Abstract
The transitional epithelium of the bladder, the urothelium, is a challenging tissue to study due to its fragility, complex cellular makeup, stratified composition, and intimate connections to both neural and connective tissue elements. With the increasing focus on the urothelium as a mechanosensory tissue with complex autocrine and paracrine signaling activities, there have arisen a number of unresolved controversies in the urothelial literature regarding whether certain important sensory and signaling proteins are expressed by the urothelium. Prominent examples of this include the transient receptor potential (TRP) family member TRPV1 and the purinergic receptor P2X3. The problem is more than one of scientific bookkeeping since studies utilizing genetic models (primarily knockout mice) claim additional credibility for urothelial functions when phenotypes are discovered. Furthermore, both of the above-mentioned receptors are important therapeutic targets for various bladder disorders including inflammatory and neuropathic pain. The reasons for the confusion about urothelial expression are manifold, but they likely include low expression levels in some cases, poor specificity of antibodies (sometimes lacking adequate controls), the presence of nonurothelial cells resident within the urothelium, and the fact that the urothelium is particularly prone to aspecific adsorption of antibodies. In this review, we attempt to summarize some of the pitfalls with currently accepted practices in this regard, as well as to describe a set of guidelines which will improve the reliability of conclusions related to urothelial expression. It is hoped that this will be of value to investigators studying the urothelium, to those attempting to interpret conflicts in the literature, and hopefully also those charged with reviewing unpublished work. These recommendations will outline a set of “baseline” and “best practice” guidelines by which both researchers and reviewers will be able to evaluate the evidence presented.
Keywords: bladder, localization, sensory, signaling, umbrella cell
Function of the Urothelium
the urothelium is a highly specialized layer of stacked epithelial cells which lines the inner surface of the mammalian urinary bladder. The most obvious function of this layer is to permit the accommodation and expulsion of large volumes of urine without allowing the components of that urine to diffuse across during prolonged storage (26). This sounds simple enough in principle and is nothing that a plastic bag cannot do. However, implicit in this one sentence description are a number of daunting physiological challenges. First, these cells must not leak, but at the same time they must be distensible enough to allow a major expansion in overall bladder surface area. To “not leak,” the urothelium must maintain a permeability barrier to osmotic and chemical gradients which can be extremely large. For example in humans, urine osmolality can be two to four times higher than that of blood, creating osmotic pressures of 5–20 atmospheres (3,800–15,200 mmHg), while urine concentrations of acid and urea can be orders of magnitude higher than that of isotonic blood plasma (25). The geometry of the bladder as a sac-shaped organ means that when filled it has a stretched and flattened urothelium with blood capillaries which infiltrate the lamina propria only a few micrometers away from the urine (25, 29). These cells must maintain a permeability barrier and tight junction integrity at the same time as they are enduring large mechanically deforming forces such as stretch (during filling and storage) and compression (during voiding).
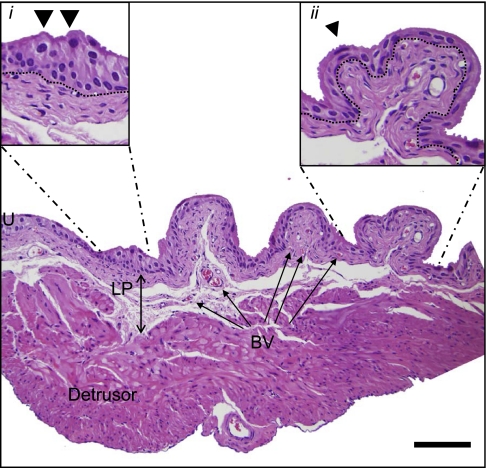
The second aforementioned urothelial attribute, namely, an ability to undergo surface area expansion, requires consideration of the geometry of the bladder as well as the unique abilities of the cells themselves. In its empty state the inner surface of the bladder is highly folded into deep rugae, which disappear as the bladder fills and unfolds. An example of this foldability when empty can be seen in Fig. 4A. The urothelium by necessity is highly distensible so that in addition to the unfolding which takes place initially, it gradually becomes thinner. Indeed, the number of cell layers which can be discriminated in tissue sections, diminishes as well (see Fig. 1). Even in a semistretched bladder with infoldings, there are regions of thickened urothelium with multiple cell layers and plump cuboidal superficial cells (Fig. 1, inset i), as well as flattened stretched urothelium apparently only two cell layers deep (Fig. 1, inset ii). Even the nucleus (indicated by the arrowhead in Fig. 1) is flattened and curves to accommodate the overall shape change of the umbrella cell.
Fig. 4.
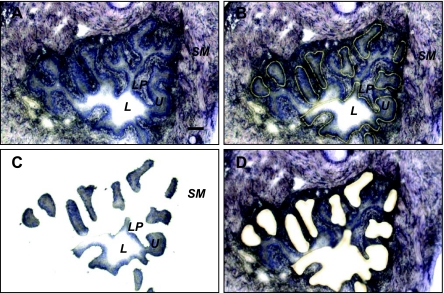
Laser-capture microdissection of urothelium from mouse bladder. Cryosections (10 μm) of normal mouse bladder were stained with Arcturus histogene stain, imaged (×20 objective) and laser microdissected on an ArcturusXT Laser Capture Microdissection System. A: intact bladder section before microdissection (L, lumen; U urothelium; LP, lamina propria; SM, smooth muscle). B: same bladder section showing laser outline before cells were removed. C: cells recovered. D: same section after urothelial cell removal. Scale bar = 100 μm.
Fig. 1.
Bladder morphology. Hematoxylin and eosin staining of fixed cryosectioned (6 μm) mouse bladder shows the trilaminar structural elements, which include urothelium (U), lamina propria (LP; double-headed arrow), and detrusor smooth muscle. Blood vessels (BV) are indicated within the LP. Inset i, expanded region of U which is compressed between folds and exhibits thickening and multiple cell layers; inset ii, expanded region of U which is much thinner and exhibits cell compression. Arrowheads point to umbrella cell nuclei, and dotted line indicates U/LP boundary. Scale bar = 100 μm.
While hydrostatic pressure imparted by slowly accumulating urine increases membrane tension making this layer thinner, it also has the effect of initiating a biochemical response in the superficial cells of the urothelium, which results in a net wave of exocytosis, causing large numbers of intracellular vesicles to traffic to and fuse with the apical membrane (31, 63, 80). This has the consequence of increasing the overall surface area of the urothelium in contact with urine and therefore the volume which can be accommodated.
