Abstract
In autosomal dominant polycystic kidney disease (ADPKD), arginine vasopressin (AVP) accelerates cyst growth by stimulating cAMP-dependent ERK activity and epithelial cell proliferation and by promoting Cl−-dependent fluid secretion. Tolvaptan, a V2 receptor antagonist, inhibits the renal effects of AVP and slows cyst growth in PKD animals. Here, we determined the effect of graded concentrations of tolvaptan on intracellular cAMP, ERK activity, cell proliferation, and transcellular Cl− secretion using human ADPKD cyst epithelial cells. Incubation of ADPKD cells with 10−9 M AVP increased intracellular cAMP and stimulated ERK and cell proliferation. Tolvaptan caused a concentration-dependent inhibition of AVP-induced cAMP production with an apparent IC50 of ∼10−10 M. Correspondingly, tolvaptan inhibited AVP-induced ERK signaling and cell proliferation. Basolateral application of AVP to ADPKD cell monolayers grown on permeable supports caused a sustained increase in short-circuit current that was completely blocked by the Cl− channel blocker CFTRinh-172, consistent with AVP-induced transepithelial Cl− secretion. Tolvaptan inhibited AVP-induced Cl− secretion and decreased in vitro cyst growth of ADPKD cells cultured within a three-dimensional collagen matrix. These data demonstrate that relatively low concentrations of tolvaptan inhibit AVP-stimulated cell proliferation and Cl−-dependent fluid secretion by human ADPKD cystic cells.
Keywords: V2 receptor, cyclic AMP, polycystic kidney disease, MAP kinase
autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disorder and accounts for ∼10% of patients with end-stage renal disease, ultimately requiring renal replacement therapy (9). Aberrant epithelial cell proliferation is responsible for inexorable growth of numerous fluid-filled cysts leading to extensive nephron loss, massively enlarged kidneys, and progressive decline in renal function. ADPKD is caused by mutations in PKD1 (∼85% of cases) and PKD2 (∼15% of cases), which encode polycystin-1 (PC-1) and PC-2, respectively. PC-1 interacts with PC-2 to form a multifunctional signaling complex that regulates intracellular Ca2+ signaling, epithelial development and repair, and is essential for maintaining a differentiated phenotype of renal epithelial cells (3, 7, 14, 26, 36, 45). The mechanism underlying renal cyst formation remains unclear; however, it is generally accepted that functional loss of the polycystins transforms tubule epithelial cells into poorly differentiated hyperplastic cells that give rise to renal cysts.
Adenosine 3′,5′-cyclic monophosphate (cAMP) agonists, including arginine vasopressin (AVP), accelerate cyst growth by stimulating both mural epithelial cell proliferation and transepithelial Cl− secretion coupled to osmotic water flow (reviewed in Ref. 38). In ADPKD cells, cAMP-dependent cell proliferation is mediated by protein kinase A activation of B-Raf, a kinase that stimulates MEK which, in turn, phosphorylates and activates the mitogen-activated protein kinase ERK (48). Translocation of activated ERK into the nucleus upregulates transcriptional activity of genes involved in cell proliferation. By contrast, B-Raf is repressed in normal kidney cells and cAMP inhibits ERK activity and cell proliferation (49). Several lines of evidence support the hypothesis that disruption of intracellular Ca2+ signaling and/or reduction in intracellular Ca2+ levels contribute to the phenotypic difference in the proliferative response to cAMP between PKD and normal renal cells (1, 24, 46, 50, 52). ADPKD cystic cells have a lower steady-state intracellular Ca2+ level compared with normal tubule cells, and a sustained reduction of intracellular Ca2+ by treatment with Ca2+ channel blockers predisposes normal renal cells to cAMP-dependent stimulation of ERK and cell proliferation (46, 50).
AVP is an important antidiuretic hormone that stimulates intracellular cAMP production by binding V2 receptors on collecting ducts and distal nephron, predominant sites for cyst formation in ADPKD and ARPKD (2, 8, 17, 32, 37, 44). Studies showed that renal cAMP is elevated in PKD animals, including pcy mice, jck mice, PCK rats, and Pkd2WS25/− mice (12, 30, 35, 47). A potential explanation for elevated renal cAMP is the overexpression and hyperactivation of V2 receptors in the cyst epithelial cells (11–13, 23, 35). ADPKD patients also have elevated plasma AVP (5, 22), a physiological response to a decline in concentrating capacity of the cystic kidneys (10, 21).
Specific V2 receptor antagonists, so-called “aquaretic agents,” block the action of AVP on collecting duct cells and are being developed for the treatment of hypotonic hyponatremia in water-retaining disorders such as the syndrome of inappropriate antidiuretic hormone secretion, liver cirrhosis, and congestive heart failure (4, 6, 29, 51). OPC-31260, a nonpeptide V2 receptor antagonist, administered to animals orthologous to human PKD, including Pkd2WS25/− mouse (ADPKD), PCK rat (ARPKD), and pcy mouse (nephronophthisis type 3), reduced renal cAMP and dramatically halted disease progression measured by reductions in kidney volume, cystic area, number of mitotic cells, and blood urea nitrogen (12, 35, 42). These results provide a strong rationale for testing V2 receptor antagonists in ADPKD patients (34).
Tolvaptan, a derivative of OPC-31260, has a higher affinity for the human V2 receptor (51) and is currently being investigated for safety and efficacy in ADPKD patients (TEMPO trials). Preliminary data suggest that tolvaptan slows ADPKD cyst growth (33); however, the mechanism has not been fully elucidated. The purpose of the current study was to examine the effects of various concentrations of tolvaptan on AVP-induced cAMP production, ERK-mediated cell proliferation, Cl− secretion, and in vitro cyst growth of human ADPKD cyst epithelial cells. The results suggest that concentrations of tolvaptan achievable in the plasma of ADPKD patients in the TEMPO trials potently inhibit AVP-induced cell proliferation and Cl−-dependent fluid secretion by human ADPKD cyst epithelial cells.
METHODS
Primary cultures of ADPKD and normal human kidney cells.
