Abstract
Antithrombin, a major anticoagulant, is robustly transported into extravascular compartments where its target proteases are largely unknown. This serpin was previously detected in human milk as complexes with matriptase, a membrane-bound serine protease broadly expressed in epithelial and carcinoma cells, and under tight regulation by hepatocyte growth factor activator inhibitor (HAI)-1, a transmembrane Kunitz-type serine protease inhibitor that forms heat-sensitive complexes with active matriptase. In the current study, we detect, in addition to matriptase-HAI-1 complexes, heat-resistant matriptase complexes generated by nontransformed mammary, prostate, and epidermal epithelial cells that we show to be matriptase-antithrombin complexes. These findings suggest that in addition to HAI-1, interstitial antithrombin participates in the regulation of matriptase activity in epithelial cells. This physiological mechanism appears, however, to largely be lost in cancer cells since matriptase-antithrombin complexes were not detected in all but two of a panel of seven breast, prostate, and ovarian cancer cell lines. Using purified active matriptase, we further characterize the formation of matriptase-antithrombin complex and show that heparin can significantly potentiate the inhibitory potency of antithrombin against matriptase. Second-order rate constants for the inhibition were determined to be 3.9 × 103 M−1s−1 in the absence of heparin and 1.2 × 105 M−1s−1 in the presence of heparin, a 30-fold increase, consistent with the established role of heparin in activating antithrombin function. Taken together these data suggest that normal epithelial cells employ a dual mechanism involving HAI-1 and antithrombin to control matriptase and that the antithrombin-based mechanism appears lost in the majority of carcinoma cells.
Keywords: coagulation, tumor cells, hepatocyte growth factor activator inhibitor-1
antithrombin, a serpin-type serine protease inhibitor, plays an essential role in hemostasis by inactivating several proteases of the coagulation system. The major target proteases of antithrombin include Factor Xa, Factor IXa, and Factor IIa (thrombin), although Factor XIa, Factor XIIa, and Factor VIIa may be additional targets (8, 54, 55). In addition to plasma and nonplasma vascular compartments, a significant amount of antithrombin is present in the extravascular compartments, either bound to the interstitial matrix or in the extravascular fluid (11, 12, 71). In our previous studies, we purified from human milk antithrombin complexes containing matriptase, a type II transmembrane serine protease (65). The presence of matriptase-antithrombin complexes in human milk indicates that antithrombin may have functions beyond its role in hemostasis and that matriptase is a physiological extravascular antithrombin target. Matriptase is expressed by various types of epithelia in almost all organ systems (50, 64). The widespread epithelial expression of matriptase is consistent with its essential roles in the formation and maintenance of epithelial integrity and function (40). Dysregulation of matriptase causes aberrant epithelial differentiation that disrupts normal function and leads to the development and progression of disease. For example, patients with autosomal recessive matriptase mutations develop ichthyotic skin with impaired degradation of the stratum corneum corneodesmosomes and abnormal hair (1–3), a syndrome also seen in matriptase hypomorphic mice whose skin express only 1% of matriptase mRNA compared with that of normal mice (38). In addition to epithelial cells, matriptase is also expressed by monocytes (29, 65), macrophages (7), mast cells (16), and neural progenitor cells (20). Matriptase can activate urokinase-type plasminogen activator in monocytes (29) and macrophage stimulating protein 1 (7). In hematopoietic cells, therefore, matriptase may be involved in initiating plasminogen activation and macrophage activation.
The presence of matriptase-antithrombin complexes in human milk provides evidence that antithrombin-mediated matriptase inhibition is a normal physiological event. The key to understanding the physiological relevance of this inhibition, however, depends on knowing where and how antithrombin inactivates matriptase. As mentioned above, matriptase is expressed by all types of epithelial cells and some leukocytes, including monocytes, macrophages, and mast cells. There are several differences in the way that matriptase activity is regulated in epithelial cells compared with leukocytes, the most prominent of which is how the enzyme is inhibited. In epithelial cells, hepatocyte growth factor activator inhibitor (HAI)-1, a Kunitz-type integral membrane serine protease inhibitor, is by far the most important matriptase inhibitor (36). The importance of HAI-1 in the regulation of matriptase function is, however, not limited to its role as a catalytic inhibitor, and indeed the loss of HAI-1 function produces a phenotype that is strikingly similar to that seen in matriptase hypomorphic mice, reinforcing the notion that HAI-1 is required for normal matriptase function (46). The unusually intimate relationship between matriptase and HAI-1 in epithelial cells is illustrated by multiple observations including their coexpression in epithelial cells (27, 39, 50), colocalization on the surface of the cells, and the unexpected role that HAI-1 plays in matriptase synthesis, intracellular trafficking, and autoactivation (48). The most striking manifestation of this unusual relationship between the protease and the protease inhibitor, however, is the observation that the defects in placental development, epidermal barrier function, and the skin inflammation that are associated with targeted deletion or mutation of HAI-1 in several animal models can be negated by the simultaneous deletion of matriptase (13, 43, 62, 63).
The situation in some nonepithelial systems appears to be very different however. For example, most leukocytes that express matriptase do not express HAI-1 or only express very low levels of the protein (7, 29, 65). This is in stark contrast to the greater than 10:1 molar excess of HAI-1 over matriptase that is typically observed in epithelial cells (H. Xu and C. Y. Lin, unpublished data). Leukocytes must, therefore, employ different mechanisms for regulating matriptase function and for inhibiting matriptase catalytic activity. The isolation of several complexes from body fluids composed of activated matriptase bound with several members of the serpin family of serine protease inhibitors suggested that these inhibitors likely play a role in controlling leukocyte-associated matriptase activity. The matriptase-antithrombin complexes isolated from human milk were, therefore, thought to come from the action of interstitial antithrombin on active matriptase elaborated from migrating leukocytes during lactation.
The tight functional relationship between HAI-1 and matriptase, combined with the significant molar excess of HAI-1 over matriptase in epithelial cells, means that high levels of this inhibitor are present at the sight of matriptase activation. This would suggest that epithelial cells likely do not need other serine protease inhibitors, such as interstitial antithrombin for efficient matriptase inhibition. We were, therefore, surprised to discover in the current study, that when high levels of matriptase activation are induced in mammary and prostate epithelial cells, in addition to the expected matriptase-HAI-1 complexes, we also detected matriptase-antithrombin complexes. These data suggest that epithelial cells also recruit antithrombin to regulate matriptase activity despite the abundance of HAI-1. This implies that the control of matriptase activity is more nuanced than we previously understood, involving additional layers of activity regulation. These observations were made using immortalized, nontransformed epithelial cells. Interestingly, when we repeated the analysis using cancer cells, we found that we were unable to detect matriptase-antithrombin complexes in the majority of tumor cell lines tested, suggesting that this additional level of matriptase regulation may be lost in cancer.
EXPERIMENTAL PROCEDURES
Cell lines and culture conditions.
184 A1N4 human mammary epithelial cells (a gift from Dr. Martha Stampfer, Berkeley, CA) were maintained in Dulbecco's modified Eagle's medium (DMEM)/HAM F-12 (Mediatech), supplemented with 0.5% fetal bovine serum (FBS), 5 μg/ml of recombinant human insulin (rh-insulin), 0.5 μg/ml of hydrocortisone, 10 ng/ml of recombinant human epidermal growth factor (rh-EGF), and 100 IU/ml penicillin-100 μg/ml streptomycin (Pen/Strep). MCF-10A human mammary epithelial cells were grown in DMEM/HAM F-12 supplemented with 5% horse serum, 0.5 μg/ml of hydrocortisone, 10 μg/ml of rh-insulin, 10 ng/ml of rh-EGF, and Pen/Strep. MTSV-1.1 B human mammary epithelial cells (a gift from Dr. Taylor-Papadimitriou, Imperial Cancer Research Fund, London, UK) were grown in Improved MEM (Richter's Mod.) (Mediatech), supplemented with 10% FBS, 10 μg/ml of rh-insulin, 5 μg/ml of hydrocortisone, and Pen/Strep. MCF-7 breast cancer cells were grown in Eagle's minimum essential medium (Quality Biology), supplemented with 10% FBS, and Pen/Strep. The immortalized human keratinocyte line HaCaT was cultured in DMEM (Cellgro, Manassas, VA) supplemented with 10% FBS (Gemini, West Sacramento, CA), 100 U/ml penicillin, and 100 μg/ml streptomycin. T-47D breast cancer cells were grown in RPMI 1640 medium (Mediatech), supplemented with 10% FBS, 9 μg/ml of rh-insulin, and Pen-Strep. RWPE-1 prostate epithelial cells were grown in Keratinocyte serum-free medium (Invitrogen), supplemented with 50 μg/ml of bovine pituitary extract (Sigma), and 5 ng/ml of rh-EGF, and Pen/Strep. LNCaP prostate cancer cells, OVCAR-3 ovarian cancer cells, OV2008 ovarian cancer cells, and RPMI 8226 multiple myeloma cells were grown in RPMI 1640 medium, supplemented with 10% FBS and Pen/Strep. DU 145 prostate cancer cells were grown in Eagle's minimum essential medium (Quality Biology), supplemented with 10% FBS and Pen/Strep. PC-3 prostate cancer cells were grown in F-12K medium (Mediatech), supplemented with 10% FBS and Pen/Strep. All cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
Antibodies and Western blot analysis.
