Abstract
Several transmembrane receptors are documented to accumulate in nuclei, some as holoreceptors and others as cleaved receptor products. Our prior studies indicate that a population of the 7-transmembrane angiotensin type-1 receptor (AT1R) is cleaved in a ligand-augmented manner after which the cytoplasmic, carboxy-terminal cleavage fragment (CF) traffics to the nucleus. In the present report, we determine the precise cleavage site within the AT1R by mass spectrometry and Edman sequencing. Cleavage occurs between Leu(305) and Gly(306) at the junction of the seventh transmembrane domain and the intracellular cytoplasmic carboxy-terminal domain. To evaluate the function of the CF distinct from the holoreceptor, we generated a construct encoding the CF as an in-frame yellow fluorescent protein fusion. The CF accumulates in nuclei and induces apoptosis in CHO-K1 cells, rat aortic smooth muscle cells (RASMCs), MCF-7 human breast adenocarcinoma cells, and H9c2 rat cardiomyoblasts. All cell types show nuclear fragmentation and disintegration, as well as evidence for phosphotidylserine displacement in the plasma membrane and activated caspases. RASMCs specifically showed a 5.2-fold increase (P < 0.001) in CF-induced active caspases compared with control and a 7.2-fold increase (P < 0.001) in cleaved caspase-3 (Asp174). Poly(ADP-ribose)polymerase was upregulated 4.8-fold (P < 0.001) in CF expressing cardiomyoblasts and colocalized with terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL). CF expression also induces DNA laddering, the gold-standard for apoptosis in all cell types studied. CF-induced apoptosis, therefore, appears to be a general phenomenon as it is observed in multiple cell types including smooth muscle cells and cardiomyoblasts.
Keywords: angiotensin II type I receptor, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling, DNA ladder, poly(ADP-ribose) polymerase, cardiomyoblast
receptor cleavage has become a commonly recognized event and often involves regulated intramembrane proteolysis (RIP) to generate an intracellular receptor fragment (13). In many cases the liberated intracellular fragment traffics to the nucleus where its function, in some instances, has been determined. In other instances, the intracellular or intranuclear function of the fragment remains obscure. For single-pass receptors, RIP is believed to involve sequential cleavage by the tumor necrosis factor-α converting enzyme metalloprotease to release the extracellular domain and the enzyme complex, γ-secretase, which cleaves within the transmembrane domain to generate an intracellular cleavage fragment.
In addition to these single-pass transmembrane receptors, several G protein-coupled receptors (GPCRs), including vasopressin 2, β2-adrenergic receptor, and endothelin B, are reported to undergo regulated limited proteolysis to produce peptides with possible bioactivity (9–11, 18, 19). An intracellular fragment is also produced from the GPCR Dfrizzled2, a postsynaptic protein that interacts with the presynaptic protein “wingless.” After endosome internalization, the intracellular domain is cleaved and translocated to the nucleus, where it is involved in transcriptional events that support synapse development (26). Furthermore, an intracellular cleavage fragment (CF) is produced from the COOH-terminus of polycystin (PC1), a noncanonical GPCR having 11 transmembrane domains (2, 24). After nuclear localization, the COOH-terminal fragment inhibits the ability of β-catenin to induce T-cell factor-dependent gene transcription and, thereby, inhibits the Wnt signaling pathway (22). The detailed processes and enzymes responsible for cleavage of most multipass receptors are not clear, though a family of intramembrane proteases referred to as IMPAS/PSH/signal peptide peptidase (SPP) are implicated (27).
Collectively, the results of these studies indicate that cleavage of receptors and other cell surface proteins, as well as accumulation of stable intracellular products, can be regulated processes that serve perhaps to further amplify or enhance effects of ligand-receptor signal transduction events initiated at the plasma membrane.
In our earlier studies using expression plasmids encoding fluorescent protein fusions of angiotensin II (ANG II) or the angiotensin type-1 receptor (AT1R), we showed that both extracellular and intracellular ANG II can induce nuclear accumulation of the AT1R, activation of cAMP response element-binding protein (CREB) and cell proliferation (3, 5). The initial studies did not differentiate between cleaved nuclear receptor versus holoreceptor. Our later studies, however, were specifically designed to determine whether the AT1R is cleaved before nuclear transport (4). A plasmid encoding a rat AT1R labeled at the amino-terminus with enhanced cyan fluorescent protein (ECFP) and at the carboxy-terminus with enhanced yellow fluorescent protein (EYFP) was employed. Image analyses of this protein in several cell types showed the two fluorescent moieties to be largely spatially colocalized in untreated cells. ANG II treatment, however, leads to a separation of the fluorescent moieties with yellow fluorescence accumulating in >30% of cellular nuclei. Immunoblot analyses of extracts and conditioned media from transfected cells showed that the CFP domain fused to the extracellular amino-terminal AT1R domain is cleaved from the membrane and that the YFP domain, together with the intracellular cytoplasmic carboxy-terminus of the AT1R, is also cleaved from the membrane-bound receptor and accumulates in the nucleus. The carboxy-terminus of the AT1R is essential for cleavage; cleavage does not occur in protein deleted with respect to this region. Overexpressed native AT1R (nonfusion) is also cleaved; the intracellular 6-kDa cytoplasmic domain (cleavage fragment, CF) accumulates to a significantly higher level with ANG II treatment.
