Abstract
Hypertension is closely associated with progressive kidney dysfunction, manifested as glomerulosclerosis, interstitial fibrosis, proteinuria, and eventually declining glomerular filtration. The postulated mechanism for development of glomerulosclerosis is barotrauma caused by increased capillary pressure, but the reason for development of interstitial fibrosis and the subsequently reduced kidney function is less clear. However, it has been hypothesized that tissue hypoxia induces fibrogenesis and progressive renal failure. This is very interesting, since recent reports highlight several different mechanisms resulting in altered oxygen handling and availability in the hypertensive kidney. Such mechanisms include decreased renal blood flow due to increased vascular tone induced by ANG II that limits oxygen delivery and increases oxidative stress, resulting in increased mitochondrial oxygen usage, increased oxygen usage for tubular electrolyte transport, and shunting of oxygen from arterial to venous blood in preglomerular vessels. It has been shown in several studies that interventions to prevent oxidative stress and to restore kidney tissue oxygenation prevent progression of kidney dysfunction. Furthermore, inhibition of ANG II activity, by either blocking ANG II type 1 receptors or angiotensin-converting enzyme, or by preventing oxidative stress by administration of antioxidants also results in improved blood pressure control. Therefore, it seems likely that tissue hypoxia in the hypertensive kidney contributes to progression of kidney damage, and perhaps also persistence the high blood pressure.
Keywords: oxygen consumption, tubular sodium transport, blood flow, nitric oxide, superoxide dismutase
the kidneys are essential for long-term regulation of arterial blood pressure, as well as for excretion of metabolic waste products and water-soluble toxins. A relatively high renal blood flow (RBF), equal to ∼25% of the cardiac output at rest, is required for effective clearance of unwanted substances from the blood. Although the kidneys receive a substantial portion of the cardiac output, renal oxygen consumption (QO2) only equals a few percent of total body QO2. This results in a surprisingly low oxygen (O2) extraction and a relatively well-oxygenated renal venous blood (22).
Intrarenal blood perfusion is highly heterogeneous, with merely 10% of RBF reaching the deeper situated medullary structures. Interestingly, the heterogeneous RBF distribution and characteristics, in combination with local differences in O2 demand, result in a distinct intrarenal O2 gradient (3, 10). The O2 tension (Po2) in the renal cortex is about 40–45 mmHg, and the lowest renal Po2 is found in inner medulla (10–15 mmHg) during normal conditions (3). The intrarenal Po2 values should be compared with that of arterial blood, which is considerably higher (80–100 mmHg). It has, therefore, been suggested, and to some extent also demonstrated, that O2 is shunted from arterial to venous blood in preglomerular vessels and vasa recta and as a result never reaches the kidney tissue (3, 19, 63). As a consequence, O2 delivery to the medulla barely matches demand, and relative hypoxia occurs as part of normal kidney function (62). However, the cortico-medullary O2 gradient is contingent upon prevailing physiological conditions, as evident from the report by Stillman et al. (112) who showed that chronic salt depletion to rats for 4 wk caused marked cortical hypoxia (112). Furthermore, the medullary oxygenation increased to levels only found in the normally well-oxygenated kidney cortex.
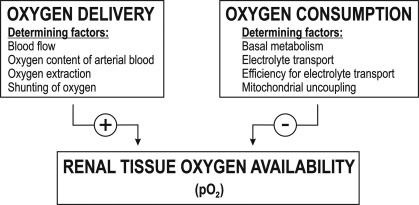
The Po2 in any tissue is ultimately the relation between O2 delivery and QO2. The O2 delivery to kidney tissue is normally fairly constant, and even if RBF increased dramatically, it would likely also increase QO2 because of the concomitantly increased glomerular filtration rate (GFR), which equals increased tubular sodium (Na+) load, and, therefore, also increased QO2 per se. In the normal kidney, ∼80% of total renal QO2 is dedicated to electrolyte transport, preferentially consisting of tubular transport of Na+ (TNa) (11). Several other O2-requiring processes also occur in the kidney, e.g., gluconeogenesis, synthesis of glucuronides, serine, acetylated metabolites, and hormones. Taken together, available data mainly suggest that QO2 is the main determinant of intrarenal Po2 within the normal range of RBF (Fig. 1). Intuitively, one might also consider that increased urinary protein leakage, and thus increased tubular energy demand for protein reabsorption, would contribute to increased kidney QO2. However, experimental data to support such statements are currently lacking.
Fig. 1.
Factors determining intrarenal oxygen availability.
