Abstract
Among the most prevalent and deadly primary brain tumors, high-grade gliomas evade complete surgical resection by diffuse invasion into surrounding brain parenchyma. Navigating through tight extracellular spaces requires invading glioma cells to alter their shape and volume. Cell volume changes are achieved through transmembrane transport of osmolytes along with obligated water. The sodium-potassium-chloride cotransporter isoform-1 (NKCC1) plays a pivotal role in this process, and previous work has demonstrated that NKCC1 inhibition compromises glioma invasion in vitro and in vivo by interfering with the required cell volume changes. In this study, we show that NKCC1 activity in gliomas requires the With-No-Lysine Kinase-3 (WNK3) kinase. Western blots of patient biopsies and patient-derived cell lines shows prominent expression of Ste-20-related, proline-alanine-rich kinase (SPAK), oxidative stress response kinase (OSR1), and WNK family members 1, 3, and 4. Of these, only WNK3 colocalized and coimmunoprecipitated with NKCC1 upon changes in cell volume. Stable knockdown of WNK3 using specific short hairpin RNA constructs completely abolished NKCC1 activity, as measured by the loss of bumetanide-sensitive cell volume regulation. Consequently, WNK3 knockdown cells showed a reduced ability to invade across Transwell barriers and lacked bumetanide-sensitive migration. This data indicates that WNK3 is an essential regulator of NKCC1 and that WNK3 activates NKCC1-mediated ion transport necessary for cell volume changes associated with cell invasion.
Keywords: cell migration, chloride transport, neurobiology, neurological diseases, sodium-potassium-chloride cotransporter isoform-1
gliomas, brain tumors thought to originate from glial cells, are among the most problematic primary cancers to treat. This is in part due to their propensity to penetrate surrounding brain tissue (23), thereby evading complete surgical resection. Unique to gliomas, tumor spread does not occur hematogenously, but rather, glioma cells invade and migrate into neighboring brain parenchyma guided by white matter tracts and blood vessels (15). Ion channel activity aids the rapid and dynamic volume and shape changes required to alter cellular volume (45). To modify cell volume, gliomas capitalize on ion release, most notably K+ and Cl−, to couple osmotically obligated water movement (43, 44). Ions must be concentrated intracellularly to favor their efflux down electrochemical gradients to provide this function, and previous research has demonstrated that sodium-potassium-chloride cotransporter isoform-1 (NKCC1) is likely responsible for Cl− accumulation in the cell, playing an integral role in hyperosmotic volume regulation and cell migration and invasion both in vitro and in vivo (9, 18). In addition, recent findings suggest that NKCC1 may also cotransport water along with ions (22), making it ideally suited to transport salt and water across the cell membrane required for cytoplasmic cell volume regulation.
To be exploited by gliomas to aid in migration and invasion, NKCC1 activity must be tightly regulated to respond to extracellular stimuli. NKCC1 phosphorylation activates NKCC1 activity through stimuli such as hormones and hyperosmotic stress (19). More recently, several upstream kinases have been identified including Ste-20-related, proline-alanine-rich kinase (SPAK) (38), and oxidative stress response kinase (OSR1) (2). SPAK and OSR1 have significant homology, overlapping functions within the cell, and both contribute to NKCC1 phosphorylation (14). They both are able to phosphorylate NKCC1 on three key threonine residues in the NH2-terminal region (16) and aid NKCC1-mediated cell volume regulation (8). The NH2-terminal region of NKCC1 serves as a scaffold with a binding motif for SPAK (13), which also happens to overlap with a binding motif of protein-phosphatase type 1 (PP1) (16). PP1 modulates NKCC1 activity through dephosphorylation and thus, decreasing its activity, especially during hypoosmotic stress (6, 11).
NKCC1 phosphorylation is also controlled by a family of kinases upstream of SPAK/OSR1 called the With-No-K (Lysine) kinases (WNK). The WNKs were first identified and cloned in 2001, but not initially linked to regulation of NKCC1 (46). Further research has indicated that the four distinct members: WNK1, WNK2, WNK3, and WNK4 (16, 30, 31, 34) contribute to tonicity regulation in neurons, epithelial cells, and the kidneys. Of particular interest to our study is the third member of this kinase family, WNK3. Holden and colleagues (24) recently isolated and characterized WNK3 expression. They noted that it had several alternatively spliced exons that are found throughout the body's tissues with particularly high expression in the brain. When coexpressed with NKCC1 in Xenopus oocytes, WNK3 bypasses NKCC1's tonicity requirements leading to maximal NKCC1-phosphorylation and transporter activity (27). In particular to cancer, WNK3 promotes cell survival in HeLa cells by decreasing apoptosis (47) and has the potential to be mutated in gliomas (35).
In this study, we investigate the contribution of WNK3 to glioma cell migration and invasion, hypothesizing that WNK3 regulation of NKCC1 enhances and enables gliomas' volume regulatory and migratory capabilities. In glioma cell lines and patient biopsies of normal and malignant brain, we find expression of SPAK, OSR1, and the WNK kinases. Furthermore, when stimulated by hyperosmolarity, we show a colocalization by immunofluorescence of NKCC1 and WNK3 and a novel physical interaction between NKCC1 and WNK3 by immunoprecipitation. Through short hairpin RNA (shRNA) knockdown, we demonstrate that WNK3 aids in glioma cell migration and is necessary for their volume regulation. These data suggest that WNK3 plays a critical role in glioma cells' ability to modify their volume for cell invasion and helps control the import of Cl− into the cell.
MATERIALS AND METHODS
Cell culture and solutions.
Experiments were performed using human glioma cells D54-MG (glioblastoma multiforme, WHO grade IV; Dr. D. Bigner, Duke University, Durham, NC), U87-MG (glioblastoma multiforme, WHO grade IV, ATCC, Manassas, VA), U251-MG (glioblastoma multiforme, WHO grade IV, ATCC), STTG1 (glioblastoma multiforme, WHO grade IV, ATCC), and GBM50 and GBM62 (locally derived glioma cell lines). HEK293 cells were obtained from ATCC. Cell were cultured under conditions previously described (18). NaCl bath solution used in volume regulation experiments (pH 7.4, osmolarity 310 ± 10 mosM) contained the following (in mmol/l): 130 NaCl, 5.0 KCl, 10.5 glucose, 32.5 HEPES, and 1 CaCl2. All reagents are from Sigma (St. Louis, MO) unless otherwise specified.
