Abstract
Despite frequent reporting of blood pressure (BP) during profound passive heat stress, both with and without a hypotensive challenge, the method by which BP is measured often varies between laboratories. It is unknown whether auscultatory and finger BP measures accurately reflect intra-arterial BP during dynamic changes in cardiac output and peripheral resistance associated with the aforementioned conditions. The purpose of this investigation was to test the hypothesis that auscultatory BP measured at the brachial artery, and finger BP measured by the Penaz method, are valid measures of intra-arterial BP during a passive heat stress and a heat-stressed orthostatic challenge, via lower body negative pressure (LBNP). Absolute (specific aim 1) and the change in (specific aim 2) systolic (SBP), diastolic (DBP), and mean BPs (MBP) were compared at normothermia, after a core temperature increase of 1.47 ± 0.09°C, and during subsequent LBNP. Heat stress did not change auscultatory SBP (6 ± 11 mmHg; P = 0.16), but Penaz SBP (−22 ± 16 mmHg; P < 0.001) and intra-arterial SBP (−11 ± 13 mmHg P = 0.017) decreased. In contrast, DBP and MBP did not differ between methods throughout heat stress. Compared with BP before LBNP, the magnitude of the reduction in BP with all three methods was similar throughout LBNP (P > 0.05). In conclusion, auscultatory SBP and Penaz SBP failed to track the decrease in intra-arterial SBP that occurred during the profound heat stress, while decreases in arterial BP during an orthostatic challenge are comparable between methodologies.
Keywords: lower body negative pressure, passive heat stress, arterial blood pressure
various methods are used to measure arterial blood pressure (BP) in the passively heat-stressed human (6, 8, 18, 20, 31, 35, 36). The most valid approach is an intra-arterial catheter connected to a calibrated transducer (BPINTRA). Although BPINTRA is the gold standard, physician assistance and supervision are often required, and there is significant cost and subject risk, resulting in BPINTRA measurements being prohibitive in most settings.
An alternative to BPINTRA is automated auscultatory BP (BPAUSC). This method is often used because of the relatively low cost, ease of use, and its capacity to be used in a variety of settings (26). However, this method is limited because each measurement requires ∼30 s, thus prohibiting the use of BPAUSC during conditions when arterial BP is rapidly changing (e.g., when a subject is approaching presyncope during an orthostatic tolerance test).
In the 1970's, noninvasive beat-by-beat BP measurements were made possible using a finger cuff coupled with Penaz technology (25). Penaz-derived BP (BPPENAZ) recordings have been validated for resting individuals (11, 29), although a large degree of variability has been noted (13, 30). Likewise, BPPENAZ is valid during acute BP changes (i.e., orthostatic stress) when subjects are normothermic (13, 24).
Because of the prohibitive nature of BPINTRA, many investigators use BPPENAZ and BPAUSC during profound passive heat stress and heat-stress coupled with hypotensive challenges (6, 8, 18, 35, 36). It is possible that the elevations in skin and core temperatures during passive heating, resulting in cutaneous vasodilation (28), alter the measurement of BP using Penaz technology. For this reason, and because BPAUSC and BPPENAZ are widely utilized in passive heat stress studies, we sought to examine the effect of passive heat stress on noninvasive measures of BP while subjects were supine, as well as during a hypotensive challenge. Specific aim 1 tested the hypothesis that BPPENAZ and BPAUSC methods provide valid measures of absolute systolic (SBP), diastolic (DBP), and mean BPs (MBP), referenced from BPINTRA, during profound passive heat stress, with and without a hypotensive challenge via lower body negative pressure (LBNP). Specific aim 2 tested the hypothesis that the changes (i.e., delta) in BPINTRA during the aforementioned conditions are appropriately tracked by changes in BPPENAZ and BPAUSC.
METHODS
Subjects.
Seven men and three women (n = 10) participated in this study; subjects' mean ± SD age, mass, and height were 42 ± 11 yr, 77.7 ± 10.9 kg, and 175 ± 7 cm, respectively. Subjects were nonsmokers, not taking medications, and were free of any known cardiovascular, metabolic, or neurological diseases. Subjects refrained from alcohol and exercise 24 h and caffeine 12 h before the study. Subjects began testing with a urine-specific gravity of <1.028. Written, informed consent was obtained from all subjects before participating in this study. Study procedures were approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas.
Instrumentation and measurements.