It is challenging to differentiate between effects of intact AT1R and AT1R-cleaved fragments in cells in which the AT1R has been overexpressed, since only a fraction of the receptor is cleaved in response to ligand treatment. The present studies were designed to test the hypothesis that the AT1R carboxy-terminus, expressed independently of the holoreceptor localizes to nucleus and has biologically significant functions. For this purpose, we employed a fusion of the AT1R carboxy-terminal CF with YFP, which permitted us to both monitor CF trafficking by image analyses and to perform functional assays.
MATERIALS AND METHODS
Plasmids.
The construction of pAT1R/EYFP is reported elsewhere (5). For construction of pAT1RCF/EYFP, the CF-encoding sequence was PCR amplified from pAT1R/EYFP using upstream primer 5′-ACGTACAAGCTTATGGGGAAGAAATTTAAAAAG-3′ and downstream primer 5′-ACGTACGGATCCAGCTCCACCTCAAAACAAGACGC-3′. The ∼200-bp product was digested with HindIII/BamHI and ligated into HindIII/BamHI-digested pEYFP-N1 (the latter from Clontech). pNLS-EYFP was constructed by PCR amplification from pEYFP-N1 (Clontech) using an upstream primer encoding GPKKKRKV, the SV40 large T-antigen nuclear localization signal (NLS) (17).
Immunocytochemistry.
Rat aortic smooth muscle cells (RASMCs, Clonetics) were plated onto 35-mm MatTek dishes and allowed to grow overnight at 37°C. Transfection was performed using 2 μg of p3XFlag/rAT1R/myc per dish with Lipofectamine 2000 and incubated at 37°C for 16 h. The following day the cells were fixed in 4% paraformaldehyde (Sigma P-6148) for 15 min at room temperature and then washed with 1× PBS three times. Cells were permeabilized by treating with 1× PBS, 0.2% saponin (Sigma) and 5% normal goat serum (Jackson ImmunoResearch) for 15 min at room temperature. Primary antibodies [rabbit monoclonal antibody (mAb) 71D10 (Cell Signaling) against myc and mouse M2 mAb F3165 against Flag (Sigma)] were incubated for 1 h at room temperature. Three washes of 1× PBS were performed. Secondary antibodies [goat anti-rabbit Alexa 488 (Molecular Probes A11008) and goat anti-mouse Alexa 555 (Molecular Probes A21422)] were then incubated for 1 h at room temperature. Cells were washed three times with 1× PBS followed by H2O. Prolong Gold w/DAPI adhesive (Molecular Probes) was applied to samples, and coverslips were allowed to set overnight.
Transfection and imaging.
Cells were plated at ∼50% confluence [5 × 105 RASMCs, 5 × 105 H9c2 cells (ATCC, rat cardiomyoblasts), 2.5 × 105 MCF-7 cells (ATCC, human breast adenocarcinoma), 3 × 105 CHO-K1 cells] on 35-mm MatTek dishes. The next day, 1 μg of pAT1RCF/EYFP or pAT1R/EYFP were added with 2.5 μg of Lipofectamine 2000 in Opti-MEM medium to cells. Media were changed at 3–4 h and cells imaged as early as 3 h posttransfection.
Annexin V-PE apoptosis assay.
Phosphotidylserine, a major membrane phospholipid, translocates to the cell surface with high frequency in apoptotic cells and is detectable with fluorescent conjugates of Annexin V. Cells were transfected as indicated immediately above. The next day, media were replaced with 1 ml of 1× binding buffer. Annexin V-PE (10 μl, Biovision) was added to each plate and incubated 5 min in the dark before imaging.
Caspase assay.
Cells were transfected as indicated above. After sulforhodamine FLICA apoptosis detection (SR-VAD-FMK, Immunochemistry Technologies), which is specific for activated caspases (caspase-1, -3, -4, -5, -6, -7, -8, and -9), three-dimensional (3D) deconvolution microscopy was performed using the Cy3 filters. Apoptosis was further quantified using the POLARstar Optima (BMG Labtech) fluorescence plate reader (black microtiter plates) using 544/590 excitation/emission filters.
3D deconvolution microscopy.
Deconvolution microscopy was performed using a Zeiss Axiovert 200M microscope, xenon light source with automated Z-axis and appropriate filters. Constrained iterative and nearest neighbors algorithms were performed using Slidebook 5.0 software (Intelligent Imaging Innovations, Denver CO). All images were captured in 3D at 0.5-μm steps through the Z axis (∼10–15 Z-axis planes captured per image) and deconvolved to render confocal images.
TUNEL and PARP assay.
Cells were plated on MatTek dishes and transfected at 85% confluence with pAT1R/EYFP or pAT1RCF/EYFP. Cells were fixed (4% paraformaldehyde, 15 min) and permeabilized (0.25% Triton X-100, 20 min) according to the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) vision kit (BioProbes, Click-iT TUNEL Alexa Fluor 647). TdT and Click-iT reactions were performed according to kit instructions. Samples were subsequently treated with primary antibodies against poly(ADP-ribose)polymerase (PARP) (214/215) cleavage site (rabbit polyclonal against human PARP, 44698G, Invitrogen) and Alexa Fluor 594 goat anti-rabbit secondary antibodies (A31632, Invitrogen). The PathScan cleaved PARP (Asp214) Sandwich ELISA kit (no. 7262, Bioke.com), a horseradish peroxiedase-based colorimetric assay was used to confirm elevated levels of cleaved PARP in cleavage fragment-expressing cells.