Cell volume measurements and proliferation.
Cell volumes were measured as previously described (9, 18) in NaCl bath solution kept at 37°C by electronic sizing with a Coulter counter Multisizer 3 (Beckman-Coulter, Miami, FL). Relative volume measurements were calculated as a ratio to the average of five baseline measurements before hyperosmotic challenge. The hyperosmotic, 10% increase in [NaCl] creates a NaCl bath solution with an osmolarity of ∼340 mosM. For proliferation assays, ∼5 × 104 cells were plated onto six-well cell culture dishes. On day 0, one well of cells was collected and cell number determined using a Coulter Counter Multisizer 3 (Beckman Coulter) to normalize cell number. Daily, samples of control and WNK3-KD cell were collected in triplicate and counted using a Coulter Counter Multisizer 3 (Beckman Coulter).
Immunocytochemistry.
D54 human glioma cells were cultured for 2–4 days on 12-mm glass coverslips (Macalaster Bicknell, New Haven, CT) in 7% serum-containing media. Medium was then aspirated, and cells were exposed to room temperature normosmotic or hyperosmotic (10% NaCl) PBS for 8 min. Cells were prepared for antibody application as in prior studies (4, 18).
Primary antibodies were then incubated overnight at 4°C. NKCC antibody (Developmental Studies Hybridoma Bank, Iowa City, IA), which recognizes both isoforms of NKCC, raised in mouse, was used at 1:1,000. Only NKCC1 is present in gliomas (9). WNK3 antibody (Novus Biologicals, Littleton, CA), raised in rabbits, was used at 1:500. The next day, cells were washed and incubated in secondary antibodies (1:500 of both goat anti-mouse Alexa 546; Invitrogen, Carlsbad, CA) and goat anti-rabbit Alexa 488 (Invitrogen) at room temperature for 90 min. Secondary antibodies were then washed off, and Hoechst was incubated at 100 μg/ml for 20 min at room temperature. Cells were then washed and mounted as described before (4, 18). Images were acquired with Slidebook 5.0 software (Intelligent Imaging Innovations, Denver, CO) using a Hamamatsu IEEE1394 digital CCD camera mounted on an Olympus IX81 microscope with an Olympus disc scanning unit to remove out-of-focus light. Images were acquired with a ×60 oil immersion lens as 10-μm stacks composed of 20 0.5-μm optical sections. Images were then interpolated, displayed in “three-view,” and normalized in Slidebook software. Confocal images were acquired using an Olympus Fluoview FV1000 microscope (Olympus, Center Valley, PA) with 405-nm, 473-nm, and 559-nm diode lasers for excitation. The colocalization tool of the FV1000 software package was used to quantify the amount of colocalization of WNK3 and NKCC1. One 1.57 μm (z-plane) was acquired of five different fields of view. Manual regions of interest (ROIs) were drawn around the cytoplasm of 5 different cells in each field of view and averaged to obtain the overlap coefficient for each image.
Western blot protocol.
Cultured cells were lysed, subjected to SDS-PAGE, and transferred to polyvinylidene difluoride paper, as in prior studies (18). The NKCC antibody was used to detect protein levels as described before (18). For other antibodies, membranes were blocked for 1 h at room temperature followed by a 2-h incubation with primary antibody in 5% milk in TBS plus Tween 20 (TBST). After three 15-min washes in TBST, membranes were placed in the appropriate horseradish peroxidase-conjugated secondary antibody at 1:2,000 (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature in 5% milk in TBST. After three more washes in TBST, blots were developed as previously described (18). The following antibodies were from Cell Signaling Technologies (Danvers, MA): WNK1 (1:1,000), WNK4 (1:3,000), SPAK (1:1,000), and OSR1 (1:1,000). WNK3 (1:5,000) was from Novus Biologicals (Littleton, CO), and the WNK2 antibody (1:10,000) was from Strategic Diagnostics (Newark, DE). WNK3 protein expression immunoreactivity was normalized to actin loading control using an Eastman Kodak Image Station 4000 MM (Kodak).
Transwell migration assay.
As in previous studies (20), 8.0-μm pore polyethylene teraphthalate track-etched membrane cell culture inserts (BD Biosciences, Sparks, MD) were prepared, and cells were allowed 5 h to migrate in the presence or absence of bumetanide. Cells were fixed, imaged, and counted as previously described (4).
shRNA and control stable cell lines.
For WNK3 knockdown, we obtained commercially available 29 mer shRNA constructs in a pRFP-C-RS vector containing either a nonsilencing, scrambled sequence (NS) or one of four sequences targeting WNK3 (Origene, Rockville, MD). The sequences are as follows: 125-GAGAGCCTCTAATCTGGTTGCTATGGAA; 126-ATGGCTCCAGAGATGTATGAAGAACACTA; 127-TTACCGTCCTTCAGGTATCAACCTCTGGA; 128-GAAGCAAGTTCTCCTAAGACAGTCATTCC.
D54 human glioma cells were transfected using the Amaxa Biosystems (Gaithersburg, MD) nucleofection technique as previously described (21).
Immunoprecipitation.
D54 glioma cells were cultured and subjected to normosmotic or hyperosmotic media (a 10% increase in [NaCl]) for 5 min before being lysed as above for the Western blot protocol. First, 500 μg of protein was diluted in 500 μl total volume of buffer, and all immunoprecipitation procedures were performed at 4°C. Prerinsed Protein-G Agarose beads (50 μl, Roche, Basel, Switzerland) were added to lysates and rotated for 30 min and spun at 14,000 rpm afterwards. Lysates were transferred to a new tube, and antibody or normal IgG was added in the following amounts: 3 μg NKCC, 1 μg WNK3, 1 μg IgG control, and allowed to rotate for 1 h. As before, 50 μl beads were added to lysates and rotated overnight. The next morning, lysates were spun as before, supernatant was removed, and beads were rinsed and spun five times. Protein from beads was eluted with 25 μl of 0.2 M glycine, pH 2.5, and incubated for 3 min with vortexing once each minute. Next, 6 μl of 6× sample buffer (18) was added, followed by 3 μl of 1 N NaOH to equilibrate pH. The entire eluate was loaded into a 4–20% precast gel (Bio-Rad, Hercules, CA) subjected to SDS-PAGE as in the above Western blot protocol.
