Abstract
Prostaglandins have been implicated as paracrine regulators of renin secretion, but the specific pathways and receptor(s) carrying out these functions have not been fully elucidated. To examine the contributions of prostanoid synthetic pathways and receptors to regulation of renin in the intact animal, we used a panel of mice with targeted disruption of several key genes: cyclooxygenase-2 (COX-2), microsomal PGE synthases 1 and 2 (mPGES1, mPGES2), EP2 and EP4 receptors for PGE2, and the IP receptor for PGI2. To activate the macula densa signal for renin stimulation, mice were treated with furosemide over 5 days and renin mRNA levels were determined by real-time RT-PCR. At baseline, there were no differences in renin mRNA levels between wild-type and the various strains of mutant mice. Furosemide caused marked stimulation of renin mRNA expression across all groups of wild-type control mice. This response was completely abrogated in the absence of COX-2, but was unaffected in mice lacking mPGES1 or mPGES2. The absence of Gs/cAMP-linked EP2 receptors had no effect on stimulation of renin by furosemide and there was only a modest, insignificant reduction in renin responses in mice lacking the IP receptor. By contrast, renin stimulation in EP4−/− mice was significantly reduced by ∼70% compared with wild-type controls. These data suggest that stimulation of renin by the macula densa mechanism is mediated by PGE2 through a pathway requiring COX-2 and the EP4 receptor, but not EP2 or IP receptors. Surprisingly, mPGES1 or mPGES2 are not required, suggesting other alternative mechanisms for generating PGE2 in response to macula densa stimulation.
Keywords: prostanoids, cyclooxygenase, kidney, renin-angiotensin system
renin secretion from renal juxtaglomerular (JG) cells is the rate-limiting step in the formation of angiotensin II and control of renin release is of critical importance for extracellular fluid volume and blood pressure regulation (4). Physiological regulation of renin release is influenced by three major mechanisms: renal sympathetic nerve activity, the renal baroreceptor, and the macula densa. For the latter, macula densa cells of the thick ascending limb of the loop of Henle sense changes in tubular sodium chloride concentration via the sodium-potassium-chloride transporter (NKCC2) located in their apical membrane (27). Macula densa cells are located in close apposition to the afferent arteriole and affect renin secretion by signaling to JG cells in response to changes in luminal sodium chloride concentration. Reduced delivery of sodium chloride to that segment stimulates renin, whereas increased delivery suppresses renin, thereby providing a direct link between tubular sodium handling and control of renin-angiotensin system activation.
Prostanoids have been long implicated in the macula densa signal for release of renin. For example, infusion of PGE2 and PGI2 into the kidney stimulates renin secretion in various ex vivo and in vitro models (3, 23, 48), and this effect depends on cAMP generation (41). These prostanoids are generated from arachidonic acid by the sequential actions of cyclooxygenases (COX-1 and COX-2) and terminal prostaglandin synthases (PGES and PGIS). COX-2 is constitutively expressed in the thick ascending limb of the loop of Henle (18). Moreover, expression of COX-2 at the macula densa is stimulated during sodium depletion (50). Pharmacological inhibition of cyclooxygenases with nonsteroidal anti-inflammatory drugs blocks renin secretion (20). This effect is recapitulated by specific inhibitors of COX-2.
PGE2 and PGI2 exert their physiological actions by signaling through specific G protein-coupled receptors: a family of E-prostanoid (EP) receptors for PGE2 and a single I-prostanoid (IP) receptor for PGI2. Since cAMP release seems to be a final common signaling pathway for renin release by the JG cell, it was presumed that PG-induced renin synthesis and secretion were likely mediated by the Gs-linked prostanoid receptors EP2, EP4, and/or IP, which all generate robust activation of adenylyl cyclase. In this regard, previous studies using various models and experimental approaches variously implicated the EP2, EP4, and IP receptors in macula densa regulation of renin (12, 32, 41). Accordingly, there remains some uncertainty regarding the specific prostaglandin(s), PG synthase(s), and cell surface receptors controlling renin by the macula densa mechanism in the intact animal. Therefore, we carried out a comprehensive examination of this mechanism using a panel of mice with targeted deletion of genes that are critical to PGE2 and PGI2 synthesis and signaling. Using these mice and their precisely matched genetic controls, we stimulated renin expression with the loop diuretic furosemide, which inhibits the NKCC2 transporter and thereby triggers the macula densa signal to stimulate renin (27). Our studies indicate critical roles for COX-2 and the EP4 receptor for PGE2 in this pathway.
MATERIALS AND METHODS
Animals.
All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of the Durham VA and Duke University Medical Centers and the University of North Carolina at Chapel Hill. COX-2−/− mice and wild-type (WT) littermates were maintained on a mixed (129/SvEv, C57BL/6) genetic background as previously described (28). mPGES1−/− mice were originally generated from ES cells derived from DBA/1 mice (45) and were back-crossed onto the C57BL/6 genetic background for greater than eight generations; WT C57BL/6 mice were used as controls. The EP2−/− mice were maintained on the C57BL/6 background and their WT littermates were used as controls. The mPGES2−/− and IP−/− mice and their littermate controls were maintained on the 129/SvEv background.