Human matriptase was detected using the monoclonal antibodies (mAb) M24 and M69. The epitope recognized by the M24 mAb likely resides in one of the noncatalytic domains of the protein and is detected in both the zymogen and activated forms of the protease. The M69 mAb recognizes an epitope that is only present in the activated form of the enzyme (4, 5) and can, therefore, specifically detect activated matriptase and does not recognize matriptase zymogen. Human HAI-1 was detected using the HAI-1-specific mAb M19 (35). A human antithrombin polyclonal antibody was purchased from R&D Systems (Minneapolis, MN). Cells were lysed with phosphate-buffered saline (PBS) containing 1.0% Triton X-100 and 1 mM 5,5′-dithiobis-(2-nitrobenzoic acid). Insoluble debris was removed by centrifugation, and the protein concentrations of the lysates were determined using the BCA protein assay reagent (Thermo Fisher Scientific, Rockford, IL) according to the manufacturer's protocol. Protein samples for Western blot analysis were diluted in 5× sample buffer. The sample buffer did not contain a reducing agent, and the samples were not boiled before SDS-PAGE unless otherwise specified. Proteins were resolved by 7.5% SDS-PAGE, transferred to BioTrace NT nitrocellulose blotting membranes (Pall, Pensacola, FL), and probed with the mAbs M24, M69, or the human antithrombin antibody. The binding of the primary antibody was detected with horseradish peroxidase-conjugated secondary antibodies (KPL, Gaithersburg, MD) and visualized using the Western Lightening Chemiluminescence Reagent Plus (Perkin-Elmer, Boston, MA).
Immunoprecipitation.
The cell lysates were incubated with mAbs immobilized on Sepharose 4B beads and rotated in a cold room for 2 h. The unbound fractions were collected and the beads were washed with 1% Triton in PBS four times. The captured proteins were eluted with 0.1 M glycine buffer, pH 2.4, and immediately neutralized with 2 M Trizma base.
Purification of active matriptase.
Enzymatically active matriptase was generated by incubating RPMI 8226 multiple myeloma cells in 150 mM phosphate buffer, pH 6.0, for 20 min. The shed active matriptase in the phosphate buffer was collected, concentrated, adjusted to pH 8.0, and applied onto a p-aminobenzamidine protease inhibitor column (GE Healthcare). After the column was washed, active matriptase was eluted with 100 mM citrate buffer, pH 4.0, and immediately adjusted to pH 6.0 with 0.1 M citrate buffer, pH 6.0. The functional concentration of purified matriptase was determined by active-site titration with antithrombin in the presence of saturating heparin pentasaccharide (54). Briefly, equal amounts of matriptase were incubated at 25°C with 0–5 nM antithrombin in the presence of 200 nM heparin in pH 7.4 kinetics buffer for a time sufficient to reach an inhibition end point based on measured rate constants for the reaction (∼5 h). Residual matriptase activity was then measured as described below and plotted as a function of the antithrombin concentration. The x-axis intercept corresponding to complete inhibition provided the active matriptase concentration.
Protease activity assay.
Matriptase activity was assessed by either gelatin zymography or using the synthetic fluorogenic peptide substrates N-t-butyloxycarbonyl-Gln-Ala-Arg-amino-4-methylcoumarin (Boc-QAR-AMC) (Sigma) or Pefafluor FXa (Centerchem). For gelatin zymography, 1 mg/ml gelatin was copolymerized in 7.5% SDS gels on which matriptase samples were separated by SDS-PAGE under nonreducing and nonboiled conditions. The gelatin gels were washed with 2% Triton X-100 three times and incubated at 37°C overnight. The gels were stained using Coomassie Brilliant Blue. For the fluorogenic peptide substrate assays, purified active matriptase alone or preincubated with antithrombin (Innovative Research, Novi, MI) in the absence or presence of heparin (Sigma) for 10 min was mixed with 250 μM Boc-QAR-AMC substrate in a reaction buffer containing 100 mM Tris·HCl (pH 8.5) and 100 μg/ml bovine serum albumin. The increase in fluorescence resulting from hydrolysis of the peptide substrate was recorded with excitation at 360 nm and emission at 480 nm using a Synergy HT multi-mode microplate reader (Bio-Tek, Winooski, VT).
Kinetics of matriptase inhibition by antithrombin.
Kinetics of matriptase inhibition by antithrombin in the absence and presence of a synthetic heparin pentasaccharide (H5) representing the binding site for antithrombin (a gift from Sanofi-Aventis) or a full-length ∼50-saccharide heparin (H50) containing this sequence were performed under pseudo-first-order conditions with an at least 10-fold molar excess of inhibitor over protease as in previous studies (52, 54). Heparins were added at a 1.2- to 2-fold molar excess over antithrombin to ensure near saturation of the inhibitor with the polysaccharide. Briefly, 100 nm antithrombin and ∼0.1 nM matriptase were incubated in a reaction volume of 100 μl with or without 120 nM H50 or 200 nm H5 at 25°C, in 0.1 M HEPES, 0.1 M NaCl, and 0.1% PEG 8000, pH 7.4 buffer. Reactions of free antithrombin and antithrombin-heparin complex were monitored by quenching reactions after varying times with 0.9 ml of 100 μm Pefafluor FXa fluorigenic substrate. Polybrene (50 μg/ml) was used to neutralize heparin when present. Residual protease activity was measured from the initial rate of substrate hydrolysis. The observed pseudo-first-order rate constants (kobs) for the inhibition reactions were determined by fitting the loss of enzyme activity to a single exponential decay function by nonlinear regression analysis. The second-order rate constants were calculated by dividing kobs by the antithrombin or the antithrombin-heparin complex concentration. In the latter case, the complex concentration was calculated from the antithrombin and heparin concentrations employed and the measured kilodaltons for the interactions using the equilibrium binding equation and kobs was corrected for the free antithrombin reaction (52, 54).
RESULTS
Formation of non-HAI-1 matriptase complexes during acid-induced matriptase activation.
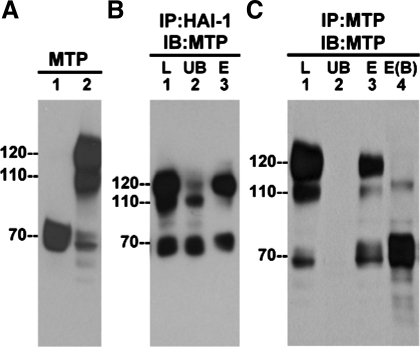
Previously we discovered that matriptase activation can be rapidly and robustly induced in cells by exposing to mildly acidic conditions, ranging from pH 5.2 to pH 6.8 (33, 66). Matriptase is present in the latent form in 184 A1N4 nontransformed, immortal mammary epithelial cells and can be detected as a 70-kDa band using the matriptase mAb M24 (Fig. 1A, lane 1). When these cells are exposed to a pH 6.0 buffer for 20 min, the vast majority of latent matriptase is converted into active matriptase that is rapidly inhibited by binding to HAI-1, leading to the formation of matriptase-HAI-1 complexes that are detected as a band at 120 kDa (Fig. 1A, lane 2). The complex is stable in SDS loading buffer without heating. With the appearance of the 120-kDa complex, the level of 70-kDa latent matriptase decreased (Fig. 1A, comparing lane 1 with lane 2 for the 70-kDa band). In addition to the 120-kDa matriptase-HAI-1 complex, we also noticed a minor matriptase complex detected as a band at 110 kDa that can be readily detected using the matriptase mAb (Fig. 1A, lane 2, 110 kDa).
Fig. 1.
