Abstract
Recent studies in humans and chimpanzees suggest that immunity can be induced to diminish the incidence of chronic hepatitis C virus (HCV) infection. However, the immunity that promotes viral recovery is poorly understood, and whether the breadth of this adaptive immunity is sufficient to overcome the substantial intergenotype antigenic diversity represents a final obstacle to demonstrating the feasibility of vaccine development. Here we demonstrate that recovery from a genotype 1 HCV infection protects chimpanzees against infection with representatives of other genotypes that exhibit up to 30% divergence at the amino acid level, including challenges with genotype 4, a mixture of genotypes 2 and 3, and a complex inoculum containing genotypes 1, 2, 3, and 4. In each instance, the level and duration of viremia were markedly reduced in comparison to the primary infection in the same animal. The data indicate that epitopes conserved between genotypes must play an essential role in immunity. The inocula used in the rechallenge studies induced typical primary infection profiles in naïve chimpanzees. Rechallenge infections were associated with rapid increases in the intrahepatic transcripts of interferon-stimulated genes, even in animals exhibiting apparent sterilizing immunity. Protective immunity was often associated with an early increase in gamma interferon transcripts in the liver and increases in intrahepatic transcripts of Mig, a T-cell chemokine that is a gamma interferon response gene. These studies are the first to show that cross-genotype immunity can be induced to HCV, demonstrating the feasibility of developing a vaccine protective against all HCV strains.
The prevalence of hepatitis C virus (HCV) infection is 1 to 2% worldwide (2). In the United States, HCV infection is the leading cause of liver failure and accounts for approximately 25% of all hepatocellular carcinoma cases (1). HCV infection also has emerged as a major problem in HIV-infected persons, approximately one-third of whom are HCV coinfected (33). Consequently, there is substantial interest in developing vaccines that might prevent viral persistence.
Since acute HCV infection is infrequently recognized in humans, much of our understanding of viral recovery derives from studies in chimpanzees, the only animal model (19, 20). Early seminal work demonstrated that chimpanzees who recovered from HCV infection could be reinfected with the same inoculum, a finding that substantially diminished hopes for vaccine development (10, 26). However, we and others have recently shown that these secondary infections often are attenuated and that viral recovery often occurs rapidly in association with brisk memory T-cell responses (3, 23). Protective immunity was observed when the primary and rechallenge inocula were closely related strains or were between genotype 1a and 1b strains (3, 36). Likewise, we found evidence of immunity that protected against viral persistence in a cohort of humans multiply exposed to genotype 1 virus (24). Most injection drug users who had cleared their initial HCV infection remained uninfected, despite ongoing exposure, and when a subsequent infection did occur, the magnitude of viremia and frequency of viral persistence were significantly lower than in controls infected for the first time (24).
Chronic HCV infections are associated with a narrow and weak T-cell response that allows the emergence of T-cell escape variants (9, 18, 35). In contrast, viral clearance is associated with an early, strong, multiantigen T-cell response that probably prevents the emergence of variants with escape mutations (8, 14, 22, 31, 32). Nonetheless, HCV has extraordinary genetic and antigenic diversity, properties along with the complexity of the viral quasispecies that have been associated with viral persistence (11, 28). Thus, it remained unclear whether the protective immunity acquired during viral clearance would be sufficiently broad to confer protection across all HCV genotypes. Here, we demonstrate the first evidence that prior infection with genotype 1 provides protective immunity to other HCV genotypes, even when animals are challenged with a highly complex mixture containing four genotypes.
MATERIALS AND METHODS
Chimpanzees.
Chimpanzees were housed at the Southwest National Primate Research Center at the Southwest Foundation for Biomedical Research. All protocols were approved by the Institutional Animal Care and Use Committee. All inocula were obtained from patients who were negative for human immunodeficiency virus (HIV) and hepatitis B virus (HBV), and use of the sera in these studies was approved by the Institutional Review Board.
ALT, antibody and TaqMan analysis.
Serum samples were collected to monitor viral RNA levels, changes in serum alanine transaminase (ALT), and antibody response to HCV proteins (ELISA Testing System 3.0; Ortho Diagnostic Systems, Raritan, N.J.). Percutaneous liver needle biopsies were taken as previously described (4). TaqMan analysis for viral RNA in the serum and liver and hepatic interferon (IFN)-stimulated gene (ISG) transcripts was performed as previously described (4, 21). The primers and probe for the HCV RNA assay were designed to detect all genotypes. The forward primer was from nucleotide positions 125 to 144 (5′-CCTICCGIGAGAGCCATAGT-3′), the reverse primer was from nucleotide positions 314 to 295 (5′-GCACTCGCAAGCACCCTATC-3′), and the probe was from nucleotide positions 173 to 151 (5′-TTCCGGTGTACTCACCGGTTCCG-3′). In addition, some samples were analyzed by a transcription-mediated amplification assay (Procleix; Chiron Corp., Emeryville, Calif.) (17, 34). The estimation of the percentage of infected cells in the liver was based on the following extrapolations: 1 μg of liver RNA represents 105 cells, and an infected cell must contain a minimum of 10 genome equivalents (GE; 2 negative strands and 8 positive strands). Thus, 104 GE/μg represents infection of 1% of the cells present in the liver biopsy sample.
