Abstract
Renal aging is characterized by structural changes in the kidney including fibrosis, which contributes to the increased risk of kidney and cardiac failure in the elderly. Studies involving healthy kidney donors demonstrated subclinical age-related nephropathy on renal biopsy that was not detected by standard diagnostic tests. Thus there is a high-priority need for novel noninvasive biomarkers to detect the presence of preclinical age-associated renal structural and functional changes. C-type natriuretic peptide (CNP) possesses renoprotective properties and is present in the kidney; however, its modulation during aging remains undefined. We assessed circulating and urinary CNP in a Fischer rat model of experimental aging and also determined renal structural and functional adaptations to the aging process. Histological and electron microscopic analysis demonstrated significant renal fibrosis, glomerular basement membrane thickening, and mesangial matrix expansion with aging. While plasma CNP levels progressively declined with aging, urinary CNP excretion increased, along with the ratio of urinary to plasma CNP, which preceded significant elevations in proteinuria and blood pressure. Also, CNP immunoreactivity was increased in the distal and proximal tubules in both the aging rat and aging human kidneys. Our findings provide evidence that urinary CNP and its ratio to plasma CNP may represent a novel biomarker for early age-mediated renal structural alterations, particularly fibrosis. Thus urinary CNP could potentially aid in identifying subjects with preclinical structural changes before the onset of symptoms and disease, allowing for the initiation of strategies designed to prevent the progression of chronic kidney disease particularly in the aging population.
Keywords: kidney, diagnostic, translational
it is well established that renal aging is associated with structural changes in the kidney including glomerulosclerosis, interstitial fibrosis, and tubular atrophy (11, 37–39). Furthermore, reduced glomerular filtration rate (GFR) and elevated urinary protein excretion are common to the elderly and are associated with increased risk of kidney failure, cardiovascular disease, and mortality (7, 25, 27). However, much less is known about the risk of adverse outcomes with age-related structural changes in the kidney. Most recently, renal aging has been highlighted in a study investigating renal structural changes in healthy kidney donors across six decades of life (28). Here, Rule et al. (28) found subclinical age-related nephropathy on implantation renal biopsy of donors, which was otherwise undetectable using conventional clinical diagnostic tests including GFR and urine albumin excretion. The investigators concluded that age-associated structural changes in the kidney may occur earlier than thought and emphasized a high-priority need for the development of urinary biomarkers to detect the presence of preclinical renal structural changes predictive of future adverse renal sequelae. Such biomarkers could aid in preventive strategies, which subsequently could delay age-related renal impairment and reduce the burden of chronic kidney disease (CKD).
C-type natriuretic peptide (CNP) is a 22-amino acid peptide, which is primarily produced by endothelial cells and possesses potent antifibrotic and antiproliferative properties through the activation of the natriuretic peptide receptor B (NPR-B) and the generation of the second messenger cGMP (8, 16, 18). We recently demonstrated that a progressive decline in circulating CNP characterizes natural aging and is strongly associated with a reciprocal increase in cardiac fibrosis which preceded cardiac dysfunction (29). Although CNP lacks natriuretic and diuretic activity, CNP may have important renal-protective properties which serve to maintain renal structure and function, such as inhibition of glomerular fibrosis after renal injury (4). Further within the kidney, CNP is locally synthesized in both mesangial and renal epithelial cells (5, 10, 17, 21). Importantly, Lewko et al. (20) have reported that injury to podocytes increases CNP production, perhaps as a compensatory protective mechanism, in cultured podocytes and in podocytes harvested from a model of experimental diabetes. In humans, studies by Cataliotti et al. (5) have demonstrated that urinary CNP is elevated in human nephrotic syndrome and that protein restriction reduces urinary CNP excretion independent of any change in circulating CNP. Taken together, these reports support the concept that CNP may not only play a role in the regulation of renal structure and function but could also serve as a biomarker for renal injury and/or renal structural changes such as fibrosis.
The objectives of this study were to determine urinary CNP excretion in the normal aging Fischer rat and its relationship to circulating CNP, renal structure including localization of renal CNP by immunohistochemistry, and renal function. We tested the hypothesis that urinary CNP would be increased in association with renal aging despite a decrease in circulating CNP. Specifically, we hypothesized that an increase in urinary CNP excretion parallels increases in structural changes such as renal fibrosis and glomerular basement membrane (GBM) thickening in the aging kidney. We also hypothesized that age-related increases in urinary CNP precedes an elevation in proteinuria. To address these hypotheses, we assessed circulating and urinary CNP in a Fischer rat model of experimental aging, which is equivalent to human aging from adolescence to the six decade of life (29), and also determined renal structural and functional adaptations to the aging process. Finally, we investigated the presence and localization of CNP by immunohistochemistry in the kidneys of young and old, healthy human kidney donors.
METHODS
Animals.
Studies were performed in 2-, 11-, and 20-mo-old male Fischer rats (Harlan Laboratories, Madison, WI, n = 8–10/age group, unless otherwise specified). The experimental study was performed in accordance with the Animal Welfare Act and with approval of the Mayo Clinic Institutional Animal Care and Use Committee.
Human renal biopsy tissue.