Formation of non-heptatocyte activator inhibitor (HAI)-1 matriptase (MTP) complexes in mammary epithelial cells. A: 184 A1N4 mammary epithelial cells were exposed to basal medium (lane 1) or a pH 6.0 buffer (lane 2) for 20 min to induce MTP activation. Cell lysates were prepared and analyzed for MTP by blotting with the MTP monoclonal antibody (mAb) M24. B: cell lysates were immunoprecipitated (IP) using HAI-1 mAb M19 beads (IP:HAI-1), and the captured proteins were eluted using pH 2.4 glycine buffer. The lysate (L), the unbound fraction (UB), and the eluted fraction (E) were analyzed by immunoblot for MTP with mAb M24 (IB:MTP). C: cell lysates were immunoprecipitated by using MTP mAb 21–9 beads and the bound proteins eluted with pH 2.4 glycine buffer (IP:MTP). Proteins samples from the lysate (L), the unbound fraction (UB), and the eluted fractions (E) were incubated with SDS sample buffer at room temperature (lane 1 through lane 3) or at 95°C (lane 4, B) for 5 min before electrophoresis and analyzed by immunoblotting for MTP with the mAb M24 (IB:MTP).
The 110-kDa matriptase complex does not appear to be a degradation product of the 120-kDa matriptase-HAI-1 complex. When the 110- and 120-kDa complexes (Fig. 1B, lane 1) were incubated with the HAI-1 mAb M19 immobilized on beads, only the 120-kDa complexes and not the 110-kDa complexes were depleted in the unbound fractions (Fig. 1B, lane 2) and could then be eluted from the beads using a pH 2.4 buffer (Fig. 1B, lane 3). Since the interaction between matriptase and HAI-1 is acid sensitive and reversible (4), the elution of the 120-kDa complex from the beads using pH 2.4 buffer results in the dissociation of matriptase from HAI-1; and although the majority of the complexes reassociate on neutralization, a proportion of the active matriptase remains dissociated after elution and neutralization. As a result, activated matriptase was detected in the eluted fraction as both 120- and 70-kDa bands (Fig. 1B, lane 3). This immunoprecipitation confirms the presence of HAI-1 in the 120-kDa matriptase complexes and suggests that the 110-kDa complex does not contain HAI-1. When an immobilized matriptase mAb was used, the 110-kDa complex, the 120-kDa matriptase-HAI-1 complexes, and 70-kDa latent matriptase (Fig. 1C, lane 1) were all efficiently immunoprecipitated (Fig. 1C, lane 2) and then eluted from the mAb (Fig. 1C, lane 3), confirming that all of them are matriptase-containing species. One striking difference between the 110-kDa complexes and 120-kDa matriptase-HAI-1 complexes is their relative heat stability. When heated at 95°C in SDS sample buffer (without reducing agents), the matriptase-HAI-1 complex was dissociated, liberating the activated matriptase, which was detected at 70-kDa (Fig. 1C, lane 4). In contrast, the 110-kDa complex appears to be relatively stable, with the heat denaturing only resulting in a slight reduction in the migration rate on SDS-PAGE (Fig. 1C, lane 4). These data confirm that the 110-kDa species is not a degradation product of the matriptase-HAI-1 complex, and the thermal stability of the complex suggests that it may be composed of activated matriptase, covalently linked to a binding partner. This led us to consider the possibility that the 110-kDa complex might contain matriptase bound with one of the serpins, the serine protease inhibitors that can bind serine proteases to form covalent, SDS-resistant complexes.
Inhibition of matriptase by serpins was demonstrated in our previous study in which three matriptase-serpin complexes were isolated from human milk (65). Among these three serpins, antithrombin was the predominant one. In the previous study, migrating leukocytes were considered to be the most likely source for the milk-derived matriptase-serpin complexes, based on the fact that monocytes and macrophages express matriptase with limited or no HAI-1 expression, leading to the hypothesis that serpins may replace HAI-1 in the control of matriptase in these hematopoietic cells.
Formation of matriptase-antithrombin complex in mammary epithelial cells during acid-induced matriptase activation.
Since antithrombin was the most prevalent serpin in the matriptase-serpin complexes found in human milk, we set out to determine whether antithrombin was the matriptase binding partner in the 110-kDa complex. Since epithelial cells do not express antithrombin, the source of the serpin would have to be the serum with which the culture medium was supplemented. We had an antibody against human antithrombin from our previous work, however, this antibody does not cross react with bovine antithrombin, and so to provide a source of human antithrombin to the cells, we incubated 184 A1N4 cells in medium containing 0.5% human serum for 10 min before acid-induced matriptase activation.
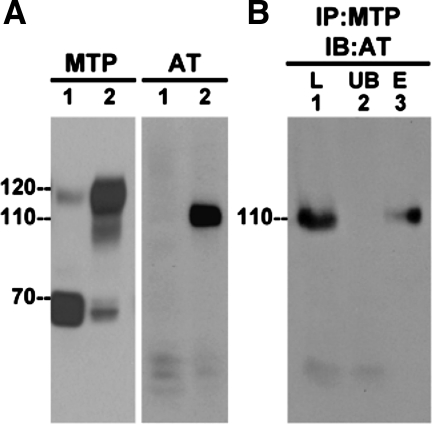
The incubation in medium supplemented with human serum did not appear to interfere with acid-induced matriptase activation since just as before, the 70-kDa latent matriptase was converted into the 120-kDa activated matriptase-HAI-1 complex after exposing these cells to a pH 6.0 buffer (Fig. 2A, MTP, lane 1 without activation and lane 2 with activation). When these samples were analyzed for the presence of antithrombin, a 110-kDa species was clearly detected by the antithrombin antibody after matriptase activation was induced (Fig. 2A, AT, lane 2). In contrast, before the induction of matriptase activation, antithrombin was only detected as a 50- to 60-kDa band of much lower intensity and there was no band at 110 kDa (Fig. 2A, AT, lane 1). To further confirm that the 110-kDa species is indeed a matriptase-antithrombin complex, we carried out immunoprecipitation-immunoblot analysis. Lysates from human-serum incubated 184 A1N4 cells that had been exposed to pH 6.0 buffer to activate the matriptase (Fig. 2B, lane 1) were incubated with immobilized matriptase mAb to deplete matriptase and matriptase-containing species (unbound fraction Fig. 2B, lane 2), and then the materials captured by the antibody-beads were eluted using a pH 2.4 buffer (Fig. 2B, lane 3). The lysate, the supernatant from the immunoprecipitation, and the materials eluted from the beads were then analyzed for the presence of antithrombin by immunoblot. As before, the antithrombin antibody detects a 110-kDa band in the acid-activated cell lysate (Fig. 2B, lane 1). Incubation with the anti-matriptase beads efficiently removed the 110-kDa species from the lysate but not the residual free antithrombin bands visible at 50–60 kDa (Fig. 2B, lane 2). The 110-kDa band was then eluted from the beads (Fig. 2B, lane 3). These data confirm that the 110-kDa species is a complex that contains both matriptase and antithrombin.
Fig. 2.
Antithrombin (AT)-mediated MTP inhibition is seen in 184 A1N4 mammary epithelial cells. A: 184 A1N4 mammary epithelial cells were exposed to culture medium containing 0.5% human serum for 10 min. Then the cells were exposed to basal medium (lane 1) or a pH 6.0 buffer (lane 2) to induce MTP activation. Cell lysates were prepared and analyzed by immunoblot for MTP species by MTP mAb M24 or for antithrombin species using a human antithrombin polyclonal antibody (AT). B: to confirm that the 110-kDa antithrombin species is MTP-AT complex, the cell lysates (L) were immunoprecipitated by MTP mAb 21–9 (IP:MTP), and the captured MTP species were eluted by pH 2.4 glycine buffer. The samples from loading (L), unbound fraction (UB), and elute fraction (E) were analyzed by immunoblotting for AT species by an AT Ab (IB:AT).
Antithrombin-mediated matriptase inhibition is a common regulatory mechanism in nontransformed epithelial cells.