Genotypic analysis.
Two different methods were used to determine the genotypes that emerged in chimpanzees receiving mixed inocula. In one assay, primers specific for each genotype were designed based on sequences obtained from the inocula. Reconstitution experiments indicated equivalent sensitivities for the different genotypes (<10 IU) and that genotypes 2, 3, and 4 could be detected simultaneously when genotype 1 was present at a 100-fold excess. In the second assay, universal primers (27) were used in nested PCRs that were conducted as multiple reactions with limiting dilutions of RNA. The product from each positive reaction was sequenced to determine the genotype. This method yielded ratios for the number of amplicons derived from each genotype.
RESULTS
Clearance of genotype 1 infection provides protective immunity to genotypes 1 to 4.
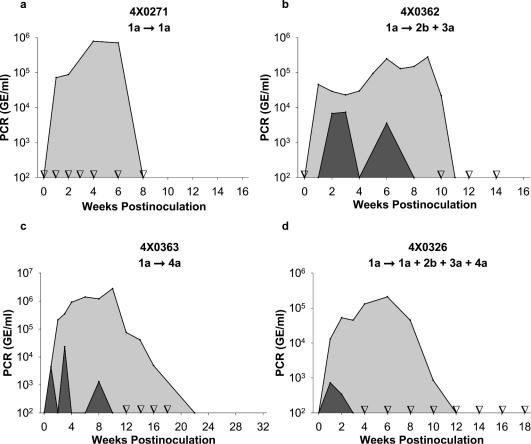
In these studies, we rechallenged four chimpanzees that had previously cleared infection with HCV genotype 1 with inocula containing genotype 1, genotype 4, a mixture of genotypes 2 and 3, and a mixture of genotypes 1, 2, 3, and 4. Despite the fact that the inocula contained very high levels of virus (>4 logs more than the average chimpanzee infectious dose), in each case, viremia during the rechallenge infection was markedly reduced in magnitude and duration in comparison to the primary infection in the same animal (Fig. 1).
FIG. 1.
Cross-genotype protective immunity to genotypes 1 to 4. Four chimpanzees were infected with the H77 strain of HCV genotype 1a. Following viral clearance, they were rechallenged with various inocula representing genotypes 1 to 4. Viremia following primary and rechallenge infection is indicated by the light and dark areas, respectively, of the graphs. Time points for the rechallenge that were tested but were PCR negative are indicated by inverted open triangles on the x axis. (a) Chimpanzee 4x0271 was rechallenged with the H77 strain of HCV genotype 1a. (b) Chimpanzee 4x0362 was rechallenged with a mixed inoculum containing genotypes 2b and 3a. (c) Chimpanzee 4x0363 was rechallenged with genotype 4a. (d) Chimpanzee 4x0326 was rechallenged with a mixture of genotypes 1a, 2b, 3a, and 4a.
Apparent sterilizing immunity following rechallenge with homologous inoculum.
Chimpanzee 4x0271 was initially infected with 1.5 × 107 GE of the H77 strain of genotype 1a. In the primary infection, viremia peaked at 106 GE/ml on week 4 and was cleared by week 8 (Fig. 1a). In contrast, clearance of viral RNA from the liver did not occur until week 16 (data not shown). One year later, after rechallenge with the identical high-dose inoculum (1.5 × 107 GE), viral RNA was undetectable in the serum and liver. Thus, this animal exhibited apparent sterilizing immunity. During rechallenge, no liver inflammation was observed as measured by increases in serum ALT levels. Although the ALT level increased to 182 IU/liter in the primary infection (the baseline ALT level was 23 IU/liter), no increase in ALT was observed following rechallenge.
Clearance of genotype 1a provides immunity to genotypes 2 and 3.