Human kidney tissue was obtained from core needle biopsy specimens from healthy living kidney donors at the time of kidney donation as previously described (28). A total of six paraffin-embedded renal biopsy specimens were examined in this study. The young group consisted of three female donors with a mean age of 19 yr (age range 18–20 yr), and an old group consisting of three 71-yr-old female donors. Minnesota research authorization was obtained for each donor, and the protocol was approved by the Institutional Review Board at Mayo Clinic (Rochester, MN).
Urine collection.
Rats were placed in metabolic cages with free access to food and water and acclimatized for 24 h. Following the acclimatization period, urine was then collected on ice for 24 h for proteinuria and CNP assessment. Urinary protein excretion was measured by Mayo Medical Laboratories on 24-h urine samples using the pyrogallol red dye-binding assay.
Acute studies for blood pressure, GFR, and plasma collection.
Rats were anesthetized (1.5% isoflurane in oxygen), and PE-50 tubing was placed into the carotid artery for blood pressure (BP) acquisition using CardioSOFT Pro software (Sonometrics, London, ON) and blood collection. The bladder was cannulated for urine collection. The jugular vein was cannulated with PE-50 tubing and was continuously infused with 2% inulin (Sigma, St. Louis, MO) in normal saline. After 60 min of equilibration, a clearance study was performed. The clearance study lasted 60 min, and urine was collected. The blood was collected at the end of the clearance study to calculate GFR, from the clearance of inulin, and for measuring plasma CNP. The collected blood was placed in EDTA tubes on ice (31) and immediately centrifuged at 2,500 rpm at 4°C for 10 min. The plasma was stored in polystyrene tubes at −80°C for future use. Inulin concentrations were measured using the anthrone method for GFR analysis, as previously described (9).
Rat renal tissue.
After the acute study, the rat kidneys were removed for total weights and were then divided into sections. A cross section of the renal tissue was preserved in 10% formalin for histological analysis of fibrosis and CNP, and smaller cube sections were preserved in 2.5% glutaraldehyde for electron microscopy (EM) analysis.
Histological analysis of fibrosis.
Fixed rat renal tissues (n = 7) were dehydrated, embedded in paraffin, and sectioned at a thickness of 4 μm. Collagen and extent of fibrosis were examined with picrosirius red staining. An Axioplan II KS 400 microscope (Carl Zeiss) was used to capture at least four randomly selected images from each slide using a ×20 objective, and KS 400 software was utilized to determine fibrotic area as a percentage of total tissue area.
EM analysis.
Rat renal tissues fixed in 2.5% glutaraldehyde were dehydrated and embedded in a resin mould. Ultrathin sections were cut according to the EM core facility procedures. The glomeruli were imaged at ×5,000 and ×8,000 magnifications using a JEM-1400 transmission electron microscope.
GBM thickness measurements.
Glomerular EM images were captured at ×5,000 magnification from each age group (n = 5) of rats. The thicknesses of the GBM were measured using the application Digital Micrograph (Gatan, Pleasanton, CA). For each rat, 20 measurements were performed by an experienced EM technician, and the data were subjected to an Excel morphometrics macro giving the mean thickness in nanometers (nm).
Plasma and urinary CNP.
As previously described, rat plasma and urinary CNP-22 (31) was determined by commercially available nonequilibrium radioimmunoassay kits from Phoenix Pharmaceutical (Mountain View, CA), using an antibody to human CNP-22 which is fully cross-reactive to rat CNP-22 . One milliliter of plasma was extracted using C18 Bond Elut cartridges. After cartridges were washed with 4 ml 100% methanol and 4 ml water, plasma was applied and the cartridges were washed. Eluates were concentrated on a Savant speed vacuum concentrator, and pellets were resuspended in 300 μl assay buffer. One hundred microliters of standards and samples were incubated with 100 μl anti-human CNP at 4°C. After 18 h, 100 μl (10,000 counts) of 125I-labeled CNP was added and incubated at 4°C for 18 h. Then, a second antibody was added to all samples to separate the free and bound fractions, and the samples were centrifuged, the free fraction was aspirated, and the bound fraction was counted on a gamma counter. A standard curve was generated and used to calculate the concentrations of the unknown samples and reported in picograms per milliliter. The range of the standard curve was 0.5–128 pg, with a lower limit of detection of 0.5 pg. Inter- and intra-assay variability were 11 and 5.2%, respectively. Recovery was 72 ± 6%. Cross-reactivity was <1% with ANP, BNP, endothelin, and adrenomedullin and 97% with CNP-53.
CNP immmunohistochemistry.
The localization of renal CNP immunoreactivity was assessed as previously reported (5, 31). Briefly, slides with paraffin-embedded rat and human renal tissues were incubated in a 60°C oven for 2 h and then deparaffinized using established laboratory procedures. After deparaffinization, slides were incubated with 0.6% hydrogen peroxide in methanol for 20 min at room temperature to block endogenous peroxidase activity, and then 5% normal goat serum was used to block nonspecific protein binding sites before the primary antibody was applied. Sections were placed in a moist chamber for 18–24 h at room temperature with the primary antibody (rabbit anti-human CNP-22, Phoenix Pharmaceutical) at a dilution of 1:500. Control slides were treated with normal rabbit serum. Sections were incubated with goat anti-rabbit IgG covalently linked to horseradish peroxidase and 3-amino-9-ethyl-carbazole substrate for peroxidase visualization and counterstained with hematoxylin to enhance nuclear detail. Stained slides were then viewed and interpreted by a renal pathologist blinded to the age groups.
