Abstract
Reduced efficacy of cardioprotective interventions in the aged female heart, including estrogen replacement, highlights the need for alternative therapeutics to reduce myocardial ischemia-reperfusion (I/R) injury in postmenopausal women. Here, we sought to determine the efficacy of protein kinase-Cε (PKCε)-mediated cardioprotection in the aged, estradiol-deficient rat heart. Infarct size and functional recovery were assessed in Langendorff-perfused hearts from adult (5 mo) or aged (23 mo) female Fisher 344 ovary-intact or ovariectomized (OVX) rats administered a PKCε-activator, receptor for activated C kinase (ψεRACK) prior to 47-min ischemia and 60-min reperfusion. Proteomic analysis was conducted on left ventricular mitochondrial fractions treated with ψεRACK prior to I/R, utilizing isobaric tags for relative and absolute quantitation (iTRAQ) 8plex labeling and tandem mass spectrometry. Real-time PCR was utilized to assess connexin 43 (Cx43) and RACK2 mRNA post-I/R. Greater infarct size in aged OVX (78%) vs. adult (37%) was reduced by ψεRACK (35%, P < 0.0001) and associated with greater mitochondrial PKCε localization (P < 0.0003). Proteomic analysis revealed three novel mitochondrial targets of PKCε-mediated cardioprotection with aging (P < 0.05): the antioxidant enzymes glutathione peroxidase (GPX) and MnSOD2, and heat shock protein 10. Finally, decreased levels of Cx43 and RACK2 mRNA seen with age were partially abrogated by administration of ψεRACK (P < 0.05). The mechanisms described here may represent important therapeutic candidates for the treatment of acute myocardial infarction in postmenopausal women and age-associated estradiol deficiency.
Keywords: ischemia, superoxide dismutase, glutathione peroxidase, heat shock protein 10, myocardial infarction
morbidity and mortality due to acute myocardial infarction are increased at the age of menopause (38), implicating the loss of estradiol (E2) in greater cardiovascular risk for older postmenopausal women (32). Epidemiological studies and clinical intervention trials have failed, however, to demonstrate cardiovascular risk reduction in older women taking hormone replacement therapy (1, 16, 30), despite experimental evidence for ischemic protection afforded by E2 replacement in ovariectomized animal models (3, 10, 24, 28, 41). These findings illustrate the need for a greater understanding of the changes contributing to impaired ischemic tolerance in the aged, E2-deficient heart and for the identification of alternative therapeutics to reduce cellular injury during myocardial infarction in postmenopausal women.
Many cardioprotective interventions known to reduce ischemia-reperfusion injury (I/R) involve signaling mechanisms that converge on the mitochondria and result in activation of protein kinase-Cε (PKCε) (19). PKCε translocates to the mitochondria during both I/R and ischemic preconditioning (IPC) (8), where it is active upon binding to its specific adaptor protein receptor for activated C kinase 2 (RACK2). Importantly, acute activation of PKCε 10 min prior to ischemia by the isozyme-specific regulatory peptide pseudo-ε RACK (ψεRACK) mimics IPC and reduces I/R injury in multiple model systems (7), including in the aged male rat heart (26). However, given our previous observations of reduced mitochondrial PKCε levels in the aged female rat heart (18) combined with the well-characterized refractoriness of the aged heart to cardioprotective intervention (21), the sufficiency of acute PKCε activation to produce cardioprotection in the aged, E2-deficient rat heart was uncertain.
Therefore, the purpose of the present study was to determine the efficacy of acute PKCε activation on reducing I/R injury in the aged, E2-deficient female rat heart. We hypothesized that I/R injury would decrease following local delivery of the PKCε-activating peptide ψεRACK in association with increased mitochondrial targeting of PKCε. Toward a mechanism of this protection, we also sought to identify novel or altered mitochondrial PKCε signaling partners mediating early cardioprotective events immediately following PKCε activation using a state-of-the-art, high-throughput quantitative proteomics approach. Given the importance of the mitochondria in the normal myocardial response to I/R injury and PKCε-mediated cardioprotection, it is logical that alterations in mitochondrial protein levels immediately following PKCε activation may also contribute to improved ischemic tolerance with age-associated E2 deficiency.
MATERIALS AND METHODS
Animal care.
Adult (5 mo, n = 19) and aged (23 mo, n = 32) female Fisher 344 rats were supplied by Harlan Sprague Dawley (Indianapolis, IN) and Taconic (Hudson, NY). Rats were housed under a 12:12-h. light-dark cycle and received food and water ad libitum. All animal handling and utilization protocols were reviewed and approved by the Penn State University Institutional Animal Care and Use Committee and adhere to American Physiology Society's Guiding Principles in the Care and Use of Animals. While we have previously demonstrated age-associated reductions in E2 levels (17, 18, 33), we also utilized bilateral ovariectomy (OVX) surgeries on a subset of aged rats to exacerbate age-associated E2 deficiency (see Refs. 17, 18, 33). Animals were allowed to recover for 4–6 wk prior to the date of experimental study, and successful surgery was confirmed at the time of death by measuring uterine weight. Ovary-intact animals were used as age-matched controls.
PKCε regulatory peptides.
A specific PKCε activator peptide (ψεRACK; C-HDAPIGYD) derived from the PKCε pseudo-RACK sequence (PKCε amino acids 85 to 92) and a PKCε inhibitory peptide (εV1–2; C-EAVSLKPT) derived from the PKCε RACK-binding sequence (PKCε amino acids 14–21) were provided by KAI Pharmaceuticals (San Francisco, CA). Peptides were conjugated reversibly to a Tat-derived carrier peptide [Tat amino acids 43–58 (C-YGRKKKRRQRRR)] by disulfide bond at the NH2 terminus.
