Abstract
G protein-coupled estrogen receptor 1 (GPER), also named GPR30, has been previously identified in the female reproductive system. In this study, GPER expression was found in the female rat myometrium by reverse transcriptase-polymerase chain reaction and immunocytochemistry. Using GPER-selective ligands, we assessed the effects of the GPER activation on resting membrane potential and cytosolic Ca2+ concentration ([Ca2+]i) in rat myometrial cells, as well as on contractility of rat uterine strips. G-1, a specific GPER agonist, induced a concentration-dependent depolarization and increase in [Ca2+]i in myometrial cells. The depolarization was abolished in Na+-free saline. G-1-induced [Ca2+]i increase was markedly decreased by nifedipine, a L-type Ca2+ channel blocker, by Ca2+-free or Na+-free saline. Intracellular administration of G-1 produced a faster and transitory increase in [Ca2+]i, with a higher amplitude than that induced by extracellular application, supporting an intracellular localization of the functional GPER in myometrial cells. Depletion of internal Ca2+ stores with thapsigargin produced a robust store-activated Ca2+ entry; the Ca2+ response to G-1 was similar to the constitutive Ca2+ entry and did not seem to involve store-operated Ca2+ entry. In rat uterine strips, administration of G-1 increased the frequency and amplitude of contractions and the area under the contractility curve. The effects of G-1 on membrane potential, [Ca2+]i, and uterine contractility were prevented by pretreatment with G-15, a GPER antagonist, further supporting the involvement of GPER in these responses. Taken together, our results indicate that GPER is expressed and functional in rat myometrium. GPER activation produces depolarization, elevates [Ca2+]i and increases contractility in myometrial cells.
Keywords: calcium imaging, membrane potential, uterine contractility
estrogens are steroid hormones with a critical role in reproduction. The effects of 17 β-estradiol, the most bioactive estrogen, are mediated by nuclear estrogen receptors (ER) α (ERα) and β (ERβ) and by the newly identified G protein-coupled estrogen receptor 1 (GPER), also named GPR30 (18, 39, 44). Several reports indicate the involvement of GPER in breast, ovarian, and uterine cancer (1, 2, 16, 17, 23, 44, 45), while the physiological role of GPER in the reproductive system is largely unknown.
In the ovary, GPER is expressed during perinatal development and mediates estrogen action on primordial follicle (47). Nongenomic actions of steroids were reported earlier in myometrium (34). However, whether or not GPER is involved in myometrial functions remains to be clarified. The present study was undertaken to investigate the effects of GPER activation on membrane potential, cytosolic Ca2+ concentration ([Ca2+]i) and contractility on rat myometrium, using the selective GPER agonist G-1 (4) and antagonist G-15 (14).
MATERIALS AND METHODS
Primary Culture of Myometrial Cells
Primary myometrial cells were prepared from rat uterus according to the procedure described by Krizsan-Agbas et al. (26) with some modifications (13). Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee. Adult (nonpregnant) female Sprague-Dawley rats (Ace Animals, Boyertown, PA) were anesthetized with urethane (1.2 g/kg ip); uterine horns were immediately excised and immersed in cold Hanks balanced salt solution (HBSS). The myometrium was rapidly separated from endometrium and stroma and then minced into fragments (about 1 mm3). Tissues were digested for 1.5 h at 37°C by collagenase type II (600 U/ml, Sigma-Aldrich, St. Louis, MO) in DMEM/F12 culture medium, followed by trituration with a sterile Pasteur pipette and filtration through a cell strainer with 70-μm pores. After collection, the cells were washed twice by centrifugation (1,000 RPM, 10 min) and resuspended in fresh DMEM/F12 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin and then plated on collagen-coated coverslips. Cultures were maintained at 37°C in a humidified atmosphere with 5% CO2; the medium was changed every 2–3 days. Myometrial cells were used for calcium imaging experiments after 3–5 days in culture.
Reverse Transcriptase-Polymerase Chain Reaction
Total RNA from rat myometrium was isolated using TRIzol reagent according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). The purity and concentration of RNA were assessed by spectrophotometrical analysis. First-strand cDNA was synthesized from 3 μg of total RNA with random hexamer primers and the SuperScript III reverse transcriptase according to the manufacturer's protocol (Invitrogen). Three microliters of 20 μl cDNA product were amplified with the following specific primers based on GPER (NM_133573) and β-actin (NM_031144) sequences: GPER: 5′-TCTGTGACCCTTTCAGCAAGTCCT-3′ (upstream), 5′-AGAAGGCTGCCAGGTTGACTATGT-3′ (downstream); β-actin: 5′-CCATTGAACACGGCATTGTCACCA-3′ (upstream), 5′-ACTCCTGCTTGCTGATCCACATCT-3′ (downstream). Amplifications were performed using Platinum Taq DNA polymerase (Invitrogen) as follows. GPER: 38 cycles of 94°C for 60 s, 58°C for 60 s, and 72°C for 60 s; β-actin: 30 cycles of 94°C for 30 s, 55°C for 45 s, and 72°C for 60 s. The polymerase chain reaction products (987 bp and 876 bp, respectively) were visualized by an ethidium bromide staining under UV light following electrophoresis on 1% agarose gel.
