Abstract
The aim of this study was to establish an immortalized human mesangial cell line similar to mesangial cells in vivo for use as a tool for understanding glomerular cell function. Mesangial cells were isolated from glomerular outgrowths from a normal human kidney, then retrovirally transfected with a temperature-sensitive SV40T antigen+human telomerase (hTERT). Mesangial cells exhibited features of compact cells with small bodies in a confluent monolayer at 33°C, but the cell shape changed to flat and stellate after 5 days in growth-restrictive conditions (37°C). Western blot and immunofluorescence analysis showed that podocyte markers (nephrin, CD2AP, podocin, Wilms' tumor-1) and an endothelial-specific molecule (VE-cadherin) were not detectable in this cell line, whereas markers characteristic of mesangial cells (α-SMA, fibronectin, and PDGFβ-R) were strongly expressed. In migration assays, a significant reduction in wound surface was observed in podocyte and endothelial cells as soon as 12 h (75 and 62%, respectively) and complete wound closure after 24 h. In contrast, no significant change was observed in mesangial cells after 12 h, and even after 48 h the wounds were not completely closed. Until now, conditionally immortalized podocyte and endothelial cell lines derived from mice and humans have been described, and this has greatly boosted research on glomerular physiology and pathology. We have established the first conditionally immortalized human glomerular mesangial cell line, which will be an important adjunct in studies of representative glomerular cells, as well as in coculture studies. Unexpectedly, mesangial cells' ability to migrate seems to be slower than for other glomerular cells, suggesting this line will demonstrate functional properties distinct from previously available mesangial cell cultures. This conditionally immortalized human mesangial cell line represents a new tool for the study of human mesangial cell biology in vitro.
Keywords: mesangial hypercellularity, mesangial proliferation, mesangial sclerosis, temperature-sensitive SV40T
the renal glomerulus is a complex microcirculation containing several different cell types, which is organized in a network of capillary loops supported by interstitial mesangium. Mesangial cells surrounded by their extracellular matrix form the central part of the glomerulus and are part of the functional component working in synchrony with endothelial cells and podocytes (8). Mesangial cells are located adjacent to endothelial cells on the opposite side of the glomerular basement membrane from podocytes. They comprise about a third of the whole glomerular cell population (22). These cells spread universally in the glomerulus in an arboreal pattern similar to a mesentery supporting capillaries and, thus, the name mesangium for this compartment. The support to the whole glomerular structure is provided by the mesangium, keeping the capillaries open and regulating filtration rate. Mesangial cells share a resemblance to pericytes, a contractile cell that appears surrounding capillaries, and smooth muscle cells, and therefore, may assist the glomerular vasculature in reacting to various physical stimuli (27, 32).
Glomerular mesangial cells both secrete and possess receptors for several growth factors and hormones, allowing for the various physiological functions in normal glomeruli. One of these receptors is the platelet-derived growth factor-β receptor (PDGFβ-R), a cell surface tyrosine kinase receptor member of the PDGF family. These growth factors are mitogens for cells of mesenchymal origin. In mesangial cells, genetic absences of PDGF or PDGFβ-R lead to the absence of glomerular mesangium (13, 29). Human mesangial cells have been demonstrated to express PDGFβ-R in the developing kidney (2, 3). Mesangial cell proliferation and accumulation of mesangial matrix are histological characteristics of various renal diseases that progress to end-stage renal failure.
In this paper, we describe the phenotypic characteristics of a human conditionally immortalized mesangial cell line in culture. We show that this cell line has characteristics of functional mesangial cells, including expression of fibronectin, α-smooth muscle actin (α-SMA), and PDGFβ-R, and an absence of podocyte and endothelial markers. To investigate the capability of the immortalized human mesangial cells to migrate in vitro, two different experiments were used: a cell monolayer wounding assay and electric cell substrate impedance-sensing (ECIS) technology.
MATERIALS AND METHODS
Induction of differentiation in mesangial cells.
Mesangial cells were isolated from a normal human kidney specimen obtained from a transplant donor kidney unsuitable for transplantation. This study has full Institutional Review Board and ethical approval for use of discarded nephrectomy specimens. Glomeruli were sieved and plated as previously described (18), and mesangial cell outgrowths were retrovirally transfected utilizing a bicistronic construct containing a temperature-sensitive simian virus 40 (tsSV40) large T antigen and human telomerase (hTERT) (20). Supernatants containing retrovirus from the packaging cell line (tsTTERT C126) were used to infect cultures of primary human mesangial cells; a freshly defrosted sieved supernatant (0.45 μm) mixed 7:3 with growth medium in addition to 8 μg/ml polybrene was used to treat cells in log-phase growth. After 24 h, fresh growth media were added and cultures were left to grow for another 3 days at 33°C before being moved to 37°C for another 4 days. Then, 0.5 mg/ml G418 (Life Technologies BRL) was added to the culture media until selection was completed (14 days). Infection was carried out at 33°C whereas selection and continuous culture were carried out at 37°C. This allows cells to proliferate at 33°C, and then thermoswitching to 37°C switches off T antigen expression, thus causing minimal interference with native cell phenotype (as successfully done previously with podocyte and endothelial cells) (24, 26). Mesangial cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (BioWhittaker), 10 U/ml streptomycin, 10 U/ml penicillin, and ITS (Sigma, Dorset, UK). Cells were grown to ∼70% confluence before they were thermoswitched to 37°C. Cells were fed with fresh media twice a week.
