Abstract
Anatomical studies have demonstrated that the vestibular nuclei project to nucleus tractus solitarius (NTS), but little is known about the effects of vestibular inputs on NTS neuronal activity. Furthermore, lesions of NTS abolish vomiting elicited by a variety of different triggering mechanisms, including vestibular stimulation, suggesting that emetic inputs may converge on the same NTS neurons. As such, an emetic stimulus that activates gastrointestinal (GI) receptors could alter the responses of NTS neurons to vestibular inputs. In the present study, we examined in decerebrate cats the responses of NTS neurons to rotations of the body in vertical planes before and after the intragastric administration of the emetic compound copper sulfate. The activity of more than one-third of NTS neurons was modulated by vertical vestibular stimulation, with most of the responsive cells having their firing rate altered by rotations in the head-up or head-down directions. These responses were aligned with head position in space, as opposed to the velocity of head movements. The activity of NTS neurons with baroreceptor, pulmonary, and GI inputs could be modulated by vertical plane rotations. However, injection of copper sulfate into the stomach did not alter the responses to vestibular stimulation of NTS neurons that received GI inputs, suggesting that the stimuli did not have additive effects. These findings show that the detection and processing of visceral inputs by NTS neurons can be altered in accordance with the direction of ongoing movements.
Keywords: semicircular canal, otolith organ, vomiting, nausea, cardiovascular regulation
nucleus tractus solitarius (NTS), a structure located in the dorsomedial portion of the caudal medulla, receives visceral afferent inputs from throughout the body and relays these signals to regions of the central nervous system that regulate autonomic functions (18, 28, 29). NTS additionally receives inputs from a variety of central nervous system regions, including the caudal portion of the vestibular nucleus complex (5, 15, 45, 47, 56). A number of homeostatic responses are affected by labyrinthine inputs, and these responses could be mediated, at least in part, through projections from the vestibular nuclei to NTS. Among these are vestibulo-sympathetic responses that adjust blood distribution in the body during postural alterations (17, 54, 55, 59), vestibulo-respiratory responses that alter respiratory muscle activity in accordance with body position in space (55), and the complex of autonomic responses that occur during motion sickness (43, 58).
Although anatomical studies have shown that the vestibular nuclei project to NTS, little is known about the effects of labyrinthine signals on NTS neuronal activity. The only prior neurophysiological study of the responses of NTS units to vestibular inputs used electrical stimulation to activate afferents from the inner ear (56). This study showed that a fraction of neurons that responded to stimulation of the abdominal or cervical portions of the vagus nerve, or that had cardiac related activity, were also inhibited or excited by electrical activation of labyrinthine afferents (56). One goal of the present experiments was to determine the effects of rotations in vertical planes that stimulate the anterior and posterior semicircular canals and otolith organs on the activity of NTS neurons, including those that receive inputs from baroreceptors or gastrointestinal (GI) afferents, or have discharges synchronized with diaphragm contractions. We tested the hypothesis that NTS neurons with inputs from different visceral afferents have distinct responses to natural vestibular stimulation.
NTS plays a critical role in the production of nausea and vomiting (16, 40). Most, if not all, emetic stimuli evoke an increase in the expression of the intermediate-early gene c-fos by NTS neurons (10, 31, 32, 39, 44). Lesions of NTS suppress or abolish vomiting elicited by a variety of different triggering mechanisms (16, 40), including vestibular stimulation (11, 13, 23, 53). There is evidence that susceptibility for nausea and emesis evoked by different triggering mechanisms is correlated. For example, individuals who are vulnerable to motion sickness often experience postoperative nausea and vomiting (1, 25, 49). These observations raise the possibility that multiple inputs that evoke vomiting converge on the same NTS neurons, such that one signal affects the processing of the other. A second objective of this study was to ascertain whether an emetic stimulus that activates GI afferents alters the responses of NTS neurons to vestibular inputs. GI afferents were stimulated using the intragastric injection of copper sulfate, which irritates the stomach lining and readily evokes vomiting (3, 12, 14, 20, 26, 27, 35, 50, 52). Copper sulfate is absorbed into the bloodstream in the intestine, but not the stomach (60). Since the copper sulfate solution was removed ∼10–15 min after injection into the stomach, it likely did not reach its transporters in the intestine, and thus was not absorbed into the bloodstream. Consequently, copper sulfate served as a reversible emetic stimulus in this study. We tested the hypothesis that motion and GI triggers for nausea and vomiting converge on the same NTS neurons by examining whether copper sulfate infusion into the stomach altered the sensitivity and response characteristics of NTS neurons to vestibular stimulation in vertical planes.
METHODS
All experimental procedures conformed to the American Physiological Society's “Guiding Principles for the Care and Use of Animals,” as well as the National Research Council Guide for the Care and Use of Laboratory Animals, and were approved by the University of Pittsburgh's Institutional Animal Care and Use Committee. Data were collected from 11 purpose-bred adult cats (Liberty Research, Waverly, NY) of either sex, weighing 2.6 to 4.9 kg. Most of the methods used in this study have been described in detail previously (19, 34, 42).
Surgeries.
Animals were anesthetized with isoflurane (5% for induction, 1.5–2.5% for maintenance) vaporized in O2, and a transducer (Millar Instruments, Houston, TX) was inserted through the femoral artery into the abdominal aorta to record blood pressure. The level of anesthesia was titrated to maintain mean blood pressure at ∼100 mmHg and to prevent spontaneous and reflexive movements. The trachea was intubated, and both femoral veins were cannulated for drug administration. The carotid arteries were dissected free of surrounding tissues and occluded bilaterally, and a ligature was placed around each artery to permit stretch of the carotid sinus (19). A catheter was inserted into the stomach through the cervical esophagus for intragastric copper sulfate injections. The C5 phrenic nerves were isolated bilaterally, secured in bipolar silver cuff electrodes that were insulated using silicone, and covered with a mixture of Vaseline and mineral oil.
The animals were placed in a stereotaxic frame with the head pitched-down 30° to vertically align the vertical semicircular canals, and supported using hip pins and a clamp placed on the dorsal process of an upper thoracic vertebra. They were subsequently decerebrated at the midcollicular level, and an occipital craniotomy was performed to expose the caudal brain stem. The caudal aspect of the cerebellum was gently retracted to permit the insertion of electrodes into the portion of the medulla near the obex.
Throughout the surgery and subsequent recording session, atropine sulfate (0.10–0.15 mg/kg) was injected intramuscularly every 3 h to decrease airway secretions, and dexamethasone (2 mg/kg) was injected intravenously every 6 h to reduce brain edema. Rectal temperature was maintained at 37–38°C using a DC-powered heat lamp and pad. After the surgery was complete, anesthesia was discontinued. Animals were paralyzed using intravenous injections of pancuronium bromide (initial injection of 0.2 mg/kg, maintained by hourly injections of 0.1–0.2 mg/kg), and artificially ventilated with room air. Tidal volume and ventilation frequency were adjusted to maintain end-tidal CO2 at 4–5%. A bilateral pneumothorax was performed to reduce ventilation-related brain movements. If hypotension occurred, mean blood pressure was increased to above 90 mmHg using an intravenous infusion of phenylephrine in saline at the rate of 0.005 to 0.01 mg·kg−1·min−1.
