Abstract
Na+ absorption is a vital process present in all living organisms. We have reported previously that lysophosphatidic acid (LPA) acutely stimulates Na+ and fluid absorption in human intestinal epithelial cells and mouse intestine by stimulation of Na+/H+ exchanger 3 (NHE3) via LPA5 receptor. In the current study, we investigated the mechanism of NHE3 activation by LPA5 in Caco-2bbe cells. LPA5-dependent activation of NHE3 was blocked by mitogen-activated protein kinase kinase (MEK) inhibitor PD98059 and U0126, but not by phosphatidylinositol 3-kinase inhibitor LY294002 or phospholipase C-β inhibitor U73122. We found that LPA5 transactivated the epidermal growth factor receptor (EGFR) and that inhibition of EGFR blocked LPA5-dependent activation of NHE3, suggesting an obligatory role of EGFR in the NHE3 regulation. Confocal immunofluorescence and surface biotinylation analyses showed that LPA5 was located mostly in the apical membrane. EGFR, on the other hand, showed higher expression in the basolateral membrane. However, inhibition of apical EGFR, but not basolateral EGFR, abrogated LPA-induced regulation of MEK and NHE3, indicating that LPA5 selectively activates apical EGFR. Furthermore, transactivation of EGFR independently activated the MEK-ERK pathway and proline-rich tyrosine kinase 2 (Pyk2). Similarly to MEK inhibition, knockdown of Pyk2 blocked activation of NHE3 by LPA. Furthermore, we showed that RhoA and Rho-associated kinase (ROCK) are involved in activation of Pyk2. Interestingly, LPA5 did not directly activate RhoA but was required for transactivation of EGFR. Together, these results unveil a pivotal role of apical EGFR in NHE3 regulation by LPA and show that the RhoA-ROCK-Pyk2 and MEK-ERK pathways converge onto NHE3.
Keywords: proline-rich tyrosine kinase 2, extracellular signal-regulated kinase
na+ absorption is a vital process present in all organisms from single cell bacteria to multicellular organisms. The Na+/H+ exchanger 3, NHE3 (Slc9a3), is highly expressed in the apical membrane of small intestine, colon, and proximal tubules of kidney, where it constitutes the major Na+-absorbing process (13, 44). NHE3 is regulated by hormones and growth factors through multiple mechanisms that include trafficking, phosphorylation, transcriptional regulation, and interaction with scaffolding and cytoskeletal proteins (13, 44). Lysophosphatidic acid (LPA) is a small, bioactive glycerophospholipid that acts as an extracellular signaling molecule in most eukaryotic tissues. Five cell surface LPA receptors and three putative LPA receptors that belong to the G protein-coupled receptor (GPCR) superfamily have been identified: LPA1–LPA8 (5). The relevance of LPA to various pathologic conditions, such as inflammation, cancer, obesity, and hypertension, is widely investigated. More recently, LPA has been implicated in electrolyte transport in epithelial cells. LPA stimulates NHE3 activity in opossum kidney (OK) cells (23). Inhibition of adenylate cyclase by LPA2 signaling is necessary for the inhibition of the cystic fibrosis transmembrane conductance regulator (CFTR)-dependent Cl− secretion in the mouse intestine (26). In addition, LPA2 signaling stimulates Cl−/OH− exchange in Caco-2 cells (36). However, unlike the regulation of CFTR by LPA, the absence of LPA2 did not appreciably affect NHE3-dependent Na+ absorption (29). Previously, it was shown that LPA5 is highly expressed in the intestine (24), and quantitative real-time PCR (qRT-PCR) of isolated intestinal epithelial cells shows that LPA receptor mRNA is expressed in the order of LPA1>LPA5>>LPA2, LPA3, LPA4 (28, 29). However, in Caco-2 and the colonal Caco-2bbe cells, LPA5 expression is low (29) and unless LPA5 is exogenously expressed, LPA is not able to stimulate NHE3 activity in Caco-2 cells (29). The stimulation of NHE3 activity by LPA5 acutely occurs within 3–5 min of treatment with low concentrations of LPA, and it requires the presence of Na+/H+ exchanger regulatory factor 2 (NHERF2), which interacts with the carboxyl terminal motif of LPA5 through its second PSD95/Dlg/ZO-1 (PDZ) domain. NHERF2 also interacts with LPA2 via the similar mechanism, and the absence of NHERF2 obliterates the effects of LPA on CFTR and NHE3 (26, 29, 35). In this study, we investigate the mechanism whereby LPA5 regulates NHE3. We report that LPA5 stimulates NHE3 activity through transactivation of the epidermal growth factor receptor (EGFR) in the apical membrane of Caco-2bbe cells and that this regulation involves proline-rich tyrosine kinase 2 (Pyk2) and mitogen-activated protein kinase kinase (MEK).1
EXPERIMENTAL PROCEDURES
Cell cultures.
Caco-2bbe (C2b) cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 50 U/ml penicillin, 50 μg/ml streptomycin, and 1× nonessential amino acids. C2b cells stably transfected with human NHE3 with a COOH-terminal vesicular stomatitis virus glycoprotein (VSVG) epitope tag, C2b/E3V, were previously described (29). LPA5 was expressed by infecting with lentivirus harboring NH2-terminal hemagglutinin (HA)-tagged human LPA5, and cells were selected with 10 μg/ml puromycin. However, the cells maintain LPA5 expression only for nine passages and all the experiments were carried out with cells of less than five passages following the initial infection with virus. The expression of LPA5 was confirmed by Western immunoblot analysis using an anti-HA antibody on the cells of the same passage that were used for each experiment.
Chemicals and materials.
LPA (18:1, 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate) was purchased from Avanti Polar Lipids (Alabaster, AL) and prepared in PBS, pH 7.2, containing 0.1% BSA. For all experiments, LPA was used at the final concentration of 1 μM for 2–3 min unless otherwise specified and the equal volume of PBS containing 0.1% BSA was added as a control. When needed, cells were pretreated with LY294002 (50 μM), U73122 (5 μM), PD98059 (20 μM), U0126 (10 μM), AG1478 (1 μM), PD153035 (1 μM), Y27632 (50 μM), or C3 (5 μg/ml), and the equal volume of DMSO was added as a vehicle control. All the antibodies were obtained from Cell Signaling (Danvers, MA), Calbiochem (La Jolla, CA), Covance (Emeryville, CA), or Santa Cruz (Santa Cruz, CA). Recombinant human epidermal growth factor (EGF) was obtained from Invitrogen (Carlsbad, CA). All other chemicals were obtained from Sigma (St. Louis, MO) or Calbiochem.
