Abstract
Urinary acidification in the collecting duct is mediated by the activity of H+-ATPases and is stimulated by various factors including angiotensin II and aldosterone. Classically, aldosterone effects are mediated via the mineralocorticoid receptor. Recently, we demonstrated a nongenomic stimulatory effect of aldosterone on H+-ATPase activity in acid-secretory intercalated cells of isolated mouse outer medullary collecting ducts (OMCD). Here we investigated the intracellular signaling cascade mediating this stimulatory effect. Aldosterone stimulated H+-ATPase activity in isolated mouse and human OMCDs. This effect was blocked by suramin, a general G protein inhibitor, and GP-2A, a specific Gαq inhibitor, whereas pertussis toxin was without effect. Inhibition of phospholipase C with U-73122, chelation of intracellular Ca2+ with BAPTA, and blockade of protein kinase C prevented the stimulation of H+-ATPases. Stimulation of PKC by DOG mimicked the effect of aldosterone on H+-ATPase activity. Similarly, aldosterone and DOG induced a rapid translocation of H+-ATPases to the luminal side of OMCD cells in vivo. In addition, PD098059, an inhibitor of ERK1/2 activation, blocked the aldosterone and DOG effects. Inhibition of PKA with H89 or KT2750 prevented and incubation with 8-bromoadenosine-cAMP mildly increased H+-ATPase activity. Thus, the nongenomic modulation of H+-ATPase activity in OMCD-intercalated cells by aldosterone involves several intracellular pathways and may be mediated by a Gαq protein-coupled receptor and PKC. PKA and cAMP appear to have a modulatory effect. The rapid nongenomic action of aldosterone may participate in the regulation of H+-ATPase activity and contribute to final urinary acidification.
Keywords: collecting duct, kidney, receptor, signaling cascade
the kidney plays a major role in the control of systemic acid-base homeostasis by reabsorbing filtered bicarbonate, generating new bicarbonate, and secreting protons largely bound to buffers such as phosphate and ammonia. The final step of urinary acidification occurs along the cortical and outer medullary collecting duct (OMCD) mainly through the action of vacuolar H+-ATPases expressed in the luminal membrane of acid-secretory type A intercalated cells (2, 8, 15, 27, 69–71). Renal acid secretion and urinary acidification are tightly regulated by a number of factors including several hormones such as endothelin, angiotensin II, and aldosterone (15, 27, 70–71). The influence of the renin-angiotensin II-aldosterone system (RAAS) on the ability of the kidney to excrete protons is well recognized. Both angiotensin II and aldosterone have been demonstrated to directly and indirectly influence transport pathways involved in acid secretion such as Na+/H+ exchangers and vacuolar H+-ATPases in different nephron segments (1, 16, 19–23, 30, 51–52, 58, 75). Aldosterone deficiency or defects in its receptor activation are causative for the hyperkalemic form of distal renal tubular acidosis (type IV dRTA) (15).
In the connecting segment and cortical collecting duct, aldosterone stimulates the activity of the electrogenic vacuolar H+-ATPase, an effect that has been proposed to be of a primarily indirect nature. Increased reabsorption of Na+ through the epithelial Na+ channel ENaC in neighboring principal cells renders the tubule lumen more negative and could, thus, facilitate proton pumping (15, 27). We have recently demonstrated that this interaction occurs mainly in the connecting tubule (40). Thus, the exact mechanism by which Na+ absorption, H+ secretion, and aldosterone effects are linked in the cortical collecting duct is not fully understood. In contrast, in the OMCD, aldosterone stimulates proton secretion and this effect occurs even in the absence of significant Na+ absorption, suggesting a direct effect on vacuolar H+-ATPase activity (65). Consistent with these observations, long-term stimulatory effects of aldosterone on vacuolar H+-ATPase activity have been described in rabbit and rat OMCDs (14, 19, 30, 52).
Cellular responses to aldosterone in the classic long-term range (i.e., responses requiring several hours or days) are mediated by alterations in gene expression driven by the binding of the steroid hormone to a cytosolic or nuclear receptor acting as a transcription factor (50, 64, 68). However, not all biological effects of aldosterone can be explained by transcriptional or genomic effects. Over the past years an increasing number of studies have identified nongenomic effects of aldosterone in both epithelial and nonepithelial tissues (for review, see refs. 17, 46–50, 64). Taken together, these studies suggest the existence of novel steroid hormone receptors or possibly classical receptors embedded in the membrane that initiate nongenomic signaling cascades (17–18, 35, 49). Activation of these putative receptors initiates several intracellular signaling cascades that appear to be tissue or cell specific, and the involvement of phospholipase C, inositol 1,4,5-trisphosphate (IP3), protein kinase C pathways as well as adenylyl cyclases, cAMP and PKA-dependent pathways has been described (17, 48–49). In addition, activation of ERK1/2 MAPK kinases has been observed (20, 41, 57). However, most of these studies were performed in cell culture models and only little information exists if similar pathways are activated in freshly isolated cells and tissues.
Recently, we described the direct stimulation of vacuolar H+-ATPase activity in freshly isolated mouse acid-secretory intercalated cells by aldosterone. In OMCD intercalated cells, aldosterone increased vacuolar H+-ATPase activity in the presence of the mineralocorticoid receptor blocker spironolactone and did not require transcription or translation. The stimulatory effect of aldosterone was associated with a transient rise in intracellular Ca2+ and required intact protein kinase C (PKC) (75). Here we further investigated the mechanism by which aldosterone stimulates vacuolar H+-ATPase activity. The results present pharmacological evidence supporting the hypothesis that aldosterone acts through both PLC/Ca2+/PKC- and cAMP/PKA-dependent pathways and requires ERK1/2 activity. Furthermore, the rapid effects of aldosterone may require a G protein-coupled membrane receptor sensitive to suramin and GP-2A but not pertussis toxin.
MATERIALS AND METHODS
Animals.
C57BL/6J mice (Jackson Laboratory) (male, 12–15 wk old, 30–35 g) were housed in climate- and humidity-controlled light-cycled rooms, fed standard rodent chow, and allowed free access to water. All studies carried out in Zurich were approved by the local Swiss Veterinary Authority (Veterinäramt, Zurich, Switzerland) and were performed according to Swiss Animal Welfare laws.
Human kidney samples.
Human kidney samples were collected from patients undergoing nephrectomy for tumor removal (Department of Urology, University of Zurich, Switzerland). Informed consent was obtained from all patients and all studies were approved by the local ethics committee. Kidney samples were taken from the apparently healthy part of the removed kidneys and placed into ice-cold medium (MEM + Earle's + l-glutamine + 25 mM HEPES, GIBCO) for transport.
Isolation of OMCDs.
OMCDs were prepared from human and mouse kidneys with a small modification, as described (72, 75). Briefly, mice were anesthetized and perfused through the left ventricle with a phosphate/sucrose buffer (140 mM sucrose/140 mM NaH2PO4, pH 7.4) with 6 mg/ml trypsin inhibitor type II-S and 250 μg/ml collagenase (Sigma). Kidneys were harvested and transferred to a dissection microscope, coronal slices 1–2 mm in thickness were prepared, and the outer medulla was dissected. Outer medulla pieces were transferred to a digestion buffer and incubated at 37°C for 30 min, washed several times with ice-cold HEPES buffer, and stored on ice until further use. Human kidney samples were cut into coronal slices, the outer medulla were identified and prepared, and small cubic pieces of about 2–3 mm in side length were incubated in the digestion buffer at 37°C for 45–60 min. After several washes with ice-cold HEPES buffer, tubules were stored on ice until further use. Human and mouse OMCDs were identified under the dissection microscope, transferred on coverslips precoated with Cell-Tak (BD Biosciences, Franklin Lakes, NJ), and allowed to adhere.
Intracellular pH measurements.
Coverslips with isolated OMCDs were transferred to a thermostatically controlled perfusion chamber (≈3 ml/min flow rate) maintained at 37°C on an inverted microscope (Zeiss Axiovert 200, Carl Zeiss, Feldbach, Switzerland) equipped with a video imaging system (Visitron, Munich, Germany) for the duration of the experiment. The isolated OMCDs were incubated in a HEPES-buffered Ringer solution containing the pH-sensitive dye BCECF-AM [2′,7′-bis(2-carboxylethyl)-5(6)-carboxyfluorescein ester] (10 μM, Molecular Probes) for 15 min and were washed to remove all nondeesterified dye.