At the end of the experiment, animals were killed by the intravenous injection of 120 mg/kg pentobarbital sodium. The brain stem was removed and fixed in 10% formaldehyde solution.
Recording procedures.
Recordings were performed from regions of the NTS and adjacent reticular formation spanning from 1.5 mm caudal to 1 mm rostral to the obex, and within 2.5 mm of the midline, using a 5 MΩ tungsten microelectrode (Frederick Haer, Bowdoin, ME). In addition to anatomical landmarks on the surface of the brain stem, the locations of respiratory neurons of the dorsal respiratory group (51) were used to estimate the boundaries of the NTS during experiments. Activity recorded from neurons was amplified by a factor of 10,000 and filtered with a band pass of 300–10,000 Hz. The output of the amplifier was sampled at 25,000 Hz using a Micro1401 mk 2 data collection system and Spike2 version 6 software (Cambridge Electronic Design, Cambridge, UK). The amplifier output was additionally fed into a window discriminator for the delineation of spikes from single units. The discriminator output was sampled at 10,000 Hz, as described above. During the post hoc data analysis following experiments, the spike detection and sorting feature of the Spike2 software was employed to segregate units if more than one was present in the recording field, and to ensure that counts of neuronal activity were accurate (see Data analysis procedures below). Activity recorded from the phrenic nerves was amplified by a factor of 10,000, filtered with a band pass of 100–10,000 Hz, integrated with a 10-ms time constant, full-wave rectified, and sampled at 1,000 Hz. We additionally sampled arterial blood pressure at 100 Hz. When vestibular stimuli were delivered, table movement was recorded using potentiometers and collected at 100 Hz.
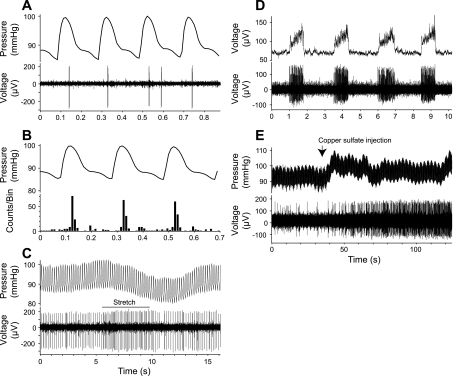
Upon encountering a unit, we recorded its spontaneous activity along with blood pressure and phrenic nerve discharges, so we could determine whether the cell had cardiovascular or respiratory related firing (Fig. 1). The carotid arteries were occluded to reduce bleeding at the decerebration site, which also eliminated stimulation of carotid sinus baroreceptors during the cardiac cycle. As an additional test to ascertain whether NTS neurons received baroreceptor inputs, we observed the effects of mechanical stretch of the carotid sinus (19), as illustrated in Fig, 1C.
Fig. 1.
Examples of different unit types, including cardiovascular units (A–C), a respiratory unit (D), and a GI unit (E). A: data traces showing arterial blood pressure (top) and nucleus tractus solitarius (NTS) unit activity (bottom). These data are from a portion of the run used to generate the averages in B. B: averaged changes in arterial blood pressure (top) and a poststimulus histogram showing associated changes in the NTS unit firing (bottom); 73 sweeps were pooled to generate traces. Averaging was triggered by maximal systolic blood pressure at the central peak. The unit tended to fire just after maximal systolic blood pressure. C: effect of mechanical stretch of the carotid sinus (indicated by horizontal line) on arterial blood pressure (top) and NTS unit activity (bottom). Carotid stretch resulted in a decrease in blood pressure and an increase in the unit's firing rate. D: comparison of phrenic nerve activity (top) to NTS unit activity (bottom). This unit fired during phrenic nerve discharges and was classified as an inspiratory neuron. E: effect of intragastric copper sulfate administration (indicated by arrow) on arterial blood pressure (top) and NTS neuronal activity (bottom). Injecting copper sulfate resulted in a transient increase in blood pressure and a sustained increase in unit firing.
We next recorded neuronal responses while rotating the entire animal about the pitch (transverse) and roll (longitudinal) axes using a servo-controlled hydraulic tilt table (NeuroKinetics, Pittsburgh, PA). Our procedures for performing vertical vestibular simulation are described in detail elsewhere (19, 34, 42). We first utilized the “wobble” stimulus, a fixed-amplitude tilt, the direction of which moves around the animal at constant speed (48), to determine whether a unit responded to vestibular stimulation. The wobble stimulus was employed for this determination, because it activates both vertical semicircular canals and otolith organs (48). We typically first delivered 0.2-Hz wobble stimuli at 5°. If these rotations were ineffective, as determined by an online calculation of the signal-to-noise ratio for responses (see Data analysis procedures for definition), we increased the stimulus amplitude to 7.5°. If a neuron did not respond to 0.2-Hz rotations at 7.5° amplitude, 0.1-Hz wobble stimulation at 10° amplitude was provided, although some units were lost before this stimulus could be delivered.
For units that responded to wobble rotations, we determined the plane of tilt that produced maximal modulation of the unit's firing rate (response vector orientation). The direction of the response vector orientation lies midway between the maximal response directions to clockwise and counterclockwise wobble stimulation, because the phase differences between stimulus and response are reversed during the two directions of rotation (48). Thus, by considering both responses, we could account for these phase differences. Subsequently, the response vector orientation was confirmed by comparing the gain of responses to rotations in the roll and pitch planes.
After a unit's response vector orientation was established, tilts in a fixed plane at or near this orientation were used to study the dynamics of the vestibular response (response gain and phase across stimulus frequencies). Response dynamics were routinely determined for the frequency range of 0.05–0.5 Hz; for some cells, rotations at 1 Hz were also delivered. The amplitude of planar stimuli was 5°-10° at frequencies ≤0.1 Hz and 5°-7.5° at frequencies >0.1 Hz.
We then tested the effects of injecting into the stomach 83 mg of copper sulfate (Sigma, St. Louis, MO) dissolved in 10 ml of distilled water on the spontaneous activity of neurons and their responses to vestibular stimulation. After we recorded the spontaneous firing rate of a unit for 5 min following copper sulfate administration, wobble and planar rotations were delivered at the same frequencies and amplitudes that were previously shown to be effective. Subsequently, the copper sulfate solution was aspirated from the stomach, and then a series of washes occurred using 10–15 ml of distilled water. In total, 60 ml of distilled water was injected and aspirated during the washing process to ensure that the copper sulfate solution was completely removed. We waited at least 10 min after testing the effects of copper sulfate infusion on the responses of an NTS neuron to vestibular stimulation before resuming recordings. It was rare to hold a unit through the stimulation battery described above and the subsequent washing process to remove copper sulfate from the stomach. As such, we were unable to determine whether a unit's spontaneous activity and responses to rotations returned to baseline levels after the copper sulfate stimulus was withdrawn.