Chronic kidney disease (CKD) can be a cause, as well as a consequence of hypertension, but blood pressure-lowering treatment reduces progression of kidney dysfunction in patients with CKD (35, 72). Hypertension alters intrarenal microcirculation, as well as metabolism, and hypertensive kidney damage is a major cause of end-stage renal disease (ESRD). Clinically, hypertensive ESRD is more commonly observed in the presence of atherosclerosis compared with ESRD arising from other etiologies (e.g., polycystic kidney disease, glomerulonephritis, and nephrolithiasis or obstruction) (6). The association of atherosclerosis with hypertensive ESRD presents the possibility that hypertensive ESRD, and possibly even hypertension per se, is caused by a primary renal microvascular and/or metabolic disorder (6). Regardless, hypertension constitutes a major risk factor for the progression of renal disease and vascular complications.
This field has been extensively studied to increase the understanding of development and progression of hypertension and its complications. Several models are widely used for studying the effects of experimental hypertension, the most common, including the spontaneously hypertensive rat (SHR), ANG II-induced slow pressor hypertension, 2-kidney, 1-clip Goldblatt hypertension (2K, 1C), Dahl salt-sensitive hypertension, deoxycorticosterone acetate-salt (DOCA-salt) hypertension, and the transgenic TG(mREN2)27 rat (Ren2).
Hypertensive kidney damage commonly presents as glomerular and tubulointerstitial damage and eventually proteinuria. Lee et al. (60) showed that injury or activation of the endothelium by hemodynamic changes resulted in increased local synthesis of angiotensinogen in the remnant rat kidney (60). This initiated a cascade of increased expression of transforming growth factor-β and matrix proteins, which contributed to the development of segmental glomerular lesions. As the development of glomerulosclerosis progresses, endothelial cells decrease in number as a result of apoptosis (46), a phenomenon that has been linked to reduced VEGF expression (45). It is now evident that hemodynamic and metabolic factors work together to induce CKD. In addition to glomerular injury, increased vascular tone in renal microvessels limits RBF. Increased ANG II and pressure-induced oxidative stress alter mitochondria and electrolyte transport efficiency, which together reduce kidney Po2 and can cause tissue hypoxia. Hypoxia is a known stimulus for fibrogenesis, and fibrosis is a common clinical finding in patients with hypertensive kidney damage. Activation of vascular and metabolic pathways that eventually result in hypoxia and interstitial fibrosis are likely to occur long before detectable clinical features of kidney damage are detectable. The role of altered O2 handling and renal tissue hypoxia for the development of hypertensive kidney damage has recently attracted attention, and the current knowledge is summarized in this review.
Hypertensive Kidney Damage
In the United States, hypertension is the second most common cause of ESRD, superseded only by diabetes (28). Johnson et al. (41) suggested division of hypertension into two stages. The first stage is primarily of nonrenal origin but is associated with renal vasoconstriction in the absence of altered renovascular structure. In the second stage, renal vasoconstriction will persist when the external stimuli are removed, likely due to afferent arteriolar damage and interstitial inflammation. As long as glomerular afferent arteriolar structures remain intact, renal autoregulation effectively prevents transmission of increases in systemic blood pressure to renal glomeruli or peritubular capillaries. This is accomplished by two intrarenal mechanisms: the afferent arteriolar myogenic response and tubuloglomerular feedback (TGF). The myogenic response is a reflex causing afferent arterioles to constrict in response to increased arterial pressure. TGF alters afferent tone in response to altered Na+ and Cl− concentrations in distal tubule as it passes the macula densa. As proposed by Johnson et al. (41), it is in stage two that patients develop salt sensitivity, renal arteriolar dysfunction, and impaired renal autoregulation. Renal arteries, including afferent arterioles, undergo pathological alterations that will compromise autoregulation (48). The endothelium becomes dysfunctional, vasodilatation is gradually impaired, and structural changes cause a shift of the autoregulatory curve to a higher set point (40). This stage constitutes a risk for developing microalbuminuria and progressive renal disease, which eventually results in ESRD. However, increased oxidative stress induced by ANG II and elevated pressure also induces increased QO2, resulting in renal tissue hypoxia, since increased O2 utilization is not compensated by increased O2 delivery. Sustained hypoxia induces fibrogenesis and tubular atrophy (43), which together with gradual renovascular dysfunction, result in progressively diminishing kidney function.
A major part of the hemodynamic, as well as the metabolic, alterations in hypertension are connected to reduced kidney tissue Po2. More than 20 yr ago, Brazy and Klotman (8) reported that proximal tubules isolated from early hypertensive SHR display higher QO2 than tubules from normotensive rats, and tubules from SHR also respond with greater increase in QO2 when stimulated by norepinephrine. Furthermore, tissue Po2 is significantly lower throughout the kidney in several models of experimental hypertension, including SHR, 2K, 1C, and ANG II-induced hypertension (82, 83, 123, 125).