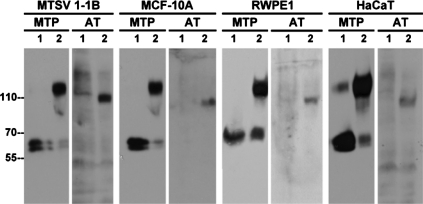
We were interested to determine whether the surprising observation that antithrombin participates in the inhibition of matriptase in 184 A1N4 cells is unique to these cells or is a common finding in nontransformed epithelial cells. To do this we used the same experimental approach to determine whether acid-induced matriptase activation results in the formation of matriptase-antithrombin complexes in a panel of four additional nontransformed epithelial cell cultures composed of the mammary epithelial cell lines MTSV-1.1B and MCF-10A, the prostate cell line RWPE1, and the keratinocyte cell line HaCaT. In all cases, exposure of the cells to pH 6.0 buffer resulted in robust matriptase activation with the generation of the 120-kDa matriptase-HAI-1 complexes (Fig. 3, MTP, comparing lanes 2 with lanes 1). Furthermore, the 110-kDa matriptase-antithrombin complex was readily detected after matriptase activation (Fig. 3, AT, comparing lanes 2 with lanes 1) suggesting that antithrombin-mediated matriptase inhibition is a common feature in epithelial cell systems. These data suggest that mammary epithelial cells are likely to be, at least in part, the in vivo source responsible for the production and secretion of matriptase-antithrombin complexes detected in human milk (65). Our study also clearly suggests that epithelial cells employ a dual mechanism for the control of matriptase activity that involves the cooperation of the serpin-type protease inhibitor antithrombin with the Kunitz-type serine protease inhibitor HAI-1.
Fig. 3.
AT-mediated MTP inhibition is commonly seen in nontumorigenic mammary and prostate epithelial cells. MTSV-1.1B and MCF 10A mammary epithelial cells, RWPE-1 prostate epithelial cells, and HaCaT keratinocytes were exposed to culture medium containing human sera for 10 min. Then these cells were exposed to basal medium (lanes 1) or a pH 6.0 buffer (lanes 2) for 20 min to induce MTP activation. Cell lysates were prepared and analyzed by Western blotting for MTP and AT.
Antithrombin-mediated matriptase inhibition is lost in many cancer cells.
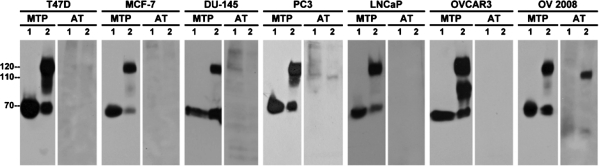
The ratio of matriptase to HAI-1 seems to be very important to the regulation of matriptase activity. Data from animal models has shown that even slight overexpression of matriptase in the skin of transgenic mice can result in tumor formation and enhanced carcinogen sensitivity, an effect that can be suppressed by concomitantly increasing the expression of HAI-1 (41). Furthermore, enhanced matriptase expression relative to HAI-1 has been also seen in many human tumors and is correlated with cancer progression and poor outcome (49, 57, 68, 70). We were very interested, therefore, to determine whether the inhibition of matriptase activity by antithrombin might represent an important additional mechanism for the suppression of matriptase activity in tumors of epithelial origin. To begin to examine this question we set out to determine whether antithrombin participates in the suppression of matriptase activity in a panel of two breast cancer cell lines (T-47D and MCF-7), three prostate cancer cell lines, (DU-145, PC3, and LNCaP), and two ovarian cancer cell lines (OVCAR3 and OV2008). Once again, we preincubated the cells in medium supplemented with human serum, induced matriptase activation by incubating them in pH 6.0 buffer, prepared lysates from control or acid-activated cells, and blotted for matriptase and for antithrombin. In all cases, as expected, robust matriptase activation was induced by exposure to an acidic environment resulting in the formation of the 120-kDa matriptase-HAI-1 complex (Fig. 4, MTP, comparing lanes 2 with lanes 1). Strikingly, however, there was no evidence for the formation of matriptase-antithrombin complexes in five of these seven cancer cell lines (Fig. 4, AT). OV2008 ovarian cancer cells were the only cancer line that the 110-kDa complex could be clearly detected. Although 110-kDa complex was generated in PC3 prostate cancer cells, its levels were very low. These data suggest that loss of antithrombin-mediated matriptase inhibition is a frequent event that may tip the balance toward proteolysis in cancer cells.
Fig. 4.
AT-mediated MTP inhibition is lost in carcinoma cells. Seven different cancer lines, as indicated, were exposed to culture media containing human sera for 10 min. These cells were then exposed to basal media (lanes 1) or a pH 6.0 buffer (lanes 2) to induce MTP activation. Cell lysates were prepared and analyzed by immunoblotting for MTP and AT.
Production and purification of active matriptase.
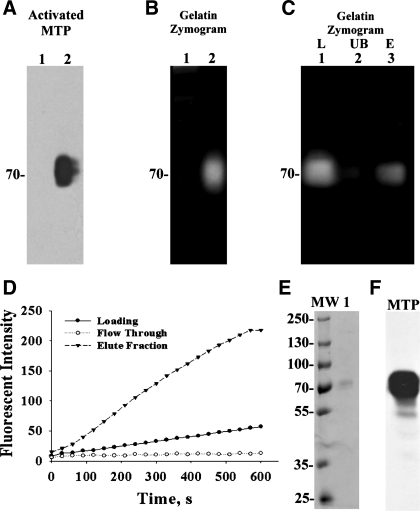
To further characterize inhibition of matriptase by antithrombin, we set out to prepare pure, active matriptase. In our previous studies, we have shown that matriptase and HAI-1 are commonly coexpressed with a HAI-1-to-matriptase molar ratio higher than 10 in most epithelial cells and carcinoma cells, and as a consequence, active matriptase is rapidly inhibited by HAI-1 in these cells. As noted above, the same is not true in leukocytes and leukocyte-derived tumors, many of which express matriptase without or with very low levels of HAI-1. When RPMI 8226 human multiple myeloma cells are incubated with pH 6.0 buffer, activation of matriptase occurs just as in epithelial/carcinoma cells. A portion of the active matriptase is rapidly shed from the cell surface and can be detected at 70 kDa by the activated matriptase-specific mAb M69 (Fig. 5A, comparing lane 2 with lane 1). A gelatinolytic activity of the corresponding size is also shed into the medium by acid-treated cells (Fig. 5B, comparing lane 2 with lane 1), which can be shown to be matriptase by the depletion of the activity from the buffer by immobilized anti-active matriptase M69 antibody beads (Fig. 5C, comparing lane 2 with lane 1). The gelatinolytic activity can then be eluted from the beads (Fig. 5C, lane 3). The active matriptase that is shed from the cells in this way provides a convenient source of active matriptase that can be purified free of protease inhibitors and other contaminants using a p-aminobenzamidine column. With the use of this approach and assaying for matriptase activity using a synthetic fluorogenic substrate Boc-Gln-Ala-Arg-AMC (Boc-QAR-AMC) (Fig. 5D) that we have used previously (34), active matriptase was isolated and purified to near homogeneity based on the observation that a single 70-kDa protein band was seen by SDS-PAGE (Fig. 5E). The purified active matriptase was also verified by immunoblot using matriptase mAb M24 (Fig. 5F).
Fig. 5.
Production and purification of active MTP. RPMI-8226 multiple myeloma cells were exposed to basal media (A and B, lanes 1) or a pH 6.0 buffer (A and B, lanes 2) for 20 min. Proteins shed into extracellular millieu were collected, concentrated, and analyzed by immunoblotting for activated MTP using the mAb M69 (A, Activated MTP) or by gelatin zymography for the gelationolytic activity (B, Gelatin Zymogram) or for amidolytic activity using synthetic fluoresent substrate (D, loading). In C, the shed 70-kDa gelatinolytic activity (lane 1) was incubated with immobilized activated MTP mAb M69. The unbound fraction (lane 2) was collected. The mAb-captured proteins were eluted by glycine buffer pH 2.4 and immediately neutralized (lane 3). These three samples were analyzed by gelatin zymography. Active MTP shed by RPMI 8226 cells was purified by protease inhibitor affinity chromatography using p-aminobenzamidine. The amidolytic activity of active MTP was used to monitor the purification (D). The eluted proteins were further analyzed by SDS-PAGE for the purity of the preparation (E, lane 1) and by immunoblot for MTP (F). MW, molelcular weight markers.
Heparin enhances antithrombin-mediated inhibition of matriptase.
