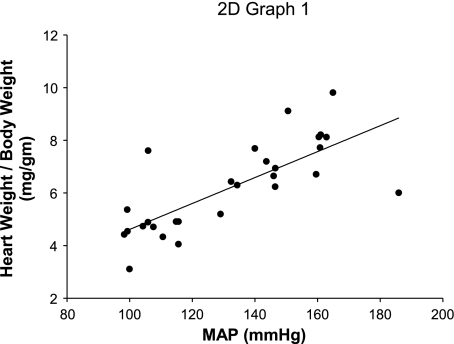
In our initial cross-transplant experiments, we established that cardiac hypertrophy depends on the level of blood pressure elevation during ANG II infusion rather than on actions of AT1 receptors in the heart (8). To assess the contribution of salt retention to cardiac hypertrophy in the Systemic KO mice, we measured heart weight-to-body weight ratios in these animals following 4 wk of ANG II infusion. Compared with a concomitant group of Systemic KO mice fed a normal-salt diet, the low-salt Systemic KO mice had markedly lower heart weights (8.0 ± 0.6 vs. 6.1 ± 0.5 mg/g; P < 0.06). Moreover, the incorporation of the low-salt Systemic KO group into the regression analysis correlating blood pressure with heart weight in our original cross-transplant experiment yielded an even tighter relationship between these parameters than we previously established (Fig. 2; R = 0.99, P < 0.0001). Thus, on a normal- or low-sodium diet, cardiac hypertrophy depends primarily on the level of blood pressure elevation.
Fig. 2.
Cardiac hypertrophy correlates tightly with blood pressure during ANG II infusion. Linear regression analysis between MAP and heart-to-body weight ratios across all normal-salt transplant cohorts (WT, Systemic KO, Kidney KO, Total KO) and the low-salt Systemic KO cohort. R = 0.99, P < 0.0001.
Hypertensive kidney injury depends on blood pressure elevation and systemic AT1 receptor activation.
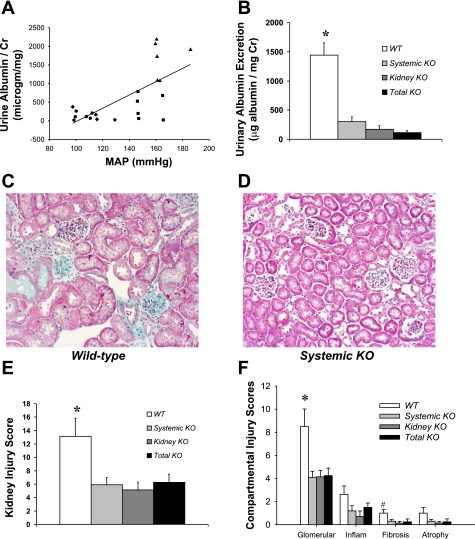
To assess functional kidney injury, we measured urinary albumin excretion following 25 days of ANG II infusion in our original four cross-transplant groups fed normal salt and in the low-salt Systemic KO group. As with cardiac hypertrophy, Systemic KO mice fed a low-salt diet had significantly reduced albuminuria compared with Systemic KO mice receiving a normal-salt diet (35 ± 10 vs. 247 ± 73 μg/mg creatinine; P = 0.05), consistent with a role for blood pressure elevation in mediating kidney injury. However, as shown in Fig. 3A, the correlation between albuminuria and blood pressure in the normal-salt cross-transplant groups (R = 0.68, P = 0.0005) was less strong than the relationship between heart weight and blood pressure that we previously reported (R = 0.84, P < 0.0001) (8), suggesting that factors other than blood pressure elevation may play a role in mediating hypertensive kidney injury. Moreover, when we plotted the levels of albuminuria separately in the four original normal-salt cross-transplant groups (Fig. 3B), we found that the WT group excreted substantial amounts of albuminuria (1,443 ± 208 μg/mg creatinine), whereas the level of albuminuria was minimal in the other three groups (P < 0.0001 for each vs. WT). Similarly, following 4 wk of ANG II infusion, the WT kidneys exhibited significant pathology marked by glomerulosclerosis, interstitial inflammation, and mild fibrosis (Fig. 3C, E, F), but kidney injury was far less severe in the other three transplant groups (Fig. 3D, E, F). Although the differences between the WT group and the other three groups in the extent of renal injury were most prominent in the glomerulus, we saw a similar pattern with respect to chronic interstitial inflammation, fibrosis, and tubular atrophy (Fig. 3F). Although the lack of significant kidney damage in the normotensive Kidney KO and Total KO groups is not surprising, the lack of substantial kidney injury in the Systemic KO group, which had blood pressure elevation on par with that of the WT group, suggests that maximal kidney injury may require not only blood pressure elevation but also activation of a pool of extrarenal, systemic AT1 receptors.