When Tigerstedt and Bergman first observed the impressive pressor effects of renal cortical extracts injected intravenously in recipient rabbits, they had just discovered one of the most powerful physiological blood pressure-regulating systems; the renin-angiotensin system (RAS) (116). ANG II influences vascular tone via two distinctly different receptors; activation of ANG II type 1 (AT1) receptors causes vasoconstriction, whereas activation of AT2-receptors induces NO release and causes vasodilation. Normally, AT1 receptors are more abundant, and constriction, therefore, dominates the vascular response to exposure to ANG II. Even short-term ANG II exposure to both rats and human vascular smooth muscle cells results in contractile dysfunction and induces structural and functional changes in rat kidneys (56, 67). These alterations result in hypertension and vascular and tubulointerstitial damage. Therefore, ANG II is commonly used to induce experimental hypertension, both via exogenous administration, as in ANG II-induced hypertension and via increased endogenous production, as in 2K, 1C. The restriction (clip) placed on the renal artery in the 2K, 1C model reduces renal perfusion pressure, which induces renin release (32). This increases ANG II in both kidneys (77) and leads to ANG II-dependent hypertension in rats (98). Furthermore, renal damage and proteinuria are improved by inhibiting ANG II signaling in SHR but unaffected by similar blood pressure-lowering treatment with the calcium channel blocker amlodipine, (21, 72). Taken together, these reports demonstrate a pivotal involvement of the RAS for the development of hypertensive kidney damage.
Determinants of Intrarenal Oxygen Availability in Hypertension
Renal O2 availability is dependent on a balance between delivery and consumption (Fig. 1). Delivery is determined by RBF and O2 extraction, but also by shunting of O2 from arterial to venous blood in preglomerular vessels. Renal QO2 is affected by mitochondrial function, electrolyte transport, and cellular QO2, processes that all can be altered by reactive oxygen species (ROS) in the kidney and renal vasculature. Increased QO2 and oxidative stress are both thought to play important roles in progression of kidney disease, theories supported by the finding that smoking is the strongest independent predictor for decline in renal function in hypertensive patients (90). The rest of this review will mainly focus on these features and on ANG II, nitric oxide (NO), hypoxia-inducible factor (HIF), and other components known to be involved in O2 metabolism and renal function.
Shunting of oxygen from arterial to venous blood in the renal vasculature.
Despite a high total RBF, the renal cortex displays a relatively low tissue Po2 (about 40–45 mmHg) (3). In two pioneering studies, Levy and colleagues (63, 64) demonstrated the existence of intrarenal shunting of O2 from arterial to venous blood in canine kidneys. Labeled erythrocytes and O2 were simultaneously injected into the renal artery, whereupon transit time to the renal venous blood was studied. It was shown that O2 could be detected before the labeled erythrocytes appeared. Erythrocytes travel in the intravascular space to end up in venous blood, and because O2 appeared prior to the simultaneously injected erythrocytes (indicating shorter traveled distances), it was concluded that O2 is shunted in the vascular structures (64). Two years later, using a similar experimental approach, Levy and Imperial (63) reported that O2 also is shunted in kidney cortex vasculature. In 1990, Schurek et al. (108) used microcoaxial needle O2-sensitive electrodes to directly measure glomerular Po2 in 54 anesthetized Munich-Wistar-Fromter rats, and estimated that glomerular Po2 was only half (46 ± 13 mmHg) of that in systemic arterial blood (90 ± 8 mmHg). When rats inhaled 100% O2, arterial Po2 increased more than 6-fold, but glomerular Po2 did not even increase twofold. It was suggested that shunting likely takes place between the countercurrent-arranged interlobular vessels (108). Later, Welch et al. (123) measured local renal Po2 in SHR and Wistar-Kyoto rats (WKY) and confirmed shunting in both strains. Using Clark-type O2 microelectrodes, they reported consistently lower Po2 in efferent arteriole and cortex compared with that in renal vein, findings that provide further support for a preglomerular O2 shunt.
For years, the significance of intrarenal arterial-venous O2 shunting remained obscure, but recently, Evans and colleagues (61) presented data suggesting that shunting contributes to dynamic regulation of intrarenal Po2. By infusing ACh into rabbit renal arteries, the group demonstrated that fractional O2 extraction decreases with increased RBF, but without alterations in renal parenchymal Po2. Hypoxic and hyperoxic ventilation affected renal Po2, but did not change the response to altered RBF. It was concluded that renal tissue Po2 is independent of RBF, a finding that provides further support for that preglomerular shunting regulates renal Po2. However, it should be noted that Welch et al. (123) did not report altered shunting of O2 in hypertensive SHR compared with normotensive WKY. Therefore, it is not likely that differences in O2 shunting contribute to the significantly lower renal tissue Po2 in SHR. However, the role of O2 shunting for regulating intrarenal Po2 in other models of hypertension remains to be determined, especially during situations that could potentially alter the physical driving forces for O2 transport between arterial and venous blood, such as increased diffusion distance due to pronounced fibrosis, or increased diffusion time due to significantly reduced blood perfusion.