Each subject was dressed in a water-perfused, tube-lined suit (Med-Eng, Ottawa, Canada) that covered the entire body, except the head, face, hands, feet, and one forearm. Each subject was then placed into an LBNP chamber, sealed at the iliac crest, while in the supine position. The suit permitted the manipulation of skin and thus core temperature by changing the temperature of the water perfusing the suit. Core temperature was measured from an ingestible pill telemetry system (HQ, Palmetto, FL). The pill was ingested immediately on arrival at the laboratory, which was ∼1.5 h before the onset of data collection. Mean skin temperature was measured in a manner similar to other studies (6, 8, 18, 35, 36) via the weighted average of six thermocouples attached to the skin (32). Heart rate was obtained from an electrocardiogram (HP Patient Monitor, Agilent, Santa Clara, CA) interfaced with a cardiotachometer (CWE, Ardmore, PA).
BP measurements.
BPINTRA was measured with a 20-gauge catheter inserted into the radial artery of the nondominant arm using sterile techniques after application of local anesthesia (∼2 ml of lidocaine). The cannula was connected to a pressure transducer (Maxxim Medical, Athens, TX) that was positioned 5 cm below the sternal notch. Calibration of the transducer was verified after the removal of each catheter by attaching a manometer to the pressure transducer and confirming calibration at 0 mmHg and at least two pressures encompassing the range exhibited by the subject.
Arterial BP was also measured by K-sound gated auscultation of the brachial artery (BPAUSC) via electrosphygmomanometry (Tango+, SunTech, Raleigh, NC) integrated with the electrocardiogram (10). An appropriate sized cuff was placed directly on the skin, underneath the water-perfused suit, and the deflation rate was set to “auto” to accommodate changes in heart rate that occur with the various perturbations. Device calibration was verified before use. Due to equipment malfunction of the Tango+ during data collection for one subject, manual BPAUSC, which correlates well with automated BPAUSC (9, 21), was obtained by a trained nurse for that individual.
Beat-by-beat artery BP was measured with an appropriate sized finger cuff attached to the hand of the same arm with the arterial catheter (BPPENAZ; Finometer Pro, FMS, Amsterdam, the Netherlands). The pulse waveform was “reconstructed” by the Finometer device to estimate brachial artery BP (11). The automated physiological calibration (i.e., servo) remained on throughout testing. Calibration periods were excluded, and the 1-s delay in the analog output of the Finometer device was accounted for before data analysis.
To avoid interruptions in beat-by-beat BPINTRA and BPPENAZ, and because BP does not differ between arms in healthy individuals (7), BPAUSC was measured on the arm opposite to BPINTRA and BPPENAZ measures.
Experimental protocol.
After instrumentation, subjects were supine for at least 10 min before normothermic measures, during which 34°C water was perfused. Subjects were then exposed to a passive heat stress by perfusing 49°C water through the water-perfused suit until core temperature increased ∼1.5°C. To aid in the achievement of a ∼1.5°C core temperature increase, evaporative heat loss was minimized using waterproof pants and jacket. To evaluate the effect of a moderate simulated orthostatic challenge on BP measurements, while remaining heat stressed, subjects underwent a moderate application of LBNP (range: 20–40 mmHg; mean: 26 ± 9 mmHg).
To evaluate the validity of each BP method during a severe hypotensive challenge, six subjects, while still heat-stressed, underwent an LBNP tolerance test until the onset of presyncopal symptoms. For this test, LBNP began at 20 mmHg and was progressively reduced by 10 mmHg every 3 min until presyncope. Presyncope was defined by the following criteria: continued self-reporting by the subject of feeling faint or nauseous, continued SBP of <80 mmHg [measured by BPAUSC, given that this is the approach often used by others (6, 8, 20, 31, 35, 36)], and/or relative bradycardia accompanied by narrowing of pulse pressure.
BPAUSC measures were obtained at baseline and after core temperature increased ∼1.5°C. During the moderate hypotensive challenge, BPAUSC was measured at least 1 min after achieving the desired LBNP. During the LBNP tolerance test, BPAUSC was measured at the end of each LBNP stage until SBP approached 90 mmHg, at which time it was continuously acquired with only minimal breaks between measures. Given the time needed for measurement of BPAUSC, coupled with rapid changes in arterial pressure at presyncope, consistent BPAUSC measures were not feasible at presyncope. To evaluate BPAUSC validity during the severe hypotensive challenge, BP was compared between all three BP methods at the final BPAUSC measure. This reading corresponded to −40 ± 17 mmHg LBNP, which typically occurred during the final minute of LBNP before termination due to presyncopal symptoms.
