Abstract
Onset of metabolic acidosis leads to a rapid and pronounced increase in expression of phosphoenolpyruvate carboxykinase (PEPCK) in rat renal proximal convoluted tubules. This adaptive response is modeled by treating a clonal line of porcine LLC-PK1-F+ cells with an acidic medium (pH 6.9, 9 mM HCO3−). Measurement of the half-lives of PEPCK mRNA in cells treated with normal (pH 7.4, 26 mM HCO3−) and acidic medium established that the observed increase is due in part to stabilization of the PEPCK mRNA. The pH-responsive stabilization was reproduced in a Tet-responsive chimeric reporter mRNA containing the 3′-UTR of PEPCK mRNA. This response was lost by mutation of a highly conserved AU sequence that binds AUF1 and is the primary element that mediates the rapid turnover of PEPCK mRNA. However, siRNA knockdown of AUF1 had little effect on the basal levels and the pH-responsive increases in PEPCK mRNA and protein. Electrophoretic mobility shift assays established that purified recombinant HuR, another AU element binding protein, also binds with high affinity and specificity to multiple sites within the final 92-nucleotides of the 3′-UTR of the PEPCK mRNA, including the highly conserved AU-rich element. siRNA knockdown of HuR caused pronounced decreases in basal expression and the pH-responsive increases in PEPCK mRNA and protein. Therefore, basal expression and the pH-responsive stabilization of PEPCK mRNA in LLC-PK1-F+ cells, and possibly in the renal proximal tubule, may require the remodeling of HuR and AUF1 binding to the elements that mediate the rapid turnover of PEPCK mRNA.
Keywords: metabolic acidosis, proximal tubule, electrophoretic mobility shift assay, siRNA knockdown
the cytosolic isoform of phosphoenolpyruvate carboxykinase (PEPCK) is encoded by the PCK1 gene (2). This enzyme catalyzes an essential cataplerotic reaction in the primary pathway of renal catabolism of glutamine (41). It is also a key regulator of the increases in renal gluconeogenesis and synthesis of ammonium and bicarbonate ions that occur during metabolic acidosis (7). The onset of acidosis causes a rapid and pronounced increase in PEPCK expression that occurs solely within the proximal convoluted tubular segment of the nephron (38). Increased levels of rat renal PEPCK mRNA are observed within 1 h following acute onset of acidosis and achieve a maximal increase of six- to sevenfold after 7 h (21). The increases in PEPCK mRNA (22) and protein (8) expression are sustained in rats that are made chronically acidotic.
Previous transcription run-off experiments performed with nuclei isolated from the kidneys of normal and acidotic rats indicated that the initial increase in PEPCK mRNA correlates with an increase in the rate of transcription (21, 22). However, the data also indicated that the sustained increase in PEPCK mRNA is due in part to mRNA stabilization. Further analysis of the mechanism of this adaptation required identification of a renal cell line that models the increase in PEPCK mRNA that occurs during metabolic acidosis. Porcine LLC-PK1 cells exhibit a number of properties that are characteristic of renal proximal tubular cells (16). However, LLC-PK1 cells are unable to synthesize glucose from pyruvate due to the lack of fructose-1,6-bisphosphatase (FBPase) (15). A gluconeogenic cell line was isolated by initially adapting LLC-PK1 cells to low-glucose medium (<0.5 mM) and then selecting with glucose-free medium containing 10 mM pyruvate. The population of cells that replicated in the selection medium expressed significant FBPase activity when subsequently grown in the absence or presence of 5 mM glucose. The isolated cells were designated as LLC-PK1-FBPase+ (13) or LLC-PK1-F+ cells. The selected cells also exhibit a 10-fold higher level of PEPCK activity (19), an enhanced rate of glutamine catabolism (13), and a higher basal rate of ammonium ion production (14) than the parental LLC-PK1 cells. Most importantly, when transferred to acidic medium (pH 6.9, 9 mM HCO3−), the LLC-PK1-F+ cells respond with a pronounced increase in ammonium ion production that correlates with increased glutaminase (14) and PEPCK (19) activities. Thus, the gluconeogenic LLC-PK1-F+ cells are a population of pH-responsive renal proximal tubule-like cells.
The LLC-PK1-F+ cells have been used to extensively characterize the pH-responsive stabilization of glutaminase (18, 26, 27, 39) and glutamate dehydrogenase (40) mRNAs. Previous studies also established that the cells model the pH-responsive increase in transcription of the PCK1 gene (20). Transfer of confluent cells to acidic medium produced a 2.5-fold increase in PEPCK mRNA that occurred following a slight delay, but with no evident change in half-life. Further studies (11, 35) demonstrated that transcriptional activation was mediated through increased phosphorylation and activation of the p38-MAPK pathway and the downstream ATF-2 transcription factor that binds to the CRE-1 element of the PCK1 promoter. However, the PEPCK mRNA turns over with a relatively short half-life (28). This process is mediated primarily by a highly conserved AU-rich sequence in its 3′-untranslated region (3′-UTR) that binds AU-factor-1 (AUF1) (17).
The conserved AU-rich sequence in the PEPCK mRNA also fits the predicted RNA binding motif for Human antigen R (HuR) (37). A primary function of HuR is to stabilize mRNAs in response to various stress conditions (10). HuR contains three conserved RNA recognition motifs (4) and a 33-amino acid hinge region that functions as a nucleocytoplasmic shuttling sequence (9). The purpose of this study was to determine whether AUF1 and HuR participate in the rapid turnover and stabilization of PEPCK mRNA during metabolic acidosis. The reported experiments establish that the pH-responsive increase in PEPCK protein observed in a new clonal line of LLC-PK1-F+ cells is mediated, in part, through stabilization of the PEPCK mRNA. Further experiments indicate that this response is reproduced in a chimeric β-globin (βG) mRNA containing the entire 3′-UTR of the rat PEPCK mRNA and requires a highly conserved AU element. In addition, electrophoretic mobility shift assays established that HuR binds with high affinity to the same AU-rich elements and CU sequence within the 3′-UTR of PEPCK mRNA that bind AUF1. Finally, siRNA knockdown of HuR expression in LLC-PK1-F+-9C cells, but not AUF1, caused pronounced decreases in basal expression and the pH-responsive increase in PEPCK mRNA and protein. Therefore, the binding of HuR to specific elements contributes to basal expression of PEPCK mRNA and protein. In addition, remodeling of HuR binding, and its possible interaction with AUF1, may mediate the pH-responsive increase in renal PEPCK expression during metabolic acidosis.
MATERIALS AND METHODS
Materials.
A pET21a-(His)6 plasmid encoding mouse HuR was obtained from Dr. Joan Steitz (Yale University). Competent Escherichia coli strain BL21-CodonPlus cells were purchased from Stratagene. Restriction endonucleases were obtained from New England Biolabs. [32P]-UTP (3,000 Ci/mmol) was purchased from PerkinElmer. Mouse monoclonal HuR (Santa Cruz Biotechnology) and β-tubulin (Sigma) antibodies and rabbit polyclonal AUF1 (Millipore) and PEPCK (Cayman) antibodies were obtained from the indicated suppliers. RiboLock Ribonuclease Inhibitor, T7 RNA polymerase and transcription buffer were purchased from Fermentas. Opti-medium and Lipofectamine RNAiMAX were obtained from Invitrogen. PCR primers and Taqman probes were synthesized by IDT and BioSearch Technologies, respectively.
Half-life analysis.
LLC-PK1-F+ cells (13) were grown at 37°C in a 5% CO2 atmosphere in DMEM-Base medium (Sigma) supplemented with penicillin/streptomycin (Sigma), 10% FBS, 5 mM glucose, 26 mM NaHCO3, 17 mM NaCl, 2 mM glutamine, 1 mM pyruvate, 3 μM phenol red, and 10 mM HEPES, pH 7.4. Acidic medium (pH 6.9) was prepared as above but contained 9 mM NaHCO3 and an additional 17 mM NaCl to maintain equivalent osmolarity. Clonal lines of LLC-PK1-F+ cells were selected by serial dilution of individual cells to allow formation of well-separated colonies. The individual colonies were segregated with cloning rings and lifted with 50 μl of trypsin. The resulting clonal lines were expanded and tested for pH responsiveness by Western blot analysis of cells maintained in normal medium vs. cells treated with acidic medium for 24 h. The 9C clonal line exhibited the greatest increase in PEPCK mRNA and protein and was used in all of the reported experiments.