Regulation of basal renal oxygen consumption.
Several factors influence basal renal QO2 (defined as QO2 nonrelated to tubular electrolyte transport), e.g., NO and ANG II (24, 50, 51). In addition to regulating vascular tone, NO participates in day-to-day regulation of renal QO2. Acute nonspecific inhibition of all nitric oxide synthases (NOS) by l-NAME in dogs resulted in significantly increased renal QO2, although GFR and therefore, TNa, both decreased (59). Therefore, the effect is likely a direct influence on basal metabolism and mitochondrial function. Similarly, using isolated tubules from rats, Deng et al. (22) showed that it is the neuronal NOS isoform that controls proximal tubular QO2 (22). Reduced NO levels increase mitochondrial QO2 and could limit renal tissue Po2. Low NO generation and activity are typical of CKD, and kidney injuries are commonly characterized by a NO/O2− imbalance (7, 103, 121). By administration of low-dose NOS inhibitors (NG-monomethyl-l-arginine and nitro-l-arginine methyl ester) to healthy volunteers and rats, Li et al. (65) recently investigated the effect of NOS inhibition on intrarenal Po2. They measured Po2 and RBF invasively using O2-sensitive optodes and laser-Doppler microfibers in anesthetized rats, and Po2 with blood oxygen level-dependent magnetic resonance imaging (BOLD-MRI) in both rats and humans. NOS inhibition reduced RBF and caused a dose-dependent decrease in preferentially medullary Po2. Reduced NO bioavailability is suspected in hypertensive kidneys, and a dysfunctional NO balance has been described in SHR, which may contribute to the development of hypertension per se. Although bradykinin, enalaprilat, and amlodipine will decrease kidney QO2 less in SHR compared with kidneys from normotensive rats, SHR kidneys still reduce kidney QO2 in response to NO donors in a similar manner as kidneys from normotensive controls, suggesting the effect is due to decreased NO production rather than to a impaired NO response (1). Ironically, NOS can also contribute to pathophysiological events by magnifying oxidative stress (132). Tetrahydrobiopterin (BH4) is a critical cofactor for NOS, and BH4 oxidation-induced deficiency is manifested as NOS uncoupling. Uncoupled NOS generates O2− rather than NO. NOS uncoupling occurs in several disease states characterized by increased oxidative stress, including hypertension. Kidneys of hypertensive Dahl salt-sensitive rats display NOS uncoupling indicated as l-NAME-inhibitable O2− production (114). Similarly, Porkert et al. (86) reported a sustained antihypertensive effect of BH4 administration to patients with poorly controlled hypertension, which was secondary to restored endothelial NO bioavailability (86). Only a minor part of the total kidney QO2 is used for production of O2− and NO in the healthy kidney. It is likely that this contribution would increase during conditions of excessive oxidative stress, such as elevated ANG II levels or inflammation, but this is merely a speculation, since there are currently no experimental data available.
ANG II affects renal tissue Po2, partly through effects on NO. ANG II reduced renal Po2 in rats and humans as measured by O2 microelectrodes or BOLD-MRI (83, 102). However, it should be noted that the rapid reduction in Po2 observed in some studies (within 10 s of ANG II administration) indicates that reduced RBF rather than increased QO2 is the mechanism altering renal Po2 during acute ANG II administration (102). ANG II-dependent hypertension enhanced TNa-dependent QO2 in thick ascending limb suspensions from rats (111). Recent in vivo data indicated that this effect was NO mediated and independent of renal perfusion pressure (82). Hypoxia in SHR is tightly linked to ANG II acting on AT1-receptors, since 2 wk of treatment with the ANG II AT1-receptor blocker candesartan normalizes renal Po2 (124). The beneficial effect of candesartan is not solely dependent on its blood pressure-lowering effects, since Po2 can only be partially restored by lowering the blood pressure to a similar level with combined treatment with the diuretic hydrochlorothiazide, hydralazine, and reserpine (124). However, in 2K, 1C rats, Po2 in postclip kidneys is maintained by ANG II acting on ANG II AT2 receptors, emphasizing the importance of intrarenal NO to maintain intrarenal Po2 (82).
Dietary salt intake influences renal Po2. One week of high Na+ intake resulted in decreased medullary Po2, and a week with low dietary Na+ increased Po2 in normotensive men, as well as in untreated hypertensive patients measured by BOLD-MRI (87). In this context, it is worth noting that ANG II may not only induce hypertension, but also predispose for subsequent salt-sensitive hypertension. After a transient ANG II infusion to rats, Lombardi et al. (67) observed that blood pressure and renal function returned to normal. However, hypertension redeveloped if the previously ANG II-treated rats were again exposed to high-Na+ diet. The authors suggested this may be due to altered renal ability to excrete the elevated salt load, possibly due to peritubular capillary loss coupled with decreased intrarenal NO formation. Stillman et al. (112) reported cortical hypoxia and medullary hyperoxia in chronically salt-depleted rats. The exact mechanism was not demonstrated, but it might include increased ANG II levels to minimize loss of plasma volume and maintain arterial pressure within the normal range.