Human tissue preparation.
Human tissue was procured from the Cooperative Human Tissue Network (CHTN) under approval of the Institutional Review Board for Human Use at the University of Alabama at Birmingham in Birmingham, AL. All human tissue was received as deidentified, frozen samples with limited pathological information. As per CHTN operating procedures, investigators are unable to obtain the key to the samples. Normal brain samples represent tissue from patients with a diagnosis of coronary artery disease, atherosclerosis, or pulmonary emboli. Tissue was prepared for Western blot analysis as above.
Statistical analysis.
For all experiments, raw data were analyzed and graphed using the Origin 7.5 software (Microcal Software), and appropriate statistical tests were chosen according to the data analyzed using GraphPad Instat (GraphPad Software) Data are reported and graphed with SD and *P < 0.05, **P < 0.01, or ***P < 0.001.
RESULTS
NKCC1 regulatory kinases are expressed in glioma cell lines and patient biopsies of normal brain and gliomas, grades I-IV.
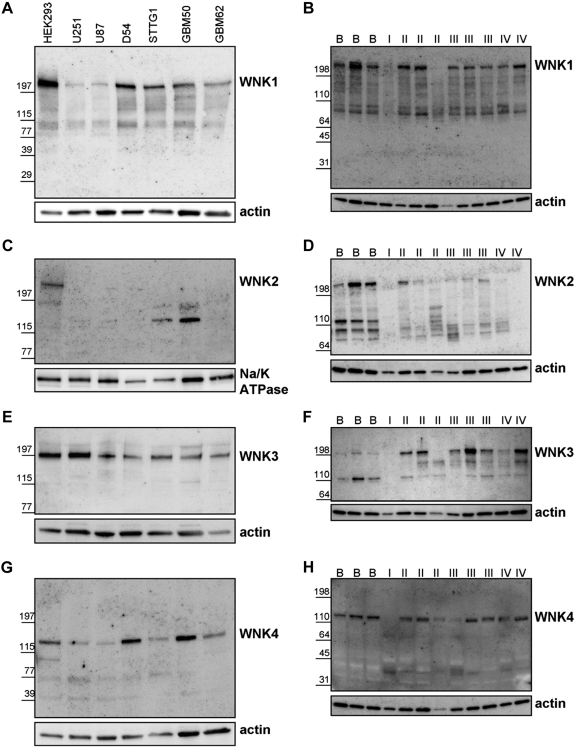
The central hypothesis in this study posits that regulation of NKCC1 by WNK3 aides in glioma cell invasion and migration. To initially examine which of the supposed kinases might regulate NKCC1, we assessed four widely used glioma cell lines (U87, U251, D54, and STTG1) and two lines recently established from patients (GBM50, GBM62) along with patient biopsies of normal and malignant brain for the presence of the six different possible regulatory kinases: SPAK, OSR1, and the four WNK kinase family members. HEK293 cells served as a positive control. We found robust and heterogeneous expression of NKCC1 (Fig. 1A), SPAK (Fig. 1C), and OSR1 (Fig. 1E) in glioma cell lines, decreased expression of SPAK (Fig. 1D) in glioma biopsies compared with normal brain, and heterogeneous expression of both NKCC1 (Fig. 1B) and OSR1 (Fig. 1F) in normal brain and glioma biopsies.
Fig. 1.
Sodium-potassium-chloride cotransporter isoform-1 (NKCC1), Ste-20-related, proline-alanine-rich kinase (SPAK), and oxidative stress response kinase (OSR1) expression in glioma cell lines and human patient biopsy tissue of normal brain and gliomas, grades I–IV. Representative Western blots of NKCC1 (A), SPAK (C), OSR1 (E), in grade IV glioma cell line whole cell lysates U251, U87, D54, and STTG1 as well as in more recent, locally derived glioma cell lines GBM50 and GBM62. HEK293 cell lysates serve as a positive control. Representative Western blots of NKCC (B), SPAK (D), and OSR1 (F) in lysates from normal human brain and glioma (grades I-IV) biopsies. (B = normal brain, I = glioma grade I, II = glioma grade II, III = glioma grade III, IV = glioma grade IV). Images are representative of at least 3 independent blots for each antibody. Actin serves as a loading control.
We also used these glioma cell lines and patient biopsies to evaluate expression of the four WNKs (Fig. 2). As assessed by Western blot, we detected heterogeneous expression of WNK1 (Fig. 2A), WNK3 (Fig. 2E), and WNK4 (Fig. 2G) across all glioma cells lines. We also detected heterogeneous expression of WNK1 (Fig. 2B) and WNK4 (Fig. 2H) in normal brain and all glioma grades. WNK2 was only expressed in the positive control cell line HEK293 near its predicted molecular mass of 242 kDa but not in glioma cell lines (Fig. 2C). Figure 2D found that WNK2 expression was reduced in malignant brain compared with normal brain patient biopsies. Finally, WNK3 expression is heterogeneous but elevated among gliomas compared with expression in normal brain (Fig. 2F). All high-grade glioma samples exhibited higher WNK3 expression than normal brain samples, and even low-grade gliomas expressed high levels of WNK3 when considering differences in protein loading (see actin loading control, Fig. 2F). Data in Figs. 1 and 2, taken together, suggest that WNK3 may be the most promising candidate that regulates NKCC1 function in these tumors. It is robustly expressed in glioma cell lines and elevated expression in glioma biopsy tissue.
Fig. 2.