Next, a chimpanzee was rechallenged with a mixture of genotypes 2 and 3, since these genotypes are considered the most divergent from genotype 1 and are frequently observed in infected individuals. Chimpanzee 4x0362 was inoculated 2.5 years after clearance of genotype 1a (strain H77) with a mixture of genotypes 2b and 3a (5 × 105 GE each). In the primary infection, viremia was cleared in week 11, while following rechallenge we observed a low-level viremia on weeks 2, 3, and 6 (Fig. 1b). Peak viremia was 40-fold lower than the level during the primary infection. Genotypic analysis of serum samples from multiple time points revealed that only genotype 3 was detectable. To validate the results from our TaqMan reverse transcription (RT)-PCR assay and to ascertain that viral clearance had occurred, testing was confirmed by using the most sensitive assay available, a non-PCR, transcription-mediated amplification (TMA) assay (Procleix; sensitivity estimated at 3.7 IU/ml). With TMA, viremia was detectable to week 8 but at no other time out to 36 weeks. Viral RNA was detected in the liver in weeks 2 and 4 at a level estimated to represent infection of less than 1 in 6,000 cells based on the number of genome equivalents per microgram of liver RNA (see Materials and Methods). In addition, no increase in serum ALT levels was observed following rechallenge, while a peak ALT value of 120 IU/liter was observed during the primary infection of this animal.
Clearance of genotype 1a provides immunity to genotype 4.
Since genotype 4 predominates in some countries such as Egypt (27), we also examined immunity to a genotype 4 inoculum. Chimpanzee 4x0363 cleared primary infection with genotype 1 at 22 weeks postinfection and was rechallenged 2.5 years later with genotype 4a (5 × 105 GE). Viremia was intermittently positive by RT-PCR at weeks 1, 3, and 8. The TMA assay detected viremia at all time points between weeks 1 and 8 but at no later time points (Fig. 1c). Peak viremia was 2.8 × 106 GE/ml during primary infection and 8.3 × 104 GE/ml upon rechallenge. The levels of hepatic viral RNA suggested that as few as 0.04% of hepatocytes were infected.
Immunity to a complex inoculum containing four genotypes.
Chimpanzee 4x0326 cleared the primary infection with genotype 1a in 14 weeks and was rechallenged 4 years later with a high-dose inoculum containing 106 GE each of genotypes 1a, 2b, 3a, and 4a. We initially anticipated that the complexity of this inoculum would induce a persistent infection; however, the animal rapidly cleared a low-level viremia (peak viremia, 7.3 × 102 GE/ml) after 2 weeks (Fig. 1d). Even with the TMA assay, viremia was detected only at weeks 1, 2, and 3 but at no other time out to 48 weeks. Based on hepatic viral RNA levels, we estimated that as few as 1 in 25,000 cells were infected. Genotypic analysis of the serum from week 1 indicated that only genotypes 1 and 4 had replicated at detectable levels and that genotype 4 dominated genotype 1 at a ratio of 3 to 1. Since the genotype 1a was our chimpanzee-adapted H77 strain and was presumably highly fit for replication in chimpanzees, the data suggest that genotype 1 was subjected to greater immunological pressure. This outcome was anticipated, since the genotype 1 was homologous to the inoculum used in the primary infection. Remarkably, of the animals receiving a cross-genotype rechallenge, this animal received the highest dose of virus with the greatest sequence diversity and was rechallenged after the longest time interval following the primary infection and yet had the lowest level and shortest duration of viremia.
Apparent sterilizing immunity to genotype 4.
Chimpanzee 4x0326 was rechallenged a second time with genotype 4 (5 × 105 GE) 5 years after the primary infection with genotype 1 and 1 year after the rechallenge with genotypes 1 to 4 (Fig. 1d). Upon reexposure to genotype 4, this animal exhibited apparent sterilizing immunity. No viremia was detected at any time by either RT-PCR or by TMA assay, and no viral RNA was detected in the liver (data not show). This result was not surprising, since this animal had previously cleared genotype 4. However, since it cleared viremia in 3 weeks and the level of viremia was very low, this experiment raises the question of whether the protection was conferred by prior exposure to genotype 4 or by a cross-reactive response to genotype 1.
Characterization of inocula in naïve chimpanzees.
