Abstract
Spinal cord injury (SCI) has serious long-term consequences on sympathetic cardiovascular regulation. Orthostatic intolerance results from insufficient baroreflex regulation (BR) of sympathetic outflow to maintain proper blood pressure upon postural changes. Autonomic dysreflexia occurs due to insufficient inhibition of spinal sources of sympathetic activity. Both of these conditions result from the inability to control sympathetic activity caudal to SCI. It is well established that limited motor ability recovers after incomplete SCI. Therefore, the goal of this study was to determine whether recovery of BR occurs after chronic, left thoracic spinal cord hemisection at either T3 or T8. Baroreflex tests were performed in rats by measuring the reflex response of left (ipsilateral) renal sympathetic nerve activity to decreases and increases in arterial pressure produced by ramped infusions of sodium nitroprusside and phenylephrine, respectively. One week after a T3 left hemisection, BR function was modestly impaired. However, 8 wk after a T3 left hemisection, BR function was normal. One week after a T8 left hemisection, BR function was significantly impaired, and 8 wk after a T8 left hemisection, BR function was significantly improved. These results indicate that BR of renal sympathetic nerve activity in rats may partially recover after spinal cord hemisections, becoming normal by 8 wk after a T3 lesion, but not after a T8 lesion. The nature of the spinal cord and/or brain stem reorganization that mediates this recovery remains to be determined.
Keywords: cardiovascular regulation, spinal cord injury, baroreflex, orthostatic hypotension, autonomic dysreflexia
cardiovascular dysregulation after spinal cord injury (SCI) manifests itself as both orthostatic hypotension (1, 6, 18, 22, 32) and autonomic dysreflexia with hypertensive crisis (13, 18, 23, 27, 33, 34). Orthostatic hypotension, following SCI, is caused by insufficient sympathetic outflow to maintain proper arterial pressure (AP) upon postural changes (1, 6, 18, 22, 32). Autonomic dysreflexia results from diminished suppression of spinally generated sympathetic activity caudal to the SCI site (13, 18, 23, 27, 33, 34).
The principal mechanism for the short-term stability of AP is the arterial baroreceptor modulation of sympathetic and parasympathetic activity (10, 15). After SCI, the destruction of descending excitatory pathways from medullary and supramedullary sites reduces or eliminates the drive to sympathetic preganglionic neurons (SPN) necessary to maintain sufficient reflex control of AP. In addition to the dizziness, nausea, and fatigue, caused by severe hypotension (7, 18), another complication of orthostatic intolerance is the inability to participate fully in rehabilitation, thereby impeding recovery (17). Additionally, the loss of tonic, descending, baroreflex regulation (BR)-independent, inhibitory systems that suppress ongoing spinally generated sympathetic activity may increase activity of SPN. This can result in autonomic dysreflexia and potentially hypertensive crisis.
Regeneration of injured central nervous system neurons is severely limited. Nevertheless, over time, animals regain some degree of motor ability after incomplete spinal lesions (3, 8, 14, 26, 35). The mechanisms responsible for this partial restoration of function are not fully known. That the degree of recovery depends on the extent of spared descending axons is clear. Recovery is also attributed to collateral sprouting and reorganization of descending and propriospinal connections. Indeed, significant structural reorganization and increased axonal connections of the corticospinal tract (CST) axons have been observed after spinal lesions in rats and mice (3, 8, 14, 30).
Within 1 wk after spinal hemisection or complete spinal transection in the rat, atrophy of SPN occurs (19, 21). However, within 1 mo after these lesions, the SPN appear morphologically restored (19). The time course of the initial lesion-induced hypotension, followed by the onset of autonomic dysreflexia, correlates with the implied reduction and restoration, respectively, of inputs to SPN (20). Thus reduction of spinal cord connections following SCI may contribute to aberrant cardiovascular regulation. Although the morphology of the SPN caudal to the spinal lesion recovers, the extent of the recovery of descending bulbospinal connections to the SPN for proper BR of sympathetic activity is not known.
While progress in understanding mechanisms mediating the restoration of motor and sensory function has been made, far less is known regarding restoration of sympathetic control after SCI. The objective of this study was to determine whether baroreflex function improved after chronic ipsilateral hemisection in rats and whether the extent of recovery depended on the rostrocaudal location of the lesion. To do this, we measured BR responsiveness of left renal sympathetic nerve activity (RSNA) in rats 1 or 8 wk after either a T3 or T8 left hemisection or sham lesion.