Homozygosity for a null mutation in the EP4 receptor gene (EP4−/−) in mice is lethal in the early postnatal period due to patent ductus arteriosus (DA) (31). However, we observed that a few of the EP4-deficient mice survived into adulthood. In these surviving animals with a mixed genetic background of 129/SvEv, C57BL/6, and DBA/2, DA closure occurred normally, independent of EP4 receptors and the characteristic changes in PGE2 after birth. One of these EP4−/− survivors was crossed with an EP4+/− sibling and, in litters born to these parents, EP4−/− mice undergoing DA closure and surviving the perinatal period were seen with higher frequency. Subsequent intercrosses of EP4−/− and EP4+/− littermates were carried out for >30 generations leading to the production of a recombinant inbred (RI) mouse line and a congenic EP4−/− line, in which there is remodeling of the DA, independent of prostanoids and the EP4 receptor. These RI-EP4−/− and their WT littermates were used for our studies.
The mouse lines used in our studies are on various genetic backgrounds including 129/SvEv, expressing two renin genes (Ren1d and Ren2), and C57BL/6 mice, expressing only the single Ren1c gene (42). Table 1 shows the genetic background and renin gene(s) expressed for each line of gene-targeted mice used in our study. Renin genotyping was performed as previously described (25) using the following primers and probe: Ren1/2 common forward TGTCCGGGAAATCCTGGAG; Ren1 reverse ACGGGGGAGATAAGATCAG; Ren2 reverse ACGGGGGAGGTAAGATTGA; Ren1/2 common probe Fam-CAGCACTGAGCCTGGTCATGTCCA-Tamra.
Table 1.
Renin gene expression in gene-targeted mouse lines
| Targeted Gene | Genetic Background | Renin Genes Expressed |
|---|---|---|
| COX-2 | C57BL/6, 129 mixed | Ren1d, Ren2 |
| EP2 | C57BL/6 | Ren1c |
| EP4 | RI* | Ren1d, Ren2 |
| IP | 129 | Ren1d, Ren2 |
| mPGES1 | C57BL/6 | Ren1c |
| mPGES2 | 129 | Ren1d, Ren2 |
COX-2, cyclooxygenase-2; EP, E-prostanoid; IP, I-prostanoid; mPGES, microsomal PGE synthase; RI*, recombinant inbred background of EP4+/+ and −/− mice, consisting of C57BL/6, 129, and DBA/2.
All experimental mice were 8–12 wk old. Mice were treated with furosemide (2.28 mmol/l in drinking water) or vehicle for 5 days, kidneys were harvested, and RNA was isolated as described below. For studies using high-salt diet, mice were fed 6% NaCl chow (Harlan-Teklad, Madison, WI) beginning 2 days before furosemide administration and for the duration of treatment.
RNA isolation and real-time RT-PCR.
Total RNA was extracted from whole kidneys using TriReagent (Sigma) according to the manufacturer's protocol. RNA was DNase-treated using Turbo DNA-free (Ambion, Austin, TX) to remove genomic DNA contamination. RNA yield was quantified by UV spectrophotometry and integrity was verified by 1% agarose gel electrophoresis and staining with ethidium bromide. Only RNA with A260/280 > 1.7 and displaying no significant degradation was used for reverse transcription.
cDNAs were synthesized from 5 μg of total RNA using random hexamers and SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). “No RT” samples lacking reverse transcriptase were prepared during each RT reaction for use as negative controls during PCR. Primers and dual-labeled probe (5′-FAM, 3′-TAMRA) targeting renin were synthesized based on previously published sequences (21). Human 18S rRNA endogenous control primer-probe set was purchased from Applied Biosystems (Foster City, CA). PCR reactions were performed in duplicate on an iCycler real-time detection system (Bio-Rad, Hercules, CA). cDNA and negative control (No RT, water) templates (1 μl) were added to 25-μl PCR reaction mixtures consisting of 1× ABI TaqMan Universal PCR master mix and either 1× human eukaryotic 18S rRNA primer-probe mix or 1× primer-probe mix for the gene of interest. Gene expression was quantified using the two standard curve method for relative quantitation (5). Briefly, a five-point dilution series of positive control cDNA was prepared, and standard curves of threshold cycle (Ct) vs. relative template concentration were generated for the gene of interest (renin) and housekeeping gene (18S rRNA). Template concentrations of unknown samples were then determined, based on their Ct values, from these standard curves. The renin primers and probe used for real-time RT-PCR in our studies recognize both Ren1 and Ren2. However, the presence of a second renin gene does not appear to affect plasma renin concentration (PRC) in mice (16).
Determination of PRC.
Mice were lightly anesthetized with isoflurane and ∼100 μl of blood were rapidly collected from the maxillary plexus into tubes containing 3 μl of 125 mM EDTA. Blood samples were maintained at room temperature and centrifuged at 3,500 rpm for 10 min to isolate plasma. Plasma samples were immediately frozen at −80°C until assay. PRC was determined by incubating plasma samples for 1 h with an excess of rat angiotensinogen derived from 48-h nephrectomized rat plasma. The incubate was then assayed for PRC using a Gamma Coat RIA kit (DiaSorin, Stillwater, MN) as previously described (33, 34).
Radiotelemetry measurements of intra-arterial pressure.
Blood pressure was measured in conscious mice by radiotelemetry using TA11PA-C10 transmitters (Data Sciences International, St. Paul, MN) as described previously (10). Briefly, mice were anesthetized with isoflurane and a pressure-sensing catheter was implanted into the left carotid artery, as previously described (6). The transducer unit was then inserted into a subcutaneous pouch along the right flank generated by blunt dissection inferiorly from the original neck incision. Mice were allowed to recover for 7 days after surgery to regain their normal circadian rhythms before experiments were initiated. During blood pressure measurements, mice were housed in a monitoring room in the animal facility where quiet is maintained and no other activities are permitted. Data were collected continuously with sampling every 5 min for 10-s intervals using Dataquest A.R.T. software (Data Sciences International).