BPINTRA was measured throughout testing, while BPPENAZ was suspended during heating to minimize finger discomfort, although the BPPENAZ cuff was not removed from the finger throughout testing. BPPENAZ was resumed at least 5 min before baseline hyperthermic measures. The initiation and completion of each BPAUSC were noted, and beat-by-beat BPPENAZ and BPINTRA data were averaged over that same time period. BPPENAZ and BPINTRA were compared during the last 5–10 s of the LBNP tolerance test (i.e., at presyncope).
Data analysis.
To examine absolute BPAUSC and BPPENAZ validity (specific aim 1), values obtained from these methods were compared with BPINTRA. To examine the ability of BPAUSC and BPPENAZ to validly track changes in BP (specific aim 2), the change in BP (i.e., delta) from normothermia to hyperthermia was compared. Likewise, changes in BP from immediately before LBNP initiation (i.e., measures at a 1.5°C core temperature increase) to measures during LBNP were compared.
Independent of the perturbation (i.e., normothermia, heat stress, or LBNP), differences in BPINTRA vs. BPAUSC and BPPENAZ were quantified with mean bias and limits of agreement using Bland-Altman plots (3). The difference between these BP measures, with a 95% probability, will lie within the respective limits of agreement of these plots. Limits of agreement were calculated by multiplying the SD of the mean difference between paired BP methods by 2 SDs (2).
Heart rate and skin and core temperatures were sampled at a minimum of 50 Hz via a data-acquisition (Biopac System, Santa Barbara, CA). Data were analyzed at baseline normothermia, after a 1.5°C core temperature increase, at moderate LBNP, at severe LBNP (i.e., corresponding to the last BPAUSC), and at presyncope (for only BPINTRA and BPPENAZ methods). A one-way repeated-measures ANOVA was used to examine differences in skin and core temperature responses between the aforementioned perturbations. Additionally, a one-way ANOVA was used to compare absolute BPINTRA to BPAUSC and to BPPENAZ at each time point (specific aim 1). A two-way (BP method × time) repeated-measures ANOVA was used to compare changes in BPINTRA to changes in BPAUSC and BPPENAZ over time (specific aim 2). Auscultatory MBP (MBPAUSC) was calculated as pulse pressure + diastolic pressure. Bonferroni post hoc tests comparing BPINTRA to BPAUSC and BPINTRA to BPPENAZ were conducted when a significant main effect or interaction was identified from the ANOVAs. Data were analyzed using SigmaStat 3.11 (Chicago, IL) with significance set at P < 0.05 and reported as means ± SD.
RESULTS
Core and mean skin temperatures increased with heating (both P < 0.001; Table 1), but there were no further increases in either of these variables during LBNP. Heart rate increased during heat stress and moderate LBNP relative to normothermia, increasing further at severe LBNP and presyncope relative to heat stress alone (all P < 0.001; Table 1).
Table 1.
Thermal and hemodynamic data while normothermic, after an ∼1.5°C core temperature increase (heat stress) and at moderate, severe, and presyncopal LBNP
| Normothermic | Heat Stress | Moderate LBNP | Severe LBNP | Presyncope | |
|---|---|---|---|---|---|
| Mean skin temperature, °C | 34.7 ± 0.5 | 39.2 ± 0.8* | 38.8 ± 1.1* | 39.0 ± 1.2* | 39.0 ± 1.2* |
| Core temperature, °C | 37.04 ± 0.42 | 38.51 ± 0.43* | 38.55 ± 0.53* | 38.73 ± 0.56* | 38.75 ± 0.57* |
| Heart rate, beats/min | 62 ± 9 | 107 ± 17* | 117 ± 15* | 134 ± 19*† | 137 ± 13*† |
Values are means ± SD; n = 10 for normothermic, heat stress, and moderate lower body negative pressure (LBNP); n = 6 for severe LBNP and presyncope conditions.
Significantly different from normothermic (P < 0.05).
Significantly different from heat stress (P < 0.05).