Fig. 3.
Kidney injury in ANG II-dependent hypertension is associated with blood pressure elevation and systemic AT1 receptor activation. A: blood pressure during ANG II infusion showed a moderate positive correlation with urinary albumin excretion across the 4 normal-salt transplant cohorts (WT = triangles, Systemic KO = squares, Kidney KO = diamonds, Total KO = circles), R = 0.68, P < 0.0005. B: urinary albumin excretion following 25 days of ANG II infusion measured by ELISA in 4 normal-salt transplant cohorts. *P ≤ 0.0001 vs. all other groups. C–D: representative kidney sections from WT (C) and Systemic KO (D) mice following 4 wk of ANG II infusion (magnification ×20). E: semiquantitative scoring of renal pathology following 4 wk of ANG II infusion in the transplant groups, n ≥ 7, *P < 0.04 vs. all other groups (scoring in arbitrary units as described in materials and methods). F: semiquantitative scoring of pathology in individual kidney compartments (Glomerular, glomerular damage; Inflam, chronic interstitial inflammation; fibrosis, interstitial fibrosis; atrophy, tubular atrophy). Vascular damage was negligible in the kidneys in this experiment. *P = 0.008 vs. Systemic KO. P = 0.02 vs. Kidney KO. P = 0.02 vs. Total KO. #P = 0.04 vs. Kidney KO. P = 0.05 vs. Systemic KO. P = 0.09 vs. Total KO.
Activation of adrenal AT1 receptors does not contribute to kidney injury induced by ANG II-dependent hypertension.
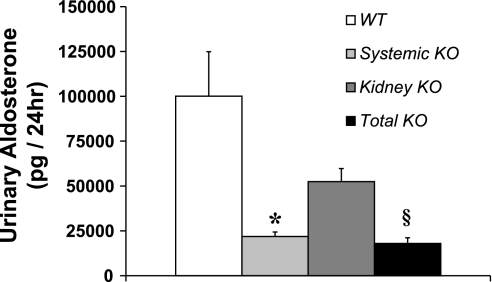
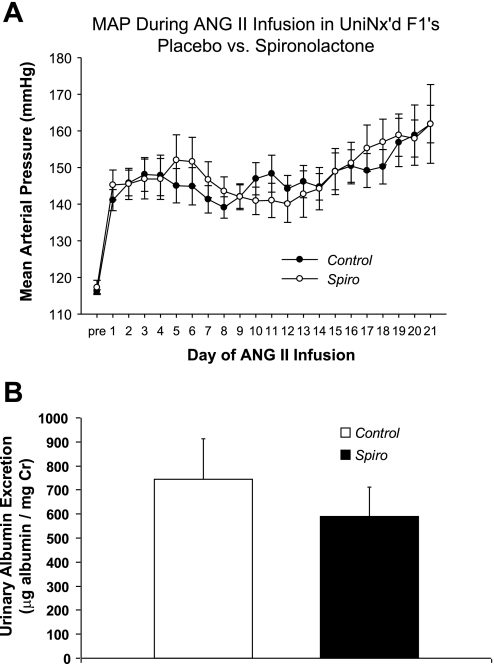
Activation of AT1 receptors in the zona glomerulosa of the adrenal gland triggers synthesis of aldosterone, which in turn has been implicated in mediating hypertensive kidney injury through blood pressure-independent mechanisms (1, 24). We therefore posited that the absence of adrenal AT1 receptors in the Systemic KO group resulted in reduced circulating aldosterone levels leading to less kidney injury in this group despite the similar blood pressure elevation in the Systemic KO and WT groups. Consistent with this possibility, we found that urinary excretion of aldosterone in the Systemic KO (21,803 ± 2,563 pg/day) and Total KO (17,951 ± 3,180 pg/day) groups, both of which lack AT1A receptors in the adrenal gland, was markedly lower than in the WT (100,085 ± 24,790 pg/day) and Kidney KO (52,450 ± 7,290 pg/day) groups, both of which express AT1A receptors in the adrenal gland (Fig. 4). Moreover, aldosterone lowers serum