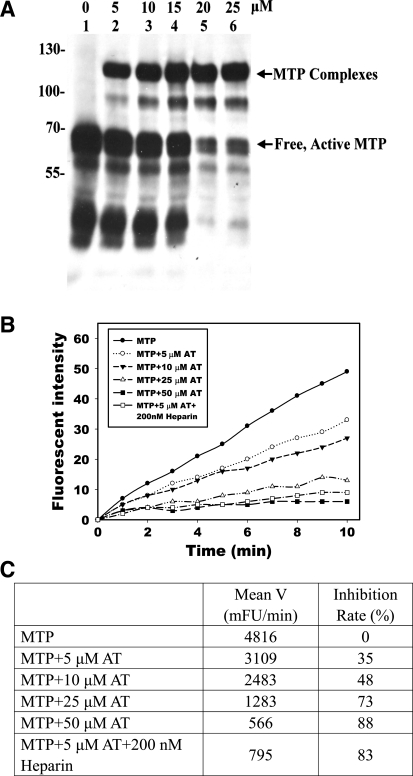
Using the purified active matriptase, we set out to characterize the inhibition of matriptase by antithrombin. Incubation of active matriptase with antithrombin resulted in the dose-dependent formation of a matriptase-antithrombin complex (Fig. 6A). The formation of a 110-kDa matriptase-antithrombin complex is consistent with the observed size of the matriptase-antithrombin complexes in body fluids and in the lysates of cells after induction of matriptase activation. Active matriptase appears to be prone to autodegradation, since in the absence of sufficient antithrombin, matriptase degradation fragments were generated. This degradation was largely suppressed in the presence of a higher concentration of antithrombin (Fig. 6A, lanes 5 and 6).
Fig. 6.
Complex formation and inhibition of active MTP by AT in vitro. Purified active MTP was incubated with increasing amounts of human AT, as indicated, and analyzed for the formation of MTP complexes with AT by immunoblotting using MTP mAb M24 (A) and for inhibition of MTP amidolytic activity using synthetic fluorescent substrate Boc-Gln-Ala-Arg-AMC (B and C). The effect of heparin (200 nM) on MTP inhibition was also determined. The proteolytic activities for the samples were determined by the rates of cleavage of the MTP substrate as indicated by the plots of the release of AMC (Fluorescent Intensity) against time (B). The inhibition was calculated by comparing the mean velocities (Mean V) of inhibitor-treated samples with MTP alone (C).
In parallel with the formation of matriptase-antithrombin complexes, incubation of active matriptase with antithrombin at pH 8.5 also resulted in a dose-dependent decrease in matriptase proteolytic activity as assessed using the synthetic, fluorogenic substrate Boc-QAR-AMC (Fig. 6, B and C). It is known that the antiprotease activity of antithrombin can be greatly enhanced by heparin or heparan sulfate. Engagement of antithrombin with a characteristic pentasaccharide binding sequence in heparin causes conformational changes in the serpin that expose the reactive center. As a consequence of this allosteric activation, heparin-like glycosaminoglycans greatly accelerate the interaction of antithrombin with target proteases (51, 53). Consistent with this literature, the addition of heparin significantly enhanced the inhibition of matriptase by antithrombin. In the presence of unfractionated heparin, the inhibition of matriptase activity by 5 μM antithrombin was almost equal to that of 50 μM antithrombin alone (Fig. 6C).
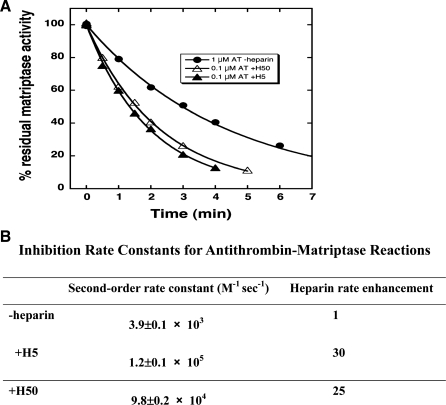
We went on to determine the inhibitory kinetics for the action of antithrombin against matriptase. Matriptase inhibition assays were performed at pH 7.4 because of the higher dissociation rate of serpin-protease complexes with increased pH and because we wanted to evaluate the kinetics at physiological pH (9). In the absence of heparin, the second-order rate constant for antithrombin against matriptase is 3.9 ± 0.1 × 103 M−1 s−1. In the presence of the heparin pentasaccharide activator of antithrombin, the rate constant increases to 1.2 ± 0.1 × 105 M−1 s−1, a 30-fold increase compared with that in the absence of heparin. A full-length heparin containing the pentasaccharide produced an experimentally indistinguishable increase in the inhibition rate constant (Fig. 7, A and B). The pentasaccharide or full-length heparin rate enhancements were unaffected by including physiological levels of calcium (5 mM) in the buffer. These findings indicate that the heparin effect is fully allosteric and not augmented by matriptase binding to heparin alongside antithrombin in a ternary bridging complex as is the case for most coagulation proteases (53, 54).
Fig. 7.
Second-order association rate constants for the reactions of free and heparin-complexed AT with MTP. Rate constants for the reactions of MTP and AT in the absence of heparin (1 μM AT-Heparin) and in the presence of heparin pentasaccharide (0.1 μM AT+H5) or full-length heparin (0.1 μM AT+H50) were measured in a physiological ionic strength, pH 7.4 buffer at 25°C under pseudo-first-order conditions by monitoring the decrease in protease activity as a function of time as detailed under experimental procedures.
DISCUSSION
Multiple protease inhibitors, including HAIs and several serpins, have been identified to inhibit matriptase in physiologically relevant in vivo settings. These protease inhibitors have either been isolated and identified in complexes with matriptase from body fluids (35, 65) or identified and characterized through the use of animal models in which the defects caused by the genetic ablation of the protease inhibitors can be rescued by the simultaneous deletion of matriptase (61, 63). The need for serpins, including antithrombin, to participate in matriptase inhibition might be attributed to circumstances such as lack of sufficient levels of the HAIs, such as in hematopoietic cells, for which blood-borne serpins may represent a convenient source for matriptase inhibition. It was something of a surprise, however, to discover that epithelial cells also employ a blood-borne serpin to inhibit matriptase even in the context of apparently overwhelming levels of HAI-1. This raises the question as to whether serpin-mediated matriptase inhibition is simply a redundant mechanism or it is an essential physiological event. It is a daunting challenge to try to address this question due to the paramount importance of antithrombin for hemostasis and the fact that targeted deletion of antithrombin in mice causes embryonic lethality due to extensive thrombosis in the myocardium and liver sinusoids (26). Nevertheless, the observation that antithrombin-mediated matriptase inhibition appears to be lost in many carcinoma cells is intriguing, particularly since lacking this mechanism could alter the balance of matriptase regulation, favoring proteolysis on the surface of the cancer cells.
Most proteases are synthesized as a zymogen and only acquire their proteolytic activity after zymogen activation. Once activated, the substrates and endogenous protease inhibitors compete for the active protease, a balance that must be closely regulated for many physiological processes. One of the most interesting features of matriptase regulation in epithelial cells is that the coupling between matriptase zymogen activation, the action of active matriptase on downstream substrates, and the inhibition of active matriptase by HAI-1 is so tight that these three events almost seem to take place at same time (15). The rapid inhibition of active matriptase by HAI-1 suggests that the inactivation of active matriptase by antithrombin must also occur very rapidly or there would not be the opportunity for the generation of antithrombin-matriptase complexes. The rapid inhibition of matriptase by antithrombin implies that HAI-1 may not be sufficient for the control of matriptase, which is surprising due to the fact that HAI-1 protein levels in most epithelial and carcinoma cells are much more abundant than matriptase with a molar ratio of HAI-1 relative to matriptase of greater than 10 to 1.
Two important facts would seem to make it necessary for epithelial cells to inactivate matriptase using other serine protease inhibitors than the highly abundant HAI-1. In polarized epithelial cells, matriptase is targeted to the basolateral membrane but HAI-1 is targeted to the entire cell surface (21, 69). As a result, a proportion of the HAI-1 may be maintained in different intracellular pools from matriptase in order for the differential subcellular trafficking and targeting. Furthermore, HAI-1 is also responsible for the inhibition of other serine proteases. We have shown, for example, that HAI-1 is responsible for the inhibition of active prostasin in keratinocytes (15, 47). In the stratum granulosum of the human epidermis, matriptase is even not a significant target protease for HAI-1 (14). HAI-1 may, therefore, be stretched too thin by its much broader cellular distribution and its diversified function. As a consequence, epithelial cells may require other serine protease inhibitor systems to execute the rapid inhibition of matriptase. Since high levels of antithrombin are present in the interstitial fluid or bound with the extracellular matrix, with its proximity to the basolateral plasma membrane where matriptase is activated (69), interstitial antithrombin may have direct access to and be available to readily inactivate active matriptase on the basolateral surface of ductal epithelial cells. Since the matriptase-antithrombin complex is secreted into body fluids, the formation of matriptase-antithrombin complex generated in this way should be followed by the internalization of the complex from the basolateral surface, transcytosis, and secretion from the apical surface into the ductal lumen in vivo. The functional relationship between matriptase and HAI-1 appears much more complex. HAI-1-mediated matriptase inhibition is also likely to occur on the basolateral surface of ductal epithelial cells, given that HAI-1 and matriptase are cosynthesized in the rough endoplasmic reticulum and a portion of HAI-1 undergoes intracellular trafficking along with matriptase to reach the basolateral surface (48). Since matriptase is prone to be activated and that its rapid activation could involve the entire cellular pool of matriptase, including but not limited to the matriptase on the cell surface in response to exogenous stimuli, such as during lactation or by extracellular acidosis (66), the cotrafficking of HAI-1 with matriptase may be critical for the control of undesired and/or premature intracellular matriptase proteolysis in the secretory pathway during a massive matriptase activation.