DNA ladder assay.
Cells were transfected as indicated above. Where indicated, cells were treated with staurosporine (5 μmol/l in DMSO) for 6 h before harvest (positive control). Cells were collected, lysed in Triton-X, treated with RNase and proteinase K, and then DNA was extracted with phenol:chloroform and isopropanol precipitated. Camptothecin-treated U937 DNA (Roche, cat. no. 11 835 246 001) served as a second positive control. Samples were electrophoresed on a 1% agarose gel with ethidium bromide to demonstrate laddering.
Statistical analyses.
Statistical P values associated with fluorometric active caspases, cleaved PARP (ELISA), and apoptotic indexes (TUNEL) were calculated using ANOVA with the Bonferroni post hoc test (GraphPad, Instat).
RESULTS
Characterization of the CF.
Our earlier published studies have shown, using both native AT1R and a double-fusion protein ECFP/AT1R/EYFP, that a population of the AT1R is cleaved and that a carboxy-terminal fragment traffics to the nucleus (4). Using the latter construct, 3D deconvolution imaging shows accumulation of YFP (but not CFP) in the nucleus. In the present study, we verify the cleavage event using small epitope tags that are even less likely to interfere with AT1R structure or function. p3XFlag/rAT1R/myc encodes a fusion protein in which Flag is upstream and in-frame and myc is downstream and in-frame with the rat AT1R. Transfection of RASMCs with p3XFlag/rAT1R/myc results in accumulation of myc/Flag immunoreactive material in trafficking vesicles within the cytoplasm and at distinct patches on the plasma membrane (red Flag + green myc epitopes = yellow plasma membrane and vesicles, Fig. 1A), whereas ANG II treatment of these transfected cells before imaging leads to accumulation of myc but not Flag immunoreactivity in nuclei (Fig. 1B). This is consistent with cleavage and trafficking of the COOH-terminus to the nucleus as we found in our previous published study.
Fig. 1.
Rat aortic smooth muscle cells (RASMCs) were transfected with p3xFlag/rAT1R/myc for 18 h then serum-starved in 0.5% FBS containing vehicle (A) or ANG II (10−8 M) (B) for 3 h. Cells were then fixed, permeabilized, and stained for Flag (Alexa Fluor 555, red) and myc (Alexa Fluor 488, green). Note that red and green fluors are largely colocalized (as yellow fluorescence) in vehicle-treated cells (arrows), whereas ANG II treatment causes a separation of the two fluorescent moieties and increased accumulation of green fluorescence on the cell surface (arrowhead) and in the nucleus (n).
We have purified the COOH-terminal fragment (Fig. 2A) and mass spectrometry analysis indicates a molecular mass of 6,102 Da (Fig. 2, B and C). Edman degradation sequencing of the six amino-terminal amino acids reveals a sequence of GKKFKK (Fig. 2D) consistent with cleavage between Leu(305) and Gly(306) within the eighth helix (15) (Fig. 2E). The observation that the AT1R is cleaved within the cytoplasmic carboxy-terminal domain and that the COOH-terminal fragment accumulates in nucleus prompted us to question the function of this fragment.
Fig. 2.
A population of the angiotensin type 1 receptor (AT1R) is cleaved, in a ligand-dependent manner, at the 7th TM domain:cytoplasmic junction to release the intracellular cytoplasmic domain. A: AT1R cleavage fragment (arrow). RASMCs were transfected with pCMV/AT1R (lanes 1, 2) or corresponding empty vector (lanes 3, 4), or mock transfected (lane 5). Twenty-four hours posttransfection, cells were treated with ANG II (10−8 mol/l) (lanes 1, 3) or vehicle (lanes 2, 4, 5) for 30 min before extracts were collected. Extracts were immunoprecipitated as described in materials and methods. The 6-kDa approximate band was selectively excised from the acrylamide gel, and material was provided to Alphalyse for Maldi-TOF MS molecular weight determination (B, C) and for NH2-terminal Edman degradation sequencing (D). E: predicted cleavage site based on molecular weight and protein sequencing is shown.
Cellular morphology.
To differentiate the roles of the AT1R holoprotein from the cleaved fragment, we constructed an expression plasmid, pAT1RCF/EYFP encoding the AT1R-cleaved fragment (see Fig. 2E for CF) fused upstream and in-frame with YFP. Transfection of this expression plasmid into a variety of cell types leads to morphological changes consistent with apoptosis in all cell types tested, some of which are reported here. The exact nature of the visible morphological changes induced by AT1RCF/EYFP are cell-type dependent.