Isolated heart preparation.
Animals were anesthetized by pentobarbital sodium (40 mg/kg body wt ip), and hearts were rapidly excised by midline thoracotomy and immediately rinsed in ice-cold (4°C) saline. Within 60 s of excision, hearts were secured via aortic cannulation to a Langendorff apparatus and perfused at constant pressure (85 mmHg), temperature (37°C), and pH (7.4) with a modified Krebs-Henseleit bicarbonate buffer containing (in mM): 1.75 CaCl2, 117.4 NaCl, 4.7 KCl, 1.2 MgSO4, 1.3 KH2PO4, 24.7 NaHCO3, 11 glucose, 0.5 pyruvate, 0.5 EDTA, and 1.2 USP units/ml heparin. Hearts were paced at 260 beats/min and a fluid-filled latex balloon inserted into the left ventricle (LV) was inflated to yield an end-diastolic pressure (EDP) of 5 to 6 mmHg. LV function was assessed using the Ponemah Physiology platform (Gould Instrument Systems, Valley View, OH).
Protocol of the isolated heart study.
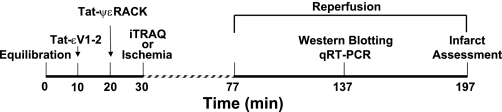
Hearts were perfused as described above for an equilibration period of 30 min duration (Fig. 1). Control hearts were perfused for the entire equilibration period, while drug-treated hearts received either 1) Tat-ψεRACK (500 nM) via the perfusate for the final 10 min of equilibration or 2) Tat-εV1–2 (1 μM) for 10 min immediately followed by coadministration of Tat-ψεRACK (500 nM) for the final 10 min of equilibration. For baseline analytical and proteomics studies, LVs were isolated, weighed, halved, and snap frozen in liquid N2 following the initial 30-min equilibration period and prior to I/R. For functional studies, hearts were subjected to 47-min global, no-flow ischemia. Pacing was resumed 1.5 min after the restoration of flow, and hearts were reperfused for either 60 min (for biochemistry studies) or 120 min (for infarct size analysis). All isolated LV sections were stored at −80°C until tissue preparation.
Fig. 1.
Protocol of the isolated heart studies. Following 30-min equilabration, hearts were either subjected to ischemia-reperfusion or were snap frozen for isobaric tags for relative and absolute quantitation (iTRAQ) processing. RACK, receptor for activated C kinase 2; qRT-PCR, real-time PCR.
Infarct size assessment.
A subset of hearts (n = 3–5 per group) underwent 47-min ischemia followed by 120-min reperfusion, and the area of infarction was assessed as described previously by Downey (31). Briefly, after reperfusion LVs were isolated, weighed, and frozen at −20°C for 30 min, LVs were then sliced transversely and stained with 1% triphenyltetrazolium chloride in phosphate buffer (pH 7.4) for 20 min at 37°C, followed by overnight incubation in 10% formalin at room temperature. Heart slices were mounted onto glass plates at 1.5-mm thickness, and both sides of each slice were digitally photographed using a camera mounted to a SZX9 microscope (Olympus). Digital images of infarcted LV were analyzed using National Institutes of Health ImageJ software.
Tissue sample preparation.
For Western blot analysis, total LV homogenates and mitochondrial subcellular protein fractions were prepared as described previously (18). All protein concentrations were determined by the method of Bradford (4). For proteomics studies, isolated mitochondrial protein homogenates were prepared exactly as previously published (17, 33), except that Tris·HCl (pH 7.4) was replaced with equivalent concentrations of HEPES (pH 7.4). This substitution was necessary to provide a primary amine-free buffer for subsequent proteomic labeling (see below). We have previously demonstrated that cytosolic contamination of the mitochondrial fraction is < 2%, as measured by lactate dehydrogenase activity (17), and no direct indications of plasma membrane contamination (i.e., identification of proteins exclusively expressed in the plasma membrane, such as ecto-5′-nucleotidase) were observed in the current proteomics studies.
Western blot analysis.
Western blot analysis was performed according to well-established procedures in our laboratory (25). Briefly, equal amounts of total, and/or mitochondrial protein sample were electrophoresed on SDS-polyacrylamide gels (Bio-Rad) and transferred to polyvinylidene difluoride membranes. Following incubation in blocking buffer (6% nonfat milk), membranes were probed with primary antibody against PKCε (Santa Cruz Biotechnology, Santa Cruz, CA), Akt and phospho (p) Akt473 (Cell Signaling), heat shock protein 10 (HSP10; Stressgen), glutathione peroxidase (GPX; Abcam), and manganese superoxide dismutase 2 (SOD2, MnSOD; Caymen Chemical). Membranes were incubated with appropriate horseradish peroxidase-linked secondary antibody, and immunoreactive bands were visualized by enhanced chemiluminescence (GE Amersham). Densitometry analysis was performed using Scion Image (NIH). Minor differences in protein loading were corrected for by SYPRO Ruby staining (Invitrogen), and densitometry values were adjusted as described previously (36). All Western blot analyses were expressed relative to the adult control group.
Isobaric tags for relative and absolute quantitation sample preparation and analysis.