potassium by driving its secretion into the mineralocorticoid-responsive distal nephron, and we found that serum potassium levels were numerically but not significantly lower in the WT (4.6 ± 0.3 mmol/l) and Kidney KO groups (4.2 ± 0.4 mmol/l) than in the Systemic KO (5.1 ± 0.9 mmol/l) and Total KO groups (4.8 ± 0.2 mmol/l). Therefore, to directly test the role of aldosterone in mediating kidney injury in our single-kidney hypertension model, we repeated the chronic ANG II infusion experiment in uninephrectomized WT mice implanted with either placebo (Control) or spironolactone (Spiro) pellets to block the actions of aldosterone. Blood pressures measured by radiotelemetry in the Control and Spiro groups remained similar throughout the ANG II infusion period (Fig. 5A) such that the average mean arterial pressures for the whole period were virtually identical [148 ± 3 vs. 149 ± 4 mmHg; P = not significant (NS)]. As shown in Fig. 5B, the Control group excreted substantial amounts of albumin following 4 wk of ANG II infusion (745 ± 169 μg/mg Cr), and aldosterone blockade did not reduce the level of albuminuria (590 ± 121 μg/mg Cr; P = NS). Moreover, the Control and Spiro groups each had moderate and similar levels of kidney pathology (10.1 ± 1.5 vs. 8.8 ± 1.6 arbitrary units; P = NS; Table 1) in the glomerular, tubulointerstitial, and vascular compartments. These data suggest that activation of AT1 receptors in the adrenal gland does not contribute to hypertensive kidney injury in this model.
Fig. 4.
Activation of systemic AT1 receptors in angiotensin II-dependent hypertension promotes aldosterone generation. Urinary aldosterone excretion measured by ELISA following 4 wk of ANG II infusion, showing higher aldosterone levels in the WT and Kidney KO groups that express AT1A receptors in the adrenal gland compared with the Systemic KO and Total KO groups that lack adrenal AT1A receptors; n ≥ 7. *P < 0.004 vs. WT. P = 0.0003 vs. Kidney KO. §P < 0.02 vs. WT. P = 0.008 vs. Kidney KO.
Fig. 5.
Effects of aldosterone blockade in uninephrectomized mice during chronic ANG II infusion. A: daily, 24-h blood pressures measured by radiotelemetry during chronic ANG II infusion in uninephrectomized WT mice implanted with vehicle (Control) or spironolactone (Spiro) pellets to block actions of aldosterone, n = 8. B: urinary albumin excretion measured by ELISA in the Control and Spiro groups following 4 wk of ANG II infusion.
Table 1.