The urothelium must also communicate information to the underlying sensory nerve fibers to reflect its degree of physical distension, so that both sympathetic and parasympathetic neurons can coordinate their activities to ensure synchronized detrusor smooth muscle contraction and urethral relaxation (17). One of the key neurotransmitters released by the urothelium in response to hydrostatic pressure induced stretch is ATP (21, 42, 80). Since there are multiple purinergic receptors expressed throughout the bladder wall, secreted ATP can elicit membrane depolarization (via P2X receptors) or G protein-coupled signaling via P2Y receptors on multiple cell types. Furthermore, metabolites of ATP including adenosine can bind to cognate receptors and initiate intracellular responses necessary for an appropriate voiding reflex (40, 82). The sensory and signal transduction functions of the urothelium are rapidly becoming hot areas for investigation as they carry the promise of providing explanations for certain syndromes of bladder pain and disease.
Structure of the Urothelium
To fulfill the many functions alluded to, mammals have evolved a highly specialized transitional epithelium which features stratified layers. Effectively, the layers on top are created by a transformation of the cells from the layers which lie below. The urothelium is therefore composed of several layers of cells which differ markedly in their morphology, function, and repertoire of expressed proteins (72).
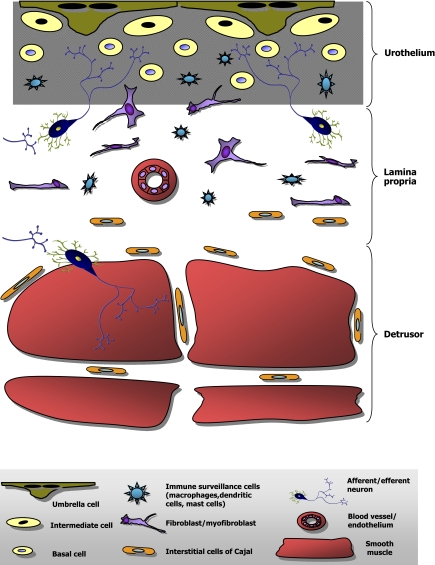
Figure 2 summarizes schematically the major cell types present in the bladder and their locations relative to the urothelium, stroma, and smooth muscle. Interfacing with the urine and sitting atop the urothelium is a single layer of highly differentiated superficial cells known as umbrella cells. These cells are large, hexagonal in shape, often multinucleated, and express a specialized multiprotein complex which forms an entirely unique paracrystalline array of particles inserted at high density into the apical membrane and covering a large proportion of its surface area (25, 72). These particles, composed of uroplakin proteins, are thought to contribute to the permeability barrier of the bladder (28, 73–74, 78) as well as to the differentiation program which creates the umbrella cell (34). Umbrella cells express high concentrations of uroplakins both in the apical membrane and within the richly abundant subapical fusiform and/or discoidal vesicles which are available for exocytosis (72).
Fig. 2.
Cellular diversity within the bladder. Simplified schematic diagram of the layers of the bladder shows the relative locations of different cell types and the complex cellular milieu near and within the urothelium.
Lying beneath the umbrella cells are two or more layers of much smaller epithelial cells known as intermediate and basal cells, which appear to be undifferentiated precursors able to undergo programmed differentiation into umbrella cells when required. Urothelial stem cells reside within this niche also and constitute ∼9% of basal cells (36). Uroplakins are expressed within all urothelial cell layers in rodents but appear to be primarily expressed in the umbrella cells of large mammals like cows and humans (72), indicating some species specificity to expression patterns. Another example of differentiation-dependent expression occurs with cytokeratins. Basal/intermediate cells express cytokeratin 17, but this protein is completely absent in umbrella cells. Conversely, umbrella cells exhibit robust expression of cytokeratin 20, which is entirely lacking in the underlying epithelium (54). An experimental model of urothelial regeneration in rats exposed to high dietary sodium saccharin showed that a differentiation-dependent switch occurs in cytokeratin expression during the process of umbrella cell renewal (54). This renewal can take place rapidly. In a model of protamine sulfate-mediated umbrella cell destruction, a functional reconstitution of the umbrella cell layer occurred within 7 days (37). Remarkably, barrier function as assessed by transepithelial resistance, and urea and water permeability was restored within 72 h, thus emphasizing its critical importance to maintaining homeostasis.
Immediately subjacent to the basal cells is the lamina propria containing basement membrane and connective tissue elements, blood vessels, and a diversity of cell types including fibroblasts, myofibroblasts, interstitial cells of Cajal, afferent nerve fibers, and immune cells (30, 49, 51, 69, 79, 81). However, some of these cells are found within the urothelium also. In particular, afferent neurons intercalate between basal and intermediate epithelial cells and reach up and appear to end in close proximity to the basolateral membrane of umbrella cells. Also, immune system cells like mast and dendritic cells can be found within the epithelium. (14, 22, 35). Thus the urothelial layer is a complex mixture of transitional epithelium, neurons, and immune surveillance myeloid cells.
How is Urothelial Protein Expression Defined?