Intracellular pH (pHi) was measured microfluorometrically by alternately exciting the dye with light at 495 and 440 nm while monitoring the emission at 532 nm with a video imaging system. Each experiment was calibrated for pHi using the nigericin/high-K+ method., and the obtained ratios were converted to pHi as described (66, 72, 75). All experiments were performed in the nominal absence of bicarbonate. The initial solution was a HEPES-buffered Ringer solution (in mM: 125 NaCl, 3 KCl, 1 CaCl2, 1.2 MgSO4, 2 KH2PO4, 32.2 HEPES, pH 7.4). Cells were acidified by using the NH4Cl (20 mM) prepulse technique and washed into a Na+-free solution [Na+ was replaced by equimolar concentrations of N-methyl-d-glucamine (NMDG)]. Intercalated cells were identified by their rapid uptake of BCECF as described previously (72). The rate of H+-ATPase activity was determined as the concanamycin-sensitive pHi alkalinization rate in the absence of Na+. Rates were calculated over the same range of pHi (6.55–6.75) for all cells studied. All chemicals were of highest purity and were purchased from Sigma (Buchs, Switzerland) and Calbiochem (Merck, Darmstadt, Germany). Chemicals were added to the solutions from stock solutions at the given concentrations for each experimental protocol (Table 1).
Table 1.
Pharmacological agents used to modulate the activity of intracellular signaling molecules
| Substance | Target |
|---|---|
| Pertussis toxin | Gi and Go G protein inhibitor |
| Suramin | G protein inhibitor. Also directly blocks purinergic and adrenergic receptors. |
| G2A | Gαq inhibitor |
| U-73122 | Inhibitor of phospholipase C |
| U-73343 | Inactive analog to U-73122 |
| BAPTA-AM | Chelator of intracellular calcium |
| Chelerythrine | Pan-PKC inhibitor |
| DOG (1,2-dioctanoyl-sn-glycerol) | Diacylglycerol analog, PKC activator |
| PD098059 | Prevents activation of mitogen-activated protein kinase kinase-1 |
| H89 {N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride} | Inhibitor of protein kinase A |
| KT5720 | Reversible and ATP-competitive inhibitor of protein kinase A |
Intrinsic buffering power and calculation of proton fluxes.
The intrinsic buffering power (βi) is defined by the amount of acid or base that has to be added to a solution to change its pH by one unit. The βi of OMCD cells was measured as described previously (7, 73), but changes in the pHi were induced by superfusion with a weak acid and the exclusion of Cl− from the bath. The cellular H+-dependent regulatory mechanisms were excluded as a buffering system by using the inhibitors concanamycin A (200 μM) and Sch28080 (2-methyl-8-(phenylmethoxy)imidazo[1,2-a]pyridine-3-acetonitrile) (Tocris Biosciences, Bristol, UK) (100 μM). The tubules were incubated either with 10 μM BCECF alone or with 10 nM aldosterone for 20 min in HEPES-buffered Ringer solution, pH 7.3. Afterward, the tubules were equilibrated with an acetate- and Cl−-free solution (in mM: 15 Ca-gluconate, 50 NMDG gluconate, 98 K-gluconate, 10 HEPES, 1.2 MgSO4, 1 K2HPO4, pH 7.3). The superfusion solution was switched to an acetate-containing but Cl−-free solution (in mM: 15 Ca-gluconate, 20 K-acetate, 50 NMDG gluconate, 98 K-gluconate, 10 HEPES, 1.2 MgSO4, 1 K2HPO4, pH 7.3) resulting in a decrease of pHi. When the cell acidification reached a plateau, the experiment was calibrated to pH 6.5 and 7.0 using the nigericin/high-K+ technique (66, 72). The βi was calculated as the ratio of the change in intracellular acetate concentration to the change in pHi: βi = ΔA/ΔpHi, where ΔpHi is the decrease in pHi resulting from weak acid addition and ΔA is the change in intracellular acetate concentration calculated from its pKa (4.74 at 37°C) and pHi.
Proton fluxes JH (in pmol·min−1·mm−1) were calculated following the equation: JH = dpHi/dt × βi × V, where dpHi/dt is the rate of H+-ATPase activity and V is cell volume. Net proton efflux is indicated by positive JH values.
Tissue preparation and treatment of rats with aldosterone and DOG.
Adult male Sprague-Dawley rats (Charles River, Wilmington, MA) were housed under standard conditions and fed a standard rodent diet. Animal studies were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care, in accordance with the National Institutes of Health, Department of Agriculture, and Association for Assessment and Accreditation of Laboratory Animal Care International requirements.
Rats were anesthetized with pentobarbital sodium (Nembutal, Abbott Laboratories, Abbott Park, IL, 50 mg/kg body wt ip) and treated using a modification of the protocol we described previously (55). A 200-μl bolus injection of 200 nM or 200 μM aldosterone (Sigma-Aldrich, St. Louis, MO) or 20 μM DOG (Cayman Chemical, Ann Arbor, MI), a cell membrane-permeable diacylglycerol analog and activator of protein kinase C, dissolved in physiological buffer was injected intravenously into the tail vein. Immediately following the bolus injection, a steady infusion of the respective drug at the same concentration was made for 15 min into the tail vein. After the infusion, the animal was perfused via the left cardiac ventricle with phosphate-buffered saline (PBS, 0.9% NaCl in 10 mM phosphate buffer, pH 7.4) followed by paraformaldehyde-lysine-periodate fixative (PLP) as previously described (54). Three control rats were injected and infused with buffer alone, and three rats each were injected and infused with aldosterone at both concentrations or with DOG as described above. Following the PLP perfusion, the kidneys were dissected, sliced, and further fixed by immersion in PLP for 4 h at room temperature and then overnight at 4°C. Kidneys were rinsed extensively in PBS the following day and stored at 4°C in PBS containing 0.02% sodium azide.
Immunofluorescence and confocal microscopy.
PLP-fixed kidney slices were cryoprotected in PBS containing 0.9 M sucrose, embedded in Tissue-Tek OCT compound 4583 (Sakura Finetek USA, Torrance, CA) and frozen at −20°C, and 5-μm sections were cut on a Leica CM3050 S cryostat (Leica Microsystems, Bannockburn, IL). Sections were rehydrated in PBS and treated with 1% (wt/vol) SDS for 4 min for retrieval of antigenic sites (9). We subsequently followed a previously described immunostaining protocol which includes a 20-min blocking step in 1% (wt/vol) bovine serum albumin in PBS, a 90-min incubation with a primary antibody against the V-ATPase 70-kDa “A” subunit (34) diluted in Dako antibody diluent (Dako, Carpinteria, CA), and a 60-min incubation with Alexa Fluor 594 goat anti-rabbit IgG (H+L) (Invitrogen, Carlsbad, CA) at 2.5 μg/ml as secondary antibody, all at room temperature (55).
Digital images were acquired using a Nikon 80i epifluorescence microscope (Nikon Instruments, Melville, NY) equipped with an Orca 100 CCD camera (Hamamatsu, Bridgewater, NJ). Images were acquired using IPLab version 3.2.4 (Scanalytics, Fairfax, VA) and final figures were produced using Adobe Photoshop version CS2 image editing software (Adobe Systems, San Jose, CA) (55).
Statistics.
Data are presented as means ± SE. One-way ANOVA with Bonferroni post test was performed when comparing means across three or more groups. All other data were tested for significance using the unpaired Student's t-test, and only results with P < 0.05 were considered as statistically significant.
RESULTS
Rapid stimulation of H+-ATPase activity in mouse OMCD intercalated cells by aldosterone.
