Abstract
Hypercholesterolemia has been suggested to have direct negative effects on myocardial function due to increased reactive oxygen species (ROS) generation and increased myocyte death. Mitochondrial permeability transition (MPT) is a significant mediator of cell death, which is enhanced by ROS generation and attenuated by exercise training. The purpose of this study was to investigate the effect of hypercholesterolemia on the MPT response of cardiac mitochondria. We tested the hypothesis that familial hypercholesterolemic (FH) pigs would have an enhanced MPT response and that exercise training could reverse this phenotype. MPT was assessed by mitochondrial swelling in response to 10–100 μM Ca2+. FH pigs did show an increased MPT response to Ca2+ that was associated with decreases in the expression of the putative MPT pore components mitochondrial phosphate carrier (PiC) and cyclophilin-D (CypD). FH also caused increased oxidative stress, depicted by increased protein nitrotyrosylation, as well as decreased levels of reduced GSH in cardiac mitochondria. Expression of the mitochondrial antioxidant enzymes manganese superoxide dismutase (MnSOD), thioredoxin-2 (Trx2), and peroxiredoxin-3 (Prx3) was greatly reduced in the FH pigs. In contrast, cytosolic catalase expression and activity were increased. However, chronic exercise training was able to normalize the MPT response in FH pigs, reduce mitochondrial oxidative stress, and return MnSOD, Trx2, Prx3, and catalase expression/activities to normal. We conclude that FH reduces mitochondrial antioxidants, increases mitochondrial oxidative stress, and enhances the MPT response in the porcine myocardium, and that exercise training can reverse these detrimental alterations.
Keywords: familial hypercholesterolemia, exercise training, antioxidant proteins
hypercholesterolemia is widely accepted as a significant risk factor for coronary artery disease. However, more recently hypercholesterolemia has also been suggested to have direct negative effects on the myocardium itself, with several studies demonstrating increased myocardial injury and decreased cardiac function following ischemia/reperfusion (IR) injury in hypercholesterolemic animals (11, 20, 43, 49). However, the molecular mechanisms by which chronically elevated cholesterol can detrimentally affect the cardiomyocyte have yet to be elucidated.
One potential mechanism by which hypercholesterolemia could induce cardiac myocyte dysfunction and death is through disruption of mitochondrial function. Many cardiotoxic stimuli lead to reactive oxygen species (ROS) generation and to Ca2+ overload of the mitochondrial matrix, which causes the opening of a large, nonspecific channel in the inner mitochondrial membrane, an event described as the mitochondrial permeability transition (MPT). MPT dissipates the proton electrochemical gradient (ΔΨm), leading to ATP depletion, further ROS production, and swelling and rupture of the mitochondria, thus releasing prodeath proteins into the cytosol (2, 13, 56). Therefore, it is not surprising that mitochondrial dysfunction has been shown to play an essential role in the pathogenesis of multiple cardiac diseases (1, 6, 24, 38, 42).
Despite this knowledge, however, very little is known about the effects of hypercholesterolemia on cardiac mitochondrial function. Studies in noncardiomyocyte cells have reported that oxidized low-density lipoprotein (LDL) can suppress mitochondrial respiration and increase mitochondrial ROS production (4, 48, 51). Moreover, liver mitochondria from LDL receptor-deficient mice exhibit increased ROS production and an enhanced MPT response (41). Hypercholesterolemia has been shown to increase mtDNA damage in cardiac mitochondria (25, 36), suggestive of mitochondrial oxidative stress. Given that hypercholesterolemia exacerbates IR injury, it is interesting that the MPT pore has been established as a critical mediator of myocardial IR injury (1, 6, 40). Taken together, these data suggest that elevated cholesterol can adversely affect cardiac mitochondrial function, but this has yet to be formally tested.
It is well known that exercise improves cardiovascular disease risk factors (45, 54). Moreover, several studies have reported that exercise can induce changes in cardiac mitochondria that would be considered beneficial. For example, exercise has been shown to decrease ROS production (50), increase mitochondria-specific antioxidant proteins (12, 58), and desensitize the MPT response (23, 34). However, these studies only examined the effects of exercise in healthy animals and whether exercise's beneficial actions on cardiac mitochondria can be recapitulated in diseased, e.g., hypercholesterolemic, animals has yet to be tested. Moreover, it is unknown whether exercise can still favorably alter the cardiac mitochondrial phenotype in a more “human-relevant” large animal such as the pig, whether it be normal or diseased.
To this end, the objective of the present study was to examine whether hypercholesterolemia detrimentally altered cardiac mitochondrial function, and, if so, whether exercise could reverse these changes. Using a genetic swine model of familial hypercholesterolemia (FH), we found that cardiac mitochondria from hypercholesterolemic pigs exhibited an exacerbated MPT response to Ca2+. Moreover, this was associated with increased oxidative stress and decreased levels of mitochondrial antioxidant enzymes, such as manganese superoxide dismutase (MnSOD), thioredoxin-2 (Trx2), and peroxiredoxin-3 (Prx3). However, chronic exercise normalized MPT responsiveness, attenuated oxidative stress, and restored MnSOD, Trx2, and Prx3 levels in the FH pigs.
MATERIALS AND METHODS
Use of animals.
All experiments were approved by the University of Missouri-Columbia Animal Care and Use Committee and conformed to the National Institutes of Health guidelines for the use and care of animals.
Porcine model of familial hypercholesterolemia.