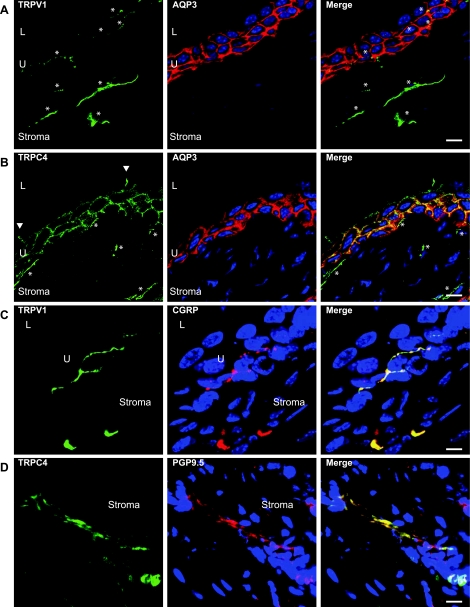
The usual tools employed by investigators interested in studying the urothelium are immunoblotting of urothelial scrapings or enzymatic digests, RT-PCR, immunohistochemistry, in situ hybridization, fluorescently activated cell sorting (FACS), and/or functional assays which often involve pharmacological approaches. In many settings, the simplest and most effective means to determine whether a protein is expressed in a particular tissue (like the urothelium) or cell type (say, umbrella cells) is to use immunohistochemistry. Seeing, after all, is believing. The advantages of this approach relate to the relative ease of the technique and the optical resolution available with modern microscopes and techniques, which include laser scanning confocal immunofluorescence (IF) microscopy. It is often possible to identify single cells, which light up or stain with an antibody, within mixed cell populations and then to colocalize with other marker proteins to confirm the assignation. This approach works particularly well when the cell type in question has a unique morphology, e.g., neurons. Examples of the utility and resolution of these techniques are shown in Fig. 3. The left panels show TRPV1 (Fig. 3, A and C) and TRPC4 (Fig. 3, B and D) immunolabeling of urothelium (U) and stroma in green. Figure 3, A and B, shows colocalization of TRPs with aquaporin-3 (AQP3), a useful robust marker of the intermediate and basal urothelial cell borders. There is clearly little to no colocalization of TRPV1 with AQP3 (Fig. 3A), while conversely there is extensive coimmunostaining of TRPC4 with urothelial cell plasma membranes (Fig. 3B, yellow in merged panel). Furthermore, both TRPs can be seen in the stroma, labeling elongated fibrous structures (asterisks). Interestingly, fine green/yellow TRPV1-positive fibers can be seen within the urothelium (Fig. 3A, asterisks). TRPC4 is also highly likely to be expressed by umbrella cells in which it appears restricted to the basolateral membrane domain, since green staining of lateral borders (Fig. 3B, arrowheads) is readily apparent, while there is no staining associated with the apical membrane. Figure 3, A and B, thus allows an assessment of TRP staining in relation to the urothelium. In addition, the particular morphology of TRP-positive structures within the stroma (and within the urothelium for TRPV1; see asterisks in Fig. 3A) suggested the possibility that these proteins were on neurons. Colocalization with neuronal marker proteins like calcitonin gene-related peptide (CGRP) and protein gene product 9.5 (PGP9.5) (Fig. 3, C and D) confirms the likelihood that both TRPV1 and TRPC4 are expressed by sensory afferents in the bladder.
Fig. 3.
Immunofluorescent localization of TRP channels in and near urothelium (U). Multicolor confocal laser scanning immunofluorescence shows the cellular locations of transient receptor potential (TRP) family members TRPV1 (A and C) and TRPC4 (B and D). Antibodies to aquaporin-3 (AQP3) were used to define the intermediate and basal urothelial cell borders (red in A and B), while TRP immunostaining is green. TRP-positive fibers are indicated by asterisks, and the TRPC4-positive lateral boundaries of an umbrella cell are highlighted by arrowheads. Right panels (C and D) show colocalization of both TRPV1 and TRPC4 (green) with neuronal marker proteins calcitonin gene-related peptide (CGRP) and protein gene product 9.5 (PGP9.5; red), indicating that TRP-positive fibers are neurons. Scale bar = 10 μm. L, lumen; U, urothelium. Figure is modified from Ref. 79.
Therefore, IF can provide convincing information on urothelial expression and can sometimes allow conclusions to be drawn about the subcellular location, for example, whether the protein is on the cell surface, within subcellular vesicles, or in the nucleus, etc. However, antibodies are often not so clear in their staining specificity and certainly require adequate controls as well as care in interpretation. The staining pattern can be heavily influenced by the way in which the tissue is prepared and fixed (56). Therefore, even the experienced investigator can be misled. The urothelium appears to be a tissue which has a surprising number of conflicting reports related to protein expression. We will examine two examples where a considerable literature exists and yet the scientific community lacks consensus on the subject of their urothelial expression. These are TRPV1 and P2X3.
TRPV1
The first description of this protein in the bladder appears to have been published in 1993, when Szallasi et al. (60) showed that isotopic resiniferatoxin (RTX; a potent capsaicin analog and specific TRPV1 agonist) could bind to membranes from homogenized rat bladders. Since then it has become apparent that TRPV1, a member of the transient receptor potential family of nonselective cation channels, is activated by heat and protons and thereby integrates stimuli which contribute to transmission of pain sensation by C-type afferent fibers (9, 62). Its involvement in normal micturition reflexes has been convincingly shown in TRPV1−/− mice, which exhibit excessive contractile activity by detrusor and altered voiding intervals (6). What is not so clear, however, is the extent of its cellular expression within the urinary bladder. Initially, TRPV1 expression was thought to be restricted to small-diameter neurons within sensory ganglia, since Northern blotting failed to detect it in any other tissue including bladder (9, 62). The first explicit description of TRPV1 as not only present, but functional within the urothelium, was by Birder et al. (5). In addition to immunolocalizing TRPV1 in rat bladder, they showed that bladder strips and primary cultured urothelial cells from rats and mice released nitric oxide (NO) and intracellular Ca2+ in response to capsaicin stimulation. Since that study, others have provided evidence to support the claim that TRPV1 is present in human urothelium. Approaches used have included immunostaining of biopsy material (39) and functional studies, including patch clamping of human urothelial cells (11). In an interesting approach designed to differentiate the influence of TRPV1 on afferent neurons from other cell types, experiments were performed in spinal cord-injured (SCI) rats. When SCI bladders were stimulated by intravesical RTX, the amplitude and duration of bladder contractions were magnified compared with controls. This implied that some of the therapeutic effects noted for capsaicin and RTX may be through actions on autonomous bladder activity rather than neurogenic in origin (24). Changes in presumptive urothelial TRPV1 activity or expression level have now been reported in patients with idiopathic and neurogenic overactive bladder (3, 45) and in transitional cell carcinoma (38).
In contrast to these studies, however, more recent ones have been unable to confirm the urothelial expression of TRPV1. Yamada et al. (76) demonstrated barely detectable PCR products for TRPV1 in isolated mouse urothelium, no specific TRPV1 antisense-cRNA hybridization to urothelium using in situ hybridization, and no IF staining of TRPV1 in the urothelium. However, IF was able to locate TRPV1-positive nerve fibers in wild-type mice which, significantly, were absent in TRPV1−/− knockout mice. In all cases, appropriate controls were included and confirmed the efficacy of the reagents and the assays. In further functional assays performed in primary cultured urothelial cells, capsaicin concentrations up to 10 μM were unable to elicit any release of intracellular Ca2+, suggesting an absence of TRPV1. Everaerts et al. (20) found similar results with no effect of 10 μM capsaicin on intracellular Ca2+ release in mouse urothelial cells and vanishingly small expression of TRPV1 by quantitative PCR. Using patch-clamp electrophysiology on freshly digested urothelial cells from guinea pig, Xu et al. (75) demonstrated TRPV4-mediated ion currents but no capsaicin-evoked currents, implying the cells lacked TRPV1. Recently, Yu et al. (79) failed to detect TRPV1 in urothelium by IF (Fig. 3, A and C), but CGRP-positive neurons were TRPV1 positive, attesting to the ability of the antibody to detect the protein. Most compellingly of all, data from a recent study employing highly innovative transgenic TRPV1 reporter mice found that TRPV1 expression was restricted to primary afferent neurons with very low-level expression in a few discrete brain areas and in a subset of arteriolar smooth muscle cells. These mice in which the TRPV1 promoter drove expression of placental alkaline phosphatase, lacZ and Cre were exquisitely specific in their ability to report low levels of TRPV1 expression (10). Furthermore, the authors confirmed the expression data using a range of approaches from calcium imaging and whole cell patch-clamp recordings to in situ hybridization. Despite the sensitivity of the reporters, they were unable to detect any expression of TRPV1 in urothelial cells.