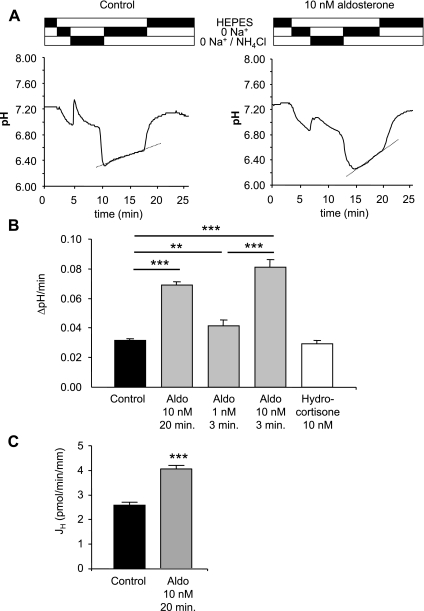
In type A acid-secretory intercalated cells of mouse OMCD, the mean initial pHi was 7.31 ± 0.01 (Table 2). pHi acidified after removal of sodium from the bath and alkalinized after the addition of an NH4Cl pulse (20 mM) (Fig. 1A). After removal of NH4Cl from the perfusion chamber, the OMCD intercalated cells were acidified to a mean pH 6.42 ± 0.01. In the continued absence of Na+ the intracellular pHi recovered slowly. The observed intracellular alkalinization rate was 0.031 ± 0.002 pHi units/min. This alkalinization is mainly due to the activity of H+-ATPases and is mostly inhibited by the specific inhibitor concanamycin as previously shown (72, 75). Readdition of Na+ to the bath led to a rapid increase of pHi to the initial value mediated by Na+/H+ exchange (Fig. 1A).
Table 2.
Summary of Na+-independent pHi alkalinization rates of single intercalated cells in freshly isolated mouse outer medullary collecting ducts
| Initial pHi | Na+-Independent pHi Recovery, ΔpH/min | Number of Cells, OMCDs, Mice | |
|---|---|---|---|
| Control | 7.31 ± 0.01 | 0.031 ± 0.002 | 122 (11/6) |
| Aldosterone 10 nM (20 min) | 7.30 ± 0.02 | 0.069 ± 0.002† | 123 (15/13) |
| Aldosterone 1 nM (3 min) | 7.39 ± 0.03 | 0.041 ± 0.004* | 32 (3/3) |
| Aldosterone 10 nM (3 min) | 7.29 ± 0.01 | 0.081 ± 0.006† | 72 (4/3) |
| Hydrocortisone | 7.26 ± 0.02 | 0.030 ± 0.002 | 82 (5/3) |
Values are means ± SE. pHi, intracellular pH; OMCDs, outer medullary collecting ducts.
P < 0.01,
P < 0.001 as compared with the respective control.
Fig. 1.
Aldosterone (Aldo) stimulates vacuolar H+-ATPase activity in freshly isolated mouse outer medullary collecting duct (OMCD) intercalated cells. A: original intracellular pH (pHi) tracing of single intercalated cells in freshly isolated mouse OMCDs. OMCDs were incubated with 10 nM aldosterone for 20 min or left untreated (control). The dotted line marks the increase in pHi measured and used to calculate ΔpH/min values. The bars above the tracing indicate solutions used. Initially, OMCD fragments were bathed in a solution containing sodium; after removal of sodium, an initial pHi acidification could be observed. Addition of NH4Cl to the bath caused a transient alkalinization, and upon its removal from the bath, a strong acidification. pHi recovered slowly in the absence of sodium and more rapidly after readdition of sodium to the bath. B: summary bar graph of intracellular Na+-independent pHi alkalinization rates of mouse single intercalated cells. OMCDs were either incubated for 20 min with 10 nM aldosterone or aldosterone was added to the superfusate during the measurement 3 min before switching to Na+-free solutions after the NH4Cl-prepulse. Hydrocortisone (10 nM) was incubated for 20 min. C: summary of net proton fluxes (JH) calculated from intracellular buffering power and ΔpH/min for control and aldosterone incubated OMCD intercalated cells. Values are presented as means ± SE. **P < 0.01, ***P < 0.001.
Preincubation of mouse OMCDs with 10 nM aldosterone for 20 min at 37°C increased the Na+-independent alkalinization rate 2–3 fold to 0.069 ± 0.002 pHi units/min as observed previously (75). To test for the immediate onset of stimulation, 10 nM aldosterone was added to the bath 3 min before the solution was switched to Na+-free conditions (75). The stimulation of H+-ATPase activity was similar to the 20-min preincubation (0.081 ± 0.006 pH units/min) (Table 2 and Fig. 1), suggesting that the nongenomic stimulation of H+-ATPase activity occurs within a very short time frame. Addition of 1 nM aldosterone in the same experimental series (3-min preincubation) led to a small but significant increase of the Na+-independent alkalization rate (0.041 ± 0.004 pH units/min, Table 2 and Fig. 1). The steroid hormone hydrocortisone had no effect at similar concentrations (10 nM) with preincubation periods of 20 min (0.030 ± 0.002 pH units/min, Table 2 and Fig. 1). Thus, aldosterone specifically stimulates H+-ATPase activity in OMCD intercalated cells in a concentration-dependent manner and this effect occurs very rapidly, consistent with a rapid and nongenomic effect (75).
We also measured intrinsic buffering power (βi) in OMCD intercalated cells. Incubation with 10 nM aldosterone for 20 min decreased βi significantly from 42.8 ± 1.5 (n = 61) to 34.0 ± 1.4 (n = 58) (P < 0.001). Net proton fluxes were calculated and found to be significantly stimulated by aldosterone (Fig. 1C).
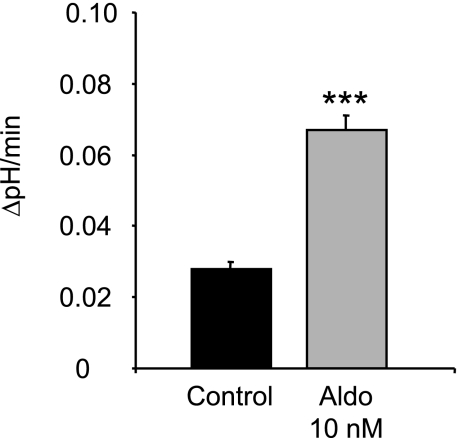
Aldosterone stimulates H+-ATPases in human OMCD cells.
Similar to the effect observed in freshly isolated mouse OMCD intercalated cells, aldosterone also stimulated H+-ATPase activity in freshly isolated human OMCDs prepared from human kidney samples after nephrectomy. The basal Na+-independent pHi recovery rate was 0.028 ± 0.002 pHi units/min (Table 3 and Fig. 2). After preincubation with 10 nM aldosterone for 20 min the intracellular pHi recovery rate increased twofold to 0.067 ± 0.004 pHi units/min (Table 3 and Fig. 2). Because of the limited access to human tissue, further studies were performed with mouse tissues. However, the results demonstrate that the stimulatory effect of aldosterone occurs also in human renal tissue and is not restricted to mouse OMCD intercalated cells.
Table 3.
Summary of Na+-independent pHi alkalinization rates of single intercalated cells in freshly isolated human outer medullary collecting ducts
| Initial pHi | Na+-Independent pHi Recovery, ΔpH/min | Number of Cells, OMCDs, Patients | |
|---|---|---|---|
| Human OMCD, control | 7.20 ± 0.01 | 0.028 ± 0.002 | 65 (5/4) |
| Human OMCD, aldosterone (10 nM) | 7.19 ± 0.02 | 0.067 ± 0.004* | 39 (3/1) |
Values are means ± SE.
P < 0.001 as compared with control.
Fig. 2.
Aldosterone stimulates vacuolar H+-ATPase activity in freshly isolated human OMCD intercalated cells. OMCDs were incubated with 10 nM aldosterone for 20 min or were left untreated (control). The summary of Na+-independent pHi alkalinization rates in single intercalated cells in freshly isolated human OMCDs in the absence and presence of aldosterone (10 nM, 20 min preincubation) is shown. Values are presented as means ± SE. ***P < 0.001.
The rapid stimulatory effect of aldosterone requires Gαq proteins and phospholipase C activity.