LV mitochondrial protein homogenates were prepared for proteomics analysis using the isobaric tags for relative and absolute quantitation (iTRAQ) 8Plex Reagent Kit (Applied Biosystems) according to the manufacturer's instructions. Briefly, 100 μg of protein from each mitochondrial sample was individually aliquoted and sequentially labeled with the iTRAQ reagents (113–119 and 121 m/z). Two-dimensional liquid chromatography (2D LC) separation and tandem mass spectrometry (MS/MS) analysis of the prepared samples was conducted at the Penn State Hershey Proteomics Core facility, specifically utilizing SCX LC separation, 15X nanoflow C18 reversed-phase LC separation, matrix-assisted laser desorption/ionization (MALDI) plate spotting, and tandem time-of-flight mass analysis.
Protein identifications and protein quantitation were determined from MS/MS spectra using the Paragon algorithm (44) of the ProteinPilot version 3.0 software package (ABI-MDSSciex), searching the NCBInr (National Center for Biotechnology Information) protein database concatenated to a reversed version of itself (as a Decoy database). Only those proteins identified at an instantaneous (local) false discovery rate < 5%, which was determined by analyzing the number of Decoy (reversed) database hits using the PSPEP (Proteomics System Performance Evaluation Pipeline) beta software tool (ABI-MDSSciex), were considered positive identifications (12).
Real-time PCR.
RNA was isolated as previously described by Chomczynski and Sacchi (9). Samples were amplified (ABI model 7300) with the reference gene cyclophilin (CyP). Forward and reverse primers for RACK2 and connexin 43 (CX43) were as follows: RACK2, 5′-GCAAATTGAAGGAAGCAGAATTG-3′, 5′-GCTGTGACGATG TTCCAAACA-3′; CX43, 5′-GCTCCTCACCAACGGCT- 3′, 5′-TTGCGGCAGGAGGAATTG-3′.
Statistical analysis.
With the exception of proteomics results, all data are presented as means ± SE and were analyzed using the Statistical Analysis System general linear models procedure. With the exception of Western blot analysis data for PKCε from baseline preischemic control hearts (two-way ANOVA), all data were analyzed by one-way ANOVA. All post hoc comparisons were analyzed using the Tukey-Kramer or Duncan method.
iTRAQ quantitative proteomics data are presented as protein expression ratios and error factors. Statistical analysis of iTRAQ quantitative protein data was automated by the Paragon algorithm of the ProteinPilot version 3.0 software package and is based on the contributing peptide ratios. An α-level of P < 0.05 was defined as statistically significant for all comparisons.
RESULTS
Baseline morphology and function.
Morphology and LV functional characteristics recorded during the equilibrium perfusion for adult, aged, and aged OVX rats are presented in Table 1. Rat weight was significantly increased by aging and further increased in aged OVX (P < 0.0001). LV weight was similarly increased in aged and aged OVX (P < 0.0001). LV developed pressure (LVDP) and first derivative of LVDP curve (−dP/dt) were significantly reduced in aged and aged OVX rats (P < 0.0001). No significant differences were observed in LV weight-to-body weight ratio, EDP, +dP/dt, and time-to-peak pressure development.
Table 1.
Baseline morphology and functional characteristics
| Characteristic | Adult | Aged | Aged OVX |
|---|---|---|---|
| No. | 19 | 16 | 16 |
| Body weight, g | 183 ± 3 | 260 ± 7* | 283 ± 10*† |
| LV weight, mg | 0.50 ± 0.02 | 0.81 ± 0.02* | 0.81 ± 0.02* |
| LV weight/body weight, mg/g | 2.73 ± 0.01 | 3.12 ± 0.03 | 2.86 ± 0.01 |
| EDP, mmHg | 5.59 ± 0.03 | 5.61 ± 0.09 | 5.66 ± 0.07 |
| LVDP, mmHg | 139.7 ± 2.9 | 131.5 ± 2.4* | 133.7 ± 4.1* |
| +dP/dt, mmHg/s | 3721 ± 146 | 3664 ± 133* | 3850 ± 195* |
| 23>dP/dt, mmHg/s | 2392 ± 52 | 2344 ± 100* | 2353 ± 107* |
| TTPK, ms | 70.0 ± 1.6 | 66.8 ± 3.1 | 66.9 ± 1.8 |
OVX, ovariectomized; LV, left ventricle; EDP, end-diastolic pressure; LVDP, left ventricular developed pressure; dP/dt, first derivative of LVDP curve; TTPK, time to peak pressure development.
Different from adult,
different from aged; P < 0.0001.
Recovery from I/R following acute PKCε activation.