As early as 3 h posttransfection, transfected RASMCs shrink and collapse; extensions retract and blatant plasma membrane blebbing occurs. This process is rapid, requiring as little as 60 min from the initial morphological changes to the final cellular disintegration (Fig. 3). No significant apoptosis occurs in cells transfected with pEYFP-N1 or pAT1R/EYFP (controls, Fig. 3A, ii). Moreover, no significant toxicity or cell death occurs in cells transfected with pNLS-EYFP, which encodes a nuclear-targeted YFP. Apoptosis resulting from AT1RCF/EYFP is not, therefore, caused by intranuclear accumulation of the YFP moiety. MCF-7 breast cancer cells show a somewhat different response, but one also consistent with apoptosis (Fig. 4). These cells, which express normal levels of p53 but lack caspase-3 protein (16), undergo observable morphological changes as early as 3 h posttransfection. The hallmark change for these cells is intense nuclear accumulation of the fusion product followed by nuclear disintegration. Remarkably, the intact nucleus in a normal-appearing transfected cell may vanish in as little as 15 min (from T = 180 to T = 195 min in Fig. 4A and from T = 60 to T = 80 min in Fig. 4B) consistent with dissolution of the nuclear envelope (12).
Fig. 3.
The AT1R carboxy-terminus induces blebbing in RASMCs. A: cells imaged 24 h posttransfection with pAT1RCF/EYFP (i), pAT1R/EYFP (green) (ii) or pNLS-EYFP (green) (iii). The carboxy-terminal fragment (i) causes dramatic morphological changes consistent with apoptosis, whereas nuclear-targeted EYFP (iii) is not toxic or apoptotic. B: AT1R COOH-terminus induces cell fragmentation as early as 3 h posttransfection with pAT1RCF/EYFP (green). Time-lapse imaging (Slidebook, volume view) of RASMCs, starting 3 h posttransfection with pAT1RCF/EYFP (green). T = 0 is the start time for image capture (3 h posttransfection). Cells progress rapidly from normal morphology to blatant cellular blebbing in as little as 60 min. Each grid square is 10 μm2.
Fig. 4.
The AT1R COOH-terminus induces rapid nuclear disintegration in the human MCF-7 breast cancer cell line. Time-lapse imaging of cells 24 h posttransfection with pAT1RCF/EYFP (green). Time (T) is in minutes; T = 0 is start time for image capture. A and B represent two independent fields of microscopic view. Note that complete dissolution of the nucleus occurs very rapidly (within 15–20 min).
Annexin V assay.
Transfection of CHO-K1 cells [which are characterized by mutant p53 sequence (14)] with pAT1RCF/EYFP is associated with an extended dramatic, visually striking, chromatin condensation and nuclear fragmentation (Fig. 5A) before cellular fragmentation (Fig. 5B), consistent with the reported morphology of CHO-K1 cells undergoing apoptosis in other studies (40). Cells show annexin V positivity (Fig. 5B, red, Fig. 5C, blue) as early as 24 h posttransfection (we did not test earlier times) consistent with apoptosis. RASMCs and MCF-7 populations similarly show annexin V-positive cells as early as 24 h posttransfection while H9c2 cells begin to show low levels at 36 h, peaking at 72 h posttransfection (not shown).
Fig. 5.
The AT1R COOH-terminus induces nuclear fragmentation and cell disintegration in CHO-K1 cells following transfection with pAT1RCF/EYFP (green). A: 48 h posttransfection with pAT1RCF/EYFP; B: 72 h posttransfection with pAT1RCF/EYFP. i, ii: Volume View mode in Slidebook; each grid is 10 μm2; 1 h elapsed between i and ii. Annexin-V-PE (red) was applied 10′ before imaging (Cy3 channel) at T = 0. iii: White outline shows perimeter of cell before disintegration. C: 24 h posttransfection with pAT1RCF/EYFP. Annexin-V-PE was applied 10′ before imaging. Image is inverted for visual contrast and clarity (Annexin V is blue, YFP is magenta).
Caspase activation.
Active caspases play a central role as executioners in apoptotic cell death and, therefore, are upregulated during apoptosis. The fluorochrome inhibitor of caspases (FLICA) reagent employed here is sulforhodaminyl-l-valylalanylaspartyl fluoromethyl ketone (SR-VAD-FMK). VAD is an amino acid sequence targeted by most caspases and will, therefore, bind to active caspases inhibiting further enzymatic activity and providing a measure of intracellular active caspases by imaging or plate-based fluorometry. pAT1RCF/EYFP-transfected RASMCs show active caspases in 43.6 ± 5% of cells by fluorescent imaging (n = 3 transfections, 300 cells counted per transfection). Under the same transfection conditions, pEYFP-N1 induces active caspases in <1% of transfected cells. Activity is both diffusely cytoplasmic (Fig. 6A, iii) and juxtaposed to nucleus (Fig. 6B, iii and iv). Exposure of live cells to FLICA led to selective labeling of cells possessing morphological changes characteristic of apoptosis. Quantitative analysis by fluorometry demonstrates an average 5.2-fold increase (n = 6 wells/plasmid, 3 independent transfection experiments, P < 0.001) in intracellular FLICA in AT1RCF/EYFP-expressing cells compared with mock transfected or those expressing AT1R/EYFP or EYFP-N1 (negative controls) (Fig. 6C) consistent with increased abundance of early, prelysis-stage apoptotic cells. By immunoblot analyses, we find the steady-state level of cleaved caspase-3 (Asp-174) (Fig. 6D, top), standardized to β-actin levels (Fig. 6D, bottom) to be increased an average of 7.2-fold (n = 3, P < 0.001) at 24, 36, and 48 h posttransfection.
Fig. 6.