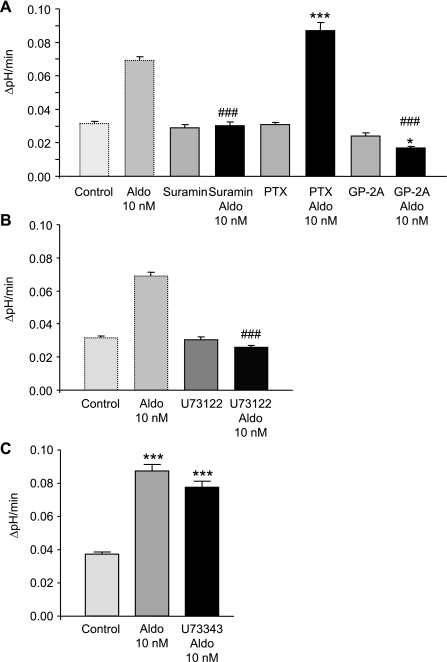
Aldosterone has been shown to require phospholipase C activity for its rapid effects in other tissues (6, 11). To investigate whether G proteins might be involved in the aldosterone-induced stimulation of H+-ATPase activity in mouse OMCDs, suramin (200 μM), a general and relatively unspecific inhibitor of G proteins (32), was used. Preincubation with suramin (200 μM) alone had no effect on the basal rates of alkalinization, whereas it prevented completely the stimulatory effect of aldosterone (Table 4 and Fig. 3). Pertussis toxin (200 ng/ml), which inactivates more specifically Gi and Go G proteins, had no effect alone and did not prevent the stimulatory effect of aldosterone (Table 4 and Fig. 3). In contrast, GP-2A (20 μM), a small peptide inhibitor of Gαq proteins (33), abolished the stimulatory effect of aldosterone, whereas it did not affect the basal activity (Table 4 and Fig. 3). Inhibition of phospholipase C activity with U-73122 (10 μM) completely impeded the stimulation by aldosterone (Table 4 and Fig. 3B). In contrast, the inactive analog U-73343 (10 μM) was unable to prevent the stimulatory effect of aldosterone (Table 4 and Fig. 3C). Thus, the rapid nongenomic action of aldosterone may require a Gαq protein-coupled membrane receptor and phospholipase C activity.
Table 4.
Summary of Na+-independent pHi alkalinization rates of single intercalated cells in freshly isolated mouse outer medullary collecting ducts in the absence and presence of different inhibitors of intracellular signaling molecules
| Initial pHi | Na+-Independent pHi Recovery, ΔpH/min | No. of Cells, OMCDs, Mice | |
|---|---|---|---|
| Suramin | 7.39 ± 0.02 | 0.029 ± 0.002 | 74 (5/3) |
| Suramin + aldosterone | 7.40 ± 0.02 | 0.030 ± 0.002§ | 51 (4/3) |
| Pertussis toxin | 7.20 ± 0.01 | 0.031 ± 0.001 | 84 (5/3) |
| Pertussis toxin + aldosterone | 7.28 ± 0.01 | 0.084 ± 0.004† | 83 (5/3) |
| GP-2A | 7.13 ± 0.01 | 0.024 ± 0.002 | 68 (4/2) |
| GP-2A + aldosterone | 7.26 ± 0.01 | 0.017 ± 0.001*§ | 92 (5/2) |
| U-73122 | 7.27 ± 0.01 | 0.031 ± 0.002 | 53 (3/3) |
| U-73122 + aldosterone | 7.26 ± 0.01 | 0.026 ± 0.001§ | 116 (8/3) |
| U-73343 + aldosterone | 7.22 ± 0.01 | 0.078 ± 0.004† | 112 (12/8) |
| BAPTA-AM | 7.42 ± 0.02 | 0.036 ± 0.002 | 68 (4/3) |
| BAPTA-AM + aldosterone | 7.35 ± 0.02 | 0.032 ± 0.002§ | 60 (5/4) |
| Chelerythrine | 7.23 ± 0.02 | 0.028 ± 0.001 | 68 (4/3) |
| Chelerythrine + aldosterone | 7.25 ± 0.01 | 0.026 ± 0.002§ | 77 (4/3) |
| DOG | 7.38 ± 0.02 | 0.060 ± 0.003† | 123 (10/5) |
| DOG + PD098059 | 7.42 ± 0.01 | 0.050 ± 0.003†§ | 87 (5/3) |
| DOG + H89 | 7.33 ± 0.03 | 0.069 ± 0.005† | 39 (3/3) |
| PD098059 | 7.23 ± 0.02 | 0.042 ± 0.003 | 86 (7/4) |
| PD098059 + aldosterone | 7.27 ± 0.01 | 0.039 ± 0.003§ | 73 (6/3) |
| H89 | 7.16 ± 0.03 | 0.028 ± 0.003 | 26 (2/2) |
| H89 + aldosterone | 7.26 ± 0.03 | 0.030 ± 0.003§ | 63 (5/3) |
| KT5720 | 7.16 ± 0.09 | 0.031 ± 0.002 | 82 (7/3) |
| KT5720 + aldosterone | 7.22 ± 0.01 | 0.036 ± 0.005§ | 114 (7/4) |
| 8-Br-cAMP | 7.43 ± 0.01 | 0.047 ± 0.002†‡ | 110 (7/3) |
| 8-Br-cAMP + chelerythrine | 7.10 ± 0.08 | 0.024 ± 0.002§ | 92 (7/3) |
Values are means ± SE. Incubation with aldosterone (10 nM) was for 20 min before measurement of alkalinization rates.
P < 0.05 and
P < 0.001, significantly different from control;
P < 0.01 and
P < 0.001, significantly different from aldosterone alone.
Fig. 3.
The stimulatory effect of aldosterone requires Gαq proteins and phospholipase C activity. A: OMCDs were incubated with the inhibitors of small G proteins suramin (200 μM), pertussis toxin (PTX, 200 ng/ml), or GP-2A (20 μM) in the absence and presence of aldosterone (10 nM). Suramin and GP-2A prevented the stimulatory effect of aldosterone, whereas PTX failed to have any effect on the stimulation. B: U-73122 (10 μM), a specific inhibitor of phospholipase C activity, prevented the effect of aldosterone. The values of control and aldosterone-treated OMCDs are shown again for comparison. C: the inactive U-73122 analog U-73343 (10 μM) could not prevent the stimulatory effect of aldosterone. Control and aldosterone values were obtained from a separate set of experiments but yielded qualitatively similar values to the previous experiments listed in Table 2 (n = 5–7 OMCDS from at least three different mice and 50–70 intercalated cells examined per condition). Values are presented as means ± SE. *P < 0.05 and ***P < 0.001 significantly different from control, ###P < 0.001 significantly different from aldosterone alone.
Intracellular calcium, protein kinase C, and ERK1/2 participate in the stimulatory action of aldosterone.
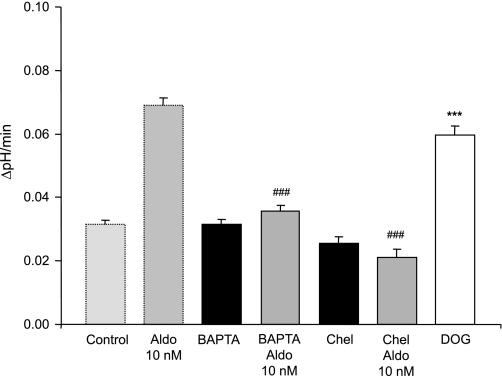
We had previously shown that aldosterone acutely and transiently raised intracellular Ca2+ in mouse OMCD intercalated cells (75). Therefore, we tested whether intracellular Ca2+ is involved in the stimulatory effect of aldosterone. Chelation of intracellular Ca2+ by preincubation of isolated OMCDs with BAPTA-AM abolished the stimulation of H+-ATPase activity without altering the basal activity (Table 4 and Fig. 4).
Fig. 4.
Aldosterone acts via intracellular Ca2+ and protein kinase C. Chelation of intracellular Ca2+ with BAPTA-AM (100 μM) prevented the aldosterone (10 nM) effect. Similarly, inhibition of protein kinase C activity with chelerythrine (Chel, 1 μM) completely blocked the stimulation by aldosterone. On the other hand, stimulation of PKC activity with DOG (1 μM) mimicked the effect of aldosterone. The values of control and aldosterone-treated OMCDs are shown again for comparison. Values are presented as means ± SE. ***P < 0.001 significantly different from control, ###P < 0.001 significantly different from aldosterone alone.
Inhibition of PKC activity with chelerythrine (1 μM) prevented completely the stimulatory effect of aldosterone in mouse OMCDs. On the other hand, incubation with the PKC activator DOG (1 μM) mimicked the effect of aldosterone and increased H+-ATPase activity twofold (Table 4 and Fig. 4).
