Abstract
Muscle contraction requires ATP and Ca2+ and, thus, is under direct control of mitochondria and the sarcoplasmic reticulum. During postnatal skeletal muscle maturation, the mitochondrial network exhibits a shift from a longitudinal (“longitudinal mitochondria”) to a mostly transversal orientation as a result of a progressive increase in mitochondrial association with Ca2+ release units (CRUs) or triads (“triadic mitochondria”). To determine the physiological implications of this shift in mitochondrial disposition, we used confocal microscopy to monitor activity-dependent changes in myoplasmic (fluo 4) and mitochondrial (rhod 2) Ca2+ in single flexor digitorum brevis (FDB) fibers from 1- to 4-mo-old mice. A robust and sustained Ca2+ accumulation in triadic mitochondria was triggered by repetitive tetanic stimulation (500 ms, 100 Hz, every 2.5 s) in FDB fibers from 4-mo-old mice. Specifically, mitochondrial rhod 2 fluorescence increased 272 ± 39% after a single tetanus and 412 ± 45% after five tetani and decayed slowly over 10 min following the final tetanus. Similar results were observed in fibers expressing mitochondrial pericam, a mitochondrial-targeted ratiometric Ca2+ indicator. Interestingly, sustained mitochondrial Ca2+ uptake following repetitive tetanic stimulation was similar for triadic and longitudinal mitochondria in FDB fibers from 1-mo-old mice, and both mitochondrial populations were found by electron microscopy to be continuous and structurally tethered to the sarcoplasmic reticulum. Conversely, the frequency of osmotic shock-induced Ca2+ sparks per CRU density decreased threefold (from 3.6 ± 0.2 to 1.2 ± 0.1 events·CRU−1·min−1·100 μm−2) during postnatal development in direct linear correspondence (r2 = 0.95) to an increase in mitochondrion-CRU pairing. Together, these results indicate that mitochondrion-CRU association promotes Ca2+ spark suppression but does not significantly impact mitochondrial Ca2+ uptake.
Keywords: excitation-contraction coupling, sarcoplasmic reticulum, ryanodine receptor, calcium signaling
skeletal muscle contraction requires ATP and Ca2+ and, thus, is under direct control of two major intracellular organelles, mitochondria and Ca2+ release units (CRUs) or triads, formed by the association of two sarcoplasmic reticulum (SR) terminal cisternae flanking a central bisecting transverse tubule (T-tubule). Mitochondria are the powerhouse of the cell, being responsible for aerobic production of cellular ATP required for myosin crossbridge cycling and Ca2+ reuptake by sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) pumps (24). CRUs are sites of excitation-contraction coupling, the process that triggers SR Ca2+ release and subsequent muscle contraction in response to an action potential propagating down the T-tubule system. Interestingly, in fully mature fast-twitch muscle fibers, the two organelles are in close proximity with each other at the level of the myofibril I band (3, 4, 22, 23, 32).
We recently reported that the association of mitochondria to CRUs occurs progressively during postnatal development in a process that involves an increase in the number of both organelles and their coordinated migration to the I band region during maturation (4). Specifically, in adult fast-twitch muscle fibers [e.g., flexor digitorum brevis (FDB) fibers from 4-mo-old mice], the A band region generally lacks mitochondria (although they are occasionally observed), especially relative to the number of mitochondria observed within the I band. On the other hand, longitudinal mitochondria within the A band region are much more commonly observed in FDB fibers from young mice (e.g., FDB fibers from 0.5- to 1-mo-old mice). This process is mediated by small (∼10 nm) electron-dense linkages, or tethers, that bridge the outer mitochondrial membrane with the SR on the side opposite to the T-tubule. The tethers provide a strong structural link, positioning mitochondria on the side of the SR opposite to Ca2+ release channels or type 1 ryanodine receptors (RYR1s) and restrict mitochondrial movement away from sites of SR Ca2+ release (4). This intimate, highly coordinated coupling of mitochondria to CRUs during postnatal development is hypothesized to provide a structural basis for localized cross talk between the two organelles (24).
Although this hypothesis has yet to be definitively demonstrated, previous studies have provided evidence for mitochondrial Ca2+ uptake (orthograde SR-mitochondrial coupling) during stimulation of adult skeletal muscle fibers in vitro (27), in situ (7, 17, 29), and in vivo (25). Additionally, mitochondrial activity inhibits local Ca2+ release events (termed Ca2+ spark suppression) from adjacent CRUs (retrograde SR-mitochondrial coupling) (for review see Ref. 24). In their capacity to generate, scavenge, and detoxify reactive oxygen (ROS)/reactive nitrogen species (RNS), CRU-paired mitochondria may create a spatially confined Ca2+/ATP/ROS domain that maintains the adjacent CRU and associated proteins (e.g., RYR1) in the proper redox state (13, 14, 19), decreasing susceptibility to Ca2+-induced Ca2+ release and, thus, Ca2+ spark activity. The nature of this bidirectional interaction has been assessed through the use of pharmacological interventions to disrupt mitochondrial function and the local redox control mechanism, to alter the local Ca2+ microdomain, and to block mitochondrial Ca2+ uptake (7, 14, 18, 19, 29).
Mitochondrial Ca2+ uptake enhances aerobic ATP production by stimulating Ca2+-dependent dehydrogenases that generate NADH (pyruvate, NAD-isocitrate, and 2-oxoglutarate dehydrogenases) and augmentation of the ATP synthetic capacity of the F1F0-ATPase (2, 5, 24). However, since the increase in mitochondrial Ca2+ during a single twitch is short-lived (<200 ms) (25) and physiological activation of skeletal muscle usually involves repetitive high-frequency neuromuscular stimulation, we hypothesized that sustained increases in mitochondrial Ca2+ occur during repetitive tetanic stimulation and that mitochondrion-CRU pairing is a prerequisite for this phenomenon. To test the impact of intimate mitochondrion-CRU coupling on bidirectional Ca2+ signaling between the SR and mitochondria, we took advantage of the fact that mitochondrion positioning changes during postnatal maturation (3, 4). Thus we characterized the degree and properties of mitochondrial Ca2+ uptake during tetanic stimulation and Ca2+ spark suppression in single skeletal muscle fibers at different times during postnatal maturation, with the expectation that the bidirectional interaction would be strengthened as mitochondrion-CRU pairing progressively increases.
MATERIALS AND METHODS
Muscle fiber preparation.
All animals were housed in a pathogen-free area at the University of Rochester, and experiments were carried out in accordance with procedures reviewed and approved by the local University Committee on Animal Resources. Mice were killed immediately prior to experiments by regulated CO2 delivery followed by cervical dislocation. Single acutely dissociated FDB muscle fibers were isolated from young (0.5- and 1-mo-old) and adult (2- and 4-mo-old) mice, as described previously (4). All single-fiber experiments were conducted at room temperature (22–23°C).
Myoplasmic Ca2+ measurements.
FDB fibers were loaded with 4 μM mag-fluo 4-AM for 25 min at room temperature and then exposed for 20 min to dye-free solution supplemented with 25 μM N-benzyl-p-toluene sulfonamide (BTS) to block contractions (8, 28). A series of repetitive high-frequency tetani (1–40 tetani elicited by 1- to 5-ms, 8-V pulses at 100 Hz for 500 ms delivered at 2.5-s intervals; Fig. 1A) were elicited using an extracellular 200 mM NaCl-filled electrode placed near the fiber of interest. No significant difference was observed in the magnitude of the first evoked Ca2+ transient elicited during the tetanus using 1- or 5-ms stimuli [peak relative change in fluorescence (ΔF/F0) = 0.56 ± 0.03 and 0.50 ± 0.04, n = 14 for 1- and 5-ms stimuli, respectively]. Thus, only single action potentials were elicited, even when 5-ms stimuli were used. For measurements of global myoplasmic Ca2+ (Fig. 1A), a rectangular region of interest was selected in mag-fluo 4-loaded fibers and excited at 480 ± 15 nm. Fluorescence emission at 535 ± 20 nm was collected at 10 kHz using a photomultiplier detection system. Data were analyzed offline using Clampfit and SigmaPlot software packages.