Gene silencing.
Lentiviral vectors (pLKO.1) containing short hairpin RNA (shRNA) targeting Pyk2 or EGFR were obtained from Sigma or Open Biosystems (Huntsville, AL), respectively. Silencing of Pyk2 or EGFR expression was performed using shRNA lentiviral particles. pLKO.1-puro was used to generate control lentivirus.
Na+-dependent intracellular pH recovery.
The Na+-dependent changes in intracellular pH (pHi) by NHE3 was determined using the ratio-fluorometric, pH-sensitive dye 2′,7′-bis-(2-carboxyethyl)-5-carboxyfluorescein acetoxymethyl ester (BCECF-AM) as previously described (39). Briefly, cells grown on Transwells (BD Falcon, Franklin Lakes, NJ) for 5–7 days postconfluence were serum starved overnight and the next morning were washed in Na+ buffer [130 mM NaCl, 20 mM HEPES, 5 mM KCl, 1 mM tetramethylammonium-PO4 (TMA-PO4), 2 mM CaCl2, 1 mM MgSO4, and 25 mM glucose] and then dye-loaded by incubating for 20 min with 6.5 μM BCECF-AM in the same solution. When inhibitors were used, the equal volume of an inhibitor or DMSO was added and incubated at room temperature during the dye-loading period. The cells were mounted on a perfusion chamber, placed on an inverted microscope, and superfused with NH4+ buffer (50 mM NH4Cl, 80 mM TMA-Cl, 20 mM HEPES, 5 mM KCl, 1 mM TMA-PO4, 2 mM CaCl2, 1 mM MgSO4, and 25 mM glucose) and subsequently with TMA+ buffer (130 mM TMA-Cl, 20 mM HEPES, 5 mM KCl, 1 mM TMA-PO4, 2 mM CaCl2, 1 mM MgSO4, and 25 mM glucose) containing 1 μM LPA or 0.1% BSA as a control. Hence, cells were preexposed to LPA or control BSA for 2–3 min before perfusion with a Na+ buffer that drives Na+-dependent pH recovery. Na+ buffer was supplemented with 50 μM Hoe 694 to inhibit NHE1 and NHE2 activities (29). Calibration of the fluorescence signal was performed using the K+/H+ ionophore nigericin as described previously (39). The microfluorometry was performed on a Nikon TE200 inverted microscope with a Nikon CFI Super Fluor ×40 objective, coupled to a Lambda 10–2 filter wheel controller equipped with a multiwavelength filter set designed for BCECF. Photometric data were acquired using Metafluor software (Molecular Devices, Sunnyvale, CA). Na+/H+ exchange rate was described by the rate of pHi recovery, which was calculated by determining slopes along the pHi recovery by linear least-squares analysis over a minimum of 9 s.
Immunoprecipitation and Western blot analysis.
Immunoprecipitation was performed as previously described (14). Briefly, C2b/E3V/LPA5 cells were washed twice in cold PBS, scraped, and lysed in lysis buffer (Cell Signaling) containing 20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM β-glycerophosphate, 2.5 mM sodium pyrophosphate, 1 mM Na2EDTA, 1 mM EGTA, 1 mM Na3VO4, 1 μg/ml leupeptin, 1% Triton X-100, and protease inhibitor cocktail tablets (Roche, Indianapolis, IN). The crude lysate was sonicated for 2 × 15 s and spun at 14,000 g for 15 min. Protein concentration was determined by the bicinchoninic acid assay (Sigma). Lysate (500 μg) was precleared by incubation with 30 μl of protein A-Sepharose beads for 1 h, and the supernatant was then incubated overnight with anti-EGFR antibody. Immunocomplex was purified by incubating with 50 μl of protein A-Sepharose beads for 1.5 h, followed by three washes in lysis buffer and two washes in PBS. All the above steps were performed at 4°C or on ice. The bound immunocomplex was eluted by incubating the protein A beads in Laemmli sample buffer for 10 min at 95°C. The proteins were then separated by SDS-PAGE and transferred to a nitrocellulose membrane for Western immunoblotting, as previously described (39).
Confocal immunofluorescence microscopy.
C2b/E3V/LPA5 cells grown 7 days postconfluence on Transwells were washed twice with cold PBS, fixed in 4% paraformaldehyde in PBS for 10 min at room temperature, permeabilized in 0.2% Triton X-100 in PBS for 5 min, and blocked in PBS containing 5% normal goat serum for 30 min at room temperature. Cells were then stained with anti-EGFR, anti-HA, or anti-villin antibodies for 1 h at room temperature. Following three washes, 10 min each, with PBS, the cells were incubated with Alexa 488-conjugated donkey anti-mouse IgG or Alexa 555-conjugated goat anti-rabbit IgG (Invitrogen) for 1 h at room temperature. After 3 × 10-min washes with PBS, the excised Transwells were mounted with ProLong Gold Antifade Reagent (Invitrogen) and observed under a Zeiss LSM510 laser confocal microscope (Zeiss Microimaging, Thornwood, NY) coupled to a Zeiss Axioplan2e with ×63 Pan-Apochromat oil lenses.
Surface biotinylation.
Surface biotinylation of LPA5, NHE3, EGFR, and Na+-K+-ATPase was performed as previously described (14). Briefly, cells grown on permeable filters were treated with 1 μM LPA or 0.1% BSA vehicle for 5 min, then were rinsed twice in PBS and incubated for 10 min in borate buffer composed of 154 mM NaCl, 7.2 mM KCl, 1.8 mM CaCl2, and 10 mM H3BO3, pH 9.0. Cells were then incubated for 40 min with 0.5 mg/ml NHS-SS-biotin (Pierce, Rockford, IL) in borate buffer. Unbound NHS-SS-biotin was quenched with Tris buffer (20 mM Tris, 120 mM NaCl, pH 7.4). Cells were then rinsed with PBS, scraped, lysed in the lysis buffer described above, and sonicated for 2 × 15 s. The lysate was agitated for 30 min and spun at 14,000 g for 15 min to remove the insoluble cell debris. Protein concentration was determined, and 1 mg of lysate was then incubated with streptavidin-agarose beads (Pierce) for 2 h. The streptavidin-agarose beads were washed three times in lysis buffer and twice in PBS. All the above procedures were performed at 4°C or on ice. Biotinylated surface proteins were then eluted by boiling the beads at 95°C for 10 min. Dilutions of the total and surface LPA5, NHE3, EGFR, and Na+-K+-ATPase were resolved by SDS-PAGE, and immunoblotted with anti-HA antibody, anti-VSVG antibody, anti-EGFR antibody, and anti-α1-subunit of Na+-K+-ATPase antibody, respectively. Densitometric analysis was performed using Scion Image software (National Institutes of Health, Bethesda, MD).