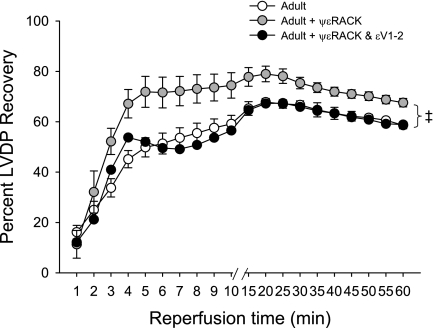
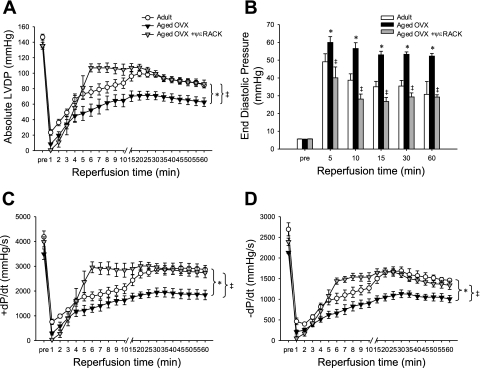
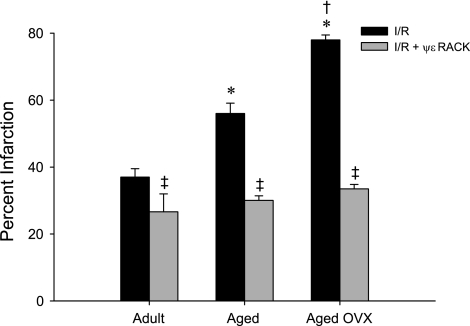
Acute activation of PKCε by administration of ψεRACK prior to ischemia improved functional recovery in the adult ovary-intact female rat heart, as indicated by improved percentage recovery of baseline LVDP during reperfusion (P < 0.0001), and this effect was blocked by coadministration of ψεRACK with the PKCε inhibitor εV1–2 (Fig. 2). Functional recovery following 47-min ischemia was reduced in the aged OVX rat heart, as indicated by reduced recovery of LVDP and reduced recovery of positive and negative dP/dt compared with adult ovary-intact rat hearts (all P < 0.0001). These reductions in functional recovery in the aged OVX rat heart were improved by administration of ψεRACK prior to ischemia (P < 0.0001, Fig. 3), with percentage recovery of baseline LVDP peaking at ∼79% (vs. 38% without ψεRACK) during reperfusion. A similar protective effect of ψεRACK on recovery of LVDP was observed in aged animals with ovaries intact (data not shown). A greater increase in EDP during reperfusion was also observed in aged OVX compared with adult ovary-intact rat hearts, and this increase was attenuated by ψεRACK treatment (P < 0.0001, Fig. 3). Infarct size was also greater in the aged ovary-intact and aged OVX rat heart compared with the adult ovary-intact rat heart. Infarct size was reduced in all three groups by ψεRACK administration prior to ischemia (Fig. 4).
Fig. 2.
Acute PKCε activation is cardioprotective in the adult female rat heart. Recovery of left ventricular developed pressure (LVDP), expressed as %recovery of baseline (preischemia) LVDP during reperfusion, following 47-min ischemia was improved by ψεRACK administration prior to ischemia. This effect was blocked by coadministration of ψεRACK with εV1–2. ‡Effect of drug; P < 0.0001.
Fig. 3.
Reduced functional recovery in the aged, estradiol (E2)-deficient rat heart is reversed by acute PKCε activation. Absolute recovery of LVDP (A), recovery of end-diastolic pressure (EDP; B), recovery of change in pressure over time (+dP/dt; C), and recovery of −dP/dt (D) were reduced in the aged overiectomized (OVX) rat heart following 47-min ischemia and were improved by ψεRACK administration prior to ischemia. Values are means ± SE (n = 10–13/group). *Effect of age+OVX, ‡effect of drug; P < 0.0001.
Fig. 4.
Increased infarct size in the aged, E2-deficient rat heart is reduced by acute PKCε activation. Following 47-min ischemia and 120-min reperfusion, infarct size (expressed as % LV mass infarcted) was greater in the aged and aged OVX rat heart compared with the adult rat heart. Infarct size was reduced in all groups by ψεRACK administration prior to ischemia. Values are means ± SE (n = 3–5/group). I/R, ischemia-reperfusion. *Effect of age, †effect of OVX, ‡effect of drug; P < 0.0001.
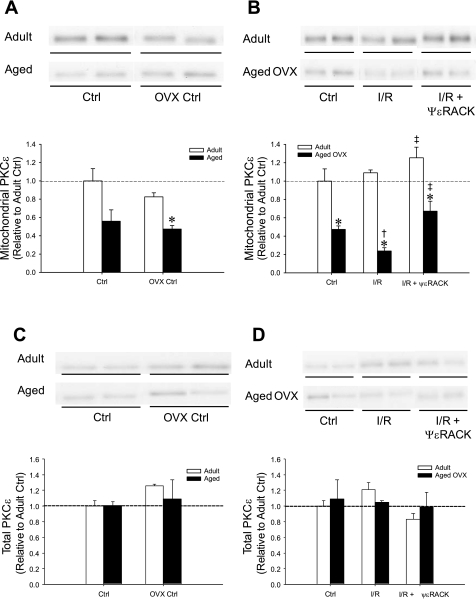
Left ventricular PKCε and Akt protein levels.
Western blot analysis was used to measure relative protein levels of mitochondrial and total PKCε. All Western blot analysis values are expressed relative to the adult ovary-intact group at the baseline preischemic control time point. Prior to I/R, mitochondrial PKCε was significantly reduced by ∼50% in aged OVX hearts (P = 0.0238; Fig. 5A). Mitochondrial PKCε was further reduced by ∼30% following I/R in aged OVX, and was increased with ψεRACK treatment in both adult ovary-intact and aged OVX following I/R (P < 0.0003, Fig. 5B). Total PKCε expression was not significantly altered in the experimental groups (Fig. 5, C and D). Total and phosphorylated Akt protein levels were significantly reduced in aged OVX vs. adult at baseline and following I/R with and without ψεRACK treatment (data not shown).
Fig. 5.
Western blot analysis demonstrating effects of age+OVX and ψεRACK on mitochondrial PKCε in hearts at baseline preischemic control (Ctrl; A) and following I/R (B) and total PKCε levels (C) at baseline preischemic control (Ctrl) and following I/R (D). Values are means ± SE (n = 3–4/group). *Effect of age+OVX, †effect of I/R, ‡effect of drug; P < 0.03.