To quantify the pH-responsive increase in PEPCK mRNA or protein, LLC-PK1-F+-9C cells were grown on six-well plates (35-mm wells) in normal medium until they were confluent. Triplicate wells of cells were either maintained in normal medium or transferred at various times to acidic medium. During the time course, the cells were fed every 12 h with fresh medium to maintain constant pH. All of the wells were harvested at the same time with 100 μl of lysis buffer (35) or with 400 μl of TRIzol reagent (Invitrogen). To measure the half-life of the endogenous PEPCK mRNA, confluent cells were incubated in normal or acidic medium for 12 h and then treated with the corresponding medium containing 8 μg/ml actinomycin D. RNA samples were isolated at 0 to 4.5 h after addition of actinomycin D. RNA concentrations were determined by measuring the absorbance at 260 nm. An initial strand of cDNA was synthesized using 1 μg of RNA, an oligo-dT18 primer, and avian myeloblastosis virus-reverse transcriptase. The cDNAs prepared from the reverse transcription reactions were used directly for quantitative real-time RT-PCR analysis. Real-time RT-PCR analysis of the endogenous porcine PEPCK mRNA was performed using 5′-CACAGGGTCTCTGGTTTTGCCCTTC-3′ as the forward primer, 5′-AAGTGAGAGGGGGAATCGTG-3′ as the reverse primer, and 5′-FAM-CGTCTGCGACGTATTTAACACCTTTGGAAAAATCTTGG-BHQ13′ as a Taqman probe. A primer set was also designed to amplify a segment within the coding region of the porcine GAPDH cDNA. For this assay, a forward primer, 5′-GATGGGCATGAACCATGAGA-3′, and a reverse primer, 5′-GGCATGGACTGTGGTCATGA-3′, were used along with a Taqman probe, 5′-CAL-Red-TGCCTCCTGTACCACCAACTGCTTGG-BHQ23′. The quantitative real-time PCR reactions were performed using Platinum qPCR Supermix-UDG (Invitrogen) in the presence of 100 nM primers, 200 nM Taqman probe, and 9 mM MgCl2 to quantify the ratio of the copy numbers of PEPCK mRNA to GAPDH mRNA. The data were then normalized to set the ratio of the two mRNAs at 0 h equal to 1.0. The log of the relative levels of PEPCK mRNA was plotted vs. time and a least squares fit of the data was used to calculate the half-life. The reported values are means ± SE of triplicate analyses of triplicate RNA samples.
Chimeric βG-PEPCK mRNAs.
LLC-PK1-F+-9C cells that express the βG-PCK-1 mRNA from a tetracycline-responsive promoter were created and maintained as described previously (17). The ExSite PCR-based site-directed mutagenesis kit (Stratagene) was used to mutate the highly conserved PCK-6 AU sequence within the 3′-UTR of the PEPCK mRNA. The sequence of the forward primer was 5′-pCGCAGACCGTACACTGCCCTTTCTTAC-3′, while that of the reverse primer was 5′-pTTTAAACATACATACTAGCAACAAAGCAACACGACTC-3′. Both primers were phosphorylated at the 5′-end and the underlined sequence corresponds to the mutated nucleotides that were introduced into the PCK-6 segment. The PCR product was treated with DpnI to digest the nonmutated parental plasmid. The mutated PCR product was ligated with T4 ligase using the Rapid Ligation Kit (MBI Fermentas) and then transformed into XL-1 blue cells. The resulting plasmid, pTβG-PCK-1-mut6, was verified by DNA sequencing and then cotransfected along with pcDNA3.1/Hygro (Invitrogen) into a clonal line of LLC-PK1-F+ cells that stably express the tTA protein (39). Clonal lines of stably transfected cells were selected by growth in medium containing 0.8 mg/ml Hygromycin B.
Cells for half-life analyses were grown in normal medium containing 50 ng/ml of doxacycline (Dox). This level of Dox is sufficient to maximally inhibit transcription from the Tet promoter. When the cells reached 90% confluence, they were transferred to normal or acidic medium minus Dox for 24 h to initiate synthesis of the βG-PCK mRNA. The cells were then treated with normal or acidic medium minus Dox for an additional 24 h. Subsequently, normal or acidic medium containing 1 μg/ml of Dox was added to rapidly inhibit transcription. The cells were incubated in the plus Dox medium for 30 min before isolating RNA for the 0- to 4.5-h time points. The RNAs were reverse transcribed and analyzed by quantitative real-time RT-PCR. An RT-PCR assay was developed to quantify the rabbit β-globin sequence within the βG-PCK cDNAs. This reaction used 5′-TCAGTGAGGGTCTGAATCACC-3′ as the forward primer and 5′-CTGCACCTGAGGAGTGAATTC-3′ as the reverse primer and included a Taqman probe 5′-FAM-CACCTTTGCTAAGCTGAGTGAACTGCAC-BHQ1–3′ that is complementary to a region between the two primers.
siRNA knockdown of HuR.
An siRNA sequence complementary to the porcine HuR mRNA was obtained from Dr. Beth Lee (Ohio State University) (23). The corresponding double-strand stealth siRNA (Invitrogen) was used to knockdown HuR. A stealth siRNA with a forward strand sequence of CACUCUGAAGUUAGAUCCUAUCACA was used to knockdown AUF1. The control siRNA was formed by annealing complementary forward, 5′-ACUACCGUUGUUAUAGGUGdTdT-3′, and reverse, 5′-CACCUAUAACAACGGUAGUdTdT-3′, ribonucleotides. This sequence is not encoded in the human, rat, or mouse genome. Transient transfection experiments were performed using cells that were grown to 70–80% confluence in six-well plates. The transfection reagent contained 0.25 ml Opti-medium, 2.5 to 5.0 μl Lipofectamine RNAiMAX, and 0.1 to 0.5 μM siRNA and was preincubated at room temperature for 20 min. The cells in each well were washed with 1 ml of PBS and covered with 1 ml of normal medium (pH 7.4) minus FBS before addition of the transfection reagent. After 6 h, the media were replaced with normal medium containing FBS. On the second day posttransfection, half of the samples and controls received normal medium while the other half were treated for 24 h with acidic medium. On the third day, the cells were harvested in 100 μl of cell lysis buffer (35) or 400 μl of TRIzol. Protein concentration was determined using a Bradford assay (3) and Western blot analyses were performed to quantify protein expression.
Western blot analysis.
Nuclei were isolated as described previously (36). Briefly, cells were lysed by incubating for 10 min on ice in a buffer containing 85 mM KCl, 5 mM PIPES, pH 8.0 and 0.5% Nonidet P-40 supplemented with Halt phosphatase inhibitor (Thermo scientific) and protease inhibitor cocktail (Sigma). The lysate was centrifuged at 3,000 rpm for 5 min and the supernatant (postnuclear fraction) was collected and the resulting pellet was resuspended in lysis buffer (nuclear fraction). The various lysates of LLC-PK1-F+-9C cells were separated on 10% SDS-polyacrylamide gels and transferred to Immobilon-F membranes (Millipore). The blots were probed with mouse monoclonal antibodies to HuR and β-tubulin and rabbit polyclonal antibodies to AUF1 and PEPCK. The proteins were detected with goat anti-rabbit 800 and goat anti-mouse 680 antibodies (LiCor) and quantified using an Odyssey Infra-red Imager. The intensities of the AUF1, HuR, and PEPCK bands were divided by the intensity of the β-tubulin band to correct for errors in sample loading. In the siRNA experiments, the samples were normalized vs. the pH 7.4 samples treated with the control siRNA.
Purification of HuR.
BL21-CodonPlus cells were used to express the (His)6-tagged HuR protein. A bacterial culture was grown on a shaker at 37°C in 500 ml of LB medium until an absorbance of 0.7 at 600 nm was reached. Recombinant protein production was induced with 1 mM IPTG for 4 h. Induced cells were centrifuged at 2,500 g for 12 min at 4°C. The pellet was resuspended in 4 ml of buffer A (300 mM KCl, 10% glycerol, 10 mM Tris, pH 8.0) containing 500 μl of protease inhibitor cocktail (Sigma) and 200 μl of Halt protease inhibitor (Pierce). The sample was sonicated four times for 1 min each at duty cycle 30 and output control 3 and the lysed cells were centrifuged at 4°C at 100,000 g for 40 min to remove cell debris.