Western blotting.
For whole cell lysates, cells were washed twice with ice-cold PBS and then scraped into ice-cold Nonidet P-40 (NP-40) extraction buffer (50 mM Tris·HCl, pH 7.5, containing 1 mM EDTA, 120 mM NaCl, 50 mM NaF, 1 mM benzamidine, 1% NP-40, 1 μM microcystin, 7.2 mM 2-mercaptoethanol, and 5 mM orthovanadate with protease phosphatase inhibitor cocktails). Cell extracts were centrifuged at 10,000 g for 5 min at 4°C, and the supernatants were stored at −80°C. SDS-PAGE using 7.5–10% acrylamide gels was performed, and protein was transferred onto polyvinylidene difluoride membranes (Millipore). Membranes were blocked with 10% BSA and then incubated with primary antibodies. Appropriate species-specific secondary horseradish peroxidase (HRP) antibodies (Amersham) were used. Luminescence was created using Femto Supersignal luminal (Pierce) before imaging in a ChemiDoc-it imaging system (UV products).
For the nephrin immunoprecipitation assay, cells were extracted in ice-cold NP-40 extraction buffer (50 mM Tris·HCl, pH 7.5, containing 1 mM EDTA, 120 mM NaCl, 50 mM NaF, 1 mM benzamidine, 1% NP-40, 1 mM microcystin, 7.2 mM 2-mercaptoethanol, 5 mM orthovanadate, and 1 mg/ml each of pepstatin, leupeptin, and antipain). Cell extracts were centrifuged at 10,000 g for 10 min at 4°C before nephrin was immunoprecipitated with mouse anti-nephrin antibody and protein G-Sepharose at 4°C. Subsequently, the beads were isolated by centrifugation and washed four times with NP-40 extraction buffer. The bound proteins were separated by SDS-PAGE followed by electrophoretic transfer to Immobilon-P membranes (Millipore, Watford, UK). The membranes were blocked in 10% (wt/vol) BSA dissolved in TBS-T (Tris-buffered saline with 0.1% Tween 20; 20 mM Tris·HCl, 137 mM NaCl, and 0.1% Tween 20) and subsequently incubated with anti-nephrin antibody followed by HRP-conjugated donkey anti-rabbit IgG. The signal was detected by enhanced chemiluminescence (Amersham Biosciences).
Bromodeoxyuridine assay.
Cell proliferation was measured using a bromodeoxyuridine (BrdU) Cell Proliferation Kit (Millipore). BrdU, a synthetic thymidine analog, can be incorporated into newly synthesized DNA, providing a test of DNA replication as an indirect measure of cell division. The assay was performed as per the manufacturer's instructions. BrdU incorporation was detected by addition of a peroxidase substrate. Spectrophotometric detection was performed at a wavelength of 450 nm.
Immunohistochemistry.
Cells cultured on glass coverslips were fixed for 10 min in 2% formaldehyde and permeabilized with 0.3% Triton X-100 in PBS for 10 min. After blocking in block solution (2% FCS+2% BSA+0.1% Tween 20 in PBS), cells were incubated with 1% primary antibody in block solution for at least 1 h. Primary antibodies were podocin (Sigma), mouse monoclonal CD2AP (Santa Cruz Biotechnology), fibronectin (Sigma), α-SMA (Sigma), SV40 large T antigen (GenTax), rabbit anti-nephrin 3 and -4 (a gift from Dr. Ashley Toye, Bristol University), nephrin monoclonals (7C1, 43C7, and 48E11; a kind gift from Dr. K Trygvasson, Stockholm, Sweden), VE-cadherin (Santa Cruz), and PDGFβ-R (Millipore). Subsequently, coverslips were washed with 4% FCS in PBS and incubated with secondary antibody at 1:100 dilutions in block solution for 30 min at room temperature. Secondary antibodies were FITC-conjugated horse anti-mouse or Texas red-conjugated goat anti-rabbit antibodies (Invitrogen). Coverslips were mounted with aqueous medium containing 4,6 diamidinophenylindole (Vectashield without DAPI; Vector laboratories) on standard glass slides. Antibody staining was visualized using a Leica DFC350 FX monochrome digital camera.
Cell migration assay.