Hypertensive kidney injury is unaffected by aldosterone blockade with spironolactone
| Kidney Injury Scores (au) | Glomerular Injury | Interstitial Inflammation | Vascular Damage | Total Injury |
|---|---|---|---|---|
| Control | 7.0 ± 0.9 | 2.2 ± 0.5 | 0.9 ± 0.3 | 10.1 ± 1.5 |
| Spironolactone | 6.2 ± 0.8 | 1.8 ± 0.6 | 0.8 ± 0.3 | 8.8 ± 1.6 |
Data are means ± SE. Hypertensive kidney injury is unaffected by aldosterone blockade with spironolactone (au, arbitrary units); n ≥ 11 per group.
Rapid blood pressure elevation with renal AT1 receptor activation promotes albuminuria.
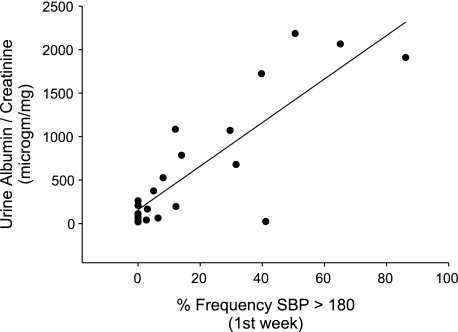
As activation of AT1 receptors in the adrenal gland did not influence the progression of renal damage in our model, we considered the alternative possibility that a difference in kidney injury between the WT and Systemic KO groups might be related to the pattern rather than the magnitude of blood pressure elevation in the two groups during ANG II-dependent hypertension. In this regard, others showed that kidney injury correlates with blood pressure elevation above a threshold beyond which the kidney fails to prevent transmission of an excess blood pressure load from the systemic circulation into the renal vasculature (2, 16). In this regard, we noted that during the first 12 days of ANG II infusion, blood pressures in the Systemic KO group rose more gradually than in the WT group. Therefore, to examine the impact on proteinuria of rapid blood pressure elevation early in the course of ANG II infusion, we performed further regression analysis in the cross-transplant groups and found that albuminuria correlated much more closely with the frequency of systolic blood pressures exceeding 180 mmHg (R = 0.84; P < 0.0001; Fig. 6) than with total blood pressure load during ANG II infusion (Fig. 3A). Indeed, the stringency of this correlation was equivalent to that previously recorded between blood pressure and cardiac hypertrophy in the cross-transplant model (8). Thus, after accounting for the capacity of the kidney to limit rapid increases in intrarenal hemodynamic pressures, we find that kidney injury, like cardiac injury, depends largely on the degree of blood pressure elevation induced by activation of renal AT1 receptors.
Fig. 6.
Kidney injury in cross-transplant groups correlates tightly with early spikes in blood pressure during ANG II-dependent hypertension. Regression analysis plotting the correlation between ANG II-induced albuminuria and the frequency of systolic blood pressures exceeding 180 mmHg during first week of ANG II infusion in 4 transplant groups (WT, Systemic KO, Kidney KO, Total KO), R = 0.84, P < 0.0001.
DISCUSSION
A fundamental role for the RAS acting through AT1 receptors in the pathogenesis of hypertension and target organ damage has been firmly established in animal models and human clinical trials (4, 6, 14, 19). However, despite widespread use of AT1 receptor blockers (ARBs) in clinical hypertension, the mechanisms through which ARBs lower blood pressure and protect the kidney from injury have not been fully elucidated. As experiments with pharmacological antagonists do not permit characterization of AT1 receptor actions in specific tissues, we combined gene-targeting and kidney cross-transplantation strategies to further explore the contributions of AT1 receptors in the kidney and in other systemic tissues to blood pressure elevation and the progression of renal disease.