ADPKD kidneys were obtained from hospitals participating in the Polycystic Kidney Research Retrieval Program with the assistance of the PKD Foundation (Kansas City, MO) and from the Biospecimen Shared Resource at the Kansas University Medical Center (KUMC). ADPKD patients who donated their discarded kidneys had a median age of 53 years, ranging from 43 to 73 years (n = 13). Since the majority of ADPKD cases are caused by mutations in PKD1 and these patients have an earlier onset of end-stage renal disease compared with patients with PKD2 mutations (54 vs. 74 years) (27), it is likely that most, if not all, of the primary ADPKD cells were derived from PKD1 kidneys. Normal regions of human kidneys, confirmed by histological examination, were collected from nephrectomy specimens. Normal kidneys withheld from transplantation as a result of anomalous vasculature were also obtained from the Midwest Transplant Network (Kansas City, KS). The protocol for the use of surgically discarded kidney tissues complies with federal regulations and was approved by the Institutional Review Board at KUMC. Primary cell cultures were prepared as previously described (40, 46). Cells were seeded and grown in T75 flasks containing DMEM/F12 (1:1) media supplemented with 5% FBS, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml sodium selenite (ITS). At 70 to 80% confluency, cells were lifted from the plastic and either frozen in culture medium containing 10% DMSO for storage in liquid N2 or seeded directly for experiments. Previously, ADPKD cells were shown to stain Dolichos biflorus agglutinin and express abundant aquaporin-2 (AQP-2), suggesting that these cells are derived primarily from collecting ducts (25, 48).
Tolvaptan.
Tolvaptan {OPC-41061; (±)-7-chloro-5-hydroxy-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5-tetra-hydro-1H-1-benzazepine} (4) was a generous gift from Otsuka Pharmaceutical (Tokushima, Japan). Tolvaptan (molecular mass of 448.94 g/mol) was dissolved in water and stored as 1,000× stock solutions in a −20°C freezer until used.
Cell proliferation measurements.
ADPKD cells (4 × 103 cells/well) were seeded into 96-well culture plates (6 wells per experimental condition) and incubated in DMEM/F12 supplemented with 1% FBS and ITS for 24 h. The serum concentration was reduced to 0.002% and ITS was removed for an additional 24 h before the experiment. For each experiment, cells were incubated in 0.002% FBS media containing 100 μM 8-Br-cAMP, AVP (10−12 to 10−6 M), 25 ng/ml EGF and/or tolvaptan (10−12 to 10−6 M) for 48 to 72 h, and then cell proliferation was determined by Promega Cell Titer 96 MTT assay (49). During the experiment, the cells remained subconfluent and did not form a polarized monolayer.
Immunoblot analysis.
Cells (0.5 × 106) were seeded onto plastic petri dishes (100-mm diameter) containing DMEM/F12 medium with 1% FBS. The serum was reduced to 0.002% when the cells were ∼75% confluent and the cells were allowed to grow for an additional 24 h. Cells were treated with tolvaptan for 30 min, and then AVP, 8-Br-cAMP, and/or EGF were added for an additional 15 min before cell lysates were prepared (48). Immunoblots were probed with antibodies for phospho-ERK (E-4), ERK1 (C-16), and ERK2 (C-14) from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary anti-rabbit and anti-mouse IgG-conjugated horseradish peroxidases were purchased from Santa Cruz Biotechnology.
Growth measurements of cultured ADPKD cysts.
Primary cultures of ADPKD cells (4 × 103 cells/well) were dispersed within ice-cold type I collagen (PureCol; Advanced BioMatrix, San Diego, CA) in wells of a 96-well culture plate (40, 41). Warming the plate to 37°C caused polymerization of the collagen, trapping the cells within the gel. A defined medium (DMEM/F12 with ITS, 5 × 10−8 M hydrocortisone, 5 × 10−5 M triiodothyronine) containing 5 μM forskolin and 5 ng/ml EGF was added for 3 days to initiate cyst growth. At this time, the outer diameters of the cysts were <100 μm. The agonists were removed and the gels were rinsed twice with defined media. To initiate the experiment, control media or media containing 10−8 M AVP alone or in combination with 10−8 M tolvaptan were added. After 5–7 days, the outer diameters of cross-sectional images of spherical cysts with distinct lumens were measured using a digital camera attached to an inverted microscope and analyzed with video analysis software. Surface area was calculated from the outer diameters and total surface area of the cysts was determined from the sum of individual cysts within each well. Cysts with diameters <100 μm were excluded from measurement. These experiments were repeated in three cell preparations from different ADPKD kidneys.
Statistics.
Data are expressed as means ± SE. Statistical significance was determined by one-way ANOVA and Student-Newman-Keuls posttest for multiple comparisons or unpaired t-test for comparison between control and treated groups.
RESULTS
Effect of AVP on cAMP-dependent proliferation of human ADPKD cells.
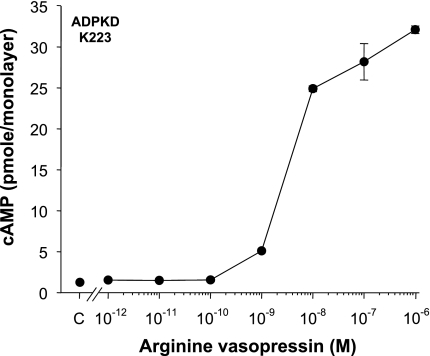
Previously, 100 mU/ml AVP (∼3.7 × 10−7 M) and 100 mU/ml desmopressin (DDAVP, 2.1 × 10−8 M), agonists that bind to V2 receptors, increased intracellular cAMP and stimulated ERK-dependent proliferation of human ADPKD cyst epithelial cells (2). Here, we tested the effect of various AVP concentrations ranging from 10−12 to 10−6 M on intracellular cAMP levels in confluent ADPKD cell monolayers. Cells were incubated for 15 min with AVP and then intracellular cAMP levels were measured using a cAMP enzyme-immunoassay system, as described previously (2). Low AVP concentrations (10−12 to 10−10 M) had no measurable effect on intracellular cAMP, whereas 10−9 to 10−6 M AVP increased intracellular cAMP levels in a concentration-dependent relationship (Fig. 1). In a composite of seven experiments, 10−9 M AVP increased cAMP levels from 0.7 ± 0.2 to 4.3 ± 0.6 pmol/monolayer (P < 0.0001), demonstrating that concentrations of AVP in the physiological range stimulate cAMP production in human ADPKD cells.