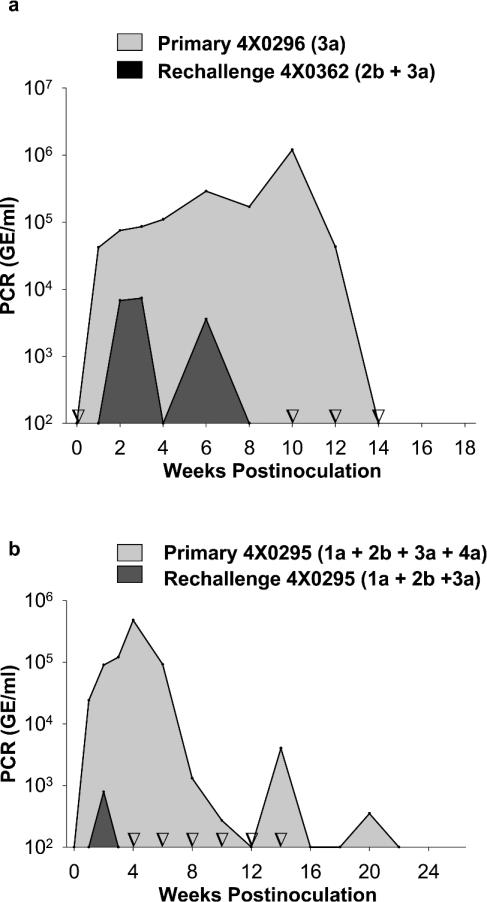
To demonstrate that the challenge viruses were capable of inducing a typical primary infection profile, we chose to examine the most divergent inocula in a naïve animal. We inoculated chimpanzee 4x0296 with the identical dose (5 × 105 GE) of the genotype 3 inoculum utilized for the rechallenge of 4x0362 (Fig. 2a). A rapid rise in viremia was noted in the first week, viremia peaked at week 10 at greater than 106 GE/ml, and viral clearance did not occur until week 14. Peak viremia was approximately 150-fold higher than that observed during rechallenge (1.1 × 106 versus 7.4 × 103 GE/ml) (Fig. 2a). ALT values peaked at 113 IU/liter on week 12, while seroconversion for anti-HCV did not occur until week 18. Viral RNA levels in the liver were also dramatically increased during the primary infection (data not shown).
FIG. 2.
Primary infection of chimpanzees with inocula used in rechallenge. (a) The genotype 3 inocula used to rechallenge 4x0362 (Fig. 1b) induced a typical acute-resolving infection profile in a naïve chimpanzee (4x0296). A graph of the viremia that occurred during the rechallenge infection of 4x0362 is superimposed on a graph of the results for the primary infection of 4x0296 to contrast the differences in the levels and duration of viremia during primary and cross-genotype rechallenge infections. (b) The four-genotype mixture (genotypes 1 to 4) used to rechallenge chimpanzee 4x0326 (Fig. 1d) induced a typical acute-resolving HCV infection in a naïve chimpanzee (4x0295). Viremia for the primary infection with the mixed-genotype inoculum containing genotypes 1a, 2b, 3a, and 4a is illustrated. Six months following clearance of the primary infection, 4x0295 was rechallenged with a mixed inoculum containing genotypes 1, 2, and 3, and the viremia resulting from this infection is depicted. Inverted open triangles indicate time points for the rechallenge that were tested but were PCR negative.
We further characterized the complex, four-genotype mixed inoculum by infecting a previously uninfected chimpanzee. The animal used in these studies (4x0295) was not entirely naïve for HCV antigens, having previously received four intrahepatic inoculations with synthetic HCV RNA derived from a noninfectious genotype 1a clone; however, no viremia was detected following these inoculations. Ten months after the final RNA inoculation, chimpanzee 4x0295 was challenged with the mixed inoculum containing genotypes 1, 2, 3, and 4. A typical primary infection profile was observed (Fig. 2b). Analysis of the serum on week 1 revealed the presence of only genotypes 1 and 4, but unlike the result of the rechallenge of 4x0326, genotype 1 dominated genotype 4 at a ratio of 11 to 1, suggesting that genotype 1 was the most fit virus in the mixed inoculum when it was not under immunological pressure (as in animal 4x0326). The data from this animal suggest that high sequence complexity in the inoculum does not necessarily predispose to a chronic infection.
A subsequent rechallenge of this animal was performed 6 months after viral clearance by using the genotype 2-plus-3 inoculum employed with 4x0362, but in this instance, the inoculum contained 1 log less of our standardized genotype 1a inoculum to test whether genotype 1 would dominate the infection or perhaps alter the immunity to genotypes 2 and 3. Since genotypes 2 and 3 were not detectable during the primary infection of this animal, it was unlikely that immunity to these viruses had developed. Following rechallenge, a low level of viremia was detected at week 2 (Fig. 2b). Genotypic analysis of the serum revealed that, as in the rechallenge of animal 4x0362, only genotype 3 could be detected. Clearly genotype 1 did not dominate the infection. The enhanced cross-genotype immunity observed in this animal in comparison to the result in animal 4x0362 may be due to the short time interval that had elapsed from clearance of the primary infection or to a prior but undetectable exposure to genotypes 2 and 3.
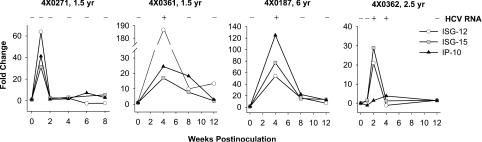
Sterilizing immunity is associated with increases in hepatic ISG expression.