It has been suggested that hypertension-induced medullary hypoxia may result from hydrogen sulfide (H2S) deficiency (4). In mammals, H2S is enzymatically generated from l-cysteine or l-homocysteine and acts as a vasodilator via ATP-sensitive potassium channels and, therefore, regulates vascular tone and blood pressure. In the kidney, H2S has been shown to increase GFR and inhibit TNa. H2S is oxidized in mitochondria in a manner dependent on O2 availability and will, therefore, accumulate under hypoxia (4). This mechanism may be adaptive, as it could potentially increase medullary perfusion and tissue Po2 in hypoxic states.
Tubular electrolyte transport efficiency.
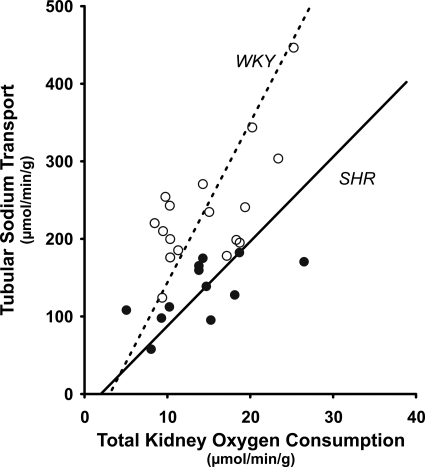
Recent studies show that renal hypoxia in hypertension is related to decreased electrolyte transport efficiency, defined as TNa achieved with a defined amount of QO2. SHRs are born normotensive but spontaneously develop hypertension at about 5 wk of age. SHR display increased renal vascular resistance, resulting in reduced RBF and an enhanced TGF response (123). With O2 electrodes, Welch et al. (123) demonstrated reduced Po2 in glomerulus, cortical proximal and distal tubules, and superficial cortical tissue in SHR compared with WKY rats. It was reported that SHR have reduced RBF, renal O2 delivery, GFR, and TNa compared with WKY. However, total kidney QO2 was not less in SHR than in WKY rats, and the authors, therefore, concluded that SHR have reduced TNa/QO2, further limiting renal Po2 (Fig. 2) (123). ANG II signaling is a likely mechanism for reduced TNa/QO2 in adult SHR, since treatment with candesartan for 2 wk normalized QO2/TNa (124). A similar reduction in TNa/QO2 has also been reported in 2K, 1C rats (127). Interestingly, blockade of ANG II AT1 receptors with candesartan was equally effective as the superoxide dismutase (SOD) mimetic tempol to restore TNa/QO2 and normalize renal Po2, highlighting elevated oxidative stress as a common mechanism to induce hypoxia in the hypertensive kidney.
Fig. 2.
Relationship between tubular sodium transport (TNa) and oxygen consumption (QO2) in spontaneously hypertensive rats (SHR) and Wistar-Kyoto control rats (WKY). [Redrawn from data originally presented by Welch et al. (123).]
Renal Protection Against Oxidative Stress
Superoxide dismutase.
Three different isoforms of SOD catalyze conversion of O2− to H2O2; mitochondrial manganese (Mn)-SOD and two isoforms of copper, zinc-SOD, located either extracellularly (EC-SOD) or intracellularly (IC-SOD) (30, 73). When glutathione is present, catalase or glutathione peroxidase further scavenges H2O2 to water. However, in the presence of Fe2+ or other trace metals, H2O2 can decompose to form OH−, commonly referred to as the Fenton reaction (26).
Dahl salt-sensitive rats have lower renal SOD and catalase activities, which correspond to elevated oxidative stress (114). The impaired vasodilation in ANG II-induced hypertension is improved by SOD treatment (88). EC-SOD−/− mice display increased renal oxidative stress and develop hypertension (126). Interestingly, this is accompanied by reduced Po2 throughout the kidneys, and both renal hypoxia and hypertension are restored by chronic antioxidant treatment with tempol. Tempol is commonly referred to as a SOD mimetic and reduces any direct effects of O2−, as well as the O2−-driven Fenton reaction (99, 100). In SHR, tempol will normalize blood pressure, renal vascular resistance, and urinary excretion of the marker for elevated oxidative stress 8-isoprostaglandin F2α (104–106).