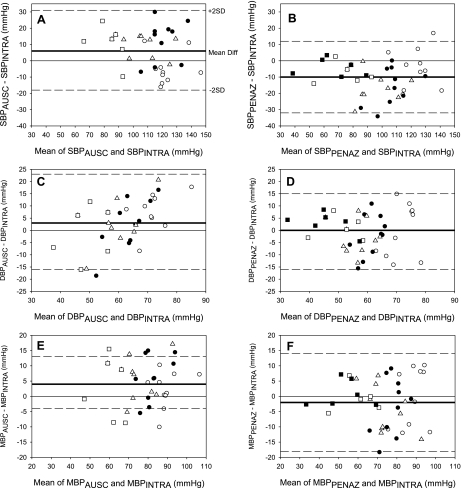
For the entire protocol, the mean difference between BPAUSC and BPINTRA ± the limits of agreement (i.e., 2 SD) was 6 ± 25 mmHg for SBP, 3 ± 19 mmHg for DBP, and 4 ± 9 mmHg for MBP (Fig. 1, A, C, and E). The mean difference between BPPENAZ and BPINTRA ± the limits of agreement was −10 ± 22 mmHg for SBP, 0 ± 16 mmHg for DBP, and −2 ± 16 mmHg for MBP (Fig. 1, B, D, and F).
Fig. 1.
Bland-Altman plot comparing systolic (SBP; A and B), diastolic (DBP; C and D), and mean arterial pressures (MBP; E and F) in intra-arterial blood pressure (BPINTRA) to auscultatory (BPAUSC; A, C, E) and Penaz BPs (BPPENAZ; B, D, F). Bold horizontal line indicates mean difference between methods; dashed horizontal lines are the limits of agreement (i.e., ±2 SD of the mean difference). ○, BP during normothermia (n = 10); ●, BP during heat stress [before lower body negative pressure (LBNP); n = 10]; ▵, BP during moderate LBNP (n = 10); □, BP during severe LBNP (n = 6); ■, BP at presyncope (n = 6).
Specific aim 1: is absolute BP measured with BPAUSC and BPPENAZ during heat-stress with and without an orthostatic challenge valid?
Normothermic Penaz SBP (SBPPENAZ), DBP (DBPPENAZ), and MBP (MBPPENAZ) and BPAUSC were not different from BPINTRA (P > 0.05); see Table 2. During heat stress, auscultatory SBP (SBPAUSC) was greater than (P = 0.042) and SBPPENAZ was less than (P = 0.008) intra-arterial SBP (SBPINTRA). There were no differences in DBP or MBP between methods (P > 0.05) while heat stressed.
Table 2.
Blood pressure measurements while normothermic, after an ∼1.5°C core temperature increase (heat stress) and at moderate, severe, and presyncopal LBNP
| Normothermic | Heat Stress | Moderate LBNP | Severe LBNP | Presyncope | |
|---|---|---|---|---|---|
| Systolic blood pressure | |||||
| Intra-arterial | 126 ± 12 | 115 ± 10 | 100 ± 16 | 79 ± 14 | 69 ± 18 |
| Ausculatory | 121 ± 12 | 127 ± 13* | 110 ± 14* | 89 ± 9* | n/a |
| Penaz | 121 ± 13 | 99 ± 15* | 86 ± 15* | 72 ± 14 | 64 ± 17 |
| Diastolic blood pressure | |||||
| Intra-arterial | 68 ± 5 | 62 ± 4 | 57 ± 7 | 49 ± 8 | 44 ± 10 |
| Ausculatory | 76 ± 8 | 65 ± 11 | 61 ± 11 | 48 ± 10 | n/a |
| Penaz | 68 ± 8 | 60 ± 7 | 55 ± 6 | 51 ± 7 | 47 ± 8 |
| Mean blood pressure | |||||
| Intra-arterial | 88 ± 7 | 79 ± 6 | 71 ± 10 | 59 ± 10 | 52 ± 12 |
| Ausculatory | 90 ± 8 | 85 ± 9 | 77 ± 11 | 62 ± 9 | n/a |
| Penaz | 88 ± 10 | 76 ± 9 | 67 ± 9 | 59 ± 9 | 53 ± 13 |
Values are means ± SD; n = 10 for normothermic, heat stress, and moderate LBNP; n = 6 for severe LBNP and presyncope conditions. n/a, The time required to obtain ausculatory blood pressure prevented a presyncope measurement.
Significantly different than intra-arterial blood pressure at the corresponding time point (P < 0.05).
SBPAUSC was greater than SBPINTRA at moderate (P = 0.008) and severe (P = 0.035) LBNP, while SBPPENAZ was less than SBPINTRA at moderate (P < 0.001) LBNP. SBPPENAZ and SBPINTRA did not differ at presyncope (P = 0.123). Auscultatory DBP (DBPAUSC), MBPAUSC, and BPPENAZ did not differ from BPINTRA during any point during LBNP (P > 0.05; see Table 2).
Specific aim 2: do BPAUSC and BPPENAZ track the change in BPINTRA that occurs with heat stress and a subsequent orthostatic challenge?
