Abstract
Background
In 2007, the Intergovernmental Panel on Climate Change (IPCC) presented a report on global warming and the impact of human activities on global warming. Later the Lancet commission identified six ways human health could be affected. Among these were not environmental factors which are also believed to be important for human health. In this paper we therefore focus on environmental factors, climate change and the predicted effects on maternal and newborn health. Arctic issues are discussed specifically considering their exposure and sensitivity to long range transported contaminants.
Methods
Considering that the different parts of pregnancy are particularly sensitive time periods for the effects of environmental exposure, this review focuses on the impacts on maternal and newborn health. Environmental stressors known to affects human health and how these will change with the predicted climate change are addressed. Air pollution and food security are crucial issues for the pregnant population in a changing climate, especially indoor climate and food security in Arctic areas.
Results
The total number of environmental factors is today responsible for a large number of the global deaths, especially in young children. Climate change will most likely lead to an increase in this number. Exposure to the different environmental stressors especially air pollution will in most parts of the world increase with climate change, even though some areas might face lower exposure. Populations at risk today are believed to be most heavily affected. As for the persistent organic pollutants a warming climate leads to a remobilisation and a possible increase in food chain exposure in the Arctic and thus increased risk for Arctic populations. This is especially the case for mercury. The perspective for the next generations will be closely connected to the expected temperature changes; changes in housing conditions; changes in exposure patterns; predicted increased exposure to Mercury because of increased emissions and increased biological availability.
Conclusions
A number of environmental stressors are predicted to increase with climate change and increasingly affecting human health. Efforts should be put on reducing risk for the next generation, thus global politics and research effort should focus on maternal and newborn health.
Keywords: climate change, environment, maternal and child health, Arctic
In 2007, the Intergovernmental Panel on Climate Change (IPCC) presented a report on global warming and the impact of human activities on the global climate (1). The global mean temperatures and ocean temperatures have increased over the last century, especially after the industrial revolution, and the global stores of ice are decreasing (Fig. 1). During the twentieth century, global sea level increased and the global ice core has diminished (Figs. 2, 3, and 4). The frequency and intensity of extreme weather events, such as draughts and heavy precipitation, have also increased over the last 30 years. At the same time, the concentrations of atmospheric carbon dioxide (CO2) and methane (CH4) are at the highest during the last 650, 000 years. The use of fossil fuels, land use and agricultural activities are responsible for increased concentrations of greenhouse gases over the last 250 years. IPCC also stated that it is extremely unlikely that the changes in global climate could have occurred without greenhouse gases from human activities. Although there are still many uncertainties about climate change, its extent and the effects of global warming, there is currently a scientific consensus that climate change is occurring (1).
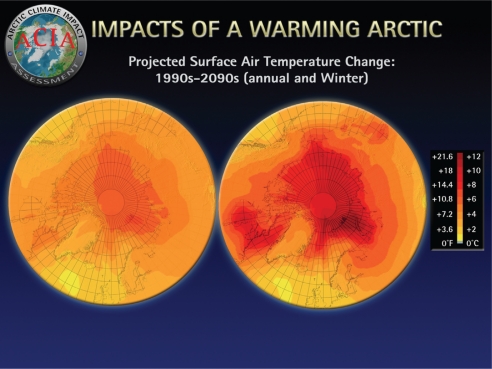
Fig. 1.
The Arctic is warming quickly (1, 35).
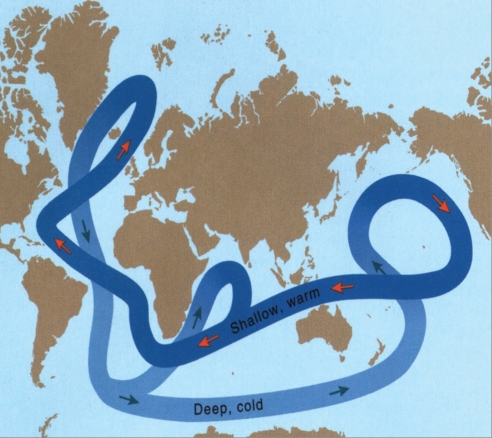
Fig. 2.
The ocean streams are important and vulnerable (11, 35).
Fig. 3.
The Greenland ice sheet dominates land ice in the Arctic. Over the past two decades, the melt area on the Greenland ice sheet has increased on average by about 0.7%/ year (or about 16% from 1979 to 2002) (35).
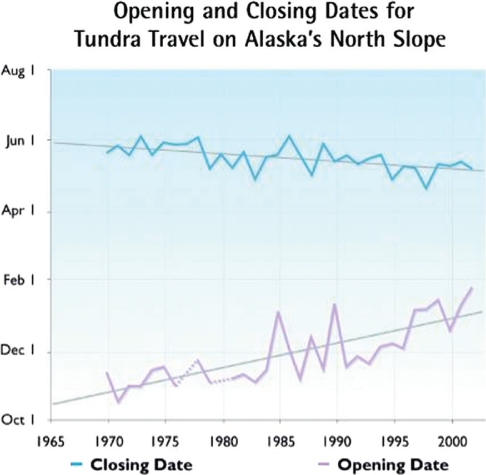
Fig. 4.
The opening and closing dates for tundra travel on Alaska's north slope are rapidly changing (11, 35).