Electrolytic lesions were made at defined coordinates by passage of a 100-μA negative current for 60 s through the recording electrode. Typically, lesions were placed 1–2 mm ventral to NTS; after a lesion was made, recordings commenced on the opposite side of the brain. These precautions ensured that damage to the brain resulting from the process of generating the lesion did not affect subsequent recordings.
Data analysis procedures.
Following experiments, all data were subjected to spike sorting using Spike2 software to ensure that counts of neuronal activity were accurate. When spike shape or amplitude changed during the course of recording from a unit, such that we were not confident about the fidelity of the data, the spurious runs were discarded. In trials in which two units were present with action potential shapes that could be reliably segregated, data from both units were subsequently analyzed, as described below.
To determine whether a unit had cardiovascular related activity, averages of neural activity were triggered from peak systolic blood pressure, as shown in Fig. 1B. In addition, a neuron was classified as a “cardiovascular unit” if its firing rate changed >30% during the decline in blood pressure, resulting from stretch of the carotid sinus (Fig. 1C). Neurons were classified as “respiratory units” if their discharges were synchronized with phrenic nerve activity, as illustrated in Fig. 1D. Three subtypes of respiratory units were distinguished: inspiratory units that fired during increases in phrenic nerve activity, expiratory units that fired between bursts of phrenic nerve activity, and pump units with respiratory related activity that disappeared when artificial ventilation was transiently removed. The latter responses were likely due to activation of pulmonary receptors when the chest was expanded by the ventilator (36). Neurons were classified as “GI units” if their firing rates increased or decreased >30% following the administration of copper sulfate, as illustrated in Fig. 1E. Some neurons had responses that combined the properties discussed above (e.g., had both cardiovascular and respiratory related activity) and were classified as “convergent neurons.” However, most units could not be classified into these four groups, and are referred to below as “unknown units.”
Neural activity recorded during whole-body rotations was binned (500 bins/cycle) and averaged over the sinusoidal stimulus period. Sine waves were fitted to responses with the use of a least-squares minimization technique (48); an initial analysis was performed using the Spike2 software, while data collection was occurring, and a post hoc final analysis was subsequently executed using MATLAB (MathWorks, Natick, MA). The response sinusoid was characterized by two parameters: phase shift from the stimulus sinusoid (subsequently referred to as phase) and amplitude relative to the stimulus sinusoid (subsequently referred to as gain). We used one primary criterion and two secondary criteria to determine whether neuronal activity was modulated by rotations (19, 34, 42). First, responses were considered significant only if the signal-to-noise ratio [calculated as in (48)] was >0.5. Data meeting this criterion were considered to represent real modulation of neuronal activity if only the first harmonic was prominent, and the responses were consistent from trial to trial. In a small number of cases, rotations were delivered at a frequency similar to that for spontaneous rhythmic activity, such that the averaged activity met the signal-to-noise criterion at that frequency, but not other rotation frequencies. In these instances, the response phases varied tremendously when runs were repeated, allowing spurious trials to be readily detected.
Statistical analyses were performed using Prism 5 software (GraphPad Software, San Diego, CA). Pooled data are presented as means ± SE. Statistical significance was assumed if P < 0.05.
Histological procedures.
A freezing microtome was used to cut the brain stem transversely at 100-μm thickness, and tissue sections were stained using thionine. Photographs of brain stem sections were captured using a digital stereomicroscope, and Motic (Xiamen, China) Images Advanced software and Adobe Illustrator software (Adobe systems, San Jose, CA) were used to generate drawings of the sections. Recording sites were reconstructed on these drawings with reference to the locations of electrolytic lesions, the relative positions of electrode tracks, and microelectrode depths.
RESULTS
We examined the effects of rotations in vertical planes on the firing rate of 222 cells located in the NTS and adjacent reticular formation. Forty-one of the neurons were classified as cardiovascular units, including 33 cells with activity synchronized to the cardiac cycle (as illustrated in Fig. 1, A and B) and eight additional cells that responded to stretch of the carotid sinus (as shown in Fig. 1C). Seventeen neurons were respiratory units, of which 13 had respiratory related activity that disappeared when the ventilator was transiently switched off, indicating that the responses were due to activation of pulmonary afferents by lung inflation. Fifteen neurons were GI units, including nine, whose firing rate decreased, and six, whose firing rate increased following the injection of copper sulfate into the stomach. Eighteen additional neurons were convergent units, most of which responded to the intragastric administration of copper sulfate (10 with increases in activity and 6 with decreases in activity). The majority of these convergent units had both cardiovascular and GI responses, while 6 had both respiratory and GI responses. The remaining 131 neurons were classified as “unknown units.”
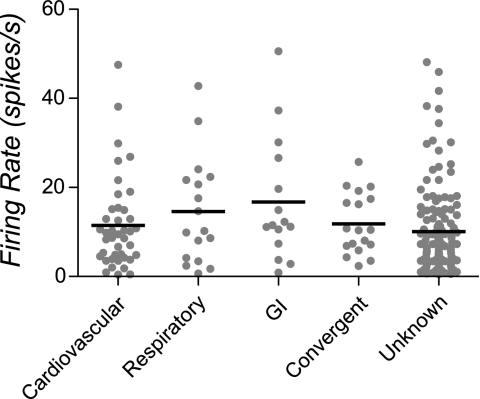
The mean spontaneous firing rate for all neurons was 11.3 ± 0.7 spikes/s. The spontaneous firing rates for each of the unit types are shown in Fig. 2; these firing rates were not shown to be significantly different using a nonparametric one-way ANOVA.
Fig. 2.
Spontaneous firing rates of units tested for responses to vestibular stimulation. Gray symbols indicate data for each neuron, whereas horizontal lines designate the median firing rate of each unit type.
Responses of NTS neurons to rotations in vertical planes.
Ninety-eight (44%) of the 222 neurons responded to whole-body rotations, including 22 cardiovascular, 11 respiratory, 14 GI, 17 convergent, and 34 unknown units. Relatively small wobble stimuli were typically effective in modulating the activity of responsive neurons: 5° for 46 units and 7.5° for 38 units. Just 14 of the 98 cells required 10° wobble stimuli to evoke responses.