METHODS
Adult, female, Sprague-Dawley rats (Charles River, Raleigh, NC), weighing between 125 and 350 g, were surgically prepared in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) using procedures approved by the Johns Hopkins University Committee on Animal Care and Use.
Chronic spinal lesions and surgical preparations.
For chronic lesion experiments, we anesthetized rats, first in a plastic chamber, and then with a nose cone, using 2% isoflurane in O2. Before chronic spinal cord hemisection surgery, we cleaned the dorsal surface of the rat with betadine. We made a 2-cm cutaneous incision centered on the vertebra overlying the site of the intended lesion (T3 or T8). The T3 spinal level underlies the T2 vertebrae, and the T8 spinal level underlies the T6 and T7 vertebrae. We used a small retractor to maintain a clear surgical field. We removed the paraspinal muscles from the dorsal surface of the exposed vertebrae using a septal elevator. After the dorsal surfaces of the vertebrae were free of muscle and connective tissue, we removed the dorsal portion of the vertebra of choice using a microrongeur to access the underlying spinal segment. We opened the dura with dura scissors, and cut the left hemisphere of the spinal cord with a 1-mm sapphire blade microknife (World Precision Instruments, Sarasota, FL). We closed the dorsal musculature and skin with sterile sutures. We used an identical technique for sham-lesioned rats, except that we did not open the dura. We manually expressed rat's bladders, when necessary, following the surgery. We monitored food and water intake daily, and saline was administered subcutaneously as needed.
For acute baroreflex experiments, we initially anesthetized rats with isoflurane, as previously described. We discontinued isoflurane after administration of α-chloralose (100 mg/kg iv, Sigma) via a left jugular cannula. This cannula was also used to administer gallamine triethiodide (see below). We maintained the depth of anesthesia at a surgical plane by supplemental doses of α-chloralose (25 mg/kg). We determined the depth of anesthesia either by corneal reflexes before and during the recovery from paralysis, or by the variability of RSNA and AP when rats were paralyzed. We monitored body temperature with a rectal probe and maintained it between 35 and 37°C with a heating pad and lamp. We cannulated the trachea for mechanical ventilation using a rodent ventilator (CWE, Ardmore, PA). We cannulated the right femoral artery for measurement of AP. We recorded AP and heart rate (HR) simultaneously with Cambridge Electronic Design Micro1401 hardware and Spike 2 software. We cannulated the left and right femoral veins for the separate administration of depressor and pressor drugs, respectively.
RSNA recording.
Preparation for RSNA recording has been described elsewhere in detail (5). We approached the left kidney via a left flank laparotomy and dissected the adrenal gland, the fat covering the psoas muscle, and the paraspinal muscles from the renal nerve, which typically was located at the junction of the aorta and the renal artery, or was found traversing the aorta. We carefully dissected the renal nerve from the renal vasculature and surrounding tissue with the aid of an operating microscope. Then we immersed the renal nerve in mineral oil and mounted it on a bipolar hook electrode connected to a differential amplifier with a band pass of 300–3,000 Hz. We further processed sympathetic activity by rectification and low-pass filtering at a time constant of 0.5 s and recorded both the unprocessed and the rectified/filtered activity with the AP and HR. We cut the distal end of the renal nerve to avoid recording afferent activity.
Baroreflex measurement.
We obtained baroreflex function curves by plotting the reflex change in RSNA to increases and decreases in AP caused by the vasodilator sodium nitroprusside (SNP, 50 μg/ml) and the α-adrenergic agonist phenylephrine (PE, 125 μg/ml), respectively, in successive ramped infusions. We administered SNP first, beginning at a rate of 2.5 ml/h, and increased the rate by 2.5 ml/h every 30 s until an AP of 60 mmHg below baseline or a maximum rate of 25 ml/h was reached. Following SNP administration, we administered PE beginning at a rate of 2.5 ml/h and increasing the rate by 2.5 ml/h every 30 s. These infusions produced an approximately linear increase in AP from 60 mmHg below baseline AP to 60 mmHg above baseline AP at a rate of ∼1.5 mmHg/s. We analyzed the RSNA from the SNP-induced nadir (60 mmHg below baseline) in AP to the PE-induced peak AP (60 mmHg above baseline). We fit baroreflex function curves to a sigmoidal function (24). We used the maximum slope of the sigmoidal curves as our measurement of the gain of the baroreflex.