Cells were seeded at confluence in six-well culture plates, allowed to adhere overnight, and then moved to 37°C and fed with standard media as mentioned above. For mesangial cells and podocytes after 3 days (and additionally at 12 days for podocytes) at 37°C, the media was changed to RPMI-1460 with 0.2% FCS and EBM with 0.2% FCS for endothelial cells. Two days later, wounds were made using sterile white pipette tips. Images from two marked in a field per well were taken using a light microscope connected to a Nikon digital camera, just before and immediately after wounding (time 0) and then every 12 h. Wound closure was quantified by counting the cells that had migrated into the wound.
Electrical cell-substrate impedance-sensing wound-healing assays.
Wound-healing assays were performed using an automated cell-monitoring system, Electrical Cell-Substrate Impedance Sensing (ECIS 1600R, Applied Biophysics, Rochester, NY), which can detect nanometer-order changes in cell-to-cell and cell-to-substrate distances separately, as we have previously described (25). Time course analysis and resistance modeling was performed using electrical impedance and was measured with the attachment mode of ECIS. The change in impedance is attributed to changes in the resistance in the paracellular pathway and the resistance between the ventral cell surface and the electrode. In nonconfluent cell layers, resistance measurements are related to the fractional area of the electrodes occupied by cell cytoplasm and can therefore be used as an indirect measurement of changes in cell morphology or wound closure.
Confluent primary and cloned mesangial cells and podocyte monolayers were cultured on ECIS plates (8W10E+; Applied Biophysics). Cells were allowed to grow in 10% FCS until fully differentiated, then washed with serum-free medium, and serum starved for 48 h. For wound-healing assays, cells were subjected to a current shot of 40-kHz frequency, and an elevated voltage pulse of 3.5-V amplitude for a 30-s duration, which caused fatality and detachment of cells located on the small active electrode, resulting in a wound that can be healed by cells which surround the small active electrode that have not been subjected to the high-voltage conditions. Wound healing was then followed by continuous resistance measurements over the electrode every 5 min for 24 h.
Statistical analysis.
A two-sample Student's t-test was used.
RESULTS
Induction of differentiation in mesangial cells.
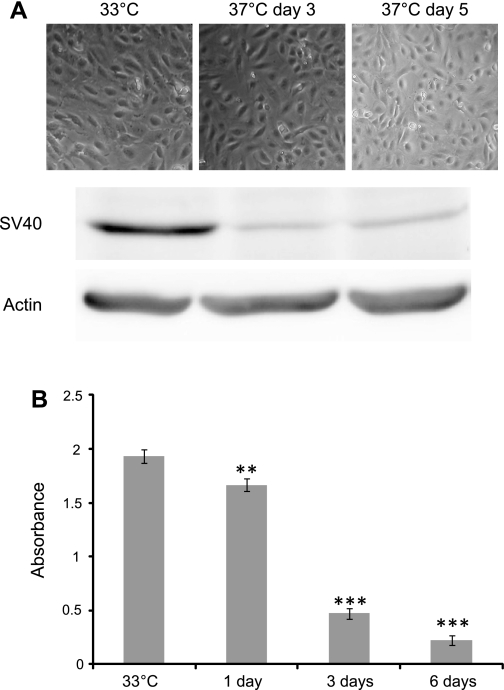
A conditionally immortalized human mesangial cell line has been developed by transfection with the temperature-sensitive SV40-T gene. These cells proliferate at the “permissive” temperature (33°C). After transfer to the “nonpermissive” temperature (37°C), they entered growth arrest and differentiated. Our data showed that at 33°C the mesangial cells had small bodies in a confluent monolayer whereas at 37°C they looked flat and stellate (Fig. 1A). Western blot analysis using an antibody to the SV40 large T antigen at both temperatures showed that SV40 was strongly expressed at 33°C, where the transgene is active and allows the cells to proliferate rapidly (Fig. 1A). Thermoswitching these cells to 37°C shows low but detectable expression of the antigen at days 3 and 5. It was not surprising to see this expression at 37°C, as the temperature-sensitive construct is designed to fully inactivate at 38.5°C (20). To measure changes in cell proliferation upon thermoswitching, a BRDU assay was carried out. This shows, as predicted, a steadily declining proliferation rate postthermoswitching to detectable but very low level proliferation at day 6 (Fig. 1B).
Fig. 1.
A: demonstration of expression of SV40 large T antigen in protein lysate extracted from putative mesangial cells (MCs) at permissive temperature (33°C) and days 3 and 5 at 37°C. Actin was used as a loading control. SV40 large T antigen protein was strongly expressed in mesangial cells at 33°C. In contrast, at 37°C either after 3 or 5 days the expression of this protein was very faint and barely detected. This indicates that the transgene was active at the permissive temperature and thermoswitching the cells to the temperature of 37°C almost completely inactivates the transgene. B: measurement of cell proliferation upon thermoswitching using a bromodeoxyuridine (BrdU) assay. This shows, as predicted, a steadily declining proliferation rate postthermoswitching to detectable but very low level proliferation at day 6. **,*** P < 0.05.
Podocyte- and endothelial-specific proteins are absent in cultured mesangial cells.