Clearly, there are some peculiar difficulties in studying this tissue. In an illuminating comparison of the performance of three different commercial antibodies against TRPV1 in rat and mouse tissues, Everaerts et al. (19) discovered that all three reacted with the urothelium, while only two of three passed the “positive control test” by labeling trigeminal ganglia. Of most interest, however, was the finding that all three antibodies to TRPV1 labeled urothelium of bladders from TRPV1−/− mice, leading the authors to conclude there was significant aspecific staining. This suggests there is some molecular or structural element, perhaps unique to the urothelium, which contributes to high rates of false positives by antibody staining. In fact, the problem of apparent nonspecificity of antibody labeling of the urothelium for many proteins appears to be widespread. It is unclear why this is, but one explanation may be that the cells are unusually viscid. Caution is therefore necessary when the results of urothelial immunohistochemistry are interpreted. Nonetheless, while antibody labeling can be problematic, investigators using a variety of other techniques have also arrived at conflicting conclusions. This indicates that some of the experimental systems employed may be misleading.
P2X3
P2X3 is an ATP-gated ion channel normally associated with sensory innervation in nerve fibers and has been strongly associated with the neurology of pain (15, 68). In 2000 and 2001, a raft of papers were published on the location of P2X3 in the bladder as well as the effect on that organ, of knocking it out (16, 18, 40, 64, 77). The first immunolocalization studies by Burnstock's group (40, 64) identified P2X3 in nerve bundles within the detrusor smooth muscle of rats and mice, but not in the urothelium. The importance of this particular purinoceptor to overall bladder function was dramatically confirmed in knockout mice, which were shown to lack a normal micturition reflex and tended to consistently overfill their bladders by 70–80% (16). At about the same time, a study of human bladder samples found that P2X3 was restricted to nerve fibers within the suburothelial region (77); however, in another study by Elneil et al. (18), the authors claimed the first identification of P2X3 in the urothelium. Both rat and human bladders appeared to be positive for P2X labeling (18). Their controls included preadsorption of primary antibody with peptide, which eliminated urothelial staining, and incubation of cryosections with secondary antibody alone, suggesting that there was genuine specificity in the binding of the primary antibody.
Since those early reports, there have been studies which claimed evidence for expression of P2X3 in urothelium of cats (7), mice (65), rabbits (67), rats (33), and humans (59, 61). Of particular interest was the apparent increase in urothelial P2X3 levels found in biopsies from patients with interstitial cystitis (59, 61). However, changes in P2X3 expression were not seen in the cat model of interstitial cystitis (7).
While the weight of published evidence would tend to favor the conclusion that P2X3 is expressed by the urothelium, the finding of significant aspecific staining of the urothelium by antibodies adds a cautionary note (19). In all of the cited studies, IF, immunohistochemistry, or immunoblotting was used to anchor the conclusion about location. The purpose of this review is to suggest that a higher burden of evidentiary proof is required to help settle some of these debates and to suggest a number of combined experimental approaches which would help in meeting this greater burden.
Structural and Technical Difficulties in Studying the Urothelium
The urothelium is unique because it is a transitional epithelium with a specific set of functions not replicated elsewhere in the body. It consists, as noted, of a heterogeneous collection of epithelia at different stages of differentiation, which include umbrella cells, intermediate cells, and basal/stem cells which clearly differ from each other in their repertoire of expressed proteins. Thus the investigator is immediately confronted with a diversity which should be taken into account when describing a protein as urothelial. Imaging modalities of high resolution such as laser-scanning confocal microscopy can differentiate antibody staining which occurs in umbrella cells vs. suburothelium (see Fig. 3). Furthermore, they can locate proteins on particular membranes and within organelles (27, 79). This approach obviously requires that high-quality antibodies are used and preferably that the cognate peptide is also available which can be used to further ensure the specificity of the antibody.
Even good antibodies may not be sufficient if the protein is present in low abundance and/or its expression is developmentally regulated or is differentiation specific. In a study of P2Y receptor expression in the bladder, Chopra et al. (13) concluded that the P2Y4 purinergic receptor was expressed in the urothelium based on functional assays and pharmacological profiling performed with rat urothelial cells in culture and based on real-time PCR of mRNA extracted from native urothelium. However, immunofluorescence was negative and immunoblotting showed that only one bladder of three gave a positive band for the protein, clearly indicating the limits of antibodies for the visual identification of P2Y4 in the urothelium (13).
The presence of nonurothelial cells within the urothelium creates yet another challenge and may complicate the interpretation of immunohistochemistry. This is particularly true if images of low resolution are obtained, e.g., by epifluorescence. Furthermore, their presence makes it virtually impossible to isolate pure urothelium for immunoblotting if the usual techniques of scraping, dissection, or enzymatic digestion are employed.
Cell culture models offer another way to study the urothelium. The urothelium is stratified and therefore has a complex multilayered phenotype. While some features of the urothelium are certainly retained in culture, the expression of differentiation markers like cytokeratins and uroplakins does not reproduce that seen in vivo (46, 58, 70). Depending on the culture conditions and the way in which cultures are established and maintained, urothelial cells can exist in a proliferative state or in a quiescent, differentiated state. Since the primary purpose of most cell culture is to facilitate cellular expansion, it is usually the former. This is clearly not the “normal” state for the urothelium. Sun has (58) proposed that typical culture conditions for the urothelium induce a phenotypic differentiation program which mimics wound healing. Further complicating interpretation of phenotype fidelity are different culture conditions used by different laboratories and the finding that urothelial lineages vary depending on the region of the bladder from which they are isolated (58). Furthermore, since primary cultured cells lack the appropriate interplay of molecular communication with cells of the mesenchyme, neurons, and smooth muscle, the repertoire of expressed proteins cannot be assumed to replicate with fidelity what is found in vivo. Cultured cells are, of course very useful for conducting functional assays, which provide information about responsiveness to various stimuli. These may be pharmacological or mechanical, for example. However, it would be best to regard most urothelial culture systems as having regenerative or myoepithelial cell properties (70) and to be circumspect when drawing conclusions about expression.