For effective inactivation of matriptase, antithrombin either from the interstitial fluids or the culture media could have bound to the surface of epithelial cells. Heparan sulfate proteoglycans (HSPGs) are likely to serve as the cell surface binding sites for antithrombin. Syndecans and glypicans are the two major cell surface HSPGs (67). For polarized epithelial cells, the basolateral plasma membrane, which is oriented toward the interstitial space, is likely the cellular location that has direct access to interstitial antithrombin. Indeed, syndecan 1 is localized at the basolateral surface of polarized epithelial cells (28). Since heparin significantly enhances the inhibitory action of antithrombin against matriptase (Figs. 6 and 7), the binding of antithrombin to cell surface HSPGs not only enhances antithrombin inhibitory potency but also brings antithrombin next to the cell surface matriptase ready for the rapid inhibition of matriptase. The cell surface antithrombin along with HAI-1 may, therefore, work in concert to inactivate cell surface matriptase in nontransformed epithelial cells. The loss of antithrombin-mediated matriptase inhibition that was seen in the majority of carcinoma cells examined in the current study indicates that these cancer cells may have lost their antithrombin binding sites on the cell surface. This loss might be the result of several different mechanisms. Reduced expression of syndecan-1 has been reported in squamous cell carcinoma of the head and neck, laryngeal cancer, and in malignant mesothelioma (24, 32, 56). Ectodomain shedding of syndecans is commonly seen in cultured cells. In cancer cells, the shedding is particularly active (42), which could cause reduced levels of syndecans on the cell surface and subsequently reduce the binding of antithrombin to cancer cells. The anticoagulant heparan sulfate contains a characteristic pentasaccharide. Aberrant modification of the HS component of HSPGs may also contribute to the loss of antithrombin-mediated matriptase inhibition. 3-O-sulfation of the central glucosamine residue of the pentasaccharide is critical for antithrombin binding (18). The sulfation is carried out by heparan sulfate 3-O-sulfotransferases (3-OSTs). Among the seven isoforms, 3-OST-2 is downregulated in many breast cancer cells, including T-47D and MCF7 via methylation-associated gene silencing (45).
Although the physiological relevance of acid-induced matriptase activation using a pH 6.0 buffer is arguable, we have previously demonstrated that acid-induced matriptase activation can be initiated by exposure to pH 6.8 (66), a condition consistent with that found with some physiological and pathological processes (17, 19, 22, 31, 44, 59, 60). We have no reason to believe that the matriptase activation induced by a pH 6.0 buffer is qualitatively different from that induced by more neutral pHs, and this approach does provide an excellent tool for the study of the HAI-1 independent mechanisms for matriptase inhibition. This is due to the very high levels of matriptase activation induced under these conditions and the ubiquity of activation among matriptase-expressing cells (66). This may in turn result from the enhanced proteolytic activity of matriptase zymogen under mildly acidic conditions (25) and the rapid intracellular acidification resulting from the acidic extracellular environment (66). Furthermore, the end products of this acid-induced matriptase activation, which consists of the matriptase-HAI-1 complex as the predominant form of activated matriptase and matriptase-antithrombin complex as a minor form, recapitulates what has been seen in human milk (35, 66). While matriptase activation can also be induced by the lysophospholipid sphingosine 1-phosphate (S1P) and the male steroid hormones androgens, both of these activation inducers are cell-type specific. S1P-induced matriptase activation was seen only in mammary epithelial cells and not in breast cancer cells (4–6), whereas androgens can induce matriptase activation only in prostate cancer cells via the androgen receptor (30). More importantly, the levels of matriptase activation induced by S1P and androgens are much lower than that produced using a pH 6 buffer. Since the matriptase-antithrombin complex only represents a small proportion of the total activated matriptase, both in body fluids and in cultured cells, it would be difficult to reliably detect matriptase-antithrombin complex formation using these two cell-type specific models of matriptase activation.
While we have demonstrated that matriptase activation is rapidly and efficiently inhibited by HAI-1 and antithrombin, free-active matriptase has been detected in the conditioned medium of cancer cells in several of our previous studies (23, 37, 58), which might seem to be at odds with our current findings. The free-active enzyme observed in our earlier studies, however, was detected based on the proteolytic activity of matriptase visualized using gelatin zymography. In one of our previous studies (4), we demonstrated that the gelatinolytic activity of free-active matriptase can be detected at very low enzyme levels; so low that the active matriptase could not be detected by immunoblot using a sensitive matriptase mAb. Furthermore, using a unique mAb specific for the activated form of matriptase, we have demonstrated that the vast majority of activated matriptase present in the conditioned media of breast cancer cells is in the form of complexes with HAI-1, with free-active matriptase being below the level of detection (6). Thus the free-active matriptase detected in our previous studies only represents an extremely small proportion of the total activated matriptase shed into the extracellular milieu. The shedding of free-active matriptase is still possible, given the fact that we are able to detect free-active matriptase by gelatin zymography or in assays using synthetic fluorescent matriptase substrates, when exposure to pH 6.0 buffer is used to induce matriptase activation (data not shown). However, the vast majority of the activated matriptase is found in complexes with HAI-1 in cell lysates with minimal levels of free-active matriptase being shed. Thus, although the detection of free-active matriptase in the conditioned medium could be at odds with our hypothesis that matriptase activation is rapidly followed by HAI-1- and antithrombin-mediated inhibition, much of this apparent discrepancy results from the differential sensitivity between assays for total activated matriptase by immunoblot analysis and the detection of proteolytic activity by gelatinolytic activity. The latter shows only the presence of free-active matriptase and not the ratio of the free-active matriptase to total activated matriptase. The presence of a trace amount of free-active matriptase is also supported by our previous studies in which free-active matriptase was not detectable by diagonal gel electrophoresis in which activated matriptase can be converted into two fragments by chemical reduction (4). Instead, the vast majority of 70-kDa matriptase shed into the conditioned medium by breast cancer cells is single-chain matriptase zymogen (4). Furthermore, although these trace amounts of free-active matriptase might be able to act on some known substrates, such as PAR2 (10), the vast majority of activated matriptase is more likely to have activated/processed downstream substrates before its inhibition by HAI-1 or antithrombin. This hypothesis is supported by our previous study in which the processes of matriptase autoactivation, activation of prostasin by the active matriptase, and then inhibition of both matriptase and prostasin by HAI-1 occur at virtually the same time (15).
In summary, we present in the current study evidence that epithelial cells utilize antithrombin as a mechanism to control matriptase. This antithrombin-mediated matriptase inhibition might take place at the basolateral plasma membrane of polarized epithelial cells in vivo, such as in lactating mammary epithelial cells. The resultant matriptase-antithrombin complex is then internalized, transcytosized, and secreted from the apical surface of the cells. This physiological mechanism is apparently lost in many carcinoma cells. It will be important to determine how the loss of antithrombin-mediated matriptase inhibition affects the activity of matriptase and its impact on cancer cells in the future.