Histology.
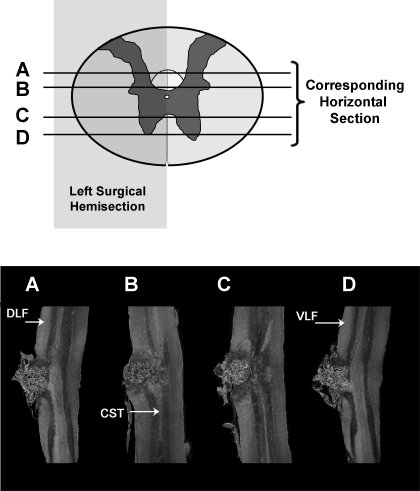
At the end of experiments, we perfused rats transcardially with buffered saline (pH 7.4), followed by 4% buffered paraformaldehyde (pH 7.4). We removed spinal cords and postfixed them in formaldehyde solution overnight. After cryoprotection in 30% sucrose for 48 h, we cut 40-μm serial, horizontal sections on a sliding microtome and mounted them on gelatin-coated glass slides. We examined lesions microscopically to verify the completeness of the hemisections (Fig. 1).
Fig. 1.
Histological horizontal sections of a representative left hemisection (T8). Horizontal lines represent the horizontal plane of the respective histological sections. DLF, dorsolateral funiculi; VLF, ventrolateral funiculi; CST, corticospinal tract. The left hemisection completely destroyed the DLF and VLF.
Data analysis.
We fit each RSNA response to changes in AP to a sigmoidal or linear function using Prism software (Graphpad, version 4.0). The sigmoidal function was described by the following equation
where y is the RSNA, x is AP, A1 is the range of RSNA, A2 is the gain coefficient, A3 is the value of x at the midpoint, and A4 is the minimum RSNA of the reflex curve (24). For one rat, a linear function was best fit to the BR function curve. We calculated the maximum gain and change in RSNA according to each fitted curve.
To construct grouped baroreflex curves, we averaged AP and corresponding RSNA data into 10-mmHg bins. To account for variations in baseline AP, we used the change from baseline as the reference for the AP bins when constructing the baroreflex curves. For the baroreflex curves, the data are expressed as mean change in AP (in mmHg) from baseline and the percent change (%Δ) in RSNA. Stable plateau values were determined when RSNA for a particular 10-mmHg AP bin did not vary by >5% from the previous RSNA value in that 10-mmHg AP bin. For the maximum RSNA plateau, this typically occurred at −50 mmHg, and for the minimum RSNA plateau at 30 mmHg. The grouped data are expressed as means ± SE. Statistical analyses employed one-way ANOVA (with Tukey's posttests). Values of P < 0.05 were considered significant.
RESULTS
Baroreflex function after chronic T8 left spinal hemisection.
In sham-lesioned rats (n = 10), mean baseline AP was 132 ± 4 mmHg, and mean HR was 470 ± 10 beats/min. In rats with T8 left hemisection (n = 9), 1 wk after lesion the mean baseline AP was 115 ± 4 mmHg and significantly reduced (P < 0.05) compared with that of sham-lesioned rats. However, HR was not significantly affected by these lesions (473 ± 16 beats/min). In another group of rats with T8 left hemisection, 8 wk after lesion, neither the mean baseline AP (124 ± 3 mmHg, n = 9) nor the mean baseline HR (411 ± 17 beats/min) was significantly different from that of sham-lesioned rats.
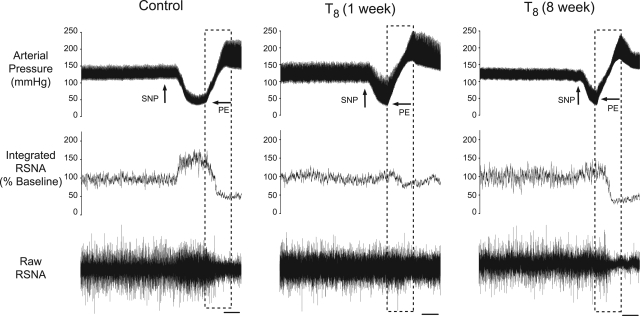
Representative tracings of baseline AP, ongoing RSNA, and baroreflex responses in a sham-lesioned rat and in T8 left-hemisectioned rats after either 1 or 8 wk are shown in Fig. 2. The ability to increase RSNA upon decreased AP was significantly impaired in both groups (Fig. 3, A and C, P < 0.05). In sham-lesioned rats, a 60-mmHg decrease in AP increased RSNA to a maximum plateau of 170 ± 11% of baseline RSNA. One week after a T8 left hemisection, a 60-mmHg decrease in AP produced an increase in RSNA to a maximum plateau of 119 ± 9% of baseline RSNA. In the group of rats in which BR testing was performed 8 wk after a T8 left hemisection, a 60-mmHg decrease in AP produced an increase in RSNA to a maximum plateau of 129 ± 9%.