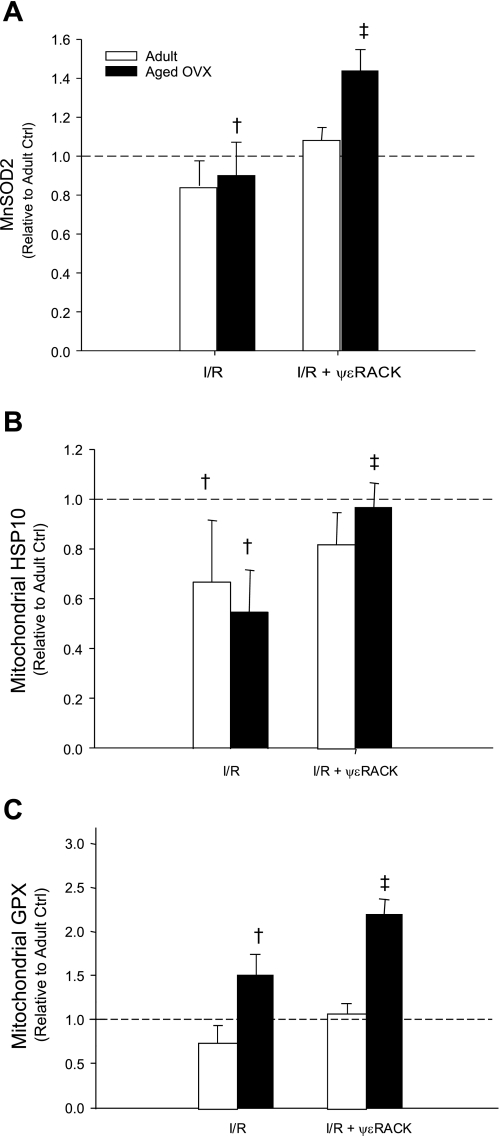
iTRAQ analysis of LV mitochondrial proteins following ψεRACK.
For the quantitative assessment of cardiac mitochondrial proteins in E2-deficient aged rats following acute PKCε activation, iTRAQ 8plex proteomics analyses were conducted. Proteins of interest were those that differed significantly in aged OVX+ψεRACK relative to aged OVX. Significant increases in SOD2, HSP10, and GPX were observed (Table 2). Subsequent Western blot analysis demonstrated increased mitochondrial protein levels for SOD2, HSP10, and GPX, respectively, following ψεRACK treatment and I/R injury in aged OVX (Fig. 6). Notably, I/R resulted in significant reductions in SOD2, HSP10, and GPX in aged OVX (P < 0.01), while I/R-induced reductions were only observed for HSP10 in adult. Levels of SOD2, HSP10, and GPX were unaffected by ψεRACK treatment in total protein homogenates (data not shown).
Table 2.
Possible downstream signaling targets of PKCε
| Unused Score | Total Score | % Coverage | Accession No. | Protein Identified | Quantitation Ratio Aged OVX + ψεRACK/Aged OVX | P Value | Error Factor | PANTHER Biological Process |
|---|---|---|---|---|---|---|---|---|
| 17.3 | 17.3 | 82.4 | gi|461731 | 10 kDa HSP10, mitochondrial | 1.11 | 0.0134 | 1.08 | Protein modification; stress response |
| 5.6 | 5.6 | 37.6 | gi|68138297 | Glutathione peroxidase | 1.21 | 0.0073 | 1.09 | Antioxidation and free radical removal |
| 15.3 | 15.3 | 50.9 | gi|8394331 | SOD2, mitochondrial | 1.28 | 0.0001 | 1.07 | Antioxidation and free radical removal |
Cardiac mitochondrial proteins demonstrating altered expression following receptor for activated C kinase (ψεRACK) treatment in the aged OVX rat heart were identified from 3 isobaric tags for relative and absolute quantitation (iTRAQ) 8plex analyses and are grouped according to associated biological processes. HSP, heat shock protein. Bold indicates that the difference in expression was significant relative to aged OVX control (n = 4/group).
Fig. 6.
Western blot analysis demonstrating effects of age+OVX and ψεRACK on SOD2 (A), heat shock protein 10 (HSP10; B), and glutathione peroxidase (GPX; C) levels following I/R. Values are means ± SE (n = 3–4/group); †effect of I/R, ‡effect of drug; P < 0.01.
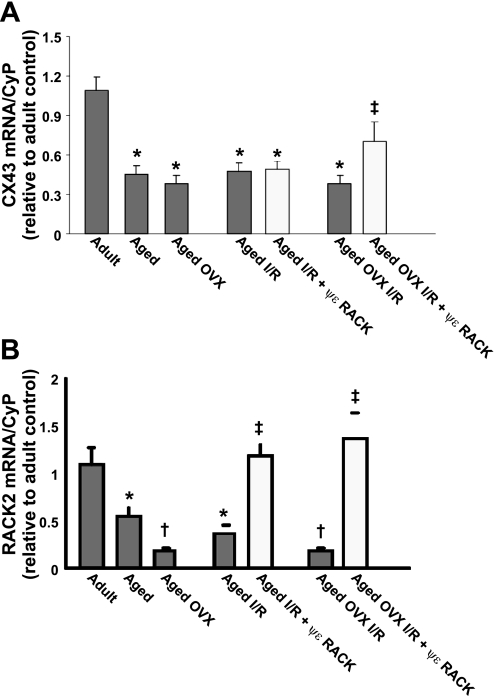
mRNA quantitation.
RNA isolated from LV's was subjected to real-time PCR using primers for RACK2 and Cx43. RACK2 mRNA levels were significantly decreased with age and OVX at baseline, while treatment with ψεRACK prior to I/R was sufficient to return RACK2 mRNA levels to those seen in adult hearts following I/R (Fig. 7A). Cx43 mRNA levels also decreased significantly with age, but no additional decrease in Cx43 levels were seen with aged OVX (Fig. 7A). Administration of ψεRACK increased Cx43 mRNA levels in aged OVX following I/R, but not in aged (Fig. 7B).