Immunocytochemistry
Myometrial cells on coverslips were fixed in 4% paraformaldehyde for 20 min. After being blocked with normal donkey serum (1:50 in PBS, 0.5% BSA, 0.4% Triton X-100), the cells were incubated with GPER/GPR30 antibody (1:200 dilution) as previously described (5). GPER antibody was a rabbit polyclonal antibody directed against the human COOH-terminus (MBL International, Woburn, MA). For control experiments, the antibody was incubated with a 10× excess of peptide at 4°C overnight. After several washes with PBS, cells were incubated with rabbit IgG Texas Red (1:30, Vector Laboratories, Burlingame, CA). The cell nuclei were stained by mounting with DAPI-Fluoromount-G (Southern Biotech, Birmingham, AL). Sections were examined under a confocal scanning laser microscope (Leica TCS SL) with excitation wavelengths set to 405 nm for DAPI and 561 nm for Texas Red, in the sequential mode.
Optical Imaging Using DiBAC4(3)
Bis-oxonol (bis-[1,3-dibutylbarbituric acid] trimethineoxonol) [DiBAC4(3)], a slow-response voltage-sensitive fluorescent dye, has been successfully used to assess relative changes in membrane potential of single cells (15). The method was similar to that previously described (8, 9). Briefly, myometrial cells were incubated for 30 min in HBSS containing 0.5 mM DiBAC4(3). The fluorescence (excitation wavelength = 480 nm, emission wavelength = 540 nm) was continuously recorded at a rate of 10 points/min. Background values (windows of identical area placed beside the cells) were subtracted. The dye partition between the cell membrane and the cytosol is a function of membrane potential. Depolarization of the membrane leads to a sequestration of the dye into cytosol and is associated with an increase in fluorescence intensity; whereas the dye concentrates in the cell membrane during hyperpolarization, leading to a decrease of fluorescence intensity in cytosol (11). Calibration of DiBAC4(3) fluorescence was performed using the Na+-K+ ionophore gramicidin in Na+-free physiological solution (11). In experiments with ionic substitution, Na+ was replaced with N-methyl-d-glucamine.
Calcium Imaging
Intracellular Ca2+ measurements were performed as previously described (5, 7, 10, 13). Briefly, cells were incubated with 5 μM fura-2 AM (Invitrogen) in HBSS at room temperature for 45 min, in the dark, washed three times with dye-free buffer, and then incubated for another 45 min to allow for complete deesterification of the dye. Coverslips were subsequently mounted in a custom-designed bath on the stage of an Eclipse TE 2000-U Nikon microscope equipped with a Roper Scientific CCD camera (Optical Apparatus, Ardmore, PA) or on a stage of a Nikon Eclipse TiE microscope equipped with a Perfect Focus System and a Photometrics CoolSnap HQ2 CCD camera (Optical Apparatus). Fura-2 AM fluorescence (emission = 510 nm), following alternate excitation at 340 and 380 nm, was acquired at a frequency of 0.33 Hz from regions of interest located on cytoplasm. Data were acquired and analyzed using a Metafluor or NIS-Elements AR 3.1 software (Optical Apparatus). The ratio of the fluorescence signals (340/380 nm) was converted to Ca2+ concentrations (21). In Ca2+-free experiments, CaCl2 was omitted and 2.5 mM EGTA was added.
Intracellular Microinjection Of Uterine Smooth Muscle Cells
Injections were performed using Femtotips II, InjectMan NI2, and FemtoJet systems (Eppendorf) as previously reported (7, 10, 13). Pipettes were back filled with an intracellular solution composed of 110 mM KCl, 10 mM NaCl, and 20 mM HEPES (pH 7.2) (22) with or without G-1. The injection time was 0.4 s at 60 hPa with a compensation pressure of 20 hPa to maintain the microinjected volume to <1% of cell volume, as measured by microinjection of a fluorescent compound (Fura-2 free acid) (22). The intracellular concentration of G-1 was determined based on the G-1 concentration in the pipette and the volume of injection.
Tissue Preparation and Tension Measurement
Uterine strips were prepared from nonpregnant Sprague-Dawley female rats according to the procedures described by Shintani et al. (42). The middle part of the uterine horn was excised in a longitudinal direction and cut into strips measuring 2 mm in width and 5 mm in length. All measurements were performed at 37°C, in Krebs solution with the following composition (in mM): 127 NaCl, 1.9 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgCl2, 26 NaHCO3, and 5 glucose, saturated with 95% O2-5% CO2.
The uterine strips were mounted vertically in a 5-ml organ bath using a force transducer (ML T0201/RAD; ADInstruments, Colorado Springs, CO) coupled to a Quad Bridge Amplifier (ADInstruments). Contractions were recorded using a PowerLab system and Chart 5 software (ADInstruments). The resting load was adjusted to ∼0.5 g to obtain a maximal response to 40 mm K+. The contractile response was analyzed by using three parameters: 1) area under the contractility curve was determined as the integrated force from the start of the induced contraction up to 10 min following the application of the compound; 2) frequency of myometrial contractions was determined as the number of contractions occurring during a 10-min period before and after the application of the compound; and 3) amplitude of myometrial contractions was defined as the distance between the peak and the initial baseline of the contractions in a period of 10 min before and after the application of the compound.