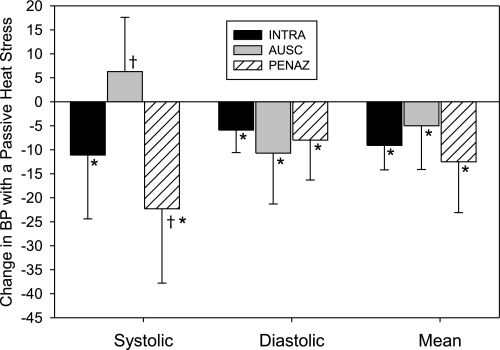
The magnitude of the decrease in SBP from normothermia to heat stress was greater for SBPPENAZ than for SBPINTRA (P = 0.002; Fig. 2). Because SBPINTRA decreased with heat stress and SBPAUSC did not (P = 0.156), the change in SBP with heat stress differed between these methods (P < 0.001). The magnitude of the decrease in DBP and MBP with heat stress was similar between measurement methods (P > 0.05; Fig. 2).
Fig. 2.
Changes from normothermia to heat stress in SBP, DBP, and MBP with BPINTRA, BPAUSC, and BPPENAZ (n = 10). Values are means ± SD. †Significantly different from BPINTRA (P < 0.05). *Significantly different from 0 (i.e., significant change from normothermic BP) (P < 0.05).
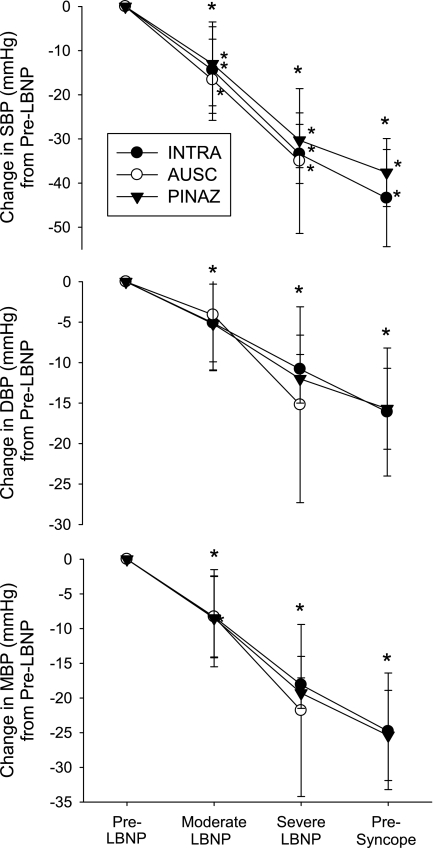
The magnitude of the reduction in SBP, DBP, and MBP throughout LBNP was not different between BP measurement methodologies (P < 0.05 for all; Fig. 3).
Fig. 3.
Decreases in SBP, DBP, and MBP from BPINTRA, BPAUSC, and BPPENAZ while heat stressed due to moderate LBNP (n = 10), severe LBNP (n = 6), and presyncope LBNP (n = 6). Values are means ± SD. *Significant change from previous condition independent of measurement method (P < 0.05).
DISCUSSION
The purpose of this study was to examine differences in arterial BP responses between three methods during a profound passive heat stress alone and in combination with a supine hypotensive challenge via LBNP. The main findings from this study are as follows: 1) during heat stress, absolute SBPAUSC is higher and SBPPENAZ is lower than SBPINTRA, and the changes in SBPAUSC and SBPPENAZ from normothermia to heat stress are different from SBPINTRA (Table 2 and Fig. 2); 2) there were no differences in MBP or DBP between methodologies when heat stressed; 3) during subsequent LBNP, the reductions in systolic, diastolic, and mean BPAUSC and BPPENAZ validly tracked the reduction in BPINTRA (Fig. 3); and 4) there is a large degree of variability in BPAUSC and BPPENAZ readings compared with BPINTRA (Fig. 1).
SBP did not differ between BP methods in normothermic conditions, but hyperthermic SBPAUSC and SBPPENAZ failed to track the decrease in SBPINTRA that occurred. This resulted in absolute SBPAUSC overestimating and SBPPENAZ underestimating actual SBPINTRA. The source of this error is not known. Although speculative, one possibility is that the large increase in limb blood flow during the heat stress (5) may affect the identification of the Korotkoff sounds by the microphone for the BPAUSC technique, resulting in earlier identification of the initial Korotkoff sound. With the Penaz approach, it is possible that large increases in skin blood flow and finger volume, which occurs with whole body heating, alters the capacity of that approach to validly measure SBP. Despite errors in SBPAUSC and SBPPENAZ measures during heat stress, slight differences in DBPs, although not significant, resulted in no overall differences in MBPs between methodologies. Regardless, the MBP data should be considered within the context of the very large degree of variability between these measures by the heat stress (see discussion below).