Fig. 7.
Real-time PCR demonstrating effects of age+OVX and ψεRACK on LV mRNA levels prior to and following I/R for RACK2 (A) and connexin 43 (Cx43; B). Values are means ± SE (n = 3–4/group). *Effect of age, †effect of OVX, ‡effect of drug; P < 0.05.
DISCUSSION
The incidence and mortality due to acute myocardial infarction is increased in women after menopause (38), and clinical and experimental evidence implicate the loss of E2 in the reduced ischemic tolerance of the aged female heart (18, 33). Acute activation of PKCε prior to ischemia is known to mimic IPC and improve ischemic tolerance through mechanisms critically involving mitochondria (19). However, the efficacy of this intervention in the aged, E2-deficient heart was unknown, given previously observed reductions in mitochondrial PKCε (18). In support of our hypotheses, acute activation of PKCε prior to ischemia by local delivery of ψεRACK peptide was associated with 1) improved functional recovery and reduced infarct size after I/R in the aged, E2-deficient rat heart, 2) increased mitochondrial targeting of PKCε following I/R, and 3) identification of candidate downstream PKCε signaling targets, which collectively suggest a role for activation of antioxidant enzymes as a mechanism of PKCε-mediated protection in the aged female heart.
Subtle alterations in morphological and baseline functional characteristics were observed with aging and E2 deficiency in female rats, including increased body weight and LV weight in response to both age and OVX. These findings are consistent with previous reports of increased adiposity in rats with E2 deficiency (23). Slight but significant reductions in LVDP and −dP/dt were also observed at baseline in aged hearts, which is consistent with age-associated reductions in myocardial contractility and relaxation (27). Ischemic tolerance was impaired in the aged, E2-deficient heart, as indicated by both reduced functional recovery and increased infarct size following I/R, and these changes were associated with reduced basal levels of mitochondrial PKCε. Further reductions in mitochondrial PKCε in aged OVX hearts following I/R may be representative of proteosomal PKCε degradation during I/R stress and may compound the basal reductions in cardioprotective reserves of the aged heart (8, 27).
Acute activation of PKCε by local delivery of ψεRACK peptide prior to ischemia mimicked IPC and reduced I/R injury in the adult heart, as indicated by improved recovery of LVDP during reperfusion, and the specificity of this response was demonstrated by the blockade of protection with coadministration of ψεRACK and the PKCε inhibitor εV1–2 peptide. As such, PKCε activation by ψεRACK was sufficient to produce cardioprotection in the aged, E2-deficient heart, reducing infarct size to similar levels as those seen in adult hearts. The protection afforded by ψεRACK was associated with increased mitochondrial targeting of PKCε following I/R. No differences were observed in total PKCε levels across groups, suggesting a mitochondrial-specific effect.
Previous studies suggest the activation of PKCε in cardioprotection limits cell death by direct phosphorylation of the voltage-dependent anion channel and adenine nucleotide translocase (2) or indirectly by interaction with multiple mitochondrial signaling targets including glycogen synthase kinase-3β (22), mitochondrial ATP-sensitive K+ channel (13), Cx43 (43), endothelial nitric oxide synthase (35), F1 ATP synthase (26), and cytochrome-c oxidase subunit IV (34). Quantitative proteomic profiling of the cardiac mitochondrial compartment using the iTRAQ 8plex approach was undertaken here to characterize additional novel or altered mitochondrial PKCε signaling partners involved in cardioprotection following ψεRACK administration in the aged, E2-deficient heart. Importantly, our proteomic analysis was conducted in ψεRACK-treated hearts prior to ischemia to identify early cardioprotective phenomenon contributing to improved ischemic tolerance with age-associated E2 deficiency.
We observed that mitochondrial HSP10, GPX, and SOD2 (MnSOD) abundance were significantly increased with ψεRACK administration in aged OVX hearts (by ∼10, 20, and 30%, respectively) by iTRAQ. Due to the brief time period of ψεRACK perfusion in these hearts (10 min), changes observed in this analysis are likely attributable to PKCε-stimulated mitochondrial translocation or import of identified proteins. Alterations in transcriptional or translational regulation are unlikely, since rapid changes in protein expression are only expected for a small subset of proteins with very short half-lives. Most mitochondrial proteins have half-lives on the order of 20 to 100 h (14), while the half-life of SOD has been reported to be ∼1.5 days (5). The vast majority of mitochondrial proteins are encoded in the nuclear chromatin, synthesized in cytosolic ribosomes, and imported into the mitochondria via membrane transport mechanisms, thus providing the possibility for rapid increases in mitochondrial protein abundance through increased translocation and mitochondrial import (45). SOD2 is one such example of a protein that is synthesized as a precursor in the cytosol and imported into the mitochondrial matrix in its mature form following proteolytic removal of its NH2-terminal leader sequence (20, 46).
Following ischemia, it is likely that improved levels of mitochondrial HSP10, GPX, and MnSOD2 observed in ψεRACK-treated aged OVX hearts are further influenced by protective effects limiting protein degradation. It is clear from the work of Zhang et al. (48) that I/R-induced alterations to the mitochondrial proteome of adult mice occur and are dependent upon severity of ischemia and specific protein abundance. How specific mitochondrial proteins are targeted for lysosomal and/or proteosomal degradation in the aged heart, and the dynamic regulation of these processes, is poorly understood and a necessary focus of future studies.