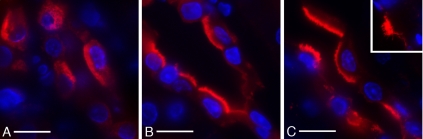
We had previously shown that acute application of aldosterone increased luminal localization of H+-ATPases in vivo (75). Here, collecting duct A-type intercalated cells of rats exhibited a similar response to aldosterone as after exposure to the cell-permeable cAMP analog CPT-cAMP (55). H+-ATPase localization following aldosterone became more polarized, and apical H+-ATPase immunostaining intensity increased markedly in most kidney regions, especially in the outer medulla (Fig. 5, A and C). Infusion of DOG had a similar effect as aldosterone alone (Fig. 5B). As previously reported for cAMP, the brighter apical staining was associated in many cells with the development of extensive microvilli that were easily detectable by conventional wide field fluorescence microscopy (Fig. 5C, see inset). The effect of aldosterone is augmented upon increasing its concentration from 200 nM to 200 μM (Fig. 5, see inset).
Fig. 5.
Aldosterone or activation of protein kinase C enhances luminal H+-ATPase. Immunofluorescence labeling for the A subunit of the V-ATPase (red) in control (A), 20 μM DOG- (B), and 200 nM aldosterone-treated (C) rat OMCD intercalated cells. The nuclei are identified by DAPI staining (blue). While intercalated cells from control animals (A) exhibit extensive cytoplasmic staining and their apical membranes have a smooth appearance, extensively stained apical microvilli are readily detectable in the treated rats, and the cytosolic domain staining is less intense. An increased response is seen in the inset of C, which shows an intercalated cell from a rat treated with a higher concentration of aldosterone (200 μM). Bar = 20 μm.
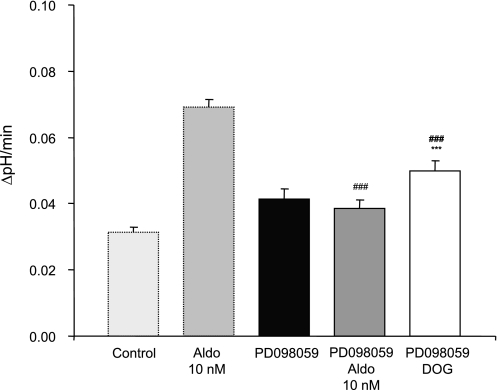
Aldosterone can interact in a nongenomic fashion with the mitogen-activated protein kinase (MAPK) signaling pathway involving ERK1/2 (20, 41, 51, 57). The stimulating effect of aldosterone on H+-ATPase activity in intercalated cells of OMCDs was completely inhibited when the activation of ERK1/2 was prevented using PD098059 (20 μM), a specific inhibitor. Preincubation with PD098059 alone had no effect on H+-ATPase activity (Table 4 and Fig. 6). To investigate the interaction between PKC and ERK1/2, we performed experiments using the PKC activator DOG (1 μM) together with the ERK1/2 inhibitor PD098059 (20 μM). The stimulation of H+-ATPase activity was attenuated (Table 4 and Fig. 6), suggesting that ERK1/2 may act downstream of PKC.
Fig. 6.
The stimulatory effect of aldosterone is mediated via ERK1/2 kinases. OMCDs were incubated with the ERK1/2 inhibitor PD098059 (20 μM) in the absence and presence of aldosterone (10 nM). PD098059 abolished the stimulatory effect of aldosterone. Coincubation of OMCDs with the PKC activator DOG (1 μM) and the ERK1/2 inhibitor PD098059 showed a small stimulation which was less pronounced than with DOG alone (see also Fig. 4). The values of control and aldosterone-treated OMCDs are shown again for comparison. Values are presented as means ± SE. ***P < 0.001 significantly different from control, ###P < 0.001 significantly different from aldosterone alone.
Protein kinase A participates in aldosterone signaling.
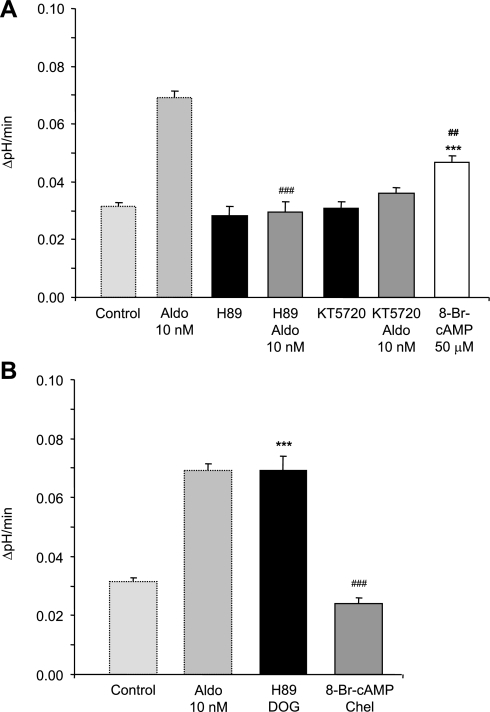
Conflicting reports exist concerning the role of cAMP and protein kinase A (PKA) activity in the rapid effects of aldosterone, suggesting that this pathway may be cell and target specific (12, 28, 38, 62). Moreover, we have previously shown that cAMP stimulates H+-ATPase activity and luminal accumulation in type A intercalated cells (55). Thus, in a last set of experiments, the role of cAMP and PKA in the aldosterone-induced stimulation of H+-ATPase activity in OMCD intercalated cells was examined. Inhibition of PKA activity with H89 (10 μM) or KT5720 (1 μM) completely prevented the aldosterone-induced stimulation of H+-ATPase activity (Fig. 7 and Table 4). In addition, incubation with the cell-permeable cAMP analog 8-bromoadenosine-cAMP (8-Br-cAMP, 50 μM) increased H+-ATPase activity but to a lesser extent than aldosterone or DOG (Table 4 and Fig. 7). Inhibition of PKA with H89 in the presence of the PKC activator DOG (1 μM) did not prevent the robust stimulation of vacuolar H+-ATPase activity that reached a similar level as with DOG alone (Fig. 7B). In contrast, activation of PKA with 8-Br-cAMP in the presence of the PKC inhibitor chelerythrine (1 μM) did not stimulate H+-ATPase activity. These results suggest that the cAMP/PKA-dependent pathway is involved in the aldosterone action and may act upstream of PKC.
Fig. 7.
Aldosterone signaling involves the cAMP-protein kinase A pathway. A: inhibition of protein kinase A activity with H89 (10 μM) or KT5720 (1 μM) completely prevented the effect of aldosterone whereas H89 or KT5720 alone had no effect on the pHi recovery rates. Incubation of OMCDs with the PKA activator 8-Bromoadenosine-cAMP (8-Br-cAMP; 50 μM and 300 μM) stimulated pHi recovery but to a lesser extent than aldosterone. B: coincubation of OMCDs with the PKC activator DOG (1 μM) and the PKA inhibitor H89 (10 μM) resulted in a robust stimulation similar to aldosterone alone demonstrating that PKC activation alone is sufficient for stimulation and that PKA does not act downstream of PKC. Activation of PKA with 8-Br-cAMP (300 μM) in the presence of the PKC inhibitor chelerythrine (1 μM) failed to stimulate H+-ATPase activity. The values of control and aldosterone treated OMCDs are shown again for comparison. Values are presented as means ± SE. ***P < 0.001 significantly different from control, ##P < 0.01 and ###P < 0.001 significantly different from aldosterone alone.
DISCUSSION
Nongenomic effects of several steroid hormones including vitamin D3, sex hormones, glucocorticoids, and aldosterone have been identified in various tissues and cells (for review, see refs. 25–26, 48–50, 59, 74). Aldosterone is mainly involved in maintaining extracellular Na+ and K+ homeostasis by regulating intestinal and renal Na+ absorption and K+ excretion (68). Moreover, in the kidney, aldosterone also regulates acid secretion by acting on proximal tubular Na+/H+ exchange and most importantly by stimulating H+-secretion along the collecting duct. Several studies have documented the chronic effects of aldosterone and aldosterone deficiency in rat and rabbit animal models as well as in cell culture and freshly isolated turtle bladder on H+-ATPase activity, the AE1 Cl−/HCO3− exchanger, and the ability of the collecting duct to excrete protons (1, 13–14, 16, 19, 30, 36, 39, 42, 52, 61, 65). Some of these effects are likely mediated by changes in transcription and translation of proteins involved in acid-base transport or acting on these transporters (36).