A 1-ml packed volume of Ni-NTA His-Bind Resin (Novagen) was added to a 10-ml Poly-Prep Chromatography Column (Bio-Rad). The column was washed with 2 ml of H2O, followed by 2 ml of buffer A. The cell supernatant was added and the column was rocked on a shaker at 4°C for 1 h. Next, the flow through from the column was collected and the column was washed with 2 ml of buffer A, followed by 2 ml of buffer A containing 50 mM imidazole to remove nonbound proteins. HuR was eluted from the column with 2-ml volumes of buffer A containing imidazole in increasing increments of 100 mM that ranged from 100 to 400 mM and detected by Western blot analysis. The fraction with 200 mM imidazole, which contained the recombinant protein, was dialyzed overnight at 4°C vs. buffer A using a Slide-A-Lyzer Dialysis Cassette with a 10,000 molecular weight cut-off (Thermo Scientific). The concentration of recombinant HuR protein was determined with a Bradford assay (3). The purity of the final preparation was assessed by SDS-PAGE stained with Coomassie-Blue and determined to be >95%.
Electrophoretic mobility shift assays.
Plasmids containing the desired DNA templates were prepared and digested as described previously (17). The DNA templates were purified on either a 1% agarose or a 10% polyacrylamide gel using either a QIAquick Gel Extraction Kit (Qiagen) or the crush and soak method, respectively. The DNA templates were transcribed with T7 RNA polymerase at 37°C for 3 h in the presence of [32P]-UTP to label the RNA transcripts. The reaction mixture contained 0.5 mM CTP, 0.5 mM GTP, 0.5 mM ATP, 0.05 mM UTP (Amersham Biosciences), 10 mM dithiothreitol, 40 units RiboLock ribonuclease inhibitor, 45 μCi [32P]-UTP, 40 units T7 RNA polymerase, transcription buffer, and the desired DNA template. The RNA transcripts were purified by phenol/chloroform/isoamyl alcohol extraction. Reactions for the gel shift assays contained 1 mM dithiothreitol, 40 units RiboLock ribonuclease inhibitor, 0.5% IGEPAL, 0.3 mg/ml tRNA (Roche), 2% glycerol, binding buffer (10 mM HEPES, 2.5 mM magnesium acetate, 25 mM potassium acetate, pH 7.4), and varying amounts of recombinant HuR protein in a final volume of 10 μl. After addition of 20,000 cpm of [32P]-labeled RNA, the reactions were incubated at room temperature for 20 min. The reactions were separated on either 5 or 8% polyacrylamide gels that were then dried and exposed to a PhosphorImager screen overnight. After the 20-min incubation, reactions with PCK-2, PCK-3, and PCK-4 RNAs were digested with 100 units of RNase T1 (Roche) for 10 min at room temperature before loading onto the gel. In addition, the latter reactions used 40,000 cpm of [32P]-labeled RNA. Competition assays were performed by addition of excess amounts of unlabeled PCK-6/7 RNA or an RNA of equal length that was transcribed from the multiple cloning site of pBluescriptII-SK(−) (pBS). The molar quantities of RNA were calculated from the added radioactivity or by measuring the absorbance at 260 nm and using an extinction coefficient calculated from the nucleotide composition.
RESULTS
pH-responsive increase in PEPCK mRNA and protein in LLC-PK1-F+-9C cells.
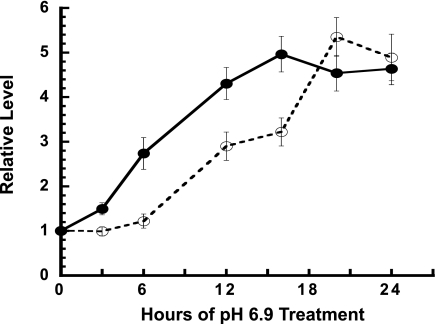
LLC-PK1-F+ cells express both cytosolic and mitochondrial PEPCK mRNAs (19). However, expression of only the cytosolic PEPCK mRNA is increased when cells are incubated with an acidic (pH 6.9, 9 mM HCO3−) medium. This treatment produced a gradual increase in PEPCK mRNA that occurred with a pronounced lag and that reached a 2.7-fold maximum after 18 h (20). However, LLC-PK1-F+ cells are a mixed population of cells. Thus, clonal lines of LLC-PK1-F+ cells were developed to reduce the inherent variance and to identify a clonal line that exhibits a greater fold increase in cytosolic PEPCK mRNA and protein. Treatment of the LLC-PK1-F+-9C clonal cells with different media in which the pH was incrementally decreased from pH 7.4 to pH 6.9 produced a graduated increase in PEPCK expression (data not shown). Since the largest increase was observed at pH 6.9, this condition was adopted to maximize the in vitro response and to facilitate characterization of the potential contribution of mRNA stabilization to the increased expression. As long as the medium was changed frequently to ensure that the pH does not decrease below 6.9, the cells grew normally and did not exhibit signs of activating a stress response. Transfer of the cells to pH 6.9 medium produced a rapid and pronounced increase (4.5-fold) in PEPCK mRNA that plateaued within 12 h (Fig. 1). Western blot analysis was used to determine whether this treatment produced a corresponding increase in PEPCK protein (Fig. 1). The blot was also probed for β-tubulin that was used as a loading control. This analysis demonstrated that the cytosolic PEPCK protein was also increased 4.5-fold in the LLC-PK1-F+-9C cells, but to achieve the maximal increase in protein required a 20-h incubation in acidic medium. The observed lag in the increase in PEPCK protein probably reflects the time required to synthesize and process the initial transcript, to transport the mature mRNA to the cytoplasm, and to complete its translation. The close correlation in the fold increases in PEPCK mRNA and protein indicates that regulation of translation is unlikely to contribute to this adaptive response.
Fig. 1.
pH-responsive increase in PEPCK protein and mRNA in LLC-PK1-F+-9C cells. Cells were grown to confluence in normal medium and then either maintained in normal (pH 7.4) medium or transferred to acidic (pH 6.9) medium for the indicated times. All of the cells were harvested at the same time and quantified for PEPCK and β-tubulin protein by Western blotting and for PEPCK and GAPDH mRNAs by real-time RT-PCR. The ratio of PEPCK protein to β-tubulin was normalized to the average of the control samples and used to calculate the fold increase in PEPCK (o—o). The PEPCK mRNA data were similarly standardized vs. the levels of GAPDH mRNA (•-•). The data are plotted as means ± SE of triplicate samples.
pH-responsive stabilization of endogenous PEPCK mRNA in LLC-PK1-F+-9C cells.
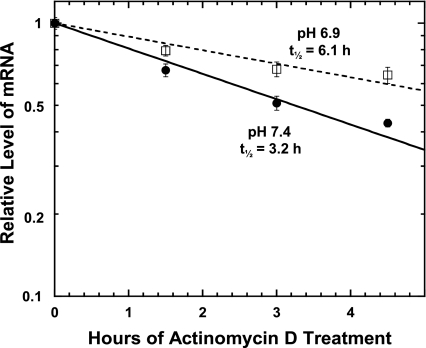
To determine whether mRNA stabilization contributes to the greater adaptive response, the half-life of the endogenous cytosolic PEPCK mRNA was quantified by using actinomycin D to inhibit transcription. RNA was isolated at various times after addition of 8 μg/ml of actinomycin D and quantified by real-time RT-PCR. Specific primers and a Taqman probe were designed using unique sequences from the 3′-UTR of the porcine PCK1 gene, which encodes the cytosolic isoform of PEPCK. The complete sequence of this gene is defined and well annotated in the pig genome database (www.ncbi.nih.gov/genome/guide/pig). Using this approach, the half-life of the endogenous PEPCK mRNA in cells maintained in normal (pH 7.4) medium was determined to be 3.2 h (Fig. 2). When the cells were treated with acidic (pH 6.9) medium for 12 h before addition of actinomycin D, the half-life of the endogenous PEPCK mRNA was increased nearly twofold to a value of 6.1 h. Therefore, mRNA stabilization accounts for a portion of the observed increase in PEPCK mRNA in LLC-PK1-F+-9C cells.
Fig. 2.