Fig. 2.
Representative tracings from rats in which baroreflex regulation (BR) testing was performed after either sham lesion, 1 wk following T8 left hemisection, or 8 wk following a T8 left hemisection. Tracings show the baseline arterial pressure (AP), left renal sympathetic nerve activity (RSNA), and the BR response in RSNA. Changes in AP for BR were induced by intravenous infusion of sodium nitroprusside (SNP) and phenylephrine (PE). Dashed boxes indicate the corresponding AP and RSNA used for baroreflex quantification. Scale bar = 30 s.
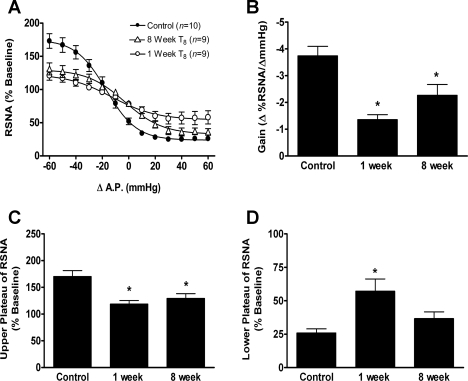
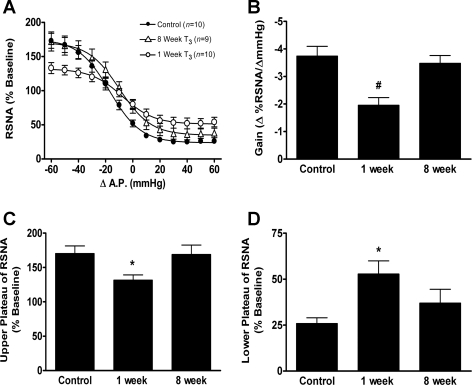
Fig. 3.
Grouped data showing the effect of T8 left hemisection (or sham lesion, n = 10) after either 1 wk (n = 9) or 8 wk (n = 9) on baroreflex relationship on left RSNA (A), maximal gain of the baroreflex response (B), maximum plateau of RSNA during baroreflex testing (C), and minimum plateau of RSNA during baroreflex testing (D). The RSNA responses were measured as percent change (%Δ) from the respective baseline RSNA before baroreflex testing. Values are means ± SE. *Significantly different from control, P < 0.05.
The ability to decrease RSNA upon increases in AP was significantly impaired in rats in which BR tests were performed 1 wk, but not 8 wk, after T8 left hemisection compared with sham-lesioned rats (Fig. 3, A and D, P < 0.05). In sham-lesioned rats, a 60-mmHg increase in AP decreased RSNA to a minimum plateau of 26 ± 3% of baseline RSNA. One week after a T8 left hemisection, a 60-mmHg increase in AP decreased RSNA to a minimum plateau of 57 ± 9% of baseline RSNA. In the group of rats in which BR testing was performed 8 wk after T8 left hemisection, a 60-mmHg increase in AP decreased RSNA to a minimum plateau of 37 ± 5% of baseline RSNA, not significantly different from that of sham-lesion rats (26 ± 3%, P > 0.05). Thus, although 8 wk after T8 left hemisection the ability to increase RSNA at decreased AP had not changed, the ability to decrease RSNA at elevated AP had improved.
One week after a T8 left hemisection, the maximum gain of the BR was significantly decreased compared with that of the sham-lesion rats and did not improve after 8 wk (Fig. 3B). In sham-lesion rats, the mean maximum BR gain was −3.7 ± 0.4% ΔRSNA/ΔAP. In the group of rats in which BR tests were performed 1 wk after T8 left hemisection, the mean maximum BR gain was −1.4 ± 0.2% ΔRSNA/ΔAP, and in the group of rats in which BR tests were performed 8 wk after T8 left hemisection, the mean maximum BR gain was −2.3 ± 0.4% ΔRSNA/ΔAP.