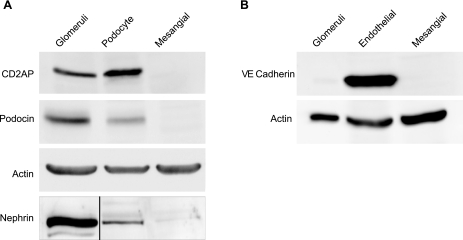
To confirm the mesangial phenotype of the cells, we examined them by Western blotting using antibodies to candidate antigens/proteins that are specific for podocytes and endothelial cells. As shown in Fig. 2A, Western blot analysis showed podocyte expression of the podocyte-specific proteins nephrin, podocin, and CD2AP, which was completely absent in the mesangial cell line. Whole glomeruli were used as a positive control. In addition, the use of VE-cadherin as an endothelial marker confirmed the lack of expression of this protein in the mesangial cell line compared with the endothelial cell controls. To further validate the lack of expression of podocyte-specific proteins in cultured mesangial cells, we performed immunostaining for podocyte markers CD2AP, podocin, and nephrin in putative mesangial cells and in podocytes. Figure 3 shows that nephrin, CD2AP, and podocin stained strongly positively at the cell surfaces and in cytoplasm of podocytes. In contrast, immunostaining of podocyte-specific proteins was negative in putative cultured mesangial cells. Normal rabbit/mouse IgG was used to rule out any nonspecific signals.
Fig. 2.
Western blot analysis of podocyte- and endothelial glomerular cell-specific proteins in cultured mesangial cells. A: expression of podocyte-specific proteins including podocin, CD2AP, and nephrin in podocytes and mesangial cells. Actin loading control is shown for podocin and CD2AP. Nephrin blotting was performed using nephrin immunopreciptation (black line indicates where irrelevant lanes have been removed). B: expression of VE-cadherin, an endothelial marker, in mesangial and endothelial cells. Actin was used as a loading control in A and B, and whole glomeruli as a positive control for all proteins except for CD2AP, where we used HEK cells as a positive control. Podocytes and not mesangial cells expressed CD2AP, podocin, and nephrin. VE-cadherin is expressed only in endothelial cells and not in mesangial cells. These results validate the staining results and provide further evidence of the establishment of a mesangial-specific cell line in culture.
Fig. 3.
Specific indirect immunofluorescence characterizes and distinguishes mesangial cells from podocytes. A–I: putative mesangial cells and previously characterized podocytes were grown separately and stained with the same antibodies. CD2AP, podocin, and nephrin staining was abundant in podocytes along the cell border and in the cytoplasm (A, D, and G); these were absent in mesangial cells (B, E, and H). Normal mouse/rabbit IgG were used to rule out nonspecific staining (C, F, and I). These results demonstrate that the mesangial cells grown in culture are distinct from podocytes. Magnification: ×40 in both cell lines.
Verifying expression of mesangial cell markers (α-SMA, fibronectin, and PDGFβ-R) in cultured mesangial cells.
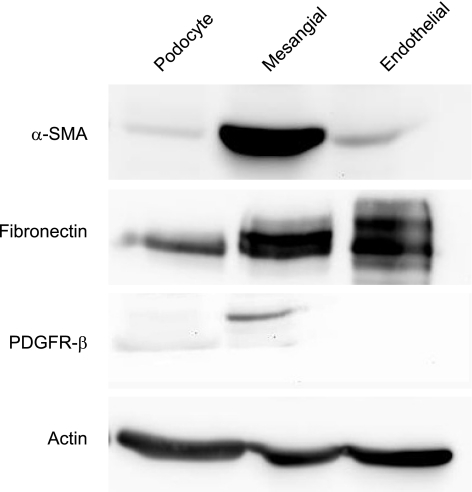
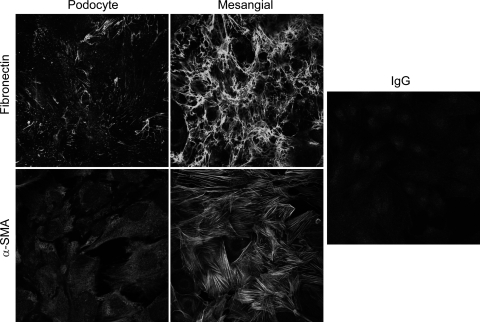
In culture, mesangial cells bear a resemblance to smooth muscle cells, expressing a variety of smooth muscle cell-associated proteins. The principle actin isoform associated with vascular smooth muscle is α-SMA; Fig. 4A showed α-SMA was abundantly expressed in the putative mesangial cells. α-SMA expression in podocytes was low but still detectable. Fibronectin is also expressed at high levels in mesangial cells compared with podocytes, where it is weakly expressed. Fibronectin was also highly expressed in the endothelial cells, as previously described (5, 17, 21). Interestingly, in glomerular cells (podocytes, endothelial, and mesangial cells), PDGFβ-R was expressed solely in mesangial cells (Fig. 4B). Immunostaining supported the above result. α-SMA and fibronectin were stained strongly positively in a linear pattern in the cytoplasm of the putative mesangial cells, which was absent in the podocyte cell line (Fig. 5).