Statistical Analysis
One-way ANOVA or two-way ANOVA were used; a P value of <0.05 was considered statistically significant.
RESULTS
GPER Expression and Immunoreactivity in Rat Myometrium
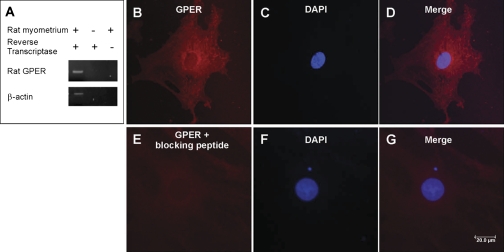
GPER mRNA was identified using reverse transcriptase-polymerase chain reaction assay as a 987 bp product in rat myometrium; β-actin (876 bp) was the internal control (Fig. 1A). Using immunocytochemical method and a GPER-specific antibody, GPER was detected in myometrial cells as red immunofluorescence in the cytosol (Fig. 1, B–D). Very weak or no immunoreactivity was present in cells incubated with the GPER antibody preabsorbed with the GPER blocking peptide, supporting the specificity of the immunostaining (Fig. 1, E–G).
Fig. 1.
G protein-coupled estrogen receptor (GPER) expression and immunoreactivity in rat myometrium. A: rat G protein estrogen receptor (GPER) mRNA was identified in rat myometrium by reverse transcriptase-polymerase chain reaction as a 987 bp band; housekeeping gene β-actin was used as an internal control. B–D: GPER immunoreactivity (red) was detected in rat myometrial cells using an antibody against human COOH terminus; cell nuclei were identified with DAPI (blue), E and F: no immunoreactivity was present in cells incubated with the antibody preabsorbed with the blocking peptide. Scale bar, 20 μm.
G-1 Depolarizes Myometrial Cells
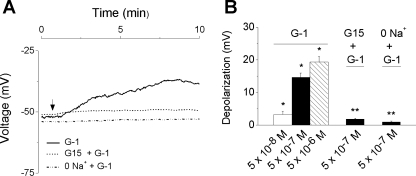
The resting membrane potential of rat myometrial cells, monitored by the voltage-sensitive dye DiBAC4(3), was −51 ± 1.8 mV (n = 74). This value is similar to that measured from uterine strips by classical electrophysiological techniques (27). Extracellular administration of G-1 (5 × 10−8 M, 5 × 10−7 M, and 5 × 10−6 M) induced a concentration-dependent membrane depolarization with a mean amplitude of 3.2 ± 0.9 (n = 12), 14.6 ± 1.3 (n = 19), and 19.2 ± 1.7 mV (n = 7), respectively (Fig. 2). The depolarization induced by G-1 (5 × 10−7 M) was prevented by pretreatment with G-15 (5 × 10−6 M, 15 min) (n = 14) (Fig. 2). G-1-induced depolarization was abolished when the cells were incubated in a Na+-free solution (n = 16), indicating the involvement of Na+ influx in G-1-induced myometrial depolarization (Fig. 2).
Fig. 2.
GPER-mediated depolarization of rat myometrial cells. A: administration of specific GPER agonist G-1 (5 × 10−7 M) produced a depolarization (solid line) that was abolished by pretreatment with the GPER antagonist G-15 (5 × 10−6 M, 15 min, dotted line) or in Na+-free saline (dashed line). The changes in voltage were assessed in cells loaded with DiBAC4(3) [bis-oxonol (bis-[1,3-dibutylbarbituric acid] trimethineoxonol)]. B: G-1 (5 × 10−8 M, 5 × 10−7 M, and 5 × 10−6 M) induced a concentration-dependent membrane depolarization by 3.2 ± 0.9 (n = 12 cells), 14.6 ± 1.3 (n = 19 cells), and 19.2 ± 1.7 mV (n = 7 cells), respectively; pretreatment with G-15 (5 × 10−6 M) or Na+-free saline reduced the response to G-1 to 1.8 ± 0.3 mV (n = 14 cells) and 0.9 ± 0.3 mV (n = 16 cells), respectively. The amplitude of depolarization has been measured on the plateau of the response 7–8 min after the administration of the compound. *Significant difference compared with the resting membrane potential; **significant difference compared with the effect of G-1 (5 × 10−7 M) (P < 0.05).
GPER Activation Increases [Ca2+]i in Myometrial Cells
Extracellular administration of G-1 elevates cytosolic Ca2+ in a concentration-dependent manner.