Finally, the physical, chemical, and/or electrostatic properties of the urothelium may make it more viscid, thus facilitating nonspecific antibody adhesion in frozen and paraffin sections. As a result of these many experimental difficulties, we would like to suggest the following guidelines be adopted to reasonably justify a claim of urothelial expression. These are divided into two levels of stringency as recognition that sometimes antibodies can give clear unequivocal results and second, depending on the aims of the study and the relative importance of the urothelial assignation, different levels of proof can be justified. However, if a claim of urothelial expression is contested by other published studies or purports to define some new capability or function of the urothelium, then the second “best practice” level should be employed for greater certainty.
Recommended Experimental Approaches for Determining Urothelial Protein Expression
Baseline.
The following techniques and controls should be used in conjunction with each other. Alone, they are insufficient for the reasons outlined earlier.
RT-PCR.
RT-PCR is a simple but essential procedure for demonstrating that the urothelium is expressing an mRNA for the protein of interest. DNA products obtained by RT-PCR should then be sequenced to confirm that they are correct. If RT-PCR fails to generate a product of the correct size, then it is necessary to confirm that the primers and other experimental details are appropriate by running it again using a positive control tissue known to express the protein in question. If the PCR works in positive controls but does not amplify a mRNA in urothelial extracts, then it can be assumed the protein is not actively being expressed at that time or is not expressed at all.
IMMUNOBLOTTING.
Immunoblotting should be performed to confirm that a protein of the correct molecular weight can be detected in urothelial scrapings or enzymatic digestions. The appropriate control in this instance would be to preadsorb the antibody against its cognate peptide and thereby demonstrate that binding to the blot is abrogated. Assuming the same antibody is used for both Western blotting and immunostaining, an assessment can then be made as to the specificity of the antibody. If many other nonspecific protein bands are present in the blot, then there is a strong possibility for nonspecific interactions with frozen or paraffin sections in immunohistochemistry. An advantage of immunoblotting is that it has the potential to detect stable proteins with long half-lives which may be transcriptionally quiescent and therefore appear negative by RT-PCR. However, this may be considered a rare event.
FACS OR MAGNETICALLY ACTIVATED CELL SORTING (MACS).
FACS or MACS offers a way to isolate a specific population or subpopulation of urothelial cells (70). Utilizing epithelial-specific cell surface markers such as uroplakin, epithelial cell adhesion molecule (EpCAM), or E-cadherin, it is possible to obtain a highly enriched fraction of urothelial cells (23). These can be then be examined in a variety of ways from functional studies (if the cells are maintained/cultured) to molecular characterization with RT-PCR and/or immunoblotting (47). Analyzing sorted cells will offer greater confidence that contaminating cells are not the reason for any positive signal obtained and is especially useful when the target protein under investigation is also expressed by nonurothelial cells in close proximity.
FUNCTIONAL ASSAYS.
Functional assays are extremely useful and have been used to successfully define not just the expression but also important properties of proteins under investigation. The primary techniques used to obtain functional data on urothelial proteins are electrophysiological or flux measurements in Ussing chambers, patch-clamp electrophysiology on isolated urothelial sheets, or urothelial cell clumps and various diverse assays which can readily be applied to cultured urothelial cells.
Perhaps the best example of functional characterization of a newly discovered protein in the urothelium was the amiloride-sensitive sodium channel, which is present in the luminal membrane (41, 43–44). Employing Ussing chambers and conventional as well as ion-sensitive microelectrodes to measure transepithelial resistance, conductance, short-circuit currents, and intracellular Na+ activity, the authors dissected the functional and regulatory signature of an ion channel which was ultimately cloned and shown to be the heteromeric epithelial sodium channel (ENaC) (8). Ussing chamber studies have also allowed dissection of other ion channel activities in the urothelium, including chloride and potassium fluxes in response to hydrostatically induced membrane stretch (66).
The patch-clamp technique for studying ion channels has also proven useful, but as noted in the earlier section on TRPV1 expression, contrasting conclusions have been arrived at even using this highly sensitive and discriminating approach. Charrua et al. (11) were able to demonstrate TRPV1 currents in human cultured urothelial cells while Xu et al. (75) did not see TRPV1 in freshly isolated guinea pig urothelial cells using the same technique.
Where there are known pharmacological reagents for affecting the activity of a target protein, it is always necessary to be mindful of the specificity (or relative lack of), the concentration at which drugs are used and the need for multiple drugs, if possible, to obtain certainty in the conclusions. The potential for off-target effects if promiscuous reagents or inappropriately high concentrations are used cannot be overstated.
Functional assays are often performed in cultured cells and can be highly informative and confirmatory. However, due to the caveats already mentioned, any functional data obtained in isolated or cultured cells to support a claim of expression should be backed by other localization techniques performed on whole tissue.
Each of these techniques provides useful information and employed collectively can provide extremely strong evidence for urothelial expression. If antibody staining is diffuse or weak, however, as may be quite legitimately found for proteins that are predominantly cytoplasmic and/or weakly expressed, the degree of confidence is weakened. Therefore, where possible the following additional techniques or approaches should be employed.
Best practice.
IMMUNOLOCALIZATION.
Immunolocalization will continue to be the major technique of choice for most investigators wishing to determine the expression of their protein in the bladder. For the results to be valid however, we recommend that the specificity of the antibody and technique be confirmed as follows. These recommendations are consistent with the more stringent requirements now being insisted upon by some scientific journals (53, 55–56). While some of the following suggestions are specific to IF, several apply also to immunoperoxidase approaches. IF does enjoy the distinct advantage that multiple markers can be immunostained simultaneously.
1) High-resolution microscopy is required; for IF, a confocal microscope should be used.
2) Dual-color colocalization with known urothelial marker proteins provides additional certainty about location; examples of proteins which can be used are uroplakins/cytokeratin 20 for umbrella cells and AQP3/cytokeratin 17 for intermediate and basal cells. This being said, it is worth noting that absolute certainty about umbrella cell plasma membrane expression can be difficult to arrive at, since it is difficult to distinguish whether a protein is in the basolateral membrane of the umbrella cell or is in the apical region of the intermediate cells which lie immediately below. Claudin 4 can be used to outline the cellular boundaries for all urothelial layers (1, 20, 54, 76, 79).