The expression of NKCC1 regulatory kinases WNK1, WNK2, WNK3 and WNK4 in glioma cell lines and human patient biopsy tissue of normal brain and gliomas, grades I–IV. Representative Western blots of WNK1 (A), WNK2 (C), WNK3 (E), and WNK4 (G) in grade IV glioma cell line whole cell lysates U251, U87, D54, and STTG1 as well as in more recent, locally derived glioma cell lines GBM50 and GBM62. HEK293 cell lysates serve as a positive control. Representative Western blots of WNK1 (B), WNK2 (D), WNK3 (F), and WNK4 (H) in lysates from normal human brain and glioma (grades I-IV) biopsies. (B = normal brain, I = glioma grade I, II = glioma grade II, III = glioma grade III, IV = glioma grade IV). Images are representative of at least 3 independent blots for each antibody. Actin and Na-K-ATPase serve as loading controls.
NKCC1 and WNK3 colocalize after hyperosmotic stress.
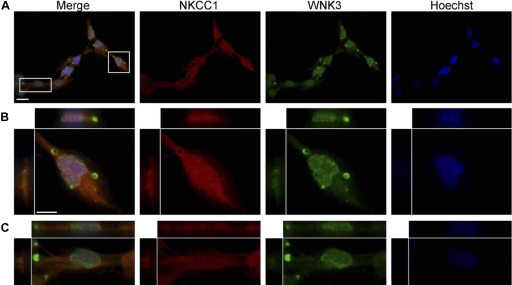
Previous research has already implicated the WNKs in cancers (35), especially WNK3 (47), and due to our results indicating higher WNK3 expression in gliomas, we focused on WNK3 as a potential regulator of NKCC1. Because NKCC1 is known to be activated by hyperosmotic stress (28, 29), we first investigated the subcellular localization of both NKCC1 and WNK3 during normosmotic (∼310 mosM) and hyperosmotic (10% increase in [NaCl], ∼340 mosM) conditions by immunocytochemistry. To examine the role of WNK3 as a functional regulator of NKCC1 activity, we elected to use the widely available D54 glioma cells as a model system. While these cells did not show the highest expression of either NKCC1 or WNK3, previous studies have shown that NKCC1 regulated their cell volume and that inhibition of NKCC1 function reduces tumor invasion in vivo (18). Also, we have generated stable NKCC1 knockdown strains of these cells (see below). Under normosmotic (∼310 mosM) condition, D54 glioma cells exhibited strong immunoreactivity for both NKCC1 (red channel) and WNK3 (green channel) throughout the cell (Fig. 3A) (cell nuclei were visualized with Hoechst, blue channel). In Fig. 3A, a representative field of view is shown. Digital magnifications of the highlighted, boxed cells are shown Fig. 3, B and C. Strikingly, WNK3 appears to localize not only diffusely across the cell body with apparent near-membrane localization but is concentrated in vacuole-like subcellular structures. We confirmed that the intense signal from the vacuole-like structures was indeed WNK3 by overlaying the DIC (differential interference contrast images) and WNK3 channel, along with no-secondary and no-primary antibody controls (arrows, Fig. 5A and data not shown, respectively). The D54 glioma cells clearly display multiple vacuole-like structures in DIC images, typical of these cells.
Fig. 3.
NKCC1 and WNK3 expression in D54 human glioma cells. A: immunofluorescent images of D54 human glioma cells exposed to normosmotic conditions, double labeled with antibodies targeting NKCC1 (red) and WNK3 (green). Nuclei are labeled with Hoechst (blue). B and C: digital magnifications of boxed cells in A. Scale bars 20 μm (A) and 10 μm (B). N = 3.
Fig. 5.
WNK3 localizes to vacuole-like subcellular compartments under normosmotic conditions. A: digital magnifications from Fig. 3B and 3C of immunofluorescent (WNK3, green) and DIC images of D54 human glioma cells under normosmotic conditions. B: digital magnifications from Fig. 4, B and C, of immunofluorescent (WNK3, green) and DIC images of D54 human glioma cells after 8 min in hyperosmotic conditions. Arrows indicate vacuole-like structures located in glioma cells. N = 3.
Sister cultures were also prepared and subjected to a hyperosmotic, 10% increase in [NaCl] condition (∼340 mosM) before immunocytological procedures were performed. After exposure to hyperosmotic conditions, WNK3 and NKCC1 colocalize throughout the cell body as is evident from the yellow signal in the merged images (Fig. 4A). As before, the Fig. 4, B and C, are digital magnifications of the highlighted, boxed cells from Fig. 4A. Not only do WNK3 and NKCC1 colocalize after hyperosmotic stress, but more importantly, WNK3 signal can be visualized as more punctuate and cytosolic throughout the cells. This suggests that upon hyperosmotic stimulation, WNK3 translocates from the vacuole-like structures to the cell cytoplasm. To confirm this assumption, we again acquired DIC images of the same cells in Fig. 4. We were able to detect vacuole-like structures in the DIC images but observed little to no WNK3 signal inside of these subcellular structures (Fig. 5, arrows). To confirm our qualitative observations, we acquired confocal images of both normosmotic and hyperosmotic conditions. Using the colocalization tool in the Olympus FV1000 software package, we obtained the overlap coefficient from ROIs of five different fields of view in each condition. The overlap coefficient is a measure of quantitative fluorophore colocalization and is relatively insensitive to differences in signal intensities. The overlap coefficient for WNK3 and NKCC1 in normosmotic was 0.1444 ± 0.0554. For hyperosmotic conditions, the overlap coefficient was 0.2335 ± 0.0130, which is a significant increase in the amount of colocalization (unpaired t-test, P = 0.0081). Taken together, Figs. 3–5 suggest that upon hyperosmotic stimulation, WNK3 is rapidly mobilized from cellular stores the cell cytosol.
Fig. 4.
NKCC1 and WNK3 expression in hyperosmotic-treated D54 human glioma cells. A: immunofluorescent images of D54 human glioma cells exposed to hyperosmotic conditions for 8 min, double labeled with antibodies targeting NKCC1 (red) and WNK3 (green). Nuclei are labeled with Hoechst (blue). B and C: digital magnifications of boxed cells in A. Scale bar 20 μm. N = 3.