Statistical analysis.
Results are expressed as means ± SE. Comparisons within groups were made by ANOVA followed by a Newman-Keuls multiple comparison test. A Pearson correlation was performed to calculate the correlation between urinary CNP excretion, renal fibrosis, GBM thickness, as well as GFR and between the urinary-to-plasma CNP ratio, renal fibrosis, and GBM thickness. GraphPad Prism software (GraphPad Software, La Jolla, CA) was used for the above calculations. Statistical significance was accepted as P < 0.05.
RESULTS
Anthropometric, renal characteristics, and hemodynamics with aging.
Body weight (BW), total kidney weight (TKW), plasma creatinine, GFR as well as mean arterial pressure (MAP) are reported in Table 1. There was a significant increase in BW and TKW at 11 mo, which was sustained at 20 mo compared with the 2-mo-old group. When TKWs were normalized to BW, there was a significant reduction in the TKW-to-BW ratio at 11 and 20 mo of age. Furthermore, while plasma creatinine was significantly increased at 11 and 20 mo compared with 2 mo of age, there was a trend for GFR to decrease at 11 and 20 mo. Importantly, there was a significant elevation in MAP only at 20 mo of age.
Table 1.
Summary of body weight, renal characteristics, and blood pressure in aging Fischer rats
| 2 mo | 11 mo | 20 mo | |
|---|---|---|---|
| BW, g | 211 ± 2 | 465 ± 5* | 445 ± 7*† |
| TKW, mg | 1,614 ± 33 | 2,763 ± 73* | 2,750 ± 54* |
| TKW:BW, mg/g | 7.67 ± 0.19 | 5.94 ± 0.15* | 6.18 ± 0.13* |
| Plasma Cr, mg/dl | 0.20 ± 0.00 | 0.39 ± 0.03* | 0.36 ± 0.02* |
| GFR, ml·min−1·kg−1 | 3.52 ± 0.29 | 2.61 ± 0.43 | 2.83 ± 0.30 |
| MAP, mmHg | 90 ± 1 | 91 ± 2 | 100 ± 3*† |
Values are means ± SE; n = 10 for all age groups.
BW, body weight; TKW, total kidney weight; Cr, creatinine; GFR, glomerular filtration rate; MAP, mean arterial pressure.
P < 0.05 vs. 2 mo.
P < 0.05 vs. 11 mo.
Renal fibrosis and glomerular ultrastructure.
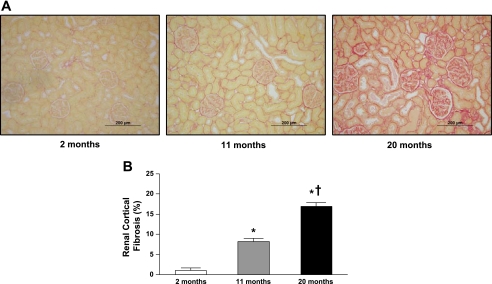
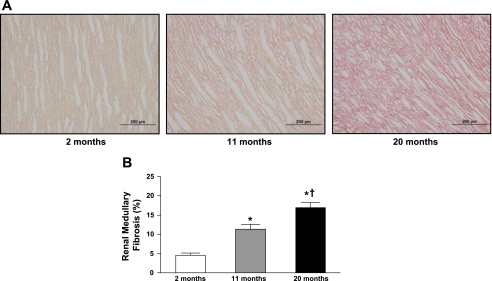
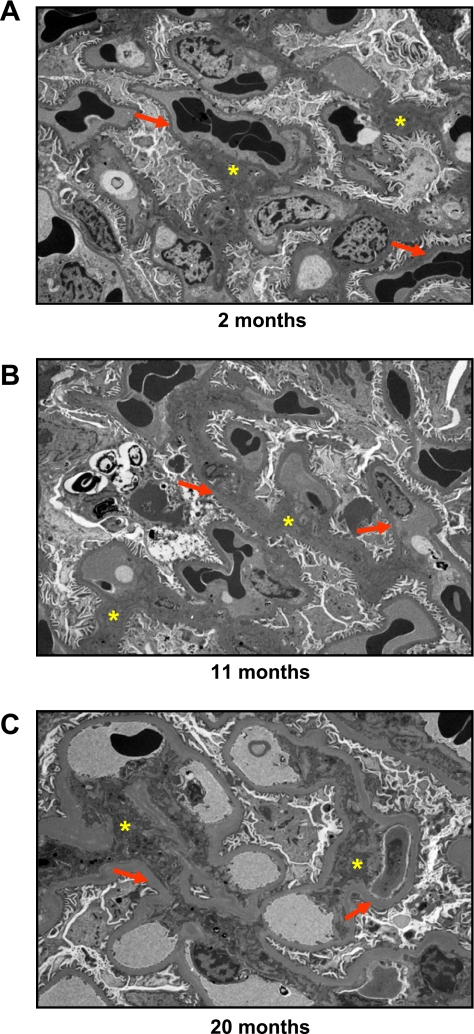
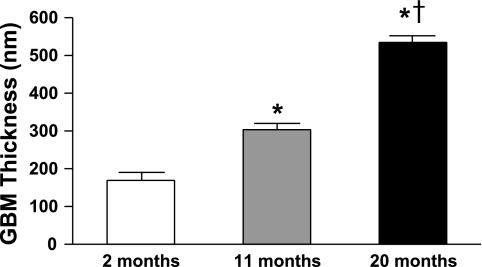
Figures 1 and 2 illustrate representative photomicrographs of the renal cortex (Fig. 1A) and medulla (Fig. 2A) stained with picrosirius red, which provide an estimate of fibrillar collagen deposition. Specifically, there was a significant and progressive increase in cortical (Fig. 1B) and medullary (Fig. 2B) interstitial collagen staining with aging. Furthermore, representative electron photomicrographs of glomeruli are shown in Fig. 3. At 2 mo (Fig. 3A), visceral epithelial cell foot processes were intact. At 11 mo (Fig. 3B), mesangial regions were mildly expanded with matrix and the capillary loop basement membranes exhibited mild thickening compared with 2 mo. At 20 mo (Fig. 3C), there was diffuse expansion of mesangial matrix. The capillary loop basement membranes were thickened and exhibited focal effacement of visceral epithelial cell foot processes compared with 2 and 11 mo of age. Results of morphometric analysis of GBM thickness are shown in Fig. 4. There was progressive and significant thickening of capillary loop basement membranes with aging, where the thickness of basement membranes at 20 mo was almost three times that of the basement membranes at 2 mo of age.