We previously showed that levels of hepatic transcripts for a set of ISGs increased sharply following HCV infection (4). Presumably due to the paracrine and autocrine amplification of the IFN response, these markers are more sensitive indicators of viral infection than RT-PCR for the viral RNA. Although viral RNA was suppressed below the level of detection in both the serum and liver when 4x0271 was rechallenged, within 1 week, a dramatic increase was observed in intrahepatic transcripts for ISG-12, ISG-15, and IP-10 (IFN-inducible protein 10), and these changes returned to baseline by week 2 (Fig. 3). We examined the changes in expression of these three ISGs in three additional animals, two from our previous studies on protective immunity to genotype 1 and animal 4x0362 (genotype 2-plus-3 rechallenge). Each animal experienced a rapid increase and decrease in ISG transcripts corresponding with the period of time that viral RNA was detectable in the liver.
FIG. 3.
Elevations of hepatic ISG transcripts following rechallenge infection. Total-cell RNA purified from liver biopsies was evaluated for changes in the expression of hepatic ISG transcripts in four animals following rechallenge. Values are indicated as fold change in comparison to a baseline sample obtained immediately prior to inoculation. The presence (+) or absence (−) of viral RNA in the liver biopsy RNA samples is indicated above the graphs.
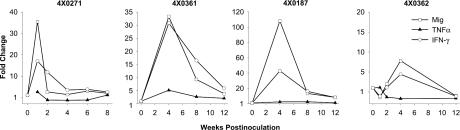
Protective immunity is associated with increases in hepatic IFN-γ transcripts.
Next, we examined intrahepatic expression of proinflammatory cytokines in the liver as a marker of the immune response during rechallenge infections. TaqMan assays were used to quantify changes in hepatic gamma IFN (IFN-γ) transcripts. Most of the animals from our previous studies that were rechallenged with genotype 1 (4x0187, 4x0198, and 4x0361) exhibited increases of 18- to 45-fold in hepatic IFN-γ transcripts following rechallenge (Fig. 4). An increase of 18-fold was also observed in the animal with apparent sterilizing immunity (4x0271). In cross-genotype rechallenges, increases in IFN-γ transcripts were seen in chimpanzees 4x0326 (genotype 1 to 4; 6.8-fold) (Fig. 4), 4x0362 (genotype 2 plus 3; 4.4-fold), and 4x0295 (genotype 1 plus 2 plus 3; 2.8-fold) but not 4x0363 (genotype 4). The failure to detect increases in IFN-γ transcripts in some animals does not preclude a role for this cytokine in viral clearance; rather, the available samples may not have been optimal for detection of the increase or the increases may not be of sufficient magnitude to be detected over the baseline level expressed in the liver.
FIG. 4.
Increases in hepatic IFN-γ and Mig transcripts are associated with protective immunity. Hepatic mRNA levels were quantified by TaqMan RT-PCR from liver RNA samples obtained following rechallenge infections with the same chimpanzees whose results are shown in Fig. 3. The data are presented as the fold change in expression in comparison to baseline samples taken before infection. TNF-α, tumor necrosis factor alpha.
To demonstrate that the increase in IFN-γ transcripts resulted in increased IFN-γ expression and induction of IFN-γ response genes, we determined the intrahepatic levels of Mig transcripts, a T-cell chemokine induced by IFN-γ. The kinetics for the increase in Mig transcripts exactly mimicked those of IFN-γ (Fig. 4). Similar to the amplification effect observed for ISGs, Mig transcripts were often elevated to a greater degree than IFN-γ itself. We also examined the intrahepatic levels of tumor necrosis factor alpha transcripts. A fivefold increase was observed in a single animal (4x0361), while the remaining animals did not have significant increases in transcripts for this cytokine.
DISCUSSION
Although some success has been reported in chimpanzees by using recombinant protein vaccines containing the envelope proteins (7), development of a vaccine for HCV remains a major challenge due to the high sequence divergence (30%) among the six major genotypes, lack of a small-animal model for testing vaccine candidates, and the incomplete understanding of protective immunity.
Bassett et al. have previously demonstrated that, following viral clearance, chimpanzees have protective immunity to rechallenge with the same genotype, that the protection can involve sterilizing immunity, that the protection can persist for up to 16 years after the primary infection, and that the protection is associated with a rapid, multiantigen memory T-cell proliferative response (3). Mehta et al. have also demonstrated that viral clearance in humans is associated with protection against chronic infection upon reexposure (24). The present studies expand on these findings, showing remarkable cross-genotype immunity to multiple genotypes even when the challenge is performed with a complex multiple genotype mixture.