In hypertensive rats with chronic renal failure induced by 5/6 nephrectomy, endothelium-dependent relaxation is impaired and O2− production increased (133). Chronic treatment with l-arginine, BH4, or SOD all reduced O2− production and restored vasorelaxation. The effects were partly additive, and it was, therefore, concluded that both increased NOS uncoupling, resulting in increased radical formation, and decreased NO production contributed to the impaired vasorelaxation (133).
Uncoupling proteins.
Uncoupling proteins (UCP) are proteins expressed in mitochondria and function as proton channels to allow proton leakage back across the inner mitochondrial membrane without creating ATP (36). It has been shown that O2− can activate UCP-2 (25, 52), possibly as a protective mechanism against excessive mitochondrial ROS formation in diabetic kidneys (31). However, leakage of protons results in elevated QO2 to sustain similar ATP production. In 2005, Bernal-Mizrachi et al. (5) demonstrated that mice with inducible UCP-1 expression in aortic smooth muscle cells developed hypertension and arteriosclerosis. These findings correlated to increased O2− production and decreased NO availability in the vessels.
Recently, Ma et al. (68) reported that UCP-2−/− mice developed hypertension when placed on high Na+ diet (8%) for 24 wk. In both wild-type and UCP-2−/− mice, the high-Na+ diet increased O2− production, decreased NO availability, enhanced phenylephrine-induced vasoconstriction, and impaired ACh-induced vasodilation. However, these alterations were significantly elevated in UCP-2−/− mice compared with wild-type mice. The authors concluded that UCP-2 is important for preventing salt-sensitive hypertension, possibly by suppressing O2− production and preserving NO bioavailability. Importantly, de Cavanagh et al. (21) showed that the increased H2O2 production, decreased mitochondrial membrane potential, and reduced expressions of NOS, Mn-SOD, and UCP-2 in SHR mitochondria were due to chronic RAS sactivation, and not due to the increased blood pressure per se.
In Dahl salt-sensitive rats, in which renal SOD is low and superoxide production is increased, chronic administration of the antioxidant α-tocopherol (vitamin E) prevented salt-sensitive hypertension and nephropathy (29). In cultured renal tubular epithelial cells, candesartan decreased O2− generation and dose-dependently restored redox balance (17). However, similar oxidative stress-lowering effects of candesartan were also observed in cultured renal epithelial cells lacking ANG II AT1 receptors. It was concluded that candesartan has a direct antioxidant effect, an effect that was not observed using any of the other highly selective AT1-receptor blockers.
Sources and Targets of Oxygen Radicals in Hypertension
ROS are generated by several enzymes as part of normal physiology. Increased production, however, can activate pathological pathways. The most common oxygen radicals are superoxide anions (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH−) (49). There are various sources for O2 radical formation. O2− is produced by mitochondria as part of normal respiration, as well as by xanthine oxidase, cyclooxygenase, lipoxygenase, uncoupled NOS, cytochrome P-450, and NADPH oxidase (27). Increased radical formation activates antioxidants to restore redox balance, and it is the balance between ROS production and antioxidant defense mechanisms that determines the oxidative stress status. There are three main targets of O2−: DNA, protein, and lipids. Extensive lipid peroxidation in biological membranes causes alterations in fluidity, permeability, and membrane potential, leading to subsequent cell rupture. When proteins are oxidized, their primary structure is altered. Structural alterations include folding, as well as charge and hydrophobia, and may cause increased aggregation and degradation. Damage to DNA by ROS includes alterations in DNA structure and chemistry, resulting in strand breakage (20, 39, 70, 94).
Pathological roles of ROS have been established in several diseases, such as ischemia-reperfusion, atherosclerosis, and renal disease (49). Furthermore, excessive oxidative stress is associated with human essential hypertension (55) and is documented in several hypertensive models, including SHR, 2K, 1C, ANG II-induced, Dahl salt-sensitive, and DOCA-salt hypertension (117). Also, there is increasing evidence that oxidative stress not only occurs in parallel with, but also contributes to and accelerates hypertension. It may be that oxidative stress, rather than elevated blood pressure per se is what reduces renal Po2 and that a defect in renal QO2 due to oxidative stress may cause exacerbation of hypertension. It should also be noted that elevated perfusion pressure per se can induce vascular ROS production via activation of NADPH oxidase in isolated femoral vessels from rats (120). However, other reports indicate that pressure-induced ROS formation is dependent on ANG II (58). ANG II-induced arterial blood pressure elevation, but not similar increase in blood pressure induced by norepinephrine, resulted in increased vascular ROS formation. Interestingly, hypoxia per se induces increased oxidative stress originating from the mitochondria (12). The mitochondria are able to sustain O2− production in the nanomolar range also during very low O2 conditions. However, mitochondrial NO production increases as O2 is reduced (13), which results in elevated levels of ONOO−, the end product when O2− reacts with NO.
Mitochondria as sources and targets of oxidative stress.