RhoA activation assay.
Activation of RhoA was determined by a modified method described by Zhang et al. (45). Cells were seeded on 60-mm culture dishes. After serum starvation for 24 h, cells were activated with LPA and lysed in ice-cold lysis/binding buffer (25 mM Tris·HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% NP-40, 1 mM DTT, 10% glycerol, and protease inhibitors). Five hundred micrograms of lysate was incubated for 1 h with glutathione-sepharose beads (GE Healthcare, Uppsala, Sweden) coupled with the Rho-binding domain of Rhotekin (GST-RBD). The construct to express GST-RBD was kindly provided by Dr. Martin Schwartz (University of Virginia, Charlottesville). Beads were washed three times with lysis/binding buffer, and the bound RhoA proteins were eluted with Laemmli sample buffer and subjected to Western blotting using an anti-RhoA antibody.
Statistical analysis.
Results are presented as means ± SE. Statistical analyses were performed by Student's t-test for paired comparison. P < 0.05 was considered significant.
RESULTS
LPA-induced NHE3 activation in C2b/E3V/LPA5 cells is MEK dependent.
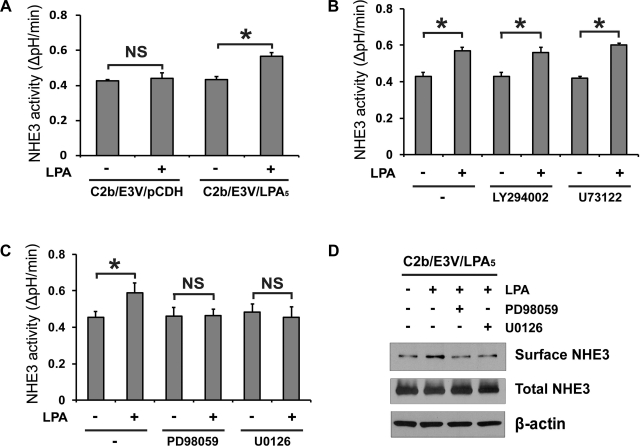
We have shown previously that LPA does not regulate NHE3 in Caco-2 and C2b cells due to low LPA5 expression in these cells (29). To circumvent this problem, we have used C2b cells expressing hNHE3V (29). Consistent with the previous study, transfection of HA-LPA5 in C2b/E3V cells resulted in a significant increase in NHE3 activity in response to 1 μM LPA, but not in control transfected cells (Fig. 1A). Major LPA signaling pathways include the phosphatidylinositol 3-kinase (PI3K)-Akt, phospholipase C (PLC)-β-Ca2+, and MEK-ERK pathways (32). Among them, PI3K is involved in LPA-induced stimulation of NHE3 in OK cells (23) and thus we first assessed the effect of PI3K inhibitor LY294002 on NHE3 activation by LPA. However, LPA-induced NHE3 activation in C2b/E3V/LPA5 cells was not affected by the presence of the PI3K inhibitor LY294002 (Fig. 1B), suggesting that PI3K does not play a key role in LPA5-induced activation of NHE3. Similarly, the PLC-β inhibitor U73122 did not block activation of NHE3 by LPA (Fig. 1B). On the other hand, the MEK inhibitor PD98059 and U0126 did not affect the basal NHE3 activity, but completely abrogated LPA-mediated activation of NHE3 (Fig. 1C). As shown previously (29), LPA increased NHE3 protein expression in the apical membrane, which was blocked by MEK inhibitors (Fig. 1D).
Fig. 1.
Lysophosphatidic acid 5 receptor (LPA5)-dependent Na+/H+ exchanger 3 (NHE3) activation in Caco-2bbe/human (h)NHE3/LPA5 cells is dependent on ERK. A: Caco-2bbe/hNHE3 (C2b/E3V) cells were transfected with lentiviral hemagglutinin (HA)-LPA5 or control virus derived from pCDH-puro vector. Serum-starved C2b/E3V/LPA5 or C2b/E3V/pCDH cells were loaded with BCECF, and NHE3 activities were determined as the rate of Na+-dependent pH recovery, ΔpH/min, in the presence or absence of 1 μM LPA for 3 min. For all experiments, cells were treated with LPA for 2–3 min. n ≥ 12; *P < 0.05. NS, not significant. B: NHE3 activity in C2b/E3V/LPA5 cells treated with or without LPA was determined in the presence of LY294002, U73122, or DMSO for 30 min. C: NHE3 activity in C2b/E3V/LPA5 cells treated with or without LPA was determined in the presence of PD98059, U0126, or DMSO. D: surface NHE3 protein abundance was assessed by surface biotinylation to determine the effects of MEK inhibitors (preincubation for 30 min) on surface NHE3 expression in C2b/E3V/LPA5 cells as described in experimental procedures. Total NHE3 and β-actin were used as loading controls. n = 3.
NHE3 regulation by LPA5 is mediated via EGFR.