The cleavage fragment induces active caspases in RASMCs. Cells were transfected with pAT1RCF/EYFP. Five hours posttransfection, cells were stained with FLICA reagent, SR-VAD, and then imaged by three-dimensional deconvolution microscopy. A and B: Cy3 filter image (i), YFP filter image (ii) and merged images (iii) from i and ii. iv: enlargement of marquis from iii. C: SR-VAD-FMK fluorometric detection of active caspases in RASMCs 24 h posttransfection or following staurosporine treatment (1 μM, 3 h). n = 10 wells/treatment, *P < 0.001. D: caspase-3 immunoblot of cell extracts at sequential times posttransfection (top). β-Actin immunoblot rehybridization filter (bottom).
Levels of active caspases were also tested and found to be elevated in MCF-7 (30.2 ± 6%) and H9c2 cells (27.2 ± 4%) [n = 3 transfections, 200 cells counted per transfection, P < 0.0001 versus control (not shown)]. As with RASMCs, pEYFP and pNLS/EYFP (controls) did not induce significant caspase activity in either of these cell types.
TUNEL and PARP.
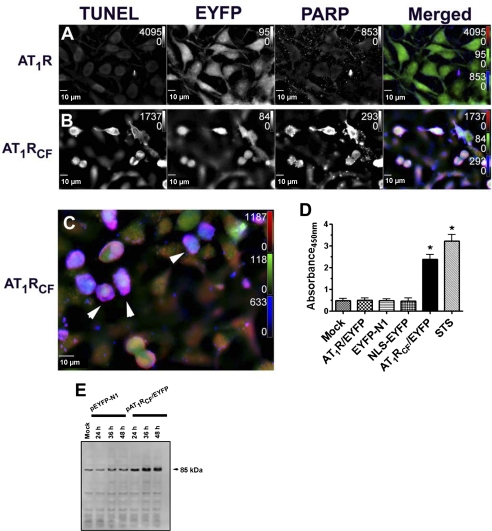
The Click-iT TUNEL assay was used to determine the level of apoptosis in this study. We show that very low levels of Alexa Fluor 647 azide-dUTP incorporate into H9c2 rat cardiomyoblasts that are transfected with pAT1R/EYFP; an average of 1.8 ± 0.8% (n = 3 transfections, 300 cells counted per transfection) of those cells transfected with pAT1R/EYFP are TUNEL positive (Fig. 7A). In contrast, under the same transfection conditions, an average of 37.4 ± 6% (n = 3 transfections, 300 cells counted per transfection) of those cells that are transfected with pAT1RCF/EYFP are visually TUNEL positive (Fig. 7, B and C).
Fig. 7.
The AT1R cleavage fragment induces DNA strand breaks and poly(ADP-ribose)polymerase (PARP) cleavage typical of late-stage apoptosis. H9c2 cardiomyoblasts were transfected with pAT1R/EYFP (control) or pAT1RCF/EYFP and fixed, permeabilized, and processed at 24 h posttransfection. A–C: cells were imaged for transfected DNA (yellow fluorescent EYFP), terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) positivity (red fluor), and PARP cleavage (blue fluor). C: enlarged merged image showing cells with high incidence of both DNA scission and PARP cleavage (arrowheads). D: PARP (Asp214) ELISA showing enhanced PARP cleavage in pAT1RCF/EYFP-transfected cells. STS, control in which cells were mock transfected and staurosporine treated for 6 h. E: cleaved PARP (Asp214) Western blot of extracts obtained from transfected cells.
PARP cleavage is an additional indicator of DNA single strand breaks. By immunocytochemistry, we find cleaved PARP to be specific to those cells that 1) express AT1RCF/EYFP, 2) display morphology consistent with apoptosis, and 3) are TUNEL positive. Colocalization of TUNEL-positive areas of the cells (red fluor) with PARP-positive regions (blue fluor) presents as magenta or deep pink. More than 30% of those cells that are transfected with pAT1RCF/EYFP are visually TUNEL positive and PARP positive (Fig. 7, B and C). Using the PARP cleavage site [214/215]-specific ELISA, we find immunoreactivity to the 85-kDa fragment to be 4.8-fold (n = 3, P < 0.001) higher in pAT1RCF/EYFP compared with mock-transfected or pAT1R/EYFP- or pEYFP-N1-transfected cells. By immunoblot analyses, we find cleaved PARP (85 kDa) to be elevated an average of at least 5.4-fold (n = 3) at each of 24, 36, and 48 h in pAT1RCF/EYFP-transfected compared with control pEYFP-N1-transfected cells.
DNA ladder assays.
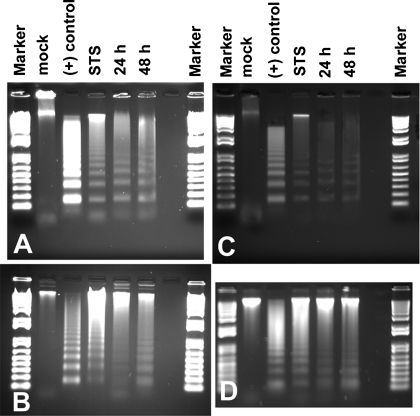
Whereas alterations in cell and nuclear morphology, caspase activation studies, and TUNEL assays are, collectively, excellent indicators of apoptosis, the gold standard is electrophoretic DNA ladder formation. We observe visible DNA laddering in CHO-K1, RASMCs, MCF-7, and H9c2 rat cardiomyoblasts at 24 and 48 h posttransfection with pAT1RCF/EYFP (Fig. 8). Mock-transfected cells show no such pattern. This also indicates that a large proportion of the transiently transfected cells very quickly respond to the transfected reagent; the AT1R cytoplasmic carboxy-terminal fragment is a powerful apoptotic reagent.