The firing rate of 93% (14/15) of GI units, and 94% (17/18) of convergent neurons was modulated by vestibular stimulation. In total, of the 31 neurons whose activity was altered by copper sulfate infusion into the stomach (15 GI and 16 convergent units), 29 (94%) responded to vertical rotations. In contrast, just 54% (22/41) of cardiovascular units, 64% (11/17) of respiratory units, and 26% (34/131) of unknown units had responses to labyrinthine inputs. A caveat, however, is that some neurons were lost during the initial battery of testing for responses to whole body rotations, before copper sulfate was injected into the stomach. Many of these lost units were unresponsive to vestibular stimulation, such that a large number of trials were required to establish whether their activity was modulated by body rotations. Most of these cells were classified as “unknown units,” although it is possible that some received GI inputs. This limitation in the data set could have resulted in an artificial elevation of the fraction of neurons with GI inputs classified as responding to vestibular stimulation.
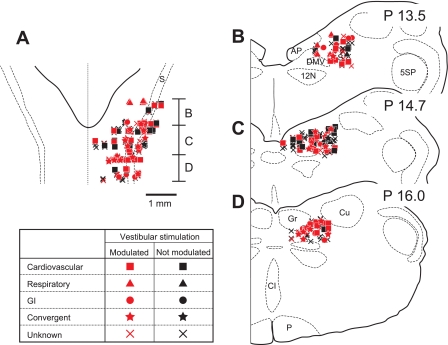
The locations in the caudal aspect of NTS and the adjacent reticular formation of the neurons that responded to vertical rotations are indicated in Fig. 3. These units were interspersed with those whose firing rates were unaffected by rotations. The population of cells sampled was located 0.25 to 2.5 mm (average of 1.49 ± 0.03 mm) from the midline, 0.15 to 2.1 mm (average of 1.03 ± 0.03 mm) from the dorsal surface of the brain stem, and 13.0 to 15.6 mm (average of 14.3 ± 0.04 mm) posterior to stereotaxic zero. In general, different unit types were intermingled, although respiratory units were located slightly more rostrally than the others (13.7 ± 0.15 mm posterior to stereotaxic zero), as confirmed by a two-way ANOVA (factors were unit type and the coordinates of unit locations) combined with Bonferroni post hoc tests.
Fig. 3.
Locations of neurons in NTS and the adjacent reticular formation that were tested for responses to vestibular stimulation. Unit locations were plotted on standard horizontal (A) and transverse (B–D) sections with reference to Berman's atlas (9). Letters beside the horizontal section show the anterior-posterior region represented by each of the transverse sections. In addition, numbers at the top of each transverse section indicate the level posterior (P) to stereotaxic zero. Different symbols are used to designate each unit type. Red symbols denote neurons whose activity was modulated by rotations in vertical planes, while black symbols denote neurons without responses to vertical vestibular stimulation. AP, area postrema; CI, inferior central nucleus; Cu, cuneate nucleus; DMV, dorsal motor nucleus of the vagus; Gr, gracile nucleus; s, solitary tract; P, pyramid; 5SP, spinal trigeminal nucleus; 12N, hypoglossal nerve; GI, gastrointestinal.
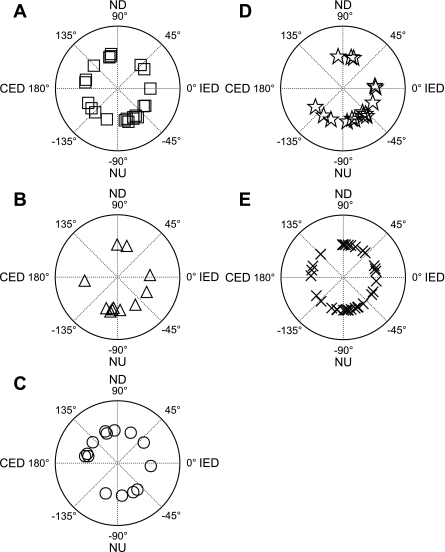
Response vector orientations were determined for all but one of the neurons that responded to vertical rotations, and are shown in Fig. 4. Of the 97 response vector orientations illustrated, 65 (67%) were closer to the pitch plane than the roll plane; two-thirds (42/65) of these cells were excited by nose-up pitch, whereas one-third were excited by nose-down pitch. The distribution of response vector orientations was similar for all unit types: 59% of cardiovascular, 73% of respiratory, 57% of GI, 75% of convergent, and 67% of unknown units had response vector orientations within 45° of the pitch plane.
Fig. 4.
Polar plots showing response vector orientations determined for the following types of NTS units: cardiovascular units (A), respiratory units (B), GI units (C); convergent units (D), and unknown units (E). Response vector orientations were determined using wobble stimuli, usually at 0.2 Hz. The response vector orientations were plotted using a head-centered coordinate system, with 0° corresponding to ipsilateral ear-down (IED) roll tilt, 90° to nose-down (ND) pitch, 180° to contralateral ear-down (CED) roll, and −90° to (NU) nose-up pitch.
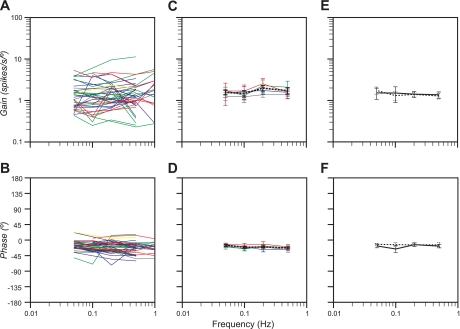
After the response vector orientation was calculated for a unit, tilts were delivered in a fixed plane near this best direction for modulating neuronal activity, to determine the dynamic properties of the cell's responses to rotations. Dynamic response properties were established over at least a stimulus decade (10-fold range of stimulus frequencies, such as 0.05–0.5 Hz) for 40 neurons. Fig. 5A illustrates the responses of a unit with unknown visceral inputs to pitch rotations at 0.05–0.5 Hz, whereas Fig. 5B shows the responses of a GI unit to roll tilts delivered at 0.05–1.0 Hz. Bode plots indicating the gain and phase of responses to fixed-plane tilts are provided in Fig. 6. Both response gain and phase were plotted with respect to stimulus position, such that a response whose phase led stimulus position by 90° was synchronous with stimulus velocity. Lines of different colors indicate the 7 cardiovascular units, 1 respiratory unit, 13 GI units, 14 convergent units, and 5 unknown units for which Bode plots were constructed. It was often difficult to collect sufficient trials to eliminate strong intrinsic rhythmic discharges from averages. For this reason, data for only a limited number of neurons with strong rhythmic activity, including cardiovascular and respiratory units, were provided in Fig. 6. The response dynamics for the majority of units were remarkably consistent: response gain stayed constant across stimulus frequencies (Fig. 6, A and C), while response phase remained near stimulus position or lagged stimulus position slightly as stimulus frequency increased (Fig. 6, B and D).
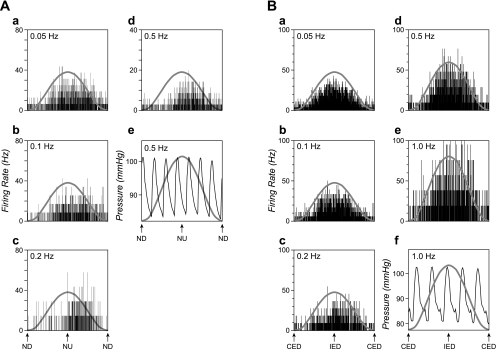
Fig. 5.