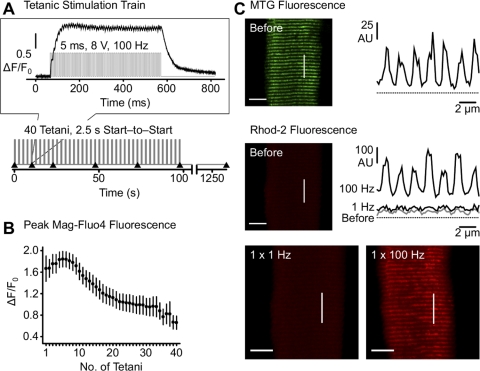
Fig. 1.
Myoplasmic and mitochondrial Ca2+ transients in flexor digitorum brevis (FDB) fibers during twitch and repetitive tetanic stimulation. A: tetanic stimulation protocol consisting of 1- to 5-ms, 8-V square-wave pulses at 100 Hz for 500 ms (top) delivered at 2.5-s intervals (0.2 duty cycle) for a total of 40 successive tetani (bottom). ▴ indicate rhod 2 image acquisition (after 1, 5, 10, 20, 30, and 40 tetani) shown in Figs. 3 and 5. Inset: increase in myoplasmic mag-fluo 4 fluorescence during the 5th tetanus of the protocol taken from a representative FDB fiber. B: peak mag-fluo 4 fluorescence (ΔF/F0) during each tetanus in FDB fibers from 1-mo-old mice. Values are means ± SE. C: representative confocal images of MitoTracker Green FM (MTG, top) and rhod 2 fluorescence (middle and bottom) in FDB fibers from a 4-mo-old mouse before and after twitch (1 Hz) or tetanic (100 Hz for 500 ms) stimulation. MTG (top right) and rhod 2 (middle right) fluorescence intensity profiles are shown along the lines of interest marked in the corresponding x-y images. AU, arbitrary units.
Electroporation in FDB muscle.
Mitochondrial-targeted ratiometric pericam (mt-pericam) (21) was electroporated into FDB muscles of adult mice, as described previously (10). Briefly, after anesthesia by intraperitoneal injection of 100 mg/kg ketamine, 10 mg/kg xylazine, and 3 mg/kg acepromazine, bovine hyaluronidase (7 μl/foot, 2 μg/μl) was injected subcutaneously into both hindlimb footpads. After 1 h, 30 μg of mt-pericam cDNA (10 μl total volume in 71 mM NaCl) were similarly injected, and the FDB muscle was electroporated (20-ms, 100 V/cm pulses at 1 Hz for 20 s) using gold-plated electrodes placed perpendicular to the long axis of the muscle, close to the proximal and distal tendons. Individual FDB fibers were isolated 1 wk after electroporation, as described previously (4).
Confocal x-y imaging and analysis of mitochondrial Ca2+ accumulation during repetitive tetanic stimulation.
Acutely dissociated FDB fibers were loaded first with 5 μM rhod 2-AM for 30 min at room temperature in Ringer solution (in mM: 146 NaCl, 5 KCl, 1 MgCl, 2 CaCl2, and 10 HEPES, pH 7.4) supplemented with 25 μM BTS to inhibit cell movement. Minimizing dye concentration and loading time at room temperature facilitated preferential mitochondrial rhod 2 loading. Fibers loaded with rhod 2 were then incubated with 300 nM MitoTracker Green (MTG; Fig. 1C) or 10 μM fluo 4-AM (Fig. 1; see Figs. 2 and 4) and then exposed for 20 min to dye-free solution supplemented with 25 μM BTS. Additional experiments were conducted in fibers loaded with 10 nM tetramethylrhodamine ethyl ester (TMRE), a cationic, mitochondrial-selective fluorescent membrane potential indicator, and 10 μM fluo 4-AM (see Fig. 5). Time series of x-y images (256 × 256 pixels/frame, 0.2 μm/pixel, 690-ms scan duration) were acquired on a confocal microscope (Nikon Eclipse C1 Plus) equipped with a ×40 1.3 numerical aperture (NA) oil objective (SuperFluor) by sequential excitation of green (MTG and fluo 4) and red (rhod 2 and TMRE) fluorophores using 488- and 543-nm lasers, respectively. Repetitive high-frequency tetanic stimulation trains (1–40 tetani, 100 Hz for 500 ms at 2.5-s intervals; Fig. 1A) were elicited using an extracellular 200 mM NaCl-filled electrode placed near the fiber of interest. Green x-y images were acquired at the beginning of the electrical stimulation, and the first red image was taken immediately after completion of the green image (690 ms after initiation of electrical stimulation). Sequential green and red x-y images were taken after 1, 5, 10, 20, 30, and 40 tetani and 1, 7.5, 15, and 20 min after the last tetanus. Complete characterization of rhod 2 (Fig. 2C) and mt-pericam (Fig. 3C) fluorescence decay following five successive tetani was assessed from x-y images taken at defined times throughout the subsequent 10 min following cessation of the final tetanus.
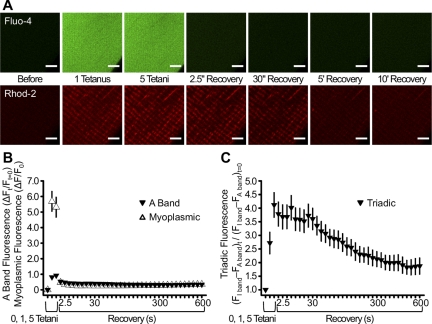
Fig. 2.
Myoplasmic and mitochondrial Ca2+ transients during tetanic stimulation. A: representative confocal images of fluo 4 (top) and rhod 2 (bottom) fluorescence in FDB fibers from 4-mo-old mice before tetanic stimulation, after 1 and 5 tetani (0.2 duty cycle), and 2.5, 30, 300, and 600 s following the 5th tetanus. Scale bars, 5 μm. B: relative change (ΔF/F0) in rhod 2 A band and fluo 4 (myoplasmic) fluorescence during and after delivery of 1 and 5 tetanic stimuli. C: normalized triadic rhod 2 fluorescence during and after delivery of 1 and 5 tetanic stimuli. Values are means ± SE; n = 17 fibers.
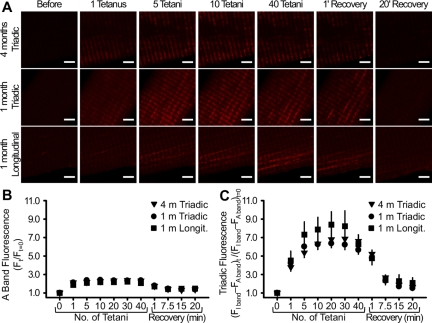
Fig. 4.
Ca2+ uptake in triadic and longitudinal mitochondria following tetanic stimulation. A: confocal images of rhod 2 fluorescence in representative FDB fibers from 4-mo-old (top) and 1-mo-old (middle and bottom) mice before stimulation, after 1, 5, 10, and 40 tetani (0.2 duty cycle), and 1, 7.5, 15, and 20 min following the last (40th) tetanus. Scale bars, 5 μm. B and C: normalized rhod 2 A band and mitochondrial rhod 2 fluorescence, respectively, during and after repetitive tetanic stimulation in FDB fibers from 4- and 1-mo-old mice. Values are means ± SE; n = 50 (4-mo-old triadic), 45 (1-mo-old triadic), and 22 [1-mo-old longitudinal (Longit)] fibers.
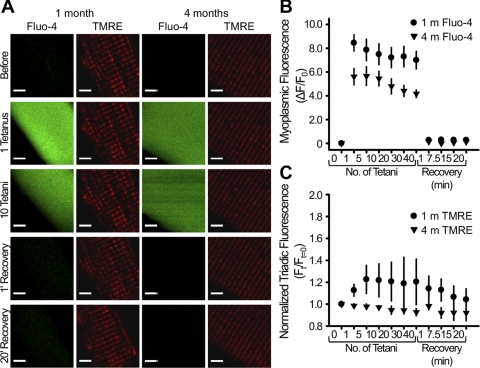
Fig. 5.