The Rapacz familial hypercholesterolemic (FH) model was developed at the University of Wisconsin by selective breeding (16, 17). These swine are characterized by a single missense mutation in the LDL receptor that decreases its affinity for LDL, resulting in elevated total cholesterol levels. Male familial hypercholesterolemic pigs were obtained from the University of Wisconsin Swine Research and Teaching Center. At 12 mo of age, the FH pigs were switched to a high-fat chow (by weight 13% protein, 21.3% fat, 41.4% total carbohydrate) and fed on this diet for 4 mo. We have found that this protocol yields greater homogeneity in the development of coronary atherosclerosis between animals than with the standard pig chow. Control, normolipidemic (NL) animals were weight-matched castrated male farm pigs fed standard pig chow (16.7% protein, 2.6% fat, 53.2% total carbohydrate). The plasma profiles for total cholesterol, LDL, high-density lipoprotein (HDL), and triglycerides for the NL and FH pigs are shown in Table 1. All four indices were significantly elevated in the FH animals.
Table 1.
Lipid profiles of normolipidemic and familial hypercholesterolemic pigs after 4 mo sedentary or 4 mo exercise training
| NL-Sed (n = 4) | FH-Sed (n = 4) | FH-Ex (n = 7) | |
|---|---|---|---|
| Total cholesterol, mg/dl | 86 ± 5.3 | 316 ± 29* | 335 ± 24* |
| HDL, mg/dl | 46 ± 1.0 | 32 ± 5.0* | 30 ± 2.0* |
| LDL, mg/dl | 50 ± 4.6 | 248 ± 28* | 271 ± 22* |
| Triglycerides, mg/dl | 16 ± 2.8 | 29 ± 3.0* | 27 ± 3.0* |
Values are expressed as means ± SE. NL-Sed, sedentary normolipidemic pigs; FH-Sed, sedentary hypercholesterolemic pigs; FH-Ex, exercised hypercholesterolemic pigs; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P < 0.05 vs. NL-Sed.
Exercise protocol.
After 4 mo on the high-fat diet, the FH pigs were switched to the standard diet and randomized to either a sedentary (FH-Sed, n = 4) or exercise (FH-Ex, n = 7) group. The FH-Ex group was trained for 4 mo using an established progressive treadmill-based training program similar to that described previously (19, 39, 57). Briefly, the pigs were subjected to treadmill training 5 days/wk for 16 wk. Having reached a plateau, training consisted of 5-min warm-up at 2.5 mph, 15 min at 5–8 mph, 60 min endurance at 4–5 mph, and a 5 min cool-down period at 2.5 mph (85 min total). The FH-Sed group was aged alongside the FH-Ex animals but did not undergo training. The plasma profiles for total cholesterol, LDL, HDL, and triglycerides in the FH pigs were not significantly altered by this exercise regimen (Table 1). The NL control pigs (NL-Sed, n = 4) were maintained in a sedentary state for the duration of the protocol. At the end of the protocol, the animals in all three groups were killed, and the heart was removed. Left-ventricular tissue was excised and used for the mitochondrial and biochemical analyses.
Mitochondrial isolation and swelling.
The isolation of cardiac mitochondrial and cytosolic fractions was carried out as previously described (1, 10). Briefly, left-ventricular tissue was homogenized using a Dounce in homogenization buffer (250 mM sucrose, 10 mM Tris pH 7.4, and 1 mM EDTA). The homogenate was centrifuged at 1000 g for 5 min to pellet the nuclei and unbroken cells/debris. The resultant supernatant was then centrifuged at 10,000 g for 10 min to pellet the mitochondria. The cytosolic fraction was then prepared by centrifuging the postmitochondrial supernatant at 20,000 g for 30 min. The mitochondrial pellet was then washed twice in EDTA-free homogenization buffer and resuspended in lysis buffer (150 mM NaCl, 10 mM Tris pH 7.4, 1 mM EDTA, and 1% Triton X-100). The yield of mitochondrial protein per gram of starting tissue was no different between the three groups (data not shown).
Mitochondrial swelling.
Mitochondria were prepared as described above and then resuspended in swelling buffer (150 mM KCl, 5 mM KH2PO4, and 10 mM Tris pH 7.4) to a final concentration of 0.25 mg/ml. Mitochondrial swelling, an index of permeability transition, was induced by the addition of CaCl2 (10–100 μM) and measured spectrophotometrically at 520 nm (1, 10). We confirmed that the MPT pore was responsible for the swelling response with 1 μM of the MPT inhibitor cyclosporine-A (data not shown).
Western blot analysis.
Mitochondrial and cytosolic proteins were resolved by SDS-PAGE using 10–15% acrylamide, transferred onto PVDF membranes, and blotted using the following commercial antibodies: ANT (sc-9299), catalase (sc-34285), GAPDH (sc-47724), nitrotyrosine (sc-32757), MnSOD (sc-18504), Prx (sc-137222), Prx3 (sc-59661), and Trx2 (sc-50336) from Santa Cruz Biotechnology; cyclophilin-D (CypD; MSA04) and cytochrome-c oxidase subunit II (MS405) from Mitosciences; and Cu/ZnSOD (07–403) from Millipore. The polyclonal phosphate carrier (PiC) antibody was custom made for us by Yenzyme. Membranes were then incubated with the appropriate alkaline phosphatase-linked secondary antibody (Santa Cruz Biotechnology) and visualized by enhanced chemifluorescence (Amersham). A Bio-Rad Gel-Doc system was used to visualize and quantify protein band densities.
Antioxidant assays.
GSH levels were determined in mitochondrial and cytosolic fractions using a commercially available luciferase-based assay from Promega. Mitochondrial and cytosolic SOD and catalase activities were also determined using commercially available assays from BioVision. All activities were then corrected for the protein concentration of each sample.
Statistical analyses.
Statistical significance was calculated by one-way ANOVA followed by Scheffé's post hoc test using StatPlus software. A P value of less than 0.05 was considered statistically significant.
RESULTS
MPT response is enhanced in cardiac mitochondria from sedentary, but not exercised, FH pigs.