As we are struggling to understand the occurring climate changes and predict future scenarios, there is considerable less knowledge about the impacts climate change will have on human health. Costello et al. asked the following question in 2009: ‘Have we yet understood the profound significance of the connection between climate change and human health?’ (2). Since then, there has been a considerable increase in the number of publications on related topics. The Lancet commission has further identified the following six ways in which climate change can affect human health: Changing patterns of disease and morbidity, food, water and sanitation, shelter and human settlements, extreme events and migration (2). The impacts of pollution and contaminants, and climate-induced changes in exposure and the effects on human health were not considered specifically. This deserves attention, considering that WHO estimates that approximately 25% of deaths in children under the age of 5 are caused by the total number of environmental factors (3). The effects of climate change and environmental pollution on the next generation might become profound, with the most sensitive members of the population being the foetus, newborns, children and women of reproductive age (2).
Climate change is particularly inter-linked with air pollution as it is both a cause (greenhouse gases) and an effect of, because increased temperatures in urban areas leads to increased concentration of air pollutants. Many strategies to reduce greenhouse gas emissions also decrease emissions of health-damaging air pollutants and precursor species. As a result of increased industrial growth, the concentrations of air pollutants are already increasing, particularly in urban areas of developing countries, and the big cities of the north (1). Big areas of the developing world, as well as the Arctic areas, are likely to experience the heaviest impact of climate change, with women bearing the greatest toll (2).
Considering that a number of studies have identified the pregnancy as a particular sensitive time period for the effects of environmental exposure, this literature review focuses on the impacts of environmental factors on the maternal and newborn health in a changing climate.
Climate change influences pollution
There seems to be growing evidence that climate change will have negative impacts on the distribution and toxicity of environmental contaminants (4–10). However, there are only a limited number of studies focused on contaminant interactions in northern areas, where there is an urgent need for more knowledge on how climate change affects chemical distribution and toxicity (11). This is especially important because the vulnerable populations are more sensitive due to other stressors such as malnourishment and disease. Temperature and precipitation, as altered by climate change, are expected to have the largest influence on the partitioning of chemical toxicants.
Anthropogenic emissions are a leading cause of climate change, and climate change is also impacting the concentration and distribution of pollutants in the atmosphere. Ozone (O3) and particulate matter (PM) are among these chemical species that cause concern (12–14).
Particles and ozone
Air quality is expected to degrade as a result of climate change, and the severity and frequency of ozone episodes are expected to increase, even though there is considerable uncertainty associated with the direction and magnitude of change for tropospheric ozone (15–18). An increased burden of disease linked to climate change is expected especially for allergy and asthma due to prolongation of the pollen seasons. An interaction between air pollution and pollen loads is expected, e.g. thunderstorms, extreme precipitation events, increased ground level ozone concentrations, increased ambient air pollution from natural and anthropogenic sources and probably an increasing number of fires (19).
Persistent toxic substances
As climate change alters primary and secondary releases of persistent organic pollutants (POPs), levels and patterns of exposure to wildlife and humans will change. Climate change is already altering food web structures in some areas, including influence on the exposure of wildlife and humans to POPs (11). The toxicity and toxicokinetics of POPs in the nature might also be altered as a direct result of changes in temperature (20).
The interactions between climate change and contaminants are complex. Increased temperature could result in increased toxicity of many persistent toxic substances (PTS) and increased mobility of chemicals in the environment. At the same time, increasing temperature could also enhance the degradation rate of pollutants in nature, leading to decreasing concentrations. Climate change is associated with changes in the regional precipitation pattern. The United Nations Environment Program (UNEP) review on organic contaminants, air pollution and climate change (19), and the overview by Noyes et al. (20) indicates that in temperate regions, reduced precipitation will increase the volatilisation of organic pollutants and also result in increased air pollution in urban areas. Climate change could also affect food safety as increasing temperatures may result in alterations in food web structures, lipid profiles and carbon flux that in turn will affect the concentrations of organic pollutants in water, soil and biota (20). Warmer climate may lead to increased use of pesticides as the number of infection- based diseases are expected to increase (21).
Within the Arctic, changes in food web structures and bioaccumulation properties of PTS are also expected as results of temperature changes (22). Recent simulation studies indicate that the bioaccumulation of some POPs will decrease in the marine environment with increasing temperature, assuming no change in food web structure and constant contaminant exposure (23). Other studies from the Arctic indicate slightly increased concentrations of PTS in the marine environment with increasing temperatures (24). It is not yet fully understood how bioaccumulation in the environment will be affected by climatic change. What we do know is that climate change will cause changes that will ultimately affect people living in the Arctic and feeding from the Arctic environment.
Indoor air pollution
More than half of the global population still cooks with wood, coal or agricultural residues on simple stoves or open fires (25). This results in exposure to a number of pollutants associated with smoke. Largely women and young children are exposed to indoor air pollution in developing as well as Arctic countries as they tend to spend more time doing indoor activities (26). People are exposed to polycyclic aromatic hydrocarbons (PAHs) when indoor, even when residing in proper houses, clearly implying that with poorer housing conditions, the indoor exposure to PAHs or PM will increase (27). In the Arctic, increasing temperatures are believed to increase the exposure to radon from the ground as the permafrost is retreating (19).
Air pollution and effects on maternal and newborn health
Evidence that poor air quality can adversely affect birth outcomes is increasing. A small number of review articles have summarised existing studies and concluded that there is likely an adverse effect of air pollution on pregnancy outcome (28). The burden of evidence seems strongest for PM of the atmospheric chemical species.
Exposure to ambient PM has been associated with adverse health effects, but the exact constituents of PM that cause disease and the mechanisms involved are unknown. Several reviews conclude that exposure to PM is strongly and consistently associated with post-neonatal respiratory mortality and less consistent with sudden infant death syndrome (28, 29). Gliniana et al. have also concluded that the available evidence is compatible with either a small adverse effect of particulate air pollution on foetal growth and duration of pregnancy or with no effect (28).