Mitochondrial membrane potential is unaltered during repetitive tetanic stimulation. A: representative confocal images of fluo 4 and tetramethylrhodamine ethyl ester (TMRE) fluorescence in FDB fibers from 1- and 4-mo-old mice before tetanic stimulation, after 1 and 10 tetani, and 1 and 20 min following the last (40th) tetanus. Scale bars, 5 μm. B and C: normalized myoplasmic fluo 4 fluorescence and triadic TMRE fluorescence, respectively, during and after repetitive tetanic stimulation in FDB fibers from 1- and 4-mo-old mice. Values are means ± SE; n = 5 fibers.
Fig. 3.
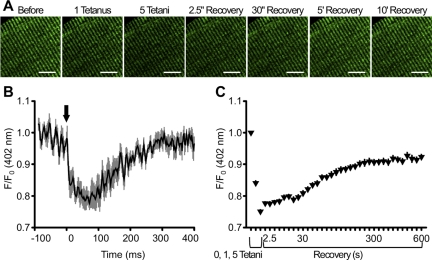
Transient mitochondrial Ca2+ uptake during twitch stimulation and sustained mitochondrial Ca2+ uptake following repetitive tetanic stimulation in mitochondrial-targeted ratiometric pericam (mt-pericam)-expressing fibers. A: representative confocal images of mt-pericam-expressing FDB fibers isolated from 4-mo-old mice before tetanic stimulation, after 1 and 5 tetani (0.2 duty cycle), and 2.5, 30, 300, and 600 s following the 5th and final tetanus. Scale bars, 10 μm. B: changes in mt-pericam fluorescence (402-nm excitation) monitored by confocal line scan imaging during twitch stimulations. Arrow indicates time (t = 0) of electrical stimulation. C: sustained mitochondrial Ca2+ increase and slow decay over 10 min following a series of 5 repetitive tetanic stimulations. Values are means ± SE; n = 6 (B) and 27 (C) fibers.
To generate a mean fluorescence value for triadic mitochondria from confocal x-y images, a 2-μm-thick line drawn along the longitudinal axis of the fiber (∼20 μm long) was used to generate a mean x-y ratio profile, with x values representing fiber length and y values representing the transversely averaged fluorescence ratio. Values for profile peaks (I band fluorescence) and troughs (A band fluorescence) following stimulation were normalized to their respective values at rest. Relative changes in triadic mitochondrial Ca2+ in response to stimulation were expressed as the difference between I and A band rhod 2 fluorescence within the same profile (I − A) and then normalized to the corresponding value prior to stimulation. For longitudinal clusters of mitochondria, profiles were centered over the A band.
Real-time confocal line scan imaging and analysis of mitochondrial Ca2+ accumulation during twitch stimulation.
Acutely dissociated, mt-pericam-expressing fibers were bathed in Ringer solution (see above) supplemented with 35 μM BTS to inhibit cell movement. A series of individual twitch transients were initially elicited using an extracellular electrode placed adjacent to the fiber of interest (1-ms pulse duration at 2 Hz). Real-time changes in relative mt-pericam fluorescence during individual twitches were monitored in confocal line scan mode (2 ms/line, 1,024 lines) across a 50-μm line drawn along the longitudinal axis of the fiber. The fiber was then stimulated with five repetitive tetani (100 Hz for 500 ms at 2.5-s intervals) followed by a series of x-y images taken over the subsequent 10 min, as described above for the rhod 2 experiments. A 402-nm laser was used to excite mt-pericam, and fluorescence emission was detected at 515 ± 30 nm. Images were acquired using a confocal microscope (Nikon Eclipse C1 Plus) equipped with a ×40 1.3 NA oil objective (SuperFluor). Real-time changes in relative mt-pericam fluorescence during twitch stimulation were assessed within a 1-μm-thick line corresponding to the mt-pericam fluorescence within the I band. Under the excitation conditions used here (402 nm), mt-pericam fluorescence decreases with an increase in Ca2+ binding (21). All x-y and line scan images were processed and analyzed offline using National Institutes of Health ImageJ and AutoQuant AutoDeblur and AutoVisualize software. All analyses were conducted on raw images, but for display purposes only, a two-dimensional blind deconvolution was used to minimize out-of-focus fluorescence.
Electron microscopy analysis.
FDB muscles were fixed in situ with 3.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) at room temperature following 1 h of exposure to isotonic or hypertonic high-Ca2+ Ringer solution (see below). Small portions of muscle were postfixed, embedded, stained en bloc, and sectioned for electron microscopy (EM), as described previously (4). CRUs and mitochondria, as well as the incidence of CRUs coupled to mitochondria, were marked and counted (Table 1) only in the subsarcolemmal region of fibers (1 μm deep) in nonoverlapping micrographs taken at ×17,700 magnification of longitudinal sections. Images were taken randomly for each fiber as it first appeared in the field of view as the grid was scanned, with care taken to avoid nuclear regions. If an individual mitochondrion extended from one band to the other or across the Z line, it was counted twice.
Table 1.
Quantitative analysis of mitochondria, CRUs, and CRU-mitochondrion pairing in subsarcolemmal regions of FDB muscle fibers
| Sampling |
A |
B |
C |
D |
|
|---|---|---|---|---|---|
| Sampling | Total Subsarcolemmal Length Analyzed, μm | No. of CRUs/100 μm | No. of Mitochondria/100 μm | No. of CRUs Associated With Mitochondria/100 μm | |
| 0.5 mo | 576 | 57 ± 34 | 50 ± 42 | 5 ± 10 (10%) | |
| 1 mo | 488 | 67 ± 43 | 50 ± 40 | 18 ± 21 (24%) | |
| 2 mo | 486 | 82 ± 46 | 60 ± 52 | 34 ± 29 (40%) | |
| 4 mo | 352 | 114 ± 46 | 112 ± 60 | 74 ± 42 (67%) |
Values are means ± SD. Values in parentheses in column D represent percentage of Ca2+ release units (CRUs) coupled (or associated) to mitochondria. Sampling consisted of 2 mice for each time point and ≥20 flexor digitorum brevis (FDB) fibers for each mouse. Frequency of CRUs in the subsarcolemmal region increases progressively with age (column B), whereas frequency of mitochondria does not change significantly at ≤2 mo of age but doubles at 4 mo of age (column C). Percentage of CRUs associated with mitochondria increases progressively from 0.5 to ≤4 mo of age (column D). Initially (0.5–1 mo of age), mitochondria are mostly grouped in clusters under the sarcolemma. Only later are they targeted to parajunctional positions. Significant increase in frequency of CRUs and mitochondria from 2 to 4 mo of age contributes to increase in CRU-mitochondrion pairs. Statistical significance was determined by Student's t-test (P < 0.0005).
Confocal imaging and analysis of osmotic shock-induced Ca2+ sparks.
FDB fibers were plated on glass coverslips in isotonic Ringer solution (in mM: 140 NaCl, 4 KCl, 1 MgSO4, 1.8 CaCl2, 5 NaHCO3, 10 glucose, and 10 HEPES, pH 7.4, ∼300 mosM), loaded with 10 μM fluo 4-AM for 60 min at room temperature, and then exposed for 10 min to dye-free solution. To induce Ca2+ sparks, fibers were immersed in hypertonic high-Ca2+ Ringer solution (in mM: 140 NaCl, 4 KCl, 1 MgSO4, 50 CaCl2, 5 NaHCO3, 10 glucose, and 10 HEPES, pH 7.4, ∼450 mosM) (34). Ca2+ sparks were recorded using a confocal microscope (Nikon Eclipse C1 Plus) equipped with a ×40 1.3 NA oil objective (SuperFluor). With the focal plane centered along the fiber periphery, a series of line scans (512 pixels × 1,024 lines, 2 ms/line) parallel to the long axis of the fiber (i.e., spanning multiple sarcomeres) were acquired. Images were analyzed offline using CaSparks automated detection software. F0 was defined as the average fluorescence level immediately before each automatically detected event. Broad event detection limits were set to identify all events with amplitudes of 0.2–6 ΔF/F, full widths at half-maximal amplitude (FWHM) of 0.5–50 μm, rise times of 0.5–100 ms, and full durations at half-maximal amplitude (FDHM) of 0.5–1,000 ms. Spark mass was calculated as amplitude ∗ 1.206 ∗ FWHM3, according to Hollingworth et al. (12), with the assumption that the scan line runs through the location of the local Ca2+ release event and that the three-dimensional volume of the event is isotropic in space and approximated by the product of three Gaussian functions. No correction was made for the contribution of out-of-focus events.