Fig. 1.
Representative histology images at ×20 objective magnification (A) and quantification of picrosirius red staining (B) of renal cortical fibrosis from 2-, 11-. and 20-mo-old Fischer rats. Values are means ± SE; n = 7 for all age groups. *P < 0.05 vs. 2 mo. †P < 0.05 vs. 11 mo.
Fig. 2.
Representative histology images at ×20 objective magnification (A) and quantification of picrosirius red staining (B) of renal medullary fibrosis from 2-, 11-, and 20-mo-old Fischer rats. Values are means ± SE; n = 7 for all age groups. *P < 0.05 vs. 2 mo. †P < 0.05 vs. 11 mo.
Fig. 3.
Representative electron micrographs at ×8,000 magnification of the glomerulus from 2-, 11-, and 20-mo-old Fischer rats. Red arrows, glomerular basement membrane; yellow stars, mesangial matrix.
Fig. 4.
Quantification of glomerular basement membrane thickness from 2-, 11-, and 20-mo-old Fischer rats. Values are means ± SE; n = 5 for all age groups. GBM, glomerular basement membrane. *P < 0.05 vs. 2 mo. †P < 0.05 vs. 11 mo.
Urinary and plasma CNP, urinary-to-plasma CNP ratio, and proteinuria.
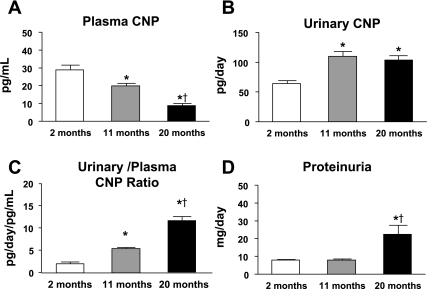
Figure 5 illustrates changes in plasma and urinary CNP with aging. Here, we observed a significant and progressive decrease in plasma CNP (Fig. 5A) between the three age groups (2 mo means ± SE: 29 ± 3 pg/ml; 11 mo means ± SE: 20 ± 1* pg/ml; 20 mo means ± SE: 9 ± 1*† pg/ml; *P < 0.05 vs. 2 mo, †P < 0.05 vs. 11 mo). In contrast, there was a significant increase in urinary CNP excretion at 11 mo which remained elevated at 20 mo (2 mo means ± SE: 64 ± 4 pg/day; 11 mo means ± SE: 110 ± 6* pg/day; 20 mo means ± SE: 103 ± 7* pg/day; *P < 0.05 vs. 2 mo) as illustrated in Fig. 5B. Further, there was also a significant and progressive increase in the urinary to plasma-to-CNP ratio (Fig. 5C: 2 mo means ± SE: 2.2 ± 0.2 pg/day/pg/ml; 11 mo means ± SE: 5.4 ± 0.3* pg/day/pg/ml; 20 mo mean ± SE: 11.7 ± 1.0*† pg/day/pg/ml; *P < 0.05 vs. 2 mo, †P < 0.05 vs. 11 mo). A significant increase in proteinuria (Fig. 5D) was observed only at 20 mo of age.
Fig. 5.
Changes in plasma C-natriuretic peptide (CNP; A), urinary CNP excretion (B), urinary-to-plasma CNP ratio (C), and proteinuria (D) between 2-, 11-, and 20-mo-old Fischer rats. Values are means ± SE; n = 10 for all age groups. *P < 0.05 vs. 2 mo. †P < 0.05 vs. 11 mo.
Urinary CNP excretion and urinary-to-plasma CNP ratio correlations.