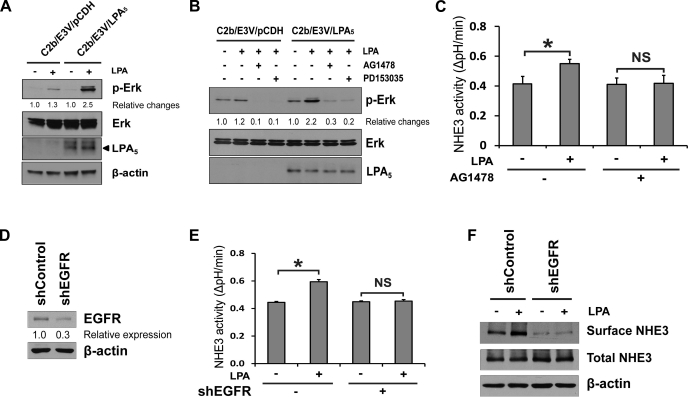
We have demonstrated previously that LPA phosphorylates ERK in Caco-2 cells via LPA2 receptor (43). To ensure that LPA5 signaling activates MEK in C2b/E3V cells, we determined phosphorylation of ERK as readout. Figure 2A shows that LPA induced a modest increase in the phosphorylation level of ERK in C2b/E3V cells, but the effect was significantly greater when LPA5 was expressed, suggesting that LPA-induced phosphorylation of ERK is primarily mediated via LPA5 in C2b/E3V/LPA5 cells. It is widely known that the receptor tyrosine kinases are transactivated by GPCRs, including LPA receptors (40). To determine whether EGFR transactivation is involved in LPA-induced ERK activation, C2b/E3V and C2b/E3V/LPA5 cells were pretreated with the EGFR inhibitors AG1478 or PD153035. As shown in Fig. 2B, both EGFR inhibitors blocked LPA-induced ERK phosphorylation. Consistent with the effects on ERK phosphorylation, AG1478 completely blocked LPA-mediated activation of NHE3, suggesting that EGFR is necessary for regulation of NHE3 by LPA5 (Fig. 2C). We confirmed the role of EGFR in LPA5-induced NHE3 regulation by knockdown of EGFR (Fig. 2D). EGFR knockdown in C2b/E3V/LPA5 cells decreased LPA-induced NHE3 activation (Fig. 2E), confirming the involvement of EGFR in this regulation. In addition, knockdown of EGFR blocked the increase in surface NHE3 abundance by LPA (Fig. 2F). Together, our data show that the presence of EGFR is obligatory for the activation of NHE3 by LPA5.
Fig. 2.
LPA5-induced NHE3 activation is mediated by EGF receptor (EGFR) transactivation. A: phosphorylation of ERK was determined in C2b/E3V/pCDH and C2b/E3V/LPA5 cells treated with LPA (2 min). p-ERK, phosphorylated ERK. β-Actin was used as a loading control. B: phosphorylation of ERK by LPA in C2b/E3V/pCDH and C2b/E3V/LPA5 cells was determined in the presence or absence of of preincubation with EGFR inhibitors AG1478 or PD153035 for 30 min. C: NHE3 activity in C2b/E3V/LPA5 cells treated with or without LPA (3 min) in the presence or absence of AG1478 determined as described in experimental procedures. n ≥ 12; *P < 0.05. D: knockdown of EGFR expression in C2b/E3V/LPA5 cells by short hairpin (sh)EGFR lentivirus is shown. EGFR expression level was decreased by 70% in shEGFR-infected cells compared with control cells. E: stimulation of NHE3 activity by LPA in C2b/E3V/LPA5 cells with EGFR knockdown was determined. n ≥ 12; *P < 0.05. F: the effect of EGFR knockdown on NHE3 protein expression in the apical membrane was determined by surface biotinylation. Total NHE3 and β-actin were used as the loading control, respectively. n = 3.
LPA transactivates EGFR in the apical membrane.
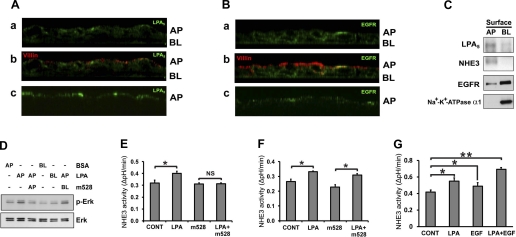
To determine the cellular locale of LPA5 and EGFR, confocal immunofluorescence microscopic analysis was performed in Triton X-100-permeabilized C2b/E3V/LPA5 cells. Figure 3 Aa shows the presence of LPA5 (green) in the apical membrane and the subapical region. HA-LPA5 staining at the basolateral membrane was low. Villin (Fig. 3Ab, red) was labeled to outline the apical side of the cell. In contrast to LPA5, EGFR labeling was evident in both apical and basolateral membrane, although the basolateral staining was more prominent (Fig. 3, Ba and Bb, green). To ensure the apical expression of LPA5 and EGFR, immunostaining was performed on nonpermeabilized cells, and anti-HA antibody or m528 anti-EGFR antibody that binds to the HA epitope of HA-LPA5 or an extracellular domain of EGFR, respectively, was added only on the apical side of the cells. The data shown in Fig. 3, Ac and Bc, confirm the apical expression of LPA5 and EGFR. The polarized expression of LPA5 and EGFR was confirmed by surface biotinylation of C2b/E3V/LPA5 cells (Fig. 3C). Western blot analysis of the apical and basolateral fractions showed that LPA5 expression was more prominent in the apical membrane in which NHE3 was present. EGFR, on the other hand, was highly expressed in the basolateral membrane. However, a weaker band was detected in the apical fraction, confirming the apical expression of EGFR seen in Fig. 3B.
Fig. 3.
LPA transactivates EGFR in the apical (AP) membrane. A: C2b/E3V/LPA5 cells grown on Transwells were fixed with (a and b) or without (c) permeabilization and stained with anti-HA antibodies (green) or anti-villin antibodies (red). Anti-villin antibodies (red) were used to mark the apical side of the cells. Vertical (x-z) sectional views perpendicular to the confocal focal plane are shown. BL, basolateral. B: C2b/E3V/LPA5 cells on Transwells were fixed with (a and b) or without (c) permeabilization. Cells were labeled with anti-EGFR monoclonal antibodies with an epitope in the cytoplasmic domain (a and b, green) or m528 anti-EGFR antibody that binds to an extracellular domain of EGFR (c, green). Vertical (x-z) sectional views are shown. C: surface biotinylation was performed to determine the polarity of LPA5 and EGFR expression in C2b/E3V/LPA5 cells as described in experimental procedures. Western blot shows protein expression in the apical and basolateral fractions. NHE3 and Na+-K+-ATPase were used as the apical and basolateral marker, respectively. n = 3. D: C2b/E3V/LPA5 cells on Transwells were treated with LPA (2 min) from either the apical or the basolateral side of the cells. To inhibit EGFR, m528 anti-EGFR antibody was applied from the apical or basolateral side and cells were gently shaken for 16 h before LPA treatment. The expression levels of phospho-ERK and total ERK were determined. Results are representative of three independent experiments. E: apical EGFR was blocked by apical application of m528 or control antibodies. NHE3 activity was determined in the presence or absence of LPA (3 min). n ≥ 8; *P < 0.05. F: basolateral EGFR was blocked by m528 or control antibodies. NHE3 activities in the presence or absence of LPA are shown. n ≥ 8; *P < 0.05. G: NHE3 activity was determined in C2b/E3V/LPA5 cells treated with 1 μM LPA, 10 ng/ml EGF, or LPA and EGF together, for 3 min. n ≥ 12; *P < 0.05; **P < 0.01.