Mitochondria are responsible for the majority of cellular energy production, but also modulate apoptosis and H2O2 signaling, implying that dysfunctional mitochondria will impair tissue function in several ways. Physiologically, only a small portion of the electrons from the mitochondrial electron transport chain will reduce O2 to form ROS such as O2−, a process referred to as leak of electrons. SHR kidneys have increased oxidative stress and concomitant mitochondrial dysfunction manifested as increased H2O2 generation, decreased membrane potential, and increased expression of UCP-2 (21). Interestingly, these alterations were prevented by chronic treatment with the ANG II AT1-receptor blocker losartan, which may indicate an additional beneficial mechanism of reducing ANG II signaling in hypertension.
Mn-SOD protects the mitochondria against excessive O2−. Mn-SOD+/− mice have reduced Mn-SOD levels and develop hypertension with age or when placed on a high-salt diet (95). Similarly, ANG II-induced hypertension is attenuated in mice overexpressing the human mitochondria-specific antioxidant enzyme thioredoxin 2 (131).
Cardiovascular disease is often accompanied by mitochondrial dysfunction characterized by increased leak of electrons. ANG II is a mediator of renal mitochondrial dysfunction (21), but until recently, the mechanisms remained unknown. In 2008, Dikalov and colleagues (24) investigated the effect of ANG II on mitochondrial respiration, membrane potential, glutathione, mitochondrial ROS, and endothelial NO. ANG II diminished mitochondrial glutathione, increased state 4 and decreased state 3 respiration, and decreased mitochondrial respiratory control ratio by inducing oxidative stress in isolated mitochondria and bovine aortic endothelial cells. These alterations were accompanied by increased mitochondrial H2O2 production, which was inhibited by the NADPH oxidase inhibitor apocynin, a peroxynitrite (ONOO−) scavenger or a PKC inhibitor. These results suggest that ANG II induces mitochondrial dysfunction via a PKC-dependent pathway by activating NADPH oxidase, resulting in increased formation of ONOO− when the generated O2− reacts with NO. Thus, the result would be reduced NO bioavailability, in addition to oxidative and nitrosative stress (24). In a more recent study from the same laboratory (23), it was demonstrated that mitochondria-targeted antioxidants mito-Tempo or mitochondrial Mn-SOD inhibit total cellular O2−, reduce cellular NADPH oxidase activity, and restore NO bioavailability. In the same study, it was showed that mito-Tempo prevented hypertension and improved endothelium-dependent vasodilation when given at the onset of ANG II infusion to mice. These results highlight the crucial involvement of mitochondrial O2− for the development of hypertension. Notably, in both ANG II-induced and DOCA salt hypertension, blood pressure was lowered by 30 mmHg by mito-Tempo. Similarly, Mn-SOD-overexpressing mice are resistant to ANG II-induced hypertension and vascular oxidative stress (23). If mitochondrial superoxide is involved in hypertension, antioxidants might currently be suboptimally administered to the sites of superoxide production. These studies suggest that specific targeting of mitochondrial superoxide may be beneficial in treatment of hypertension.
Renal NADPH oxidase in hypertension.
It is likely that NADPH oxidase and its subunits are pivotal for increasing oxidative stress in the vasculature and kidneys and so make a major contribution to the pathogenesis of hypertension. ANG II acting on AT1 receptors activates NADPH oxidase to generate O2−, which induces increased expression of the subunits p22phox and Nox-1 and reduced expression of EC-SOD and Nox-4 (14). However, adverse effects of ANG II via AT1 receptors are to some extent counteracted by AT2 receptor-mediated effects, i.e., decreased expression of p22phox, Nox-1, and p67phox (14). ANG II-induced O2− generation by NADPH oxidase results in reduced renal tissue Po2, mainly due to significantly lower TNa/QO2, which is completely prevented by administration of tempol (74, 125). Chabrashvili et al. (15) showed in 2002 that all main components of NADPH oxidase are expressed in SHR kidneys and that there is a prominent increase in p47phox in vasculature, macula densa, and distal nephron of SHR kidneys compared with WKY controls (15). They also demonstrated that the increase preceded the development of hypertension. In a subsequent study, the same group reported that high-Na+ intake increased O2− generation and enhanced renal expression and activity of NADPH oxidase, concomitant with reduced renal expression of intracellular IC-SOD and Mn-SOD (47). Similarly, Adler and Huang (1, 2) reported ANG II-mediated O2− production in SHR renal cortex, combined with enhanced expression of NADPH oxidase components and EC-SOD deficiency (1, 2). They suggested that reduced NO bioavailability in the hypertensive SHR kidney results in intrarenal hypoxia, which contributes to renal fibrosis and other injury. Accordingly, increased NO bioavailability by reducing oxidative stress would improve renal Po2. Further support for reduced NO in SHR kidneys is provided by studies reporting that the increased TGF response in SHR is not further elevated by specific neuronal NOS blockade using 7-nitroindazole (128). Antioxidants will reduce blood pressure and renal vascular resistance in SHR. The mechanism likely involves NO since systemic NOS blockade by l-NAME prevents the antihypertensive effect of tempol in this animal model (104). Importantly, tempol and the ANG II AT1 receptor blocker candesartan restore TGF (128, 129), suggesting that ANG II stimulates ROS generation and reduces NO in SHR. In 2006, Modlinger et al. (74) used sophisticated small interfering RNA in vivo against p22phox in the slow pressor model of ANG II-induced hypertension in rats to markedly reduce blood pressure. It is likely that this intervention interfered with normal ANG II signaling to improve renal Po2. However, a causal link between the improved renal Po2 and the concomitant reduction in arterial blood pressure has yet to be described, but certainly deserves further attention.