Statistics.
To enable comparison between different fibers within each experiment, confocal images were recorded using identical laser power and photomultiplier sensitivity and processed using identical values for contrast and brightness. Statistical significance between relative changes in myoplasmic fluo 4 fluorescence and mitochondrial rhod 2 and mt-pericam fluorescence (P < 0.05) and Ca2+ spark spatiotemporal properties (P < 0.001) was determined using Kruskal-Wallis one-way ANOVA with Dunn's post hoc tests.
RESULTS
Sustained mitochondrial Ca2+ accumulation following tetanic stimulation in rhod 2-loaded FDB fibers.
We monitored mitochondrial Ca2+ accumulation immediately following low- and high-frequency electrical stimulation in single mouse FDB fibers in which rhod 2 was preferentially loaded into mitochondria. In resting fibers, basal rhod 2 fluorescence was low, and no detectable change in rhod 2 fluorescence was collected in x-y confocal images 690 ms after twitch stimulation (Fig. 1C). In contrast, a robust increase in I band-delimited mitochondrial rhod 2 fluorescence (i.e., MTG-positive areas) was observed following a single high-frequency tetanus (100 Hz for 500 ms) sufficient to produce a sustained myoplasmic Ca2+ signal detected using mag-fluo 4 that decayed rapidly following termination of stimulation (Fig. 1, A and C). Specifically, each high-frequency tetanus evoked a rapid, robust, and sustained global myoplasmic Ca2+ transient (Fig. 1A, inset). The average rise time of the first twitch reached a peak within just a few milliseconds (3.86 ± 0.02 ms), and summation during the first tetanus resulted in a peak change in relative mag-fluo 4 fluorescence (ΔF/F0 = 1.66 ± 0.24) that occurred within a few tens of milliseconds after initiation of the train (33.8 ± 1.4 ms) and remained elevated throughout the tetanus. Consistent with rapid termination of release and efficient Ca2+ clearance, mag-fluo 4 fluorescence abruptly decayed after termination of the tetanic stimulation train. Subsequent tetani elicited largely the same response as the first train, with a progressive reduction in peak amplitude after ∼15–20 tetani (Fig. 1B).
To more rigorously quantify the time course of the increase and decay of the mitochondrial rhod 2 signal compared with the myoplasmic Ca2+ transient, FDB fibers from adult mice were coloaded with myoplasmic (fluo 4) and mitochondrial (rhod 2) Ca2+ dyes and stimulated by a series of five successive tetani (100 Hz for 500 ms at 2.5-s intervals; Fig. 1A). The myoplasmic Ca2+ signal (relative change in fluo 4 fluorescence or ΔF/F0) was maximal during the first tetanus (5.69 ± 0.63 ΔF/F0) and decayed rapidly to near-baseline levels (0.48 ± 0.05 ΔF/F0) after completion of the fifth tetanus (Fig. 2, A and B). As indicated above, a large and sustained increase in I band-delimited rhod 2 fluorescence occurred after the first tetanus (Fig. 2A, bottom). A minimal increase in A band-delimited rhod 2 fluorescence with kinetics identical to those of fluo 4 was presumably due to a low-level myoplasmic rhod 2 dye (Fig. 2B). Given that the rhod 2 signal within the I band region represents the sum of mitochondrial and myoplasmic components and that mitochondria are not normally present within the A band region of FDB fibers from adult mice (4), we used the difference between I band and A band rhod 2 fluorescence (I − A) as an index of the relative changes in Ca2+ within mitochondria located in the I band and associated with the CRU (4) (referred to here as “triadic mitochondria”). This analysis revealed a significant increase in the triadic mitochondrial Ca2+ signal after only one tetanus (2.72 ± 0.39) that was further increased after five successive tetani (4.12 ± 0.45). Importantly, the triadic mitochondrial Ca2+ signal remained significantly elevated over baseline during the subsequent 10 min, even in the absence of further stimulation (Fig. 2C). The results in Fig. 2 reveal a remarkable difference between the activation and decay kinetics of the myoplasmic and triadic mitochondrial Ca2+ signals following repetitive tetanic stimulation.
Mitochondrial Ca2+ accumulation following twitch and tetanic stimulation in mt-pericam-expressing FDB fibers.
Despite the marked difference between the time courses of the fluo 4 and triadic rhod 2 signals and the corrections made for the triadic rhod 2 signal (I − A) in Fig. 2, a potential contribution of low-level myoplasmic dye is a limitation of the rhod 2 experiments. To overcome this limitation and more rigorously evaluate changes in mitochondrial Ca2+ during single-twitch and repetitive high-frequency tetanic stimulation, we expressed mt-pericam in FDB muscle by in vivo electroporation. Because mt-pericam is specifically targeted to mitochondria, it enables assessment of mitochondria Ca2+ in the absence of any contamination from concurrent changes in myoplasmic Ca2+. Consistent with prior studies (25), we observed rapid and short-lived (<200 ms) mitochondrial-specific Ca2+ uptake during a twitch stimulation in confocal line scan measurements of mt-pericam fluorescence in expressing FDB fibers [−ΔF(402 nm)/F0(402 nm) = 0.19 ± 0.02; Fig. 3B]. On the other hand, five successive high-frequency tetani (100 Hz for 500 ms at 2.5 s intervals) elicited a larger (0.25 ± 0.01) and more sustained increase in mitochondrial Ca2+, taking >10 min to fully decay to baseline (Fig. 3, A and C). These latter results are essentially identical to those observed for rhod 2-loaded fibers in Fig. 2, confirming a robust and sustained Ca2+ increase in triadic mitochondria following only several brief tetani.
Sustained Ca2+ accumulation in triadic and longitudinal mitochondria following repetitive tetanic stimulation.
FDB fibers from young (1-mo-old) mice exhibit triadic mitochondria and longitudinal clusters of subsarcolemmal and intermyofibrillar mitochondria (4). To directly assess mitochondrial Ca2+ uptake in triadic and longitudinal mitochondria, we compared the magnitude and time course of changes in mitochondrial rhod 2 fluorescence following repetitive tetanic stimulation (40 tetani, 100 Hz, 500 ms, 2.5-s intervals) in FDB fibers from young (1-mo-old) and adult (4-mo-old) mice. Consistent with results shown in Figs. 1 and 2, robust triadic mitochondrial Ca2+ accumulation was observed in FDB fibers from 4-mo-old mice following the first tetanus, was increased for the next 5–10 tetani, and remained elevated even after 40 tetani (3.78 ± 0.42, 5.36 ± 0.52, 6.32 ± 0.54, and 6.62 ± 0.55, respectively; Fig. 4, A and C). Only a minimal A band-associated rhod 2 signal was observed in FDB fibers from young (1-mo-old) and adult (4-mo-old) mice (Fig. 4B).
Unexpectedly, the time course of mitochondrial Ca2+ uptake and decay was similar for CRU-paired triadic and longitudinal mitochondria in fibers from young mice (Fig. 4, A and C). The prominent triadic and longitudinal mitochondrial Ca2+ signals remained elevated throughout the repetitive tetanic stimulation protocol, persisted to about the same level for >1 min following the 40th tetanus, exhibited half times of decay of ∼7 min, and required 20 min to return completely to baseline levels (Fig. 4, A and C). The extremely slow decay of the triadic and longitudinal mitochondrial Ca2+ signals is in direct contrast to the rapid decay of the myoplasmic Ca2+ signal following the termination of tetanic stimulation. Thus, repetitive tetanic stimulation triggers a similar sustained accumulation of Ca2+ in the triadic and longitudinal mitochondrial populations.
Mitochondrial Ca2+ accumulation occurs in the absence of mitochondrial depolarization.