Intra-uterine growth retardation and pre-term birth are associated with PM (30, 31). There is only one available study that describes an association between PM exposure during pregnancy and birth defects, reporting a possible increased risk of isolated atrial septal defects (32). There is too limited evidence to conclude on a possible effect of PM on stillbirth risk (28). Whether the reported association between PM and intra-uterine growth retardation really results from an effect of PM or from air pollutants trapped in the PM or correlated with PM values such as PAHs remains to be elucidated (28).
Heinrich et al. (33) concluded that although the mechanisms of air pollution effects are not completely understood, children, infants and pregnant women need specific protection against exposure to fine particles. Especially, the first months after birth might be of particular sensitivity due to immature and developing organs.
Despite the growing knowledge on the effects of maternal exposure to PM and the effects on the birth outcome, there are still considerable knowledge gaps regarding more sensitive time periods during pregnancy, making preventive actions harder to accomplish (28).
Ozone is described as an extremely reactive chemical that has been shown to have harmful effects on human health, crop production and natural areas (8). The negative health effects are somewhat similar to the effects of PM, or at least they are to a large extent associated.
Nitrogen dioxide (NO2) is the air pollutant most frequently used as a surrogate for traffic-related pollution in prospective studies, both in adults and in children (34). This is due to the fact that outdoor NO2 levels correlate well with pollutants generated by traffic, they can be easily measured using passive samplers and they are routinely measured by air quality networks that allow for correction for seasonality. A recent study suggests that pre-natal exposure to airborne PAHs adversely affects children's cognitive development by 5 years of age, with potential implications for school performance (35).
PTS and health effects
Persistent toxic substances are inorganic or organic compounds. Some of them (e.g. heavy metals) occur naturally, but the majority originates from past and current human activities. Most of them are persistent in nature, fat soluble and toxic to living organism also in very low concentrations (11). As a result of their chemical properties, many PTS bioaccumulate in organisms and magnify in food chain, resulting in high concentrations in species on top of the food chain. Even though many POPs were phased out of production many years ago, they remain as hazardous waste in old industrial equipment for ages and can easily be remobilised in nature. A large proportion of electronic waste from industrial countries ends up in recycling stations in Asia and Africa due to lower costs and less strict waste handling legislation. High levels of polychlorinated biphenyls (PCBs), brominated flame retardants (PBDEs), toxic metals, dioxins/furans (PCDDs/PCDFs) and PAHs have been found in such dumping sites (22). Recently, a study from India reported significantly higher concentrations of non-dioxin like PCBs and total PCBs in breast milk from mothers living close to a waste dumping site than in breast milk from a reference area (26).
The effects of long-term environmental exposure to pollutants are often complicated to investigate as the mechanism of actions in humans are well characterised. Humans are also exposed not only to one contaminant at a time but to a complex mixture of substances. The time between exposure and outcome is often long and the potential endpoints may have large normal variability (e.g. birth weight) or being a complex disease (e.g. cancer). The effects can also be transgenerational. Comprehensive data on the distribution and health effects of PTSs in the northern hemisphere are slowly emerging, but data for the southern hemisphere are scarce. A number of countries in the southern hemisphere are defined as developing countries, where socioeconomic and health characteristics of the population differ from those in the industrialised countries (3). Hunger, malnutrition, lack of proper housing and sanitation and high unemployment rates result in poor health status and high incidence of communicable diseases. Most seriously affected are women and children. Population migration and rapid urbanisation with its wide range of anthropogenic activities also contribute to the environmental degradation and pollution, resulting in extreme susceptibility and vulnerability of the population to negative impact of PTS.
Climate change, PTS and maternal and child health outcomes in Arctic populations
There is a concern that climate change will affect the health situation of people in Arctic regions. POPs are transported to the polar regions from urbanised/industrialised areas via atmospheric and ocean transport (11, 19, 21). Several places also have substantial local exposure to contaminants through storage of PCB oils from former military complexes and industrial waste (11, 36). The environmental stores and possible effects on biological exposure have been thoroughly discussed in different reports from The Arctic Monitoring and Assessment Programme (11) since 1993. Recently, some decreasing human levels of the classical compounds, e.g. PCBs and the DDT group, are presented through new trend studies of the Russian Arctic (37). The declining levels might be associated not only with global agreements on reduced emissions (19), but also with local public health information campaigns (36). Even so, the issue of increased emission of Mercury associated with temperature change remains (38). As we can calculate that approximately 50% of the global Mercury emission is anthropogenic and the remaining part is natural evaporation from oceans, big rivers and ice cores, there will probably be an increase in exposure during the next 30–50 years, associated with increased impact from the bacteria responsible for the methylisation of Mercury into the biologically active Methyl Mercury (MeHg) (38).
Health of Arctic indigenous populations and the possibilities for good health studies
In general, the health status of indigenous people of the Arctic countries is characterised by lower life expectancy, higher infant mortality, higher rates of infectious diseases (particularly among children) and much higher incidences of injuries and suicide, when compared with the non-indigenous populations of these countries (11). It is likely that the higher prevalence of tobacco use, more sedentary lifestyles and consumption of more calorie-rich and nutrient-poor store-bought foods have contributed to an increasing burden of chronic diseases. With or without a climate change, health intervention strategies and population health in the Arctic will only improve with better information and better cooperation between all players. A full understanding of ethnic-specific health status can only arise if common health status indicators are selected and assessed both at short and long term. This will require that all relevant authorities and organisations in each country work closely together.