Averaged responses of two NTS units to sinusoidal rotations at different frequencies. Each histogram contains 500 bins (bin width varies from 40 ms at 0.05 Hz to 2 ms at 1 Hz). A sine wave superimposed on each trace shows table movement. A: responses of a unit with unknown visceral inputs to 5° rotations in the pitch plane. The number of sweeps averaged for each trace were 4 in a, 6 in b, 7 in c, and 91 in d. Panel e shows blood pressure recordings during one of the trials used to produce d; body tilt did not produce alterations in blood pressure. B: averaged responses of another unit with GI inputs to sinusoidal rotations in the roll plane. Rotation amplitudes were 7.5° at all frequencies but 1 Hz (e), where 5° rotations were delivered. Numbers of sweeps averaged for each trace were 7 in a, 9 in b, 12 in c, 26 in d, and 26 in e. Panel f shows blood pressure recordings during one of the trials used to produce e.
Fig. 6.
Bode plots illustrating the dynamic properties of NTS neuronal responses to tilt at multiple frequencies. Responses were plotted relative to stimulus position. A and B: individual Bode plots for each cell where responses to rotations were measured over at least a stimulus decade. Lines of different colors are used to designate each unit type, where red denotes cardiovascular units, yellow denotes respiratory units, green denotes GI units, blue denotes convergent units, and gray denotes unknown units. C and D: averages of the responses shown in A and B for the frequency range of 0.05–0.5 Hz; error bars designate means ± SE. The same colors were used to designate each unit type as in A and B. Since a Bode plot was generated for only one respiratory neuron, average data were not provided. Dashed lines in C and D indicate averaged data for all neurons. E and F: average Bode plots for 10 neurons with GI inputs whose responses to rotations were recorded before (solid lines) and after (dashed lines) the intragastric administration of copper sulfate.
Effects of intragastric copper sulfate infusion on the responses of NTS neurons to rotations in vertical planes.
The responses of 40 neurons to wobble stimuli were recorded before and after the intragastric administration of copper sulfate. The spontaneous activity of 28 of these neurons was altered >30% after copper sulfate infusion into the stomach; firing rate increased in half of the neurons and decreased in the other half. Injection of copper sulfate often produced a transient change in blood pressure, as illustrated in Fig. 1E. However, we classified a unit as receiving GI inputs only if a sustained alteration in firing rate was observed, which was independent of perturbations in blood pressure. The firing rates of the other 12 neurons, which included 7 cardiovascular, 2 convergent, and 3 unknown units, remained relatively stable after copper sulfate was delivered.
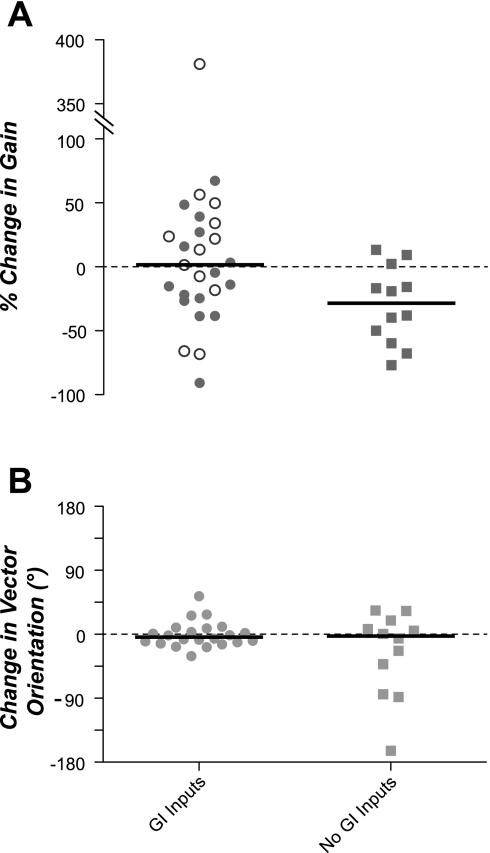
Fig. 7A shows the effects of irritating the stomach lining with copper sulfate on the gain of responses to wobble stimuli, which were delivered at the same frequency and amplitude in both sets of trials. The response gain for most neurons changed <30% after copper sulfate was administered (median of 27%), although the effects on a few cells were pronounced. The responsiveness to vestibular stimulation of approximately half of the NTS neurons with GI inputs increased after copper sulfate infusion, whereas the other half became less sensitive to whole body rotations. As such, the median percentage change in response gain for this population of neurons was very small: 5%. A Wilcoxon signed-rank test showed that this value was not significantly different from 0 (P = 0.94). Such variability in neuronal responses to repeated rotational stimulation is evident in the vestibular nuclei and is likely not biologically significant (34, 41). In contrast, the responsiveness to vestibular stimulation of NTS neurons without GI inputs typically decreased (median of 29%) after copper sulfate was administered. This value was significantly different from 0 (P < 0.01, Wilcoxon signed-rank test).
Fig. 7.
Effect of intragastric copper sulfate infusion on the gain of responses to wobble stimulation (A) and response vector orientation (B). In each panel, responses were segregated depending on whether a unit's spontaneous firing rate changed >30% after copper sulfate administration (GI inputs) or remained stable (no GI inputs). In A, ○ designate neurons with GI inputs whose firing rate increased after copper sulfate injection, whereas ● indicate neurons whose firing rate decreased. Horizontal lines designate median values.
Fig. 7B shows the effects of the intragastric administration of copper sulfate on the response vector orientations of NTS neurons. For neurons with GI inputs, the response vector orientations were nearly identical before and after copper sulfate was infused. The median change was 4°, while the response vector orientation of only one cell was altered more than 45°. The median change in response vector orientations for neurons lacking GI inputs was 3°, although three cells had variations >45°. The median value for neither population of cells was significantly different from 0 (P = 0.46 for neurons with GI inputs and P = 0.37 for neurons lacking GI inputs, Wilcoxon signed-rank test).
It was possible to hold 10 neurons with GI inputs long enough to generate Bode plots before and after the infusion of copper sulfate into the stomach. Copper sulfate administration had no appreciable effect on the response dynamics of the neurons (Fig. 6, E and F). Both prior to and following copper sulfate delivery, the response gain remained consistent across stimulus frequencies. The response phase was within 25° of stimulus position at all frequencies, both before and after copper sulfate administration.