Data analysis.
Data are presented as means ± SE. For furosemide-treated WT groups (designated as 100% in mRNA expression studies), renin mRNA expression values for all WT animals of that group were averaged and this mean was set at 100%. Expression in all other groups was expressed relative to the furosemide-treated WT group.
Group sizes for experimental and WT control mice were as follows: COX-2, n = 3–9; EP2, n = 4; EP4, n = 3–4; IP, n = 7–10; EP4/IP high-salt, n = 4–8; mPGES1/mPGES2, n = 3–7. Some group sizes were small due to limited availability of gene-targeted mice, but provided sufficient statistical power to resolve differences between groups. Differences between groups were assessed by unpaired t-test and a P value of <0.05 was considered significant. Statistical analyses were performed using GraphPad Prism software.
RESULTS
To begin to examine prostanoid synthetic pathways involved in macula densa control of renin, we first treated WT and COX-2−/− mice with furosemide or vehicle and measured changes in kidney renin mRNA expression. At baseline, renin mRNA levels were similar in COX-2-deficient mice and WT controls (3.2 ± 1.6 vs. 7.8 ± 2.2%; expressed as percent of furosemide-treated WT; P = 0.076). Furosemide treatment caused a robust stimulation of renin expression in WT animals from 9.1 ± 2.6 to 100 ± 17.3% (P = 0.002). In contrast, renin mRNA expression in COX-2−/− animals was largely unaffected by furosemide (3.7 ± 1.8 vs. 3.9 ± 1.9%) and therefore renin expression after furosemide was significantly lower in the COX-2-deficient mice than their controls (3.9 ± 1.9 vs. 100.0 ± 17.3%, P = 0.002; Fig. 1). Thus, in line with previous studies (8, 15, 17, 44, 47), we find that COX-2 is essential for chronic stimulation of renin by the macula densa mechanism.
Fig. 1.
Furosemide stimulation of renin mRNA expression in the kidneys of wild-type (WT; filled bars) and cyclooxygenase (COX)-2−/− (open bars) mice. Relative expression of renin mRNA was measured by real-time RT-PCR in kidneys of mice treated with either vehicle or furosemide and is presented as percent of the furosemide-stimulated WT value. *P < 0.05 vs. WT by unpaired t-test.
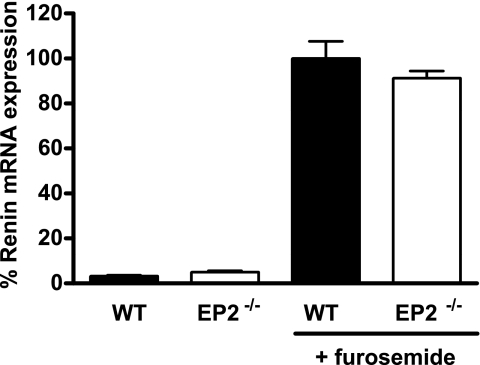
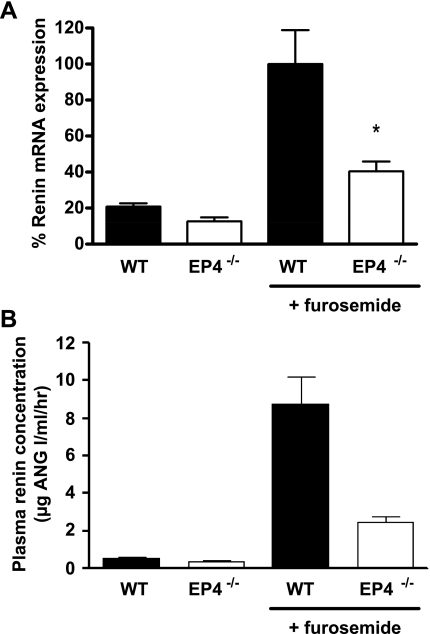
We used EP2−/− and EP4−/− mice to examine the relative roles of these receptors in furosemide-induced renin mRNA expression. At baseline, there was no difference in renin mRNA levels between the EP2-deficient mice and their genetically matched WT controls (5.19 ± 1.19 vs. 3.31 ± 0.85%, P = 0.25). Chronic furosemide treatment caused significant and equivalent stimulation of renin mRNA in both groups (91.4 ± 6.3 vs. 100.0 ± 7.6%, P = 0.42; Fig. 2). Renin mRNA levels in EP4−/− animals at baseline were not different from those in their WT controls (12.6 ± 3.9 vs. 20.9 ± 3.0%, P = 0.08). Compared with genetically matched EP4+/+ controls, the renin mRNA response to furosemide was markedly attenuated in EP4-deficient animals (40.5 ± 10.8 vs. 100.0 ± 18.8%, P = 0.03; Fig. 3A). To determine whether the deficit in kidney renin mRNA expression in EP4−/− mice was associated with a change in circulating levels of renin, we measured PRC in these mice and their EP4+/+ controls. We found that basal PRC was significantly lower in EP4−/− mice than in their WT controls (0.38 ± 0.04 vs. 0.56 ± 0.08 mg ANG I·μl−1·h−1, P = 0.037; Fig. 3B). Furthermore, the furosemide-stimulated increase in PRC was markedly attenuated in EP4−/− compared with WT animals (2.48 ± 0.31 vs. 8.77 ± 1.42 mg ANG I·μl−1·h−1, P = 0.001).