Immunoprecipitation with WNK3 reveals a physical interaction with NKCC1.
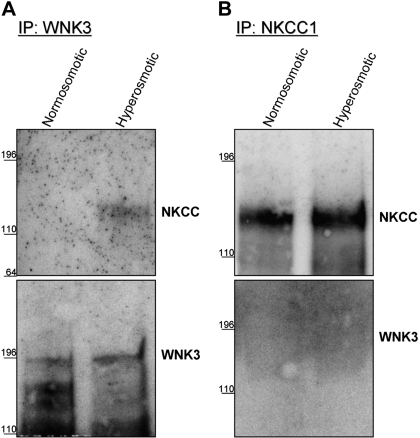
Although immunocytochemistry can indicate proximity of two proteins, it is limited in resolution and cannot definitively demonstrate physical interactions between proteins. To determine whether NKCC1 and WNK3 physically interact, cell lysates of sister cultures of D54 glioma cells were prepared after 5 min in either normosmotic or hyperosmotic conditions. We first used an affinity pulldown assay where antibody targeting WNK3 was used to pulldown WNK3 from both normosmotic (standard culture conditions, see materials and methods) and hyperosmotic (10% increase in [NaCl]) whole cell lysates. After immunoprecipitation (IP) with WNK3, a 120-kDa NKCC1 band could only be visualized by Western blot in the hyperosmotic condition (Fig. 6A) but not in the normosmotic control condition. Although NKCC1 only coimmunoprecipitated with WNK3 under hyperosmotic condition, WNK3 was present in approximately equal amounts in both conditions. We performed the converse experiment using a monoclonal antibody targeting NKCC and found that WNK3 did not co-IP with NKCC1 in either the normosmotic or hyperosmotic conditions (Fig. 6B) despite roughly equal amounts of NKCC1 being isolated from normosmotic and hyperosmotic treated glioma cells. Control IP experiments without primary antibody or normal IgG (rabbit, WNK3; mouse, NKCC1) confirmed that the bands in Fig. 6 were indeed WNK3 and NKCC1 (data not shown). This suggests that the NKCC1 antibody might be interfering with the physical interaction between WNK3 and NKCC1. These data reinforce the immunocytological findings in Figs. 3 and 4 suggesting that WNK3 translocates from vacuole-like structures to the cell cytoplasm upon hyperosmotic stimulation in D54 glioma cells.
Fig. 6.
NKCC1 coimmnuoprecipitates with WNK3 after hyperosmotic stress. A: immunoprecipitation of WNK3 coimmunoprecipitates NKCC1 only after hyperosmotic treatment. Equal amounts of WNK3 were pulled down. B: after immunoprecipitation of NKCC1, no WNK3 was detected despite equal amounts of NKCC1 being pulled down. Blots are representative of n = 3 experiments for WNK3 immunoprecipitation (A) and n = 5 for NKCC1 immunoprecipitation (B).
WNK3 regulation of NKCC1 is required for volume regulation in human glioma cells.
Having established that WNK3 interacts with NKCC1 under hyperosmotic stimulation, we sought to evaluate whether this interaction leads to a functional regulation of NKCC1 activity. Numerous studies have demonstrated that one of the important functions of NKCC1 is regulatory volume increase (RVI), which is used to reestablish normal cell volume after cells have been shrunk by exposure to hyperosmotic conditions (9, 18, 32, 37, 42). We generated a stable knockdown of WNK3 using shRNAs (WNK3-KD) in D54 glioma cells. Compared with the nonsilencing, scrambled shRNA transfected (NS) cells used as controls, the WNK3-KD cell line showed significantly reduced WNK3 expression as assessed by densitometry quantifications from Western blots (Fig. 7, D and E). The knockdown of WNK3 did not affect cell viability as rates of cell proliferation were unchanged (data not shown). When subjected to a hyperosmotic, 10% increase in [NaCl], NS glioma cells initially shrunk by 8–10% and regulated their volume back to baseline within 40 min, (Fig. 7A). As previously described (18), RVI was mediated by NKCC1 and was completely abolished by the presence of 200 μM bumetanide, a known NKCC1 inhibitor. In contrast, WNK3-KD cells lacked any sign of RVI, and hence, had lost the ability to regulate cellular volume when challenged hyperosmotically (Fig. 7B). The inability of the WNK3-KD cells to regulate their volume was unaffected by the presence of bumetanide. At 40 min postchallenge, the average normalized mean cell volume (MCV) of vehicle-treated NS cells (0.9975 ± 0.0228) was significantly different from bumetanide-treated NS cells (0.9119 ± 0.0063), vehicle-treated WNK-KD cells (0.9167 ± 0.0256), and bumetanide-treated WNK3-KD cells (0.9000 ± 0.017). These data not only suggest that WNK3 is necessary for RVI in human glioma cells, but that its presence is required for the normal response of NKCC1 under hyperosmotic conditions.
Fig. 7.
WNK3 knockdown abolishes regulatory volume increase (RVI) in human glioma cells. A: average normalized mean cell volumes (MCVs, n = 5,000–10,000 cells) of NS glioma cells undergoing RVI after hyperosmotic (15 mM NaCl) challenge in the presence or absence of 200 μM bumetanide. Experiments were performed three independent times. B: average normalized MCVs for WNK3-KD cells undergoing RVI after hyperosmotic challenge in the presence or absence of bumetanide. Experiments were performed three independent times. C: average NS and WNK3-KD cell normalized MCVs at 40 min post-hyperosmotic challenge. One-way ANOVA, P = 0.001, Tukey-Kramer post hoc test, **P < 0.01. D: image of Western blot showing WNK3 expression in NS and WNK3-KD cell lysates. Actin serves as a loading control. Blots are representative of N = 8 experiments from four different cell lysate preparations. E: normalized WNK3 expression in NS and WNK3-KD glioma cell lines. Unpaired t-test, P = 0.0009.
WNK3-KD inhibits glioma cell migration.