Our initial work with the cross-transplant model demonstrated that AT1 receptors in the kidney are the primary AT1 receptor tissue pool responsible for mediating blood pressure elevation in the setting of RAS activation (8). Salt balance studies in those experiments also indicated that activation of renal AT1 receptors promotes sodium retention during the initial phase of ANG II-dependent hypertension. However, in those earlier studies, we did not directly explore whether this enhanced sodium reabsorption during chronic ANG II infusion could account for the similar chronic blood pressure augmentation in Systemic KO mice expressing AT1 receptors only in the kidney and WT controls. In the present experiments, we addressed this problem by feeding a low-salt diet to Systemic KO mice during ANG II infusion to abrogate the putative effects of ANG II-mediated sodium retention. We find that low-salt Systemic KO animals have a dramatically attenuated chronic hypertensive response compared with normal-salt Systemic KO controls. Indeed, according to this model, salt reabsorption driven by renal AT1 receptor activation accounts for all of the blood pressure elevation accruing to actions of these receptors during the first 11 days of ANG II infusion. This result is consistent with our previous report that salt excretion is restricted during the first week of ANG II infusion in mice expressing AT1 receptors in the kidney compared with animals lacking this receptor pool. The effect of salt balance and, by inference, intravascular volume is so profound that blood pressures in the low-salt Systemic KO mice are significantly lower than in normal-salt Systemic KO controls on every recorded day of chronic ANG II infusion. Thus, as predicted, activation of renal AT1 receptors plays a fundamental role in the pathogenesis of hypertension by facilitating sodium reabsorption in the kidney. The current findings are also consistent with the results of our previously published studies of non-ANG II-infused transplant animals in which salt loading increased blood pressures only in the Kidney KO and Total KO groups, suggesting that the deficiency of renal AT1 receptors in these groups led to suppressed baseline blood pressures due to inappropriate sodium losses in the nephron (9).
Although effects of renal AT1 receptors on sodium handling account for fully 20–30 mmHg of the blood pressure increase during the ANG II infusion period, our experiments also identify a surprising sodium-independent contribution of AT1 receptors in the kidney to hypertension that is particularly evident during the second and third weeks of ANG II infusion. Specifically, beginning on day 12 of ANG II, blood pressures in the Systemic KO group receiving a low-salt diet progressively diverge from those of the normal-salt Total KO mice lacking AT1 receptors in all tissues. Indeed, by the third week of ANG II infusion, 37% of the blood pressure increase in the Systemic KO mice relative to their baseline persists despite removal of sodium from the diet. We speculate that this increase may be due to direct effects of ANG II on the renal vasculature to enhance systemic vascular resistance as AT1 receptors are expressed prominently on both vascular endothelium and vascular smooth muscle cells (VSMC). Nevertheless, we have not directly measured systemic vascular resistance in the current experiments, and discriminating the contributions of AT1 receptors within individual components of the renal vasculature to hypertension awaits more incisive approaches. We also acknowledge that the minor AT1 receptor isoform, AT1B, remains intact in all four experimental groups, so that our current data do not model the contribution of AT1B actions to salt retention. Nevertheless, in our earlier study (8), blood pressures in the Total KO group rose only 6 mmHg during ANG II infusion, and this increase presumably reflects a small but measurable contribution of AT1B signals to ANG II-induced blood pressure elevation.
The correlation between blood pressure elevation and target organ damage has been elegantly characterized by Griffin and Bidani (2, 3, 16, 17) in previous studies. We find that the blunting of the hypertensive response in the Systemic KO mice fed a low-salt diet leads to a mitigation in ANG II-induced cardiac hypertrophy, confirming previous reports that cardiac enlargement is driven by blood pressure elevation rather than activation of AT1 receptors in the heart (8, 27). Accordingly, lowering blood pressures in our model by adjusting the diet can ameliorate ANG II-induced remodeling of the heart despite the complete absence of cardiac AT1 receptors in the Systemic KO cohorts.
In the current experiments, blood pressure during chronic ANG II infusion also correlates positively with kidney injury. However, the association is less uniform than that seen between blood pressure and cardiac hypertrophy. As others posited (2), this finding may be related to the kidney's ability to limit the transmission of elevated systemic blood pressures to the renal vasculature. Consistent with this possibility, we find that the correlation between urinary albumin excretion and early surges in blood pressure that may transgress the kidney's threshold for preventing local hemodynamic injury is quite robust (Fig. 6), matching the linearity between blood pressure and cardiac hypertrophy (8).