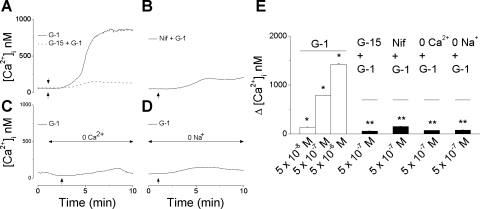
The mean basal [Ca2+]i of rat myometrial cells was 68 ± 1.7 nM (n = 167 cells). Extracellular administration of G-1 (5 × 10−8 M, 5 × 10−7 M, and 5 × 10−6 M) induced a concentration-dependent increase in [Ca2+]i with a mean amplitude of 137 ± 1.6 nM (n = 9 cells), 783 ± 4.2 nM (n = 26 cells), and 1,421 ± 7.3 nM (n = 12 cells), respectively (Fig. 3, A and E). G-1-induced increase in [Ca2+]i occurred slowly, starting 1–2 min after administration of G-1, and reached a plateau after 6–7 min; a representative example is shown in Fig. 3A. The increase in [Ca2+]i produced by G-1 (5 × 10−7 M) was largely abolished (59 ± 4 nM) by pretreatment with G-15 (5 × 10−6 M, 15 min), a GPER antagonist (10) (Fig. 3, A and E). In the presence of nifedipine, a L-type Ca2+ channel blocker (10−6 M, 10 min pretreatment), application of G-1 (5 × 10−7 M) increased [Ca2+]i only by 152 ± 2.6 nM (n = 18 cells) (Fig. 3, B and E). The effect was significantly reduced compared with G-1 alone (P < 0.5), indicating the involvement of Ca2+ entry through plasmalemmal Ca2+ channels. Indeed, in Ca2+-free saline, administration of G-1 (5 × 10−7 M) elevates [Ca2+]i only by 74 ± 0.9 nM (n = 11 cells) (Fig. 3, C and E). Similarly, in Na+-free saline, G-1 (5 × 10−7 M) increases [Ca2+]i only by 81 ± 3 nM (n = 15 cells) (Fig. 3, D and E).
Fig. 3.
GPER-mediated cytosolic Ca2+ concentration ([Ca2+]i) increase in rat myometrial cells. A: G-1 (5 × 10−7 M) induced a robust increase in [Ca2+]i (solid line) in fura-2 AM-loaded myometrial cells, which was markedly diminished by pretreatment with G-15 (5 × 10−6 M, 15 min), nifedipine (10−6 M, 10 min pretreatment) (B), Ca2+-free saline (C), or Na+-free saline (D) (n = 12–25 cells for each condition). E: extracellular administration of G-1 (5 × 10−8 M, 5 × 10−7 M, and 5 × 10−6 M) induced a concentration-dependent increase in [Ca2+]i by (n = 47 cells); the amplitude of [Ca2+]i was measured on the plateau of the response, 6–7 min after the administration of the agonist. *Significant difference compared with basal [Ca2+]i; **significant difference compared with the effect of G-1 (5 × 10−7 M) in regular saline (P < 0.05).
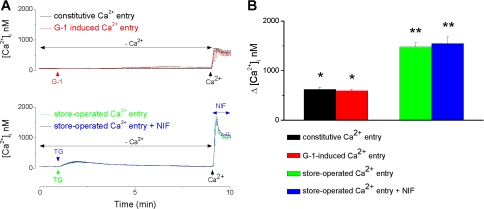
We also assessed the participation of store-operated calcium entry (SOCE) in the response to G-1. Addition of 2 mM Ca2+ after 10 min in Ca2+-free saline elicited a constitutive Ca2+ entry with an amplitude of 625 ± 44 nM (n = 23 cells); in the same conditions, G-1 (5 × 10−7 M) produced an increase in [Ca2+]i by 593 ± 36 nM (n = 31 cells), which was not statistically different from the constitutive Ca2+ entry (Fig. 4A). In myometrial cells, treatment with thapsigargin (1 μM), an inhibitor of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase, followed by addition of Ca2+ produced a robust SOCE seen as an increase in [Ca2+]i by 1,479 ± 82 nM (n = 17 cells). The amplitude of SOCE was not significantly affected by treatment with nifedipine (10−6 M); the increase in [Ca2+]i was 1,545 ± 138 nM (n = 12 cells) after nifedipine versus 1,479 ± 82 nM in the absence of nifedipine (Fig. 4).
Fig. 4.
Store-operated calcium entry is not involved in G-1 mediated increase in [Ca2+]i. A: examples of actual Ca2+ responses produced in myometrial cells when Ca2+ was added to the saline after 10 min in Ca2+-free saline (constitutive Ca2+ entry; black trace), after administration of G-1 (5 × 10−7 M, G-1-induced Ca2+ entry; red trace) or treatment with thapsigargin (TG, 10−6 M; store-operated Ca2+ entry) in the absence (green trace) or presence (blue trace) of nifedipine (10−6 M). B: comparison of the amplitude of Ca2+ response produced by different conditions: G-1 increased [Ca2+]i by 593 ± 36 (n = 31 cells), which was not statistically different from the constitutive Ca2+ entry (625 ± 44 nM) (n = 23 cells), while much lower than the store-operated Ca2 entry (1,479 ± 82 nM; n = 17 cells); the response was not sensitive to nifedipine (1,545 ± 138 nM: n = 12 cells). *Significant difference compared with basal [Ca2+]i; **significant difference compared with the constitutive or G-1-induced Ca2+ entry (P < 0.05).
Intracellular microinjection of G-1 elevates [Ca2+]i.