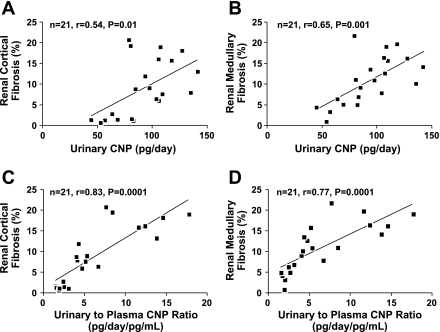
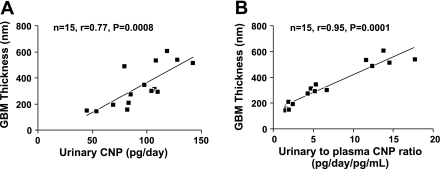
Figures 6 illustrates a positive correlation among urinary CNP, renal cortical fibrosis (Fig. 6A; n = 21, r = 0.54, P = 0.01), and renal medullary fibrosis (Fig. 6B; n = 21, r = 0.65, P = 0.001). There was no correlation between urinary CNP and GFR (n = 30, r = 0.01, P = 0.97). Furthermore, there was a strong positive correlation among urinary-to-plasma CNP ratio, renal cortical fibrosis (Fig. 6C; n = 21, r = 0.83, P = 0.0001), and renal medullary fibrosis (Fig. 6D; n = 21, r = 0.77, P = 0.0001). Figure 7 illustrates a strong positive correlation among GBM thickness, urinary CNP (Fig. 7A; n = 15, r = 0.77, P = 0.0008), and urinary-to-plasma CNP ratio (Fig. 7B; n = 15, r = 0.95, P = 0.0001).
Fig. 6.
Correlations between urinary CNP excretion and renal cortical (A) and medullary (B) fibrosis, as well as between urinary-to-plasma CNP ratio and renal cortical (C) and medullary (D) fibrosis.
Fig. 7.
Correlations between GBM thickness and urinary CNP excretion (A) as well as between GBM thickness and urinary-to-plasma CNP ratio (B).
Immunohistochemical localization of renal tissue CNP.
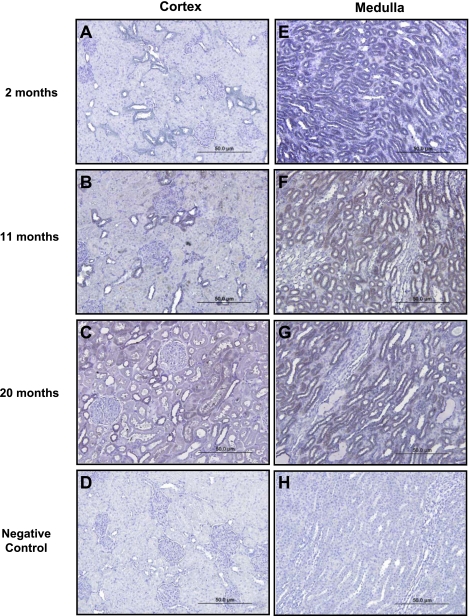
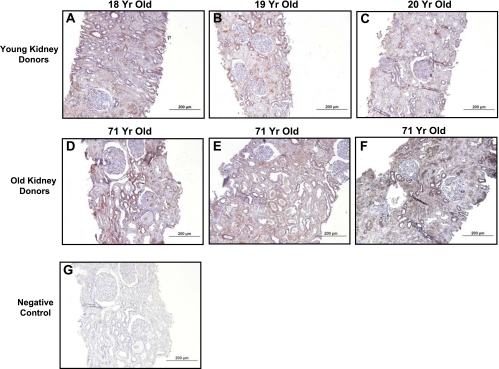
Figure 8 illustrates immunohistochemical localization of CNP in the renal cortex (A–C) and the medulla (E–G) in Fischer rats at 2, 11, and 20 mo of age. At 2 mo of age, there was no significant staining of proximal tubules and staining for CNP was localized to distal tubules within the renal cortex. At 11 mo of age, CNP staining within the renal cortex was predominantly localized to distal tubules, with faint staining of proximal tubules. At 20 mo, strong staining for CNP was observed within distal tubules and focally within proximal tubules of the renal cortex. Strong staining for CNP was observed within distal tubules of the renal medulla and did not appreciably change with age. Additionally, Fig. 9 demonstrates immunohistochemical localization of CNP in young (A–C) and old (D–F) biopsy specimens from healthy human kidney donors. In biopsies obtained from young kidney donors, CNP staining was predominantly localized to distal tubules, with relatively weak, focal staining observed within proximal tubules. In biopsies obtained from old kidney donors (D–F), strong staining of CNP was observed within both distal and proximal tubules.
Fig. 8.
Representative images of CNP immunohistochemical localization in renal cortical (A–C) and medullary (E–G) tissue from 2-, 11-, and 20-mo-old Fischer rats at ×20 objective magnification. D and H: negative controls.
Fig. 9.
CNP immunohistochemical localization in renal tissue from young human donors (A–C) and old human donors (D–F) at ×20 objective magnification. G: negative control.
DISCUSSION
The current study reports for the first time the selective and significant increase in urinary CNP excretion during aging and its strong association with renal fibrosis and GBM thickening, which occurred before the onset of significant proteinuria or BP elevation. Importantly, the increase in urinary CNP excretion observed with renal aging occurred together with a significant increase in the urinary-to-plasma CNP ratio and decrease in circulating CNP and renal function. Furthermore, we also report similar distribution and intensity patterns of CNP localization by immunohistochemistry in young and old rat and human kidneys. Specifically, we observed strong distal and proximal tubular CNP staining in the old kidney of both species. Thus these findings provide evidence that urinary CNP and its ratio with plasma CNP may represent a novel biomarker for early renal structural changes during aging before the appearance of clinical signs.