The results of this investigation were not anticipated. In prior studies of HCV and other chronic viral infections, single-amino-acid mutations were sufficient to eliminate protective immunity (9, 18, 35, 37). Thus, we were surprised to detect a protective effect against an inoculum whose amino acid composition differed by more than 25% from the original infecting strain. This result can be explained in part by the breadth of the T-cell response in acute resolving and chronic infections. Chronic HCV infections are thought to be associated with a limited T-cell response that allows the emergence of T-cell escape variants (9, 18, 35), while viral clearance is thought to be associated with a broad multiantigen T-cell response that prevents the emergence of escape variants (8, 14, 22, 31, 32). The findings in this study indicate that effective memory responses can be generated to a wide array of HCV epitopes such that protection spans across the divergence seen in different genotypes. We were also surprised that a complex viral inoculum comprised of four different genotypes was rapidly cleared, since preliminary studies demonstrated an association between viral persistence and the complexity of the HCV quasispecies (11, 28). Although the biological basis for these findings remains unknown, the results should encourage efforts to develop vaccines to prevent HCV persistence.
In these rechallenge studies, ideally each inoculum would have been put into several naïve chimpanzees to demonstrate its infectivity and to provide parallel clinical profiles. However, the limited availability and high cost of chimpanzees in research made this impossible. Fortunately, the primary infection of each of these animals was studied as the optimal control for each rechallenge. In addition, primary infections were characterized for the most divergent inoculum (the genotype 3 inoculum) and the most complex inoculum (the mixture containing genotypes 1 to 4). In addition, the magnitude of the differences observed between primary and rechallenge studies obviates concerns that factors other than the initial infection generated the apparent protective effect. Likewise, by exceeding the average chimpanzee infectious dose by four orders of magnitude, we ensured that each inoculum was infectious.
A number of laboratories have demonstrated the direct antiviral effects of IFN-α (5, 12, 16, 21) and IFN-γ (6, 13, 21) by using the HCV subgenomic replicon system, but at this time, the mechanism by which alpha/beta or gamma (type I or type II) IFN inhibits HCV replication is not understood. In our present experiments, no significant increases in IFN-α or IFN-β transcripts were observed; nonetheless, large increases in ISG transcripts were detected, findings that are consistent with previous microarray studies on primary infection with HCV (4). Increases in ISGs in the absence of detectable increases in type I IFNs are likely due to the potency of these cytokines and the amplification of the response when using ISGs as the downstream readout. We have hypothesized that the type I IFN response plays an important role in limiting viral replication and spread in the liver during primary infection until an adaptive immune response can eliminate infected cells (4, 19). The importance of type I IFNs in the context of rechallenge infection may be less significant, since the memory T-cell response emerges early and results in low-level or undetectable viremia.
The results from replicon studies demonstrating that IFN-γ has direct antiviral activity for HCV (6, 13, 21), together with the results presented here, suggest that IFN-γ may be an important mediator of T-cell function in viral clearance. Increases in hepatic IFN-γ transcripts during viral clearance in primary infections have been observed (R. E. Lanford and C. Bigger, unpublished data), and here we demonstrated increases of over 40-fold during rechallenge infection. Others have noted increases in IFN-γ expression by T cells and/or IFN-γ transcripts in the liver during HCV primary or rechallenge infections and have speculated on their importance in viral clearance as well (23, 25, 29, 31). We also demonstrated a concomitant increase in Mig transcripts. Analysis of Mig levels is an especially useful approach to demonstrating the biological activity resulting from the increase in IFN-γ transcripts, since it is a T-cell chemokine that may be involved in clearance and an IFN-γ-inducible gene. Since viral clearance often occurs in the absence of an increase in the level of ALT during primary infection and none of the rechallenge animals experienced flares in ALT levels during viral clearance, it is possible that clearance is mediated by a noncytolytic mechanism that may be dependent on IFN-γ and possibly other cytokines. Such a role for IFN-γ in noncytolytic viral clearance has been demonstrated by Guidotti et al. in studies involving transgenic mice replicating HBV (15). However, since the number of infected hepatocytes is low during rechallenge, viral clearance could occur by a cytolytic mechanism without a significant rise in the level of ALT. Clearance during primary infection also often occurs without a significant rise in the ALT level or when the rise in ALT does not correspond temporally with the drop in viral RNA levels. Still, the percentage of hepatocytes that are infected during acute infection may often be too low to give rise to a pronounced rise in the level of ALT if cytolytic clearance occurred over several weeks. One possibility is that both cytolytic and noncytolytic mechanisms are involved in viral clearance. Recent studies by Summers et al. (30) suggest the potential for a complex interplay between cytolytic and noncytolytic mechanisms in viral clearance in the woodchuck HBV model. The precise role of different cytokines in clearance of HCV RNA awaits further studies. A complete understanding of the mechanism of viral clearance is not required for the development of an efficacious vaccine, and the present studies provide encouragement that protection against all HCV genotypes should be possible with the appropriate vaccine.