Further analysis of kidney injury and albuminuria following 4 wk of ANG II infusion in individual cross-transplant groups also revealed the surprising finding that the Systemic KO mice lacking AT1 receptors outside the kidney were protected from hypertensive renal damage despite achieving a chronic blood pressure elevation on par with WT controls. This finding may well relate to the slower pace of blood pressure increase in the Systemic KO group. However, we have not exhaustively tested the alternative possibility that activation of a pool of nonrenal systemic AT1 receptors somehow exacerbates kidney injury in the setting of hypertension. While the lack of significant kidney damage in the Kidney KO group precludes a contribution of systemic AT1 receptors to renal injury in the absence of hypertension, several tissue pools of systemic AT1 receptors could conceivably potentiate kidney damage following the initial injury induced by blood pressure elevation. First, as aldosterone has been shown to directly mediate kidney damage (5, 15), we initially posited that activation of AT1 receptors in the adrenal gland in our model might contribute to renal injury by driving synthesis of aldosterone. However, when we blocked the actions of aldosterone in uninephrectomized mice infused with ANG II, we saw no changes in the pattern of blood pressure elevation or associated renal damage (Fig. 5, Table 1). This finding does not diminish the known important contributions of aldosterone to kidney injury, as aldosterone blockade in humans protects against CKD (7), and our model may not manifest sufficiently robust kidney damage to allow detection of these renoprotective effects of aldosterone inhibition. Nevertheless, AT1 receptor activation in the adrenal gland does not appear to account for the excess in kidney injury seen in the WT compared with Systemic KO groups in our model.
We considered several other systemic nonrenal pools of AT1 receptors that could mediate hypertensive kidney injury in the current model. First, as AT1 receptors are expressed on immune cell lineages, we carefully explored the contribution of this AT1 receptor pool to hypertensive kidney injury in a separate manuscript (10). We found that activation of AT1 receptors on bone marrow-derived cells is paradoxically protective with regard to ANG II-induced renal damage. That study raised the possibility that global AT1 receptor blockade may be having unintended detrimental effects that are obscured by the benefits of renal AT1 receptor blockade thereby highlighting the urgency for a greater understanding of the roles of individual tissue pools of AT1 receptors in regulating target organ damage.
Another important pool of systemic AT1 receptors that could modulate kidney injury lies in the nervous system (12, 23). However, we previously determined that the transplanted kidneys in our model are denervated thus minimizing inputs from the sympathetic nervous system (9). A differential contribution of neuronal AT1 receptors to kidney injury in our WT and Systemic KO groups therefore seems unlikely unless AT1 receptors in the central nervous system are impacting kidney damage indirectly through stimulation of systemic inflammatory pathways (21). Barring this possibility, two other systemic pools of AT1 receptors that could influence ANG II-induced kidney damage lie in the endothelium and VSMCs. In this regard, Henke et al. (18) showed that abrogating important inflammatory signaling pathways activated by ANG II specifically in the vascular endothelium provides impressive protection from hypertensive kidney injury. Nevertheless, as endothelial and VSM AT1 receptors are present in kidney tissues and in nonrenal systemic tissues, more incisive approaches will be required to identify the contributions of endothelial or VSM AT1 receptors outside the kidney to the progression of ANG II-induced renal disease.
In summary, these studies demonstrate that activation of AT1 receptors in the kidney mediates blood pressure elevation not only by facilitating renal sodium reabsorption but also through sodium-independent mechanisms. Early in the course of ANG II infusion, surges in blood pressure that may exceed the kidney's capacity to prevent transmission of systemic pressures into the renal vasculature are associated with proteinuria and pathologic renal injury. Maximal kidney damage in this context may also require activation of a pool of nonrenal systemic AT1 receptors unrelated to the synthesis of aldosterone. These experiments highlight the complex relationship between RAS activation and target organ damage and illustrate how gene-targeting strategies can be coupled with alternative approaches to discriminate functions of individual AT1 receptor pools. Understanding the roles of these receptor pools should facilitate development of more targeted therapeutics in hypertension.