Contaminants play a role in the current health status of indigenous populations in many areas of the Arctic but must be assessed together with other important factors, e.g. education, economic well-being, cultural strength, community engagement, lifestyle choices, genetic susceptibility and availability of public health services.
Conducting studies to give advice to the public health authorities in the Arctic is difficult due to a variety of limiting factors: exposure to complex contaminant mixtures, small population size, contaminant–nutrient interactions, genetic factors, confounding factors and health priorities. The climate change adds even more difficulties to the assessment of contaminants and health outcomes. Indigenous populations in the Arctic are exposed to mixtures of contaminants primarily through the food chain. It is clear from studies of chemical mixtures that data derived from single chemical experiments cannot be used to predict the risk resulting from exposure to complex mixtures of POPs. Interactions between components of the mixture not only modify the disposition of individual components but also their dose–response relationship for various developmental endpoints. These interactions, coupled with differences in nutrient levels, could explain some of the differences between different ongoing cohort studies (11).
Environmental POPs and health in the Arctic
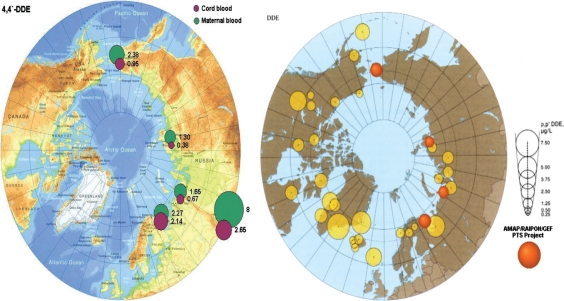
Selected results from the monitoring programme in AMAP are visualised in Figs. 5 and 6, (11). The figures clearly demonstrate the body burden s of PTS in indigenous Greenlandic, Canadian and Russian populations from the Arctic. Many POPs, including PCBs, PCDDs, PCDFs and pesticides, can mimic hormone activities. As potential endocrine disrupters, they are suspected to be capable of increasing the risk of cancer, birth defects and reproductive, neurodevelopmental disorders and immune system effects. To date, no clear evidence for adverse endocrine-related human health effects of POPs has been obtained at the individual or population level. However, data from studies on wildlife species, laboratory animals and biomarker effects in vitro have strengthened the need for further research to address the potential impacts of endocrine disruptors on human populations (11). There have been associations found in individual cohort studies between fish consumption, POPs exposure and head circumference, birth weight, duration of pregnancy and infant growth, but the relationships differ between studies (11). It is clear that a number of confounding factors such as the diet, individual susceptibility to contaminants and co-variation of other contaminants may alter the results in human PTS studies. It is therefore recommended that as many as possible of these factors are taken into account. Results from the PCB studies conducted in the Faroe Islands and Nunavik so far suggest that pre-natal exposure to PCBs is related to a relatively specific profile of cognitive impairments in children. Among the cognitive functions assessed, effects have been most clearly demonstrated on executive functions and speed of information processing. Those effects can be responsible for the small decreases in IQ observed in most studies. Verbal abilities and visual recognition memory are also likely to be impaired. Several recent studies in Arctic Canada confirm and support the relationship between contaminant exposure and depressed immunity (39). Both PCBs and DDE are associated with a higher incidence rate of acute otitis media and respiratory tract infections in Inuit children during the first 6 months of life. Concentrations of lymphocytes and immunoglobulin A have been found to be depressed in comparative studies of breast-fed babies and bottle-fed babies. The effectiveness of vaccination programmes among Inuit children and children from the Faroe Islands appears to be compromised by perinatal exposure to PCBs (as a marker of POPs) (40). New research with piglets supports these findings and indicates that transplacental POPs’ exposure leads to a reduction in antibody response. Findings from a Russian Arctic cohort add evidence that higher levels of maternal blood PCBs might be associated with more frequent occurrences of low birth weight, premature births, stillbirths and menstrual irregularities (36). The cohort is being assessed for the 6-years child follow-up these days.
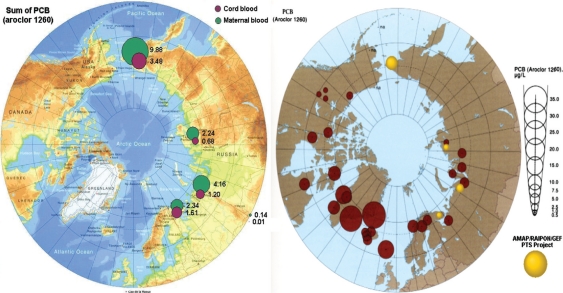
Fig. 5.
The Arctic Monitoring and Assessment Programme (AMAP). Circumpolar levels of PCBs (μg/L maternal blood) (11).
Fig. 6.
The Arctic Monitoring and Assessment Programme (AMAP). Circumpolar levels of the 4,4'DDE metabolite of DDT(μg/L maternal blood) (11).
New research results with pigs, which have a similar reproductive system to humans, indicate that exposure of sperm to environmentally pertinent organochlorine mixtures in vitro adversely affects oocyte development, sperm fertility and embryonic development. However, a comparison of existing population studies do not reveal any definitive or consistent relationships (11).
The multi-national INUENDO study has indicated some links between POP exposure and biomarkers of male reproductive function (41). Associations were found between high PCB153 serum levels and low sperm counts, decreased sperm motility and damage to sperm chromatin integrity in some of the sub-populations studied. In spite of these effects, fertility was not related to POPs except in Inuit. There are also open questions related to the role of genetic background, lifestyle and/or diet nutrition factors such as trace elements/antioxidants (e.g. Se) that may interfere with the possible adverse health effects of POPs (41).