Baroreflex function after chronic T3 left spinal hemisection.
In rats with T3 left hemisection (n = 10), 1 wk after lesion, the mean baseline AP was 118 ± 4 mmHg, not significantly different compared with that of sham-lesioned rats (132 ± 4 mmHg, P > 0.05). HR was not significantly affected by these lesions (482 ± 20 beats/min). In another group of rats with T3 left hemisection, 8 wk after lesion, neither the mean baseline AP (125 ± 6 mmHg, n = 9) nor the mean baseline HR (471 ± 24 beats/min) was significantly different from that of sham-lesioned rats.
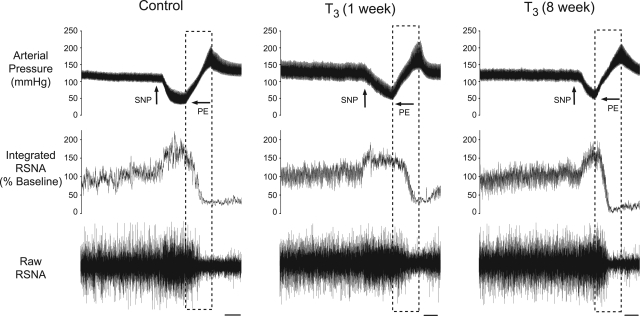
Representative tracings of baseline AP, ongoing RSNA, and baroreflex responses in a sham-lesioned rat and in T3 left-hemisectioned rats after either 1 or 8 wk are shown in Fig. 4. The ability to increase RSNA upon decreased AP was significantly impaired in the group of rats in which BR testing was performed 1 wk after T3 left hemisection, compared with responses in sham-lesioned rats (Fig. 5, A and C, P < 0.05). One week after a T3 left hemisection, a 60-mmHg decrease in AP produced an increase in RSNA to a maximum plateau of 132 ± 8% of baseline RSNA. Eight weeks after a T3 left hemisection, the ability to increase RSNA upon decreases in AP had improved. In this group of rats, a 60-mmHg decrease in AP produced an increase in RSNA to a maximum plateau of 169 ± 14% and was not significantly different from that of the sham-lesioned rats (170 ± 11%, P > 0.05).
Fig. 4.
Representative tracings from rats in which BR testing was performed after either sham lesion, 1 wk following T3 left hemisection, or 8 wk following a T3 left hemisection. Tracings show the baseline AP, left RSNA, and the BR response in RSNA. Changes in AP for BR were induced by intravenous infusion of SNP and PE. Dashed boxes indicate the corresponding AP and RSNA used for baroreflex quantification. Scale bar = 30 s.
Fig. 5.
Grouped data showing the effect of T3 left hemisection (or sham lesion, n = 10) after either 1 wk (n = 10) or 8 wk (n = 9) on baroreflex relationship on left RSNA (A), maximal gain of the baroreflex response (B), maximum plateau of RSNA during baroreflex testing (C), and minimum plateau of RSNA during baroreflex testing (D). The RSNA responses were measured as %Δ from the respective baseline RSNA before baroreflex testing. Values are means ± SE. Significantly different from *control, and #both control and 8 wk: P < 0.05.
The ability to decrease RSNA upon increases in AP was significantly impaired in rats in which BR tests were performed 1 wk, but not 8 wk, after T3 left hemisection compared with that of sham-lesioned rats (Fig. 5, A and D, P < 0.05). One week after a T3 left hemisection, a 60-mmHg increase in AP decreased RSNA to a minimum plateau of 53 ± 7% of baseline RSNA. In the group of rats in which BR testing was performed 8 wk after T3 left hemisection, a 60-mmHg increase in AP decreased RSNA to a minimum plateau of 37 ± 8% of baseline RSNA and was not significantly different from that of sham-lesion rats (26 ± 3%, P > 0.05). Thus 8 wk after T3 left hemisection, the ability to both increase RSNA at decreased AP and the ability to decrease RSNA at elevated AP had improved.