Fig. 1.
Effect of arginine vasopressin (AVP) on intracellular cAMP levels in human autosomal dominant polycystic kidney disease (ADPKD) cyst epithelial cells. Cells cultured from a human ADPKD kidney (K223) were grown as confluent cell monolayers (n = 4 wells/condition) and treated for 15 min with AVP; concentrations ranging from 10−12 to 10−6 M. Intracellular cAMP levels were determined by an enzyme immunoassay and expressed in picromoles per cell monolayer. In a composite of 7 experiments, the addition of 10−9 M AVP increased intracellular cAMP from 0.7 ± 0.2 to 4.3 ± 0.6 pmol/monolayer, P < 0.0001.
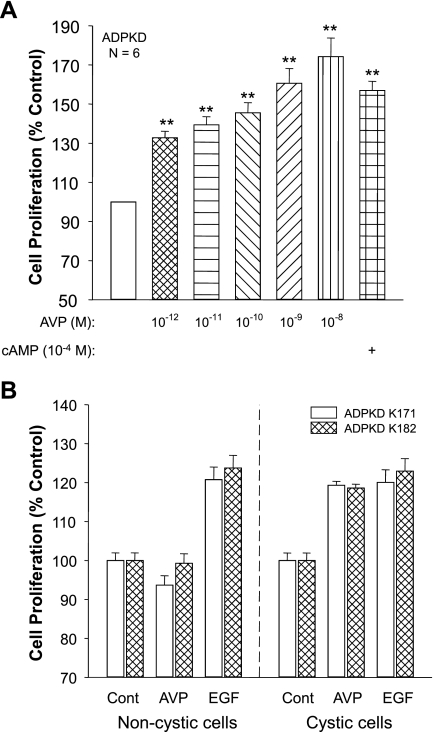
Incubation of ADPKD cells for 2 or 3 days with 10−12 M AVP, a concentration 1,000-fold lower than was required to induce a measurable increase in intracellular cAMP, caused a significant increase in cell proliferation (Fig. 2A). The reason for the discrepancy in the concentration relationship between the effect of AVP on intracellular cAMP and cell proliferation is unclear. It is well-documented that compartmentalization of the cAMP signal provides specificity for a cellular response to ligands binding to G protein-coupled receptors (16). It is possible that 10−12 M AVP increases cAMP in a cellular compartment in close proximity of a protein complex involved in activation of cell proliferation; however, this concentration may be insufficient to increase total cellular cAMP to a level that can be detected by standard enzyme-immunoassay methods. The difference in the incubation periods for the cAMP assays (15 min) and the proliferation assays (48–72 h) may also account for the disparity. In a composite of six experiments, 10−9 M AVP increased ADPKD cell proliferation 60.6 ± 7.6% (P < 0.001) above the control-treated cells (set to 100%). The increase in cell proliferation induced by AVP was comparable to the effect of 100 μM 8-bromo-cAMP, a cell membrane-permeable form of cAMP.
Fig. 2.
Effect of AVP on the proliferation of cystic and noncystic cells cultured from ADPKD kidneys. A: epithelial cells cultured from the surface cysts on 6 end-staged ADPKD kidneys were incubated in absence and presence of various concentrations of AVP or 10−4 M 8-Br-cAMP, a membrane-permeable form of cAMP. Cell proliferation (means ± SE) was determined by a Promega MTT proliferation assay and normalized to untreated control (open bar). **P < 0.001. B: primary cultures were generated from tubule epithelial cells from noncystic regions, confirmed by histology examination, and surface cysts of 2 early-stage ADPKD kidneys (K171 and K182). Cells were incubated with either 25 ng/ml epidermal growth factor (EGF), a mitogenic factor, or 3.7 × 10−7 M AVP. AVP stimulated proliferation of cystic cells but not noncystic “normal tubule” cells, whereas EGF increased the proliferation of both cystic and noncystic cells.
To determine whether AVP stimulates the proliferation of noncystic tubule cells, epithelial cells were cultured from normal-appearing tissue of two early-stage ADPKD kidneys and compared with cells derived from adjacent cortical cysts (46). These ADPKD patients had relatively normal renal function; however, one kidney was removed from each patient for the treatment of severe pain. EGF stimulated the proliferation of both cystic and noncystic cells (Fig. 2B); by contrast, AVP stimulated the proliferation of only the cystic cells. Treatment with AVP had no effect on noncystic cells from kidney #182 and caused a small decrease in proliferation of noncystic cells from kidney #171. These results are consistent with previous studies showing that cAMP stimulates proliferation of cyst epithelial cells but not noncystic cells from early-stage human ADPKD kidneys (46).
Effect of tolvaptan on AVP-dependent cAMP production and ERK-dependent proliferation of human ADPKD and normal human kidney cells.
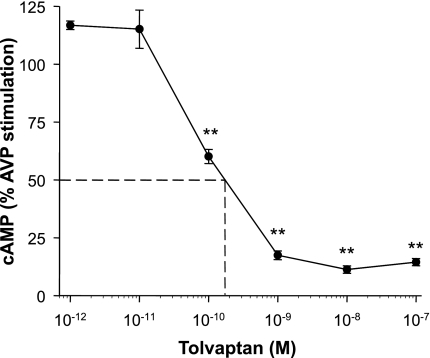
ADPKD cells were incubated with tolvaptan for 30 min before the addition of 10−9 M AVP for an additional 15 min. Lowest concentrations of tolvaptan (10−12 and 10−11 M) caused a slight increase in intracellular cAMP above the effect of AVP alone (set to 100%); however, these increases were not significant. By contrast, higher concentrations of tolvaptan inhibited the effect of AVP with an apparent IC50 of ∼2 × 10−10 M (Fig. 3) and the maximal inhibitory effect was achieved with a concentration as low as 10−9 M tolvaptan.
Fig. 3.