The invalid measurement of absolute SBPAUSC and SBPPENAZ during heat stress persisted throughout the hypotensive challenge (Table 2). This appeared to be independent of sex (data not shown), but a formal analysis is not warranted due to the small number of women tested (n = 3). It is important to emphasize that the magnitude of the reduction in SBP during LBNP was similar between methodologies (specific aim 2, Fig. 3). This latter observation suggests that differences in absolute SBPPENAZ, SBPAUSC, and SBPINTRA during a heat-stressed hypotensive challenge (Table 2) are due to the effect of heat stress alone and are not due to the hypotensive challenge per se. This is reinforced by data showing that SBPPENAZ appropriately measures SBP during a hypotensive challenge when individuals are normothermic (13, 24).
Particularly noticeable is the large degree of variability in the difference between the two noninvasive measures of BP and BPINTRA (Fig. 1). This level of variability has been previously reported in a variety of other testing situations (12–14, 17, 27, 33). Standards for the American Association for the Advancement of Medical Instrumentation criteria requires a mean difference of <5 mmHg, along with a standard deviation < 8 mmHg between measurement methods (1). According to these criteria, both auscultation and Penaz measurements of absolute BP are unacceptable during heat stress and a simulated orthostatic challenge (Fig. 1), as the standard deviation of these techniques were > 8 mmHg. This unacceptable degree of variability is consistent with the standards of the British Hypertension Society, in which at least 50% of the measurements between compared devices must have a difference ≤ 5 mmHg (23). Only 14% of SBPAUSC, 24% of DBPAUSC, and 26% of MBPAUSC measures throughout the protocol differed by ≤ 5 mmHg from the corresponding BPINTRA. Similarly 26% of SBPPENAZ, 36% of DBPPENAZ, and 36% of MBPPENAZ measures throughout the protocol differed ≤ 5 mmHg from the corresponding BPINTRA. Given this large degree of variability, the lack of mean differences between methodologies for DBP and MBP is misleading.
The Bland-Altman plots also reveal that the variability in device validity is independent of BP. As BP decreased with heat stress and the subsequent orthostatic challenge, the difference in BPINTRA and BPAUSC or BPPENAZ remained highly variable. Also, it is important to point out that the variability occurred not only between subjects, but also within a given subject. For example, the difference between MBPAUSC and intra-arterial MBP in one subject was −4 mmHg at rest, 11 mmHg when heat stressed, 1 mmHg during moderate LBNP, and −9 mmHg during severe LBNP. This emphasizes the point that applying a correction factor to either an individual or a group would not be appropriate. This large degree of variability should be considered when obtaining BP noninvasively.
Criteria for test termination during LBNP or upright tilt often includes a consistent SBP of <70–90 mmHg (6, 8, 18, 20, 31, 35, 36), as well as a 15- to 20-mmHg decrease in SBP (8, 15). Therefore, the validity of both absolute BPs and the changes in BP are important to appropriately identify presyncope and thus test termination during LBNP or similar orthostatic testing. The present data suggest that, if using absolute SBP as the sole termination criteria, the length of an orthostatic tolerance test in heat-stressed individuals may vary, depending on the BP measurement method (Table 2). However, if the termination criteria are based on the magnitude of the decrease in SBP, the present data suggest that decreases in SBPPENAZ appropriately reflect decreases in SBPINTRA (Fig. 3).