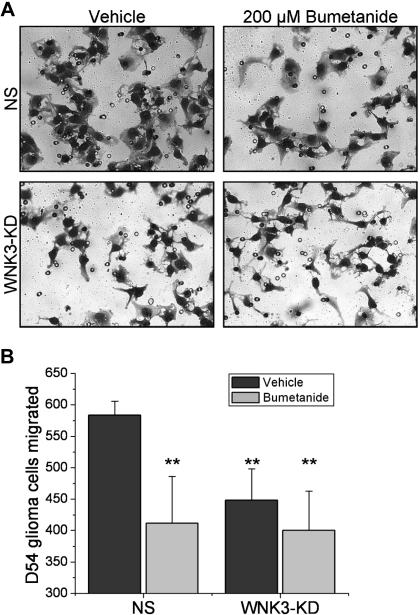
It has previously been suggested that NKCC1 and the above-described volume regulation by NKCC1 are essential to maintain normal cell invasion, allowing cells to reduce their volume to pass across narrow barriers to regain normal cell volume thereafter. As a consequence one would expect that WNK3-KD cells, showing impaired RVI, should also display impairments in cell invasion. Therefore, we assessed NS and WNK3-KD cells' ability to migrate three-dimensionally across Transwell barriers, which mimic the spatial constraints of the brain. The barriers require cells to pass through 8-μm pores by chemotactic attraction to vitronectin. After migrating for 5 h in the presence or absence of 200 μM bumetanide, the number of cells migrated across the Transwell barrier was quantified. Figure 8A shows representative images, used for counting cells that have migrated, of both NS and WNK3-KD cells in the presence and absence of bumetanide. After four independent experiments, the average number of vehicle-treated NS glioma cells that had migrated was 583.75 ± 22.321, whereas the average for bumetanide-treated NS cells was 448.5 ± 49.856. The average number WNK3-KD cells migrated was quite similar for both the vehicle (411.5 ± 74.56) and bumetanide (400.25 ± 62.782) treatments, which were comparable to the bumetanide-treated NS cells. The average number of NS cells migrated in the vehicle condition was significantly different from all other conditions (Fig. 8B). These data suggest that loss of WNK3 impairs glioma cell invasion to an equivalent degree as the pharmacological inhibition of NKCC1, again suggesting that WNK3 is an essential regulator of NKCC1 activity in glioma cell volume regulation and cell invasion.
Fig. 8.
WNK3 knockdown inhibits glioma cell invasion. A: representative DIC images of NS and WNK3-KD cells in the presence and absence of 200 μM bumetanide that have migrated through an 8-μm pore Transwell barrier for 5 h. B: average number of NS and WNK3-KD glioma cells migrated across an 8-μm pore Transwell barrier. Experiments were performed in triplicate and repeated four independent times. One-way ANOVA, P = 0.002, Tukey-Kramer post hoc test, **P < 0.01.
DISSCUSSION
Changes in cell volume are increasingly recognized as an important biology required for cancer cell invasion (3, 4, 10) and metastasis. Ion channels and transporters have emerged as essential molecular components, with the NKCC1 transporter taking a prominent role in establishing the required transmembrane ionic gradient used by cells to regulate their volume (5, 18, 33, 40, 44). However, the interdependence of cell invasion and cell volume changes and, most importantly, the regulation of this process, is poorly understood. In this study, we embarked on an initial examination of NKCC1 regulation, cell volume, and the ensuing effects on cell invasion using human malignant gliomas as a model system. Volume changes are particularly important for the dissemination of malignant gliomas, which exclusively use extracellular routes of invasion. In glioma cell lines and human biopsy tissues, we show expression of several NKCC1 regulatory kinases. These include SPAK, OSR1 and WNKs 1, 3, and 4, which are implicated in the phosphorylation of NKCC1. We demonstrate a unique role for WNK3 as the major regulator in human gliomas. More specifically, we observe that following a hyperosmotically induced decrease in cell volume, WNK3 interacts with and most likely phosphorylates NKCC1. A physical interaction of NKCC1 and WNK3 could be demonstrated by immunofluorescent colocalization and by immunoprecipitation. The downregulation of WNK3 by shRNA knockdown causes a loss of NKCC1 function which, in turn, compromises cell volume changes associated with cell invasion.
WNKs as regulators of cell volume.
WNKs have previously been implicated in volume control in tissues tasked with fluid transport such as the kidney (48, 49) and epithelium (8). More recently, they have also emerged as important regulators in the brain (7, 14, 32) where they appear to influence the neuronal Cl− gradient, which establishes whether the activation of GABA receptors is excitatory or inhibitory. More importantly, the WNKs have been implicated in Cl− flux in various cells types and are able to phosphorylate and regulate SPAK, OSR1, and other WNK family members (1, 12, 28, 29, 48). Finally, they have been described in a variety of different cancers to modulate proliferation, expression, and activity of several chloride-cation transporters, including NKCC, aide in the evasion of apoptosis, and enhance invasion and migration (35).
From these studies, our findings that WNK3 regulates NKCC1 activity may not be entirely surprising; however, we unexpectedly found that in gliomas, WNK3 only could associate with and regulates NKCC1 after a decrease in cell volume. We induced such a decrease experimentally by exposing cells to hyperosmotic conditions. We envision that as invading cells reduce their volume to squeeze through narrow passages, they similarly recruit WNK3 to activate NKCC1-mediated transport thereby reequilibrating normal cell volume. As such, we implicate WNK3 as an important feedback regulator of cell volume in a hydrodynamic model of cell invasion facilitated by dynamic changes in cell volume. Our experiments that show a disruption of volume regulation in WNK3-KD cells support this notion.
WNK kinases in cancer.