Fig. 2.
Furosemide stimulation of renin mRNA expression in the kidneys of WT (filled bars) and EP2−/− (open bars) mice. Relative expression of renin mRNA was measured by real-time RT-PCR in kidneys of mice treated with either vehicle or furosemide and is presented as percent of the furosemide-stimulated WT value.
Fig. 3.
Furosemide stimulation of renin in WT (filled bars) and EP4−/− (open bars) mice. A: relative expression of renin mRNA was measured by real-time RT-PCR in kidneys of mice treated with either vehicle or furosemide and is presented as percent of the furosemide-stimulated WT value. B: plasma renin concentration was measured by radioimmunoassay. *P < 0.05 vs. WT by unpaired t-test.
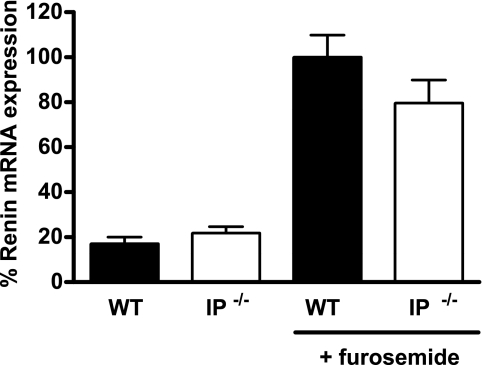
Previous studies also implicated the GS-linked IP receptor for PGI2 in macula densa control of renin (12). Thus, we also compared the renin response to furosemide in mice lacking the IP receptor for PGI2 and genetically matched WT control mice. At baseline, renin mRNA levels were very similar in the groups of IP+/+ and −/− animals (21.8 ± 2.9 vs. 17.1 ± 2.9% vehicle, P = 0.28). Likewise, furosemide caused marked stimulation of renin in both IP-deficient and WT mice (79.8 ± 10.2 vs. 100.0 ± 9.8% furosemide, P = 0.17; Fig. 4).
Fig. 4.
Furosemide stimulation of renin mRNA expression in the kidneys of WT (filled bars) and IP−/− (open bars) mice. Relative expression of renin mRNA was measured by real-time RT-PCR in kidneys of mice treated with either vehicle or furosemide and is presented as percent of the furosemide-stimulated WT value.
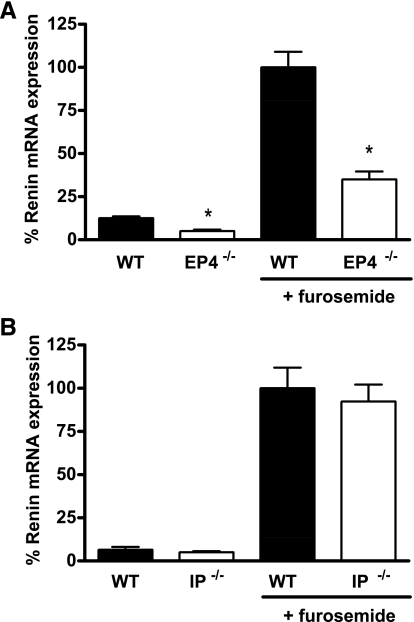
Changes in blood pressure also impact renin release and expression via the renal baroreceptor. Since furosemide is a potent diuretic that may reduce blood pressure and perhaps activate renal baroreceptor mechanisms, we carried out additional experiments wherein EP4- and IP-deficient mice, along with their WT controls, were fed a high-salt (6% NaCl) diet during the period of furosemide administration to attenuate reductions of extracellular fluid volume. In general, the patterns of renin responses were similar to the studies with conventional sodium intake. There was no difference in renin levels between the IP+/+ and −/− animals on the high-salt diets at baseline (3.7 ± 0.35 vs. 3.1 ± 1.2% vehicle, P = 0.62) or after stimulation with furosemide, where they were virtually identical (92.2 ± 8.1 vs. 100.0 ± 16.6%, P = 0.69; Fig. 5B). By contrast, renin mRNA levels were significantly lower in EP4−/− animals than controls at baseline with high-salt feeding (5.1 ± 0.8 vs. 12.6 ± 1.0%, P < 0.001; Fig. 5A). During high-salt treatment, furosemide stimulation of renin was significantly attenuated in the EP4-deficient mice compared with WT controls (35.0 ± 4.7 vs. 100.0 ± 9.1%, P < 0.001).
Fig. 5.
Furosemide stimulation of renin mRNA expression in the kidneys of EP4−/− (A) and IP−/− (B) mice on a high-salt diet. Relative expression of renin mRNA was measured by real-time RT-PCR in kidneys of WT (filled bars) and EP4−/− or IP−/− (open bars) mice treated with either vehicle or furosemide while consuming a high-salt (6% NaCl) diet. Data are presented as percent of the furosemide-stimulated WT value. *P < 0.05 vs. WT by unpaired t-test.
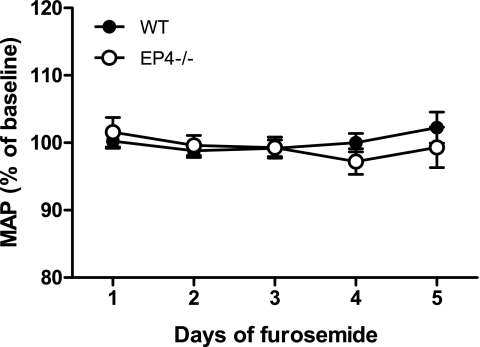
To ensure that there were no changes in blood pressure associated with the regimen of furosemide administration, we measured blood pressure in EP4−/− mice and their WT littermates by radiotelemetry at baseline and during 5 days of furosemide treatment. Under basal conditions, 24-h mean arterial pressure (MAP) was not significantly different between WT (116 ± 2 mmHg) and EP4−/− mice (112 ± 2 mmHg, P = 0.12). As shown in Fig. 6, furosemide treatment did not significantly affect daily MAP in either group, compared with their respective baseline values.