Fig. 8.
Expression of the AT1RCF induces DNA laddering. CHO-K1 (A), RASMCs (B), MCF-7 (C), and H9c2 cardiomyoblasts (D) were transfected with pAT1RCF/EYFP, and DNA was extracted at indicated times posttransfection. STS (treated for 6 h, 1 μM). Positive control is from Roche (apoptotic U937 cells, lyophilized). Marker is 1 kb Plus DNA ladder.
ANG II-induced apoptosis.
Our prior published study showed that ANG II induces CF formation (4), and the present study shows that the CF is apoptotic for several cell types. This pleads the question, is ANG II apoptotic for naive (untransfected) cultured cells and if so, how does it compare with the CF in terms of apoptotic potential? To address this question, we treated H9c2 cardiomyoblasts with ANG II (10−8 mol/l) and performed multipoint time-lapse image capture over a 4-h period. We find that some cells undergo visible morphological changes consistent with apoptosis (e.g., manifest membrane blebbing) as early as 140 min after ANG II treatment (Fig. 9, A–D). Apoptosis assays performed at 24 h after ANG II treatment demonstrate a fourfold ANG II increase in DNA fragmentation by TUNEL index from 1.8 ± 0.6% (control) to 7.2 ± 4% (n = 3 experiments, 300 cells/treatment) (Fig. 9, E and F) Apoptosis is completely blocked by pretreatment of the cells with losartan (10−7 mol/l) for 15 min before ANG II addition (Fig. 9F). Also note that AT1R transfection in the absence of ANG II treatment is not apoptotic.
Fig. 9.
ANG II induces rapid apoptosis in select H9c2 cardiomyocytes. A–D: H9c2 cells were treated with ANG II (10−8 mol/l) and imaged at times 0, 40, 100, and 140 min after ANG II application (×60). Arrows show membrane blebbing characteristic of apoptosis. E: cells were imaged for TUNEL positivity (dark cells, arrows); n are normal nuclei of nonapoptotic cells (×40). F: apoptotic index was quantified by TUNEL. Note that full-length receptor is not apoptotic in absence of ANG II, but ANG II treatment and CF expression do induce apoptosis.
DISCUSSION
A diverse array of receptors are subject to RIP or alternative cleavage mechanisms, with resulting production of extracellular and accumulation of intracellular and intranuclear products that trigger physiological and pathophysiological consequences. We have not identified the enzymes responsible for cleavage of the AT1R, though our earlier studies show that EDTA and orthophenanthroline inhibit cleavage suggesting that a metalloprotease could be involved in one step of the cleavage (4). One family of proteins, the IMPAS/PSH/signal peptide peptidases (27), cleave polytopic membrane proteins such as presenilin and could also be involved in AT1R cleavage to generate the CF.
Interestingly, intracellular cleavage products from many diverse receptors and transmembrane proteins including the receptor for advanced glycation end products (7), the amyloid precursor protein (1, 29, 39), and the low-density lipoprotein receptor-related protein (33) correlate with nuclear transport, cell death, and apoptosis, consistent with the action of AT1RCF. Whereas each of these transmembrane proteins has been associated with nuclear transport and apoptosis, an underlying homology in the sequences of the cleaved peptides is not readily apparent. Nor is there any unambiguous reason why regulated proteolysis of these particular diverse receptors might be linked to cell death. Further investigation of the CF-mediated cascade and apoptotic pathways (intrinsic vs. extrinsic) activated by the AT1RCF may be instructive in formulating a thesis.
The molecular mass of the CF-EYFP fusion (AT1RCF/EYFP) is ∼36 kDa permitting passive translocation of the fragment into the nucleus via the nuclear pore complex (37). Active transport is not required. However, facilitated transport may occur and may enhance accumulation of the fusion protein in the nucleus. Studies from the Morinelli et al. (28) and Raizada and colleagues (25) laboratories have shown that the cytoplasmic membrane-proximal region of the COOH-terminus possesses a NLS (sequence KKFKKY). Mutation of this sequence (K307Q) dramatically reduces receptor translocation to the nucleus (28). We have also mutated this sequence within the context of the COOH-terminal fragment fusion. Mutation of K307Q within the context of the COOH-terminal fragment does not eliminate but significantly reduces nuclear accumulation of the fragment (not shown). The reduction in nuclear accumulation depends on cell type. Dramatic reduction occurs in CHO-K1 cells, whereas reduction to approximately one-third of nonmutant nuclear levels occurs in MCF-7 cells. Mutation of G306Q (control) has no impact on nuclear localization of the CF fusion. It appears that the NLS is not necessary but may augment translocation of the CF to the nucleus.