While several lines of evidence exist to support the possible role of the antioxidant SOD2 as a cardioprotective PKCε target in adult hearts, similar evidence for GPX is less prevalent. Narrow rises in Ca2+, such as may be seen in IPC, caused increased activity of both GPX and SOD2 in rat liver mitochondria (42). IPC in isolated male mouse hearts was cardioprotective and caused increased mitochondrial SOD2 abundance, as well as reduced cytochrome c release following I/R (20). Also, the SOD-mimetic EUK-8 was cardioprotective in isolated hearts from adult OVX female rats (47). Perhaps of greatest interest is the report of increased SOD activity following pharmacological preconditioning with the adenosine A1 receptor agonist CCPA in isolated rat hearts (15), since PKCε is known to be activated downstream of the A1 receptor (11). It is likely that increases in GPX and SOD2 immediately following PKCε activation may serve to combat increased reactive oxygen species production in the aged female heart (29). In contrast, HSP10 is a stress response and chaperone protein shown to regulate mitochondrial procaspase-3 activation, and thus the initiation of apoptosis, through the formation of a complex with HSP60 in the intermembrane space (40). While speculative, it is also interesting to note that HSPs have recently been implicated in mitochondrial import of PKCε during I/R (6), and thus may contribute to observed increases in mitochondrial PKCε localization following ψεRACK demonstrated here.
Finally, we interrogated the influence of acute PKCε activation as a mechanism to affect gene transcription as part of a delayed cardioprotective response in our model. Consistent with our previous findings (33), RACK2 mRNA levels were reduced with aging, which likely contributes to age-associated reductions in PKCε localization (17, 33). A significant increase in RACK2 mRNA levels with administration of ψεRACK in both aged and aged OVX animals returned RACK2 mRNA to levels seen in adult hearts following I/R. Cx43 mRNA levels also increased with administration of ψεRACK in hearts isolated from aged OVX. Given evidence that PKC may influence Cx43 and RACK2 transcription (33, 37, 39), our results point to a possible regulatory role of PKCε in the transcriptional regulation of genes that contribute to delayed cardioprotection (33). However, since we did not directly assess functional endpoints for delayed cardioprotection, our interpretation is speculative. Independent effects of ψεRACK on RACK2 or Cx43 mRNA levels, independent of ischemia, were also not determined.
Perspectives and Significance
Improved ischemic tolerance resulting from acute PKCε activation by ψεRACK was associated with increased mitochondrial targeting of PKCε following I/R. Identification of novel candidate downstream PKCε signaling targets in mitochondria suggests a role for the regulation of oxidative stress through the activation of antioxidant enzymes as a mechanism of PKCε-mediated cardioprotection in the aged female heart. Additional effects of acute PKCε activation on cardioprotective gene transcription suggest a role for PKCε in regulation of immediate and possibly delayed mechanisms of cardioprotection with aging (RACK2 transcription). While our results provide strong rationale for the efficacy of acute PKCε activation to improve ischemic tolerance with postmenopausal E2 deficiency, there exist known limitations of extrapolating results discerned from a rodent model to postmenopausal women. Future confirmatory approaches will be necessary to clinically validate the observations described here and are the necessary next step in developing clinically useful strategies to treat ischemic heart disease in aged women.
GRANTS
This study was supported by National Institutes of Health Grants RO1-HL-091097-01A2S1 and RC2-AA-019403-01 American Heart Association Grant-in-Aid and Pharmaceuticals Grant-in-Aid (all to D. H. Korzick).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.S.L., S.J.J., and D.H.K. performed experiments; T.S.L., S.J.J., and D.H.K. analyzed data; T.S.L., S.J.J., and D.H.K. interpreted results of experiments; T.S.L. and D.H.K. prepared figures; T.S.L. drafted manuscript; T.S.L., S.J.J., and D.H.K. approved final version of manuscript; D.H.K. conception and design of research; D.H.K. edited and revised manuscript.
REFERENCES
- 1.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291: 1701–1712, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cε interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res 92: 873–880, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth EA, Marchesi M, Kilbourne EJ, Lucchesi BR. 17β-estradiol as a receptor-mediated cardioprotective agent. J Pharmacol Exp Ther 307: 395–401, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Broeyer FJ, van Aken BE, Suzuki J, Kemme MJ, Schoemaker HC, Cohen AF, Mizushima Y, Burggraaf J. The pharmacokinetics and effects of a long-acting preparation of superoxide dismutase (PC-SOD) in man. Br J Clin Pharmacol 65: 22–29, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budas GR, Churchill EN, Disatnik M, Sun L, Mochly-Rosen D. Mitochondrial import of PKCε is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res 88: 83–92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res 55: 523–536, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Budas GR, Mochly-Rosen D. Mitochondrial protein kinase Cε (PKCε): emerging role in cardiac protection from ischaemic damage. Biochem Soc Trans 35: 1052–1054, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Delyani JA, Murohara T, Nossuli TO, Lefer AM. Protection from myocardial reperfusion injury by acute administration of 17β-estradiol. J Mol Cell Cardiol 28: 1001–1008, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev 12: 181–188, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 4: 207–214, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Garg V, Hu K. Protein kinase C isoform-dependent modulation of ATP-sensitive K+ channels in mitochondrial inner membrane. Am J Physiol Heart Circ Physiol 293: H322–H332, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hare JF, Hodges R. Turnover of mitochondrial inner membrane proteins in hepatoma monolayer cultures. J Biol Chem 257: 3575–3580, 1982 [PubMed] [Google Scholar]