Exposure to POPs may contribute to the development of metabolic syndrome. The endocrine-disrupting properties of several contaminants, especially dioxin-like compounds, can affect glucose and lipid metabolism that in turn affect the onset of metabolic syndrome. Genetic factors and lifestyle are also important determinants of metabolic syndrome. The dramatic increase in the rate of diabetes among Inuit and Alaskan Natives may be affected by multiple factors, but the role played by contaminants in obesity, metabolism and diabetes warrants new, comprehensive studies (42).
Toxic elements and human health in the Arctic
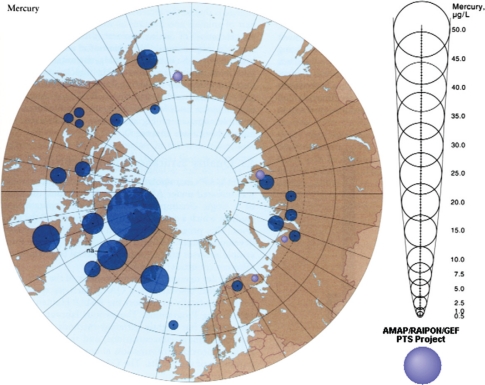
Fig. 7 visualises the Mercury burden of Arctic people, especially the Greenlandic indigenous populations. The growing foetus and newborn children are especially sensitive to the toxic effects of environmental mercury (Hg) and lead (Pb), providing a special concern for the effects of emissions due to climate change. Animal studies indicate that exposure to environmentally relevant mixtures of POPs and metals has significant effects on reproduction. Exposures led to decreases in maternal weight gain, weight gain in offspring and increased mortality rates in pups prior to weaning (11). Interactions between MeHg and POPs in mixtures warrant further study as lower levels of MeHg administered alone have been found to lead to more polar bear pup mortality than that found in mixture studies containing higher levels of MeHg (11).
Fig. 7.
The Arctic Monitoring and Assessment Programme (AMAP). Circumpolar levels of Mercury (μg/L maternal blood) (11).
Potential neurobehavioural effects associated with MeHg exposure have been found in the Faroe Islands in the domains of verbal function, visuomotor integration and attention (43). However, the most consistent marker of pre-natal MeHg exposure is delayed auditory processing assessed from brainstem auditory event potentials. Because of the inconsistencies between studies, there is a need for additional well-conducted prospective studies to elucidate the specific growth and neurobehavioural effects of MeHg and to assess the impacts of differences in maternal diet during pregnancy on susceptibility to MeHg exposure. Recent studies in the Faroe Islands, Greenland and Nunavik all indicate that Hg can affect circulatory parameters such as pulse pressure, heart rate and heart rate variability, blood pressure, hypertension and atherosclerosis, imposing higher risk, e.g. pre-eclampsia during pregnancy (11). Pre-natal exposures to MeHg may also affect the development of cardiovascular homeostasis. If these preliminary findings are confirmed, the estimated attributable burden of diseases due to contaminant exposure might increase. Even small relative risks have a large impact on diseases having high incidence and mortality and could affect policy development on safe levels of exposure. Confirmation of these findings in other studies is needed as the current findings have potentially significant implications for Hg intervention policies. In addition, more research is needed to determine the relationship between changes in risk of cardiovascular disease and changes in diet among Arctic indigenous populations (1).
Lead is well known to adversely affect neurodevelopment and behaviour in children. Until recently, studies of behavioural effects of Pb in children have only confirmed effects from post-natal exposure, not from pre-natal exposure. New studies with children from Nunavik have shown that cord blood Pb concentrations were related to observational measures of inattention, even at cord blood Pb concentrations below 10 µg/dL (1). The new data indicate that behavioural effects of low prenatal Pb exposure are likely to be observed when testing protocols that include sensitive measures of behaviour. Some new associations have been reported in Arctic Russia between spontaneous abortions and Hg levels in blood (11). No negative associations were found between maternal exposure to nickel and the risk of delivering a newborn with malformations of the genital organs.
Nutrients, contaminants and health in the Arctic
Nutrients and antioxidants found in seafood are thought to be capable of attenuating the effects of some environmental pollutants, especially Hg, but the hypothesis has not been adequately tested in humans. Very high intakes of Se, a well-studied antioxidant, may actually have had a negative impact on the visual system in Nunavik children, instead of being beneficial or protective against Hg neurotoxicity (44). Further research on the relationships between nutrients, especially Se, and contaminants (especially Hg in the context of climate change) is needed. Vitamin A and n-3 fatty acids are known to be capable of modulating immune function. Several organochlorine compounds have been shown to alter vitamin A homeostasis in a number of species (11). New data for pre-school Inuit children from Nunavik indicate that vitamin A deficiency is a risk factor for acute respiratory infections and otitis media among Inuit children from Nunavik (45). Imbalances or deficiencies of certain PUFAs of the n-3 and n-6 series are also thought to contribute to shorter duration of pregnancy and a wide range of childhood difficulties, including ADHD or related symptoms, disruptive behaviours and learning difficulties. Nutritional factors including antioxidants and PUFAs can alter responses to contaminant exposures and could account for differences in the results found in the two compatible Seychelles Child Development and the Faroe Islands cohort studies (46).
Genetics and health in the Arctic
Some contaminants (PCBs and MeHg) and some nutrients (vitamin A) are able to directly affect gene expression (47). These findings have consequences for health outcomes and may indicate modes of action of some of these entities and how they interact. This research is really at the starting point but might get increasing importance in a changing climate. The possibility that organochlorines adversely affect the genome by decreasing global genomic methylation is intriguing and should be examined in other circumpolar populations (11).