Effect of treatment with acidic medium on the half-life of the endogenous PEPCK mRNA in LLC-PK1-F+-9C cells. Confluent cultures of cells were treated for 12 h in either normal, pH 7.4 (•——•) or acidic, pH 6.9 (□——□) medium. RNA was isolated at various times after addition of 8 μg/ml actinomycin D. The relative levels of PEPCK and GAPDH mRNAs were quantified by real-time RT-PCR. The level of PEPCK mRNA was divided by the corresponding level of GAPDH mRNA. The log of the normalized data was then plotted against the time after the addition of actinomycin D. The reported data are means ± SE of duplicate experiments each of which contained triplicate samples for each time point.
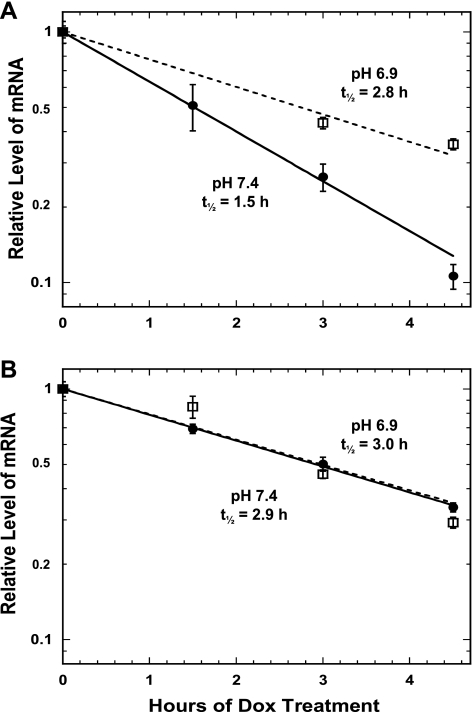
A tet-off promoter system was used to establish that the pH-responsive stabilization is mediated by a specific element within the 3′-UTR of the PEPCK mRNA. LLC-PK1-F+-9C cells that stably express high levels of a tTA protein were used to select a line that also expresses a chimeric β-globin-PCK-1 (βG-PCK-1) mRNA. This construct contains a tetracycline-responsive promoter, the coding region of the rabbit β-globin gene, and the full-length 3′-UTR of the rat PEPCK mRNA. The level of the chimeric mRNA is increased by growing the cells in normal medium minus Dox. Subsequent addition of Dox causes a prompt inhibition of transcription and the rapid decay of the βG-PCK-1 mRNA (t1/2 = 1.5 h; Fig. 3A). When the cells were treated with acidic medium (9 mM HCO3−, pH 6.9), the βG-PCK-1 mRNA is stabilized nearly twofold and now decays with a half-life of 2.8 h. Previous studies established that the rapid decay of the βG-PCK-1 mRNA in normal medium results from the presence of multiple instability elements that bind AUF1 (17). A short 22-nucleotide segment (PCK-6) that contains a highly conserved AU sequence was identified as the primary element that mediates this instability. Mutation of this sequence within the βG-PCK-1 construct caused a twofold increase in the half-life of the resulting βG-PCK-1-mut6 mRNA (t1/2 = 2.9 h) in pH 7.4 medium (Fig. 3B) and no further stabilization when the cells were treated with pH 6.9 medium (t1/2 = 3.0 h). Therefore, the highly conserved AU sequence within this segment is also necessary for the pH-responsive stabilization of the PEPCK mRNA.
Fig. 3.
Effect of treatment with acidic medium on the half-life of chimeric β-globin (βG)-PCK mRNAs in LLC-PK1-F+-9C cells. Half-lives of βG-PCK-1 (A) and βG-PCK-1-mut6 (B) mRNAs. Confluent cultures of cells were treated with either normal, pH 7.4 (•——•) or acidic, pH 6.9 (□——□) medium. RNA was isolated at various times after addition of 1 μg/ml doxacycline (Dox). The relative levels of PEPCK and GAPDH mRNAs were quantified by real-time RT-PCR. The level of PEPCK mRNA was divided by the corresponding level of GAPDH mRNA. The log of the normalized data was then plotted against the time after the addition of Dox. The reported data are means ± SE of triplicate samples for each time point.
Effect of siRNA knockdown of AUF1 on PEPCK levels in LLC-PK1-F+-9C cells.
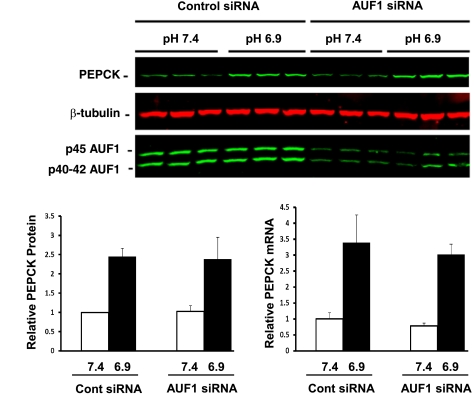
LLC-PK1-F+ cells express only the p40, p42, and p45 isoforms of AUF1. Overexpression of a recombinant p37 isoform of AUF1 had no effect on the basal level or the pH-responsive increase in PEPCK protein (data not shown). Transient transfection of a siRNA directed against a sequence common to all four of the porcine AUF1 mRNAs was used to determine the effect of decreased expression on PEPCK expression in LLC-PK1-F+-9C cells. Preliminary experiments established that maximal knockdown of AUF1 protein was achieved at 72-h posttransfection with a final concentration of 100 nM siRNA. Western blot analysis (Fig. 4) indicated that this treatment produced a 65% decrease in the three isoforms of AUF1 protein. Cells transfected with 100 nM control siRNA exhibited no effect on AUF1 expression or on the basal levels or the pH-responsive increases of PEPCK mRNA and protein compared with untreated cells (data not shown). Similarly, the knockdown of AUF1 had little effect on the basal levels of both PEPCK mRNA and protein. In addition, the pH-responsive increases were not affected when the AUF1 protein levels were decreased by the siRNA. This observation indicates that the binding of AUF1 to PEPCK mRNA is not the sole mediator of the rapid turnover of PEPCK mRNA or the pH-responsive increases in PEPCK mRNA and protein that occurs in LLC-PK1-F+-9C cells under conditions that model a metabolic acidosis.
Fig. 4.
siRNA knockdown of AUF1 has little effect on the basal level or the pH-responsive increase in PEPCK mRNA and protein. LLC-PK1-F+-9C cells were transfected with 100 nM control siRNA or 100 nM AUF1 siRNA. The transfected cells were treated with either normal (pH 7.4) or acidic (pH 6.9) medium for 24 h and then harvested with lysis buffer or TRIzol. Western blot analysis was used to quantify the levels of PEPCK, β-tubulin, and AUF1 proteins. The levels of PEPCK protein at pH 7.4 (open bars) and at pH 6.9 (filled bars) were normalized relative to the level of β-tubulin. Real-time qRT-PCR was used quantify the levels of PEPCK and GAPDH mRNAs. The levels of PEPCK mRNA at pH 7.4 (open bars) and at pH 6.9 (filled bars) mRNAs were normalized relative to the level of GAPDH mRNA. The normalized data are plotted as means ± SE of triplicate samples.
Binding of recombinant HuR to the 3′-UTR of PEPCK mRNA.
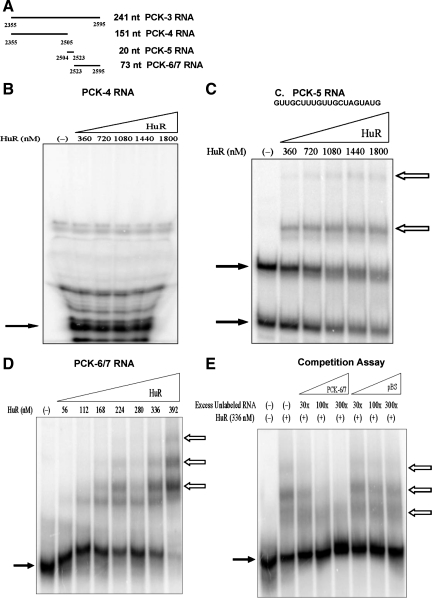
HuR is an mRNA binding protein that contributes to the stabilization of mRNAs that contain certain AU-rich elements. Therefore, electrophoretic mobility shift assays were performed to determine whether recombinant HuR binds to specific sites within the 3′-UTR of PEPCK mRNA. The initial assay used the PCK-2 or PCK-3 RNAs that contain the 5′- or 3′-half of the 3′-UTR of PEPCK mRNA, respectively. RNA:HuR complex formation was observed with the PCK-3 RNA, but not PCK-2 RNA (data not shown). Multiple deletions of PCK-3 RNA were then tested for the ability to bind HuR. PCK-4 and PCK-5 RNAs comprise the 5′-end of the PCK-3 RNA (Fig. 5A). PCK-4 RNA did not bind to HuR (Fig. 5B), but the PCK-5 RNA formed two shifted bands with HuR that suggest that multiple RNA:protein complexes are formed (Fig. 5C). The PCK-5 RNA is a short GU-rich segment. However, a higher concentration of HuR (360–1,800 nM) was required to form a shift with the PCK-5 RNA than with the PCK-3 RNA (56–392 nM). This observation suggests that HuR may bind with higher affinity to elements that are contained within the 3′-end of the PCK-3 RNA. Therefore, HuR binding to the combined PCK-6/7 RNA was analyzed.