GRANTS
This study was supported by National Institutes of Health (NIH) R01CA123223 (to M. D. Johnson and C.-Y. Lin), by NIH R37HL-39888 (to S. T. Olson), and by the Maryland Cigarette Restitution Fund Program (to C.-Y. Lin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Alef T, Torres S, Hausser I, Metze D, Tursen U, Lestringant GG, Hennies HC. Ichthyosis, follicular atrophoderma, and hypotrichosis caused by mutations in ST14 is associated with impaired profilaggrin processing. J Invest Dermatol 129: 862–869, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Avrahami L, Maas S, Pasmanik-Chor M, Rainshtein L, Magal N, Smitt J, van MJ, Shohat M, Basel-Vanagaite L. Autosomal recessive ichthyosis with hypotrichosis syndrome: further delineation of the phenotype. Clin Genet 74: 47–53, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, Rainshtein L, Ben AD, Lurie R, Pasmanik-Chor M, Indelman M, Zvulunov A, Saban S, Magal N, Sprecher E, Shohat M. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet 80: 467–477, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benaud C, Dickson RB, Lin CY. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem 268: 1439–1447, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Benaud C, Oberst M, Hobson JP, Spiegel S, Dickson RB, Lin CY. Sphingosine 1-phosphate, present in serum-derived lipoproteins, activates matriptase. J Biol Chem 277: 10539–10546, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Benaud CM, Oberst M, Dickson RB, Lin CY. Deregulated activation of matriptase in breast cancer cells. Clin Exp Metastasis 19: 639–649, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Bhatt AS, Welm A, Farady CJ, Vasquez M, Wilson K, Craik CS. Coordinate expression and functional profiling identify an extracellular proteolytic signaling pathway. Proc Natl Acad Sci USA 104: 5771–5776, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bjork I, Olson ST. Antithrombin. A bloody important serpin. Adv Exp Med Biol 425: 17–33, 1997 [PubMed] [Google Scholar]
- 9. Calugaru SV, Swanson R, Olson ST. The pH dependence of serpin-proteinase complex dissociation reveals a mechanism of complex stabilization involving inactive and active conformational states of the proteinase which are perturbable by calcium. J Biol Chem 276: 32446–32455, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Camerer E, Barker A, Duong DN, Ganesan R, Kataoka H, Cornelissen I, Darragh MR, Hussain A, Zheng YW, Srinivasan Y, Brown C, Xu SM, Regard JB, Lin CY, Craik CS, Kirchhofer D, Coughlin SR. Local protease signaling contributes to neural tube closure in the mouse embryo. Dev Cell 18: 25–38, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carlson TH, Atencio AC, Simon TL. In vivo behavior of radioiodinated rabbit antithrombin III. Demonstration of a noncirculating vascular compartment. J Clin Invest 74: 191–199, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carlson TH, Simon TL, Atencio AC. In vivo behavior of human radioiodinated antithrombin III: distribution among three physiologic pools. Blood 66: 13–19, 1985 [PubMed] [Google Scholar]
- 13. Carney TJ, von der HS, Sonntag C, Amsterdam A, Topczewski J, Hopkins N, Hammerschmidt M. Inactivation of serine protease Matriptase1a by its inhibitor Hai1 is required for epithelial integrity of the zebrafish epidermis. Development 134: 3461–3471, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Chen CJ, Wu BY, Tsao PI, Chen CY, Wu MH, Chan YL, Lee HS, Johnson MD, Eckert RL, Chen YW, Chou F, Wang JK, Lin CY. Increased matriptase zymogen activation in inflammatory skin disorders. Am J Physiol Cell Physiol 300: C406–C415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen YW, Wang JK, Chou FP, Chen CY, Rorke EA, Chen LM, Chai KX, Eckert RL, Johnson MD, Lin CY. Regulation of the matriptase-prostasin cell surface proteolytic cascade by hepatocyte growth factor activator inhibitor-1 (HAI-1) during epidermal differentiation. J Biol Chem 285: 31755–31762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng MF, Jin JS, Wu HW, Chiang PC, Sheu LF, Lee HS. Matriptase expression in the normal and neoplastic mast cells. Eur J Dermatol 17: 375–380, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Chernew I, Braude AI. Depression of phagocytosis by solutes in concentrations found in the kidney and urine. J Clin Invest 41: 1945–1953, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desai UR, Petitou M, Bjork I, Olson ST. Mechanism of heparin activation of antithrombin. Role of individual residues of the pentasaccharide activating sequence in the recognition of native and activated states of antithrombin. J Biol Chem 273: 7478–7487, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Edlow DW, Sheldon WH. The pH of inflammatory exudates. Proc Soc Exp Biol Med 137: 1328–1332, 1971 [DOI] [PubMed] [Google Scholar]
- 20. Fang JD, Chou HC, Tung HH, Huang PY, Lee SL. Endogenous expression of matriptase in neural progenitor cells promotes cell migration and neuron differentiation. J Biol Chem 286: 5667–5679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Godiksen S, Selzer-Plon J, Pedersen ED, Abell K, Rasmussen HB, Szabo R, Bugge TH, Vogel LK. Hepatocyte growth factor activator inhibitor-1 has a complex subcellular itinerary. Biochem J 413: 251–259, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol 121: 345–353, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Ihara S, Miyoshi E, Ko JH, Murata K, Nakahara S, Honke K, Dickson RB, Lin CY, Taniguchi N. Prometastatic effect of N-acetylglucosaminyltransferase V is due to modification and stabilization of active matriptase by adding beta 1–6 GlcNAc branching. J Biol Chem 277: 16960–16967, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Inki P, Joensuu H, Grenman R, Klemi P, Jalkanen M. Association between syndecan-1 expression and clinical outcome in squamous cell carcinoma of the head and neck. Br J Cancer 70: 319–323, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inouye K, Yasumoto M, Tsuzuki S, Mochida S, Fushiki T. The optimal activity of a pseudozymogen form of recombinant matriptase under the mildly acidic ph and low ionic strength conditions. J Biochem 147: 485–492, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Ishiguro K, Kojima T, Kadomatsu K, Nakayama Y, Takagi A, Suzuki M, Takeda N, Ito M, Yamamoto K, Matsushita T, Kusugami K, Muramatsu T, Saito H. Complete antithrombin deficiency in mice results in embryonic lethality. J Clin Invest 106: 873–878, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kataoka H, Suganuma T, Shimomura T, Itoh H, Kitamura N, Nabeshima K, Koono M. Distribution of hepatocyte growth factor activator inhibitor type 1 (HAI-1) in human tissues. Cellular surface localization of HAI-1 in simple columnar epithelium and its modulated expression in injured and regenerative tissues. J Histochem Cytochem 47: 673–682, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Kato M, Saunders S, Nguyen H, Bernfield M. Loss of cell surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchyme-like cells. Mol Biol Cell 6: 559–576, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kilpatrick LM, Harris RL, Owen KA, Bass R, Ghorayeb C, Bar-Or A, Ellis V. Initiation of plasminogen activation on the surface of monocytes expressing the type II transmembrane serine protease matriptase. Blood 108: 2616–2623, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Kiyomiya KI, Lee MS, Tseng IC, Zuo H, Barndt RJ, Johnson MD, Dickson RB, Lin CY. Matriptase activation and subsequent shedding with HAI-1 is induced by steroid sex hormones in human prostate cancer cells but not in breast cancer cells. Am J Physiol Cell Physiol 291: C40–C49, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Korting HC, Hubner K, Greiner K, Hamm G, Braun-Falco O. Differences in the skin surface pH and bacterial microflora due to the long-term application of synthetic detergent preparations of pH 55 and pH 70. Results of a crossover trial in healthy volunteers. Acta Derm Venereol 70: 429–431, 1990 [PubMed] [Google Scholar]
- 32. Kumar-Singh S, Jacobs W, Dhaene K, Weyn B, Bogers J, Weyler J, Van ME. Syndecan-1 expression in malignant mesothelioma: correlation with cell differentiation, WT1 expression, and clinical outcome. J Pathol 186: 300–305, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Lee MS, Tseng IC, Wang Y, Kiyomiya K, Johnson MD, Dickson RB, Lin CY. Autoactivation of matriptase in vitro: requirement for biomembrane and LDL receptor domain. Am J Physiol Cell Physiol 293: C95–C105, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Lee SL, Dickson RB, Lin CY. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem 275: 36720–36725, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Lin CY, Anders J, Johnson M, Dickson RB. Purification and characterization of a complex containing matriptase and a Kunitz-type serine protease inhibitor from human milk. J Biol Chem 274: 18237–18242, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Lin CY, Tseng IC, Chou FP, Su SF, Chen YW, Johnson MD, Dickson RB. Zymogen activation, inhibition, and ectodomain shedding of matriptase. Front Biosci 13: 621–635, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Lin CY, Wang JK, Torri J, Dou L, Sang QA, Dickson RB. Characterization of a novel, membrane-bound, 80-kDa matrix-degrading protease from human breast cancer cells. Monoclonal antibody production, isolation, and localization. J Biol Chem 272: 9147–9152, 1997 [PubMed] [Google Scholar]