An increasingly recognized manifestation of natural aging is organ fibrogenesis, which often precedes overt organ dysfunction such as in myocardial aging in which ventricular fibrosis is widespread and occurs before systolic or diastolic dysfunction (29). As aging progresses, myocardial fibrosis increases and impairment in systolic and diastolic function occurs most likely due to a progressive loss in cardiomyocyte number, rarefaction of the coronary microvasculature, and accumulation of replacement fibrosis (15, 23, 36). In the current study, aging was associated with a significant increase in renal fibrosis in both the cortex and medulla at 11 mo, which was similar to the situation seen in the heart of aging rats (29). Furthermore, at 11 mo EM revealed that mesangial regions were mildly expanded with matrix and that the GBM exhibited significant thickening compared with at 2 mo. Importantly, these changes in the extracellular matrix (ECM), at the level of the glomeruli, were not associated with any changes in BP or proteinuria. Consistent with our hypothesis, these renal structural changes at 11 mo were accompanied by a significant increase in urinary CNP excretion despite a significant decrease in circulating CNP. The mechanism of this elevation in urinary CNP excretion observed is most likely multifactorial. Such factors include 1) an increase in renal CNP production; 2) blunted tubular reabsorption of CNP; and/or 3) reduced CNP degradation by the zinc-dependent enzyme neprilysin due to a zinc deficiency often seen with aging (13, 26). However, the rise in urinary CNP excretion is unlikely due to an increased clearance of circulating CNP by the glomerulus, as plasma CNP was significantly reduced and GFR tended to decrease. Together, the reciprocal changes in urinary and plasma CNP levels resulted in a significant elevation in the urinary-to-plasma CNP ratio.
Previous studies have underscored the importance of the kidney as a site for CNP production beyond the endothelium. Specifically, CNP has been reported to be present in the rat kidney (10, 33), mouse podocytes (20), canine tubular cells (22), and human renal cells (5, 19, 21). Furthermore, studies have also demonstrated that gene expression of NPR-B, CNP's primary receptor, is abundant in rodent and human kidneys (3, 20, 32). Here, we demonstrated progressive CNP immunostaining in distal and proximal cortical tubules of the aging rat kidney. Importantly, the presence and localization of CNP immunostaining was also present in the renal tissue from young and old healthy living kidney donors. This CNP immunostaining in the human kidney had similar distribution and intensity patterns of that seen in the rat kidney, with specifically more intense staining in the old kidney of both species. Studies have increasingly supported a fibro-inhibiting role for CNP/NPR-B signaling in terms of both suppressing collagen production and reducing cell proliferation. Particularly, in vivo studies have reported that exogenous CNP inhibited myofibroblast proliferation and activation (6, 34), prevented neointimal formation and thickening after arterial injury (12, 35), and suppressed post-acute myocardial infarction-induced cardiac fibrosis (30). Furthermore, in vitro studies have demonstrated that CNP possesses robust antifibrotic and antiproliferative properties in young rat and adult human cardiac fibroblasts (16, 29). Moreover, others have reported that CNP suppresses human glomerular mesangial cell proliferation and type IV collagen secretion in vitro (24) and potently inhibits glomerular mesangial cell proliferation and matrix accumulation of type IV collagen after renal injury in vivo (4), which is highly relevant to the current study.
Here, we observed that early widespread renal fibrosis and glomerular structural changes at 11 mo were associated with increased urinary CNP excretion. Based upon the known biology of CNP, one might speculate that this increase in urinary CNP excretion, which occurred before the onset of an elevation in proteinuria or BP, may reflect a compensatory production and secretion of CNP from renal tubular cells to suppress pathophysiological fibrogenesis and to preserve glomerular structure in the aging kidney. Importantly, renal fibrosis is a multifactorial and dynamic process in which many cellular events are occurring simultaneously. These events include and are not limited to 1) an increase in ECM production cause by an imbalance between profibrotic cytokines such as transforming growth factor (TGF)-β and antifibrotic peptides such as CNP; 2) a decrease in collagen degradation due to an imbalance between matrix metalloproteinases and tissue inhibitors of metalloproteinases; and 3) mesangial and fibroblast activation. As renal fibrogenesis is a complex process involving numerous factors and pathways, additional studies will be needed to define CNP's role during this process in the aging kidney.
When we extended our studies from 11 mo to the more advanced age of 20 mo, we further supported our hypothesis of a parallel between elevated urinary CNP excretion and progressive renal structural changes. This was now accompanied by a significant increase in proteinuria as well as BP. Specifically, we observed more extensive and diffuse expansion of the mesangial matrix at 20 mo together with markedly thickened GBM and focal effacement of visceral epithelial cell foot processes. Additionally, fibrosis was also further increased in the cortex and medulla at 20 mo, and, despite decreased plasma CNP and GFR, urinary CNP excretion remained elevated in association with these renal structural alterations. Interestingly, normal glomeruli predominantly synthesize type IV collagen (1), which is a major structural component of basement membranes (2), and its production has been shown to be suppressed by CNP (4, 24). Therefore, we postulate that the maintenance of this elevation in urinary CNP excretion at 20 mo may in part serve as a compensatory protective mechanism to suppress the production of various types of renal collagen, which is consistent with other studies involving renal injury (4, 24).