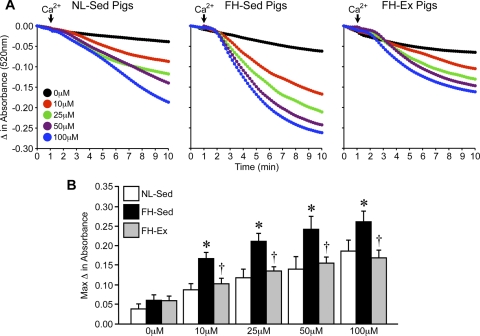
To assess whether the MPT response is exacerbated in hypercholesterolemic hearts, we isolated mitochondria from left-ventricular tissue from NL-Sed, FH-Sed, and FH-Ex pigs and subjected them to swelling. The starting absorbances at 520 nm were not different between NL-Sed, FH-Sed, and FH-Ex mitochondria (0.76 ± 0.02, 0.83 ± 0.04, and 0.72 ± 0.06 AU, respectively). The addition of Ca2+ induced a concentration-dependent decrease in absorbance at 520 nm in cardiac mitochondria from NL pigs, indicative of swelling (Fig. 1, A and B). In cardiac mitochondria from the sedentary FH animals, the swelling response was greatly exacerbated for all concentrations of Ca2+ (Fig. 1, A and B), suggestive of an increased sensitivity of the MPT pore to Ca2+. However, mitochondria from the chronically exercised FH pigs exhibited Ca2+-induced swelling responses that were no different from those observed in the NL pigs (Fig. 1, A and B).
Fig. 1.
Mitochondrial permeability transition is enhanced in cardiac mitochondria from sedentary but not exercised familial hypercholesterolemic (FH) pigs. A: swelling induced by 10–100 μM CaCl2 in cardiac mitochondria from sedentary normolipidemic (NL-Sed; n = 4) and sedentary (FH-Sed; n = 4) and exercised (FH-Ex, n = 7) FH pigs (left, middle, and right, respectively). B: maximum change in absorbance from mitochondrial swelling shown in A. Graphs are shown as means ± SE with *P < 0.05 vs. NL-Sed and †P < 0.05 vs. FH-Sed.
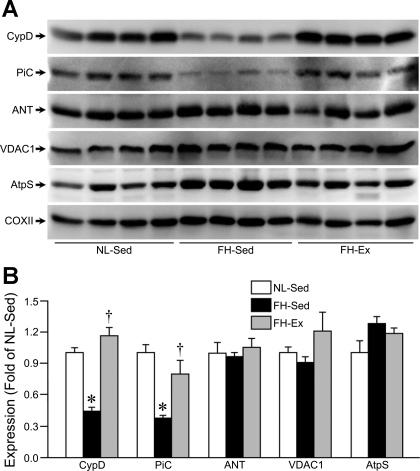
Despite this enhanced MPT reaction in the FH-Sed pigs, we actually observed decreases in the mitochondrial PiC and CypD, 2 putative components of the MPT pore, in the FH-Sed cardiac mitochondria compared with NL controls (Fig. 2, A and B). Moreover, the reductions in CypD and PiC were no longer observed in the mitochondria from the FH-Ex pigs (Fig. 2, A and B). These changes would appear to be specific as the levels of another MPT protein, the adenine nucleotide translocase (ANT), were unaltered (Fig. 2, A and B). Other major mitochondrial proteins, such as the voltage-dependent anion channel (VDAC), ATP synthase, and cytochrome-c oxidase were also unaffected in the FH pigs (Fig. 2A).
Fig. 2.
Cyclophilin-D and the mitochondrial phosphate carrier are downregulated in cardiac mitochondria from sedentary but not exercised FH pigs. A: Western blot analysis for the putative mitochondrial permeability transition (MPT) pore components cyclophilin-D (CypD), mitochondrial phosphate carrier (PiC), and adenine nucleotide translocase (ANT) in mitochondrial lysates from NL-Sed, FH-Sed, and FH-Ex hearts (n = 4 in each group). The voltage-dependent anion channel (VDAC), ATP synthase (AtpS), and cytochrome-c oxidase subunit II (COXII) were also blotted for. B: quantification of the mitochondrial protein expression data. Graphs are shown as means ± SE with *P < 0.05 vs. NL-Sed. †P < 0.05 vs. FH-Sed.
Cardiac mitochondrial oxidative stress is enhanced in sedentary, but not exercised, FH pigs.
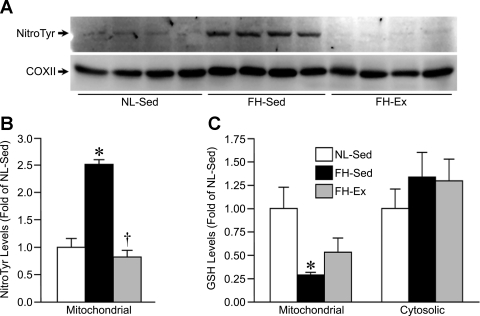
One mechanism that would lead to increased sensitivity to MPT is elevated ROS (5, 14, 52). To assess this, we blotted the mitochondrial lysates from NL-Sed, FH-Sed, and FH-Ex pig hearts for nitrotyrosine, a marker of oxidative stress. As shown in Fig. 3A, a greater degree of nitrotyrosylation of a specific band (∼70 kDa) was observed in the FH-Sed mitochondria compared with the NL-Sed mitochondria. However, the nitrosylation of this protein band was significantly reduced to near-normal levels in the FH-Ex mitochondria (Fig. 3, A and B). We found no evidence of altered nitrotyrosylation in the cytosolic fractions of any of the groups (data not shown). As a second marker of oxidative stress, we determined the levels of GSH in both the mitochondrial and cytosolic fractions of the three groups of hearts. GSH was significantly depleted in cardiac mitochondria from FH-Sed pigs compared with the NL-Sed controls (Fig. 3C). However, this decrease in GSH was no longer significant in the FH-Ex mitochondria, although a significant difference was also not observed with respect to the FH-Sed animals either (Fig. 3C). There was no difference in cytosolic GSH levels between the 3 groups (Fig. 3C).