Fig. 5.
Recombinant Human antigen R (HuR) binds to PCK-5 RNA and PCK-6/7 RNA, but not PCK-4 RNA. A: PCK-4 RNA, PCK-5 RNA, and PCK-6/7 RNA are fragments of the PCK-3 RNA. Electrophoretic mobility shift assays were performed with PCK-4 RNA (B), PCK-5 RNA (C), and PCK-6/7 RNA (D). The [32P]-labeled RNAs were incubated with increasing concentrations of recombinant HuR. PCK-4 RNA was digested with RNase T1 before electrophoresis. Samples were run on 8% native polyacrylamide gels. The solid arrows indicate the digested or unbound RNA and the outlined arrows indicate the RNA:protein complexes. C: nucleotide sequence of PCK-5 RNA. E: competition assay was performed by incubating a constant amount of [32P]-labeled PCK-6/7 RNA and recombinant HuR with increasing quantities of unlabeled PCK-6/7 RNA or pBS RNA. Samples were run on 5% native polyacrylamide gels. The solid arrows indicate the unbound RNA and the outlined arrows indicate the RNA:protein complexes.
PCK-6/7 RNA contains the 73 nucleotides that comprise the 3′-end of the PEPCK mRNA (Fig. 5A). HuR formed at least three separate complexes with the PCK-6/7 RNA (Fig. 5D). A competition assay was performed to establish the specificity of HuR binding to the PCK-6/7 RNA. When excess unlabeled PCK-6/7 RNA was added, the apparent binding of HuR to the [32P]-labeled RNA decreased (Fig. 5E). However, HuR binding was not affected when the same excess of an unlabeled nonspecific RNA (pBS) was added to the reaction. The pBS RNA is a 73-nucleotide segment that was transcribed from the multiple cloning site of the pBluescriptII-SK(−) plasmid.
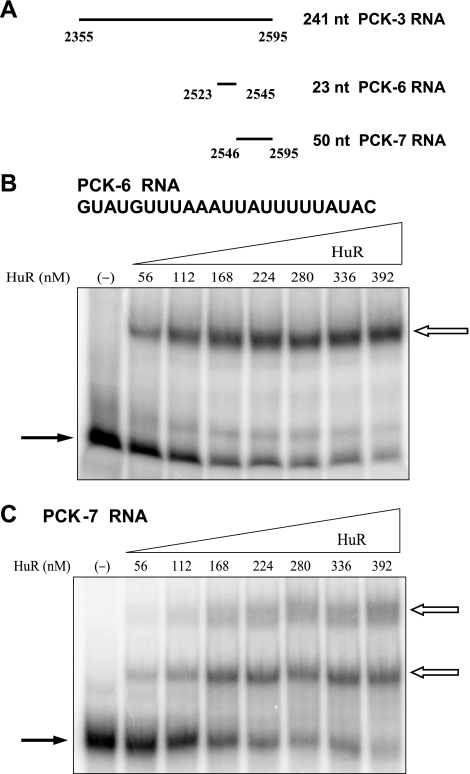
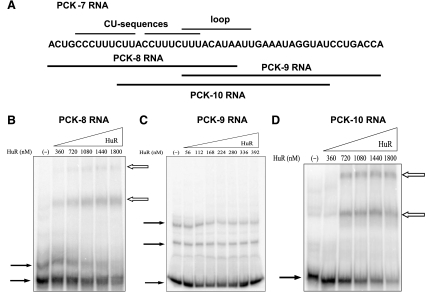
Next, the individual PCK-6 and PCK-7 RNAs were tested and found to form complexes with HuR (Fig. 6). PCK-6 RNA formed only a single shifted complex. The PCK-6 RNA contains 23 nucleotides and includes a highly conserved AU sequence. By contrast, the PCK-7 RNA formed at least two separate bands, suggesting that HuR may bind to multiple sites within the PCK-7 RNA. The PCK-7 RNA contains 50 nucleotides and includes two CU sequences, an AU-rich region, and a predicted stem-loop structure that contains a conserved AU-rich loop sequence. Three deletion constructs of the PCK-7 RNA were used to further localize HuR binding activity. The PCK-8 RNA contains the two CU sequences, PCK-9 RNA contains the 3′-AU-rich region, and PCK-10 RNA contains the predicted stem-loop structure (Fig. 7A). Both PCK-8 RNA and PCK-10 RNA formed two shifted complexes with HuR, but the PCK-9 RNA did not interact with HuR (Fig. 7). Thus, both the CU sequences and the predicted stem-loop structure may form multiple RNA:protein complexes with HuR. However, a higher concentration range of HuR was again required to detect binding to the PCK-8 and PCK-10 RNAs compared with the PCK-6/7 RNA (360–1,800 nM HuR vs. 56–392 nM HuR). This observation indicates that the combined binding sites within the PCK-7 RNA may interact to synergistically enhance the binding of HuR.
Fig. 6.
Recombinant HuR binds to PCK-6 RNA and PCK-7 RNA. A: PCK-6 RNA and PCK-7 RNA comprise the 3′-end of the PCK-3 RNA. Electrophoretic mobility shift assays were performed with PCK-6 RNA (B) and PCK-7 RNA (C). [32P]-labeled PCK-6 RNA or PCK-7 RNA was incubated with increasing concentrations of recombinant HuR protein. Samples were run on 5% native polyacrylamide gels. The solid arrows indicate the unbound RNA and the outlined arrows indicate the RNA:protein complexes. B: nucleotide sequence of PCK-6 RNA.
Fig. 7.
Recombinant HuR binds to PCK-8 RNA and PCK-10 RNA but not PCK-9 RNA. A: schematic diagram lists the nucleotide sequence of PCK-7 RNA. PCK-8 RNA, PCK-9 RNA, and PCK-10 RNA are deletion constructs that contain the indicated nucleotides from PCK-7 RNA. Electrophoretic mobility shift assays were performed with PCK-8 RNA (B), PCK-9 RNA (C), and PCK-10 RNA (D). The [32P]-labeled RNAs were incubated with increasing concentrations of recombinant HuR. Samples were run on 8% native polyacrylamide gels. The solid arrows indicate the unbound RNA and the outlined arrows indicate the RNA:protein complexes.
Binding of recombinant HuR to mutated constructs of PCK-7 RNA.