To assess potential effects of mitochondrial Ca2+ uptake on the mitochondrial membrane potential, we simultaneously monitored myoplasmic fluo 4 fluorescence and mitochondrial TMRE fluorescence during repetitive tetanic stimulation (Fig. 5). Similar to MTG fluorescence intensity profiles (Fig. 1C), FDB fibers loaded with TMRE exhibit clear double rows of transverse red fluorescence, consistent with efficient loading of the dye into I band-delimited triadic mitochondria. The repetitive tetanic stimulation protocol used in Figs. 1–4 to trigger robust and sustained mitochondrial Ca2+ uptake failed to significantly alter steady-state triadic TMRE fluorescence in FDB fibers from young (1-mo-old) and adult (4-mo-old) mice (Fig. 5, A and C). Thus, mitochondrial Ca2+ uptake during repetitive tetanic stimulation does not result in a sustained depolarization of the mitochondrial membrane potential. However, because the single x-y TMRE images shown in Fig. 5A were taken between each tetanus, transient changes in mitochondrial membrane potential that may have occurred during tetanic stimulation would have escaped detection. Nevertheless, the maintenance of robust electrically evoked SR Ca2+ release (Fig. 5B) and steady-state triadic mitochondrial TMRE fluorescence (Fig. 5C) throughout the repetitive tetanic stimulation protocol demonstrates that sarcolemmal and mitochondrial membrane potentials remained intact throughout these experiments.
Triadic and longitudinal mitochondria are continuous and structurally tethered to the SR.
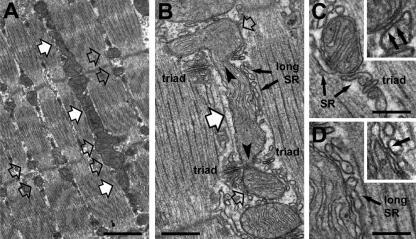
We previously demonstrated that mitochondrial disposition changes during postnatal maturation of fast-twitch skeletal fibers from mice (4). Initially (at 0.5 mo of age), most mitochondria are clustered longitudinally between myofibrils or under the sarcolemma. Numbers of mitochondria and CRUs increase substantially over the subsequent few months (1–4 mo). During this time, mitochondria progressively move to the I band adjacent to CRUs (triadic mitochondria), forming two distinct transversal networks, one on either side of the Z line (Fig. 6A, open arrows). Longitudinal rows of intermyofibrillar mitochondria, frequent at 0.5–1 mo of age, are still present occasionally in fast-twitch fibers even at 2–4 mo of age (Fig. 6A, white arrows). A more detailed EM characterization of the longitudinal mitochondrial population enabled determination of morphological features that may explain the functional data shown in Fig. 4. First, longitudinal mitochondria are structurally continuous with triadic mitochondria (Fig. 6B, arrowheads). Second, triadic and longitudinal mitochondria are closely associated with the SR: triadic mitochondria are in close proximity to CRUs at the I band (Fig. 6, B and C), while longitudinal mitochondria are surrounded by nonjunctional SR (Fig. 6, B and D) at the A band (Fig. 6B). Importantly, high-magnification EM analysis revealed that triadic and longitudinal mitochondria are structurally tethered to the SR by small electron-dense strands that appear to hold the two organelles together, creating a restricted ∼10- to 15-nm-wide subcellular space (Fig. 6, C and D, black arrows).
Fig. 6.
Triadic and longitudinal mitochondria are continuous and structurally tethered to the sarcoplasmic reticulum (SR). A: representative electron micrograph (longitudinal section) of an FDB fiber from a 4-mo-old mouse highlighting location of longitudinal (white arrows) and triadic (open arrows) mitochondria. B: representative electron micrograph highlighting 1) continuity (arrowheads) between triadic (open arrows) and longitudinal (white arrow) mitochondria and 2) association with the longitudinal SR (long SR, small black arrows). C and D: under higher magnification, small electron-dense strands (insets, black arrows) tether mitochondria to the triad or the longitudinal (long) SR. Scale bars, 1 μm (A), 0.5 μm (B), and 0.25 μm (C and D).
Osmotic shock induces changes in subsarcolemmal structure.
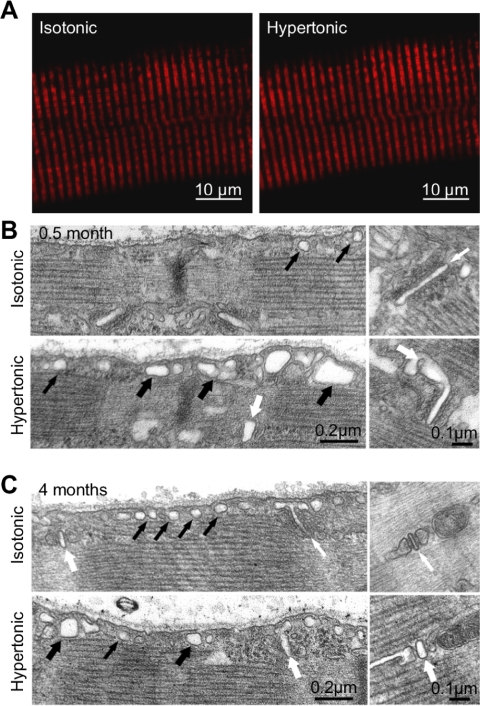
Exposure to hyperosmotic solution elicits peripherally located local Ca2+ release events (or “Ca2+ sparks”) in skeletal muscle fibers (34). Here, we determined the effect of osmotic shock on muscle fiber ultrastructure. This treatment did not grossly alter triadic mitochondrial localization, since the characteristic double-row banded pattern of MitoTracker Red CMXRos (MTR) fluorescence was retained in FDB fibers from 4-mo-old mice following exposure to hypertonic high-Ca2+ Ringer solution (Fig. 7A). More detailed EM analyses confirmed the absence of major ultrastructural changes within the fiber interior following osmotic shock in fibers from 0.5- or 4-mo-old mice. On the other hand, osmotic shock induced detectable changes within subsarcolemmal regions of the fiber perimeter (i.e., within 1 μm under the surface membrane in EM images; Fig. 7, B and C). Specifically, exposure to hypertonic high-Ca2+ Ringer solution induced swelling of T-tubules and caveolae just beneath the surface membrane, the extent of which was variable within a given field of view. Importantly, osmotic shock produced very similar structural changes in FDB fibers from 0.5- and 4-mo-old mice. The only significant difference between the two postnatal time points was a higher percentage of fibers from young (0.5-mo-old) mice exhibiting subsarcolemmal and intermyofibrillar clusters of mitochondria, consistent with the progressive relocation of mitochondria from longitudinal rows to parajunctional positions within the I band (Table 1) during postnatal development, as described previously (4).
Fig. 7.
Exposure to hypertonic high-Ca2+ osmotic shock induces swelling of T-tubules and caveolae. A: representative confocal images of MitoTracker Red CMXRos (MTR) fluorescence in a FDB fiber from a 4-mo-old mouse before (left) and after (right) exposure to hypertonic high-Ca2+ osmotic shock. B and C: electron micrographs (longitudinal sections) of FDB fibers from 0.5- and 4-mo-old mice exposed to isotonic or hypertonic high-Ca2+ solution before fixation. Left: subsarcolemmal regions; right: T-tubule profiles within triads at higher magnification. Small black arrows, caveolae; large black arrows, dilated caveolae; small white arrows, T-tubules; large white arrows, dilated T-tubules.
Altered frequency and spatiotemporal properties of osmotic shock-induced Ca2+ sparks during postnatal maturation.
We compared the incidence and properties of Ca2+ sparks induced by exposure to hypertonic high-Ca2+ (450 mosM) Ringer solution at different stages of postnatal maturation (0.5, 1, 2, and 4 mo) that exhibit marked differences in mitochondrion-CRU tethering (4). After exposure to hypertonic high-Ca2+ solution, FDB fibers of all ages exhibited local Ca2+ release events (Fig. 8A). Activity persisted for the subsequent hour of experimentation, primarily at the fiber periphery. Indeed, triads potentially involved in mediating peripherally localized Ca2+ sparks were found within a few micrometers of the sarcolemma (Fig. 7, B and C, Table 1). Because the external solution contained Ca2+, a contribution of Ca2+ influx cannot be excluded. However, the quantal nature of the events argues against nonspecific membrane tears.