Fig. 6.
Daily mean arterial pressure (MAP; 24 h) in EP4−/− and WT control mice during 5 days of furosemide treatment. Daily means are expressed as percent of baseline MAP for each group of mice.
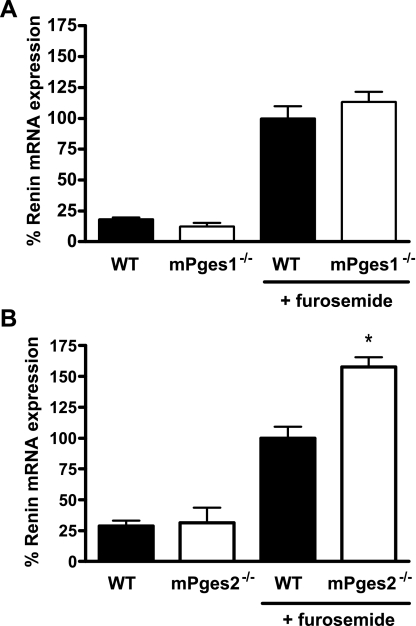
To identify the key pathways for PGE2 synthesis linked to macula densa signaling, we tested renin responses in mice lacking the two putative PGE synthase enzymes: mPGES1 and mPGES2. At baseline, there were no differences in levels of renin mRNA between groups of mPGES1+/+ or −/− (12.5 ± 4.7 vs. 18.2 ± 3.0%, P = 0.33; Fig. 7A) or mPGES2+/+ or −/− animals (31.4 ± 12.2 vs. 28.6 ± 4.5%, P = 0.84; Fig. 6B). Following stimulation with furosemide, renin mRNA expression was increased in all of the groups. While there was no difference in renin mRNA levels between mPGES1−/− mice and their genetically matched controls (113.3 ± 18.0 vs. 100.0 ± 9.9%, P = 0.50; Fig. 7A), the renin response in mPGES2−/− animals was exaggerated compared with mPGES2+/+ controls (157.5 ± 8.1 vs. 100 ± 9.1%, P = 0.0015; Fig. 7B).
Fig. 7.
Furosemide stimulation of renin in mPGES1−/− (A) and mPGES2−/− (B) mice. Relative expression of renin mRNA was measured by real-time RT-PCR in kidneys of WT (filled bars) or mPGES−/− (open bars) mice treated with either vehicle or furosemide and is presented as percent of the furosemide-stimulated WT value. *P < 0.05 vs. WT by unpaired t-test.
DISCUSSION
The renin-angiotensin system (RAS) is a critical mediator of body fluid homeostasis and blood pressure. In the circulating RAS, the level of renin is a rate-limiting step determining the downstream activity of the system. As such, the mechanisms regulating renin expression at the JG apparatus provide major physiological control of the RAS. The macula densa is one of the key pathways controlling renin, linking renin release to sodium chloride concentrations in the distal nephron. A role for prostaglandins in stimulation of renin at the macula densa has been long recognized (46), but the precise molecular mechanisms underlying prostaglandin-dependent regulation of renin have not been clearly identified. In this regard, the identity of the specific prostanoid receptors and synthetic pathways transmitting signals triggering renin expression and release at the macula densa remains a matter of debate. Accordingly, we used a panel of genetically modified mice with precisely matched genetic controls to address this question. Our results clearly demonstrate that the EP4 receptor for PGE2 plays the major role in regulating chronic expression of renin via the macula densa mechanism.
The initial step for generating prostanoids from arachidonic acid is mediated by cyclooxygenase enzymes, COX-1 and COX-2. In our studies, we find that COX-2 is absolutely required for renin stimulation by the macula densa, as the increase in expression of renin mRNA after furosemide is completely abrogated in COX-2-deficient mice (Fig. 1). This observation is consistent with previous studies demonstrating impaired renin responses to both acute and chronic stimuli with COX-2 inhibition or in mice lacking COX-2 (9, 26, 49). The requirement for COX-2 in the macula densa pathway indicates a role for one or more prostanoid moieties as signaling molecules.
More than 30 years ago, the capacities for pharmacological doses of PGE2 or PGI2 to stimulate renin release were demonstrated (13). Since cAMP has been determined to be a final intracellular mediator triggering renin release in JG cells, it follows that the prostanoid(s) in this pathway would signal via Gs proteins, which are linked to adenylate cyclase that generates cAMP upon activation. In this regard, the EP2 and EP4 receptors for PGE2 and the IP receptor for PGI2 all signal via Gs (3, 21), and all three receptors have been implicated in control of renin at the macula densa (9, 25, 28). For example, studies by Fujino and associates (12) suggested a dominant role for the IP receptor in renin regulation. They showed that, after renal artery stenosis induced by clipping, IP-deficient mice had an attenuated renin response and were resistant to the development of hypertension, indicating a key role for the IP receptor in the baroreceptor pathway for renin release. Moreover, they also found that stimulation of renin in response to furosemide and low-salt diet was also diminished in mice lacking IP receptors, whereas responses in mice lacking EP2 or EP4 receptors were normal. Nusing et al. (32) also reported a blunted renin response to furosemide in IP-deficient mice. By contrast, we found a very modest, nonsignificant reduction in furosemide-induced renin expression in mice lacking IP receptors. Moreover, when mice were maintained on a high-salt diet to prevent volume depletion, renin levels after furosemide were stimulated to levels that were virtually identical in IP-deficient and control mice. The reasons for the apparent discrepancy between our findings and previous studies are not completely clear. Differences in genetic background of the experimental mice used in the various studies are likely a contributing factor.