While the pathophysiological role of CNP remains to be defined in the kidney during aging as well as the mechanism of its production and activation, urinary CNP appears to be a possible biomarker which parallels early age-related renal structural changes, particularly cortical and medullary fibrosis and GBM thickening. Such a concept of urinary CNP excretion as a biomarker, particularly in various human diseases, is supported by key human studies. Cataliotti et al. (5) has shown that nephrotic syndrome subjects had significantly higher urinary CNP excretion compared with normal subjects, which subsequently decreased to a greater extent than concomitant reductions in proteinuria in response to a low-protein diet. Moreover, Mattingly et al. (21) has reported that urinary CNP excretion is elevated in congestive heart failure, while Gulberg et al. (14) demonstrated that urinary CNP excretion was increased in cirrhotic patients with impaired renal function but not in cirrhotic patients with normal renal function. Taken together, these studies clearly demonstrate the potential usefulness of urinary CNP excretion in disease states with abnormal renal structure and function. However, to date it is unknown whether urinary CNP could be a useful biomarker for progressive structural changes, including fibrosis, within the aging kidney and should be confirmed in human studies.
There are several limitations to the current study. Additional studies are warranted to define the urinary and circulating relationship of the CNP system during the normal aging process in humans to provide evidence that the current findings have clear clinical relevance. Furthermore, more comprehensive studies are needed beyond aging alone in patients with diseases that are often accompanied by renal fibrosis and remodeling such as hypertension or other renal disease states. These studies would confirm the utility of urinary CNP as a sensitive and specific biomarker for renal structural changes and/or dysfunction. We also need to determine in experimental aging whether physiological and/or pharmacological replacement therapy with CNP can prevent these age-associated renal structural changes in association with lower urinary CNP excretion. Such studies would aid in further elucidating the mechanism involved in the elevation of urinary CNP excretion. The results from these potential studies could support efforts to identify subjects with preclinical renal changes, allowing for the initiation of strategies designed to prevent the progression of CKD particularly in an aging population.
In conclusion, renal aging is characterized by significant renal cortical and medullary fibrosis, together with mild ultrastructural changes in the glomeruli, in 11-mo-old Fischer rats before the onset of proteinuria or the elevation of BP. Importantly, these renal structural changes are paralleled by an increase in urinary CNP excretion despite a reduction in plasma CNP and renal function. With aging to 20 mo, these structural alterations significantly progressed and urinary CNP excretion remained elevated despite further reductions in plasma CNP. Furthermore, immunohistochemistry of the rat and human kidney also demonstrated increased CNP staining in both the aging rat and human renal tubules. These studies support a possible role for urinary CNP and its ratio with plasma CNP as a potential novel biomarker for age-related structural changes in the kidney, warranting further experimental and human studies in aging and renal disease.
GRANTS
This study was supported by Grants RO1 HL36634, RO1 HL83231, RO1 DK90358, and PO1 HL76611 from the National Institutes of Health and the Mayo Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors acknowledge the outstanding contributions of Gerald E. Harders and Sharon M. Sandberg. Part of this work was presented at the 2011 World Congress of Nephrology in Vancouver, Canada.
REFERENCES
- 1. Bergijk EC, Munaut C, Baelde JJ, Prins F, Foidart JM, Hoedemaeker PJ, Bruijn JA. A histologic study of the extracellular matrix during the development of glomerulosclerosis in murine chronic graft-versus-host disease. Am J Pathol 140: 1147–1156, 1992 [PMC free article] [PubMed] [Google Scholar]
- 2. Butkowski RJ, Wieslander J, Kleppel M, Michael AF, Fish AJ. Basement membrane collagen in the kidney: regional localization of novel chains related to collagen IV. Kidney Int 35: 1195–1202, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Canaan-Kuhl S, Jamison RL, Myers BD, Pratt RE. Identification of “B” receptor for natriuretic peptide in human kidney. Endocrinology 130: 550–552, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Canaan-Kuhl S, Ostendorf T, Zander K, Koch KM, Floege J. C-type natriuretic peptide inhibits mesangial cell proliferation and matrix accumulation in vivo. Kidney Int 53: 1143–1151, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Cataliotti A, Giordano M, De Pascale E, Giordano G, Castellino P, Jougasaki M, Costello LC, Boerrigter G, Tsuruda T, Belluardo P, Lee SC, Huntley B, Sandberg S, Malatino LS, Burnett JC., Jr CNP production in the kidney and effects of protein intake restriction in nephrotic syndrome. Am J Physiol Renal Physiol 283: F464–F472, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Chrisman TD, Garbers DL. Reciprocal antagonism coordinates C-type natriuretic peptide and mitogen-signaling pathways in fibroblasts. J Biol Chem 274: 4293–4299, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 8. D'Souza SP, Davis M, Baxter GF. Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther 101: 113–129, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Davidson WD, Sackner MA. Simplification of the anthrone method for the determination of inulin in clearance studies. J Lab Clin Med 62: 351–356, 1963 [PubMed] [Google Scholar]