Acknowledgments
This work was supported by NIH grants U19 AI40035 and P51 RR13986.
We thank V. Shyamala (Chiron Corp.) for performing the Procleix assays.
REFERENCES
- 1.Alter, H. J., and M. Houghton. 2000. Hepatitis C virus and eliminating post-transfusion hepatitis. Nat. Med. 6:1082-1086. [DOI] [PubMed] [Google Scholar]
- 2.Alter, H. J., and L. B. Seeff. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 20:17-35. [DOI] [PubMed] [Google Scholar]
- 3.Bassett, S. E., B. Guerra, K. Brasky, E. Miskovsky, M. Houghton, G. R. Klimpel, and R. E. Lanford. 2001. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 33:1479-1487. [DOI] [PubMed] [Google Scholar]
- 4.Bigger, C. B., K. M. Brasky, and R. E. Lanford. 2001. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J. Virol. 75:7059-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 6.Cheney, I. W., V. C. H. Lai, W. D. Zhong, T. Brodhag, S. Dempsey, C. Lim, Z. Hong, J. Y. N. Lau, and R. C. Tam. 2002. Comparative analysis of anti-hepatitis C virus activity and gene expression mediated by alpha, beta, and gamma interferons. J. Virol. 76:11148-11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choo, Q.-L., G. Kuo, R. Ralston, A. Weiner, D. Chien, G. Van Nest, J. Han, K. Berger, K. Thudium, C. Kuo, J. Kansopon, J. McFarland, A. Tabrizi, K. Ching, B. Moss, L. B. Cummins, M. Houghton, and E. Muchmore. 1994. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. USA 91:1294-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10:439-449. [DOI] [PubMed] [Google Scholar]
- 9.Erickson, A. L., Y. Kimura, S. Igarashi, J. Eichelberger, M. Houghton, J. Sidney, D. McKinney, A. Sette, A. L. Hughes, and C. M. Walker. 2001. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 15:883-895. [DOI] [PubMed] [Google Scholar]
- 10.Farci, P., H. J. Alter, S. Govindarajan, D. C. Wong, R. Engle, R. R. Lesniewski, I. K. Mushahwar, S. M. Desai, R. H. Miller, N. Ogata, and R. H. Purcell. 1992. Lack of protective immunity against reinfection with hepatitis C virus. Science 258:135-140. [DOI] [PubMed] [Google Scholar]
- 11.Farci, P., A. Shimoda, A. Coiana, G. Diaz, G. Peddis, J. C. Melpolder, A. Strazzera, D. Y. Chien, S. J. Munoz, A. Balestrieri, R. H. Purcell, and H. J. Alter. 2000. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288:339-344. [DOI] [PubMed] [Google Scholar]
- 12.Frese, M., T. Pietschmann, D. Moradpour, O. Haller, and R. Bartenschlager. 2001. Interferon-α inhibits hepatitis C virus subgenomic RNA replication by an MxA-independent pathway. J. Gen. Virol. 82:723-733. [DOI] [PubMed] [Google Scholar]
- 13.Frese, M., V. Schwarzle, K. Barth, N. Krieger, V. Lohmann, S. Mihm, O. Haller, and R. Bartenschlager. 2002. Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology 35:694-703. [DOI] [PubMed] [Google Scholar]
- 14.Grüner, N. H., T. J. Gerlach, M. C. Jung, H. M. Diepolder, C. A. Schirren, W. W. Schraut, R. Hoffmann, R. Zachoval, T. Santantonio, M. Cucchiarini, A. Cerny, and G. R. Pape. 2000. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J. Infect. Dis. 181:1528-1536. [DOI] [PubMed] [Google Scholar]
- 15.Guidotti, L. G., K. Ando, M. V. Hobbs, T. Ishikawa, L. Runkel, R. D. Schreiber, and F. V. Chisari. 1994. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc. Natl. Acad. Sci. USA 91:3764-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo, J. T., V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75:8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson, J. B., K. Smith, C. Knott, A. Korpela, A. Simmons, E. Piwowar-Manning, S. McDonough, L. Mimms, and J. M. Vargo. 2002. Sensitivity of the Procleix HIV-1/HCV assay for detection of human immunodeficiency virus type 1 and hepatitis C virus RNA in a high-risk population. J. Clin. Microbiol. 40:2387-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantzanou, M., M. Lucas, E. Barnes, H. Komatsu, G. Dusheiko, S. Ward, G. Harcourt, and P. Klenerman. 2003. Viral escape and T cell exhaustion in hepatitis C virus infection analysed using class I peptide tetramers. Immunol. Lett. 85:165-171. [DOI] [PubMed] [Google Scholar]