Alternatively, other studies implicated PGE2 and its EP2 and EP4 receptors in macula densa signaling. For instance, using a clever biosensor, Peti-Peterdi and associates (35) showed that PGE2 is released from the basolateral surface of macula densa cells in response to reductions in luminal salt content. Using isolated, perfused kidneys from genetically modified mice, Schweda and associates (41) showed that renin release stimulated by exogenous PGE2 depended on EP2 and EP4 receptors. In a survey of mouse lines lacking individual prostanoid receptors, Nusing and associates (32) found that renin stimulation after furosemide was attenuated in both EP4- and IP-deficient mice, but the effect was more pronounced in the EP4-deficient mice. Similarly, our data are also consistent with a major role for the EP4 receptor in macula densa signaling. However, as discussed above, we do not find evidence for a significant role of IP receptor in this process. This is especially apparent in the studies of furosemide administration during high-salt feeding where there was no appreciable effect of deleting the IP receptor, yet there was a marked attenuation of the renin response in the EP4-deficient line.
Furosemide triggers the macula densa signal for renin release by inhibiting the NKCC2 transporter. However, it is theoretically possible that changes in body fluid volumes associated with furosemide administration might affect renal perfusion pressure, which could impact renin release through the baroreceptor mechanism. As discussed above, we used high-salt feeding as one approach to avoid such confounding effects in our experiments. We also directly measured blood pressure in EP4−/− mice and their WT controls by radiotelemetry before and during the period of furosemide administration. Using this approach, we did not detect a significant change in blood pressure in either group of animals during the 5-day course of furosemide treatment (Fig. 6). These findings suggest that the changes in renin mRNA expression and PRC we observed are primarily due to activation of the macula densa pathway.
Furosemide may also have direct effects on JG cells independent of the macula densa mechanism. For example, Castrop and associates (7) demonstrated that a second isoform of the Na-K-2Cl cotransporter, NKCC1, is expressed by JG cells where it functions to suppress basal renin release in mice. They found that furosemide stimulated renin release from primary cultures of JG cells from WT mice but not from NKCC1−/− mice. Although it is possible that the modest residual renin response we observed in EP4−/− mice might be due to a direct inhibitory effect of furosemide on NKCC1 in JG cells, this response would have to include a linkage to COX-2 since it is completely extinguished in COX-2-deficient mice. Another possible explanation for this finding is upregulation of renin secretion by non-PGE2-mediated mechanisms in response to furosemide treatment. Furosemide acts on macula densa cells to increase generation of nitric oxide (2), which may activate soluble guanylyl cyclase in JG cells, leading to increased levels of cGMP. Phosphodiesterase-3 degrades cAMP, the second messenger that mediates JG cell renin release, and its activity is inhibited by cGMP. Thus, in response to furosemide, elevated levels of cGMP may indirectly stimulate renin release, in the absence of EP4 receptors, by preventing the breakdown of cAMP (1, 40).
Recent evidence indicates that JG cells may not be the only sources of renin in the kidney. These studies suggest that renin may be synthesized and secreted in the distal nephron, in the connecting tubule and collecting duct (CD) in particular (14, 36). While the mechanisms regulating expression of renin in the CD have not been clearly delineated, they appear to differ markedly from those controlling renin release by JG cells. In particular, responses to salt loading are generally opposite to those in JG cells, whereas effects of furosemide to increase renin synthesis by the CD have not been reported (37). While preliminary studies suggest that prostaglandins may contribute to regulation of renin at non-JG sites (38), the role of prostaglandin synthetic enzymes and receptors in CD renin secretion is not known. Since our measurements of renin expression were carried out in samples of whole kidney, it is possible that non-JG sources may have contributed to the overall quantity of renin expression that we detected. Nonetheless, the relative quantity of renin produced in the CD appears to be substantially less than the JG contribution and any confounding effects of these measurements would likely be minimal.
The enzymatic pathway for synthesis of PGE2 from arachidonic acid requires cyclooxygenase and a PGE synthase. Both COX-2 and the major known PGE synthase, microsomal PGE synthase 1 (mPGES1), have been localized to macula densa cells (18, 35, 43). As discussed above, genetic deletion of COX-2 completely eliminates macula densa signaling. To identify the PGE synthase participating in this pathway, we tested renin responses in mice lacking either of the two putative mPGE synthases, mPGES1 or mPGES2. We found no diminution of renin response to furosemide in either the mPGES1- or mPGES2-deficient lines. Furthermore, stimulation of renin mRNA expression by furosemide was actually enhanced in the mPGES2-deficient mice (Fig. 6B). This finding is consistent with our recent report showing that mPGES2 does not function as a PGE synthase in vivo and that mPGES2−/− mice have significant elevations of circulating PGE2 levels due to reduced expression of 15-prostaglandin dehydrogenase, the primary enzyme responsible for PGE2 metabolism (22). Thus, although we clearly showed that the EP4 receptor for PGE2 primarily mediates macula densa triggering of renin, these data indicate that neither of the previously identified mPGES enzymes are required for this response. While there is another putative PGE synthase, cytosolic PGE synthase, studies by our group and others indicate that this enzyme is also unlikely to catalyze PGE2 synthesis in vivo (29). These findings suggest that alternative synthetic pathways for PGE2 must exist in macula densa cells. Generation of PGE2 at the macula densa may be mediated by an as yet unidentified PGE synthase enzyme, a cytosolic glutathione S transferase enzyme (30), or may occur through nonenzymatic generation of PGE2 from endoperoxide precursors (24, 39).