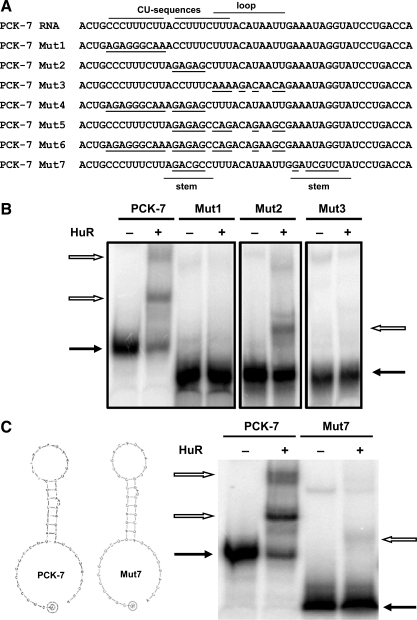
Seven different mutated constructs were designed to further assess the function of the individual binding elements within the PCK-7 RNA. RNAdraw software (www.rnadraw.com) predicts that the PCK-7 RNA will form the stem-loop structure illustrated in Fig. 8C. Also illustrated are the various nucleotides that were mutated in the individual PCK-7 Mut1 to Mut7 constructs (Fig. 8A). The constructs were designed to test the effects of individual or combined sets of mutations that alter the individual CU sequences, the highly conserved sequence within the loop, and the importance of the predicted secondary structure. Each of these elements was mutated separately in the PCK-7 Mut1 to Mut3 constructs. Of the three RNAs, only the PCK-7 Mut2 construct formed a complex with HuR (Fig. 8B). This construct contains mutations in the second CU sequence. Thus, this sequence is not essential for the binding of HuR to the PCK-7 RNA. However, the second CU sequence is essential to form the predicted stem-loop structure. Thus, HuR may primarily recognize specific sequence motifs and not the predicted secondary structure. By contrast, the lack of binding to the PCK-7 Mut1 and Mut3 RNAs indicate that the initial CU sequence and the conserved loop sequence are likely binding sites for HuR. The complete loss of HuR binding associated with the mutation of either site suggests that the two sites may interact to form a required structure or to synergistically enhance the binding of HuR. Consistent with this conclusion, none of the additional constructs that contained mutations in multiple regions (PCK-7 Mut4 through Mut6) were able to interact with HuR (data not shown). The PCK-7 Mut7 RNA contains complementary mutations in the two sequences that form the stem of the predicted stem-loop structure. The mutations were designed using the RNAdraw software so that the PCK-7 and PCK-7 Mut7 RNAs were predicted to form the same stem-loop structure (Fig. 8C). The observation that the latter RNA only forms a weak interaction with HuR further supports the conclusion that the predicted stem-loop structure is not the primary determinant of HuR binding. In summary, HuR binds primarily to the conserved pH response element within the PCK-6 segment and interacts synergistically with two additional sites in the PCK-7 segment.
Fig. 8.
Recombinant HuR binds to 2 distinct sites within the PCK-7 RNA. A: schematic diagram lists the sequences of the PCK-7 RNA and the Mut1 through Mut7 constructs. It also illustrates the regions that contain the CU sequences and the stem and loop sequences. The nucleotides modified in the 7 different mutated constructs of PCK-7 RNA are underlined. B: electrophoretic mobility shift assays were performed using a constant concentration of recombinant HuR and equivalent amounts of [32P]-labeled PCK-7 RNA or the Mut1, Mut2, or Mut3 RNAs. The panel was constructed from 3 separate gels, each of which used the labeled PCK-7 RNA as a positive control. C: schematic diagram illustrates the secondary structure of the PCK-7 RNA and PCK-7 Mut7 RNA as predicted by RNAdraw. An electrophoretic mobility shift assay was performed using a constant concentration of recombinant HuR and equivalent amounts of [32P]-labeled PCK-7 RNA or the PCK-7 Mut7 RNA. Samples were run on 5% native polyacrylamide gels. The solid arrows indicate the unbound RNA and the outlined arrows indicate the RNA:protein complexes.
Effect of siRNA knockdown of HuR on PEPCK levels in LLC-PK1-F+-9C cells.
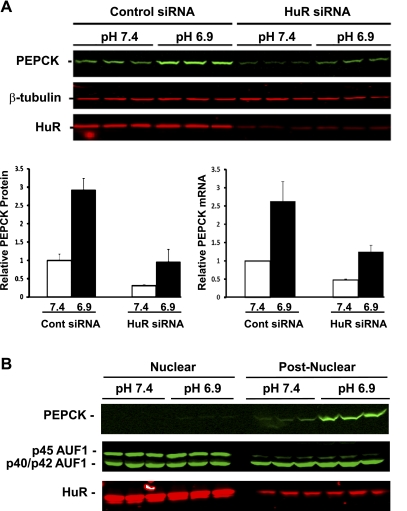
Transient transfection of an siRNA specific for porcine HuR (23) was used to determine the effect of decreased expression of HuR on the basal level and the pH-responsive increase in PEPCK expression. Preliminary experiments established that maximal knockdown of HuR protein was achieved with 20 nM siRNA. Western blot analysis (Fig. 9A) established that this treatment produced an 85% decrease in the level of HuR protein in cells that were either maintained in normal medium or treated with acidic medium. Cells transfected with 20 nM control siRNA exhibited no decrease in HuR compared with untreated cells. Treatment of the transfected control cells with acidic medium for 24 h produced a 2.9-fold increase in PEPCK protein. The knockdown of HuR produced pronounced decreases in both the basal and the induced levels of PEPCK protein. Similar changes were observed in the levels of PEPCK mRNA. These observations strongly support the conclusions that the binding of HuR to PEPCK mRNA contributes to both the basal expression and the pH-responsive increase in PEPCK mRNA stabilization in LLC-PK1-F+-9C cells.
Fig. 9.
Role of HuR in the pH-responsive stabilization of PEPCK mRNA. A: siRNA knockdown of HuR has pronounced effects on the basal level and the pH-responsive increase in PEPCK mRNA and protein. LLC-PK1-F+-9C cells were transfected with 20 nM control siRNA or 20 nM HuR siRNA. The transfected cells were treated with either normal (pH 7.4) or acidic (pH 6.9) medium for 24 h and then harvested with lysis buffer or TRIzol. Western blot analysis was used to quantify the levels of PEPCK, β-tubulin, and HuR proteins. The levels of PEPCK protein at pH 7.4 (open bars) and at pH 6.9 (filled bars) were normalized relative to the level of β-tubulin. Real-time qRT-PCR was used to quantify the levels of PEPCK and GAPDH mRNAs. The levels of PEPCK mRNA at pH 7.4 (open bars) and at pH 6.9 (filled bars) mRNAs were normalized relative to the level of GAPDH mRNA. The normalized data are plotted as means ± SE of triplicate samples. B: effect of pH on the nuclear cytoplasmic distribution of HuR and AUF1. Triplicate plates of confluent LLC-PK1-F+-9C cells were treated with normal (pH 7.4) or acidic (pH 6.9) medium for 18 h and then lysed and separated into nuclear and postnuclear fractions. Western blot analysis was used to quantify the levels of PEPCK, HuR, and AUF1 proteins.
While HuR is normally located predominantly in the nucleus, it translocates to the cytoplasm in response to various stress conditions such as heat shock (12), UV irradiation (43), amino acid starvation (44), chronic ethanol exposure (33), hypoxia (29), and ATP depletion (24). Therefore, nuclei and postnuclear fractions were isolated to determine whether treatment of LLC-PK1-F+-9C cells with pH 6.9 medium causes the redistribution of HuR and/or AUF1 (Fig. 9B). Treatment of cells with acidic medium for 18 h caused a pronounced increase in PEPCK protein that is recovered solely in the postnuclear fraction. As expected, only 12% of the HuR protein was recovered in the postnuclear fraction. Furthermore, the proportion of HuR recovered in this fraction was unchanged when the cells were treated with acidic medium. Similarly, only 11% of the total p45 AUF was recovered in the postnuclear fraction. By contrast, nearly 60% of the combined p40/p42 AUF1 was cytosolic. However, treatment of cells for 18 h with acidic medium again had no effect on the subcellular distribution of AUF1. Identical results were obtained when the cells were treated with pH 6.9 medium for 24 or 48 h (data not shown). Thus, a remodeling of the binding of HuR and AUF1 to the 3′-UTR of PEPCK mRNA during acidosis is not driven by changes in the relative cytosolic concentrations of the two RNA binding proteins.
DISCUSSION
Previous nuclear run-off assays indicated that increased transcription and mRNA stabilization contribute to the increased expression of rat renal PEPCK that occurs during chronic acidosis (21, 22). LLC-PK1-F+ cells are the only available cell line that models the increase in transcription of the renal PCK1 gene that occurs during acidosis (13). The newly developed clonal line of LLC-PK1-F+-9C cells provides an improved model of the in vivo response. The latter cells exhibit a more rapid and more pronounced increase in PEPCK mRNA that more closely approximates the temporal changes observed in rats that are made acutely acidotic (21, 22). The reported data also indicate that the pH-responsive increase in PEPCK expression in the LLC-PK1-F+-9C cells is mediated, in part, by at least a twofold stabilization of the PEPCK mRNA. The pH-responsive stabilization observed for the endogenous PEPCK mRNA is reproduced in a chimeric βG mRNA that contains the entire 3′-UTR of the rat PEPCK mRNA. The more rapid turnover of the chimeric mRNA may be due to differences in the sequences of the porcine and rat 3′-UTRs or the selective and more effective inhibition that is achieved with the Dox-responsive promoter. The PCK-6 segment of the rat PEPCK mRNA contains a 17-nucleotide AU sequence that has a high degree of similarity to the 16-nucleotide AU sequence that mediates the pH-responsive stabilization of the glutaminase mRNA (26). In addition, 11 of the 17 nucleotides (UUAAAUUAUUU) are fully conserved within the 3′-end of the 3′-UTR of all 27 of the mammalian PCK1 genes that have been sequenced. Disruption of this AU sequence by introduction of seven GC nucleotides resulted in a twofold stabilization of the βG-PCK-1 mRNA. The finding that the βG-PCK-1-mut6 mRNA still turns over with a relative fast half-life (∼3 h) is consistent with the presence of multiple instability elements in the 3′-UTR of the PEPCK mRNA (17). However, mutation of this element also prevented a further stabilization of the chimeric mRNA when the cells were transferred to an acidic medium. Therefore, this highly conserved sequence is the primary element that mediates the pH-responsive stabilization of the PEPCK mRNA.