Fig. 4.
Expression of mesenchymal markers α-smooth muscle actin (SMA) and fibronectin and platelet-derived growth factor receptor (PDGFβ-R). The figure shows α-SMA and fibronectin were abundantly expressed in mesangial cells, whereas α-SMA expression in podocytes was low and was entirely absent in glomerular endothelial cells. Fibronectin was expressed in the glomerular endothelial cell as well and was weakly expressed in podocytes. Actin was used as a loading control. PDGFβ-R was expressed exclusively in mesangial cells.
Fig. 5.
Indirect immunofluorescence shows expression of the mesenchymal markers fibronectin and α-SMA in mesangial cells and podocytes. Both fibronectin and α-SMA were highly expressed in the mesangial cells whereas in podocytes were barely detectable. Mouse IgG was used to rule out nonspecific staining. Magnifications: ×40 in both cell lines.
Migration assay.
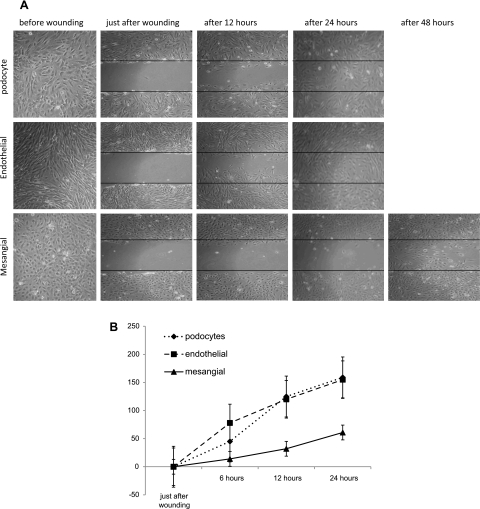
In a migration assay involving podocytes, mesangial, and endothelial cells, wounded cultures of confluent, growth-arrested cells (Fig. 6, A and B) showed a marked reduction in wound surface in podocytes (62%, SD = 37.16) (P = 0.045) and in endothelial cells (68%, SD = 28.51) (P = 0.034) as soon as 12 h and complete wound closure after 24 h. In contrast, there was no significant decrease in wound closure observed in mesangial cell culture after 12 h, reaching ∼40% at the end of the first day, and even after 48 h the mesangial cell wounds were not completely closed. This result indicates that the mesangial cells have less migration activity in contrast to their neighbors in glomeruli.
Fig. 6.
A: wound healing assay. Podocytes, glomerular endothelial, and mesangial cells were seeded at confluence into 6-well culture plates and allowed to adhere overnight. Then, the medium was altered to 0.2% FCS to reduce cell proliferation. Two days afterward, wounds were made. Images from 2 marked fields/well were taken under phase-contrast examination, just before wounding, immediately after wounding (0), and after 12, 24, and 48 h postwounding. Wounds in podocytes and endothelial cells wells were completely closed after 24 h, and unexpectedly in mesangial cells the wound was not completely closed even after 48 h. B: proportion of cells invading the scratch-made wound at different time points (after 6, 12, and 24 h); unexpectedly, mesangial cells migrate more slowly than podocytes and endothelial cells.
ECIS wound-healing assays.
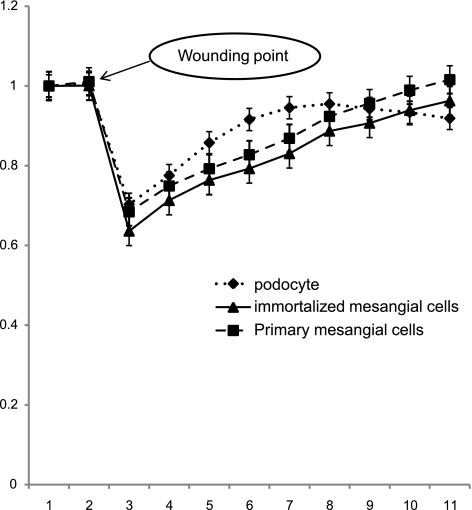
To confirm the cell migration data, we utilized ECIS to wounded cells, measured recovery in real time, and compared podocytes with mesangial cells both primary culture and immortalized. Primary and cloned mesangial cells, and podocytes cultured on ECIS 8W10E+ plates, were subjected to an elevated voltage pulse of 40-kHz frequency, 3.5-V amplitude for 30-s duration, and resistance was measured over 24 h. The application of the high-field pulse led to a drastic decrease in cell resistance in the three cell lines, indicating loss of cells over the electrode (Fig. 7). As cells migrated back onto the electrode, podocytes showed increasing resistance over time, reaching peak resistance values just below that of nonwounded cells after 6 h, whereas both primary and cloned mesangial cells took ∼12 h to reach the same level. Resistance of mesangial cells continued to increase thereafter, due to continued proliferation of those cells. Interestingly, this supports our result in the scratch assay (Fig. 6).