Invasive pressures were obtained at the radial artery, whereas noninvasive pressures were either measured at the brachial artery, for BPAUSC, or reconstruction to the brachial artery from the finger, for the BPPENAZ. Due to pulse-wave amplification, SBP may be slightly greater in the radial vs. brachial artery (22), while amplification is more pronounced when the reference vessel is the aorta (19). It is important to point out that, regardless of measurement site, MBP is not affected by pulse-wave amplification (22). While subjects were normothermic, there was no evidence of pulse-wave amplification (Table 2). However, pulse-wave amplification is affected by a variety of factors, such as increased heart rate and vasodilation (4, 34). It is, therefore, possible that some of the observed differences in SBP while heat stressed were due to increases in heart rate and vasodilation affecting pulse-wave amplification. To investigate if the observed differences in SBP were due to differences between radial and brachial artery pressures profiles, BPINTRA waveforms were reconstructed to reflect brachial artery waveforms with the same technology used to reconstruct Finometer waveforms (BeatScope version 1.1, FinaPres Medical Systems, Arnhem, the Netherlands). Reconstructed SBPINTRA was 8 ± 3 mmHg lower (P < 0.001) than the original SBPINTRA, and this decrease was independent of perturbation (i.e., normothermia, heat stress, and LBNP; P = 0.096). Consequently, the absolute values of reconstructed SBPINTRA and SBPPENAZ were similar during heat stress and at moderate LBNP (P = 0.35 and 0.22, respectively). Despite the effects of reconstructing SBPINTRA on the interpretation of the findings, we chose to use non-reconstructed data for the primary analysis of BPINTRA. Justification for this selection is that most laboratories that have the capacity to measure BPINTRA will not have the capability to reconstruct these radial pressures. In those laboratories, decisions regarding the effects of a perturbation, such as LBNP tolerance, will be based on the non-reconstructed pressure readings. Independent of this, the large variability in MAP illustrated in Fig. 1 would remain after accounting for pulse-wave amplification, given similar MBPs across the arterial tree.
Limitiations.
Throughout the study, subjects were severely passively heated and in a supine position. The magnitude of increase in core temperature was chosen to be consistent with numerous studies using similar levels of heating (6, 8, 18, 35, 36). During profound heat stress, such as the one employed, skin and limb blood flow responses are influenced by body position (supine vs. upright) and activity level (rest vs. exercise) (16). Given this, coupled with the possibility that the inaccuracies of SBPAUSC and SBPPENAZ during passive heat stress were due to changes in skin and limb blood flow during heat stress, the results may be different if heat stress occurred while subjects were upright or during dynamic exercise or at different body positions. Therefore, the present findings are specific to the employed level of passive heat stress and LBNP while subjects are supine and are not necessarily representative of differing experimental conditions.
Conclusions.
With the exception of SBP, both BPPENAZ and BPAUSC track absolute BPINTRA across all perturbations. However, there is a large degree of variability between both BPINTRA and BPPENAZ, as well as BPINTRA and BPAUSC. This large degree of variability should be considered when noninvasively measuring BP during heat stress alone and in combination with an orthostatic challenge. Finally, the relative decreases in BPINTRA during an orthostatic challenge while subjects are heat stressed are appropriately tracked with BPAUSC and BPPENAZ.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL61388 and HL84072.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge the volunteers for their time. The assistance of Kim Hubing and Jena Langlois is appreciated.
REFERENCES
- 1. Association for the Advancement of Medical Instrumentation In: American National Standard, Electronic or Automated Sphygmomanometers. Arlington, VA: AAMI, 1993, p. 40 [Google Scholar]
- 2.Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med 26: 217–238, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310, 1986 [PubMed] [Google Scholar]
- 4.Bos WJ, van den Meiracker AH, Wesseling KH, Schalekamp MA. Effect of regional and systemic changes in vasomotor tone on finger pressure amplification. Hypertension 26: 315–320, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Brothers RM, Wingo JE, Hubing KA, Crandall CG. Methodological assessment of skin and limb blood flows in the human forearm during thermal and baroreceptor provocations. J Appl Physiol 109: 895–900, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durand S, Cui J, Williams KD, Crandall CG. Skin surface cooling improves orthostatic tolerance in normothermic individuals. Am J Physiol Regul Integr Comp Physiol 286: R199–R205, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Eguchi K, Yacoub M, Jhalani J, Gerin W, Schwartz JE, Pickering TG. Consistency of blood pressure differences between the left and right arms. Arch Intern Med 167: 388–393, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Engelke KA, Doerr DF, Crandall CG, Convertino VA. Application of acute maximal exercise to protect orthostatic tolerance after simulated microgravity. Am J Physiol Regul Integr Comp Physiol 271: R837–R847, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Furtado EC, Ramos Pdos S, Araujo CG. Blood pressure measurement during aerobic exercise: subsidies for cardiac rehabilitation. Arq Bras Cardiol 93: 45–52, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Glasser SP, Ramsey MR., 3rd An automated system for blood pressure determination during exercise. Circulation 63: 348–353, 1981 [DOI] [PubMed] [Google Scholar]