- 19.Lanford, R. E., and C. Bigger. 2002. Advances in model systems for hepatitis C virus research. Virology 293:1-9. [DOI] [PubMed] [Google Scholar]
- 20.Lanford, R. E., C. Bigger, S. Bassett, and G. R. Klimpel. 2001. The chimpanzee model of hepatitis C virus infections. ILAR J. 42:117-126. [DOI] [PubMed] [Google Scholar]
- 21.Lanford, R. E., B. Guerra, H. Lee, D. R. Averett, B. Pfeiffer, D. Chavez, L. Notvall, and C. Bigger. 2003. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(I)-poly(C), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J. Virol. 77:1092-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Major, M. E., K. Mihalik, M. Puig, B. Rehermann, M. Nascimbeni, C. M. Rice, and S. M. Feinstone. 2002. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J. Virol. 76:6586-6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta, S. H., A. Cox, D. R. Hoover, X. H. Wang, Q. Mao, S. Ray, S. A. Strathdee, D. Vlahov, and D. L. Thomas. 2002. Protection against persistence of hepatitis C. Lancet 359:1478-1483. [DOI] [PubMed] [Google Scholar]
- 25.Nascimbeni, M., E. Mizukoshi, M. Bosmann, M. E. Major, K. Mihalik, C. M. Rice, S. M. Feinstone, and B. Rehermann. 2003. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J. Virol. 77:4781-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prince, A. M., B. Brotman, T. Huima, D. Pascual, M. Jaffery, and G. Inchauspe. 1992. Immunity in hepatitis C infection. J. Infect. Dis. 165:438-443. [DOI] [PubMed] [Google Scholar]
- 27.Ray, S. C., R. R. Arthur, A. Carella, J. Bukh, and D. L. Thomas. 2000. Genetic epidemiology of hepatitis C virus throughout Egypt. J. Infect. Dis. 182:698-707. [DOI] [PubMed] [Google Scholar]
- 28.Ray, S. C., Y. M. Wang, O. Laeyendecker, J. R. Ticehurst, S. A. Villano, and D. L. Thomas. 1999. Acute hepatitis C virus structural gene sequences as predictors of persistent viremia: hypervariable region 1 as a decoy. J. Virol. 73:2938-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su, A. I., J. P. Pezacki, L. Wodicka, A. D. Brideau, L. Supekova, R. Thimme, S. Wieland, J. Bukh, R. H. Purcell, P. G. Schultz, and F. V. Chisari. 2002. Genomic analysis of the host response to hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 99:15669-15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summers, J., A. R. Jilbert, W. Yang, C. E. Aldrich, J. Saputelli, S. Litwin, E. Toll, and W. S. Mason. 2003. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc. Natl. Acad. Sci. USA 100:11652-11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thimme, R., J. Bukh, H. C. Spangenberg, S. Wieland, J. Pemberton, C. Steiger, S. Govindarajan, R. H. Purcell, and F. V. Chisari. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Nat. Acad. Sci. USA 99:15661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas, D. L. 2002. Hepatitis C and human immunodeficiency virus infection. Hepatology 36:201-209. [DOI] [PubMed] [Google Scholar]
- 34.Vargo, J., K. Smith, S. Wang, C. Fang, S. McDonough, C. Giachetti, S. Caglioti, R. Gammon, D. Gilbert, J. Brooks Jackson, W. Richards, S. Stramer, and L. Mimms. 2002. Clinical specificity and sensitivity of a blood-screening assay for detection of HIV-1 and HCV RNA. Transfusion 42:876-885. [DOI] [PubMed] [Google Scholar]
- 35.Weiner, A., A. L. Erickson, J. Kansopon, K. Crawford, E. Muchmore, A. L. Hughes, M. Houghton, and C. M. Walker. 1995. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc. Natl. Acad. Sci. USA 92:2755-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner, A. J., X. Paliard, M. J. Selby, A. Medina-Selby, D. Coit, S. Nguyen, J. Kansopon, C. L. Arian, P. Ng, J. Tucker, C. T. Lee, N. K. Polakos, J. Han, S. Wong, H. H. Lu, S. Rosenberg, K. M. Brasky, D. Chien, G. Kuo, and M. Houghton. 2001. Intrahepatic genetic inoculation of hepatitis C virus RNA confers cross-protective immunity. J. Virol. 75:7142-7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, O. O., P. T. Sarkis, A. Ali, J. D. Harlow, C. Brander, S. A. Kalams, and B. D. Walker. 2003. Determinants of HIV-1 mutational escape from cytotoxic T lymphocytes. J. Exp. Med. 197:1365-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]