Previous studies established that concurrent or competitive binding of AUF1 and HuR may determine the half-life of various target mRNAs that contain AU-rich elements (25). Therefore, a primary purpose of this study was to test the hypothesis that binding of AUF1 and HuR to specific elements in the 3′-UTR of PEPCK mRNA may mediate its rapid turnover and contribute to the increase in renal PEPCK expression during metabolic acidosis. Previous electrophoretic mobility shift analyses established that p40-AUF1, a protein that usually facilitates the rapid turnover of mRNAs (5, 6), binds to multiple segments of the 3′-UTR of PEPCK mRNA that contribute to its instability (17).
The four isoforms of AUF1 are derived by alternative splicing of the initial transcript of a single gene. LLC-PK1-F+-9C cells express high levels of the p40-, p42-, and p45-AUF1. As a result, it was difficult to achieve a high degree of knockdown of the three proteins. A maximal knockdown of 65% was achieved using 100 nM siRNA. Use of lower or higher concentrations of the siRNA was less effective. The threefold decrease in AUF1 expression had little effect on the basal levels or the pH-responsive increases in PEPCK mRNA or protein. It is uncertain whether a greater decrease in AUF1 would alter PEPCK expression. Thus, the binding, observed in vitro, may still contribute to the turnover of the PEPCK mRNA.
Experiments using RNA electrophoretic mobility shift assays identified multiple segments of the 3′-UTR of PEPCK mRNA that bind HuR with high affinity and specificity. All of the interactions were mapped to the 92 nucleotides that constitute the 3′-end of the 3′-UTR. The initial 5′-end of this segment, the PCK-5 RNA, contains a 20-nucleotide GU-rich sequence that binds HuR with modest affinity (Fig. 5C). Incubation of [32P]-labeled PCK-5 RNA with 1–2 μM HuR was not sufficient to produce a half-maximal shift. By contrast, the adjacent 23-nucleotide segment, the PCK-6 RNA, binds HuR with greater affinity and forms a single shifted complex. Formation of a half-maximal shift with PCK-6 RNA required only a 100–200 nM concentration of HuR (Fig. 6B). The highly conserved AU sequence within the PCK-6 RNA is an excellent fit to the binding motif for HuR that was recently predicted by bioinformatic analysis of target mRNA sequences identified by microarray analysis of immunoprecipitated HuR:RNA complexes (30).
The remaining 3′-segment, PCK-7 RNA, formed two high-affinity complexes that also required 100–200 nM HuR to shift half of the [32P]-labeled RNA (Fig. 6C). Analyses of multiple deletion constructs indicate that HuR binds specifically to the initial CU sequence and the AU-rich sequence that constitutes the 11-base loop within the predicted stem-loop structure. This conclusion was supported by analysis of the seven mutated constructs of the PCK-7 mRNA. HuR did not bind to the five constructs that had mutations in the initial CU sequence and/or the loop sequence, but did bind to the two constructs that lacked mutations in the two regions. The 11-nucleotide loop sequence (UUUACAUAAUU) is also highly conserved. This sequence is identical in 11 of the 27 fully sequenced mammalian PCK1 genes including human, rat, mouse, and pig. Most of the remaining mammalian PCK1 genes contain only one or two base substitutions within this sequence. Analysis of the deletion constructs also suggests that HuR primarily recognizes specific nucleotide sequences and not the predicted secondary structure. Overall, these data are consistent with previous experiments that indicate that HuR preferentially binds to AU-rich and U-rich elements in mRNA (1, 31).
Binding activity was also determined using the combined PCK-6/7 RNA that contains all of the high-affinity binding sites for HuR. This RNA formed at least three separate shifted bands (Fig. 5D). The observed formation of larger complexes with increasing concentrations may indicate that HuR binds independently and with slightly different affinities to multiple sites on a single PCK-6/7 RNA. Alternatively, HuR may form homomultimers after binding to a single site on the PCK-6/7 RNA. Further studies established that the observed binding of HuR to the [32P]-labeled PCK-6/7 RNA is specific (Fig. 5E).
The HuR siRNA used in the reported experiments was previously shown to effectively knockdown expression of HuR in LLC-PK1 cells (23). Transient transfection using 20 nM of this sequence in a Stealth siRNA consistently decreased the level of HuR to 15% of normal in LLC-PK1-F+-9C cells. This decrease in HuR expression resulted in pronounced decreases in both the basal level and the pH-responsive increase in PEPCK protein (Fig. 9). These data suggest that the observed binding of HuR functions primarily to enhance the stability of the PEPCK mRNA. In addition, the observed binding interactions of HuR also contribute to the pH-responsive stabilization of the PEPCK mRNA during metabolic acidosis. The residual increases in PEPCK mRNA and protein probably result from the activation of transcription of the PCK1 gene that occurs when the cells are transferred to acidic medium (11, 35).
In response to multiple stress conditions, a large proportion of HuR is translocated from the nucleus to the cytoplasm (12, 24, 29, 33, 43, 44). By contrast, treatment of LLC-PK1-F+-9C cells with acidic medium did not produce an increase in the level of HuR recovered in the postnuclear fraction. This observation suggests that the cells tolerate this level of decrease in medium pH without activating a stress response. It also indicates that an alternative mechanism may mediate the stabilization of the PEPCK mRNA during acidosis. Recent studies established that dissociation of HuR from its target mRNAs in response to ionizing radiation occurs without nuclear translocation and is instead mediated by phosphorylation of HuR by the checkpoint kinase, Chk2 (32). Activation of the p38-MAPK and the downstream MK2 kinase has also been suggested to phosphorylate HuR and to enhance its affinity for AU-rich elements (42). Previous studies established that activation of this signaling pathway mediates the increase in transcription of the PCK1 gene when these cells are treated with acidic medium (11, 35). Therefore, the phosphorylation of nuclear HuR and its association with newly synthesized PEPCK mRNA may be the primary mechanism by which this mRNA is stabilized during onset of acidosis.
Recent microarray (34) and proteomic (8) analyses indicate that the expression of a large number of mRNAs and proteins is increased or decreased in the kidney in response to metabolic acidosis and that alterations in mRNA stability may be the primary mechanism that mediates the remodeling of the renal proteome (8). Thus, the further characterization of the mechanism of the pH-responsive stabilization of the PEPCK mRNA is an important paradigm for understanding how the kidney responds to changes in acid-base balance.