One week after a T3 left hemisection, the maximum gain of the BR was significantly decreased compared with that of the sham-lesion rats and had significantly improved after 8 wk (Fig. 5B). In the group of rats in which BR tests were performed 1 wk after T3 left hemisection, the mean maximum BR gain was −2.0 ± 0.3% ΔRSNA/ΔAP, and this was significantly decreased compared with that of sham-lesioned rats (−3.7 ± 0.4% ΔRSNA/ΔAP, P < 0.05). In the group of rats in which BR tests were performed 8 wk after T3 left hemisection, the mean maximum BR gain was −3.5 ± 0.3% ΔRSNA/ΔAP and significantly (P < 0.05) greater than mean BR gain of rats 1 wk after T3 left hemisection.
DISCUSSION
In this study, we show that recovery of BR function after chronic spinal cord lesions occurs in the rat, and that the degree of recovery depends on both the recovery time and the rostrocaudal location of the lesion. We observed modest BR impairment in rats 1 wk after an upper thoracic (T3) left hemisection and complete recovery in rats 8 wk after this lesion. In rats with midthoracic (T8) hemisection, BR was profoundly impaired after 1 wk and modestly, yet significantly, improved after 8 wk. Thus this study provides important new physiological data to show that, similar to motor and sensory function, sympathetic cardiovascular control of AP also has the potential to improve after SCI.
The spontaneous recovery of sensory and motor function after SCI has been well documented in the rat and mouse (2, 3, 8, 14, 35). After spinal transection in the rat, the CST collateralizes extensively (3, 11, 16, 28, 35). Restoration of function is attributed not only to this neuronal sprouting, but also to the reorganization of new and spared spinal connections, leading to “rerouting” of pathways (3, 8, 31). For example, 1 wk after spinal hemisection, ipsilateral retrograde labeling of spinally projecting brain stem neurons is significantly diminished. However, after 10 wk, brain stem labeling was completely restored, and propriospinal labeling was increased (8). The restoration of brain stem labeling was attributed to the reorganization of long and short propriospinal connections. Hindlimb electromyograph recordings in the rat showed a restoration of cortical stimulated activity 12 wk after a CST lesion (3). New contacts of sprouting CST axons onto long descending propriospinal neurons in the upper cervical spinal cord are thought to bridge across the lesion site, thereby improving motor function (3, 8, 9).
In this study, we measured the BR responsiveness of left RSNA after a mid- or upper thoracic left hemisection. Thus we refer to the left spinal pathways as “ipsilateral”. In rats with these lesions, BR modulation of sympathetic activity after spinal hemisection was derived in one of two possible ways. The first is via descending contralateral (right-sided) axons that cross the midline caudal to the hemisection. The second possible way is via collaterals of descending ipsilateral axons that cross the midline rostral to the hemisection to synapse on contralateral neurons, or synaptic antecedents to SPN. These axons then recross the midline caudal to the hemisection to synapse on SPN. Our laboratory has previously shown that the pathways responsible for BR-induced increases in RSNA at reduced AP descend bilaterally, and the pathways responsible for BR-independent, tonic inhibition of ongoing spinally generated sympathetic activity mainly descend ipsilaterally (36). In that study, we found that, after an acute T8 left hemisection, rats retained the ability to increase left RSNA upon decreases in AP. However, we also found that the ability to decrease RSNA at elevated AP was attenuated, and that this was due to reduced descending tonic inhibition of ongoing, spinally generated RSNA.
One week after a T8 left hemisection, BR-induced increases in RSNA were profoundly impaired and did not significantly improve even after 8 wk. Thus, although in the previous study acute left hemisection at T8 did not significantly affect increases in RSNA at decreased AP, we now show that the BR-mediated increases in RSNA at low AP apparently deteriorate within the first week following the lesion, and after 8 wk the BR-mediated increase in RSNA remains attenuated.
We also observed modest impairment of the BR regulation of RSNA 1 wk after a T3 left hemisection and, in this case, full recovery after 8 wk. Although we have previously shown in rats with acute spinal lesions that the pathways responsible for the tonic inhibition of spinal sympathetic activity are primarily ipsilateral, data from the present experiments provide evidence that contralateral pathways responsible for descending inhibition, as well as descending excitation exist, and that these pathways cross the cord caudal to T3.