3) Antibodies should be preadsorbed with the cognate peptide before immunostaining to demonstrate that positive cellular staining is eliminated. While this is highly recommended, it is sometimes impossible in situations where a company will not provide a peptide or the amino acid sequence used to raise the antibody. Therefore, sources which can provide such controls are to be strongly preferred. Indeed, Saper editorializes (55) that such antibodies are “not fit for scientific work” and the policies of that journal prohibit publication of uncharacterized antibodies. It is always safer for the investigator to go with well-characterized and documented antibodies whose efficacy is ensured.
4) If antibody staining is diffuse or faint and does not clearly localize to defined structures (such as cell membranes or intracellular vesicles) or tissue layers, then it may be nonspecific. At a minimum, tissues/organs which are known to express the protein should be obtained and the antibody tested on these to confirm its efficacy and specificity (positive control). In addition, and this point needs to be emphasized, urothelial staining should be specific; i.e., there should not be a generalized equivalent degree/intensity of staining in the lamina propria and/or smooth muscle. It is almost certain that even where a protein is expressed in multiple strata of the bladder wall, there will be differences in its concentration or distribution. Figure 3B illustrates this point since TRPC4 is clearly present in the plasma membrane of all three urothelial cell layers but in addition has a distinctive distribution within the stroma.
5) Tissue sections should be stained with secondary antibody alone to ensure it is not providing a nonspecific signal (secondary antibody control).
6) Sections should always be counterstained with a nuclear label (DAPI or TOPRO-3) and a cell border label such as rhodamine phalloidin for actin staining; this allows precise definition of the urothelium and its boundary (localization control).
IN SITU HYBRIDIZATION.
In situ hybridization of labeled complementary probes to endogenously expressed cellular RNA allows precise definition of the tissue distribution, as well as the temporal expression, of any RNA species of interest. In this way it can become a powerful discriminatory tool. In situ hybridization makes it possible to examine the RNA transcripts within individual cells, such as the urothelium. In situ hybridization was used to demonstrate TRPV4 expression in basal urothelial cells and an apparent lack of urothelial expression for TRPV1 (76). Thus it is possible to use the technique to detect RNAs that are present in only a fraction of cells. Furthermore, it is a technique with extremely high specificity and does not rely on the use of antibodies for primary recognition. Indeed, it can be a powerful confirmatory assay to validate the specificity of antibody binding patterns (53). In some cases, it may be difficult to positively identify low-abundance transcripts.
KNOCKOUT ANIMALS.
If available, the specificity of antibody staining in both immunoblots and immunofluorescence should be tested in animals engineered to lack the functional gene and therefore protein of interest. This provides an absolute reference and is incontrovertible evidence for the fidelity of antibody binding. In cases where a knockout animal has not been generated or is not viable, there are now available strategies based on the Cre/loxP system for producing urothelial-specific knockouts. Wu (50, 83) has utilized the unique specificity of uroplakin expression in the urothelium to create Cre-recombinase transgenic mice in which expression of Cre is driven by the uroplakin II promoter. The availability of these animals means that in theory any protein of interest expressed by the urothelium can be conditionally ablated. In cases where “floxed” mice are not available, this may require the generation of mice engineered to contain flanking loxP sites within critical regions of the gene of interest. However, such efforts would be well worth pursuing to settle some of the more controversial debates regarding sensory and neuronal properties suggested for the urothelium.
LASER-CAPTURE MICRODISSECTION.
The development of microscope-based laser-dissection systems has revolutionized our ability to remove whole cells and cell groupings intact from complex three-dimensional tissues composed of morphologically distinct cell types. Thus it presents itself as a powerful tool for the particular problem being discussed here. Figure 4 illustrates the potential for isolation of highly purified urothelium. Stained paraffin or frozen sections of bladder can be visualized under magnification (Fig. 4A) and specific cells identified and laser captured. Figure 4B shows the whole bladder tissue section mounted on PEN membrane slides following UV laser cutting. The cells of interest are still in place. These regions are then captured on an LCM cap (Fig. 4C) leaving behind the majority of the tissue section (Fig. 4D). From the isolated cells, mRNA can be extracted, amplified, and then real-time quantitative PCR used to identify whether the mRNA for the protein of interest is being expressed and at what level. This technique has been used successfully to identify allelic loss in urothelial cancer (12) and to investigate host urothelial responses to bacterial infection (52). It represents an exciting new methodology for resolving some of the existing controversies about urothelial expression of sensory proteins. Admittedly, capturing large areas of urothelium en bloc carries the risk of coisolation of other cell types, but the enrichment for the urothelium with no contribution of contaminating cells from the lamina propria still makes this a highly discriminating approach.
ELECTRON MICROSCOPY.
Electron microscopy (EM) provides a degree of morphological resolution which is unparalleled. In addition to allowing specific cellular identification, e.g., umbrella cells, subcellular structures can also be readily identified. EM in combination with immunogold labeling can positively identify antigens present on urothelial cells and on structures like cytoplasmic vesicles within those cells. In one study, uroplakin III present on the membranes of intracellular discoidal vesicles in umbrella cells were shown to exocytose to the plasma membrane in response to a stretch stimulus (63), and the same group later showed that this was a Rab11a-dependent process using immunogold to identify both uroplakin III and Rab11a in the same vesicles (32). Immunogold localization in thin-section EM has been used to assess disease-related changes in expression or distribution of urothelial proteins (48) and to confirm the presence of specific gap junction proteins like connexin 43 within interstitial cells of the suburothelium (57). Not all antibodies will work with this technique, so careful selection of reagents and optimization of protocols are required. As with all antibody-based techniques, appropriate controls and specific labeling of cellular structures with gold particles are required.
Concluding Thoughts
Long dismissed as a simple inert barrier designed to hold urine for long periods, the urothelium has begun to surprise us with its dynamic properties and its complex interactions with other functional components of the bladder. It is an exciting time to be deciphering its mysteries since sensory and signaling features hint at fascinating neuroepithelial properties (2, 4). However, our full understanding of these functions is hampered by current uncertainties over whether it really expresses important molecules like TRPV1 and P2X3. Our recommendation is that multiple experimental approaches be employed, which when taken together, “build the case” for urothelial expression until the evidence becomes compelling. An overreliance on the fidelity of antibodies is perhaps the biggest common mistake and, in the absence of independent confirmatory assays, has led to much uncertainty in the field. Convergent data from other assays like in situ hybridization should become mandatory if knockouts are not available. It is hoped that these recommendations will adjust expectations to a higher threshold of proof and that they will help both investigators and reviewers decide whether claims of urothelial expression are reasonably warranted.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK083299 to W. G. Hill.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Bryce MacIver and Dr. John Mathai for helpful critiques in the preparation of this manuscript. We also thank Maximilian von Bodungen for excellent technical support.