GRANTS
This research was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-37124 and DK-43704 awarded to N. P. Curthoys.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Abe R, Sakashita E, Yamamoto K, Sakamoto H. Two different RNA binding activities for the AU-rich element and the poly(A) sequence of the mouse neuronal protein mHuC. Nucleic Acids Res 24: 4895–4901, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beale EG, Chrapkiewicz NB, Scoble HA, Metz RJ, Quick DP, Noble RL, Donelson JE, Biemann K, Granner DK. Rat hepatic cytosolic phosphoenolpyruvate carboxykinase (GTP). Structures of the protein, messenger RNA, and gene. J Biol Chem 260: 10748–10760, 1985 [PubMed] [Google Scholar]
- 3. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 4. Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science 265: 615–621, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Buzby JS, Brewer G, Nugent DJ. Developmental regulation of RNA transcript destabilization by A + U-rich elements is AUF1-dependent. J Biol Chem 274: 33973–33978, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Buzby JS, Lee SM, Van Winkle P, DeMaria CT, Brewer G, Cairo MS. Increased granulocyte-macrophage colony-stimulating factor mRNA instability in cord versus adult mononuclear cells is translation-dependent and associated with increased levels of A + U-rich element binding factor. Blood 88: 2889–2897, 1996 [PubMed] [Google Scholar]
- 7. Curthoys NP. Renal ammonium ion production and excretion. In: The Kidney: Physiology and Pathophysiology, 4th Edition, edited by Alpern RJ, Hebert SC. San Diego, CA: Elsevier, 2007, p. 1601–1619 [Google Scholar]
- 8. Curthoys NP, Taylor L, Hoffert JD, Knepper MA. Proteomic analysis of the adaptive response of rat renal proximal tubules to metabolic acidosis. Am J Physiol Renal Physiol 292: F140–F147, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Fan XC, Steitz JA. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc Natl Acad Sci USA 95: 15293–15298, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J 17: 3448–3460, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feifel E, Obexer P, Andratsch M, Euler S, Taylor L, Tang A, Wei Y, Schramek H, Curthoys NP, Gstraunthaler G. p38 MAPK mediates acid-induced transcription of PEPCK in LLC-PK1-FBPase+ cells. Am J Physiol Renal Physiol 283: F678–F688, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Gallouzi IE, Brennan CM, Stenberg MG, Swanson MS, Eversole A, Maizels N, Steitz JA. HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc Natl Acad Sci USA 97: 3073–3078, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gstraunthaler G, Handler JS. Isolation, growth, and characterization of a gluconeogenic strain of renal cells. Am J Physiol Cell Physiol 252: C232–C238, 1987 [DOI] [PubMed] [Google Scholar]
- 14. Gstraunthaler G, Holcomb T, Feifel E, Liu W, Spitaler N, Curthoys NP. Differential expression and acid-base regulation of glutaminase mRNAs in gluconeogenic LLC-PK1-FBPase+ cells. Am J Physiol Renal Physiol 278: F227–F237, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Gstraunthaler G, Pfaller W, Kotanko P. Lack of fructose-1,6-bisphosphatase activity in LLC-PK1 cells. Am J Physiol Cell Physiol 248: C181–C183, 1985 [DOI] [PubMed] [Google Scholar]
- 16. Gstraunthaler GJ. Epithelial cells in tissue culture. Renal Physiol Biochem 11: 1–42, 1988 [DOI] [PubMed] [Google Scholar]
- 17. Hajarnis S, Schroeder JM, Curthoys NP. 3′-Untranslated region of phosphoenolpyruvate carboxykinase mRNA contains multiple instability elements that bind AUF1. J Biol Chem 280: 28272–28280, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hansen WR, Barsic-Tress N, Taylor L, Curthoys NP. The 3′-nontranslated region of rat renal glutaminase mRNA contains a pH-responsive stability element. Am J Physiol Renal Fluid Electrolyte Physiol 271: F126–F131, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Holcomb T, Curthoys NP, Gstraunthaler G. Subcellular localization of PEPCK and metabolism of gluconeogenic substrains of renal cell lines. Am J Physiol Cell Physiol 268: C449–C457, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Holcomb T, Liu W, Snyder R, Shapiro R, Curthoys NP. Promoter elements that mediate the pH response of PCK mRNA in LLC-PK1-F+ cells. Am J Physiol Renal Fluid Electrolyte Physiol 271: F340–F346, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Hwang JJ, Curthoys NP. Effect of acute alterations in acid-base balance on rat renal glutaminase and phosphoenolpyruvate carboxykinase gene expression. J Biol Chem 266: 9392–9396, 1991 [PubMed] [Google Scholar]
- 22. Hwang JJ, Perera S, Shapiro RA, Curthoys NP. Mechanism of altered renal glutaminase gene expression in response to chronic acidosis. Biochemistry 30: 7522–7526, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Jeyaraj S, Dakhlallah D, Hill SR, Lee BS. HuR stabilizes vacuolar H+-translocating ATPase mRNA during cellular energy depletion. J Biol Chem 280: 37957–37964, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeyaraj SC, Dakhlallah D, Hill SR, Lee BS. Expression and distribution of HuR during ATP depletion and recovery in proximal tubule cells. Am J Physiol Renal Physiol 291: F1255–F1263, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J 23: 3092–3102, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laterza OF, Curthoys NP. Specificity and functional analysis of the pH-responsive element within renal glutaminase mRNA. Am J Physiol Renal Physiol 278: F970–F977, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Laterza OF, Hansen WR, Taylor L, Curthoys NP. Identification of an mRNA-binding protein and the specific elements that may mediate the pH-responsive induction of renal glutaminase mRNA. J Biol Chem 272: 22481–22488, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Laterza OF, Taylor L, Unnithan S, Nguyen L, Curthoys NP. Mapping and functional analysis of an instability element in phosphoenolpyruvate carboxykinase mRNA. Am J Physiol Renal Physiol 279: F866–F873, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Levy NS, Chung S, Furneaux H, Levy AP. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem 273: 6417–6423, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Lopez de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci USA 101: 2987–2992, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma WJ, Chung S, Furneaux H. The Elav-like proteins bind to AU-rich elements and to the poly(A) tail of mRNA. Nucleic Acids Res 25: 3564–3569, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Masuda K, Abdelmohsen K, Kim MM, Srikantan S, Lee EK, Tominaga K, Selimyan R, Martindale JL, Yang X, Lehrmann E, Zhang Y, Becker KG, Wang JY, Kim HH, Gorospe M. Global dissociation of HuR-mRNA complexes promotes cell survival after ionizing radiation. EMBO J 30: 1040–1053, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McMullen MR, Cocuzzi E, Hatzoglou M, Nagy LE. Chronic ethanol exposure increases the binding of HuR to the TNFalpha 3′-untranslated region in macrophages. J Biol Chem 278: 38333–38341, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nowik M, Lecca MR, Velic A, Rehrauer H, Brandli AW, Wagner CA. Genome-wide gene expression profiling reveals renal genes regulated during metabolic acidosis. Physiol Genomics 32: 322–334, 2008 [DOI] [PubMed] [Google Scholar]
- 35. O'Hayre M, Taylor L, Andratsch M, Feifel E, Gstraunthaler G, Curthoys NP. Effects of constitutively active and dominant negative MAPK kinase (MKK) 3 and MKK6 on the pH-responsive increase in phosphoenolpyruvate carboxykinase mRNA. J Biol Chem 281: 2982–2988, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Pan YX, Chen H, Kilberg MS. Interaction of RNA-binding proteins HuR and AUF1 with the human ATF3 mRNA 3′-untranslated region regulates its amino acid limitation-induced stabilization. J Biol Chem 280: 34609–34616, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ray D, Kazan H, Chan ET, Castillo LP, Chaudhry S, Talukder S, Blencowe BJ, Morris Q, Hughes TR. Rapid and systematic analysis of the RNA recognition specificities of RNA-binding proteins. Nat Biotechnol 27: 667–670, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Schoolwerth AC, deBoer PA, Moorman AF, Lamers WH. Changes in mRNAs for enzymes of glutamine metabolism in kidney and liver during ammonium chloride acidosis. Am J Physiol Renal Fluid Electrolyte Physiol 267: F400–F406, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Schroeder JM, Ibrahim H, Taylor L, Curthoys NP. Role of deadenylation and AUF1 binding in the pH-responsive stabilization of glutaminase mRNA. Am J Physiol Renal Physiol 290: F733–F740, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Schroeder JM, Liu W, Curthoys NP. pH-responsive stabilization of glutamate dehydrogenase mRNA in LLC-PK1-F+ cells. Am J Physiol Renal Physiol 285: F258–F265, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Taylor L, Curthoys NP. Glutamine metabolism: role in acid-base balance. Biochem Molec Biol Ed 32: 291–304, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Tran H, Maurer F, Nagamine Y. Stabilization of urokinase and urokinase receptor mRNAs by HuR is linked to its cytoplasmic accumulation induced by activated mitogen-activated protein kinase-activated protein kinase 2. Mol Cell Biol 23: 7177–7188, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang W, Furneaux H, Cheng H, Caldwell MC, Hutter D, Liu Y, Holbrook N, Gorospe M. HuR regulates p21 mRNA stabilization by UV light. Mol Cell Biol 20: 760–769, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yaman I, Fernandez J, Sarkar B, Schneider RJ, Snider MD, Nagy LE, Hatzoglou M. Nutritional control of mRNA stability is mediated by a conserved AU-rich element that binds the cytoplasmic shuttling protein HuR. J Biol Chem 277: 41539–41546, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.