- 11.Guelen I, Westerhof BE, Van Der Sar GL, Van Montfrans GA, Kiemeneij F, Wesseling KH, Bos WJ. Finometer, finger pressure measurements with the possibility to reconstruct brachial pressure. Blood Press Monit 8: 27–30, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Hunyor SN, Flynn JM, Cochineas C. Comparison of performance of various sphygmomanometers with intra-arterial blood-pressure readings. Br Med J 2: 159–162, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imholz BP, Settels JJ, van der Meiracker AH, Wesseling KH, Wieling W. Non-invasive continuous finger blood pressure measurement during orthostatic stress compared to intra-arterial pressure. Cardiovasc Res 24: 214–221, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Imholz BP, van Montfrans GA, Settels JJ, van der Hoeven GM, Karemaker JM, Wieling W. Continuous non-invasive blood pressure monitoring: reliability of Finapres device during the Valsalva manoeuvre. Cardiovasc Res 22: 390–397, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Jarvis SS, Pawelczyk JA. The location of the human volume indifferent point predicts orthostatic tolerance. Eur J Appl Physiol 109: 331–341, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Johnson JM, Rowell LB, Brengelmann GL. Modification of the skin blood flow-body temperature relationship by upright exercise. J Appl Physiol 37: 880–886, 1974 [DOI] [PubMed] [Google Scholar]
- 17.Jones RD, Brown AG, Roulson CJ, Smith ID, Chan SC. The upgraded Finapres 2300e. A clinical evaluation of a continuous noninvasive blood pressure monitor. Anaesthesia 47: 701–705, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Keller DM, Low DA, Wingo JE, Brothers RM, Hastings J, Davis SL, Crandall CG. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. J Physiol 587: 1131–1139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroeker EJ, Wood EH. Comparison of simultaneously recorded central and peripheral arterial pressure pulses during rest, exercise and tilted position in man. Circ Res 3: 623–632, 1955 [DOI] [PubMed] [Google Scholar]
- 20.Lewis NC, Atkinson G, Lucas SJ, Grant EJ, Jones H, Tzeng YC, Horsman H, Ainslie PN. Diurnal variation in time to presyncope and associated circulatory changes during a controlled orthostatic challenge. Am J Physiol Regul Integr Comp Physiol 299: R55–R61, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Livi R, Teghini L, Cagnoni S, Scarpelli PT. Simultaneous and sequential same-arm measurements in the validation studies of automated blood pressure measuring devices. Am J Hypertens 9: 1228–1231, 1996 [DOI] [PubMed] [Google Scholar]
- 22.McDonald DA. Contours of pressure and flow waves in arteries In: McDonald's Blood Flow in Arteries (5th ed.), edited by Nichols WW, O'Rourke MF. New York: Oxford University Press, 2005 [Google Scholar]
- 23.O'Brien E, Petrie J, Littler W, de Swiet M, Padfield PL, Altman DG, Bland M, Coats A, Atkins N. An outline of the revised British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens 11: 677–679, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 13: 647–655, 1989 [DOI] [PubMed] [Google Scholar]
- 25. Penaz J. Photoelectric measurement of blood pressure, volume and flow in the finger. In: Proceedings of the Conference Committee of the 10th International Conference on Medicine and Biological Engineering, Dresden, 1973, p. 104 [Google Scholar]
- 26.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals. 1. Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 111: 697–716, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Porter KB, O'Brien WF, Kiefert V, Knuppel RA. Finapres: a noninvasive device to monitor blood pressure. Obstet Gynecol 78: 430–433, 1991 [PubMed] [Google Scholar]
- 28.Rowell LB. Thermal stress. In: Human Circulation: Regulation During Physical Stress. London: Oxford University Press, 1986, p. 174–212 [Google Scholar]
- 29.Schutte AE, Huisman HW, van Rooyen JM, Malan NT, Schutte R. Validation of the Finometer device for measurement of blood pressure in black women. J Hum Hypertens 18: 79–84, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Silke B, McAuley D. Accuracy and precision of blood pressure determination with the Finapres: an overview using re-sampling statistics. J Hum Hypertens 12: 403–409, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Taneja I, Moran C, Medow MS, Glover JL, Montgomery LD, Stewart JM. Differential effects of lower body negative pressure and upright tilt on splanchnic blood volume. Am J Physiol Heart Circ Physiol 292: H1420–H1426, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Turjanmaa V. Determination of blood pressure level and changes in physiological situations: comparison of the standard cuff method with direct intra-arterial recording. Clin Physiol 9: 373–387, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Wesseling KH, Settels JJ, van der Hoeven GM, Nijboer JA, Butijn MW, Dorlas JC. Effects of peripheral vasoconstriction on the measurement of blood pressure in a finger. Cardiovasc Res 19: 139–145, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol 291: R1443–R1448, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol 93: 85–91, 2002 [DOI] [PubMed] [Google Scholar]