Fig. 3.
Cardiac mitochondrial oxidative stress is enhanced in sedentary but not exercised FH pigs. A: Western blotting for protein nitrotyrosylation in mitochondrial lysates from NL-Sed, FH-Sed, and FH-Ex pig hearts (n = 4 in each group). COXII was used as a loading control. B: quantification of the nitrotyrosine data. C: reduced GSH in mitochondrial and cytosolic fractions from NL-Sed, FH-Sed, and FH-Ex pig hearts (n = 4 in each group). Graphs are shown as means ± SE with *P < 0.05 vs. NL-Sed and †P < 0.05 vs. FH-Sed.
Mitochondrial antioxidant capacity is impaired in sedentary but not exercised FH pigs.
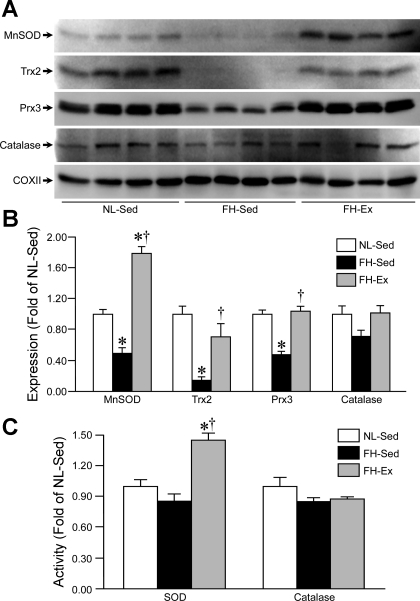
To assess the mechanism behind the increase in mitochondrial oxidative stress in the FH-Sed hearts and the protection afforded by exercise, we next examined the levels and activities of various antioxidant proteins in the mitochondrial and cytosolic fractions of the NL-Sed, FH-Sed, and FH-Ex hearts. Blotting of mitochondrial lysates demonstrated profound decreases in the levels of the mitochondria-specific antioxidant enzymes MnSOD, Trx2, and Prx3 in the FH-Sed hearts compared with the NL-Sed controls (Fig. 4, A and B). In the FH-Ex group, however, Trx2 and Prx3 levels were no different from the NL-Sed mitochondria, and MnSOD expression was actually further increased (Fig. 4, A and B). Mitochondrial catalase expression was not significantly different between the three groups (Fig. 4, A and B). Somewhat surprisingly, given the striking changes in protein levels, we found no significant alterations in SOD activity between the NL-Sed and FH-Sed mitochondria (Fig. 4C). However, mitochondrial SOD activity was significantly elevated in the FH-Ex hearts (Fig. 4C). Mitochondrial catalase activity was not significantly different between the three groups (Fig. 4C).
Fig. 4.
Mitochondrial antioxidant capacity is impaired in sedentary, but not exercised, FH pigs. A: Western blotting for manganese superoxide dismutase (MnSOD), thioredoxin-2 (Trx2), peroxiredoxin-3 (Prx3), and catalase in mitochondrial lysates from NL-Sed, FH-Sed, and FH-Ex pig hearts (n = 4 in each group). COXII was used as a loading control. B: quantification of the antioxidant expression data. C: Mitochondrial SOD and catalase activities in the NL-Sed, FH-Sed, and FH-Ex pig hearts (n = 4 in each group). Graphs are shown as means ± SE with *P < 0.05 vs. NL-Sed and †P < 0.05 vs. FH-Sed.
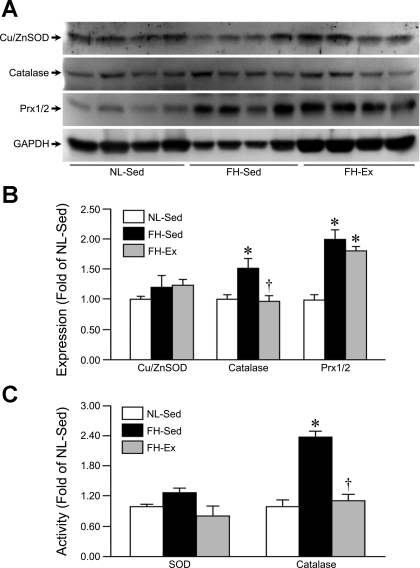
We also analyzed the three main antioxidant enzymes in the cytosolic compartment of the NL-Sed, FH-Sed, and FH-Ex pig hearts, Cu/ZnSOD, catalase, and Prx1/2. Expression levels of Cu/ZnSOD were unaltered in either of the FH groups (Fig. 5, A and B), as was cytosolic SOD activity (Fig. 5C). Cytosolic catalase expression and activity were elevated in the FH-Sed hearts; however, in FH-Ex animals, both of these levels returned to normal (Fig. 5A–C). Prx1/2 protein levels were significantly increased in the cytosolic fractions of the FH-Sed hearts and remained elevated in the FH-Ex hearts (Fig. 5, A and B).
Fig. 5.
Cytosolic catalase levels and activity are augmented in sedentary but not exercised FH pigs. A: Western blot analysis for copper/zinc superoxide dismutase (Cu/ZnSOD), catalase, and peroxiredoxin-1/2 (Prx1/2) in cytosolic lysates from NL-Sed, FH-Sed, and FH-Ex pig hearts (n = 4 in each group). GAPDH was used as a loading control. B: quantification of the antioxidant expression data. C: cytosolic SOD and catalase activities in the NL-Sed, FH-Sed, and FH-Ex pig hearts (n = 4 in each group). Graphs are shown as means ± SE with *P < 0.05 vs. NL-Sed and †P < 0.05 vs. FH-Sed.