Experimental evidence suggests that dramatic apoptosis may account for loss of cardiac myocytes in heart failure (20, 21, 30–32, 34, 36). Also, several studies have established the existence of a relationship among ANG II, hypertrophy of cardiac myocytes, apoptosis, and heart failure (8, 31, 35). Indeed, several published reports suggest that ANG II is apoptotic for various cell types in vivo and in vitro. In a recent study, ANG II was found to increase the apoptotic index of H9c2 cardiomyoblasts, as we show in the present study (23). In other studies, AT1R receptor blockade and angiotensin-converting enzyme inhibition have been found effective in blocking ANG II-induced damage to glomeruli (38) and myocardium (6) in animal models. Since the AT1R CF is a powerful apoptotic reagent and is produced in an ANG II-induced manner, we suggest that ANG II-dependent apoptosis observed by other investigators is mediated in part by the AT1R cleavage fragment. Furthermore, the effectiveness of AT1 receptor blockers and angiotensin-converting enzyme inhibitors may reflect, at least in part, inhibition of CF formation through inhibition of AT1R activation by ANG II and subsequent cleavage.
Since the consensus estimate, based largely on DNA end-labeling, for apoptotic myocytes involved in end-stage heart failure is a modest 0.2%, Olivetti and colleagues (32) suggest that perhaps apoptosis and necrosis combined contribute to cell death in the final stages. Certainly, this seems reasonable since products generated by apoptotic cells may be toxic to surrounding cells. But, in addition, our studies presented here suggest that, depending on the apoptotic trigger and the cell type, the time required to proceed from the initiating event to complete cell dissolution may be quite short (<3 h in some cases). If we assume that apoptotic myocytes of failing hearts (∼0.2% of the left ventricular myocytes) are detectable (by TUNEL or morphologically) for only a 6-h period after which they die, an average level of 0.2% apoptotic cells over a 3-wk period could lead to an impressive 17% reduction in functioning myocytes.
Whereas the present report documents in vitro apoptosis resulting from expression of a recombinant AT1R CF, our previous published studies have shown that ANG II augments cleavage of the native AT1 receptor and nuclear trafficking of the CF, which precedes apoptotic cell death. Our observations that ANG II augments AT1 receptor cleavage and transport to the nucleus as well as our studies, which show that the CF is a powerful apoptotic reagent in a number of cell types including cardiomyoblasts, suggest that the CF could be involved in cardiomyocyte death associated with heart failure. Moreover, the results of these studies indicate that cleavage of receptors and other cell surface proteins, as well as accumulation of stable intracellular products, can be regulated processes that serve perhaps to further amplify or enhance effects of ligand-receptor signal transduction events initiated at the plasma membrane.
GRANTS
This work was supported by the Ochsner Clinic Foundation and National Heart, Lung, Blood Institute Grant HL-072795 (to J. L. Cook and R. N. Re).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Barten DM, Albright CF. Therapeutic strategies for Alzheimer's disease. Mol Neurobiol 37: 171–186, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bertuccio CA, Chapin HC, Cai Y, Mistry K, Chauvet V, Somlo S, Caplan MJ. Polycystin-1 C-terminal cleavage is modulated by polycystin-2 expression. J Biol Chem 284: 21011–21026, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cook JL, Mills SJ, Naquin R, Alam J, Re RN. Nuclear accumulation of the AT1 receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J Mol Cell Cardiol 40: 696–707, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Cook JL, Mills SJ, Naquin RT, Alam J, Re RN. Cleavage of the angiotensin II type 1 receptor and nuclear accumulation of the cytoplasmic carboxy-terminal fragment. Am J Physiol Cell Physiol 292: C1313–C1322, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Cook JL, Re R, Alam J, Hart M, Zhang Z. Intracellular angiotensin II fusion protein alters AT1 receptor fusion protein distribution and activates CREB. J Mol Cell Cardiol 36: 75–90, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Fabris B, Candido R, Bortoletto M, Toffoli B, Bernardi S, Stebel M, Bardelli M, Zentilin L, Giacca M, Carretta R. Stimulation of cardiac apoptosis in ovariectomized hypertensive rats: potential role of the renin-angiotensin system. J Hypertens 29: 273–281 [DOI] [PubMed] [Google Scholar]
- 7. Galichet A, Weibel M, Heizmann CW. Calcium-regulated intramembrane proteolysis of the RAGE receptor. Biochem Biophys Res Commun 370: 1–5, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez A, Ravassa S, Lopez B, Beaumonta J, Diez J. Renin-angiotensin-aldosterone system and cardiomyocyte apoptosis in hypertensive heart disease. In: The Local Cardiac Renin-Angiotensin Aldosterone System, edited by Frohlich E, Re R. New York: Springer, 2009, p. 143–150 [Google Scholar]