Effect of tolvaptan on intracellular cAMP levels in ADPKD cells stimulated with AVP. ADPKD cells were incubated for 30 min in the absence or presence of various concentrations of tolvaptan ranging from 10−12 to 10−7 M. AVP (10−9 M) was added to the bathing solution for an additional 15 min, and then cAMP was extracted from the cells and measured using a cAMP enzyme immunoassay (2). The apparent IC50 for tolvaptan to inhibit the effect of AVP on intracellular cAMP in human ADPKD cyst epithelial cells appears to be ∼0.2 nM. Data are means ± SE (n = 3 ADPKD cell preparations). **P < 0.001 compared with AVP alone (set to 100%).
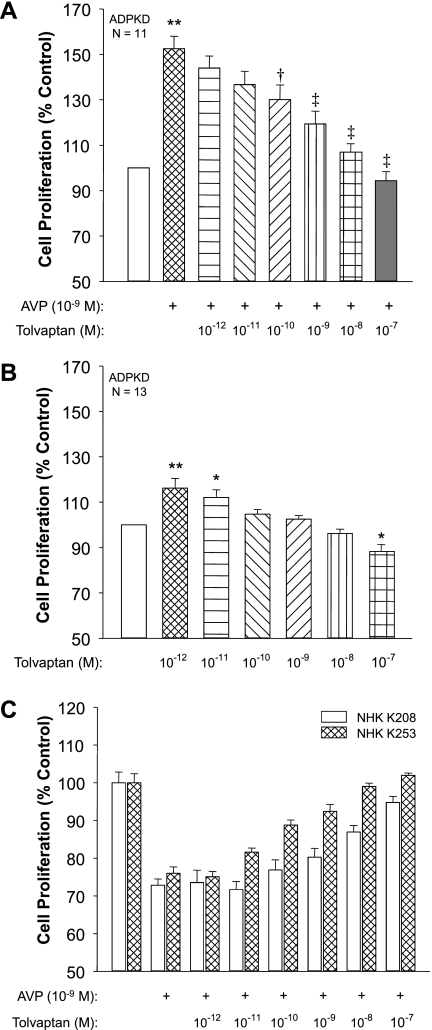
In cell proliferation assays, AVP increased ADPKD cell proliferation 52% above control (Fig. 4A) and the addition of tolvaptan inhibited AVP-induced cell proliferation with a similar dose relationship as its effect on intracellular cAMP. Stimulation of cell proliferation by AVP was decreased 30% (P < 0.05) and 43% (P < 0.001) by addition of 10−10 and 10−9 M tolvaptan, respectively, and was completely blocked by 10−8 M tolvaptan. In the absence of AVP, tolvaptan had a biphasic effect on cell proliferation (Fig. 4B). The lowest concentrations of tolvaptan caused a small, but significant, increase in ADPKD cell proliferation, suggesting that the compound may be a partial agonist. By contrast, the highest concentration of tolvaptan (10−7 M) reduced proliferation below baseline. In NHK cells, AVP inhibited cell proliferation by ∼25% (Fig. 4C), consistent with the effect of cAMP (49), and the addition of tolvaptan blocked the AVP effect with cell proliferation returning to baseline with the highest tolvaptan concentration.
Fig. 4.
Effect of tolvaptan on AVP-induced proliferation of human ADPKD and normal human kidney (NHK) cells. A: ADPKD cells (n = 11 kidney preparations) were incubated for 72 h with control media or 10−9 M AVP in the absence or presence of various concentrations of tolvaptan. **P < 0.001 compared with control-treated cells. †P < 0.05 and ‡P < 0.001 compared with cells treated with AVP alone. B: in the absence of AVP, low concentrations of tolvaptan caused a small increase in ADPKD cell proliferation, whereas higher concentrations inhibited proliferation below the basal rate. *P < 0.01 and **P < 0.001 compared with untreated cells. C: AVP decreased the proliferation rate of epithelial cells cultured from NHK (K208 and K253). Tolvaptan blocked the effect of AVP on NHK cell proliferation, consistent with inhibition of cAMP production induced by V2 receptor.
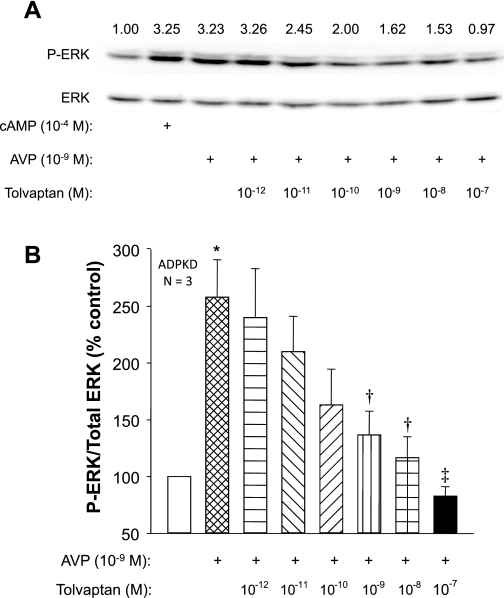
To determine whether the effect of tolvaptan to inhibit proliferation was associated with changes in ERK activation, we measured phosphorylated ERK (P-ERK) and total ERK in ADPKD cells incubated with 10−9 M AVP in the absence or presence of tolvaptan. Treatment with AVP for 15 min increased P-ERK to the same level as 10−4 M 8-Br-cAMP (Fig. 5A). Incubation with tolvaptan for 30 min before the addition of AVP inhibited the effect of AVP on ERK phosphorylation, whereas there was no consistent effect on total ERK levels. In a composite of three experiments, 10−9 M tolvaptan caused a significant decrease in P-ERK/ERK and 10−8 M tolvaptan completely blocked AVP-induced ERK activation (Fig. 5B).
Fig. 5.
Effect of AVP and tolvaptan on ERK activation in human ADPKD cells. A: representative immunoblots of phosphorylated ERK (P-ERK) and total ERK in ADPKD cells using antibodies to P-ERK (Y204) and total ERK. Cells were incubated with 10−9 M AVP or 10−4 M 8-Br-cAMP for 15 min. Tolvaptan was added for 30 min before the addition of AVP. Numbers above the bands indicate the ratio of P-ERK/total ERK. B: relative ERK activity (P-ERK/total ERK) represented as a percent of the untreated ADPKD cells (set to 100%); n = 3 cell preparations. *P < 0.05 compared with untreated control. †P < 0.05 and ‡P < 0.01 compared with cells treated with AVP alone.