DISCUSSION
This study was the first to characterize the responses of NTS neurons to whole-body tilts that activate vestibular receptors. It made use of rotations in vertical planes that stimulate both the anterior and posterior semicircular canals and otolith organs; the only vestibular end organs that were unaffected were the horizontal semicircular canals. Over one-third of NTS units responded to vertical vestibular stimulation, including those with inputs from baroreceptors activated during the cardiac cycle or by stretch of the carotid sinus, GI receptors activated by infusion of the emetic drug copper sulfate into the stomach, and pulmonary receptors activated by expansion of the chest cavity during artificial ventilation. The characteristics of the responses of most NTS neurons to labyrinthine stimulation were similar: they were typically most prominent during head movements in the sagittal plane, were aligned with head position in space (as opposed to the velocity of head movements), and were similar in magnitude when low- and high-frequency rotations were delivered. Such characteristics of responses to head movements are similar to those of otolith organ afferents (2, 22). As such, the firing rates of some NTS neurons are altered in accordance with head position during head-up or head-down rotations, presumably affecting the responses of the cells to visceral inputs.
The activities of both sympathetic nerves (57) and respiratory muscles (46) are modulated by vestibular inputs elicited by head movements in the transverse plane, which raises the possibility that NTS neurons are components of the neural pathways that mediate these responses. Furthermore, the dynamics of the responses of NTS neurons to vertical vestibular stimulation are similar to those of vestibulo-sympathetic reflexes (57). Neurons in the rostral ventrolateral medulla that relay labyrinthine inputs to sympathetic preganglionic neurons also have response dynamics that closely resemble those of NTS neurons (19). These findings suggest that NTS neurons may be components of the neural pathways that mediate vestibular influences on the sympathetic nervous system and blood pressure.
Another possibility is that the convergence of vestibular and baroreceptor signals on NTS neurons regulates the gain of the baroreceptor reflex. A recent study in conscious cats suggested that baroreceptor reflexes are amplified following the elimination of vestibular inputs, as indicated by the observation that rostral ventrolateral medulla (RVLM) neurons were more likely to exhibit cardiac related activity subsequent to a bilateral labyrinthectomy (8). An increase in the baroreceptor reflex gain following vestibular lesions would explain previous observations that instability in blood pressure during postural alterations dissipates within a week after the loss of labyrinthine inputs and vestibulo-sympathetic responses (33). Further studies are thus warranted to characterize the influences of the vestibular system on the processing of baroreceptor inputs by the brain stem.
This study also demonstrated that NTS neurons whose firing rate was altered by the intragastric infusion of copper sulfate responded to rotations in vertical planes. Since copper sulfate is an emetic compound (3, 12, 14, 20, 26, 27, 35, 50, 52), presumably some of these cells were components of the neural circuit that produces nausea and vomiting. As such, the present findings support older lesion studies that showed that motion sickness-related vomiting is mediated through vestibular inputs to NTS (11, 13, 23, 53). Contrary to our expectation, infusion of copper sulfate into the stomach did not appreciably alter the responses to vestibular stimulation of NTS neurons with GI inputs. Thus, the present findings do not support the hypothesis that emetic stimuli have additive effects on the activity of neurons that elicit nausea and vomiting, such that one stimulus increases a neuron's responsiveness to a second input. However, such interactions could occur downstream from NTS. Emesis can be evoked in animals with all parts of the nervous system removed except the medulla and spinal cord (38), whereas nausea is mediated through ascending projections from NTS to the parabrachial nucleus, hypothalamus, and forebrain structures (58). Since discrete neural projections from NTS likely are responsible for triggering nausea and vomiting, any interplay between emetic stimuli likely differs in the two pathways, because it is not evident in recordings from NTS neurons.
Since NTS neurons receive direct inputs from the caudal portion of the vestibular nucleus complex (5, 15, 45, 47, 56), it is reasonable to speculate that this projection mediates the responses of vestibular nucleus neurons to rotations in vertical planes. However, although some NTS neurons were activated at short latency by electrical stimulation of the vestibular nerve, the response latencies of other units were very long (56), suggesting that multisynaptic pathways additionally convey labyrinthine inputs to NTS. The parabrachial nucleus receives vestibular inputs (4, 6) and has interconnections with NTS (21, 24, 30), raising the possibility that the responses to body rotations recorded in this study were mediated in part through a vestibular nucleus-parabrachial-NTS pathway.
Copper sulfate infusion into the stomach attenuated the responses to vestibular stimulation of neurons whose spontaneous firing rate was unaffected by the emetic stimulus. This finding could be related to the noxious nature of copper sulfate, which likely evokes visceral pain along with nausea and vomiting, as evidenced by the observation that transient increases in blood pressure often occurred when copper sulfate was administered (see Fig. 1E). It is feasible that neurons comprising the multisynaptic pathways that convey vestibular signals to NTS were inhibited by these noxious inputs, thereby attenuating their responses to labyrinthine inputs. For example, noxious GI inputs affect the activity of parabrachial neurons (37), which may participate in transmitting vestibular signals to NTS. Additional experiments are needed to determine whether other noxious inputs also affect the responses of NTS neurons to vestibular inputs.
It seems unlikely that the blood pressure changes during copper sulfate infusion were primarily responsible for the alterations in firing rate of NTS neurons elicited by this stimulus. Most neurons whose firing rate changed >30% following the administration of copper sulfate were not responsive to mechanical stretch of the carotid sinus, which should have provided a powerful stimulus to baroreceptors, suggesting that these cells were insensitive to baroreceptor inputs. In addition, blood pressure alterations during copper sulfate infusion were small and transient, whereas the responses of neurons to this stimulus were sustained. Furthermore, even if a component of the responses to infusion of copper sulfate had been related to blood pressure alterations, our interpretation of the data would not have been substantially altered. This is because the firing rates of NTS neurons receiving a variety of types of visceral inputs were affected similarly by rotational stimuli that activated vestibular receptors.
It is also feasible that the responses of NTS neurons to whole-body rotations were due to activation of visceral afferents as the organs shifted in the body cavity. However, several lines of evidence argue against this possibility. First, small-amplitude rotations, which should have provided only a minimal stimulus to visceral afferents, were effective in modulating the activity of NTS neurons. Second, the responses of NTS neurons to body tilts were similar when low-frequency and high-frequency rotations were delivered. High-frequency tilts should have provided a weaker stimulus for visceral afferents than low-frequency rotations that better facilitated the shifting of the viscera in the body cavity. Third, neurophysiological studies employing electrical stimulation to excite vestibular afferents (56), as well as anatomical tracing experiments (5, 15, 45, 47, 56), have demonstrated that a substantial labyrinthine input is conveyed to NTS, which should provide for robust modulation of NTS unit activity during postural alterations. Another potential concern was that whole-body rotations altered blood distribution in the body and blood pressure, which resulted in baroreceptor-mediated changes in the activity of NTS neurons. Although large body movements produce such fluid shifts (54, 59), the small-amplitude rotations delivered in these experiments did not affect blood pressure, as evidenced in panel e of Fig. 5A, e and f of Fig. 5B. Therefore, it seems likely that the changes in activity of NTS neurons elicited by whole-body in this study resulted from the activation of vestibular afferents.