The biological actions of these steroid hormones are mediated not only by changes in transcription but also by much faster nongenomic mechanisms. Distinct rapid effects of aldosterone on acid-base transport in several nephron segments and kidney-derived cell lines have been described. H+-ATPase activity is stimulated by aldosterone in freshly isolated rat late proximal tubule and mouse OMCD intercalated cells (44, 75), and Na+/H+ exchanger activity increased in the rat late proximal tubule (45), MDCK, and M1 cell lines (20, 22, 51). Similarly, aldosterone stimulates Na+/H+ exchange in freshly isolated colonic crypts (76). In contrast, in the thick ascending limb, aldosterone acutely inhibits bicarbonate absorption (23).
The intracellular signaling cascades mediating these nongenomic effects of aldosterone have been partly investigated and appear to differ between different cell types. However, most work has been done in cell culture models such as M1, MDCK, or vascular smooth muscle cells but much less information is available from freshly isolated cells and tissues. This may be important because it is well documented that cell lines harbor or loose characteristics not found or present in the native tissues (77). Thus, we investigated the signaling cascade mediating the stimulatory effect of aldosterone on H+-ATPase activity in freshly isolated mouse OMCD intercalated cells. We demonstrate that aldosterone stimulates within minutes H+-ATPase activity in mouse and human outer medullary collecting duct cells. Moreover, we show that in rat outer medullary collecting duct, aldosterone induces a rapid translocation of H+-ATPase to the luminal pole in vivo. These data suggest that the effect is not species specific and may also be relevant in humans.
We had recently shown that the stimulatory effect of aldosterone persists in the presence of the partial mineralocorticoid receptor antagonist spironolactone and does not require the mineralocorticoid receptor, or transcription and translation (75). The stimulation was observed after incubation with 10 nM aldosterone for 20 min. Here we showed that stimulation could be observed even after immediate application and at a concentration as low as 1 nM. The effect of aldosterone was specific and not observed with the corticosteroid hydrocortisone.
It has been postulated that specific receptors for steroid hormones may exist that initiate their nongenomic effects (6, 46). Some of the nongenomic effects of aldosterone and corticosterone involve membrane-bound mineralocorticoid receptors (25–26, 35). Moreover, GPR30, an orphan G protein-coupled receptor, has been recently identified as a receptor for estrogens and shown to mediate some of its nongenomic effects (31, 56, 67). GPR30 may also mediate the nongenomic effects of aldosterone in some tissues (24). Consistently, we found that the rapid stimulatory effect of aldosterone was inhibited by suramin and GP-2A, inhibitors of small G proteins. Suramin is an unspecific inhibitor of G proteins, whereas GP-2A specifically blocks Gαq proteins (33). Pertussis toxin was without effect, suggesting that Go and Gi proteins are not involved and that the aldosterone effect involves Gαq proteins. In addition to G protein inhibition, blockade of phospholipase C activity by U-73122 also prevented the stimulatory effect of aldosterone. This effect of U-73122 was not achieved with the inactive analog U-73343. Recently, a direct stimulatory effect of U-73122 on human PLC has been described (37); however, it is unlikely that this occurred in our experiments since U-73122 had no effect on untreated OMCDs. Similarly, other groups have described evidence for a nongenomic receptor-mediated activation of intracellular signaling cascades by aldosterone that were abolished by inhibitors of G proteins and PLC (11, 22, 43, 76).
The effect of aldosterone on H+-ATPase activity was further mediated by Ca2+ and protein kinase C stimulation as evident from 1) the lack of stimulation after chelation of intracellular Ca2+ with BAPTA, 2) the previous observation that aldosterone induces an acute and transient rise in intracellular Ca2+ (75), 3) the inhibition of stimulation with the PKC inhibitor chelerythrine, and 4) the fact that the diacylglycerol analog DOG mimicked the stimulatory effect of aldosterone. Direct interactions of aldosterone with protein kinase C have been shown (3) but are unlikely to mediate the effect in OMCD intercalated cells since upstream inhibition of PLC completely abolishes the stimulatory effect. In some tissues and cells, the nongenomic effects of aldosterone involve activation of ERK1/2 MAPK kinases (20, 41, 57). In agreement with these observations, blockade of ERK1/2 phosphorylation by PD098059 strongly reduced the stimulation of H+-ATPase activity. Furthermore, it appears that ERK1/2 acts downstream of PKC, because the concomitant incubation of OMCDs with DOG and PD098059 failed to stimulate H+-ATPases. This blockade, however, was not complete, which may indicate either that ERK was not fully inhibited by PD098059 or that an alternative ERK-independent pathway may exist. Thus, H+-ATPase activity in OMCD acid-secretory type A intercalated cells is stimulated by a PLC/Ca2+/PKC-dependent pathway.
We recently described a very similar stimulatory pathway for angiotensin II in mouse OMCD cells (58), suggesting that it may represent a common pathway of H+-ATPase stimulation in these cells. Stimulation of H+-ATPase activity has also been demonstrated in a cell line derived from the rat inner medullary collecting duct requiring intracellular Ca2+, PLC, and PKC activity (53, 60, 63). Similar observations have been made in the epididymis (5). The activation of protein kinase C is sufficient to induce the rapid translocation of H+-ATPases to the luminal side of type A intercalated cells as demonstrated by the DOG in vivo experiments. Consistently, we have previously shown that disruption of the microtubular network with colchicine abolishes the stimulatory effect of aldosterone (75) and colchicine administration in vivo disrupts H+-ATPase trafficking (10). Whether calcium is also directly required for the trafficking and insertion of H+-ATPases into the membrane remains to be further examined.
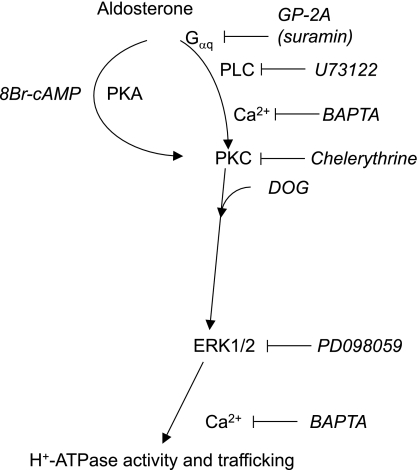
We and others have recently shown that the cAMP-dependent pathway stimulates H+-ATPase activity in renal intercalated cells and induces its translocation into the apical membrane (4, 55). Similarly, blockade with two different inhibitors of a protein kinase A-dependent pathway reduced the stimulatory effect of aldosterone. Incubation of OMCDs with the cAMP analog 8-Br-cAMP stimulated H+-ATPase activity but to a lesser extent than the PKC-dependent pathway. The stimulation produced by 50 μM 8-Br-cAMP could not be increased with higher concentrations, indicating that maximal stimulation had been reached. However, pharmacologic stimulation of the PKC-dependent cascade appears to be sufficient for the increase in H+-ATPase activity because DOG in the presence of H89 enhanced vacuolar H+-ATPase activity. In contrast, activation of PKA with 8-Br-cAMP and concomitant inhibition of PKC with chelerythrine prevented the stimulation of H+-ATPase activity, suggesting that PKA requires intact PKC to be effective and that PKA may act upstream of PKC (see hypothetical model Fig. 8). Taken together, it appears that PKA may rather have a modulatory effect and that PKC may transmit the main signal. A mild stimulatory effect of a PKA-dependent pathway on bicarbonate absorption has been described previously in perfused rabbit outer medullary collecting duct and may thus reflect a physiologically relevant route of regulation of vacuolar H+-ATPase activity (29).
Fig. 8.
Model of nongenomic signaling of aldosterone in mouse OMCD intercalated cells to stimulate H+-ATPase activity. Aldosterone stimulates H+-ATPase activity through a major pathway requiring Gαq proteins, phospholipase C, intracellular Ca2+, PKC, and ERK1/2. A second interlinked pathway involves cAMP and PKA. The PKA-dependent pathway is likely upstream of PKC and ERK1/2 because inhibition of this pathway blocks the cAMP/PKA-dependent stimulation of H+-ATPase activity. Chelation of Ca2+ may interfere at several stages because Ca2+ may be involved in stimulation of PKC isoforms as well as in the exocytic insertion of H+-ATPases into the membrane, which has been shown earlier to occur during stimulation of H+-ATPase activity (58, 75). Inhibitors and activators of signaling molecules used in this study are shown in italics.