Fig. 8.
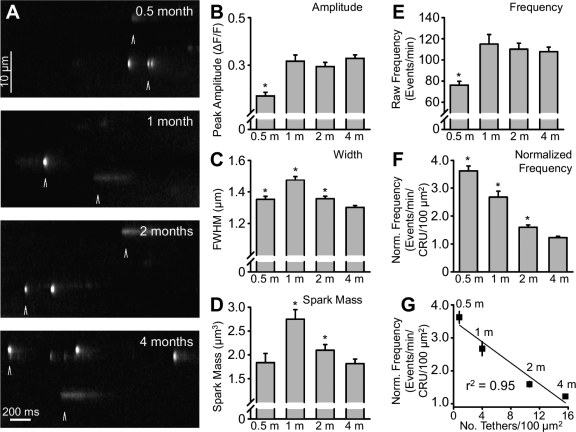
Properties of osmotic shock-induced Ca2+ sparks in FDB fibers during postnatal development. A: representative x-t line scan images of Ca2+ sparks in FDB fibers from 0.5-, 1-, 2-, and 4-mo-old mice. For display purposes, images were processed using a Gaussian smoothing function and median filter and then normalized to background. Arrows mark representative “Ca2+ sparks” and “Ca2+ bursts.” B: peak Ca2+ spark amplitude. C: full width at half-maximal amplitude (FWHM). D: Ca2+ spark mass calculated as amplitude ∗ 1.206 ∗ FWHM3 (12). E and F: raw Ca2+ spark frequency and Ca2+ spark frequency normalized to Ca2+ release unit (CRU) density, with 21 ± 5, 43 ± 9, 69 ± 12, and 88 ± 25 CRUs/100 μm2 used for fibers from 0.5-, 1-, 2-, and 4-mo-old mice, respectively (4). G: correlation between normalized Ca2+ spark frequency and tether density, with 0.8, 4.0, 10.6, and 15.6 tethers/100 μm2 used for fibers from 0.5-, 1-, 2-, and 4-mo-old mice, respectively. Values are means ± SE; n = 875 (0.5-mo-old), 710 (1-mo-old), 1,286 (2-mo-old), and 2,278 (4-mo-old) events. *P < 0.001 vs. 4-mo-old mice.
Ca2+ spark frequency and morphology varied throughout postnatal maturation. At all ages, brief (∼20 to 30 ms) local Ca2+ release events (termed Ca2+ sparks) with spatiotemporal properties similar to evoked Ca2+ sparks identified by others in mammalian skeletal muscle preparations (6, 13, 14, 34) were observed (Fig. 8A). In addition, longer-duration (>100 ms) events (Fig. 8A), analogous to previously termed Ca2+ bursts (34), were also detected. However, since the spatiotemporal properties of the short- and long-duration events exhibited similar changes with age, all subsequent analyses were conducted on the entire population of events and, hereafter, are collectively referred to as Ca2+ sparks.
Mean peak Ca2+ spark amplitude, spatial width, and mass at the different postnatal ages (0.5, 1, 2, and 4 mo) are summarized in Fig. 8, B–D. Mean peak Ca2+ spark amplitude was significantly lower in fibers from 0.5-mo-old mice (0.3 ± 0.01 ΔF/F) than in fibers from 1-, 2-, and 4-mo-old mice, which exhibited similar average peak amplitudes (0.4 ± 0.01, 0.4 ± 0.01, and 0.4 ± 0.01 ΔF/F, respectively; Fig. 8B). A similar postnatal trend was also observed for average Ca2+ spark rise time (data not shown). In contrast, average FWHM peaked at 1 mo (38.2 ± 1.0 ms) and was reduced at 0.5, 2, and 4 mo (29.5 ± 0.8, 33.3 ± 0.7, and 33.9 ± 0.5 ms, respectively; Fig. 8C). Calculated spark mass [amplitude ∗ 1.206 ∗ FWHM3 (12)] was greater at 1 mo than at all other time points (1.8 ± 0.2, 2.8 ± 0.2, 2.1 ± 0.1, and 1.8 ± 0.1 μm3 for 0.5, 1, 2, and 4 mo, respectively; Fig. 8D).
The most dramatic effect of postnatal maturation was on Ca2+ spark frequency (Fig. 8, E–G). Raw Ca2+ spark frequency followed the identical pattern of amplitude and FDHM (data not shown), being lowest at 0.5 mo (76.1 ± 3.7 events/min) and significantly higher in FDB fibers from 1-, 2- and 4-mo-old mice (115.0 ± 9.1, 110.1 ± 5.7, and 107.8 ± 4.3 events/min, respectively; Fig. 8E). This trend is consistent with the increase in the CRU number and density during postnatal maturation in subsarcolemmal (i.e., <1 μm from the sarcolemma; Table 1) and internal regions of myofibers (4). To evaluate Ca2+ spark frequency per CRU, we normalized raw Ca2+ spark frequencies to the CRU density at each developmental stage. Osmotic shock-induced Ca2+ sparks in our experiments occurred primarily at the fiber periphery, within 5 μm from the surface membrane. Given this relatively large spatial range from the surface membrane, we normalized the raw Ca2+ spark frequencies using the corresponding “internal” CRU densities at each developmental stage (4). Significantly, Ca2+ spark frequency per CRU density decreased progressively throughout postnatal development (3.6 ± 0.2, 2.7 ± 0.2, 1.6 ± 0.1, and 1.2 ± 0.1 events/min/CRU/100 μm2 at 0.5, 1, 2, and 4 mo, respectively; Fig. 8F). More importantly, a strong linear correlation (r2 = 0.95; Fig. 8G) was observed between the normalized Ca2+ spark frequency and the mitochondrion-CRU tether density determined previously (4).
DISCUSSION
This study provides important new information with regard to SR-mitochondrial communication in skeletal muscle. 1) Mitochondrial Ca2+ uptake was robust and long-lived (lasting several minutes) following repetitive tetanic stimulation (Figs. 2–4) in the absence of sustained depolarization of the mitochondrial membrane potential. 2) Unexpectedly, sustained mitochondrial Ca2+ uptake following repetitive tetanic stimulation was similar for triadic and longitudinal mitochondrial populations (Fig. 4), even though the two different populations are located at substantially different positions relative to the CRU. 3) Sustained mitochondrial Ca2+ uptake was not accompanied by a steady-state change in mitochondrial membrane potential (Fig. 5). 4) As observed previously for CRU-coupled mitochondria (4), high-resolution EM identified restricted sites of contact between longitudinal mitochondria and the nonjunctional SR that are mediated by electron-dense tethers (Fig. 6). 5) A progressive increase in retrograde suppression of local SR Ca2+ release during postnatal maturation occurs in direct correspondence with a parallel increase in mitochondrial triadic positioning (Fig. 8). These findings indicate that mitochondrial tethering to the CRU is more critical for Ca2+ spark suppression than for sustained mitochondrial Ca2+ uptake during tetanic stimulation. The relationship of these findings to prior published studies and their implications for skeletal muscle function are discussed in detail below.
Implications of sustained mitochondrial Ca2+ accumulation following tetanic stimulation.
Consistent with prior studies (25), mitochondrial Ca2+ uptake was transient (<200 ms) following a single electrically evoked twitch (Fig. 3B). On the other hand, mitochondria in FDB fibers from young (1-mo-old) and adult (4-mo-old) mice exhibited robust and sustained Ca2+ uptake following repetitive tetanic stimulation (Figs. 2–4). Importantly, we confirmed sustained mitochondrial Ca2+ uptake following repetitive tetanic stimulation in rhod 2-loaded (Figs. 2 and 4) and mt-pericam-expressing (Fig. 3) fibers. In the absence of calibration of these signals, the absolute level of the mitochondrial Ca2+ increase during tetanic stimulation remains unknown. However, the actual increase in mitochondrial free Ca2+ is likely to be rather modest, given the lack of a detectable depolarization of the mitochondrial membrane potential, a characteristic of mitochondrial Ca2+ overload, or overt muscle fiber damage following the prolonged repetitive tetanic stimulation used in this study.