To determine the polarity of EGFR transactivation by LPA, cells were treated with LPA from either apical or basolateral side of the cells that were grown in Transwells (Fig. 3D). Apical LPA increased the phosphorylation level of ERK, but basolaterally applied LPA showed no effect, consistent with the apical expression of LPA5 shown earlier (Fig. 3, A and C). To determine whether apical or basolateral EGFR is transactivated by LPA, EGFR was blocked using neutralizing anti-EGFR antibody m528 from either the apical or the basolateral compartment (31). Inhibition of apical EGFR blocked ERK phosphorylation induced by apical LPA. However, inhibition of basolateral EGFR failed to block ERK phosphorylation. Consistent with the effects on ERK phosphorylation, inhibition of apical EGFR by m528 blocked stimulation of NHE3 activity by LPA in C2b/E3V/LPA5 cells (Fig. 3E). On the contrary, inhibition of basolateral EGFR did not affect NHE3 activation (Fig. 3F).
It was shown previously that activation of EGFR in the basolateral membrane of enterocytes activates NHE3 in Caco-2 cells and rabbit ileum (16, 19). To examine whether the apical EGFR also regulates NHE3, NHE3 activity was determined following apical addition of EGF. Figure 3G shows that apical EGF activated NHE3 activity, although its effect was small compared with the effect by LPA. Interestingly, when LPA and EGF were added concomitantly, we observed enhanced stimulation of NHE3 in an additive manner. These studies indicate that LPA5, which is predominantly expressed in the apical membrane of C2b cells, transactivates apical EGFR, but not basolateral EGFR. However, transactivation of EGFR by LPA5 does not saturate EGFR in the apical membrane.
LPA-induced NHE3 activation in C2b/E3V/LPA5 cells is Pyk2 dependent.
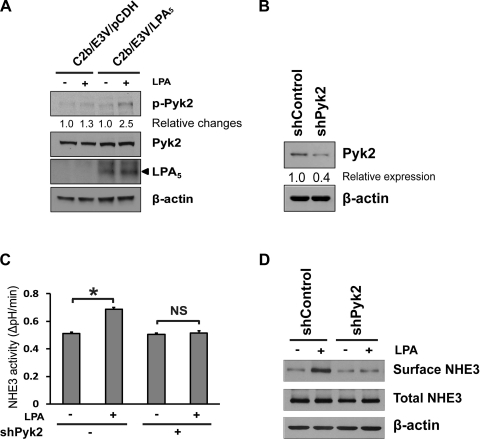
It was shown that Pyk2 is activated by LPA (1) and that Pyk2 is necessary for regulation of NHE3 by acidification (27, 38). We examined whether LPA activates Pyk2 by determining phosphorylation of Pyk2 at Tyr402 (25). LPA phosphorylated Pyk2 in C2b/E3V/LPA5 cells, but not in control transfected C2b/E3V cells (Fig. 4A). We next investigated whether Pyk2 plays a role in LPA-induced NHE3 regulation by knockdown of Pyk2 (Fig. 4B). Pyk2 knockdown in C2b/E3V/LPA5 cells attenuated LPA-induced NHE3 activation (Fig. 4C), demonstrating the importance of Pyk2 in this regulation. Consistent with MEK and EGFR inhibition, Pyk2 knockdown blocked the increase in NHE3 apical abundance by LPA (Fig. 4D).
Fig. 4.
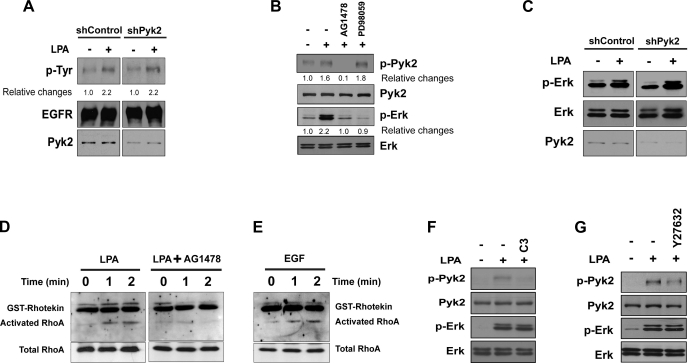
Proline-rich tyrosine kinase 2 (Pyk2) is involved in LPA5-dependent NHE3 activation. A: phosphorylation of Pyk2 (p-Pyk2) was determined in C2b/E3V/pCDH and C2b/E3V/LPA5 cells. The expression levels of Pyk2 and β-actin are shown as loading controls. B: C2b/E3V/LPA5 cells were treated with shControl or shPyk2. Western blot shows that Pyk2 expression level was decreased by 60% by shPyk2. C: NHE3 activity in C2b/E3V/LPA5 treated with shControl or shPyk2 was determined in the presence or absence of LPA (3 min). n ≥ 12; *P < 0.05. D: the effect of Pyk2 knockdown on surface NHE3 protein abundance was determined by surface biotinylation. Total NHE3 and β-actin were used as the loading control, respectively. n = 3.
LPA5-dependent transactivation of EGFR independently activates ERK and Pyk2.
A previous study using Pyk2-deficient fibroblasts showed that Pyk2 is necessary for activation of EGFR by LPA (1). However, in our study, knockdown of Pyk2 did not affect phosphorylation of EGFR, indicating that Pyk2 is not involved in EGFR activation by LPA5 in C2b/E3V/LPA5 cells (Fig. 5A). On the other hand, inhibition of EGFR by AG1478 completely blocked phosphorylation of Pyk2, indicating that Pyk2 is regulated by EGFR (Fig. 5B, lane 3). We next examined whether Pyk2 is regulated by MEK or vice versa. Inhibition of MEK by PD98059 did not diminish Pyk2 phosphorylation by LPA (Fig. 5B, lane 4). To our surprise, ERK phosphorylation was not altered by Pyk2 knockdown (Fig. 5C), suggesting that Pyk2 and MEK do not influence each other.
Fig. 5.