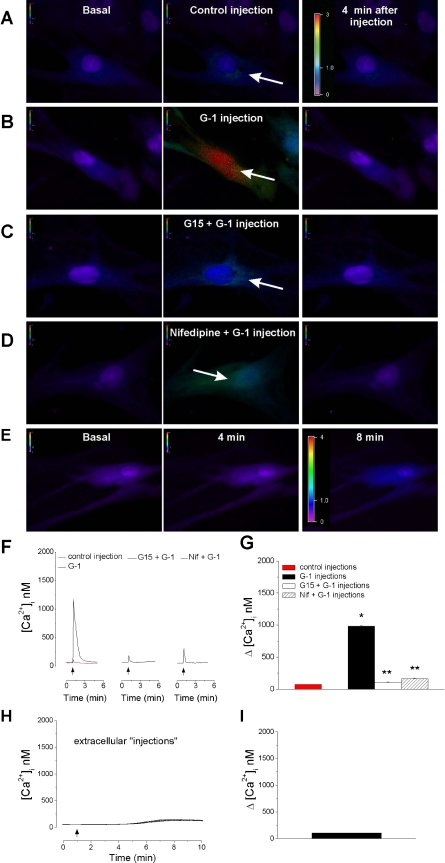
Intracellular microinjection of control buffer induced a small elevation in [Ca2+]i with a mean amplitude of 72 ± 1.6 nM in rat myometrial cells (n = 10 cells) (Fig. 5, A, F, and G). Microinjection of G-1 (5 × 10−7 M final concentration inside the cell) produced a robust and transitory increase in [Ca2+]i by 983 ± 8 nM (n = 10 cells) (Fig. 5, B, F, and G). The effect occurred faster and had a higher amplitude than that induced by extracellular application (seconds vs. minutes) (P < 0.05) (for comparison see Fig. 5F and Fig. 3A). Pretreatment with G-15 (5 × 10−6 M, 15 min) prevented the effects of intracellularly injected G-1 (Fig. 5, C, F, and G) (n = 11 cells); the small increase in [Ca2+]i produced by G-1 administration in the presence of the antagonist was not significantly different from that in control injection. Pretreatment with nifedipine (10−6 M, 10 min) markedly reduced the G-1-induced increase in [Ca2+]i to 102 ± 8 nM (Fig. 5, D, F, and G) (n = 8 cells). When G-1 (5 × 10−5 M in the pipette) was applied extracellularly by Femtotips, in the close vicinity (1–2 μm) of the cell membrane induced a slow increase in [Ca2+]i by 103 + 2.6 nM with a plateau at 5–8 min after administration (n = 6 cells) (Fig. 5, E, H, and I).
Fig. 5.
Intracellular microinjection of G-1 increases [Ca2+]i in rat myometrial cells. A–D: representative examples of changes in fura-2 fluorescence ratio (340/380 nm) before, during, and 4 min after injection; the fluorescence ratio scale (0–3) is illustrated in each panel and magnified in A; the arrows indicate the injection site. A: microinjection of control buffer induced a minimal change in fluorescence ratio. B: microinjection of G-1 (5 × 10−7 M final concentration inside the cell) induced a marked increase in fluorescence ratio that was prevented by pretreatment with G-15 (5 × 10−6 M) (C). D: microinjection of G-1 (5 × 10−7 M) in cells pretreated with nifedipine, a L-type Ca2+ channel blocker, produced a minimal change in fluorescence ratio. E: representative examples of changes in fura-2 AM fluorescence ratio (340/380 nm) before, at 4 min, and 8 min after extracellular administration of G-1 by Femtotips in the vicinity of the membrane; the fluorescence ratio scale (0–4) is illustrated in each panel. F: representative tracings of Ca2+ responses induced by microinjection of control buffer (left, red trace), G-1 (left, black trace), G-1 in cells pretreated with G-15 (middle trace), or nifedipine (right). G: comparison of changes in [Ca2+]i during injection of control buffer (72 ± 1.6 nM, n = 10 cells), G-1 (983 ± 8 nM; n = 10 cells), and G-1 in the presence of G-15 (102 ± 8; n = 11 cells) or nifedipine (163 ± 9 nM; n = 8 cells). *Significant difference compared with the injection of control buffer; **significant difference compared with the effect of G-1 alone (P < 0.05). H: averaged changes in [Ca2+]i (n = 6 cells) induced by extracellular injection of G-1. I: G-1 induced a slow increase in [Ca2+]i by 103 ± 2.6 nM at 5–8 min after administration.
G-1 Increases Uterine Strips Contractility
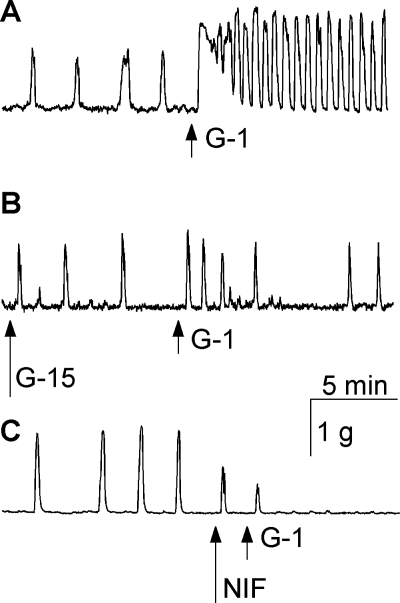
In basal conditions, the contractility of rat uterine strips was characterized by the following parameters: 1) the area under the contractility curve was 96 ± 3.2 g/10 min, 2) the frequency of myometrial contractions was 5.4 ± 0.8 contractions/10 min, and 3) the amplitude of myometrial contractions was 1.06 + 0.09 g. Administration of G-1 (5 × 10−7 M) increased the area under the contractility curve by 862 ± 4.1%, the frequency of myometrial contractions by 426 ± 3.4% and the amplitude of contractions by 52 ± 0.7% (n = 6 experiments) compared with basal conditions. In addition, a transitory increase in basal tone was also observed. A representative trace is shown in Fig. 6A. Pretreatment of the uterine strips with G-15 (5 × 10−6 M, 10 min) or nifedipine (10−6 M) abolished the effect of G-1 on contractility (Fig. 6, B and C). In addition, nifedipine also abolished the basal contractions (Fig. 6C).