REFERENCES
- 1. Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol 287: F305–F318, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology 65: 400–405, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Birder LA, Kanai AJ, Cruz F, Moore K, Fry CH. Is the urothelium intelligent? Neurourol Urodyn 29: 598–602, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA 98: 13396–13401, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol 287: F1084–F1091, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31: 5067–5077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charrua A, Reguenga C, Cordeiro JM, Correiade-Sa P, Paule C, Nagy I, Cruz F, Avelino A. Functional transient receptor potential vanilloid 1 is expressed in human urothelial cells. J Urol 182: 2944–2950, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Cheng L, MacLennan GT, Zhang S, Wang M, Pan CX, Koch MO. Laser capture microdissection analysis reveals frequent allelic losses in papillary urothelial neoplasm of low malignant potential of the urinary bladder. Cancer 101: 183–188, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Chopra B, Gever J, Barrick SR, Hanna-Mitchell AT, Beckel JM, Ford AP, Birder LA. Expression and function of rat urothelial P2Y receptors. Am J Physiol Renal Physiol 294: F821–F829, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christmas TJ, Rode J. Characteristics of mast cells in normal bladder, bacterial cystitis and interstitial cystitis. Br J Urol 68: 473–478, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol 567: 621–639, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407: 1011–1015, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Daly DM, Collins VM, Chapple CR, Grundy D. The afferent system and its role in lower urinary tract dysfunction. Curr Opin Urol 21: 268–274, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Elneil S, Skepper JN, Kidd EJ, Williamson JG, Ferguson DR. Distribution of P2X1 and P2X3 receptors in the rat and human urinary bladder. Pharmacology 63: 120–128, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Everaerts W, Sepulveda MR, Gevaert T, Roskams T, Nilius B, De Ridder D. Where is TRPV1 expressed in the bladder, do we see the real channel? Naunyn Schmiedebergs Arch Pharmacol 379: 421–425, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Everaerts W, Vriens J, Owsianik G, Appendino G, Voets T, De Ridder D, Nilius B. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol 298: F692–F701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol 505: 503–511, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gardiner RA, Seymour GJ, Lavin MF, Strutton GM, Gemmell E, Hazan G. Immunohistochemical analysis of the human bladder. Br J Urol 58: 19–25, 1986 [DOI] [PubMed] [Google Scholar]
- 23. Genheimer CW, Guthrie KI, Shokes JE, Bruce AT, Quinlan SF, Sangha N, Ilagan RM, Basu J, Burnette T, Ludlow JW. Increased urothelial cell detection in the primary bladder smooth muscle cell cultures with dual MACS/qRT-PCR approach. Appl Immunohistochem Mol Morphol 19: 184–189, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Gevaert T, Vandepitte J, Ost D, Nilius B, De Ridder D. Autonomous contractile activity in the isolated rat bladder is modulated by a TRPV1 dependent mechanism. Neurourol Urodyn 26: 424–432, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Hicks RM. The mammalian urinary bladder: an accommodating organ. Biol Rev Camb Philos Soc 50: 215–246, 1975 [DOI] [PubMed] [Google Scholar]
- 26. Hicks RM, Ketterer B, Warren RC. The ultrastructure and chemistry of the luminal plasma membrane of the mammalian urinary bladder: a structure with low permeability to water and ions. Philos Trans R Soc Lond B Biol Sci 268: 23–38, 1974 [DOI] [PubMed] [Google Scholar]
- 27. Hill WG, Meyers S, von Bodungen M, Apodaca G, Dedman JR, Kaetzel MA, Zeidel ML. Studies on localization and function of annexin A4a within urinary bladder epithelium using a mouse knockout model. Am J Physiol Renal Physiol 294: F919–F927, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu P, Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, Sun TT. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol 283: F1200–F1207, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Inoue T, Gabella G. The interface between epithelium and lamina propria in the rat urinary bladder. Arch Histol Cytol 55 Suppl: 157–163, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Johnston L, Woolsey S, Cunningham RM, O'Kane H, Duggan B, Keane P, McCloskey KD. Morphological expression of KIT positive interstitial cells of Cajal in human bladder. J Urol 184: 370–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khandelwal P, Ruiz WG, Apodaca G. Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway. EMBO J 29: 1961–1975, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khandelwal P, Ruiz WG, Balestreire-Hawryluk E, Weisz OA, Goldenring JR, Apodaca G. Rab11a-dependent exocytosis of discoidal/fusiform vesicles in bladder umbrella cells. Proc Natl Acad Sci USA 105: 15773–15778, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim JC, Yoo JS, Park EY, Hong SH, Seo SI, Hwang TK. Muscarinic and purinergic receptor expression in the urothelium of rats with detrusor overactivity induced by bladder outlet obstruction. BJU Int 101: 371–375, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Kong XT, Deng FM, Hu P, Liang FX, Zhou G, Auerbach AB, Genieser N, Nelson PK, Robbins ES, Shapiro E, Kachar B, Sun TT. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol 167: 1195–1204, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem 55: 443–452, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Kurzrock EA, Lieu DK, Degraffenried LA, Chan CW, Isseroff RR. Label-retaining cells of the bladder: candidate urothelial stem cells. Am J Physiol Renal Physiol 294: F1415–F1421, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, Zeidel ML. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol 283: F242–F253, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Lazzeri M, Vannucchi MG, Spinelli M, Bizzoco E, Beneforti P, Turini D, Faussone-Pellegrini MS. Transient receptor potential vanilloid type 1 (TRPV1) expression changes from normal urothelium to transitional cell carcinoma of human bladder. Eur Urol 48: 691–698, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Lazzeri M, Vannucchi MG, Zardo C, Spinelli M, Beneforti P, Turini D, Faussone-Pellegrini MS. Immunohistochemical evidence of vanilloid receptor 1 in normal human urinary bladder. Eur Urol 46: 792–798, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol 163: 2002–2007, 2000 [PubMed] [Google Scholar]
- 41. Lewis SA, Diamond JM. Na+ transport by rabbit urinary bladder, a tight epithelium. J Membr Biol 28: 1–40, 1976 [DOI] [PubMed] [Google Scholar]