Fig. 7.
Measurement of cell monolayer resistance after wounding using cell impedance analyzer (electric cell substrate impedance sensing; ECIS). The curve shows the resistance measurements of primary and cloned mesangial cells and podocyte monolayers postwounding using ECIS technology. Cells were grown in ECIS 8W1E+ plates, submitted to an elevated voltage pulse of 40-kHz frequency and 3.5-V amplitude for a 30-sec duration. This led to detachment and loss of cells located on the small active electrode, leading to a wound normally healed by cells around the small active electrode that have not been subjected to the elevated voltage pulse. Wound healing was then followed by continuous resistance measurements for 12 h. The curves show a sharp drop in resistance following the high-voltage pulse. Resistance in podocytes increased more quickly than in mesangial cells (primary and cloned) to approach the prewounding level at ∼6 and 12 h, respectively, postwounding.
DISCUSSION
Conditionally immortalized podocyte and endothelial cell lines derived from both mice and humans have been described, and their use has greatly boosted research on glomerular physiology and pathology. We have established the first conditionally immortalized human glomerular mesangial cell line, which will be an important adjunct in studies of representative glomerular cells, as well as in coculture studies.
Mesangial cell culture is a useful model for carrying out many experiments. However, ordinary mesangial cell culture can only be acquired from primary culture of isolated glomeruli, which cannot be maintained viable for a long time. Thus it is not easy to examine the long-term reaction of mesangial cells to different stimuli in primary culture. To study the response of mesangial cells to different stimuli for longer periods of time, an established mesangial cell line is essential. In 1988, MacKay et al. (16) established clonal lines of mouse mesangial cells. However, they were derived from transgenic mice. Ten years later, Nitta et al. (19) established a culture line of transformed immortalized rat mesangial cells which can be grown for prolonged time, and they showed that these cells have the phenotypic characteristics of primary mesangial cells in culture. In 2000, Kida (11) had successfully immortalized rat glomerular mesangial cells by transfecting E6 and E7 genes via electroporation, and a human mesangial cell line was reported by Sraer et al. (20) transformed with SV40-T, without the ability to switch off SV40.
In this study, we report the phenotypic characteristics of a conditionally immortalized human mesangial cell line derived from normal human kidney. Western blot results showed that the transfected mesangial cells displayed expression of tsSV40 at 33°C, which allows cells to proliferate. In contrast, thermoswitching mesangial cells to 37°C switches off T antigen expression, thus causing minimal interference with the native cell phenotype, although the expression of tSV40 at 37°C is still detectable at a low level after 5 days. The tsSV40T construct is designed to optimally inactivate at a temperature of 38.5°C. However, we did not subject the cells to this temperature as there would be the risk of heat shock. Additionally, we know from our previous experience with this construct in mammalian cells, namely, podocytes and endothelial cells, that thermoswitching to 37°C is sufficient to completely stop proliferation within 24–48 h (24, 26). In growth-permissive conditions, the cells exhibit features of compact cells with small bodies in a confluent monolayer. In contrast, cells showed a different morphology after 5 days in growth-restrictive conditions: they were larger, flatter, and had a stellate shape. These results are consistent with a differentiated morphology of the mesangial cells when grown under this condition (19, 30).
However, the number of reported proteins specifically expressed in mesangial cells is limited. The Western blot results supported by immunohistochemistry analysis demonstrated that in human glomeruli, α-SMA expression was seen predominantly in mesangial cells, whereas in podocytes its expression was faint and barely detected, and in endothelial cells α-SMA was completely absent. Distinct from γ and β, which are present in various cell types, the existence of α-SMA has been interpreted as an indicator for smooth muscle-derived tissues (28). In agreement with our data, several studies have suggested that α-SMA is specifically expressed in proliferating mesangial cells in the injured glomerulus (1, 10); thus this molecule is regarded as an appropriate marker for mesangial cells. Most reports agree that podocytes do not produce fibronectin, which in the glomeruli appears to be almost exclusively produced by endothelial and mesangial cells (5, 30, 31). As expected, the data obtained suggested that fibronectin was abundantly expressed in mesangial and endothelial cells, while in podocytes was weakly expressed. Early in nephrogenesis, mesangial cells are recognized by their expression of a variety of markers, including α-SMA and PDGFβ-R (15, 23). PDGFβ-R is vital for mesangial cell formation (29); our results show that in human glomeruli PDGFβ-R was expressed exclusively in mesangial cells and not in podocytes or endothelial cells. This finding is similar to in vivo data of Hiroyuki et al. (6), where it was noted that PDGFβ-R expression is increased in mesangial regions.