Besides its well-documented effects on renal Na+ and K+ transport, aldosterone is an important regulator of renal acid secretion acting mainly on H+-ATPases along the collecting duct. The significance of the stimulatory effect of aldosterone is highlighted by the fact that various inborn or acquired syndromes associated either with aldosterone deficiency or with insufficient aldosterone signaling in the kidney are invariably associated with distal renal tubular acidosis and hyperkalemia (15). It is possible that the rapid nongenomic and slower genomic stimulatory effects of aldosterone on H+-ATPase activity may interact in several ways as is becoming increasingly clear for the effects of aldosterone on the major Na+-transporting mechanisms in the collecting duct (43, 64). Thus, mechanisms identified here may not only lead to the rapid stimulation of H+-ATPase activity but may also contribute subsequently to the activation of genomic events.
In summary, aldosterone rapidly stimulates H+-ATPase activity in acid-secretory OMCD intercalated cells from mouse and human kidney and induces the net translocation of pumps to the luminal membrane. This nongenomic effect is mediated via a PLC/Ca2+/PKC-dependent pathway but is interconnected with the cAMP-PKA cascade. ERK1/2 kinases are essential for stimulation. A membrane Gαq protein-coupled receptor may be involved in initiating these cascades. The rapid nongenomic stimulation of H+-ATPases by aldosterone may be part of the biological action of aldosterone in the kidney and contribute to the increase in acid secretion and control of systemic acid-base homeostasis.
GRANTS
This study was supported by Swiss National Research Foundation Grants 31-068318 and 31-109677/1 (to C. A. Wagner). This work was also supported by National Institutes of Health (NIH) Grants DK-73266 (to T. G. Păunescu) and DK-42956 (to D. Brown). The Microscopy Core Facility of the Program in Membrane Biology received additional support from the Boston Area Diabetes and Endocrinology Research Center (NIH DK-57521) and from the Center for the Study of Inflammatory Bowel Disease (NIH DK-43351).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Al-Awqati Q, Norby LH, Mueller A, Steinmetz PR. Characteristics of stimulation of H+ transport by aldosterone in turtle urinary bladder. J Clin Invest 58: 351–358, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci USA 86: 5429–5433, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alzamora R, Harvey BJ. Direct binding and activation of protein kinase C isoforms by steroid hormones. Steroids 73: 885–888, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Alzamora R, Thali RF, Gong F, Smolak C, Li H, Baty CJ, Bertrand CA, Auchli Y, Brunisholz RA, Neumann D, Hallows KR, Pastor-Soler NM. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the A subunit in kidney cells. J Biol Chem 285: 24676–24685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+-ATPase recycling. J Biol Chem 280: 8452–8463, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Boldyreff B, Wehling M. Rapid aldosterone actions: from the membrane to signaling cascades to gene transcription and physiological effects. J Steroid Biochem Mol Biol 85: 375–381, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Bourgeois S, Meer LV, Wootla B, Bloch-Faure M, Chambrey R, Shull GE, Gawenis LR, Houillier P. NHE4 is critical for the renal handling of ammonia in rodents. J Clin Invest 120: 1895–1904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown D, Hirsch S, Gluck S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature 331: 622–624, 1988 [DOI] [PubMed] [Google Scholar]
- 9. Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS). Histochem Cell Biol 105: 261–267, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Brown D, Sabolic I, Gluck S. Colchicine-induced redistribution of proton pumps in kidney epithelial cells. Kidney Int Suppl 33: S79–S83, 1991 [PubMed] [Google Scholar]
- 11. Christ M, Douwes K, Eisen C, Bechtner G, Theisen K, Wehling M. Rapid effects of aldosterone on sodium transport in vascular smooth muscle cells. Hypertension 25: 117–123, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Christ M, Gunther A, Heck M, Schmidt BM, Falkenstein E, Wehling M. Aldosterone, not estradiol, is the physiological agonist for rapid increases in cAMP in vascular smooth muscle cells. Circulation 99: 1485–1491, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Damasco MC, Ansaldo M, Malnic G. Effects of adrenalectomy and acute replacement by corticosteroids on distal acidification. Can J Physiol Pharmacol 67: 607–614, 1989 [DOI] [PubMed] [Google Scholar]
- 14. DuBose TD, Jr, Caflisch CR. Effect of selective aldosterone deficiency on acidification in nephron segments of the rat inner medulla. J Clin Invest 82: 1624–1632, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DuBose TD, Jr, Alpern RJ. Renal tubular acidosis. In: The Metabolic and Molecular Bases of Inherited Disease ( 8th ed.), edited by Scriver CR, Beaudet AL, Sly WS, Valle D. New York: McGraw-Hill, 2001, p. 4983–5021 [Google Scholar]
- 16. Eiam-Ong S, Kurtzman NA, Sabatini S. Regulation of collecting tubule adenosine triphosphatases by aldosterone and potassium. J Clin Invest 91: 2385–2392, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falkenstein E, Christ M, Feuring M, Wehling M. Specific nongenomic actions of aldosterone. Kidney Int 57: 1390–1394, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones–a focus on rapid, nongenomic effects. Pharmacol Rev 52: 513–556, 2000 [PubMed] [Google Scholar]
- 19. Garg LC, Narang N. Effects of aldosterone on NEM-sensitive ATPase in rabbit nephron segments. Kidney Int 34: 13–17, 1988 [DOI] [PubMed] [Google Scholar]
- 20. Gekle M, Freudinger R, Mildenberger S, Schenk K, Marschitz I, Schramek H. Rapid activation of Na+/H+-exchange in MDCK cells by aldosterone involves MAP-kinase ERK1/2. Pflügers Arch 441: 781–786, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Gekle M, Golenhofen N, Oberleithner H, Silbernagl S. Rapid activation of Na+/H+ exchange by aldosterone in renal epithelial cells requires Ca2+ and stimulation of a plasma membrane proton conductance. Proc Natl Acad Sci USA 93: 10500–10504, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gekle M, Silbernagl S, Oberleithner H. The mineralocorticoid aldosterone activates a proton conductance in cultured kidney cells. Am J Physiol Cell Physiol 273: C1673–C1678, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Good DW, George T, Watts BA., III Aldosterone inhibits HCO3− absorption via a nongenomic pathway in medullary thick ascending limb. Am J Physiol Renal Physiol 283: F699–F706, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Gros R, Ding Q, Sklar LA, Prossnitz EE, Arterburn JB, Chorazyczewski J, Feldman RD. GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension 57: 442–451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grossmann C, Gekle M. New aspects of rapid aldosterone signaling. Mol Cell Endocrinol 308: 53–62, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Grossmann C, Ruhs S, Seiferth A, Gekle M. Interaction between mineralocorticoid receptor and cAMP/CREB signaling. Steroids 75: 539–543, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Hamm LL, Alpern RJ. Cellular mechanisms of renal tubular acidification. In: The Kidney: Physiology and Pathophysiology ( 3rd ed.), edited by Seldin DW, Giebisch G. Philadelphia, PA: Lippincott Williams & Wilkins, 2000, p. 1935–1979 [Google Scholar]
- 28. Haseroth K, Gerdes D, Berger S, Feuring M, Gunther A, Herbst C, Christ M, Wehling M. Rapid nongenomic effects of aldosterone in mineralocorticoid-receptor-knockout mice. Biochem Biophys Res Commun 266: 257–261, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Hays S, Kokko JP, Jacobson HR. Hormonal regulation of proton secretion in rabbit medullary collecting duct. J Clin Invest 78: 1279–1286, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hays SR. Mineralocorticoid modulation of apical and basolateral membrane H+/OH−/HCO3− transport processes in the rabbit inner stripe of outer medullary collecting duct. J Clin Invest 90: 180–187, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hewitt SC, Deroo BJ, Korach KS. Signal transduction. A new mediator for an old hormone? Science 307: 1572–1573, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Hohenegger M, Waldhoer M, Beindl W, Boing B, Kreimeyer A, Nickel P, Nanoff C, Freissmuth M. Gsalpha-selective G protein antagonists. Proc Natl Acad Sci USA 95: 346–351, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hunt RA, Bhat GJ, Baker KM. Angiotensin II-stimulated induction of sis-inducing factor is mediated by pertussis toxin-insensitive G(q) proteins in cardiac myocytes. Hypertension 34: 603–608, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8: 124–136, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102: 19204–19207, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khadouri C, Marsy S, Barlet-Bas C, Cheval L, Doucet A. Short-term effect of aldosterone on NEM-sensitive ATPase in rat collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 257: F177–F181, 1989 [DOI] [PubMed] [Google Scholar]