Fig. 6.
G-1 increases uterine contractility. A–C: representative examples of contractility recordings in rat uterine strips. A: administration of G-1 (5 × 10−7 M) increased the frequency and amplitude of uterine contractions. B: pretreatment with G-15 (5 × 10−6 M, 10 min) prevented the effect of G-1. C: nifedipine (10−6 M) abolished the basal contractions and prevented the effect of G-1 on uterine strips contractility.
DISCUSSION
GPER has been identified in several tissues including uterus (25, 31). Recent evidence indicate that the receptor is involved in endometrial pathology (24, 43, 45, 46), while the role of GPER in myometrium is largely unexplored. Recently, GPER has been proposed to act as a negative regulator of ERα-dependent uterine growth in response to estradiol (20). However, GPER-deficient mice did not seem to have an impaired uterine function (32). In this study, we examined the role of GPER in nonpregnant rat myometrium, by assessing the effect of selective GPER ligands, previously characterized (4, 14), on [Ca2+]i, membrane potential, and uterine contractility.
First, we detected the presence of GPER expression in nonpregnant rat myometrium using reverse transcriptase-polymerase chain reaction and immunocytochemistry. GPER immunoreactivity was identified intracellularly, as reported in several other cell types (12, 33, 39). Next, using a slow-response voltage-sensitive dye, we found that administration of G-1, a GPER-selective agonist (4), produced a concentration-dependent depolarization of rat myometrial cells. G-1-induced depolarization was abolished in a Na+-free saline, indicating the involvement of Na+ influx in the response. In myometrial cells, Na+ influx occurs trough voltage-gated Na+ channels and nonselective cation channels from transient receptor potential channels family (TRPC). All TRPCs except for TRPC3 are expressed in nonpregnant rat myometrium (3), while the human myometrium lacks TRPC2 (50). The specific Na2+ influx pathway(s) associated with GPER activation remains to be clarified.
In myometrium, similar to other excitable tissues, depolarization due to various stimuli leads to an opening of plasmalemmal voltage-sensitive Ca2+ channels, followed by an increase in cytoplasmatic Ca2+ concentration and contraction (49). With respect to the concentrations of GPER ligands used, notable differences were reported in different cellular systems and between endogenously expressing and GPER-transfected cells. Several recent publications, including ours (2, 5, 28), indicate that in primary cells, G-1 activates GPER in micromolar range. In transfected cells, which overexpress the receptor, lower concentrations of agonists may be sufficient to activate the receptor (4).
Our calcium imaging experiments indicate that extracellular administration of G-1 induced a delayed and sustained increase in [Ca2+]i. Since Ca2+ entry in rat uterus occurs almost entirely via L-type Ca2+ channels (49), we examined the [Ca2+]i response to G-1 in the presence of nifedipine, a L-type Ca2+ channel blocker. Pretreatment with nifedipine markedly decreased G-1-induced increase in [Ca2+]i, supporting the involvement of L-type Ca2+ channels in the response. In Ca2+-free saline, the response to G-1 was markedly reduced, indicating a limited or lack of participation of sarcoplasmic reticulum. SOCE is an important Ca2+ influx pathway in many nonexcitable and some excitable cells (36). We examined an eventual participation of SOCE in the response to G-1. Depletion of the internal Ca2+ stores by treatment with thapsigargin, an inhibitor of sarcoplasmic-endoplasmic reticulum Ca2+-ATPase, evoked a robust SOCE, which was not affected by treatment with nifedipine. G-1-induced [Ca2+]i response was similar to the constitutive Ca2+ entry and did not seem to involve a SOCE mechanism. Previously, we reported that GPER activation by G-1 produced an increase in [Ca2+]i in neurons (5) and breast cancer cells (2). Similarly, other reports indicate that GPER activation enhanced [Ca2+]i in cells endogenously expressing or transfected with the receptor (4, 35 ,39).