Our results are in contrast to those of Lannergren et al. (18), who reported negligible mitochondrial Ca2+ uptake in toe fibers following tetanic stimulation. There are several possible explanations for this difference. Unlike the study of Lannergren et al., our experiments were conducted in the presence of BTS to block contraction (8, 28) and minimize movement artifacts. Given that BTS inhibits myosin ATPase activity (28), total ATP consumption during tetanic stimulation would be reduced in our experiments. As a consequence, there is a reduced need for mitochondrial ATP production and subsequent dissipation of the mitochondrial membrane potential, thus potentially enhancing the driving force for Ca2+ uptake. Alternatively, BTS may increase Ca2+ availability for mitochondrial Ca2+ uptake by reducing crossbridge cycling and Ca2+ binding to troponin C.
On the other hand, our results are consistent with those of Bruton et al. (7), who demonstrated significant mitochondrial Ca2+ accumulation during tetanic stimulation of mouse extensor digitorum longus and soleus muscle fibers, and Shkryl et al. (29), who reported mitochondrial Ca2+ uptake during caffeine-induced Ca2+ release in permeabilized extensor digitorum longus and soleus fibers. Furthermore, Aydin and colleagues (1) reported significant mitochondrial Ca2+ uptake in myopathic FDB fibers from Tfam (mitochondrial transcription factor A) knockout mice, although only a slight increase was observed in control fibers after 25 tetani. The smaller increase in mitochondrial Ca2+ uptake in control fibers observed by Aydin et al. could be due to the three- to four-times-longer duration of dye loading used in the prior study, resulting in significantly elevated resting rhod 2 fluorescence levels and, thus, a smaller relative increase in rhod 2 fluorescence upon stimulation. In any event, our results are the first to demonstrate significant mitochondrial Ca2+ accumulation in intact wild-type FDB fibers using a physiological tetanic stimulation paradigm.
The combination of efficient mitochondrial Ca2+ uptake and slow Ca2+ removal results in mitochondria functioning as “temporal Ca2+ integrators” during tetanic stimulation. Consequently, mitochondrial Ca2+ accumulation is sustained following repetitive tetanic stimulation and, thus, ideally suited to promote prolonged Ca2+ activation of ATP generation (20, 31) precisely when it is most needed, i.e., under conditions of intense muscle activity.
Potential mechanisms for sustained Ca2+ accumulation into triadic and longitudinal mitochondria following tetanic stimulation.
Since mitochondria in the I band are tethered to CRUs (4), we initially expected Ca2+ uptake to be greater in triadic than longitudinal mitochondria. Thus, our demonstration of similar mitochondrial Ca2+ uptake for both mitochondrial populations was unexpected. What mechanisms could account for comparable activity-dependent mitochondrial Ca2+ uptake in triadic and longitudinal mitochondrial populations? As one possibility, longitudinal and triadic mitochondria may respond to global Ca2+ signals, rather than restricted Ca2+ release microdomains within the immediate vicinity of RYRs located in the CRU. Indeed, triadic mitochondria are positioned on the side of the CRU opposite to the site of Ca2+ release, such that the minimal distance between the site of release and the closest outer mitochondrial membrane is usually ∼130 nm (4). For the few triadic mitochondria that run along the side of triads and extend into the longitudinal population (Fig. 6B), this distance may be somewhat reduced. Simulations of Ca2+ release in an isotropic medium indicate that Ca2+ release microdomains dissipate markedly only a few tens of nanometers and almost entirely ∼100 nm away from the point of release (30). Thus, mitochondria will experience a significant Ca2+ release microdomain only in cases where the distance between RYRs and the mitochondrial membrane is significantly <100 nm. Such cases are extremely rare in adult fibers but somewhat more frequent in developing muscle.
Given the low affinity of mitochondrial Ca2+ uptake mechanisms and location of mitochondria outside the RYR Ca2+ release microdomain, Ca2+ uptake by triadic and longitudinal mitochondria may involve a unique form of SR-mitochondrial communication. For example, continuity is observed between triadic and longitudinal mitochondrial matrixes, indicating that the two populations are not structurally separated. Thus, Ca2+ uptake by one population may influence the other via intramatrix transfer. In addition to a direct continuity, the SR is connected to triadic and longitudinal mitochondria by small strands or tethers that hold the two organelles together. Tethers create a restricted ∼10- to 15-nm-wide interorganelle space, referred to here as “SR-mitochondrial nanodomains” (Fig. 9). Although RYR Ca2+ release channels are unlikely to be present within these nanodomains, SERCA expression, which is extremely high throughout the nonjunctional SR (9, 15), should be high. Thus, mitochondrial Ca2+ uptake following tetanic stimulation could, in part, involve Ca2+ accumulation within SR-mitochondrial nanodomains during SERCA unidirectional reverse flux (or backflux) (27a, 27b). SERCA Ca2+ backflux, which is enhanced by ADP, may be sufficient (following repetitive tetanic stimulation) to limit or even surpass SR Ca2+ uptake and, thus, allow for accumulation of Ca2+ on the myoplasmic side of the SR membrane. By virtue of being tethered to nonjunctional SR (4), triadic and longitudinal mitochondria could experience a similar SERCA backflux during repetitive tetanic stimulation sufficient to activate mitochondrial Ca2+ uptake. Regardless of the mechanism, our observation that Ca2+ uptake following tetanic stimulation is similar for triadic and longitudinal mitochondria indicates that targeting to the CRU is not required for sustained mitochondrial Ca2+ uptake.
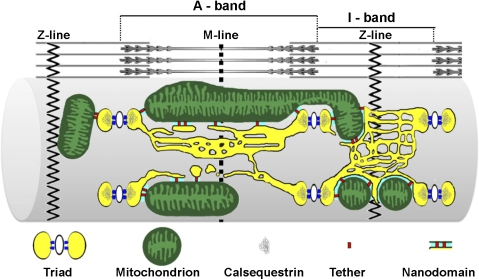
Fig. 9.
Schematic representation of intimate interactions between SR and both triadic and longitudinal mitochondria. Triadic and longitudinal mitochondria are associated with the SR by small strands or tethers, which hold the membranes of the 2 organelles close together, creating a restricted subcellular space ∼10–15 nm wide, referred to as the “SR-mitochondrial nanodomain.” Yellow, SR; white, T-tubules; blue, ryanodine receptor 1 feet; green, mitochondria; gray, calsequestrin; red, SR-mitochondrial tethers; cyan, nanodomains.
Increased mitochondrion-CRU coupling correlates with Ca2+ spark suppression.
Our results suggest that mitochondrion-CRU targeting plays a more important role in retrograde suppression of local Ca2+ release. Retrograde suppression of local Ca2+ sparks in mammalian skeletal muscle (13, 14, 19) is facilitated by close proximity of mitochondria to CRUs, although mitochondria-independent mechanisms of Ca2+ spark suppression may also be at play (16). In support of the former, we found that the frequency of osmotic shock-induced Ca2+ sparks per CRU density decreased threefold in FDB fibers during postnatal maturation, in direct correspondence with a parallel increase in mitochondrion-CRU pairing and tether density. Although this reduction in Ca2+ spark frequency could also reflect other changes in the CRU that occur during development, these findings are consistent with the local redox control mechanism for mitochondrial-mediated inhibition of local SR Ca2+ release championed by Shirokova and colleagues (13, 14, 19).
How might osmotic shock preferentially overcome Ca2+ spark suppression on the fiber periphery? Swelling of caveolae and T-tubules at the periphery may disrupt dihydropyridine receptor inhibition of spontaneous RyR1 Ca2+ release. In addition, changes in subsarcolemmal structure induced by osmotic shock may interfere with proper redox signaling within the CRU. Specifically, we found that osmotic shock produced dramatic structural changes in caveolae, small (50- to 100-nm) Ω-shaped invaginations of the sarcolemma (26). These structural changes in caveolae may disrupt scaffolding of critical signal transduction elements (e.g., caveolin-3 and neuronal nitric oxide synthase) (11, 26, 33) that enhance local ROS/RNS stress. Tethering of mitochondria to the CRU would ensure that mitochondrial ROS/RNS detoxification systems are strategically positioned to minimize this insult, although potentially less so at the fiber periphery, where caveolae are located. Future work is needed to test the validity of this hypothesis and to determine if the strong correlation between Ca2+ spark frequency and mitochondrial CRU tethering also holds for other triggers of Ca2+ sparks in skeletal muscle (e.g., strenuous exercise).