- 10. Dean AD, Vehaskari VM, Greenwald JE. Synthesis and localization of C-type natriuretic peptide in mammalian kidney. Am J Physiol Renal Fluid Electrolyte Physiol 266: F491–F496, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Floege J, Hackmann B, Kliem V, Kriz W, Alpers CE, Johnson RJ, Kuhn KW, Koch KM, Brunkhorst R. Age-related glomerulosclerosis and interstitial fibrosis in Milan normotensive rats: a podocyte disease. Kidney Int 51: 230–243, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Furuya M, Miyazaki T, Honbou N, Kawashima K, Ohno T, Tanaka S, Kangawa K, Matsuo H. C-type natriuretic peptide inhibits intimal thickening after vascular injury. Ann NY Acad Sci 748: 517–523, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Gandhi MS, Deshmukh PA, Kamalov G, Zhao T, Zhao W, Whaley JT, Tichy JR, Bhattacharya SK, Ahokas RA, Sun Y, Gerling IC, Weber KT. Causes and consequences of zinc dyshomeostasis in rats with chronic aldosteronism. J Cardiovasc Pharmacol 52: 245–252, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gulberg V, Moller S, Henriksen JH, Gerbes AL. Increased renal production of C-type natriuretic peptide (CNP) in patients with cirrhosis and functional renal failure. Gut 47: 852–857, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. The cardiac microvasculature in hypertension, cardiac hypertrophy and diastolic heart failure. Curr Vasc Pharmacol 6: 292–300, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Horio T, Tokudome T, Maki T, Yoshihara F, Suga S, Nishikimi T, Kojima M, Kawano Y, Kangawa K. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology 144: 2279–2284, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Kalra PR, Clague JR, Coats AJ, Anker SD, Poole-Wilson PA, Struthers AD. C-type natriuretic peptide production by the human kidney is blunted in chronic heart failure. Clin Sci (Lond) 118: 71–77, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science 252: 120–123, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Lai KN, Leung JC, Yandle TG, Fisher S, Nicholls MG. Gene expression and synthesis of natriuretic peptides by cultured human glomerular cells. J Hypertens 17: 575–583, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Lewko B, Endlich N, Kriz W, Stepinski J, Endlich K. C-type natriuretic peptide as a podocyte hormone and modulation of its cGMP production by glucose and mechanical stress. Kidney Int 66: 1001–1008, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Mattingly MT, Brandt RR, Heublein DM, Wei CM, Nir A, Burnett JC., Jr Presence of C-type natriuretic peptide in human kidney and urine. Kidney Int 46: 744–747, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Nir A, Beers KW, Clavell AL, Wei CM, Heublein DM, Dousa TP, Burnett JC., Jr CNP is present in canine renal tubular cells and secreted by cultured opossum kidney cells. Am J Physiol Regul Integr Comp Physiol 267: R1653–R1657, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res 68: 1560–1568, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Osawa H, Yamabe H, Kaizuka M, Tamura N, Tsunoda S, Baba Y, Shirato K, Tateyama F, Okumura K. C-Type natriuretic peptide inhibits proliferation and monocyte chemoattractant protein-1 secretion in cultured human mesangial cells. Nephron 86: 467–472, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Peralta CA, Katz R, Sarnak MJ, Ix J, Fried LF, De Boer I, Palmas W, Siscovick D, Levey AS, Shlipak MG. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol 22: 147–155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prasad AS, Fitzgerald JT, Hess JW, Kaplan J, Pelen F, Dardenne M. Zinc deficiency in elderly patients. Nutrition 9: 218–224, 1993 [PubMed] [Google Scholar]
- 27. Rifkin DE, Katz R, Chonchol M, Fried LF, Cao J, de Boer IH, Siscovick DS, Shlipak MG, Sarnak MJ. Albuminuria, impaired kidney function and cardiovascular outcomes or mortality in the elderly. Nephrol Dial Transplant 25: 1560–1567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC, Stegall MD. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 152: 561–567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sangaralingham SJ, Huntley BK, Martin FL, McKie PM, Bellavia D, Ichiki T, Harders GE, Chen HH, Burnett JC., Jr The aging heart, myocardial fibrosis, and its relationship to circulating C-type natriuretic peptide. Hypertension 57: 201–207, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soeki T, Kishimoto I, Okumura H, Tokudome T, Horio T, Mori K, Kangawa K. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol 45: 608–616, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittelkow MR, Burnett JC., Jr Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol Heart Circ Physiol 263: H1318–H1321, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Suga S, Nakao K, Mukoyama M, Arai H, Hosoda K, Ogawa Y, Imura H. Characterization of natriuretic peptide receptors in cultured cells. Hypertension 19: 762–765, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Suzuki E, Hirata Y, Hayakawa H, Omata M, Kojima M, Kangawa K, Minamino N, Matsuo H. Evidence for C-type natriuretic peptide production in the rat kidney. Biochem Biophys Res Commun 192: 532–538, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Tao J, Mallat A, Gallois C, Belmadani S, Mery PF, Nhieu JT, Pavoine C, Lotersztajn S. Biological effects of C-type natriuretic peptide in human myofibroblastic hepatic stellate cells. J Biol Chem 274: 23761–23769, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Ueno H, Haruno A, Morisaki N, Furuya M, Kangawa K, Takeshita A, Saito Y. Local expression of C-type natriuretic peptide markedly suppresses neointimal formation in rat injured arteries through an autocrine/paracrine loop. Circulation 96: 2272–2279, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res 27: 341–348, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis 17: 302–307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang H, Fogo AB. Cell senescence in the aging kidney. J Am Soc Nephrol 21: 1436–1439, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND, Silva FG. The aging kidney. Kidney Int 74: 710–720, 2008 [DOI] [PubMed] [Google Scholar]