DISCUSSION
The purpose of this study was to investigate the effects of hypercholesterolemia and aerobic exercise training on cardiac mitochondrial function in a swine model of FH. The major findings of this study are that FH enhances the MPT response, increases mitochondrial oxidative stress, and impairs the mitochondrial, but not the cytosolic, antioxidant system. Importantly, chronic exercise training rescues, for the most part, the increased oxidative stress, the MPT alterations, and impairment of the mitochondrial oxidant defenses associated with chronic hypercholesterolemia.
Although MPT has been studied in many other cardiac disease models (1, 6, 10, 24, 38, 42), to the best of our knowledge, our study is the first to examine the effects of chronic hypercholesterolemia on the MPT response in cardiac mitochondria. Previously, Oliveira et al. (41) reported that liver mitochondria from mice lacking the LDL receptor exhibit an exacerbated MPT response to Ca2+; however, they did not examine whether cardiac mitochondria exhibited the same phenotype. In the present study, we used pigs that have a missense mutation in the LDL receptor, which we would expect to phenocopy the LDL receptor-deficient mice. We did not observe any significant changes in the baseline absorbance between the sedentary NL and FH cardiac mitochondria, which would indicate that the FH-Sed mitochondria are not preswollen. However, similar to the mitochondria from the LDL receptor knockout mice, we did observe a significant sensitization of the MPT response for any given concentration of Ca2+, suggesting that the threshold for MPT pore activation is substantially lowered in the cardiac mitochondria from the sedentary FH pigs. Thus, it would appear that while mitochondria are not actively undergoing (or have undergone) MPT per se in the FH-Sed hearts, they are “primed” such that any additional cardiac insult would result in a more robust MPT response and ultimately more cardiac myocyte death. Such a paradigm would certainly explain why hypercholesterolemic hearts exhibit much greater infarct sizes following IR (11, 20, 43, 49).
The simplest explanation for such a change in MPT responsiveness is that the expression levels of one or more components of the MPT pore are upregulated in cardiomyocytes from the FH-Sed pigs, as has been reported for hyperglycemia (33) and cardiac hypertrophy (35). Interestingly, however, we found that the expression of the two main MPT pore components PiC and CypD was actually decreased in the mitochondria from sedentary hypercholesterolemic pigs, despite ANT, VDAC, ATP synthase, and cytochrome-c oxidase levels remaining unchanged. This would suggest that in an attempt to counteract the enhanced responsiveness of the MPT pore in the hypercholesterolemic state, the myocardium is actively reducing the levels of the pore's componentry.
The fact that the enhanced MPT response in the FH-Sed mitochondria appears to be independent of protein expression suggests that hypercholesterolemia is somehow enhancing the sensitivity of the MPT pore to Ca2+. In this regard, the most plausible scenario is one in which oxidant levels are elevated, as oxidants have been well documented to decrease the threshold of activation of the MPT pore in response to Ca2+ (5, 14, 52). Specifically, ROS are believed to modify key thiol groups on the MPT pore (5, 13, 14). This, in turn, may facilitate the effects of Ca2+ by displacing adenine nucleotides, which inhibit the pore, from ANT (14). Alternatively, oxidative stress can induce the translocation of the MPT regulator CypD to the inner membrane, where it presumably interacts with the MPT pore (7). Several studies have reported increased incidence of oxidative stress in the hearts of hypercholesterolemic animals and humans (7, 21, 25, 36). Thus, we investigated whether the myocardial tissues of FH-Sed pigs showed any signs of increased oxidative stress compared with the normocholesterolemic controls. Indeed, Western blot analysis did show that FH-Sed hearts had significantly more protein nitrotyrosylation in the mitochondrial, but not the cytosolic, fraction. Consistent with this finding, we also observed a marked decrease in the levels of reduced glutathione in the FH-Sed mitochondria. Again, however, cytosolic GSH levels were not affected, indicating that the oxidative stress was limited to the mitochondria of these hearts. Intriguingly, we only observed an increase in nitrotyrosylation of one single mitochondrial band at ∼70 kDa. The exact nature of this protein is currently unknown, but it is interesting that Brookes and Darley-Usmar (3) identified the 70-kDa glutamate dehydrogenase as a mitochondrial target of peroxynitrite-induced nitrotyrosylation. Needless to say, this is something that we are currently pursuing using a proteomic approach.
Concomitant with the changes in redox status, we observed profound reductions in the expression of the mitochondria-specific antioxidant enzymes MnSOD, Trx2, and Prx3 in the FH-Sed hearts. The reductions in Trx2 and Prx3 are particularly compelling in light of the fact that GSH levels are also reduced, as Jones' group has demonstrated that the Trx2/Prx3/GSH antioxidant system is tightly coupled (60). Thus, it would appear that the reductions in these critical proteins are most likely the reason for the increased oxidative stress and the exacerbated MPT response in the hypercholesterolemic hearts. Indeed, mice genetically deficient for MnSOD, Trx2, or Prx3 exhibit enhanced oxidant production and, in the case of the MnSOD+/− mice, more pronounced MPT responses (26, 31, 44). Conversely, overexpression of MnSOD, Trx2, or Prx3 can attenuate MPT (18, 28, 32). That being said, we did not observe a significant decrease in mitochondrial SOD activity. Why this is the case is not entirely clear, especially given the marked reduction in protein levels, but may be due to sensitivity of the assay (see below). Interestingly, the expression of the cytosolic antioxidant enzymes catalase and Prx1/2 was increased in the hypercholesterolemic animals, and catalase activity was concomitantly elevated. Most likely, this is a mechanism by which the heart tries to compensate for the impairment of the mitochondrial antioxidant system. Thus, it would appear that chronic hypercholesterolemia appears to selectively destroy the mitochondrial oxidant defense system in cardiac myocytes, although the reasons as to why this selectivity occurs remain to be elucidated.