Perspectives and Significance
The data provided in this manuscript show that vestibular inputs elicited by head-up or head-down rotations of the body modulate the firing rates of some NTS neurons, including those with inputs from baroreceptors, pulmonary receptors, and GI receptors. The characteristics of these responses were similar to those of vestibulo-sympathetic (57) and vestibulo-respiratory responses (46), and to those of neurons in brain stem regions, such as the RVLM that convey labyrinthine inputs to sympathetic preganglionic neurons (19). As such, NTS neurons may contribute to producing vestibulo-sympathetic and vestibulo-respiratory responses. It is also possible that vestibular inputs to NTS subserve another role than modulating sympathetic and respiratory activity in accordance with body position in space, such as gating visceral inputs during movement and changes in posture. It has been suggested that visceral inputs play an important role in deciphering spatial orientation, and complement inputs from the inner ear, which can provide ambiguous cues about the location of the body in space and its trajectory (7). Thus, it is feasible that vestibular inputs to NTS amplify visceral signals when the visceral inputs are needed to track ongoing postural changes. Regardless of the functional relevance of labyrinthine inputs to NTS, future studies should consider that homeostasis is challenged during behaviors involving body motion in space, and that the detection and processing of visceral inputs can be altered in the context of movement. As such, studies of NTS neurons in awake, behaving animals may shed new insights into homeostatic regulation. In addition, experiments in human subjects should address whether changes in body position affect homeostatic responses following stimulation of visceral receptors.
GRANTS
This work was supported by Grants R01-DC00693 and R01-DC03732 from the National Institute on Deafness and Other Communication Disorders of the National Institutes of Heath (NIH). Core support was provided by National Institutes of Health Grant P30-DC05205.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Bret Boyle, Michael Catanzaro, Lucy Cotter, Kenneth Hobbs, Jonathan Janiczek, Daniel Miller, Sonya Puterbaugh, and Sarah Weber for assistance in data collection and analysis.
REFERENCES
- 1. Aftab S, Khan AB, Raza G. Assessment of risk factors for postoperative nausea and vomiting. J Coll Physicians Surg—Pakistan 18: 137–141, 2008 [PubMed] [Google Scholar]
- 2. Anderson JH, Blanks RHI, Precht W. Response characteristics of semicircular canal and otolith systems in the cat. I. Dynamic responses of primary vestibular fibers. Exp Brain Res 32: 491–507, 1978 [DOI] [PubMed] [Google Scholar]
- 3. Ariumi H, Saito R, Nago S, Hyakusoku M, Takano Y, Kamiya H. The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett 286: 123–126, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Balaban CD. Vestibular nucleus projections to the parabrachial nucleus in rabbits: Implications for vestibular influences on the autonomic nervous system. Exp Brain Res 108: 367–381, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Balaban CD, Beryozkin G. Vestibular nucleus projections to nucleus tractus solitarius and the dorsal motor nucleus of the vagus nerve: Potential substrates for vestibulo-autonomic interactions. Exp Brain Res 98: 200–212, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Balaban CD, McGee DM, Zhou J, Scudder CA. Responses of primate caudal parabrachial nucleus and Kolliker-Fuse nucleus neurons to whole body rotation. J Neurophysiol 88: 3175–3193, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Balaban CD, Yates BJ. Vestibulo-autonomic interactions: A teleologic perspective. In: Anatomy and Physiology of the Central and Peripheral Vestibular System, edited by Highstein SM, Fay RR, Popper AN. Heidelberg, Germany: Springer, 2004, p. 286–342 [Google Scholar]
- 8. Barman SM, Sugiyama Y, Suzuki T, Cotter LA, Destefino VJ, Reighard DA, Cass SP, Yates BJ. Rhythmic activity of neurons in the rostral ventrolateral medulla of conscious cats: effect of removal of vestibular inputs. Am J Physiol Regul Integr Comp Physiol 301: R937–R946, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berman AI. The Brain Stem of the Cat. Madison, WI: University of Wisconsin Press, 1968 [Google Scholar]
- 10. Boissonade FM, Davison JS. Effect of vagal and splanchnic nerve section on fos expression in ferret brain stem after emetic stimuli. Am J Physiol Regul Integr Comp Physiol 271: R228–R236, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Borison HL, Borison R. Motion sickness reflex arc bypasses the area postrema in cats. Exp Neurol 92: 723–737, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Bountra C, Bunce K, Dale T, Gardner C, Jordan C, Twissell D, Ward P. Anti-emetic profile of a non-peptide neurokinin NK1 receptor antagonist, CP-99,994, in ferrets. Eur J Pharmacol 249: R3–R4, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Brizzee K. The central nervous system connections involved in motion induced emesis. In: Motion and Space Sickness, edited by Crampton GH. Boca Raton, FL: CRC Press, 1990, p. 9–27 [Google Scholar]
- 14. Brizzee KR, Marshall KR. Developmental studies on emetic response to tartar emetic and copper sulfate in the cat. Proc Soc Exp Biol Med 103: 839–842, 1960 [DOI] [PubMed] [Google Scholar]
- 15. Cai YL, Ma WL, Li M, Guo JS, Li YQ, Wang LG, Wang WZ. Glutamatergic vestibular neurons express fos after vestibular stimulation and project to the NTS and the PBN in rats. Neurosci Lett 417: 132–137, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Carpenter DO. Neural mechanisms of emesis. Can J Physiol Pharmacol 68: 230–236, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Carter JR, Ray CA. Sympathetic responses to vestibular activation in humans. Am J Physiol Regul Integr Comp Physiol 294: R681–R688, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Cechetto DF. Central representation of visceral function. Fed Proc 46: 17–23, 1987 [PubMed] [Google Scholar]
- 19. Destefino VJ, Reighard DA, Sugiyama Y, Suzuki T, Cotter LA, Larson MG, Gandhi NJ, Barman SM, Yates BJ. Responses of neurons in the rostral ventrolateral medulla (RVLM) to whole-body rotations: Comparisons in decerebrate and conscious cats. J Appl Physiol 110: 1699–1707, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Endo T, Nemoto M, Minami M, Yoshioka M, Saito H, Parvez SH. Changes in the afferent abdominal vagal nerve activity induced by cisplatin and copper sulfate in the ferret. Biog Amine 11: 399–407, 1995 [Google Scholar]
- 21. Felder RB, Mifflin SW. Modulation of carotid sinus afferent input to nucleus tractus solitarius by parabrachial nucleus stimulation. Circ Res 63: 35–49, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol 39: 996–1008, 1976 [DOI] [PubMed] [Google Scholar]
- 23. Fox RA, Corcoran M, Brizzee KR. Conditioned taste aversion and motion sickness in cats and squirrel monkeys. Can J Physiol Pharmacol 68: 269–278, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res 319: 229–259, 1984 [DOI] [PubMed] [Google Scholar]