- 42. Lewis SA, Lewis JR. Kinetics of urothelial ATP release. Am J Physiol Renal Physiol 291: F332–F340, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Lewis SA, Wills NK. Apical membrane permeability and kinetic properties of the sodium pump in rabbit urinary bladder. J Physiol 341: 169–184, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lewis SA, Wills NK. Localization of the aldosterone response in rabbit urinary bladder by electrophysiological technics. Ann NY Acad Sci 372: 56–63, 1981 [DOI] [PubMed] [Google Scholar]
- 45. Li M, Sun Y, Simard JM, Chai TC. Increased transient receptor potential vanilloid type 1 (TRPV1) signaling in idiopathic overactive bladder urothelial cells. Neurourol Urodyn 30: 606–611, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Liebert M, Wedemeyer G, Chang JH, Stein JA, McKeever PE, Carey TE, Flint A, Steplewski Z, Buchsbaum DJ, Wahl RL. Comparison of antigen expression on normal urothelial cells in tissue section and tissue culture. J Urol 144: 1288–1292, 1990 [DOI] [PubMed] [Google Scholar]
- 47. Lieu DK, Degraffenried LA, Isseroff RR, Kurzrock EA. Beta1 integrin expression pattern in transitional urothelium does not allow for efficient stem cell enrichment as in other epithelia. Tissue Eng 13: 263–270, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Limas C, Cutler B, Lange P. Ultrastructural localization of blood group antigen A in normal and neoplastic urothelium. Histopathology 18: 1–10, 1991 [DOI] [PubMed] [Google Scholar]
- 49. McCloskey KD. Interstitial cells in the urinary bladder—localization and function. Neurourol Urodyn 29: 82–87, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Mo L, Cheng J, Lee EY, Sun TT, Wu XR. Gene deletion in urothelium by specific expression of Cre recombinase. Am J Physiol Renal Physiol 289: F562–F568, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Rasmussen H, Rumessen JJ, Hansen A, Smedts F, Horn T. Ultrastructure of Cajal-like interstitial cells in the human detrusor. Cell Tissue Res 335: 517–527, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Reigstad CS, Hultgren SJ, Gordon JI. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J Biol Chem 282: 21259–21267, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Rhodes KJ, Trimmer JS. Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci 26: 8017–8020, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Romih R, Jezernik K, Masera A. Uroplakins and cytokeratins in the regenerating rat urothelium after sodium saccharin treatment. Histochem Cell Biol 109: 263–269, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol 493: 477–478, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol 465: 161–163, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int 90: 118–129, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Sun TT. Altered phenotype of cultured urothelial and other stratified epithelial cells: implications for wound healing. Am J Physiol Renal Physiol 291: F9–F21, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Sun Y, Chai TC. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol 171: 448–452, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Szallasi A, Conte B, Goso C, Blumberg PM, Manzini S. Characterization of a peripheral vanilloid (capsaicin) receptor in the urinary bladder of the rat. Life Sci 52: P221–P226, 1993 [DOI] [PubMed] [Google Scholar]
- 61. Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR. P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int 93: 1344–1348, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543, 1998 [DOI] [PubMed] [Google Scholar]
- 63. Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 13: 830–846, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21: 5670–5677, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Walczak JS, Price TJ, Cervero F. Cannabinoid CB1 receptors are expressed in the mouse urinary bladder and their activation modulates afferent bladder activity. Neuroscience 159: 1154–1163, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Wang EC, Lee JM, Johnson JP, Kleyman TR, Bridges R, Apodaca G. Hydrostatic pressure-regulated ion transport in bladder uroepithelium. Am J Physiol Renal Physiol 285: F651–F663, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest 115: 2412–2422, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wirkner K, Sperlagh B, Illes P. P2X3 receptor involvement in pain states. Mol Neurobiol 36: 165–183, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int 91: 89–93, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Woodman JR, Mansfield KJ, Lazzaro VA, Lynch W, Burcher E, Moore KH. Immunocytochemical characterisation of cultures of human bladder mucosal cells. BMC Urol 11: 5, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. Uroplakins in urothelial biology, function, and disease. Kidney Int 75: 1153–1165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wu XR, Manabe M, Yu J, Sun TT. Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J Biol Chem 265: 19170–19179, 1990 [PubMed] [Google Scholar]
- 74. Wu XR, Sun TT. Molecular cloning of a 47 kDa tissue-specific and differentiation-dependent urothelial cell surface glycoprotein. J Cell Sci 106: 31–43, 1993 [DOI] [PubMed] [Google Scholar]
- 75. Xu X, Gordon E, Lin Z, Lozinskaya IM, Chen Y, Thorneloe KS. Functional TRPV4 channels and an absence of capsaicin-evoked currents in freshly-isolated, guinea-pig urothelial cells. Channels (Austin) 3: 156–160, 2009 [DOI] [PubMed] [Google Scholar]
- 76. Yamada T, Ugawa S, Ueda T, Ishida Y, Kajita K, Shimada S. Differential localizations of the transient receptor potential channels TRPV4 and TRPV1 in the mouse urinary bladder. J Histochem Cytochem 57: 277–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yiangou Y, Facer P, Ford A, Brady C, Wiseman O, Fowler CJ, Anand P. Capsaicin receptor VR1 and ATP-gated ion channel P2X3 in human urinary bladder. BJU Int 87: 774–779, 2001 [DOI] [PubMed] [Google Scholar]
- 78. Yu J, Manabe M, Wu XR, Xu C, Surya B, Sun TT. Uroplakin I: a 27-kD protein associated with the asymmetric unit membrane of mammalian urothelium. J Cell Biol 111: 1207–1216, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yu W, Hill WG, Apodaca G, Zeidel ML. Expression and distribution of transient receptor potential (TRP) channels in bladder epithelium. Am J Physiol Renal Physiol 300: F49–F59, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yu W, Khandelwal P, Apodaca G. Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical surface of bladder umbrella cells. Mol Biol Cell 20: 282–295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yu W, Robson SC, Hill WG. Expression and distribution of ectonucleotidases in mouse urinary bladder. PLoS One 6: e18704, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yu W, Zacharia LC, Jackson EK, Apodaca G. Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol 291: C254–C265, 2006 [DOI] [PubMed] [Google Scholar]
- 83. Zhou H, Liu Y, He F, Mo L, Sun TT, Wu XR. Temporally and spatially controllable gene expression and knockout in mouse urothelium. Am J Physiol Renal Physiol 299: F387–F395, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]