Exercise training has been shown to have beneficial effects on cardiac mitochondrial function in healthy animals. Extensive data from Powers' laboratory have shown that exercise can decrease mitochondrial ROS production, upregulate MnSOD expression, and decrease the amplitude of Ca2+-induced MPT in cardiac mitochondria from healthy animals (12, 15, 23). Many other laboratories have recapitulated these findings (22, 36, 50, 58). Yet very few studies have tested whether such effects are still viable in the hypercholesterolemic animal. Thus, we subsequently investigated whether exercise training could improve the MPT response of our more clinically relevant FH animals. Indeed, exercised FH animals exhibited MPT responses that were similar to the normolipidemic pigs, as opposed to the exaggerated responses seen in the FH-Sed animals. Interestingly, exercise also normalized the changes in PiC and CypD expression observed in the FH-Sed pigs, again, indicating that the changes in MPT responsiveness were independent of protein changes. Oxidative stress was again investigated, since it was shown to negatively affect the MPT response. FH-Ex animals had significantly less nitrotyrosylation of the 70-kDa mitochondrial protein, and the reduction in mitochondrial GSH levels observed in the FH-Sed pigs was not as pronounced in the exercised animals. Thus, exercise was able to attenuate the enhanced mitochondrial oxidation in the hypercholesterolemic hearts.
Chronic exercise also had a profound influence on antioxidant expression in the hypercholesterolemic pigs. The reductions in mitochondrial Trx2 and Prx3 seen in the sedentary FH pigs were completely normalized in the exercised FH pigs. In addition, MnSOD levels were actually increased in the FH-Ex hearts to a point beyond that seen even in normocholesterolemic animals, with a concomitant elevation in activity. The improvement in MnSOD expression and activity with exercise training has been described in several studies (23, 29). However, there have been no previous studies demonstrating modulation of Trx2 and Prx3 by training. Certainly, this global maintenance of the mitochondrial antioxidant system (MnSOD, Trx2, Prx3, GSH) would provide a mechanism by which exercise training is able to ameliorate the deleterious effects of hypercholesterolemia on mitochondrial function.
The effect of exercise training on catalase expression and activity is especially controversial, with studies reporting either no changes (22, 59) or increases (30, 50) in catalase expression/activity. In the present study, the increases in catalase expression and activity seen in the cytosol from the sedentary FH hearts were seemingly paradoxically abrogated in the exercised hearts. However, this would further indicate that the responses observed in the FH-Sed animals are, indeed, a compensatory mechanism, such that the restoration of the mitochondrial antioxidant system by exercise and reduction in oxidative stress would mean that the cytosolic catalase would not have to be recalibrated. Cytosolic Prx1/2 expression was not affected by exercise training, remaining elevated to the same extent as in the sedentary hearts. This would suggest that changes in Prx1/2 levels are a direct response to the chronic cholesterolemia, rather than a secondary response to the impairment of the mitochondrial antioxidants, and that they do not play a significant role in the mitoprotective effects of exercise.
It should be noted that exercise itself did not alter blood cholesterol and triglyceride levels in the FH pigs (as shown in Table 1), indicating that its beneficial effects were at the level of the cardiac myocyte and not simply a secondary response to an altered blood cholesterol profile. This is consistent with previous studies in either LDL-R or ApoE knockout mice, which showed no reduction in cholesterol levels with chronic exercise (46, 47), and presumably is a reflection of the fact that the genetic lesion, i.e., LDL-R mutation, is still in place regardless of the activity state of the animal. Hence, it would appear that chronic exercise still exerts a “mitoprotective” effect in the hypercholesterolemic animal. This is significant, as it would imply that exercise could still exert an infarct-sparing effect in hypercholesterolemic animals, whereas other cardioprotective interventions, such as preconditioning and postconditioning are no longer effective in this context (21, 53, 55).
Study limitations.
As with any study, there are limitations that should be noted. First, during the exercise regimen, the FH pigs, which were previously on a high-fat diet, were switched back to normal diet. The rationale for this was to mimic human clinical trials and the standard of care for patients with coronary heart disease, in which exercise is initiated at the same time, as diet is altered. However, this still leaves the question of whether exercise alone can still exert its beneficial effects independently of diet. Whether this is indeed the case is the subject of ongoing studies in our laboratory. The second limitation is that all the pigs used in this study were castrated, which, in itself, can influence cardiac function and susceptibility to cardiac disease. A third limitation is the SOD activity assay. Even though we observed a >70% decrease in MnSOD protein levels, we did not see a significant drop in mitochondrial SOD activity. Although activity enhancing posttranslational modifcations cannot be ruled out at this juncture, it is most likely that the SOD assay was not sensitive enough to detect negative changes, i.e., our baseline activity in the NL-Sed mitochondria was just at the threshold of detection, such that any decrease would not register. Unfortunately, because of the limits in our sample quantities, we were not able to increase the amount of protein in each assay reaction.
Perspectives and Significance
While the detrimental effects of chronically elevated cholesterol are traditionally thought to primarily affect the vasculature, our data provide evidence that the underlying myocardium can also be profoundly affected, especially at the mitochondrial level. These findings provide a potential mechanism as to why hypercholesterolemic animals and patients suffer a greater myocardial infarct following coronary occlusion. Moreover, the fact that exercise can overcome these adverse mitochondrial changes suggests that it may be a useful, cost-effective tool to protect the hypercholesterolemic myocardium. Indeed, the American College of Sports Medicine/Americab Heart Association guidelines recommend at least 30 min of moderate intensity exercise 5 days/wk, with up to 90 min being recommended for additional benefits (www.acsm.org), an intensity level and time duration comparable to that undergone by the pigs in our present study.