Pyk2 and ERK are independently activated by LPA5. A: phosphorylation of EGFR was assessed by determining Tyr phosphorylation levels of immunoprecipitated EGFR in C2b/E3V/LPA5 cells treated with shControl or shPyk2. B: the effects of AG1478 or PD98059 on Pyk2 or ERK phosphorylation were determined by Western blotting. Results are representative of three independent experiments. C: phosphorylation of ERK in cells treated with shControl or shPyk2 was determined by Western blotting. Results are representative of three independent experiments. D: activation of RhoA by LPA in C2b/E3V/LPA5 cells in the presence or absence of AG1478 was determined by Rhotekin pulldown. Activated RhoA bound to glutathione S-transferase (GST)-Rhotekin at different time intervals was resolved by SDS-PAGE, transferred, and blotted using an anti-RhoA antibody to determine the extent of RhoA activation. Total RhoA was determined as a loading control. n = 3. E: activation of RhoA by EGF was determined as described above. n = 3. F and G: C2b/E3V/LPA5 cells were treated with LPA in the presence or absence of C3 toxin (pretreated for 16 h, F) or Y27632 (pretreated for 2 h, G). Phosphorylated and total Pyk2 and ERK were determined. n = 3.
LPA activates Pyk2 via Rho GTPase in IEC-18 intestinal epithelial cells (41). To determine whether LPA activates RhoA in C2b cells, we determined RhoA activation by using GST-RBD. LPA rapidly activated RhoA as shown in Fig. 5D, left. Surprisingly, the presence of AG1478 ablated RhoA activation (Fig. 5D), suggesting that RhoA activation is dependent on EGFR. To ensure that EGFR is capable of activating RhoA, Rhotekin pulldown assay was performed in cells treated with EGF. Figure 5E shows that activation of EGFR by EGF resulted in activation of RhoA, demonstrating that EGFR can directly activate RhoA in C2b cells. Next, we determined whether LPA5 signaling activates Pyk2 via a Rho GTPase-dependent pathway. Inhibition of RhoA with the C3 exoenzyme blocked phosphorylation of Pyk2 but not ERK (Fig. 5F). Consistently, Y27632, a Rho-associated kinase (ROCK) inhibitor, inhibited phosphorylation of Pyk2, but not phosphorylation of ERK (Fig. 5G). These results suggest that LPA activates Pyk2 through the EGFR-RhoA-ROCK pathway and that Pyk2 and MEK are independently regulated by LPA5 via EGFR.
DISCUSSION
Recent studies have shown that LPA has stimulatory effects on fluid absorption in the intestine (29). Using cultured C2b cells, we showed previously that LPA stimulates Na+ absorption by NHE3 when LPA5 is transfected in these cells (29). In addition, our in vivo data using LPA2-null or NHERF2-null mice have been consistent with the notion that LPA5 is the main LPA receptor in the intestine mediating Na+ and fluid absorption (29). In the present study, we extended our study by investigating the mechanism of LPA5-elicited stimulation of NHE3. We found that EGFR plays a central role in LPA5-dependent regulation of NHE3 in C2b cells. Several receptor tyrosine kinases, including members of the EGFR family, are rapidly transactivated following GPCR stimulation. The mechanism of EGFR transactivation has been a subject of many studies, and multiple mechanisms have been proposed (40). EGFR is generally thought to be located in the basolateral membrane of polarized epithelial cells (3, 34). Previous studies have shown the role of the basolateral EGFR in regulation of electrolyte transport in intestinal epithelial cells (17, 19). However, it is becoming more evident that EGFR is found at the apical membrane; EGFR is found at the luminal surface of enterocytes in the sucking rat ileum and pig intestine as well as gastric mucosal oxyntic cells (9, 18). Although the functions of apical EGFR are poorly characterized, activation of apical EGFR decreases paracellular permeability of oxyntic mucosal cells and increases tolerance to apical acid (4). Interestingly, overexpression of EGFR in LLC-PK1 cell sorts a fraction of EGFR to the apical membrane where it transduces cellular signaling to enhance cell proliferation (21). Despite the detection of EGFR in both apical and basolateral membranes of C2b cells, the basolateral expression was much higher. On the other hand, LPA5 showed an opposite expression profile with a prevalence for the apical expression and the application of LPA to the apical but not the basolateral side of C2b cells activated ERK. Importantly, LPA5 transactivated EGFR in the apical membrane but not the basolateral EGFR. The pairing up of LPA5 with apically located EGFR probably makes it spatially more efficient for rapid signal transduction, given their close proximity. We showed previously that LPA5 interacts with NHERF2 through its carboxyl terminal motif that specially binds to the second PDZ domain of NHERF2 (29). Interestingly, the first PDZ domain of NHERF1 interacts with EGFR to stabilize the receptor expression at the cell surface (22). Our unpublished data indicate that NHERF2 also interacts with EGFR, which is not unexpected given the high degree of similarity between NHERF1 and NHERF2. In intestinal epithelial cells, NHERF2 is predominantly expressed in the apical or near the apical membrane, and hence it remains an intriguing possibility that NHERF2 tethers LPA5 and EGFR together.
Transactivation of EGFR often involves Src and ectodomain shedding of a transmembrane precursor of EGFR ligand (10, 40). Consistently, we found that inhibition of Src and metalloproteinases by PP2 and TAPI-1, respectively, blocked auto-phosphorylation of EGFR induced by LPA (data not shown). In addition to Src, Pyk2 has been implicated in linking GPCRs to EGFR, although other studies showed that Pyk2 is recruited by transactivated EGFR (1, 33). In addition, Pyk2 is involved in acid-induced activation of NHE3 and Src (27). In the current study, knockdown of Pyk2 blocked LPA5-dependent activation of NHE3. However, knockdown of Pyk2 did not affect LPA-induced phosphorylation of EGFR. Instead, inhibition of EGFR ablated phosphorylation of Pyk2, indicating that Pyk2 lies downstream of EGFR. It was shown that LPA5 couples with G12/13 to activate RhoA and ROCK in B103 neuroblastoma cells (24). Consistent with this earlier study, LPA rapidly activated RhoA in C2b/E3V cells, and C3 transferase or Y27632 attenuated LPA-induced Pyk2 phosphorylation, indicating that LPA5 activates Pyk2 via the Rho-ROCK pathway. It is generally assumed that GPCRs directly activate RhoA via G proteins. However, we found that the presence of EGFR was necessary for the activation of RhoA in C2b cells, suggesting that LPA activates RhoA via transactivation of EGFR. This unexpected finding was affirmed by demonstrating that activation of RhoA by EGF implying that RhoA is a downstream effect of EGFR. In agreement with our current findings, LPA activates Rho in Swiss 3T3 cells via EGFR (8).