As regulators of NKCC1, the WNK kinases are in a unique position to regulate ion import into the cell and adjust cell volume in different extracellular conditions. Since the WNKs are sensitive to tonicity changes in the extracellular milieu, they are positioned to play a role in cancer where the volume changes associated with migration and invasion are accomplished through ion flux. Altered WNK expression levels have also been implicated in several studies examining cancers, including gliomas. WNK2 has been shown to inhibit cell proliferation through the modulation of the MEK1/ERK1 pathway in HeLa cells (36). In addition, two recent epigenetic studies in gliomas and meningiomas revealed a suppression of WNK2. In meningiomas, Jun and colleagues (26) showed that in higher grade tumors, over 70% had aberrantly methylated CpG islands in the promoter region of WNK2. They also found abnormal methylation in the WNK2 promoter in several glioma samples. The Costello laboratory has also produced a study showing a high degree of epigenetic silencing of WNK2 in over 30 infiltrative gliomas and that introducing functional WNK2 to glioma cells reduced their capacity to form colonies (25). Along with WNK2, Verissimo et al. (47) showed that WNK3 interacts with heat shock protein 70 in HeLa cells and increases cell survival by acting through caspase-3. Our observations are in accordance with these studies, as we showed a decrease in WNK2 in higher grade gliomas and an absence of it in our cell lines. From the findings of Verissimo et al., elevated WNK3 in gliomas would aid in their ability to be resistant to the standard treatments of chemotherapy and radiation.
Interaction of WNK3 and NKCC1.
Many of the interactions between the WNK kinases and SPAK/OSR1 have been detailed previously. For example, WNK1 and WNK4 are known to interact with each and even coregulate (16, 30). Both SPAK and OSR1 have binding domains located in the NH2-terminal region of NKCC1, and WNK1 can also interact with SPAK (28, 29, 34). Although one recent study showed a co-IP of SPAK and WNK3 and implicated WNK3 as a chloride-sensing kinase that regulated NKCC activity (39), another demonstrated that the brain isoform of WNK3 acted in tandem with SPAK instead of direct phosphorylation (17). Neither of these studies investigated the interaction of WNK3 and NKCC1 but only the phosphorylation of NKCC1. To our knowledge, this study demonstrates a possible novel physical interaction of WNK3 and NKCC1, though we did not determine whether this interaction occurred by direct binding of WNK3 to NKCC1 or was facilitated by an adaptor protein such as SPAK/OSR1. Increased colocalization and co-IP of NKCC1 with WNK3 were observed only after a hyperosmotic, 10% increase in [NaCl] that lasted no longer than 10 min in both immunocytological and IP procedures. These data could indicate a physical interaction of WNK3 and NKCC1 whether direct of via adaptor protein such as SPAK/OSR1. This time course would allow for the activation by phosphorylation of NKCC1 by regulatory kinases and would position WNK3 ideally as the Cl−/volume-sensitive kinase (27, 39).
Furthermore, one group demonstrated that NKCC1 could be maximally phosphorylated and activated by WNK3 when both WNK3 and NKCC1 were coexpressed in a Xenopus oocytes overexpression system. This phosphorylation allows for the activation and maximal rate of ion movement through NKCC1 (27). Although they did not examine the volume of the Xenopus oocytes they transfected, they most likely experienced an increase in cell volume, especially in light of our findings in Fig. 7 demonstrating the dependency of NKCC1 activation on WNK3. Additionally, recent evidence showing that water moves through the pore of NKCC1 (22) uniquely positions WNK3 to aid in cell volume regulation through regulation of NKCC1 activity. In the context of gliomas and other cancers, the ability to metastasize, migrate, and invade surrounding tissue away from the original tumor site is a key phenotype. Considering that blocking Cl− efflux (20, 41) or influx (18) inhibits glioma migration, it is possible that WNK3 is in an unique position to modulate cell migration. We also show in Fig. 8 that WNK3 contributes to glioma migration to the same level as NKCC1. This suggests that WNK3's effect on glioma migration is solely through its regulatory control of NKCC1. Understanding WNK3's role in aiding glioma migration and invasion will be pivotal in future studies examining WNK3 function both in vitro and in vivo.
GRANTS
This work was supported by National Institutes of Health Grants RO1-036692 and RO1-031234 to H. Sontheimer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author (s).
ACKNOWLEDGMENTS
We greatly appreciate Katie Haas for critical editing of this manuscript. The NKCC antibody developed by Forbush and Lytle was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
REFERENCES
- 1. Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci USA 103: 10883–10888, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen W, Yazicioglu M, Cobb MH. Characterization of OSR1, a member of the mammalian Ste20p/germinal center kinase subfamily. J Biol Chem 279: 11129–11136, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Chen Z, Zhang Z, Gu Y, Bai C. Impaired migration and cell volume regulation in aquaporin 5-deficient SPC-A1 cells. Respir Physiol Neurobiol 176: 110–117, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Cuddapah VA, Sontheimer H. Molecular interaction and functional regulation of ClC-3 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) in human malignant glioma. J Biol Chem 285: 11188–11196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cuddapah VA, Sontheimer H. Ion channels and tranporters in cancer. 2. Ion channels and the control of cancer cell migration. Am J Physiol Cell Physiol. First published in May 4, 2011; doi: 10.1152/ajpcell.00102.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Darman RB, Flemmer A, Forbush B. Modulation of ion transport by direct targeting of protein phosphatase Type I to the Na-K-Cl cotransporter. J Biol Chem 276: 34359–34362, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Delpire E, Austin TM. Kinase regulation of Na+-K+-2Cl− cotransport in primary afferent neurons. J Physiol 588: 3365–3373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J 409: 321–331, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Ernest NJ, Sontheimer H. Extracellular glutamine is a critical modulator for regulatory volume increase in human glioma cells. Brain Res 1144: 231–238, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ernest NJ, Weaver AK, Van Duyn LB, Sontheimer HW. Relative contribution of chloride channels and transporters to regulatory volume decrease in human glioma cells. Am J Physiol Cell Physiol 288: C1451–C1460, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gagnon KB, Delpire E. Multiple pathways for protein phosphatase 1 (PP1) regulation of Na-K-2Cl cotransporter (NKCC1) function: the N-terminal tail of the Na-K-2Cl cotransporter serves as a regulatory scaffold for Ste20-related proline/alanine-rich kinase (SPAK) AND PP1. J Biol Chem 285: 14115–14121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290: C134–C142, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gagnon KB, England R, Delpire E. A single binding motif is required for SPAK activation of the Na-K-2Cl cotransporter. Cell Physiol Biochem 20: 131–142, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Geng Y, Hoke A, Delpire E. The Ste20 kinases Ste20-related proline-alanine-rich kinase and oxidative-stress response 1 regulate NKCC1 function in sensory neurons. J Biol Chem 284: 14020–14028, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery 39: 235–250, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Gimenez I. Molecular mechanisms, and regulation of furosemide-sensitive Na-K-Cl cotransporters. Curr Opin Nephrol Hypertens 15: 517–523, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Glover M, Zuber AM, O'Shaughnessy KM. Renal and brain isoforms of WNK3 have opposite effects on NCCT expression. J Am Soc Nephrol 20: 1314–1322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haas BR, Sontheimer H. Inhibition of the sodium-potassium-chloride cotransporter isoform-1 reduces glioma invasion. Cancer Res 70: 5597–5606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haas M, Forbush B., III The Na-K-Cl cotransporters. J Bioenerg Biomembr 30: 161–172, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Habela CW, Ernest NJ, Swindall AF, Sontheimer H. Chloride accumulation drives volume dynamics underlying cell proliferation and migration. J Neurophysiol 101: 750–757, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Habela CW, Olsen ML, Sontheimer H. ClC3 is a critical regulator of the cell cycle in normal and malignant glial cells. J Neurosci 28: 9205–9217, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamann S, Herrera-Perez JJ, Zeuthen T, Alvarez-Leefmans FJ. Cotransport of water by the Na+-K+-2Cl(−) cotransporter NKCC1 in mammalian epithelial cells. J Physiol 588: 4089–4101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst 99: 1583–1593, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Holden S, Cox J, Raymond FL. Cloning, genomic organization, alternative splicing and expression analysis of the human gene WNK3 (PRKWNK3). Gene 335: 109–119, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Hong C, Moorefield KS, Jun P, Aldape KD, Kharbanda S, Phillips HS, Costello JF. Epigenome scans and cancer genome sequencing converge on WNK2, a kinase-independent suppressor of cell growth. Proc Natl Acad Sci USA 104: 10974–10979, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jun P, Hong C, Lal A, Wong JM, McDermott MW, Bollen AW, Plass C, Held WA, Smiraglia DJ, Costello JF. Epigenetic silencing of the kinase tumor suppressor WNK2 is tumor-type and tumor-grade specific. Neuro Oncol 11: 414–422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kahle KT, Rinehart J, de Los HP, Louvi A, Meade P, Vazquez N, Hebert SC, Gamba G, Gimenez I, Lifton RP. WNK3 modulates transport of Cl− in and out of cells: implications for control of cell volume and neuronal excitability. Proc Natl Acad Sci USA 102: 16783–16788, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kahle KT, Rinehart J, Ring A, Gimenez I, Gamba G, Hebert SC, Lifton RP. WNK protein kinases modulate cellular Cl− flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology (Bethesda) 21: 46, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol 70: 329–355, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Kahle KT, Wilson FH, Lalioti M, Toka H, Qin H, Lifton RP. WNK kinases: molecular regulators of integrated epithelial ion transport. Curr Opin Nephrol Hypertens 13: 557–562, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Lenertz LY, Lee BH, Min X, Xu BE, Wedin K, Earnest S, Goldsmith EJ, Cobb MH. Properties of WNK1 and implications for other family members. J Biol Chem 280: 26653–26658, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Lindinger MI, Hawke TJ, Lipskie SL, Schaefer HD, Vickery L. K(+) transport and volume regulatory response by NKCC in resting rat hindlimb skeletal muscle. Cell Physiol Biochem 12: 279–292, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Lui VC, Lung SS, Pu JK, Hung KN, Leung GK. Invasion of human glioma cells is regulated by multiple chloride channels including ClC-3. Anticancer Res 30: 4515–4524, 2010 [PubMed] [Google Scholar]
- 34. McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev 91: 177–219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moniz S, Jordan P. Emerging roles for WNK kinases in cancer. Cell Mol Life Sci 67: 1265–1276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moniz S, Verissimo F, Matos P, Brazao R, Silva E, Kotelevets L, Chastre E, Gespach C, Jordan P. Protein kinase WNK2 inhibits cell proliferation by negatively modulating the activation of MEK1/ERK1/2. Oncogene 26: 6071–6081, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Ong SB, Shah D, Qusous A, Jarvis SM, Kerrigan MJ. Stimulation of regulatory volume increase (RVI) in avian articular chondrocytes by gadolinium chloride. Biochem Cell Biol 88: 505–512, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Piechotta K, Garbarini N, England R, Delpire E. Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl− cotransporter in the nervous system: evidence for a scaffolding role of the kinase. J Biol Chem 278: 52848–52856, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Ponce-Coria J, San Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los HP, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA 105: 8458–8463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med 16: 107–121, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Ransom CB, O'Neal JT, Sontheimer H. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci 21: 7674–7683, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ross SB, Fuller CM, Bubien JK, Benos DJ. Amiloride-sensitive Na+ channels contribute to regulatory volume increases in human glioma cells. Am J Physiol Cell Physiol 293: C1181–C1185, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Sontheimer H. Ion channels and amino acid transporters support the growth and invasion of primary brain tumors. Mol Neurobiol 29: 61–71, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sontheimer HW. An unexpected role for ion channels in brain tumor metastasis. Exp Biol Med (Maywood) 233: 779–791, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soroceanu L, Manning TJ, Jr, Sontheimer H. Modulation of glioma cell migration and invasion using Cl− and K+ ion channel blockers. J Neurosci 19: 5942–5954, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verissimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene 20: 5562–5569, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Verissimo F, Silva E, Morris JD, Pepperkok R, Jordan P. Protein kinase WNK3 increases cell survival in a caspase-3-dependent pathway. Oncogene 25: 4172–4182, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Yang CL, Zhu X, Ellison DH. The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest 117: 3403–3411, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zagorska A, Pozo-Guisado E, Boudeau J, Vitari AC, Rafiqi FH, Thastrup J, Deak M, Campbell DG, Morrice NA, Prescott AR, Alessi DR. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J Cell Biol 176: 89–100, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]