Effect of tolvaptan on AVP-induced Cl− secretion by human ADPKD cell monolayers.
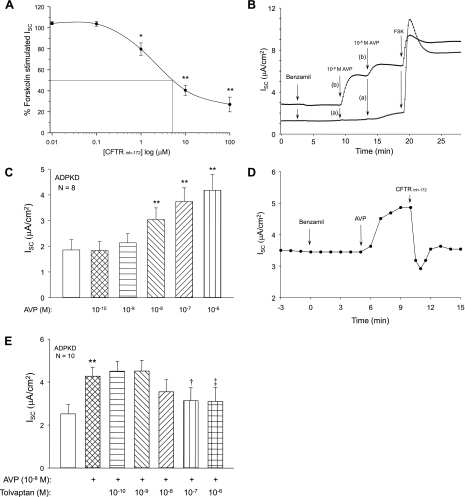
Accumulation of fluid within the cysts of ADPKD kidneys is driven by cAMP-dependent transcellular Cl− secretion mediated by apical CFTR Cl− channels (38, 40). To determine the effects of AVP and tolvaptan on anion secretion, ADPKD cell monolayers were grown on permeable supports, which were mounted in modified Ussing chambers for measurement of short-circuit current (ISC), as described previously (19). Forskolin, a cAMP agonist, stimulated ISC by ADPKD cells and the subsequent apical addition of CFTRinh-172, an inhibitor of the CFTR Cl− channel, blocked forskolin-stimulated ISC with an apparent IC50 of 5 μM (Fig. 6A). V2R is predominantly expressed on the basolateral surface of collecting duct cells. Recently, V2R was also shown to localize to the primary cilium on the apical aspect of collecting duct cells (20). Here, we compared the effects of apical and basolateral AVP on anion secretion by human ADPKD cell monolayers. Benzamil (10 μM), a potent amiloride derivative, was added to the apical medium to eliminate the potential contribution of the epithelial Na+ channel ENaC to ISC (Fig. 6B). Basolateral addition of 10−9 M AVP caused a marked increase in ISC and 10−8 M AVP caused a further increase. By contrast, there was no effect of 10−9 AVP added to the apical media and 10−8 M apical AVP caused only a small increase in ISC. These data confirm that basolateral, but not apical, AVP potently stimulates anion secretion by human ADPKD cells. In other experiments, AVP was added sequentially to the basolateral fluid for final concentrations ranging from 10−10 to 10−6 M and steady-state ISC was recorded (∼5 min; Fig. 6C). In the composite of eight experiments, there was no change in ISC at low concentrations, but concentrations of AVP at or above 10−8 M caused a consistent increase in anion secretion. Addition of 5 μM apical CFTRinh-172 completely blocked the effect of AVP, confirming that AVP-induced anion secretion was dependent on apical CFTR Cl− channel (Fig. 6D). To investigate the effect of tolvaptan on Cl− secretion, ADPKD cell monolayers were stimulated with 10−8 M AVP and after a new steady-state ISC was reached increasing concentrations of tolvaptan were added (Fig. 6E). Basolateral addition of tolvaptan (≥10−8 M) inhibited AVP-induced Cl− secretion by human ADPKD cyst epithelial cells.
Fig. 6.
Effect of tolvaptan on AVP-induced Cl− secretion by human ADPKD cell monolayers. Short-circuit current (ISC) was measured across ADPKD cell monolayers mounted in Ussing chambers using a dual voltage-clamp device as described previously (39). A: 10 μM forskolin added to the basolateral fluid maximally stimulated ISC, set to 100%. Addition of apical CFTR Cl− channel blocker CFTRinh-172 inhibited cAMP-dependent anion secretion with an apparent IC50 of 5 μM. In a composite of 3 monolayers, 5 μM CFTRinh-172 inhibited 100% of the AVP-induced ISC. B: ISC tracings for 2 ADPKD cell monolayers treated with either apical (a) or basolateral (b) AVP. Apical 10 μM benzamil, an inhibitor of the epithelial Na+ channel, was added to block Na+ transport. Basolateral (b) addition of 10−9 M and 10−8 M AVP increased ISC. By contrast, the addition of apical (a) 10−9 M AVP had no effect and 10−8 M AVP caused only a small increase in ISC. Maximal current induced by 10 μM basolateral forskolin was similar between the 2 monolayers. C: various concentrations of AVP were added sequentially to the basolateral media after a new steady-state ISC was reached. D: anion secretion induced by 10−8 M AVP was completely blocked by 5 μM CFTRinh-172, confirming that AVP increases transepithelial Cl− secretion by ADPKD monolayers. E: in a summary of 10 monolayers, AVP-stimulated Cl− secretion was inhibited by increasing concentrations of tolvaptan sequentially added to the basolateral media. *P < 0.05 and **P < 0.001 compared with control. †P < 0.01 and ‡P < 0.001 compared with AVP-stimulated ISC.
Effect of tolvaptan on AVP-dependent cyst formation of ADPKD cells in vitro.
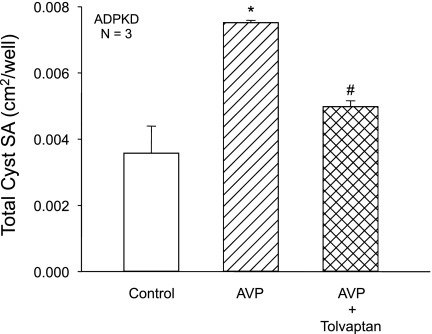
To investigate the effect of tolvaptan on AVP-dependent cell proliferation and fluid secretion under conditions that more closely resemble cyst growth in situ, ADPKD cells were cultured within a polymerized collagen gel and stimulated to form cysts. Cysts developed within a collagen matrix from clonal growth of individual cells treated with EGF and forskolin. After cyst formation was initiated, EGF and forskolin were removed, and AVP alone or in combination with tolvaptan were added to the bathing media. AVP increased the total surface area (SA) of the ADPKD cysts (diameter ≥100 μm) per well (Fig. 7). Addition of 10−8 M tolvaptan significantly reduced total SA, demonstrating that V2 receptor antagonism inhibits both cell proliferation and Cl−-dependent fluid secretion by ADPKD cysts stimulated with AVP.