As mesangial cells are located next to endothelial cells and opposite of podocytes across the glomerular basement membrane, in glomeruli we used podocytes and endothelial cells as a control to exclude contamination of the putative mesangial cells with these cells. In the putative mesangial cells, four podocyte antibodies were used to prove lack of podocyte markers podocin and nephrin, which were described in 2002 in a wild-type human podocyte cell line as podocyte-specific molecules (24), and CD2-associated protein, which as reported by Li et al. (14) is restricted to podocytes in glomeruli. As expected, our results showed that they are absent in cultured mesangial cells and expressed specifically in podocytes. The fourth podocyte-specific molecule is Wilms' tumor 1 protein (WT1), a transcriptional factor expressed early during kidney development, and upon further differentiation its expression is downregulated except in podocytes of mature glomeruli; this also stained positively in the nucleus of podocytes and not in mesangial cells. To distinguish mesangial cells from endothelial cells in culture, we used VE-cadherin as a marker for endothelial cells, as reported by Satchell et al. (26) in 2006. Our data demonstrated that VE-cadherin expression was completely absent in mesangial cells, whereas in endothelial cells it was strongly positive. These results prove that in glomeruli, CD2AP, nephrin, podocin, and WT1 were exclusively expressed in podocytes and VE-cadherin was expressed in endothelial cells but neither of these markers was expressed in mesangial cells; therefore, these serve as excellent markers to differentiate mesangial cells from their neighbors in glomeruli.
Mesangial injury is a feature of many human renal diseases (12). In both human disease and animal models, mesangial cells respond to injury with a well-characterized pattern of cell proliferation, matrix expansion, and phenotypic alteration. Fundamental to the restoration of glomerular architecture after injury is cell migration, in which mesangial progenitor cells having their origin in the extraglomerular mesangium migrate into the glomerular periphery (7). By a combined process of proliferation and migration, these cells have the potential to repopulate the entire injured mesangium (9). To explore the ability of the immortalized human mesangial cells to migrate in vitro, we used two different experiments, a cell layer-wounding assay and ECIS technology.
In a migration assay in wounded cultures of confluent, growth-arrested cells, in podocyte and endothelial cells, a significant reduction in wound surface was observed as soon as 12 h, and wounds were completely closed by 24 h. Surprisingly, in mesangial cells there was no significant change in the wound size at 12 h, and this reached 30% by 24 h. These findings were consistent with Dubus et al. (4), who showed that in mesangial cells the wounds were closed by ∼40% at day 3. Real-time measurement of mesangial monolayer resistance, such as that offered by ECIS, is a potent means for fast discovering transitory, and even slight, changes in mesangial cell layer integrity. The fall in resistance reflects a decline in current across the monolayer measured by ECIS. As demonstrated in Fig. 6, both primary and cloned mesangial cells migrate slower than podocytes to reach the prewounding level after ∼12 and 6 h, respectively. However, the cloned and particularly the primary mesangial cells seem to cross the prewounding level after ∼13 h postwounding, and this may be because to some extent these cells preserve the ability to proliferate even after 5 days at 37°C.
This new mesangial cell line has a limited proliferation capacity at 37°C compared with previous immortalized and primary cultures. Those models may therefore have overemphasized the migratory capacity of mesangial cells in vitro due to their excessive proliferation. This is important because it is known that mediators affecting proliferation can be dissociated from those mediating migration in renal injury (7), and therefore our cell line may be a more accurate tool for studying mesangial cell migration in injury models and distinguish this from proliferative repair.
There are several facts to support our argument that cells generated in culture are indeed immortalized mesangial cells. 1) Cell morphology and size are different from other glomerular cells in culture. 2) Immunofluorescence and Western blot examination showed that they express proteins (α-SMA, fibronectin, and PDGFβ-R) that are normally expressed strongly in mesangial cells in glomeruli. 3) Immunofluorescence and Western blotting showed that the putative mesangial cells do not express podocyte- or endothelial-specific molecules. 4) Distinct from podocytes and endothelial cells, mesangial cells tend to continue proliferation when grown at 37°C despite downregulation of SV40 expression. 5) Mesangial cells migrate more slowly than podocytes and endothelial cells, a novel finding suggesting this will be a model with distinct functional properties compared with previous models.