In conclusion, PGE2 generated through COX-2 and activating its EP4 receptor comprise the paracrine signaling pathway at the macula densa regulating expression and release of renin by the JG cell. A role for the EP4 receptor to trigger renin release and promote increased blood pressure seems paradoxical in view of its effects as a putative dilator receptor in the systemic vasculature (11, 19). Nonetheless, such complex actions of the prostanoid system have been observed in other tissues, highlighting the importance of local actions of these mediators in shaping global physiological responses. Finally, our studies suggest that PGE2 can be generated in sufficient quantities to activate this signal in the absence of mPGES1, the major PGE synthase in the kidney, indicating the presence of other pathways for generation of PGE2 in macula densa cells.
GRANTS
This work was supported by National Institutes of Health Grant DK069896 and by funding from the Medical Research Service of the Department of Veterans' Affairs and the Edna and Fred L. Mandel Center for Hypertension and Atherosclerosis Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Beierwaltes WH. cGMP stimulates renin secretion in vivo by inhibiting phosphodiesterase-3. Am J Physiol Renal Physiol 290: F1376–F1381, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Beierwaltes WH. Selective neuronal nitric oxide synthase inhibition blocks furosemide-stimulated renin secretion in vivo. Am J Physiol Renal Fluid Electrolyte Physiol 269: F134–F139, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Beierwaltes WH, Schryver S, Sanders E, Strand J, Romero JC. Renin release selectively stimulated by prostaglandin I2 in isolated rat glomeruli. Am J Physiol Renal Fluid Electrolyte Physiol 243: F276–F283, 1982 [DOI] [PubMed] [Google Scholar]
- 4. Bie P, Damkjaer M. Renin secretion and total body sodium: pathways of integrative control. Clin Exp Pharmacol Physiol 37: e34–e42, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25: 169–193, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics 5: 89–97, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Castrop H, Lorenz JN, Hansen PB, Friis U, Mizel D, Oppermann M, Jensen BL, Briggs J, Skott O, Schnermann J. Contribution of the basolateral isoform of the Na-K-2Cl− cotransporter (NKCC1/BSC2) to renin secretion. Am J Physiol Renal Physiol 289: F1185–F1192, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng HF, Wang JL, Zhang MZ, Miyazaki Y, Ichikawa I, McKanna JA, Harris RC. Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J Clin Invest 103: 953–961, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng HF, Wang JL, Zhang MZ, Wang SW, McKanna JA, Harris RC. Genetic deletion of COX-2 prevents increased renin expression in response to ACE inhibition. Am J Physiol Renal Physiol 280: F449–F456, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 54: 652–658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foudi N, Kotelevets L, Louedec L, Leseche G, Henin D, Chastre E, Norel X. Vasorelaxation induced by prostaglandin E2 in human pulmonary vein: role of the EP4 receptor subtype. Br J Pharmacol 154: 1631–1639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujino T, Nakagawa N, Yuhki K, Hara A, Yamada T, Takayama K, Kuriyama S, Hosoki Y, Takahata O, Taniguchi T, Fukuzawa J, Hasebe N, Kikuchi K, Narumiya S, Ushikubi F. Decreased susceptibility to renovascular hypertension in mice lacking the prostaglandin I2 receptor IP. J Clin Invest 114: 805–812, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerber JG, Branch RA, Nies AS, Gerkens JF, Shand DG, Hollifield J, Oates JA. Prostaglandins and renin release. II. Assessment of renin secretion following infusion of PGI2,E2 and D2 into the renal artery of anesthetized dogs. Prostaglandins 15: 81–88, 1978 [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension 57: 594–599, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greenberg SG, Lorenz JN, He XR, Schnermann JB, Briggs JP. Effect of prostaglandin synthesis inhibition on macula densa-stimulated renin secretion. Am J Physiol Renal Fluid Electrolyte Physiol 265: F578–F583, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Hansen PB, Yang T, Huang Y, Mizel D, Briggs J, Schnermann J. Plasma renin in mice with one or two renin genes. Acta Physiol Scand 181: 431–437, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Harding P, Sigmon DH, Alfie ME, Huang PL, Fishman MC, Beierwaltes WH, Carretero OA. Cyclooxygenase-2 mediates increased renal renin content induced by low-sodium diet. Hypertension 29: 297–302, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94: 2504–2510, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hristovska AM, Rasmussen LE, Hansen PB, Nielsen SS, Nusing RM, Narumiya S, Vanhoutte P, Skott O, Jensen BL. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension 50: 525–530, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Imanishi M, Kawamura M, Akabane S, Matsushima Y, Kuramochi M, Ito K, Ohta M, Kimura K, Takamiya M, Omae T. Aspirin lowers blood pressure in patients with renovascular hypertension. Hypertension 14: 461–468, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Ito S, Carretero OA, Abe K, Beierwaltes WH, Yoshinaga K. Effect of prostanoids on renin release from rabbit afferent arterioles with and without macula densa. Kidney Int 35: 1138–1144, 1989 [DOI] [PubMed] [Google Scholar]