- 38. List K, Currie B, Scharschmidt TC, Szabo R, Shireman J, Molinolo A, Cravatt BF, Segre J, Bugge TH. Autosomal ichthyosis with hypotrichosis syndrome displays low matriptase proteolytic activity and is phenocopied in ST14 hypomorphic mice. J Biol Chem 282: 36714–36723, 2007 [DOI] [PubMed] [Google Scholar]
- 39. List K, Hobson JP, Molinolo A, Bugge TH. Co-localization of the channel activating protease prostasin/(CAP1/PRSS8) with its candidate activator, matriptase. J Cell Physiol 213: 237–245, 2007 [DOI] [PubMed] [Google Scholar]
- 40. List K, Kosa P, Szabo R, Bey AL, Wang CB, Molinolo A, Bugge TH. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. Am J Pathol 175: 1453–1463, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, Burke B, Nielsen BS, Gutkind JS, Bugge TH. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev 19: 1934–1950, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J 277: 3876–3889, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Mathias JR, Dodd ME, Walters KB, Rhodes J, Kanki JP, Look AT, Huttenlocher A. Live imaging of chronic inflammation caused by mutation of zebrafish Hai1. J Cell Sci 120: 3372–3383, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Mauro T, Holleran WM, Grayson S, Gao WN, Man MQ, Kriehuber E, Behne M, Feingold KR, Elias PM. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch Dermatol Res 290: 215–222, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Miyamoto K, Asada K, Fukutomi T, Okochi E, Yagi Y, Hasegawa T, Asahara T, Sugimura T, Ushijima T. Methylation-associated silencing of heparan sulfate D-glucosaminyl 3-O-sulfotransferase-2 (3-OST-2) in human breast, colon, lung and pancreatic cancers. Oncogene 22: 274–280, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Nagaike K, Kawaguchi M, Takeda N, Fukushima T, Sawaguchi A, Kohama K, Setoyama M, Kataoka H. Defect of hepatocyte growth factor activator inhibitor Type 1/serine protease inhibitor, Kunitz type 1 (Hai-1/Spint1) leads to ichthyosis-like condition and abnormal hair development in mice. Am J Pathol 173: 1464–1475, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Netzel-Arnett S, Currie BM, Szabo R, Lin CY, Chen LM, Chai KX, Antalis TM, Bugge TH, List K. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem 281: 32941–32945, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Oberst MD, Chen LY, Kiyomiya KI, Williams CA, Lee MS, Johnson MD, Dickson RB, Lin CY. Hepatocyte growth factor activator inhibitor 1 (HAI-1) regulates activation and expression of matriptase, a membrane-bound serine protease. Am J Physiol Cell Physiol 289: C462–C470, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Oberst MD, Johnson MD, Dickson RB, Lin CY, Singh B, Stewart M, Williams A, al Nafussi A, Smyth JF, Gabra H, Sellar GC. Expression of the serine protease matriptase and its inhibitor HAI-1 in epithelial ovarian cancer: correlation with clinical outcome and tumor clinicopathological parameters. Clin Cancer Res 8: 1101–1107, 2002 [PubMed] [Google Scholar]
- 50. Oberst MD, Singh B, Ossandon M, Dickson RB, Johnson MD, Lin CY. Characterization of matriptase expression in normal human tissues. J Histochem Cytochem 51: 1017–1025, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Olson ST, Bjork I. Regulation of thrombin activity by antithrombin and heparin. Semin Thromb Hemost 20: 373–409, 1994 [DOI] [PubMed] [Google Scholar]
- 52. Olson ST, Bjork I, Shore JD. Kinetic characterization of heparin-catalyzed and uncatalyzed inhibition of blood coagulation proteinases by antithrombin. Methods Enzymol 222: 525–559, 1993 [DOI] [PubMed] [Google Scholar]
- 53. Olson ST, Chuang YJ. Heparin activates antithrombin anticoagulant function by generating new interaction sites (exosites) for blood clotting proteinases. Trends Cardiovasc Med 12: 331–338, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Olson ST, Swanson R, Raub-Segall E, Bedsted T, Sadri M, Petitou M, Herault JP, Herbert JM, Bjork I. Accelerating ability of synthetic oligosaccharides on antithrombin inhibition of proteinases of the clotting and fibrinolytic systems. Comparison with heparin and low-molecular-weight heparin. Thromb Haemost 92: 929–939, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Petersen LC, Karpf DM, Agerso H, Hermit MB, Pelzer H, Persson E, Nichols TC, Ezban M. Intravascular inhibition of factor VIIa and the analogue NN1731 by antithrombin. Br J Haematol 152: 99–107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pulkkinen JO, Penttinen M, Jalkanen M, Klemi P, Grenman R. Syndecan-1: a new prognostic marker in laryngeal cancer. Acta Otolaryngol (Stockh) 117: 312–315, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Saleem M, Adhami VM, Zhong W, Longley BJ, Lin CY, Dickson RB, Reagan-Shaw S, Jarrard DF, Mukhtar H. A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev 15: 217–227, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Shi YE, Torri J, Yieh L, Wellstein A, Lippman ME, Dickson RB. Identification and characterization of a novel matrix-degrading protease from hormone-dependent human breast cancer cells. Cancer Res 53: 1409–1415, 1993 [PubMed] [Google Scholar]
- 59. Shum WW, Da SN, Brown D, Breton S. Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. J Exp Biol 212: 1753–1761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Simmen HP, Battaglia H, Giovanoli P, Blaser J. Analysis of pH, pO2 and pCO2 in drainage fluid allows for rapid detection of infectious complications during the follow-up period after abdominal surgery. Infection 22: 386–389, 1994 [DOI] [PubMed] [Google Scholar]
- 61. Szabo R, Hobson JP, Christoph K, Kosa P, List K, Bugge TH. Regulation of cell surface protease matriptase by HAI2 is essential for placental development, neural tube closure and embryonic survival in mice. Development 136: 2653–2663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Szabo R, Kosa P, List K, Bugge TH. Loss of matriptase suppression underlies spint1 mutation-associated ichthyosis and postnatal lethality. Am J Pathol 174: 2015–2022, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Szabo R, Molinolo A, List K, Bugge TH. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene 26: 1546–1556, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Szabo R, Wu Q, Dickson RB, Netzel-Arnett S, Antalis TM, Bugge TH. Type II transmembrane serine proteases. Thromb Haemost 90: 185–193, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Tseng IC, Chou FP, Su SF, Oberst M, Madayiputhiya N, Lee MS, Wang JK, Sloane DE, Johnson M, Lin CY. Purification from human milk of matriptase complexes with secreted serpins: mechanism for inhibition of matriptase other than HAI-1. Am J Physiol Cell Physiol 295: C423–C431, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tseng IC, Xu H, Chou FP, Li G, Vazzano AP, Kao JP, Johnson MD, Lin CY. Matriptase activation, an early cellular response to acidosis. J Biol Chem 285: 3261–3270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tumova S, Woods A, Couchman JR. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int J Biochem Cell Biol 32: 269–288, 2000 [DOI] [PubMed] [Google Scholar]
- 68. Vogel LK, Saebo M, Skjelbred CF, Abell K, Pedersen ED, Vogel U, Kure EH. The ratio of Matriptase/HAI-1 mRNA is higher in colorectal cancer adenomas and carcinomas than corresponding tissue from control individuals. BMC Cancer 6: 176–183, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang JK, Lee MS, Tseng IC, Chou FP, Chen YW, Fulton A, Lee HS, Chen CJ, Johnson MD, Lin CY. Polarized epithelial cells secrete matriptase as a consequence of zymogen activation and HAI-1-mediated inhibition. Am J Physiol Cell Physiol 297: C459–C470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Warren M, Twohig M, Pier T, Eickhoff J, Lin CY, Jarrard D, Huang W. Protein expression of matriptase and its cognate inhibitor HAI-1 in human prostate cancer: a tissue microarray and automated quantitative analysis. Appl Immunohistochem Mol Morphol 17: 23–30, 2008 [DOI] [PubMed] [Google Scholar]
- 71. Xu Y, Slayter HS. Immunocytochemical localization of endogenous anti-thrombin III in the vasculature of rat tissues reveals locations of anticoagulantly active heparan sulfate proteoglycans. J Histochem Cytochem 42: 1365–1376, 1994 [DOI] [PubMed] [Google Scholar]