Fig. 7.
Effect of tolvaptan on in vitro cyst growth of ADPKD cells cultured within collagen gel. ADPKD cells were seeded within a collagen gel and media containing 5 μM forskolin and 5 ng/ml EGF were added for 3 days to initiate cyst formation. Agonists were removed and gels were incubated with control media or media containing 10−8 M AVP ± 10−8 M tolvapatan for 5–7 days. Total surface area (SA) of cysts (≥100 μm) per well was measured. AVP increased total SA of the cysts, demonstrating that AVP accelerates cyst growth in vitro, and tolvaptan significantly inhibited AVP-induced cyst formation. *P < 0.01 compared with control. #P < 0.05 compared with AVP alone.
DISCUSSION
AVP is secreted by the posterior pituitary gland in response to changes in extracellular fluid osmolality. Binding of AVP to V2 receptors on the basolateral membrane of principal cells of the collecting duct increases intracellular cAMP, causing activation and insertion of AQP-2 water channels into the apical membrane. Enhanced water permeability of the collecting duct allows water in the glomerular filtrate to be reabsorbed and returned to the circulation. Maintenance of water excretion by the kidneys depends on an appropriate plasma AVP level to correctly regulate osmotic water reabsorption.
In ADPKD and ARPKD, renal cysts originate from collecting ducts, where V2 receptors are predominantly expressed (2, 32, 37). AVP stimulates cAMP production in human ADPKD and ARPKD cells through G protein-coupled receptor activation of adenylyl cyclases. Several signaling pathways have been implicated in PKD pathogenesis; however, cAMP has been shown to play a central role in cyst growth by stimulating both cell proliferation and transepithelial fluid secretion. In PCK rats, a model orthologous to human ARPKD, suppression of plasma AVP by simply increasing water intake reduced renal cAMP and decreased the level of ERK activation, cell proliferation, and disease progression (23). OPC-31260, which antagonizes V2 receptors, also reduced renal cAMP and dramatically halted disease progression in four different genetic models of PKD (12, 35, 42). Wang et al. (43) confirmed that the effect of the V2 receptor antagonist was due to inhibition of the renal effects of AVP by eliminating vasopressin expression in the PCK rat. PCK rats were crossed with Brattleboro rats that lack AVP due to a mutation in the vasopressin gene. Elimination of AVP reduced renal cAMP accumulation, ERK activity, cell proliferation, and fibrosis; and the kidneys were essentially free of cysts. Administration of DDAVP by osmotic minipump restored cystic disease in the AVP-deficient PCK rats, providing unequivocal evidence for the roles of AVP and cAMP on PKD progression.
Tolvaptan is an orally administered, potent, and highly selective V2 receptor antagonist that increases free water clearance and has been shown to correct hyponatremia (serum sodium levels below 135 mmol/l) associated with heart failure, cirrhosis, or syndrome of inappropriate antidiuretic hormone secretion (4, 6, 29, 51). The affinity of tolvaptan for V2 receptors is 1.8-fold greater than AVP (Ki 0.43 vs. 0.78 nM) and 29-fold greater than V1A receptor (Ki 12.3 nM); and tolvaptan does not appear to bind to V1B receptors (51). In patients with heart failure, daily delivery of 15–60 mg of tolvpatan (titrated according to response) for up to 30 days improved serum sodium levels. The drug was well-tolerated with only a few adverse events such as thirst and dry mouth.
Pharmacokinetic parameters have been evaluated after oral administration of 30 mg tolvaptan once daily for 7 days (15). The mean peak plasma concentration was 283 ng/ml (630 × 10−7 M) achieved 2 h after drug delivery. The mean area under the plasma concentration-time curve over the 24-h dosage interval was 3,087 ng·h−1·ml−1. The mean minimum plasma concentration of tolvaptan at the end of the once daily dosage interval was 48 ng/ml (1.07 × 10−7 M) and the average plasma concentration at steady state was 129 ng/ml (2.87 × 10−7 M). Free concentration of tolvaptan may be much lower than total plasma concentration since the drug is highly bound to plasma protein (28).
TEMPO (Tolvaptan Efficacy and safety in Management of Polycystic kidney disease and its Outcomes) 2/4 is a multi-center, open-label randomized dose-ranging study to investigate the long-term effects of tolvaptan on patient safety, tolerability, and efficacy. Mean total kidney volume (TKV) was measured by magnetic resonance imaging and by changes in glomerular filtration rate (eGFR). After a dose titration phase, patients were randomized into two groups receiving oral delivery of tolvaptan twice per day in split doses of either 45/15 mg (low) or 60/30 mg (high). Target urine osmolality for tolvaptan inhibition of renal AVP activity was below 300 mosmol/kgH2O. Preliminary results from the 3-yr treatment indicate that tolvaptan decreased TKV growth and slowed the decline of eGFR in ADPKD patients (33). On withdrawal of tolvaptan, TKV resumed at the expected rate of growth while GFR appeared to remain stable. TEMPO 3/4 is a placebo-controlled double-blind trial, involving oral delivery of tolvaptan twice per day in split doses 45/15, 60/30, and 90/30 mg for 3 yr.
In the current study, we found that 1 × 10−9 M AVP increased intracellular cAMP from 0.7 ± 0.2 to 4.3 ± 0.6 pmol/monolayer, increased the level of P-ERK, and accelerated the proliferation of ADPKD cyst epithelial cells. Tolvaptan inhibited AVP-induced cAMP production with an apparent IC50 of ∼0.2 nM (Fig. 3), similar to the reported Ki for tolvaptan binding to V2 receptors. Tolvaptan (10−10 to 10−9 M) decreased P-ERK/ERK (Fig. 5) and inhibited ADPKD cell proliferation (Fig. 4A). These concentrations had no apparent effect on basal cell proliferation (Fig. 4B). Interestingly, 10−7 M tolvaptan caused a small, but significant, decrease in basal cell proliferation. The mechanism for the inhibitory effect of tolvaptan in the absence of AVP is unclear; however, it is possible that at high concentrations, the drug inhibits endogenous activity of the V2R or that the drug has additional anti-proliferative effects.