The vulnerable Arctic people and implications for policy making
Changing the energy system to stabilise the climate is likely to have a wide variety of effects that are not directly related to greenhouse gas emissions, including human health, macroeconomy, ecosystems, agricultural yields and employment patterns (1–3, 38).
Food safety is a crucial issue for the pregnant population in a changing climate. Available food without environmental contamination can be provided through a good monitoring of hazardous substances in food (36) (Fig. 8). Very good results have been reached through dietary advice for young people and pregnant women in the Faroe Island (3, 36). The perspective for the next 1–2 generations will possibly be a change in exposure patterns; decrease in organic substance exposure through global collaboration and cleanup of local sources (11, 19, 36) but increased exposure to Mercury because of increased emissions and increased methylation (38).
Fig. 8.
Grandmothers dilemma, also known as the Arctic dilemma: feeding the child with the very best dietary items, without knowing the contaminant content (11).
The different stages of the pregnancy provide special challenges. The embryological period is especially sensitive to environmental impact, with Thalidomide as a tragic example (48). After the placentation, the placenta barrier still allows crossing of different metals and POPs (49). The change in zoonosis patterns of the polar regions due to climate change will increase the risk of exposure to new bacteria and viruses for the unborn child (50) (Fig. 9). The brain development is also ongoing throughout the pregnancy, leading to harmful effects from, e.g. Mercury and Lead on the brain development, even after birth and the first part of childhood (44). As a consequence of this, a systematic implementation of mother–child cohorts to discover complications or delayed development of children is highly warranted and ongoing (11, 49).
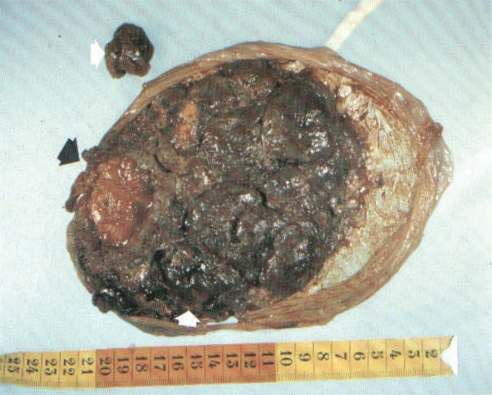
Fig. 9.
The placenta is a very vulnerable organ for environmental impact, especially from smoking, infectious agents, andcontaminants. This picture is taken after a premature delivery of a very dysmature baby from a mother smoking 30 cigarettesdaily during pregnancy (Odland, private picture).
As the latest reports on trends in biological levels of POPs are promising (37), the scenario for the next generations in the Arctic is worrying (38). The situation for the unborn child during the different stages of pregnancy is especially problematic because of the change in maternal exposure related to changes in external temperature (38). Some worsening conditions for the embryo and the foetus might be prevented through detailed trend studies connected to good dietary advice for the pregnant women (36, 38). Even so, good and safe food is also associated with good socioeconomic conditions and specific challenges to secure the diet for the pregnant population (11). The gender perspective must have a high focus and priority for the coming research on climate change and human health. The security for the unborn child must have top priority in the future and we strongly recommend that the next IPCC report focuses more closely on this topic.
Conflict of interest
The authors declare that there were no financial competitive interests and no scientific conflicts of interest.
References
- 1.IPCC. Climate Change 2007. Impacts, adaptation and vulnerability. Contribution of working group II to the fourth assessment report of the Intergovernmental Panel on Climate Change. 2007. Available from http://www.ipcc.ch/publications_and_data/ar4/wg2/en/contents.html.
- 2.Costello A, Abbas M, Allen A, Ball S, Bell S, Bellamy R, et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. The Lancet. 2009;373:1693–733. doi: 10.1016/S0140-6736(09)60935-1. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Protecting Health from Climate Change. 2008. Available from http://www.who.int/world-health-day/toolkit/report_web.pdf.