GRANTS
This work was supported by funding from National Institutes of Health Grant DK087893-01, the Medical Research Service of the Veterans Administration, and by the Edna and Fred L. Mandel Center for Hypertension and Atherosclerosis Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors acknowledge outstanding administrative support from Norma Barrow.
REFERENCES
- 1. Arima S, Kohagura K, Xu HL, Sugawara A, Abe T, Satoh F, Takeuchi K, Ito S. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J Am Soc Nephrol 14: 2255–2263, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 44: 595–601, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bidani AK, Griffin KA, Picken M, Lansky DM. Continuous telemetric blood pressure monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol Renal Fluid Electrolyte Physiol 265: F391–F398, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Brown NJ, Nakamura S, Ma L, Nakamura I, Donnert E, Freeman M, Vaughan DE, Fogo AB. Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int 58: 1219–1227, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Brunner HR, Laragh JH, Baer L, Newton MA, Goodwin FT, Krakoff LR, Bard RH, Buhler FR. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med 286: 441–449, 1972 [DOI] [PubMed] [Google Scholar]
- 7. Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 1: 256–262, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 55: 99–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359: 995–1003, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest 106: 103–106, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension 43: 364–369, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Gavras H, Lever AF, Brown JJ, Macadam RF, Robertson JI. Acute renal failure, tubular necrosis, and myocardial infarction induced in the rabbit by intravenous angiotensin II. Lancet 2: 19–22, 1971 [DOI] [PubMed] [Google Scholar]
- 15. Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 98: 1063–1068, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Griffin KA, Abu-Amarah I, Picken M, Bidani AK. Renoprotection by ACE inhibition or aldosterone blockade is blood pressure-dependent. Hypertension 41: 201–206, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Griffin KA, Bidani AK. Progression of renal disease: renoprotective specificity of renin-angiotensin system blockade. Clin J Am Soc Nephrol 1: 1054–1065, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN. Vascular endothelial cell specific NF-κB suppression attenuates hypertension-induced renal damage. Circ Res 101: 268–276, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA 92: 3521–3525, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res 107: 263–270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michel F, Ambroisine ML, Duriez M, Delcayre C, Levy BI, Silvestre JS. Aldosterone enhances ischemia-induced neovascularization through angiotensin II-dependent pathway. Circulation 109: 1933–1937, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Morris M, Li P, Callahan MF, Oliverio MI, Coffman TM, Bosch SM, Diz DI. Neuroendocrine effects of dehydration in mice lacking the angiotensin AT1a receptor. Hypertension 33: 482–486, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Rocha R, Chander PN, Zuckerman A, Stier CT., Jr Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension 33: 232–237, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Spurney RF, Ibrahim S, Butterly D, Klotman PE, Sanfilippo F, Coffman TM. Leukotrienes in renal transplant rejection in rats. Distinct roles for leukotriene B4 and peptidoleukotrienes in the pathogenesis of allograft injury. J Immunol 152: 867–876, 1994 [PubMed] [Google Scholar]
- 26. Spurney RF, Ruiz P, Pisetsky DS, Coffman TM. Enhanced renal leukotriene production in murine lupus: role of lipoxygenase metabolites. Kidney Int 39: 95–102, 1991 [DOI] [PubMed] [Google Scholar]
- 27. van Kats JP, Methot D, Paradis P, Silversides DW, Reudelhuber TL. Use of a biological peptide pump to study chronic peptide hormone action in transgenic mice. Direct and indirect effects of angiotensin II on the heart. J Biol Chem 276: 44012–44017, 2001 [DOI] [PubMed] [Google Scholar]