- 15.Hochhauser E, Kaminski O, Shalom H, Leshem D, Shneyvays V, Shainberg A, Vidne BA. Role of adenosine receptor activation in antioxidant enzyme regulation during ischemia-reperfusion in the isolated rat heart. Antioxid Redox Signal 6: 335–344, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA 280: 605–613, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Hunter J, Korzick D. Age- and sex-dependent alterations in PKC-ERK1/2 signaling in rat myocardium. Mech Ageing Dev 126: 535–550, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen deficiency decreases ischemic tolerance in aged rat myocardium: roles of PKCδ,PKCε, Akt, and GSK3β. Am J Physiol Regul Integr Comp Physiol 292: R800–R809, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res 70: 222–230, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Jin ZQ, Zhou HZ, Cecchini G, Gray MO, Karliner JS. MnSOD in mouse heart: acute responses to ischemic preconditioning and ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 288: H2986–H2994, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res 66: 233–244, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113: 1535–1549, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura M, Irahara M, Yasui T, Saito S, Tezuka M, Yamano S, Kamada M, Aono T. The obesity in bilateral ovariectomized rats is related to a decrease in the expression of leptin receptors in the brain. Biochem Biophys Res Commun 290: 1349–1353, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kolodgie FD, Farb A, Litovsky SH, Narula J, Jeffers LA, Lee SJ, Virmani R. Myocardial protection of contractile function after global ischemia by physiologic estrogen replacement in the ovariectomized rat. J Mol Cell Cardiol 29: 2403–2414, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Korzick DH, Hunter JC, McDowell MK, Delp MD, Tickerhoof MM, Carson LD. Chronic exercise improves myocardial inotropic reserve capacity through α1-adrenergic and protein kinase C-dependent effects in Senescent rats. J Gerontol A Biol Sci Med Sci 59: 1089–1098, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Korzick DH, Kostyak JC, Hunter JC, Saupe KW. Local delivery of PKCε-activating peptide mimics ischemic preconditioning in aged hearts through GSK-3β but not F1-ATPase inactivation. Am J Physiol Heart Circ Physiol 293: H2056–H2063, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Lakatta EG, Sollott SJ. Perspectives on mammalian cardiovascular aging: humans to molecules. Comp Biochem Physiol A Mol Integr Physiol 132: 699–721, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Lee TM, Lin MS, Chou TF, Tsai CH, Chang NC. Adjunctive 17β-estradiol administration reduces infarct size by altered expression of canine myocardial connexin43 protein. Cardiovasc Res 63: 109–117, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Lesnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys 420: 287–297, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 349: 523–534, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Miki T, Cohen MV, Downey JM. Failure of N-2-mercaptopropionyl glycine to reduce myocardial infarction after 3 days of reperfusion in rabbits. Basic Res Cardiol 94: 180–187, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res 75: 478–486, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Novotny JL, Simpson AM, Tomicek NA, Lancaster TL, Korzick DH. Rapid estrogen receptor-α activation improves ischemic tolerance in aged female rats through a novel PKCε-dependent mechanism. Endocrinology 150: 889–896, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Ogbi M, Johnson JA. Protein kinase Cε interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem J 393: 191–199, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ping P, Takano H, Zhang J, Tang XL, Qiu Y, Li RC, Banerjee S, Dawn B, Balafonova Z, Bolli R. Isoform-selective activation of protein kinase C by nitric oxide in the heart of conscious rabbits: a signaling mechanism for both nitric oxide-induced and ischemia-induced preconditioning. Circ Res 84: 587–604, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms ε and η in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res 81: 404–414, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Rigas AC, Ozanne DM, Neal DE, Robson CN. The scaffolding protein RACK1 interacts with androgen receptor and promotes cross-talk through a protein kinase C signaling pathway. J Biol Chem 278: 46087–46093, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115: e69–e171, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Salameh A, Krautblatter S, Baessler S, Karl S, Rojas Gomez D, Dhein S, Pfeiffer D. Signal transduction and transcriptional control of cardiac connexin43 up-regulation after α1-adrenoceptor stimulation. J Pharmacol Exp Ther 326: 315–322, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J 18: 2040–2048, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sbarouni E, Iliodromitis EK, Bofilis E, Kyriakides ZS, Kremastinos DT. Short-term estrogen reduces myocardial infarct size in oophorectomized female rabbits in a dose-dependent manner. Cardiovasc Drugs Ther 12: 457–462, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Schild L, Plumeyer F, Reiser G. Ca2+ rise within a narrow window of concentration prevents functional injury of mitochondria exposed to hypoxia/reoxygenation by increasing antioxidative defence. FEBS J 272: 5844–5852, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Schwanke U, Konietzka I, Duschin A, Li X, Schulz R, Heusch G. No ischemic preconditioning in heterozygous connexin43-deficient mice. Am J Physiol Heart Circ Physiol 283: H1740–H1742, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, Hunter CL, Nuwaysir LM, Schaeffer DA. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics 6: 1638–1655, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Stojanovski D, Johnston AJ, Streimann I, Hoogenraad NJ, Ryan MT. Import of nuclear-encoded proteins into mitochondria. Exp Physiol 88: 57–64, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Wispe JR, Clark JC, Burhans MS, Kropp KE, Korfhagen TR, Whitsett JA. Synthesis and processing of the precursor for human mangano-superoxide dismutase. Biochim Biophys Acta 994: 30–36, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Xu Y, Armstrong SJ, Arenas IA, Pehowich DJ, Davidge ST. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. Am J Physiol Heart Circ Physiol 287: H165–H171, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Liem DA, Mueller M, Wang Y, Zong C, Deng N, Vondriska TM, Korge P, Drews O, MacLellan WR, Honda H, Weiss JN, Apweiler R, Ping P. Altered proteome biology of cardiac mitochondria under stress conditions. J Proteome Res 7: 2204–2214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]