- 25. Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg 102: 1884–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Gardner CJ, Armour DR, Beattie DT, Gale JD, Hawcock AB, Kilpatrick GJ, Twissell DJ, Ward P. GR205171: A novel antagonist with high affinity for the tachykinin NK1 receptor, and potent broad-spectrum anti-emetic activity. Regul Pept 65: 45–53, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Gonsalves S, Watson J, Ashton C. Broad spectrum antiemetic effects of CP-122,721, a tachykinin NK1 receptor antagonist, in ferrets. Eur J Pharmacol 305: 181–185, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Grill HJ, Hayes MR. The nucleus tractus solitarius: A portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Intl J Obes 33 Suppl 1: S11–S15, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Fortuna MG, Kanbar R. Central CO2 chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol 108: 995–1002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Horn CC, Ciucci M, Chaudhury A. Brain fos expression during 48 h after cisplatin treatment: Neural pathways for acute and delayed visceral sickness. Auton Neurosci 132: 44–51, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ito H, Nishibayashi M, Maeda S, Seki M, Ebukuro S. Emetic responses and neural activity in young musk shrews during the breast-feeding/weaning period: Comparison between the high and low emetic response strains using a shaking stimulus. Exp Anim 54: 301–307, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ. Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J Appl Physiol 86: 1552–1560, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Jian BJ, Shintani T, Emanuel BA, Yates BJ. Convergence of limb, visceral, and vertical semicircular canal or otolith inputs onto vestibular nucleus neurons. Exp Brain Res 144: 247–257, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Lang IM, Sarna SK, Shaker R. Gastrointestinal motor and myoelectric correlates of motion sickness. Am J Physiol Gastrointest Liver Physiol 277: G642–G652, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol 443: 55–77, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Michl T, Jocic M, Schuligoi R, Holzer P. Role of tachykinin receptors in the central processing of afferent input from the acid-threatened rat stomach. Reg Peptides 102: 119–126, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Res 647: 255–264, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Miller AD, Ruggiero DA. Emetic reflex are revealed by expression of the immediate-early gene c-fos in the cat. J Neurosci 14: 871–888, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller AD, Umezaki T, Nakazawa K, Shiba K, Siniaia MS, Zheng Y. Recovery of retching after lesions involving the nucleus of the solitary tract. Neurosci Res 31: 77–80, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Miller DM, Cotter LA, Gandhi NJ, Schor RH, Cass SP, Huff NO, Raj SG, Shulman JA, Yates BJ. Responses of caudal vestibular nucleus neurons of conscious cats to rotations in vertical planes, before and after a bilateral vestibular neurectomy. Exp Brain Res 188: 175–186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller DM, Reighard DA, Mehta AS, Kalash R, Yates BJ. Responses of thoracic spinal interneurons to vestibular stimulation. Exp Brain Res 195: 89–100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Money KE. Motion sickness. Physiol Rev 50: 1–39, 1970 [DOI] [PubMed] [Google Scholar]
- 44. Onishi T, Mori T, Yanagihara M, Furukawa N, Fukuda H. Similarities of the neuronal circuit for the induction of fictive vomiting between ferrets and dogs. Auton Neurosci 136: 20–30, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Porter JD, Balaban CD. Connections between the vestibular nuclei and brain stem regions that mediate autonomic function in the rat. J Vestib Res 7: 63–76, 1997 [PubMed] [Google Scholar]
- 46. Rossiter CD, Hayden NL, Stocker SD, Yates BJ. Changes in outflow to respiratory pump muscles produced by natural vestibular stimulation. J Neurophysiol 76: 3274–3284, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Ruggiero DA, Mtui EP, Otake K, Anwar M. Vestibular afferents to the dorsal vagal complex: Substrate for vestibular-autonomic interactions in the rat. Brain Res 743: 294–302, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Schor RH, Miller AD, Tomko DL. Responses to head tilt in cat central vestibular neurons. I. Direction of maximum sensitivity. J Neurophysiol 51: 136–146, 1984 [DOI] [PubMed] [Google Scholar]
- 49. Thomas M, Woodhead G, Masood N, Howard R. Motion sickness as a predictor of postoperative vomiting in children aged 1–16 years. Paediatr Anaesth 17: 61–63, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Verbalis JG, Richardson DW, Stricker EM. Vasopressin release in response to nausea-producing agents and cholecystokinin in monkeys. Am J Physiol Regul Integr Comp Physiol 252: R749–R753, 1987 [DOI] [PubMed] [Google Scholar]
- 51. von Euler C, Hayward JN, Marttila I, Wyman RJ. Respiratory neurones of the ventrolateral nucleus of the solitary tract of cat: Vagal input, spinal connections and morphological identification. Brain Res 61: 1–22, 1973 [DOI] [PubMed] [Google Scholar]
- 52. Wang SC, Borison HL. Copper sulphate emesis; a study of afferent pathways from the gastrointestinal tract. Am J Physiol 164: 520–526, 1951 [DOI] [PubMed] [Google Scholar]
- 53. Wilpizeski CR, Lowry LD, Goldman WS. Motion-induced sickness following bilateral ablation of area postrema in squirrel monkeys. Laryngoscope 96: 1221–1225, 1986 [DOI] [PubMed] [Google Scholar]
- 54. Wilson TD, Cotter LA, Draper JA, Misra SP, Rice CD, Cass SP, Yates BJ. Vestibular inputs elicit patterned changes in limb blood flow in conscious cats. J Physiol 575: 671–684, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yates BJ, Bronstein AM. The effects of vestibular system lesions on autonomic regulation: Observations, mechanisms, and clinical implications. J Vestib Res 15: 119–129, 2005 [PubMed] [Google Scholar]
- 56. Yates BJ, Grelot L, Kerman IA, Balaban CD, Jakus J, Miller AD. Organization of vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem. Am J Physiol Regul Integr Comp Physiol 267: R974–R983, 1994 [DOI] [PubMed] [Google Scholar]
- 57. Yates BJ, Miller AD. Properties of sympathetic reflexes elicited by natural vestibular stimulation: Implications for cardiovascular control. J Neurophysiol 71: 2087–2092, 1994 [DOI] [PubMed] [Google Scholar]
- 58. Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: An update. Brain Res Bul 47: 395–406, 1998 [DOI] [PubMed] [Google Scholar]
- 59. Yavorcik KJ, Reighard DA, Misra SP, Cotter LA, Cass SP, Wilson TD, Yates BJ. Effects of postural changes and removal of vestibular inputs on blood flow to and from the hindlimb of conscious felines. Am J Physiol Regul Integr Comp Physiol 297: R1777–R1784, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zimnicka AM, Ivy K, Kaplan JH. Acquisition of dietary copper: a role for anion transporters in intestinal apical copper uptake. Am J Physiol Cell Physiol 300: C588–C599, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]