Conclusions and future perspectives.
In summary, we provide new insights into bidirectional SR-mitochondrial communication in skeletal muscle. Sustained mitochondrial Ca2+ uptake following tetanic stimulation is not restricted to triadic mitochondria and does not result in sustained mitochondrial depolarization. Conversely, a strong correlation exists between the progressive increase in mitochondrion-CRU tethering during postnatal maturation and a parallel reduction in susceptibility to Ca2+ spark activation, consistent with Ca2+ spark suppression being mediated by a retrograde signal from energized mitochondria to the adjacent CRU (13, 14, 19). Thus, mitochondrial tethering to the CRU is more critical for Ca2+ spark suppression than for sustained mitochondrial Ca2+ uptake. The results provide evidence for differential bidirectional SR-mitochondrial Ca2+ cross talk in skeletal muscle and justify future investigations to determine the role of this signaling mechanism in exercise, fatigue, and muscle disease. In addition, the importance of bidirectional SR-mitochondrial signaling may differ between fast- and slow-twitch muscle fibers, as each differs with respect to mitochondrial content and positioning, relative oxidative vs. glycolytic activities, and ROS production/scavenging capacities.
GRANTS
This research was supported by National Institutes of Health Grants AR-044657 (to R. T. Dirksen) and T32 DE-07202 (to A. E. Rossi), a Telethon Research Grant (GGP08153 to F. Protasi), and the Academia Dei Lincea Fund (to L. Wei).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Drs. Werner Melzer and Daniel Ursu (Department of Applied Physiology, University of Ulm, Ulm, Germany) for kindly providing access to and instruction in the use of their CaSparks analysis software and Dr. Graham Lamb for making the initial suggestion of SERCA backflux as a potential mechanism for privileged SR-mitochondrial communication in muscle.
REFERENCES
- 1. Aydin J, Andersson DC, Hanninen SL, Wredenberg A, Tavi P, Park CB, Larsson NG, Bruton JD, Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Hum Mol Genet 18: 278–288, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol 34: 1259–1271, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bolanos P, Guillen A, Rojas H, Boncompagni S, Caputo C. The use of CalciumOrange-5N as a specific marker of mitochondrial Ca2+ in mouse skeletal muscle fibers. Pflügers Arch 455: 721–731, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Boncompagni S, Rossi AE, Micaroni M, Beznoussenko GV, Polishchuk RS, Dirksen RT, Protasi F. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol Biol Cell 20: 1058–1067, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817–C833, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Brown LD, Rodney GG, Hernandez-Ochoa E, Ward CW, Schneider MF. Ca2+ sparks and T tubule reorganization in dedifferentiating adult mouse skeletal muscle fibers. Am J Physiol Cell Physiol 292: C1156–C1166, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruton J, Tavi P, Aydin J, Westerblad H, Lannergren J. Mitochondrial and myoplasmic [Ca2+] in single fibres from mouse limb muscles during repeated tetanic contractions. J Physiol 551: 179–190, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol 4: 83–88, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Chu A, Saito A, Fleischer S. Preparation and characterization of longitudinal tubules of sarcoplasmic reticulum from fast skeletal muscle. Arch Biochem Biophys 258: 13–23, 1987 [DOI] [PubMed] [Google Scholar]
- 10. DiFranco M, Neco P, Capote J, Meera P, Vergara JL. Quantitative evaluation of mammalian skeletal muscle as a heterologous protein expression system. Protein Expr Purif 47: 281–288, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem 272: 25437–25440, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Hollingworth S, Peet J, Chandler WK, Baylor SM. Calcium sparks in intact skeletal muscle fibers of the frog. J Gen Physiol 118: 653–678, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Isaeva EV, Shirokova N. Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal muscle fibres. J Physiol 547: 453–462, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isaeva EV, Shkryl VM, Shirokova N. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. J Physiol 565: 855–872, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jorgensen AO, Kalnins V, MacLennan DH. Localization of sarcoplasmic reticulum proteins in rat skeletal muscle by immunofluorescence. J Cell Biol 80: 372–384, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klein MG, Schneider MF. Ca2+ sparks in skeletal muscle. Prog Biophys Mol Biol 92: 308–332, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Lannergren J, Bruton JD. Mitochondrial Ca2+ in mouse soleus single muscle fibres in response to repeated tetanic contractions. Adv Exp Med Biol 538: 557–562, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Lannergren J, Westerblad H, Bruton JD. Changes in mitochondrial Ca2+ detected with Rhod-2 in single frog and mouse skeletal muscle fibres during and after repeated tetanic contractions. J Muscle Res Cell Motil 22: 265–275, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Martins AS, Shkryl VM, Nowycky MC, Shirokova N. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J Physiol 586: 197–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70: 391–425, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Nagai T, Sawano A, Park ES, Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc Natl Acad Sci USA 98: 3197–3202, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ogata T, Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec 248: 214–223, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Ramesh V, Sharma VK, Sheu SS, Franzini-Armstrong C. Structural proximity of mitochondria to calcium release units in rat ventricular myocardium may suggest a role in Ca2+ sequestration. Ann NY Acad Sci 853: 341–344, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Rossi AE, Boncompagni S, Dirksen RT. Sarcoplasmic reticulum-mitochondrial symbiosis: bidirectional signaling in skeletal muscle. Exerc Sport Sci Rev 37: 29–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rudolf R, Mongillo M, Magalhaes PJ, Pozzan T. In vivo monitoring of Ca2+ uptake into mitochondria of mouse skeletal muscle during contraction. J Cell Biol 166: 527–536, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schlegel A, Volonte D, Engelman JA, Galbiati F, Mehta P, Zhang XL, Scherer PE, Lisanti MP. Crowded little caves: structure and function of caveolae. Cell Signal 10: 457–463, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Sembrowich WL, Quintinskie JJ, Li G. Calcium uptake in mitochondria from different skeletal muscle types. J Appl Physiol 59: 137–141, 1985 [DOI] [PubMed] [Google Scholar]
- 27a. Shannon TR, Ginsburg KS, Bers DM. Reverse mode of the sarcoplasmic reticulum calcium pump and load-dependent cytosolic calcium decline in voltage-clampedcardiac ventricular myocytes. Biophys J 78: 322–333, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27b. Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res 91: 594–600, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Shaw MA, Ostap EM, Goldman YE. Mechanism of inhibition of skeletal muscle actomyosin by N-benzyl-p-toluenesulfonamide. Biochemistry 42: 6128–6135, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Shkryl VM, Shirokova N. Transfer and tunneling of Ca2+ from sarcoplasmic reticulum to mitochondria in skeletal muscle. J Biol Chem 281: 1547–1554, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Stern MD. Buffering of calcium in the vicinity of a channel pore. Cell Calcium 13: 183–192, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Territo PR, Mootha VK, French SA, Balaban RS. Ca2+ activation of heart mitochondrial oxidative phosphorylation: role of the F0/F1-ATPase. Am J Physiol Cell Physiol 278: C423–C435, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Vendelin M, Beraud N, Guerrero K, Andrienko T, Kuznetsov AV, Olivares J, Kay L, Saks VA. Mitochondrial regular arrangement in muscle cells: a “crystal-like” pattern. Am J Physiol Cell Physiol 288: C757–C767, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Venema VJ, Ju H, Zou R, Venema RC. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem 272: 28187–28190, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Weisleder N, Collet C, Zhou J, Chu Y, Hirata Y, Zhao X, Pan Z, Brotto M, Cheng H, Ma J. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat Cell Biol 7: 525–530, 2005 [DOI] [PubMed] [Google Scholar]