- 9. Grantcharova E, Furkert J, Reusch HP, Krell HW, Papsdorf G, Beyermann M, Schulein R, Rosenthal W, Oksche A. The extracellular N terminus of the endothelin B (ETB) receptor is cleaved by a metalloprotease in an agonist-dependent process. J Biol Chem 277: 43933–43941, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Grantcharova E, Reusch HP, Grossmann S, Eichhorst J, Krell HW, Beyermann M, Rosenthal W, Oksche A. N-terminal proteolysis of the endothelin B receptor abolishes its ability to induce EGF receptor transactivation and contractile protein expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 26: 1288–1296, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Grossmann S, Higashiyama S, Oksche A, Schaefer M, Tannert A. Localisation of endothelin B receptor variants to plasma membrane microdomains and its effects on downstream signalling. Mol Membr Biol 26: 279–292, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Hara S, Halicka HD, Bruno S, Gong J, Traganos F, Darzynkiewicz Z. Effect of protease inhibitors on early events of apoptosis. Exp Cell Res 223: 372–384, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Hass MR, Sato C, Kopan R, Zhao G. Presenilin: RIP and beyond. Semin Cell Dev Biol 20: 201–210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu T, Miller CM, Ridder GM, Aardema MJ. Characterization of p53 in Chinese hamster cell lines CHO-K1, CHO-WBL, and CHL: implications for genotoxicity testing. Mutat Res 426: 51–62, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Huynh J, Thomas WG, Aguilar MI, Pattenden LK. Role of helix 8 in G protein-coupled receptors based on structure-function studies on the type 1 angiotensin receptor. Mol Cell Endocrinol 302: 118–127, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 273: 9357–9360, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Kalderon D, Roberts BL, Richardson WD, Smith AE. A short amino acid sequence able to specify nuclear location. Cell 39: 499–509, 1984 [DOI] [PubMed] [Google Scholar]
- 18. Kojro E, Fahrenholz F. Ligand-induced cleavage of the V2 vasopressin receptor by a plasma membrane metalloproteinase. J Biol Chem 270: 6476–6481, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Kojro E, Postina R, Gilbert S, Bender F, Krause G, Fahrenholz F. Structural requirements for V2 vasopressin receptor proteolytic cleavage. Eur J Biochem 266: 538–548, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Kumar D, Jugdutt BI. Apoptosis and oxidants in the heart. J Lab Clin Med 142: 288–297, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Kumar D, Kirshenbaum LA, Li T, Danelisen I, Singal PK. Apoptosis in adriamycin cardiomyopathy and its modulation by probucol. Antioxid Redox Signal 3: 135–145, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Lal M, Song X, Pluznick JL, Di Giovanni V, Merrick DM, Rosenblum ND, Chauvet V, Gottardi CJ, Pei Y, Caplan MJ. Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signaling. Hum Mol Genet 17: 3105–3117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu JJ, Li DL, Zhou J, Sun L, Zhao M, Kong SS, Wang YH, Yu XJ, Zhou J, Zang WJ. Acetylcholine prevents angiotensin II-induced oxidative stress and apoptosis in H9c2 cells. Apoptosis 16: 94–103, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell 10: 57–69, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Lu D, Yang H, Shaw G, Raizada MK. Angiotensin II-induced nuclear targeting of the angiotensin type 1 (AT1) receptor in brain neurons. Endocrinology 139: 365–375, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science 310: 1344–1347, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moliaka YK, Grigorenko A, Madera D, Rogaev EI. Impas 1 possesses endoproteolytic activity against multipass membrane protein substrate cleaving the presenilin 1 holoprotein. FEBS Lett 557: 185–192, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Morinelli TA, Raymond JR, Baldys A, Yang Q, Lee MH, Luttrell L, Ullian ME. Identification of a putative nuclear localization sequence within ANG II AT(1A) receptor associated with nuclear activation. Am J Physiol Cell Physiol 292: C1398–C1408, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Nakayama K, Ohkawara T, Hiratochi M, Koh CS, Nagase H. The intracellular domain of amyloid precursor protein induces neuron-specific apoptosis. Neurosci Lett 444: 127–131, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335: 1182–1189, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Narula J, Kolodgie FD, Virmani R. Apoptosis and cardiomyopathy. Curr Opin Cardiol 15: 183–188, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med 336: 1131–1141, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Polavarapu R, An J, Zhang C, Yepes M. Regulated intramembrane proteolysis of the low-density lipoprotein receptor-related protein mediates ischemic cell death. Am J Pathol 172: 1355–1362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sabbah HN. Apoptotic cell death in heart failure. Cardiovasc Res 45: 704–712, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Sadoshima J, Izumo S. Molecular characterization of angiotensin II–induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res 73: 413–423, 1993 [DOI] [PubMed] [Google Scholar]
- 36. Singal PK, Iliskovic N, Li T, Kumar D. Adriamycin cardiomyopathy: pathophysiology and prevention. FASEB J 11: 931–936, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Terasaki M, Campagnola P, Rolls MM, Stein PA, Ellenberg J, Hinkle B, Slepchenko B. A new model for nuclear envelope breakdown. Mol Biol Cell 12: 503–510, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tuncdemir M, Ozturk M. The effects of angiotensin-II receptor blockers on podocyte damage and glomerular apoptosis in a rat model of experimental streptozotocin-induced diabetic nephropathy. Acta Histochem 2011. January 24 [DOI] [PubMed] [Google Scholar]
- 39. Venugopal C, Pappolla MA, Sambamurti K. Insulysin cleaves the APP cytoplasmic fragment at multiple sites. Neurochem Res 32: 2225–2234, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Yu A, McMaster CR, Byers DM, Ridgway ND, Cook HW. Stimulation of phosphatidylserine biosynthesis and facilitation of UV-induced apoptosis in Chinese hamster ovary cells overexpressing phospholipid scramblase 1. J Biol Chem 278: 9706–9714, 2003 [DOI] [PubMed] [Google Scholar]