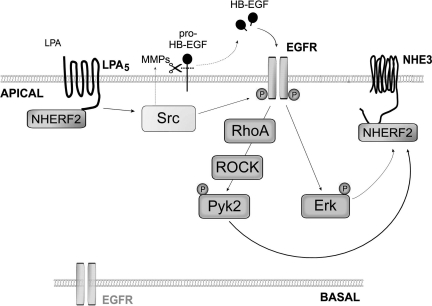
LPA has been described to induce exocytic trafficking of NHE3 to the apical membrane in a NHERF2 dependent manner (29). This regulation is acute occuring within 2–3 min of LPA treatment. We found that increased NHE3 expression in the apical membrane of C2b cells is maintained for at least 15 min after removal of LPA (data not shown). However, whether LPA specifically alters the rate of NHE3 removal from the apcial membrane is not known. In the current study, we found that LPA5-induced activation of Pyk2 and ERK is associated with increased NHE3 abundance at the apical surface. In addition, we found that phosphorylation of Pyk2 was not blocked by MEK inhibition, indicating that Pyk2 and ERK are activated via two distinct pathways. Based on these findings, we propose a model that LPA5 signaling transactivates EGFR, which then activates the RhoA-ROCK-Pyk2 and Ras-MEK-ERK pathways (Fig. 6). However, it remains unclear how ERK or Pyk2 regulates NHE3 activity and surface abundance in response to LPA. Regulation of NHE3 activity is dependent on the actin cytoskeleton, and RhoA/ROCK stimulates NHE3 activity by formation of stress fiber that increases NHE3 apical membrane abundance (20, 42). Pyk2 is a member of the focal adhesion kinase (FAK) family, and it regulates actin cytoskeletal organization in combination with FAK (6). Thus, it seems feasible that Pyk2 inhibition may contribute to disruption of the actin cytoskeleton, resulting in decreased NHE3 expression in the apical membrane and hence decreased NHE3 activity. The Ras-MEK-ERK pathway has been the subject of intense study because mutations of the signaling components of this pathway are a hallmark of several human diseases (11). ERK has also been implicated to play a role in regulation of membrane protein trafficking by phosphorylation (7, 15). The p90 kDa ribosomal S6 kinases (RSKs) are a family of Ser/Thr kinases that lie downstream of ERK (2). Sustained acidification activates NHE1 in AP-1 cells via an ERK-dependent pathway, and RSK phosphorylates NHE1 in response to serum (30, 37). Recently, starfish NHE, which is a homolog of mammalian NHE3, was shown to be phosphorylated by RSK at Ser-590, -606, and -673 in the COOH terminus of starfish NHE (12). Given the evolutionary link between starfish NHE and mammalian NHE3, it is possible that NHE3, which contains several R-X-X-pS/T motifs, is also phosphorylated by RSKs. Probable points of cellular communication by Pyk2 and ERK in stress fiber formation and NHE3 phosphorylation, respectively, are in conceptual agreement with our present data that either Pyk2 knockdown or MEK inhibition similarly blocks activation of NHE3 by LPA without a sign of redundancy.
Fig. 6.
Putative model depicting the mechanism of NHE3 regulation by LPA5-mediated signaling. LPA5 transactivates EGFR in the apical membrane, which activates the RhoA-RhoA-associated kinase (ROCK)-Pyk2 and the Ras-MEK-ERK pathways that converge onto NHE3. HB, heparin-binding; MMP, matrix metalloproteinases; NHERF, Na+/H+ exchanger regulatory factor 2.
In summary, we report here that LPA5 transactivates apical EGFR, followed by two independent activations of ERK and Pyk2. This work highlights the importance of apical EGFR in regulation of NHE3 that EGFR in the apical membrane plays a pivotal role in regulation of NHE3 by activation of the RhoA-ROCK-Pyk2 and Ras-MAPK pathways.
GRANTS
This work was supported by National Institutes of Health Grants (NIH) DK-061418 and DK-061418S1. P. He was supported by a postdoctoral fellowship from the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Agnieszka Bialkowska for help with lentiviral infection. We acknowledge the Emory Digestive Disease Research Development Center (supported by NIH Grant DK-064399) for the use of the confocal microscope.
Footnotes
This article is the topic of an Editorial Focus by Weiqiang Zhang and Anjaparavanda P. Naren (46).
REFERENCES
- 1. Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem 276: 20130–20135, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol 9: 747–758, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bishop WP, Wen JT. Regulation of Caco-2 cell proliferation by basolateral membrane epidermal growth factor receptors. Am J Physiol Gastrointest Liver Physiol 267: G892–G900, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Chen MC, Solomon TE, Kui R, Soll AH. Apical EGF receptors regulate epithelial barrier to gastric acid: endogenous TGF-α is an essential facilitator. Am J Physiol Gastrointest Liver Physiol 283: G1098–G1106, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 50: 157–186, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Du QS, Ren XR, Xie Y, Wang Q, Mei L, Xiong WC. Inhibition of PYK2-induced actin cytoskeleton reorganization, PYK2 autophosphorylation and focal adhesion targeting by FAK. J Cell Sci 114: 2977–2987, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Giovannardi S, Forlani G, Balestrini M, Bossi E, Tonini R, Sturani E, Peres A, Zippel R. Modulation of the inward rectifier potassium channel IRK1 by the Ras signaling pathway. J Biol Chem 277: 12158–12163, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Gohla A, Harhammer R, Schultz G. The G-protein G13 but not G12 mediates signaling from lysophosphatidic acid receptor via epidermal growth factor receptor to Rho. J Biol Chem 273: 4653–4659, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Gonnella PA, Siminoski K, Murphy RA, Neutra MR. Transepithelial transport of epidermal growth factor by absorptive cells of suckling rat ileum. J Clin Invest 80: 22–32, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 20: 1594–1600, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144: 646–674, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Harada K, Fukuda E, Hirohashi N, Chiba K. Regulation of intracellular pH by p90Rsk-dependent activation of an Na(+)/H(+) exchanger in starfish oocytes. J Biol Chem 285: 24044–24054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He P, Yun CC. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol 2010: 238080, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He P, Zhang H, Yun CC. IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J Biol Chem 283: 33544–33553, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrlich A, Klinman E, Fu J, Sadegh C, Lodish H. Ectodomain cleavage of the EGF ligands HB-EGF, neuregulin1-beta, and TGF-alpha is specifically triggered by different stimuli and involves different PKC isoenzymes. FASEB J 22: 4281–4295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Janecki AJ, Montrose MH, Zimniak P, Zweibaum A, Tse CM, Khurana S, Donowitz M. Subcellular redistribution is involved in acute regulation of the brush border Na+/H+ exchanger isoform 3 in human colon adenocarcinoma cell line Caco-2. Protein kinase C-mediated inhibition of the exchanger. J Biol Chem 273: 8790–8798, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Keely SJ, Uribe JM, Barrett KE. Carbachol stimulates transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T84 cells. Implications for carbachol-stimulated chloride secretion. J Biol Chem 273: 27111–27117, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Kelly D, McFadyen M, King TP, Morgan PJ. Characterization and autoradiographic localization of the epidermal growth factor receptor in the jejunum of neonatal and weaned pigs. Reprod Fertil Dev 4: 183–191, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Khurana S, Nath SK, Levine SA, Bowser JM, Tse CM, Cohen ME, Donowitz M. Brush border phosphatidylinositol 3-kinase mediates epidermal growth factor stimulation of intestinal NaCl absorption and Na+/H+ exchange. J Biol Chem 271: 9919–9927, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Kurashima K, D'Souza S, Szaszi K, Ramjeesingh R, Orlowski J, Grinstein S. The apical Na(+)/H(+) exchanger isoform NHE3 is regulated by the actin cytoskeleton. J Biol Chem 274: 29843–29849, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Kuwada SK, Lund KA, Li XF, Cliften P, Amsler K, Opresko LK, Wiley HS. Differential signaling and regulation of apical vs. basolateral EGFR in polarized epithelial cells. Am J Physiol Cell Physiol 275: C1419–C1428, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Lazar CS, Cresson CM, Lauffenburger DA, Gill GN. The Na+/H+ exchanger regulatory factor stabilizes epidermal growth factor receptors at the cell surface. Mol Biol Cell 15: 5470–5480, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee-Kwon W, Kawano K, Choi JW, Kim JH, Donowitz M. Lysophosphatidic acid stimulates brush border Na+/H+ exchanger 3 (NHE3) activity by increasing its exocytosis by an NHE3 kinase A regulatory protein-dependent mechanism. J Biol Chem 278: 16494–16501, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem 281: 23589–23597, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature 376: 737–745, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med 202: 975–986, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li S, Sato S, Yang X, Preisig PA, Alpern RJ. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest 114: 1782–1789, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin S, Wang D, Iyer S, Ghaleb AM, Shim H, Yang VW, Chun J, Yun CC. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology 136: 1711–1720, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M, Lamprecht G, de Jonge HR, Tse M, Donowitz M, Hogema BM, Chun J, Seidler U, Yun CC. Lysophosphatidic acid stimulates the intestinal brush border Na(+)/H(+) exchanger 3 and fluid absorption via LPA(5) and NHERF2. Gastroenterology 138: 649–658, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malo ME, Li L, Fliegel L. Mitogen-activated protein kinase-dependent activation of the Na+/H+ exchanger is mediated through phosphorylation of amino acids Ser770 and Ser771. J Biol Chem 282: 6292–6299, 2007 [DOI] [PubMed] [Google Scholar]
- 31. McCole DF, Keely SJ, Coffey RJ, Barrett KE. Transactivation of the epidermal growth factor receptor in colonic epithelial cells by carbachol requires extracellular release of transforming growth factor-alpha. J Biol Chem 277: 42603–42612, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 3: 582–591, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Schauwienold D, Sastre AP, Genzel N, Schaefer M, Reusch HP. The transactivated epidermal growth factor receptor recruits Pyk2 to regulate Src kinase activity. J Biol Chem 283: 27748–27756, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Scheving LA, Shiurba RA, Nguyen TD, Gray GM. Epidermal growth factor receptor of the intestinal enterocyte. Localization to laterobasal but not brush border membrane. J Biol Chem 264: 1735–1741, 1989 [PubMed] [Google Scholar]
- 35. Singh AK, Riederer B, Krabbenhoft A, Rausch B, Bonhagen J, Lehmann U, de Jonge HR, Donowitz M, Yun C, Weinman EJ, Kocher O, Hogema BM, Seidler U. Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice. J Clin Invest 119: 540–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singla A, Dwivedi A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of lysophosphatidic acid (LPA) mediated stimulation of intestinal apical Cl−/OH− exchange. Am J Physiol Gastrointest Liver Physiol 298: G182–G189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takahashi E, Abe J, Gallis B, Aebersold R, Spring DJ, Krebs EG, Berk BC. p90(RSK) is a serum-stimulated Na+/H+ exchanger isoform-1 kinase. Regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J Biol Chem 274: 20206–20214, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Tsuganezawa H, Sato S, Yamaji Y, Preisig PA, Moe OW, Alpern RJ. Role of c-SRC and ERK in acid-induced activation of NHE3. Kidney Int 62: 41–50, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Wang D, Sun H, Lang F, Yun CC. Activation of NHE3 by dexamethasone requires phosphorylation of NHE3 at Ser663 by SGK1. Am J Physiol Cell Physiol 289: C802–C810, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol 4: 651–657, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Wu SS, Chiu T, Rozengurt E. ANG II and LPA induce Pyk2 tyrosine phosphorylation in intestinal epithelial cells: role of Ca2+, PKC, and Rho kinase. Am J Physiol Cell Physiol 282: C1432–C1444, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Yang X, Huang HC, Yin H, Alpern RJ, Preisig PA. RhoA required for acid-induced stress fiber formation and trafficking and activation of NHE3. Am J Physiol Renal Physiol 293: F1054–F1064, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA, Shim H. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol 289: C2–C11, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Zhang H, Wang D, Sun H, Hall RA, Yun CC. MAGI-3 regulates LPA-induced activation of ERK and RhoA. Cell Signal 19: 261–268, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang W, Naren AP. Bioactive phospholipid signaling influences NHE3-dependent intestinal Na+ and fluid absorption: a macromolecular complex perspective. Focus on “Lysophosphatidic acid 5 receptor induces activation of Na+/H+ exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells” Am J Physiol Cell Physiol (August 17, 2011). doi:10.1152/ajpcell.00303.2011 [DOI] [PMC free article] [PubMed]