GRANTS
This study was partly supported by the Nephrotic Syndrome Trust (www.nstrust.co.uk). R. Lennon was supported by the Wellcome Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1. Alpers CE, Hudkins KL, Gown AM, Johnson RJ. Enhanced expression of “muscle-specific” actin in glomerulonephritis. Kidney Int 41: 1134–1142, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Alpers CE, Seifert RA, Hudkins KL, Johnson RJ, Bowen-Pope DF. Developmental patterns of PDGF B-chain, PDGF-receptor, and alpha-actin expression in human glomerulogenesis. Kidney Int 42: 390–399, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Alpers CE, Seifert RA, Hudkins KL, Johnson RJ, Bowen-Pope DF. PDGF-receptor localizes to mesangial, parietal epithelial, and interstitial cells in human and primate kidneys. Kidney Int 43: 286–294, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Dubus I, L'Azou B, Gordien M, Delmas Y, Labouyrie JP, Bonnet J, Combe C. Cytoskeletal reorganization by mycophenolic acid alters mesangial cell migration and contractility. Hypertension 42: 956–961, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Ekblom P. Formation of basement membranes in the embryonic kidney: an immunohistological study. J Cell Biol 91: 1–10, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fellstrom B, Klareskog L, Heldin CH, Larsson E, Ronnstrand L, Terracio L, Tufveson G, Wahlberg J, Rubin K. Platelet-derived growth factor receptors in the kidney–upregulated expression in inflammation. Kidney Int 36: 1099–1102, 1989 [DOI] [PubMed] [Google Scholar]
- 7. Haseley LA, Hugo C, Reidy MA, Johnson RJ. Dissociation of mesangial cell migration and proliferation in experimental glomerulonephritis. Kidney Int 56: 964–972, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Herrera GA. Plasticity of mesangial cells: a basis for understanding pathological alterations. Ultrastruct Pathol 30: 471–479, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Hugo C, Shankland SJ, Bowen-Pope DF, Couser WG, Johnson RJ. Extraglomerular origin of the mesangial cell after injury. A new role of the juxtaglomerular apparatus. J Clin Invest 100: 786–794, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest 87: 847–858, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kida T. Immortalization of rat kidney glomerular mesangial cell and its coculture with glomerular epithelial cell. Biotechnol Bioprocess Eng 5: 92–98, 2000 [Google Scholar]
- 12. Klahr S, Schreiner G, Ichikawa I. The progression of renal disease. N Engl J Med 318: 1657–1666, 1988 [DOI] [PubMed] [Google Scholar]
- 13. Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Li C, Ruotsalainen V, Tryggvason K, Shaw AS, Miner JH. CD2AP is expressed with nephrin in developing podocytes and is found widely in mature kidney and elsewhere. Am J Physiol Renal Physiol 279: F785–F792, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125: 3313–3322, 1998 [DOI] [PubMed] [Google Scholar]
- 16. MacKay K, Striker LJ, Elliot S, Pinkert CA, Brinster RL, Striker GE. Glomerular epithelial, mesangial, and endothelial cell lines from transgenic mice. Kidney Int 33: 677–684, 1988 [DOI] [PubMed] [Google Scholar]
- 17. Madri JA, Roll FJ, Furthmayr H, Foidart JM. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol 86: 682–687, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mundel P, Reiser J, Kriz W. Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol 8: 697–705, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Nitta K, Horiba N. An immortalized rat mesangial cell line. In Vitro Cell Dev Biol Anim 33: 156–157, 1997 [DOI] [PubMed] [Google Scholar]
- 20. O'Hare MJ, Bond J, Clarke C, Takeuchi Y, Atherton AJ, Berry C, Moody J, Silver AR, Davies DC, Alsop AE, Neville AM, Jat PS. Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc Natl Acad Sci USA 98: 646–651, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oberley TD, Mosher DF, Mills MD. Localization of fibronectin within the renal glomerulus and its production by cultured glomerular cells. Am J Pathol 96: 651–662, 1979 [PMC free article] [PubMed] [Google Scholar]
- 22. Olivetti G, Anversa P, Rigamonti W, Vitali-Mazza L, Loud AV. Morphometry of the renal corpuscle during normal postnatal growth and compensatory hypertrophy. A light microscope study. J Cell Biol 75: 573–585, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ricono JM, Xu YC, Arar M, Jin DC, Barnes JL, Abboud HE. Morphological insights into the origin of glomerular endothelial and mesangial cells and their precursors. J Histochem Cytochem 51: 141–150, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Saleem MA, Zavadil J, Bailly M, McGee K, Witherden IR, Pavenstadt H, Hsu H, Sanday J, Satchell SC, Lennon R, Ni L, Bottinger EP, Mundel P, Mathieson PW. The molecular and functional phenotype of glomerular podocytes reveals key features of contractile smooth muscle cells. Am J Physiol Renal Physiol 295: F959–F970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Satchell SC, Tasman CH, Singh A, Ni L, Geelen J, von Ruhland CJ, O'Hare MJ, Saleem MA, van den Heuvel LP, Mathieson PW. Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int 69: 1633–1640, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J 1: 272–281, 1987 [DOI] [PubMed] [Google Scholar]
- 28. Skalli O, Vandekerckhove J, Gabbiani G. Actin-isoform pattern as a marker of normal or pathological smooth-muscle and fibroblastic tissues. Differentiation 33: 232–238, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 8: 1888–1896, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Sraer JD, Delarue F, Hagege J, Feunteun J, Pinet F, Nguyen G, Rondeau E. Stable cell lines of T-SV40 immortalized human glomerular mesangial cells. Kidney Int 49: 267–270, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Wartiovaara J, Stenman S, Vaheri A. Changes in expression of fibroblast surface antigen (SFA) during cytodifferentiation and heterokaryon formation. Differentiation 5: 85–89, 1976 [DOI] [PubMed] [Google Scholar]
- 32. Yamanaka N. Development of the glomerular mesangium. Pediatr Nephrol 2: 85–91, 1988 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.