- 22. Jania LA, Chandrasekharan S, Backlund MG, Foley NA, Snouwaert J, Wang IM, Clark P, Audoly LP, Koller BH. Microsomal prostaglandin E synthase-2 is not essential for in vivo prostaglandin E2 biosynthesis. Prostaglandins Other Lipid Mediat 88: 73–81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jensen BL, Schmid C, Kurtz A. Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am J Physiol Renal Fluid Electrolyte Physiol 271: F659–F669, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Keeting PE, Dong DS, Fu SC, Lysz TW. Rat lens prostaglandin generation proceeds by the nonenzymatic degradation of PGH2 endoperoxide. Exp Eye Res 44: 261–268, 1987 [DOI] [PubMed] [Google Scholar]
- 25. Kim HS, Lee G, John SW, Maeda N, Smithies O. Molecular phenotyping for analyzing subtle genetic effects in mice: application to an angiotensinogen gene titration. Proc Natl Acad Sci USA 99: 4602–4607, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim SM, Chen L, Mizel D, Huang YG, Briggs JP, Schnermann J. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol 292: F415–F422, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Lapointe JY, Laamarti A, Bell PD. Ionic transport in macula densa cells. Kidney Int Suppl 67: S58–S64, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Lovgren AK, Jania LA, Hartney JM, Parsons KK, Audoly LP, Fitzgerald GA, Tilley SL, Koller BH. COX-2-derived prostacyclin protects against bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 291: L144–L156, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Lovgren AK, Kovarova M, Koller BH. cPGES/p23 is required for glucocorticoid receptor function and embryonic growth but not prostaglandin E2 synthesis. Mol Cell Biol 27: 4416–4430, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murakami M, Nakatani Y, Tanioka T, Kudo I. Prostaglandin E synthase. Prostaglandins Other Lipid Mediat 68–69: 383–399, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Nguyen M, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, Anderson PA, Malouf NN, Koller BH. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature 390: 78–81, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Nusing RM, Treude A, Weissenberger C, Jensen B, Bek M, Wagner C, Narumiya S, Seyberth HW. Dominant role of prostaglandin E2 EP4 receptor in furosemide-induced salt-losing tubulopathy: a model for hyperprostaglandin E syndrome/antenatal Bartter syndrome. J Am Soc Nephrol 16: 2354–2362, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Adenylyl cyclase isoform v mediates renin release from juxtaglomerular cells. Hypertension 49: 618–624, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Decreased intracellular calcium stimulates renin release via calcium-inhibitable adenylyl cyclase. Hypertension 49: 162–169, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest 112: 76–82, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens 3: 96–104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol 289: F632–F637, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robben JH, Fenton RA, Vargas SL, Schweer H, Peti-Peterdi J, Deen PM, Milligan G. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int 76: 1258–1267, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Salmon JA, Smith DR, Flower RJ, Moncada S, Vane JR. Further studies on the enzymatic conversion of prostaglandin endoperoxide into prostacyclin by porcine aorta microsomes. Biochim Biophys Acta 523: 250–262, 1978 [DOI] [PubMed] [Google Scholar]
- 40. Sayago CM, Beierwaltes WH. Nitric oxide synthase and cGMP-mediated stimulation of renin secretion. Am J Physiol Regul Integr Comp Physiol 281: R1146–R1151, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Schweda F, Klar J, Narumiya S, Nusing RM, Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol 287: F427–F433, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Sigmund CD, Gross KW. Structure, expression, and regulation of the murine renin genes. Hypertension 18: 446–457, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Tomida T, Numaguchi Y, Matsui H, Toki Y, Ito T, Okumura K, Hayakawa T. Altered expression of prostacyclin synthase in a subset of the thick ascending limb cells and mesangial cells in 5/6-nephrectomized rats. Hypertens Res 24: 411–419, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Traynor TR, Smart A, Briggs JP, Schnermann J. Inhibition of macula densa-stimulated renin secretion by pharmacological blockade of cyclooxygenase-2. Am J Physiol Renal Physiol 277: F706–F710, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, Carter D, Thomas NA, Durtschi BA, McNeish JD, Hambor JE, Jakobsson PJ, Carty TJ, Perez JR, Audoly LP. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci USA 100: 9044–9049, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vander AJ. Direct effects of prostaglandin on renal function and renin release in anesthetized dog. Am J Physiol 214: 218–221, 1968 [DOI] [PubMed] [Google Scholar]
- 47. Wang JL, Cheng HF, Harris RC. Cyclooxygenase-2 inhibition decreases renin content and lowers blood pressure in a model of renovascular hypertension. Hypertension 34: 96–101, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Webber PC, Larsson C, Anggard E, Hamberg M, Corey EJ, Nicolaou KC, Samuelsson B. Stimulation of renin release from rabbit renal cortex by arachidonic acid and prostaglandin endoperoxides. Circ Res 39: 868–874, 1976 [DOI] [PubMed] [Google Scholar]
- 49. Yang T, Endo Y, Huang YG, Smart A, Briggs JP, Schnermann J. Renin expression in COX-2-knockout mice on normal or low-salt diets. Am J Physiol Renal Physiol 279: F819–F825, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol 274: F481–F489, 1998 [DOI] [PubMed] [Google Scholar]