- 4.Bell ML, Goldberg R, Hogrefe C, Kinney PL, Knowlton K, Lynn B, et al. Climate change, ambient ozone, and health in 50 US cities. Climatic Change. 2007;82:61–76. [Google Scholar]
- 5.Buckman AH, Brown SB, Small J, Muir DCG, Parrott J, Solomon KR, et al. Role of temperature and enzyme induction in the biotransformation of polychlorinated biphenyls and bioformation of hydroxylated polychlorinated biphenyls by rainbow trout (Oncorhynchus mykiss) Environ Sci Technol. 2007;41:3856–63. doi: 10.1021/es062437y. [DOI] [PubMed] [Google Scholar]
- 6.Dentener F, Stevenson D, Ellingsen K, Van Noije T, Schultz M, Amann M, et al. The global atmospheric environment for the next generation. Environ Sci Technol. 2006;40:3586–94. doi: 10.1021/es0523845. [DOI] [PubMed] [Google Scholar]
- 7.Hogrefe C, Lynn B, Civerolo K, Ku JY, Rosenthal J, Rosenzweig C, et al. Simulating changes in regional air pollution over the eastern United States due to changes in global and regional climate and emissions. J Geophys Res -Atmos. 2004;109 [Google Scholar]
- 8.Knowlton K, Rosenthal JE, Hogrefe C, Lynn B, Gaffin S, Goldberg R, et al. Assessing ozone-related health impacts under a changing climate. Environ Health Perspect. 2004;112:1557–63. doi: 10.1289/ehp.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macdonald RW, Harner T, Fyfe J. Recent climate change in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data. Sci Total Environ. 2005;342:5–86. doi: 10.1016/j.scitotenv.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 10.Patra RW, Chapman JC, Lim RP, Gehrke PC, Sunderam RM. Effects of temperature on ventilatory behavior of fish exposed to sublethal concentrations of endosulfan and chlorpyrifos. Environ Toxicol Chem. 2009;28:2182–90. doi: 10.1897/08-532.1. [DOI] [PubMed] [Google Scholar]
- 11.Hansen JC, Odland JO, et al., editors. AMAP. Oslo, Norway: Arctic Monitoring and Assessment Programme (AMAP); 2009. AMAP Assessment 2009: Human Health in the Arctic; p. Xiv+254. [Google Scholar]
- 12.Monteiro A, Miranda AI, Borrego C, Vautard R. Air quality assessment for Portugal. Sci Total Environ. 2007;373:22–31. doi: 10.1016/j.scitotenv.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Anderson GB, Bell ML. Does one size fit all? The suitability of standard ozone exposure metric conversion ratios and implications for epidemiology. J Expo Sci Environ Epidemiol. 2010;20:2–11. doi: 10.1038/jes.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aw J, Kleeman MJ. Evaluating the first-order effect of intraannual temperature variability on urban air pollution. J Geophys Res-Atmos. 2003;108 [Google Scholar]
- 15.Ebi KL, Mills DM, Smith JB, Grambsch A. Climate change and human health impacts in the United States: an update on the results of the US National Assessment. Environ Health Perspect. 2006;114:1318–24. doi: 10.1289/ehp.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racherla PN, Adams PJ. Sensitivity of global tropospheric ozone and fine particulate matter concentrations to climate change. J Geophys Res-Atmos. 2006;111 [Google Scholar]
- 17.Racherla PN, Adams PJ. US Ozone Air Quality under Changing Climate and Anthropogenic Emissions. Environ Sci Technol. 2009;43:571–7. doi: 10.1021/es800854f. [DOI] [PubMed] [Google Scholar]
- 18.Shea KM, Truckner RT, Weber RW, Peden DB. Climate change and allergic disease. J Allergy Clin Immunol. 2008;122:443–53. doi: 10.1016/j.jaci.2008.06.032. quiz 454–5. Review. [DOI] [PubMed] [Google Scholar]
- 19.Stockholm Convention. Climate change and POPs. Predicting the Impacts. 2009. Available from http://www.unep.org/civil-society/Portals/59/Documents/Greenroom/events/Climate_and_POPs_final.pdf.
- 20.Noyes PD, McElwee MK, Miller HD, Clark BW, Van Tiem LA, Walcott KC, et al. The toxicology of climate change: environmental contaminants in a warming world. Environ Int. 2009;35:971–86. doi: 10.1016/j.envint.2009.02.006. Review. [DOI] [PubMed] [Google Scholar]
- 21.Epstein PR. Climate change and human health. New Engl J Med. 2005;353:1433–6. doi: 10.1056/NEJMp058079. [DOI] [PubMed] [Google Scholar]
- 22.Borgå K, Saloranta TM, Ruus A. Simulating climate change-induced alterations in bioaccumulation of organic contaminants in an Arctic marine food web. Environ Toxicol Chem. 2010;29:1349–57. doi: 10.1002/etc.159. [DOI] [PubMed] [Google Scholar]
- 23.Hallanger IG, Ruus A, Herzke D, Warner NA, Evenset A, Heimstad ES, et al. Influence of season, location, and feeding strategy on bioaccumulation of halogenated organic contaminants in Arctic marine zooplankton. Environ Toxicol Chem. 2011;30:77–87. doi: 10.1002/etc.362. [DOI] [PubMed] [Google Scholar]
- 24.Smith KR. Indoor air pollution in developing countries: recommendations for research. Indoor Air. 2002;12:198–207. doi: 10.1034/j.1600-0668.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Global health observatory database. 2010. Available from http://apps.who.int/ghodata/ [cited 25 February 2011]
- 26.Choi H, Perera F, Pac A, Wang L, Flak E, Mroz E, et al. Estimating individual-level exposure to airborne polycyclic aromatic hydrocarbons throughout the gestational period based on personal, indoor, and outdoor monitoring. Environ Health Perspect. 2008;116:1509–18. doi: 10.1289/ehp.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gliniana SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. Does particulate air pollution contribute to infant death? A systematic review. Environ Health Perspect. 2004;112:1365–71. doi: 10.1289/ehp.6857. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacasaña M, Esplugues A, Ballester F. Exposure to ambient air pollution and prenatal and early childhood health effects. Eur J Epidemiol. 2005;20:183–99. doi: 10.1007/s10654-004-3005-9. Review. [DOI] [PubMed] [Google Scholar]