- 37. Klein RR, Bourdon DM, Costales CL, Wagner CD, White WL, Williams JD, Hicks SN, Sondek J, Thakker DR. Direct activation of human phospholipase C by its well known inhibitor u73122. J Biol Chem 286: 12407–12416, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koppel H, Christ M, Yard BA, Bar PC, van der Woude FJ, Wehling M. Nongenomic effects of aldosterone on human renal cells. J Clin Endocrinol Metab 88: 1297–1302, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Kornandakieti C, Tannen RL. H+ transport by the aldosterone-deficient rat distal nephron. Kidney Int 25: 629–635, 1984 [DOI] [PubMed] [Google Scholar]
- 40. Kovacikova J, Winter C, Loffing-Cueni D, Loffing J, Finberg KE, Lifton RP, Hummler E, Rossier B, Wagner CA. The connecting tubule is the main site of the furosemide-induced urinary acidification by the vacuolar H+-ATPase. Kidney Int 70: 1706–1716, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Krug AW, Grossmann C, Schuster C, Freudinger R, Mildenberger S, Govindan MV, Gekle M. Aldosterone stimulates epidermal growth factor receptor expression. J Biol Chem 278: 43060–43066, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Krug AW, Papavassiliou F, Hopfer U, Ullrich KJ, Gekle M. Aldosterone stimulates surface expression of NHE3 in renal proximal brush borders. Pflügers Arch 446: 492–496, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Le Moellic C, Ouvrard-Pascaud A, Capurro C, Cluzeaud F, Fay M, Jaisser F, Farman N, Blot-Chabaud M. Early nongenomic events in aldosterone action in renal collecting duct cells: PKCalpha activation, mineralocorticoid receptor phosphorylation, and cross-talk with the genomic response. J Am Soc Nephrol 15: 1145–1160, 2004 [PubMed] [Google Scholar]
- 44. Leite-Dellova DC, Malnic G, Mello-Aires M. Genomic and nongenomic stimulatory effect of aldosterone on H+-ATPase in proximal S3 segments. Am J Physiol Renal Physiol 300: F682–F691, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Leite-Dellova DC, Oliveira-Souza M, Malnic G, Mello-Aires M. Genomic and nongenomic dose-dependent biphasic effect of aldosterone on Na+/H+ exchanger in proximal S3 segment: role of cytosolic calcium. Am J Physiol Renal Physiol 295: F1342–F1352, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Losel R, Feuring M, Wehling M. Non-genomic aldosterone action: from the cell membrane to human physiology. J Steroid Biochem Mol Biol 83: 167–171, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Losel R, Schultz A, Wehling M. A quick glance at rapid aldosterone action. Mol Cell Endocrinol 217: 137–141, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol 4: 46–55, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev 83: 965–1016, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Losel RM, Feuring M, Falkenstein E, Wehling M. Nongenomic effects of aldosterone: cellular aspects and clinical implications. Steroids 67: 493–498, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Markos F, Healy V, Harvey BJ. Aldosterone rapidly activates Na+/H+ exchange in M-1 cortical collecting duct cells via a PKC-MAPK pathway. Nephron Physiol 99: p1–p9, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Mujais SK. Effects of aldosterone on rat collecting tubule N-ethylmaleimide-sensitive adenosine triphosphatase. J Lab Clin Med 109: 34–39, 1987 [PubMed] [Google Scholar]
- 53. Nicoletta JA, Ross JJ, Li G, Cheng Q, Schwartz J, Alexander EA, Schwartz JH. Munc-18–2 regulates exocytosis of H+-ATPase in rat inner medullary collecting duct cells. Am J Physiol Cell Physiol 287: C1366–C1374, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Paunescu TG, Da Silva N, Marshansky V, McKee M, Breton S, Brown D. Expression of the 56-kDa B2 subunit isoform of the vacuolar H+-ATPase in proton-secreting cells of the kidney and epididymis. Am J Physiol Cell Physiol 287: C149–C162, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298: F643–F654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Rossol-Haseroth K, Zhou Q, Braun S, Boldyreff B, Falkenstein E, Wehling M, Losel RM. Mineralocorticoid receptor antagonists do not block rapid ERK activation by aldosterone. Biochem Biophys Res Commun 318: 281–288, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Rothenberger F, Velic A, Stehberger PA, Kovacikova J, Wagner CA. Angiotensin II stimulates vacuolar H+-ATPase activity in renal acid-secretory intercalated cells from the outer medullary collecting duct. J Am Soc Nephrol 18: 2085–2093, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Schmidt BM. Rapid non-genomic effects of aldosterone on the renal vasculature. Steroids 73: 961–965, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Schwartz JH, Masino SA, Nichols RD, Alexander EA. Intracellular modulation of acid secretion in rat inner medullary collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 266: F94–F101, 1994 [DOI] [PubMed] [Google Scholar]
- 61. Sebastian A, Sutton JM, Hulter HN, Schambelan M, Poler SM. Effect of mineralocorticoid replacement therapy on renal acid-base homeostasis in adrenalectomized patients. Kidney Int 18: 762–773, 1980 [DOI] [PubMed] [Google Scholar]
- 62. Sheader EA, Wargent ET, Ashton N, Balment RJ. Rapid stimulation of cyclic AMP production by aldosterone in rat inner medullary collecting ducts. J Endocrinol 175: 343–347, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Slotki I, Schwartz JH, Alexander EA. Interrelationship between cell pH and cell calcium in rat inner medullary collecting duct cells. Am J Physiol Cell Physiol 265: C432–C438, 1993 [DOI] [PubMed] [Google Scholar]
- 64. Stockand JD. New ideas about aldosterone signaling in epithelia. Am J Physiol Renal Physiol 282: F559–F576, 2002 [DOI] [PubMed] [Google Scholar]
- 65. Stone DK, Seldin DW, Kokko JP, Jacobson HR. Mineralocorticoid modulation of rabbit medullary collecting duct acidification. A sodium-independent effect. J Clin Invest 72: 77–83, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18: 2210–2218, 1979 [DOI] [PubMed] [Google Scholar]
- 67. Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146: 624–632, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Verrey F. Early aldosterone action: toward filling the gap between transcription and transport. Am J Physiol Renal Physiol 277: F319–F327, 1999 [DOI] [PubMed] [Google Scholar]
- 69. Wagner CA, Devuyst O, Bourgeois S, Mohebbi N. Regulated acid-base transport in the collecting duct. Pflügers Arch 458: 137–156, 2009 [DOI] [PubMed] [Google Scholar]
- 70. Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar H+-ATPase. Physiol Rev 84: 1263–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Wagner CA, Geibel JP. Acid-base transport in the collecting duct. J Nephrol Suppl 5: S112–S127, 2002 [PubMed] [Google Scholar]
- 72. Wagner CA, Lukewille U, Valles P, Breton S, Brown D, Giebisch GH, Geibel JP. A rapid enzymatic method for the isolation of defined kidney tubule fragments from mouse. Pflügers Arch 446: 623–632, 2003 [DOI] [PubMed] [Google Scholar]
- 73. Watts BA, 3rd, Good DW. Apical membrane Na+/H+ exchange in rat medullary thick ascending limb. pH-dependence and inhibition by hyperosmolality. J Biol Chem 269: 20250–20255, 1994 [PubMed] [Google Scholar]
- 74. Wehling M. Specific, nongenomic actions of steroid hormones. Annu Rev Physiol 59: 365–393, 1997 [DOI] [PubMed] [Google Scholar]
- 75. Winter C, Schulz N, Giebisch G, Geibel JP, Wagner CA. Nongenomic stimulation of vacuolar H+-ATPases in intercalated renal tubule cells by aldosterone. Proc Natl Acad Sci USA 101: 2636–2641, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Winter DC, Schneider MF, O'Sullivan GC, Harvey BJ, Geibel JP. Rapid effects of aldosterone on sodium-hydrogen exchange in isolated colonic crypts. J Membr Biol 170: 17–26, 1999 [DOI] [PubMed] [Google Scholar]
- 77. Zhuang Z, Marshansky V, Breton S, Brown D. Is caveolin involved in normal proximal tubule function? Presence in model PT systems but absence in situ. Am J Physiol Renal Physiol 300: F199–F206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]