Anatomic data suggest that neurons in the rostroventrolateral medulla (RVLM) project bilaterally to the intermediolateral cell column of the spinal cord with an ipsilateral predominance (4, 25), and crossing occurs mainly at the level of the spinal cord (29). However, whereas pathways from RVLM to the superior cervical ganglion cells are bilateral, pathways to adrenal medulla are ipsilateral (4, 25). Although histological data suggest distinct differences in laterality, glutamate microinjection into the RVLM elicits identical adrenal nerve and superior cervical nerve responses, regardless of laterality, and this is attributed to the likelihood that the pathways are polysynaptic (25). While pharmacological activation of the brain stem nuclei and transynaptic tracer studies provides solid evidence that networks of crossing axons for the regulation of sympathetic activity exist, it does not necessarily prove that the pathways are of functional significance in the intact rat.
Acute T8 left hemisection has been shown to disinhibit tonic, spinally generated RSNA (36), and we now show that this impairment persists for at least 1 wk after a T8 lesion. However, because we also observed significantly improved inhibition of RSNA at increased AP in rats 8 wk after T8 left hemisection, crossed inhibitory pathways may descend in the intact spinal cord below T8, but their effect is too weak to significantly suppress the ongoing, contralateral, spinally generated sympathetic activity. Thus the improvement of inhibition of RSNA at elevated AP 8 wk after T8 left hemisection may be mediated by a strengthening of existing descending inhibitory pathways that are, indeed, bilateral and cross the spinal cord caudal to the lesion. Another, less likely explanation for the improvement in the ability to suppress spinal activity is that descending, ipsilateral axons cross and create new synapses on contralateral interneurons located rostral to the hemisection. The axons of these interneurons could then recross the spinal cord caudal to the hemisection to affect ipsilateral RSNA.
Although descending, sympathoexcititory RVLM pathways are bilateral, and we have shown that the BR-induced excitation of RSNA at reduced AP is mediated bilaterally as well, the organization of pathways involved in descending inhibition of tonic spinally generated sympathetic activity (and, therefore, the level of sympathetic inhibition experienced at elevated AP) is not well known. Because our laboratory has previously shown that acute, contralateral hemisections (right side) fail to affect the overall changes in left RSNA upon changes in AP (36), the crossing axons likely play a minor role in BR-mediated maximum increases and decreases in RSNA in the intact rat. Thus contralateral descending inhibitory pathways are most likely present before spinal injury, although the contribution to sympathetic regulation is significant only after impairment of the main ipsilateral pathway.
Perspectives and Significance
This study suggests that partial recovery of sympathetically regulated cardiovascular function is possible after SCI. Although modest improvement of motor and sensory function has been shown in rats and mice, data on the extent of, or even the possibility of, recovery of cardiovascular regulation are limited. The locations of spinal cord lesions have a direct consequence on the severity of orthostatic hypotension and/or autonomic dysreflexia after SCI (12). Thus this study provides important new physiological information showing that, like somatomotor and somatosensory systems, plasticity of spinal sympathetic systems also exists. It will be important to develop treatments that can augment the plasticity of sympathetic systems. Additionally, it will also be important to determine whether treatments already shown to improve somatomotor and somatosensory function also improve the recovery of sympathetic cardiovascular function after SCI.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant HL-16315 (to L. P. Schramm). M. R. Zahner was supported by NHLBI Training Grant 2-T32-HL-007581.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Aslan SC, Randall DC, Donohue KD, Knapp CF, Patwardhan AR, McDowell SM, Taylor RF, Evans JM. Blood pressure regulation in neurally intact human vs. acutely injured paraplegic and tetraplegic patients during passive tilt. Am J Physiol Regul Integr Comp Physiol 292: R1146–R1157, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci 23: 1988–1996, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 7: 269–277, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: implications for the central control of cardiovascular regulation. J Comp Neurol 499: 840–859, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chau D, Kim N, Schramm LP. Sympathetically correlated activity of dorsal horn neurons in spinally transected rats. J Neurophysiol 77: 2966–2974, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma 23: 1713–1725, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Claydon VE, Steeves JD, Krassioukov A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 44: 341–351, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14: 69–74, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley KC, Zaporozhets E, Schmidt BJ. Propriospinal neurons are sufficient for bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J Physiol 586: 1623–1635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dampney RA, Coleman MJ, Fontes MA, Hirooka Y, Horiuchi J, Li YW, Polson JW, Potts PD, Tagawa T. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol 29: 261–268, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Fouad K, Pedersen V, Schwab ME, Brosamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol 11: 1766–1770, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Furlan JC, Fehlings MG, Shannon P, Norenberg MD, Krassioukov AV. Descending vasomotor pathways in humans: correlation between axonal preservation and cardiovascular dysfunction after spinal cord injury. J Neurotrauma 20: 1351–1363, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Gao SA, Ambring A, Lambert G, Karlsson AK. Autonomic control of the heart and renal vascular bed during autonomic dysreflexia in high spinal cord injury. Clin Auton Res 12: 457–464, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Gulino R, Dimartino M, Casabona A, Lombardo SA, Perciavalle V. Synaptic plasticity modulates the spontaneous recovery of locomotion after spinal cord hemisection. Neurosci Res 57: 148–156, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Heymans C, Neil E. Reflexogenic Areas of the Cardiovascular System. Boston, MA: Little, Brown, 1958 [DOI] [PubMed] [Google Scholar]
- 16.Hill CE, Beattie MS, Bresnahan JC. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol 171: 153–169, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Illman A, Stiller K, Williams M. The prevalence of orthostatic hypotension during physiotherapy treatment in patients with an acute spinal cord injury. Spinal Cord 38: 741–747, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Krassioukov A, Claydon VE. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog Brain Res 152: 223–229, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Krassioukov AV, Weaver LC. Morphological changes in sympathetic preganglionic neurons after spinal cord injury in rats. Neuroscience 70: 211–225, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Krenz NR, Weaver LC. Changes in the morphology of sympathetic preganglionic neurons parallel the development of autonomic dysreflexia after spinal cord injury in rats. Neurosci Lett 243: 61–64, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Llewellyn-Smith IJ, Weaver LC. Changes in synaptic inputs to sympathetic preganglionic neurons after spinal cord injury. J Comp Neurol 435: 226–240, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Mathias CJ. Orthostatic hypotension and paroxysmal hypertension in humans with high spinal cord injury. Prog Brain Res 152: 231–243, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Mayorov DN, Adams MA, Krassioukov AV. Telemetric blood pressure monitoring in conscious rats before and after compression injury of spinal cord. J Neurotrauma 18: 727–736, 2001 [DOI] [PubMed] [Google Scholar]
- 24.McDowall LM, Dampney RA. Calculation of threshold and saturation points of sigmoidal baroreflex function curves. Am J Physiol Heart Circ Physiol 291: H2003–H2007, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Moon EA, Goodchild AK, Pilowsky PM. Lateralisation of projections from the rostral ventrolateral medulla to sympathetic preganglionic neurons in the rat. Brain Res 929: 181–190, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Mountney A, Zahner MR, Lorenzini I, Oudega M, Schramm LP, Schnaar RL. Sialidase enhances recovery from spinal cord contusion injury. Proc Natl Acad Sci U S A 107: 11561–11566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osborn JW, Taylor RF, Schramm LP. Chronic cervical spinal cord injury and autonomic hyperreflexia in rats. Am J Physiol Regul Integr Comp Physiol 258: R169–R174, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Pan B, Kim EJ, Schramm LP. Increased close appositions between corticospinal tract axons and spinal sympathetic neurons after spinal cord injury in rats. J Neurotrauma 22: 1399–1410, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Shahar T, Palkovits M. Cross over of forebrain and brainstem neuronal projections to spinal cord sympathetic preganglionic neurons in the rat. Stress 10: 145–152, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Soares S, Barnat M, Salim C, von Boxberg Y, Ravaille-Veron M, Nothias F. Extensive structural remodeling of the injured spinal cord revealed by phosphorylated MAP1B in sprouting axons and degenerating neurons. Eur J Neurosci 26: 1446–1461, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Stelzner DJ, Cullen JM. Do propriospinal projections contribute to hindlimb recovery when all long tracts are cut in neonatal or weanling rats? Exp Neurol 114: 193–205, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil 81: 506–516, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Weaver LC, Marsh DR, Gris D, Brown A, Dekaban GA. Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention. Prog Brain Res 152: 245–263, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Weaver LC, Verghese P, Bruce JC, Fehlings MG, Krenz NR, Marsh DR. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J Neurotrauma 18: 1107–1119, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A 98: 3513–3518, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahner MR, Schramm LP. Spinal regions involved in baroreflex control of renal sympathetic nerve activity in the rat. Am J Physiol Regul Integr Comp Physiol 300: R910–R916, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]