GRANTS
The National Heart, Lung, and Blood Institute Grants HL094404 (to C. P. Baines) and HL052490 (to M. H. Laughlin and D. K. Bowles) supported this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the expert technical assistance of Jan Ivey, David Harah, and Pam Thorne.
REFERENCES
- 1.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol 72: 61–80, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Brookes PS, Darley-Usmar VM. Role of calcium and superoxide dismutase in sensitizing mitochondria to peroxynitrite-induced permeability transition. Am J Physiol Heart Circ Physiol 286: H39–H46, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Cheng J, Cui R, Chen CH, Du J. Oxidized low-density lipoprotein stimulates p53-dependent activation of proapoptotic Bax leading to apoptosis of differentiated endothelial progenitor cells. Endocrinology 148: 2085–2094, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Chernyak BV, Bernardi P. The mitochondrial permeability transition pore is modulated by oxidative agents through both pyridine nucleotides and glutathione at two separate sites. Eur J Biochem 238: 623–630, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem 277: 34793–34799, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem J 302: 321–324, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csont T, Bereczki E, Bencsik P, Fodor G, Görbe A, Zvara A, Csonka C, Puskás LG, Sántha M, Ferdinandy P. Hypercholesterolemia increases myocardial oxidative and nitrosative stress thereby leading to cardiac dysfunction in apoB-100 transgenic mice. Cardiovasc Res 76: 100–109, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dhar SK, Xu Y, Chen Y, St Clair DK. Specificity protein 1-dependent p53-mediated suppression of human manganese superoxide dismutase gene expression. J Biol Chem 281: 21698–21709, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emter CA, Baines CP. Cardiovascular remodeling in aortic-banded miniature swine is altered by aerobic interval training. Am J Physiol Heart Circ Physiol 299: H1348–H1356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferdinandy P. Myocardial ischaemia/reperfusion injury and preconditioning: effects of hypercholesterolemia/hyperlipidemia. Br J Pharmacol 138: 283–285, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA, Powers SK. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J 22: 2862–2871, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46: 821–831, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J Biol Chem 272: 3346–3354, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Hamilton KL, Powers SK, Sugiura T, Kim S, Lennon S, Tumer N, Mehta JL. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol 281: H1346–H1352, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Hasler-Rapacz J, Prescott MF, Von Linden-Reed J, Rapacz JM, Jr, Hu Z, Rapacz J. Elevated concentrations of plasma lipids and apolipoproteins B, C-III, and E are associated with the progression of coronary artery disease in familial hypercholesterolemic swine. Arterioscler Thromb Vasc Biol 15: 583–592, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Hasler-Rapacz J, Ellegren H, Fridolfsson AK, Kirkpatrick B, Kirk S, Andersson L, Rapacz J. Identification of a mutation in the low density lipoprotein receptor gene associated with recessive familial hypercholesterolemia in swine. Am J Med Genet 76: 379–386, 1998 [PubMed] [Google Scholar]
- 18.He M, Cai J, Go YM, Johnson JM, Martin WD, Hansen JM, Jones DP. Identification of thioredoxin-2 as a regulator of the mitochondrial permeability transition. Toxicol Sci 105: 44–50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaps CL, Jeffery EC, Laine GA, Price EM, Bowles DK. Effects of exercise training and hypercholesterolemia on adenosine activation of voltage-dependent K+ channels in coronary arterioles. J Appl Physiol 105: 1761–1771, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshida S, Nishida M, Yamashita N, Igarashi J, Hori M, Kamada T, Kuzuya T, Tada M. Amelioration of severity of myocardial injury by a nitric oxide donor in rabbits fed a cholesterol-rich diet. J Am Coll Cardiol 27: 902–909, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Iliodromitis EK, Andreadou I, Prokovas E, Zoga A, Farmakis D, Fotopoulou T, Ioannidis K, Paraskevaidis IA, Kremastinos DT. Simvastatin in contrast to postconditioning reduces infarct size in hyperlipidemic rabbits: possible role of oxidative/nitrosative stress attenuation. Basic Res Cardiol 105: 193–203, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol 289: R1564–R1572, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol 294: H928–H935, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 12: 908–916, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Knight-Lozano CA, Young CG, Burow DL, Hu ZY, Uyeminami D, Pinkerton KE, Ischiropoulos H, Ballinger SW. Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation 105: 849–854, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2+/− mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci USA 98: 2278–2283, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419: 316–321, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Kowaltowski AJ, Netto LE, Vercesi AE. The thiol-specific antioxidant enzyme prevents mitochondrial permeability transition. Evidence for the participation of reactive oxygen species in this mechanism. J Biol Chem 273: 12766–12769, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Lawler JM, Kwak HB, Kim JH, Suk MH. Exercise training inducibility of MnSOD protein expression and activity is retained while reducing prooxidant signaling in the heart of senescent rats. Am J Physiol Regul Integr Comp Physiol 296: R1496–R1502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lennon SL, Quindry J, Hamilton KL, French J, Staib J, Mehta JL, Powers SK. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol 96: 1299–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Li L, Shoji W, Takano H, Nishimura N, Aoki Y, Takahashi R, Goto S, Kaifu T, Takai T, Obinata M. Increased susceptibility of MER5 (peroxiredoxin III) knockout mice to LPS-induced oxidative stress. Biochem Biophys Res Commun 355: 715–721, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Loor G, Kondapalli J, Iwase H, Chandel NS, Waypa GB, Guzy RD, Vanden Hoek TL, Schumacker PT. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim Biophys Acta 1813: 1382–1394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lumini-Oliveira J, Magalhães J, Pereira CV, Moreira AC, Oliveira PJ, Ascensão A. Endurance training reverts heart mitochondrial dysfunction, permeability transition and apoptotic signaling in long-term severe hyperglycemia. Mitochondrion 11: 54–63, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Marcil M, Bourduas K, Ascah A, Burelle Y. Exercise training induces respiratory substrate-specific decrease in Ca2+-induced permeability transition pore opening in heart mitochondria. Am J Physiol Heart Circ Physiol 290: H1549–H1557, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Matas J, Young NT, Bourcier-Lucas C, Ascah A, Marcil M, Deschepper CF, Burelle Y. Increased expression and intramitochondrial translocation of cyclophilin-D associates with increased vulnerability of the permeability transition pore to stress-induced opening during compensated ventricular hypertrophy. J Mol Cell Cardiol 46: 420–430, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Mercer JR, Cheng KK, Figg N, Gorenne I, Mahmoudi M, Griffin J, Vidal-Puig A, Logan A, Murphy MP, Bennett M. DNA damage links mitochondrial dysfunction to atherosclerosis and the metabolic syndrome. Circ Res 107: 1021–1031, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzler B, Hu Y, Dietrich H, Xu Q. Increased expression and activation of stress-activated protein kinases/c-Jun NH2-terminal protein kinases in atherosclerotic lesions coincide with p53. Am J Pathol 156: 1875–1886, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, Sweeney HL, Robbins J, Molkentin JD. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med 14: 442–447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation 89: 2308–2314, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Oliveira HC, Cosso RG, Alberici LC, Maciel EN, Salerno AG, Dorighello GG, Velho JA, de Faria EC, Vercesi AE. Oxidative stress in atherosclerosis-prone mouse is due to low antioxidant capacity of mitochondria. FASEB J 19: 278–280, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Oliveira PJ, Seica R, Coxito PM, Rolo AP, Palmeira CM, Santos MS, Moreno AJ. Enhanced permeability transition explains the reduced calcium uptake in cardiac mitochondria from streptozotocin-induced diabetic rats. FEBS Lett 554: 511–514, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Osipov RM, Bianchi C, Feng J, Clements RT, Liu Y, Robich MP, Glazer HP, Sodha NR, Sellke FW. Effect of hypercholesterolemia on myocardial necrosis and apoptosis in the setting of ischemia-reperfusion. Circulation 120: S22–S30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pérez VI, Lew CM, Cortez LA, Webb CR, Rodriguez M, Liu Y, Qi W, Li Y, Chaudhuri A, Van Remmen H, Richardson A, Ikeno Y. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic Biol Med 44: 882–892, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Prasad DS, Das BC. Physical inactivity: a cardiovascular risk factor. Indian J Med Sci 63: 33–42, 2009 [PubMed] [Google Scholar]
- 46.Pynn M, Schäfer K, Konstantinides S, Halle M. Exercise training reduces neointimal growth and stabilizes vascular lesions developing after injury in apolipoprotein e-deficient mice. Circulation 109: 386–392, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Ramachandran S, Penumetcha M, Merchant NK, Santanam N, Rong R, Parthasarathy S. Exercise reduces preexisting atherosclerotic lesions in LDL receptor knock out mice. Atherosclerosis 178: 33–38, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Roy Chowdhury SK, Sangle GV, Xie X, Stelmack GL, Halayko AJ, Shen GX. Effects of extensively oxidized low-density lipoprotein on mitochondrial function and reactive oxygen species in porcine aortic endothelial cells. Am J Physiol Endocrinol Metab 298: E89–E98, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Scalia R, Gooszen ME, Jones SP, Hoffmeyer M, Rimmer DM, Trocha SD, Huang PL, Smith MB, Lefer AM, Lefer DJ. Simvastatin exerts both anti-inflammatory and cardioprotective effects in apolipoprotein E-deficient mice. Circulation 103: 2598–2603, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Starnes JW, Barnes BD, Olsen ME. Exercise training decreases rat heart mitochondria free radical generation but does not prevent Ca2+-induced dysfunction. J Appl Physiol 102: 1793–1798, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Takabe W, Li R, Ai L, Yu F, Berliner JA, Hsiai TK. Oxidized low-density lipoprotein-activated c-Jun NH2-terminal kinase regulates manganese superoxide dismutase ubiquitination: implication for mitochondrial redox status and apoptosis. Arterioscler Thromb Vasc Biol 30: 436–441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeyama N, Matsuo N, Tanaka T. Oxidative damage to mitochondria is mediated by the Ca2+-dependent inner-membrane permeability transition. Biochem J 294: 719–725, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang XL, Takano H, Xuan YT, Sato H, Kodani E, Dawn B, Zhu Y, Shirk G, Wu WJ, Bolli R. Hypercholesterolemia abrogates late preconditioning via a tetrahydrobiopterin-dependent mechanism in conscious rabbits. Circulation 112: 2149–2156, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas NE, Baker JS, Davies B. Established and recently identified coronary heart disease risk factors in young people: the influence of physical activity and physical fitness. Sports Med 33: 633–650, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Ueda Y, Kitakaze M, Komamura K, Minamino T, Asanuma H, Sato H, Kuzuya T, Takeda H, Hori M. Pravastatin restored the infarct size-limiting effect of ischemic preconditioning blunted by hypercholesterolemia in the rabbit model of myocardial infarction. J Am Coll Cardiol 34: 2120–2125, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72: 19–44, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Woodman CR, Ingram D, Bonagura J, Laughlin MH. Exercise training improves femoral artery blood flow responses to endothelium-dependent dilators in hypercholesterolemic pigs. Am J Physiol Heart Circ Physiol 290: H2362–H2368, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med 189: 1699–1706, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu X, Zhao W, Wan W, Ji LL, Powers AS, Erikson JM, Zhang JQ. Exercise training combined with angiotensin II receptor blockade reduces oxidative stress after myocardial infarction in rats. Exp Physiol 95: 1008–1015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H, Go YM, Jones DP. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch Biochem Biophys 465: 119–126, 2007 [DOI] [PubMed] [Google Scholar]