- 29.Jedrychowski W, Bendkowska I, Flak E, Penar A, Jacek R, Kaim I, et al. Estimated risk for altered fetal growth resulting from exposure to fine particles during pregnancy: an epidemiologic prospective cohort study in Poland. Environ Health Perspect. 2004;112:1398–402. doi: 10.1289/ehp.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker JD, Mendola P, Woodruff TJ. Preterm birth after the Utah Valley Steel Mill closure: a natural experiment. Epidemiology. 2008;19:820–3. doi: 10.1097/EDE.0b013e3181883d5d. [DOI] [PubMed] [Google Scholar]
- 31.Gilboa SM, Mendola P, Olshan AF, Langlois PH, Savitz DA, Loomis D, et al. Relation between ambient air quality and selected birth defects, seven county study, Texas, 1997–2000. Am J Epidemiol. 2005;162:238–52. doi: 10.1093/aje/kwi189. [DOI] [PubMed] [Google Scholar]
- 32.Heinrich J, Slama R. Fine particles, a major threat to children. Int J Hyg Environ Health. 2007;210:617–22. doi: 10.1016/j.ijheh.2007.07.012. Epub 4 September 2007. [DOI] [PubMed] [Google Scholar]
- 33.Diaz-Sanchez D, Proietti L, Polosa R. Diesel fumes and the rising prevalence of atopy: an urban legend? Curr Allergy Asthma Rep. 2003;3:146–52. doi: 10.1007/s11882-003-0027-4. [DOI] [PubMed] [Google Scholar]
- 34.Edwards SC, Jedrychowski W, Butscher M, Camann D, Kieltyka A, Mroz E, et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children's intelligence at 5 years of age in a prospective cohort study in Poland. Environ Health Perspect. 2010;118:1326–31. doi: 10.1289/ehp.0901070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ACIA. Arcic Climate Impact Assessment. Cambridge University Press; 2004. Impacts of a Warmer Arctic. [Google Scholar]
- 36.Arctic Monitoring and Assessment Programme (AMAP) Final Report. Oslo: 2004. Persistent Toxic Substances, Food Security and Indigenous Peoples of the Russian North; p. 192 p. AMAP Report 2004: 2. [Google Scholar]
- 37.Rylander C, Sandanger TM, Petrenya N, Konoplev A, Bojko E, Odland JO. Indications of decreasing human PTS concentrations in North West Russia. Global Health Action. 2011;4 doi: 10.8427/gha.v4i0.8427. 8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AMAP. Arctic Monitoring and Assessment Programme (AMAP) Oslo: Arctic Pollution 2011; p. vi+38. ISBN-13978-82-7971-066-0. [Google Scholar]
- 39.Dallaire E, Ayotte P, Pereg D, Dery S, Dumas P, Langlois S, Dewailly E. Determinants of plasma concentrations of perfluorooctane sulfonate and brominated compounds in Nunavik Inuit Adults (Canada) Environ Sci Technol. 2009;43:5130–6. doi: 10.1021/es9001604. [DOI] [PubMed] [Google Scholar]
- 40.Heilmann C, Budtz-Jørgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ Health Perspect. 2010;118:1434–8. doi: 10.1289/ehp.1001975. Epub 1 June 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonde JP, Toft G, Rylander L, Rignell-Hydbom A, Giwercman A, Spano M, et al. INUENDO. Fertility and markers of male reproductive function in Inuit and European populations spanning large contrasts in blood levels of persistent organochlorines. Environ Health Perspect. 2008;116:269–77. doi: 10.1289/ehp.10700. Review. Erratum in: Environ Health Perspect. 2008 Mar; 116: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen JC, Deutch B, Odland JØ. Dietary transition and contaminants in the Arctic: emphasis on Greenland. Circumpolar Health Suppl. 2008;2:10–25. doi: 10.1080/22423982.2007.11864604. [DOI] [PubMed] [Google Scholar]
- 43.Jensen TK, Grandjean P, Jørgensen EB, White RF, Debes F, Weihe P. Effects of breast feeding on neuropsychological development in a community with methylmercury exposure from seafood. J Expo Anal Environ Epidemiol. 2005;15:423–30. doi: 10.1038/sj.jea.7500420. [DOI] [PubMed] [Google Scholar]
- 44.Boucher O, Muckle G, Saint-Amour D, Dewailly E, Ayotte P, Jacobson SW, et al. The relation of lead neurotoxicity to the event-related potential P3b component in Inuit children from arctic Québec. Neurotoxicology. 2009;30:1070–7. doi: 10.1016/j.neuro.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cameron C, Dallaire F, Vézina C, Muckle G, Bruneau S, Ayotte P, et al. Neonatal vitamin A deficiency and its impact on acute respiratory infections among preschool Inuit children. Can J Public Health. 2008;99:102–6. doi: 10.1007/BF03405454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodman M, Barraj LM, Mink PJ, Britton NL, Yager JW, Flanders WD, et al. Estimating uncertainty in observational studies of associations between continuous variables: example of methylmercury and neuropsychological testing in children. Epidemiol Perspect Innov. 2007;4:9. doi: 10.1186/1742-5573-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shirakawa AK, Nagakubo D, Hieshima K, Nakayama T, Jin Z, Yoshie O. 1,25-dihydroxyvitamin D3 induces CCR10 expression in terminally differentiating human B cells. J Immunol. 2008;180:2786–95. doi: 10.4049/jimmunol.180.5.2786. [DOI] [PubMed] [Google Scholar]
- 48.Lancaster PA. Causes of birth defects: lessons from history. Congenit Anom. 2011;51:2–5. doi: 10.1111/j.1741-4520.2010.00311.x. [DOI] [PubMed] [Google Scholar]
- 49.Hansen S, Nieboer E, Odland JØ, Wilsgaard T, Veyhe AS, Sandanger TM. Levels of organochlorines and lipids across pregnancy, delivery and postpartum periods in women from Northern Norway. J Environ Monit. 2010;12:2128–37. doi: 10.1039/c0em00346h. [DOI] [PubMed] [Google Scholar]
- 50.Odland JO, Nieboer E, Romanova N, Thomassen Y. Elements in placenta and pregnancy outcome in arctic and subarctic areas. Int J Circumpolar Health. 2004;63:169–87. doi: 10.3402/ijch.v63i2.17703. [DOI] [PubMed] [Google Scholar]