GPCR are active structural proteins located usually in the cell membrane. However, increasing evidence indicates that angiotensin II receptors are active not only when stimulated from outside but also from inside the cells (6, 37, 38). We recently reported that intracellular angiotensin II activates myometrial cells via an intracrine mechanism (13). Also, we characterized the intracellular activation of cannabinoid CB1 receptors by anandamide (10). With respect to GPER, the subcellular localization has yet to be firmly established; both intracellular and plasma membrane localizations have been reported (16, 33, 39, 41). Initially, GPER was shown to be uniquely localized to the endoplasmic reticulum in cells transiently or constitutively expressing GPER (39). In contrast, other studies using morphological and functional assays showed that GPER is expressed only in the plasma membrane of GPER-transfected cells (16, 29, 44) or of CA2 pyramidal neurons of the rat hippocampus (19). In myometrial cells, G-1 administered intracellularly by microinjection produced a faster and transitory increase in [Ca2+]i, with a higher amplitude than that induced by extracellular administration of G-1 in the same final concentration. Since G-1 is a membrane permeant compound, we also applied G-1 on the extracellular side of the membrane by microinjection, using Femtotips. In this case, we noted a small and delayed increase in [Ca2+]i, whose shape and kinetics were similar to that produced by bath application of G-1. We attribute the differences in kinetics and amplitude of responses to intracellular versus extracellular administration of the agonist to the location of GPER in myometrial cells. Our calcium imaging and immunocytochemistry results support an intracellular location of functional GPER. This is in agreement with a recent report which found that GPER is localized intracellularly in other tissues (12). The response was abolished by pretreatment with G-15, a GPER antagonist, indicating that this response is receptor specific.
Increase of [Ca2+]i and stimulation of myosin light chain kinase (MLCK) via Ca2+-calmodulin is essential for myometrial contraction. To identify the effect of GPER activation on uterine contraction, the next series of experiments were carried out on rat uterine strips. Administration of G-1 caused a robust stimulant effect on uterine strip contractility by increasing the frequency and amplitude of uterine contractions. Similarly, estrogen has been reported to stimulate uterine contractility in perfused nonpregnant human or swine uterus (30, 40) or in artificially inseminated sows (48). Taken together, our results support the involvement of GPER activation in myometrial depolarization and contraction. Similarly, a very recent report, published when the present study was under review, indicates that GPER activation is associated with increased contractility of the human myometrium and the receptor is most likely to be involved in myometrium physiology during pregnancy (28).
GRANTS
This study was supported by National Institutes of Health Grants HL-90804 (to E. Brailoiu) and 5R01CA127731-03 (to J. B. Arterburn and T. I. Oprea). A. A. Tica and O. S. Tica were funded by Sunset Molecular Discovery, Santa Fe, NM.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A. A. Tica, E. C. Dun, O. S. Tica, X. Gao, and E. Brailoiu performed experiments; A. A. Tica, E. C. Dun, and O. S. Tica analyzed data; A. A. Tica, X. Gao, and G. C. Brailoiu prepared figures; A. A. Tica and G. C. Brailoiu drafted manuscript; A. A. Tica, E. C. Dun, O. S. Tica, X. Gao, J. B. Arterburn, G. C. Brailoiu, T. I. Oprea, and E. Brailoiu approved final version of manuscript; J. B. Arterburn, G. C. Brailoiu, T. I. Oprea, and E. Brailoiu edited and revised manuscript; E. Brailoiu conception and design of research; E. Brailoiu interpreted results of experiments.
ACKNOWLEDGEMENTS
We thank Mary Dun and Elena Deliu for technical assistance and Dr. Patrick Piggot for facilitating access to the confocal microscope.
Present address of A. A. Tica and O. S. Tica: Dept. of Pharmacology, School of Medicine, University of Medicine and Pharmacy, Craiova, Romania.
REFERENCES
- 1. Albanito L, Lappano R, Madeo A, Chimento A, Prossnitz ER, Cappello AR, Dolce V, Abonante S, Pezzi V, Maggiolini M. G-protein-coupled receptor 30 and estrogen receptor-alpha are involved in the proliferative effects induced by atrazine in ovarian cancer cells. Environ Health Perspect 116: 1648–1655, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. Ariazi EA, Brailoiu E, Yerrum S, Shupp HA, Slifker MJ, Cunliffe HE, Black MA, Donato AL, Arterburn JB, Oprea TI, Prossnitz ER, Dun NJ, Jordan VC. The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer Res 70: 1184–1194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Babich LG, Ku CY, Young HW, Huang H, Blackburn MR, Sanborn BM. Expression of capacitative calcium TrpC proteins in rat myometrium during pregnancy. Biol Reprod 70: 919–924, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2: 207–212, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 193: 311–321, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Brailoiu E, Filipeanu CM, Tica A, Toma CP, de Zeeuw D, Nelemans SA. Contractile effects by intracellular angiotensin II via receptors with a distinct pharmacological profile in rat aorta. Br J Pharmacol 126: 1133–1138, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brailoiu E, Hooper R, Cai X, Brailoiu GC, Keebler MV, Dun NJ, Marchant JS, Patel S. An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J Biol Chem 285: 2897–2901, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brailoiu GC, Brailoiu E, Chang JK, Dun NJ. Excitatory effects of human immunodeficiency virus 1 Tat on cultured rat cerebral cortical neurons. Neuroscience 151: 701–710, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brailoiu GC, Gurzu B, Gao X, Parkesh R, Aley PK, Trifa DI, Galione A, Dun NJ, Madesh M, Patel S, Churchill GC, Brailoiu E. Acidic NAADP-sensitive calcium stores in the endothelium: agonist-specific recruitment and role in regulating blood pressure. J Biol Chem 285: 37133–37137, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brailoiu GC, Oprea TI, Zhao P, Abood ME, Brailoiu E. Intracellular CB1 cannabinoid receptors are activated by anandamide. J Biol Chem 86: 29166–29174, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brauner T, Hulser DF, Strasser RJ. Comparative measurements of membrane potentials with microelectrodes and voltage-sensitive dyes. Biochim Biophys Acta 771: 208–216, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Cheng SB, Graeber CT, Quinn JA, Filardo EJ. Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids 76: 892–896, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Deliu E, Tica AA, Motoc D, Brailoiu GC, Brailoiu E. Intracellular angiotensin II activates rat myometrium. Am J Physiol Cell Physiol 301: C559–C565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol 5: 421–427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ebner TJ, Chen G. Use of voltage-sensitive dyes and optical recordings in the central nervous system. Prog Neurobiol 46: 463–506, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 148: 3236–3245, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol 80: 231–238, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16: 70–84, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun 346: 904–910, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Gao F, Ma X, Ostmann AB, Das SK. GPR30 activation opposes estrogen-dependent uterine growth via inhibition of stromal ERK1/2 and estrogen receptor alpha (ERα) phosphorylation signals. Endocrinology 152: 1434–1447, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 22. Guse AH, Berg I, da Silva CP, Potter BV, Mayr GW. Ca2+ entry induced by cyclic ADP-ribose in intact T-lymphocytes. J Biol Chem 272: 8546–8550, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Huang GS, Gunter MJ, Arend RC, Li M, Arias-Pulido H, Prossnitz ER, Goldberg GL, Smith HO. Co-expression of GPR30 and ERbeta and their association with disease progression in uterine carcinosarcoma. Am J Obstet Gynecol 203: 242 e241–e245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ignatov T, Eggemann H, Semczuk A, Smith B, Bischoff J, Roessner A, Costa SD, Kalinski T, Ignatov A. Role of GPR30 in endometrial pathology after tamoxifen for breast cancer. Am J Obstet Gynecol 203: 595 e599–e516, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 150: 1722–1730, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Krizsan-Agbas D, Pedchenko T, Smith PG. Neurotrimin is an estrogen-regulated determinant of peripheral sympathetic innervation. J Neurosci Res 86: 3086–3095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuriyama H, Suzuki H. Changes in electrical properties of rat myometrium during gestation and following hormonal treatments. J Physiol 260: 315–333, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maiti K, Paul JW, Read M, Chan EC, Riley SC, Nahar P, Smith R. G-1-activated membrane estrogen receptors mediate increased contractility of the human myometrium. Endocrinology 152: 2448–2455, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Mizukami Y. In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: from discovery to functions in vivo. Endocr J 57: 101–107, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Mueller A, Siemer J, Schreiner S, Koesztner H, Hoffmann I, Binder H, Beckmann MW, Dittrich R. Role of estrogen and progesterone in the regulation of uterine peristalsis: results from perfused non-pregnant swine uteri. Hum Reprod 21: 1863–1868, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Olde B, Leeb-Lundberg LM. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab 20: 409–416, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80: 34–41, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 149: 4846–4856, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Perusquia M. Nongenomic action of steroids in myometrial contractility. Endocrine 15: 63–72, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol 70: 165–190, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Putney JW. The physiological function of store-operated calcium entry. Neurochem Res 36: 1157–1165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Re RN. The lysosomal action of intracrine angiotensin II. Focus on “Intracellular angiotensin II activates rat myometrium”. Am J Physiol Cell Physiol 301: C553–C554, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Re RN, Cook JL. Mechanisms of disease: intracrine physiology in the cardiovascular system. Nat Clin Pract Cardiovasc Med 4: 549–557, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Richter ON, Bartz C, Dowaji J, Kupka M, Reinsberg J, Ulrich U, Rath W. Contractile reactivity of human myometrium in isolated non-pregnant uteri. Hum Reprod 21: 36–45, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Morris JF, Prossnitz ER, Kawata M. Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology 148: 5842–5850, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Shintani Y, Hirano K, Nakayama T, Nishimura J, Nakano H, Kanaide H. Mechanism of trypsin-induced contraction in the rat myometrium: the possible involvement of a novel member of protease-activated receptor. Br J Pharmacol 133: 1276–1285, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol 196: 386 e381–e389; discussion 386 e389–e311, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146: 624–632, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol 20: 631–646, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Wang A, Ji L, Shang W, Li M, Chen L, White RE, Han G. Expression of GPR30, ERalpha and ERbeta in endometrium during window of implantation in patients with polycystic ovary syndrome: a pilot study. Gynecol Endocrinol 27: 251–255, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Wang C, Prossnitz ER, Roy SK. G protein-coupled receptor 30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology 149: 4452–4461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Willenburg KL, Knox RV, Kirkwood RN. Effect of estrogen formulation and its site of deposition on serum PGFM concentrations, uterine contractility, and time of ovulation in artificially inseminated weaned sows. Anim Reprod Sci 80: 147–156, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Wray S, Kupittayanant S, Shmygol A, Smith RD, Burdyga T. The physiological basis of uterine contractility: a short review. Exp Physiol 86: 239–246, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Yang M, Gupta A, Shlykov SG, Corrigan R, Tsujimoto S, Sanborn BM. Multiple Trp isoforms implicated in capacitative calcium entry are expressed in human pregnant myometrium and myometrial cells. Biol Reprod 67: 988–994, 2002 [DOI] [PubMed] [Google Scholar]