In HELA cells expressing cloned human V2 receptors, tolvaptan (10−10 to 10−9 M) had no effect on AVP-induced cAMP production, whereas higher concentrations strongly inhibited cAMP production (51). In the present study, we examined a broader range of tolvaptan concentrations in human ADPKD cells. Surprisingly, vanishingly low concentrations of tolvaptan (10−12 to 10−11 M) increased cAMP ∼20% above the effect of AVP alone (Fig. 3) and stimulated basal cell proliferation (Fig. 4B), suggesting that tolvaptan at low concentrations could be a partial agonist for the V2 receptor. However, these effects of tolvaptan did not appear to prevent the concentration-dependent decrease in cell proliferation (Fig. 4A) and ERK activation (Fig. 5) when administered with AVP. In recent studies of human subjects (TEMPO and the Study of Ascending Levels of Tolvaptan in Hyponatremia), tolvaptan administration over a range of doses consistently decreased urine osmolality and increased the rate of urine flow acutely and over the longer term. Moreover, there are no reports of unanticipated antidiuresis following the administration of tolvaptan. It would appear that doses of tolvaptan used in these trials are sufficient to chronically inhibit V2 receptors, reduce urine osmolality, and increase urine volume above baseline levels; the expected effects were tolvaptan to lower cAMP levels in collecting ducts.
The remarkable appearance of an end-stage ADPKD kidney is due to the accumulation of fluid within the hundreds or thousands of cysts that grossly expand the TKV. Fluid secretion by human ADPKD cells is associated with an increase in apically negative transepithelial potential difference (Vte) and positive ISC, consistent with active anion secretion (19). Apical application of diphenylamine-2-carboxylate, a Cl− channel blocker, decreases ISC, depolarizes Vte, and inhibits net fluid secretion. Here, we show that basolateral, but not apical, AVP increases ISC across ADPKD cell monolayers (Fig. 6B) and that apical addition of the highly selective CFTR Cl− channel blocker CFTRinh-172 blocks AVP-induced ISC (Fig. 6D), confirming that basolateral AVP induces Cl− secretion by cystic cells via apical CFTR Cl− channels (18, 19, 31, 40). Tolvaptan (concentrations ≥10−8 M) inhibits AVP-stimulated Cl− secretion by human ADPKD cell monolayers (Fig. 6E) and decreases in vitro cyst growth of ADPKD cells cultured within a three-dimensional collagen matrix (Fig. 7). The capacity for tolvaptan to inhibit fluid secretion may be as important as its effect on cell proliferation. Inhibition of fluid secretion may limit the expansion of existing cysts and allow net fluid absorption to reduce cyst size and TKV.
In summary, concentrations of tolvaptan likely to be attained in the plasma of ADPKD patients by the dosing regimen of the TEMPO studies inhibit AVP-induced activation of the B-Raf/MEK/ERK pathway and cell proliferation and decrease both AVP-stimulated Cl− secretion and in vitro cyst growth of ADPKD cells. These data in human cyst epithelial cells aid in understanding the cellular mechanisms underlying the action of tolvaptan and offer additional support for the use of tolvaptan in the treatment of renal cyst progression in patients with ADPKD.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK081579 and P50DK057301 (to D. P. Wallace) and Otsuka Pharmaceutical.
DISCLOSURES
Part of this work was supported by a grant from Otsuka Pharmaceutical. Dr. Hiroyuki Fujiki is an employee of Otsuka Pharmaceutical.
ACKNOWLEDGMENTS
Portions of this work have previously been published in abstract form (J Am Soc Nephrol 18: 362A, 2007). The authors thank Dr. Jared Grantham for helpful suggestions during the preparation of the manuscript.
REFERENCES
- 1. Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD). Hepatology 49: 160–174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, Helmkamp GM, Jr, Grantham JJ. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int 66: 964–973, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Boucher CA, Ward HH, Case RL, Thurston KS, Li X, Needham A, Romero E, Hyink D, Qamar S, Roitbak T, Powell S, Ward C, Wilson PD, Wandinger-Ness A, Sandford RN. Receptor protein tyrosine phosphatases are novel components of a polycystin complex. Biochim Biophys Acta 1812: 1225–1238, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costello-Boerrigter LC, Boerrigter G, Burnett JC., Jr Pharmacology of vasopressin antagonists. Heart Fail Rev 14: 75–82, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Danielsen H, Pedersen EB, Nielsen AH, Herlevsen P, Kornerup HJ, Posborg V. Expansion of extracellular volume in early polycystic kidney disease. Acta Med Scand 219: 399–405, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Decaux G, Soupart A, Vassart G. Nonpeptide arginine-vasopressin antagonists: the vaptans. Lancet 371: 1624–1632, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Delmas P. Polycystins: from mechanosensation to gene regulation. Cell 118: 145–148, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Devuyst O, Burrow CR, Smith BL, Agre P, Knepper MA, Wilson PD. Expression of aquaporins-1 and -2 during nephrogenesis and in autosomal dominant polycystic kidney disease. Am J Physiol Renal Fluid Electrolyte Physiol 271: F169–F183, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Gabow PA, Kaehny WD, Johnson AM, Duley IT, Manco-Johnson M, Lezotte DC, Schrier RW. The clinical utility of renal concentrating capacity in polycystic kidney disease. Kidney Int 35: 675–680, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Gattone VH, 2nd, Maser RL, Tian C, Rosenberg JM, Branden MG. Developmental expression of urine concentration-associated genes and their altered expression in murine infantile-type polycystic kidney disease. Dev Genet 24: 309–318, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M. A transcriptional network in polycystic kidney disease. EMBO J 23: 1657–1668, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Hauptman PJ, Zimmer C, Udelson J, Shoaf SE, Mallikaarjun S, Bramer SL, Orlandi C. Comparison of two doses and dosing regimens of tolvaptan in congestive heart failure. J Cardiovasc Pharmacol 46: 609–614, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Houslay MD, Milligan G. Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem Sci 22: 217–224, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, Leonhard WN, van de Wal A, Ward CJ, Verbeek S, Deruiter MC, Breuning MH, de Heer E, Peters DJ. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet 13: 3069–3077, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Magenheimer BS, St John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, Maser RL, Grantham JJ, Calvet JP. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na+,K+,2Cl− cotransporter-dependent cystic dilation. J Am Soc Nephrol 17: 3424–3437, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Mangoo-Karim R, Ye M, Wallace DP, Grantham JJ, Sullivan LP. Anion secretion drives fluid secretion by monolayers of cultured human polycystic cells. Am J Physiol Renal Fluid Electrolyte Physiol 269: F381–F388, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Marion V, Schlicht D, Mockel A, Caillard S, Imhoff O, Stoetzel C, van Dijk P, Brandt C, Moulin B, Dollfus H. Bardet-Biedl syndrome highlights the major role of the primary cilium in efficient water reabsorption. Kidney Int 79: 1013–1025, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Martinez-Maldonado M, Yium JJ, Eknoyan G, Suki WN. Adult polycystic kidney disease: studies of the defect in urine concentration. Kidney Int 2: 107–113, 1972 [DOI] [PubMed] [Google Scholar]
- 22. Michalski A, Grzeszczak W. The effect of hypervolemia on electrolyte level and and level of volume regulating hormones in patients with autosomal dominant polycystic kidney disease. Pol Arch Med Wewn 96: 329–343, 1996 [PubMed] [Google Scholar]
- 23. Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, Wallace DP. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 17: 2220–2227, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol 17: 1015–1025, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Nguyen AN, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol 18: 46–57, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Parnell SC, Magenheimer BS, Maser RL, Rankin CA, Smine A, Okamoto T, Calvet JP. The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun 251: 625–631, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Pei Y. Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin Pract 118: c19–c30, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Plosker GL. Tolvaptan. Drugs 70: 443–454, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Serradeil-Le Gal C, Lacour C, Valette G, Garcia G, Foulon L, Galindo G, Bankir L, Pouzet B, Guillon G, Barberis C, Chicot D, Jard S, Vilain P, Garcia C, Marty E, Raufaste D, Brossard G, Nisato D, Maffrand JP, Le Fur G. Characterization of SR 121463A, a highly potent and selective, orally active vasopressin V2 receptor antagonist. J Clin Invest 98: 2729–2738, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, Beier DR, Ibraghimov-Beskrovnaya O. Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol 17: 2821–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Sullivan LP, Wallace DP, Grantham JJ. Chloride and fluid secretion in polycystic kidney disease. J Am Soc Nephrol 9: 903–916, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Thomson RB, Mentone S, Kim R, Earle K, Delpire E, Somlo S, Aronson PS. Histopathological analysis of renal cystic epithelia in the Pkd2WS25/− mouse model of ADPKD. Am J Physiol Renal Physiol 285: F870–F880, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Torres VE, Chapman AB, Grantham JJ, Watnick TJ, Ouyang J, Krasa HB, Czerwiec FS. TEMPO 2/4 update: changes in ADPKD total kidney volume and eGFR with 3 years of tolvaptan and after withdrawal. J Am Soc Nephrol 21: 528A, 2010 [Google Scholar]
- 34. Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang JJ, Czerwiec FS. Rationale and design of the TEMPO (tolvaptan efficacy and safety in management of autosomal dominant polycystic kidney disease and its outcomes) 3–4 study. Am J Kidney Dis 57: 692–699, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH., 2nd Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Vandorpe DH, Chernova MN, Jiang L, Sellin LK, Wilhelm S, Stuart-Tilley AK, Walz G, Alper SL. The cytoplasmic C-terminal fragment of polycystin-1 regulates a Ca2+-permeable cation channel. J Biol Chem 276: 4093–4101, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Verani RR, Silva FG. Histogenesis of the renal cysts in adult (autosomal dominant) polycystic kidney disease: a histochemical study. Mod Pathol 1: 457–463, 1988 [PubMed] [Google Scholar]
- 38. Wallace DP. Cyclic AMP-mediated cyst expansion. Biochim Biophys Acta 1812: 1291–1300, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wallace DP, Christensen M, Reif G, Belibi F, Thrasher B, Herrell D, Grantham JJ. Electrolyte and fluid secretion by cultured human inner medullary collecting duct cells. Am J Physiol Renal Physiol 283: F1337–F1350, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Wallace DP, Grantham JJ, Sullivan LP. Chloride and fluid secretion by cultured human polycystic kidney cells. Kidney Int 50: 1327–1336, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Wallace DP, Quante MA, Reif GA, Nivens E, Ahmed F, Hempson SJ, Blanco G, Yamaguchi T. Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am J Physiol Renal Physiol 295: F1463–F1471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang X, Gattone V, 2nd, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 16: 846–851, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu G, D'Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93: 177–188, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Xia S, Li X, Johnson T, Seidel C, Wallace DP, Li R. Polycystin-dependent fluid flow sensing targets histone deacetylase 5 to prevent the development of renal cysts. Development 137: 1075–1084, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17: 178–187, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Yamaguchi T, Nagao S, Kasahara M, Takahashi H, Grantham JJ. Renal accumulation and excretion of cyclic adenosine monophosphate in a murine model of slowly progressive polycystic kidney disease. Am J Kidney Dis 30: 703–709, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, Rome LA, Sullivan LP, Grantham JJ. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 57: 1460–1471, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 279: 40419–40430, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, Yamada Y, Tsujimae K, Aoyama M, Kotosai K, Ogawa H, Yamashita H, Kondo K, Tominaga M, Tsujimoto G, Mori T. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther 287: 860–867, 1998 [PubMed] [Google Scholar]
- 52. Yang J, Zhang S, Zhou Q, Guo H, Zhang K, Zheng R, Xiao C. PKHD1 gene silencing may cause cell abnormal proliferation through modulation of intracellular calcium in autosomal recessive polycystic kidney disease. J Biochem Mol Biol 40: 467–474, 2007 [DOI] [PubMed] [Google Scholar]