Conscious rats with ANG II-induced hypertension have impaired vasodilation when stimulated by ACh, calcium ionophore A23187, or nitroglycerin (80). Interestingly, Nishiyama et al. (80) demonstrated that tempol reduced blood pressure and renal vascular resistance, but only in the presence of NO. Furthermore, Welch et al. (127) reported that the tempol-induced reduction of oxidative stress in 2K, 1C partially corrected renal cortical hypoxia, independently of reduction in blood pressure (127). Tempol, but not candesartan, restored Po2 and TNa/QO2 in early 2K,1C. As 2K, 1C hypertension progresses, we have reported that the direct ANG II involvement declines, and the impact of oxidative stress increases as a result of a self-sustained viscous cycle (83). Acute tempol administration still improved blood pressure and RBF more effectively than candesartan in the clipped kidney of 12-mo hypertensive 2K, 1C rats (83).
Renal O2− is increased in the medulla in ANG II-dependent hypertensive rats (125). Interestingly, ANG II-induced O2− enhanced thick ascending limb TNa related QO2, which was normalized by tempol (111). In mice with ANG II-induced hypertension, NADPH oxidase inhibition blocked O2− generation and lowered blood pressure (92). It has been suggested that part of the ANG II effect on O2− generation is mediated by activation of the immune system. Guzik et al. (34) reported T-cell activation in ANG II-infused mice, an effect that was abolished in NADPH oxidase-deficient mice.
Generation of O2− is also increased in kidneys of hypertensive Dahl salt-sensitive rats on high-salt diet. This can be inhibited by l-NAME, suggesting the O2− is due to NOS uncoupling (114). In contrast, l-NAME administered to control rats increased O2− production from the NOS. NOS-related ROS production was increased in aortas of mice with DOCA-salt hypertension (57). Using p47phox−/−, neuronal NOS−/− (nNOS−/−) and endothelial NOS−/− (eNOS−/−) mice, Landmesser et al. (57) demonstrated that DOCA hypertension increased ROS generation from NADPH oxidase, leading to oxidation of BH4, eNOS uncoupling, and further elevated ROS production.
A very challenging idea was proposed by Chen et al. (18) in 2005 when they demonstrated that hypoxia per se limits NADPH-dependent O2− production in kidney homogenates from hypertensive SHR, as well as normotensive WKY control rats (18). One may speculate that it is an evolutionary benefit to have a last line of defense against exacerbated ROS production that would otherwise cause acute toxicity.
Consequences of Renal Hypoxia
Normal kidney function requires an adequate hypoxic gene response to counteract reduced Po2. Hypoxia-inducible factors (HIF)-1 and -2 are transcription factors that are activated during hypoxia and regulate the hypoxic gene response (109). HIF-1α is degraded by an O2-dependent mechanism and accumulates during hypoxia to form an active heterodimer with the β-subunit (84). HIF activation has been reported to be beneficial, as well as causative of glomerular injury and renal fibrosis (37, 122). Possibly, HIF activation occurs in parallel with renal injury, but it mainly acts to prevent damage. In the kidney, HIF mediates protective pathways, such as erythropoietin, heme-oxygenase (HO)-1 and peroxisome proliferator-activated receptor α-regulated enzyme (16). Activated HIF is also a regulator of several O2-sensitive genes in the kidney, e.g., NOS and cyclooxygenase-2. However, oxidative stress impairs renal O2 sensing, as evident from the lack of increased HIF-1α and HO-1 staining in kidneys from streptozotocin-diabetic and Cohen diabetes-sensitive rats (96). Furthermore, Katavetin et al. (44) demonstrated that d-glucose, but not l-glucose, significantly blunted hypoxia-induced upregulation of VEGF mRNA in immortalized rat proximal tubular cells. Interestingly, they also demonstrated that H2O2 blunted this response, whereas α-tocopherol restored the response also during high d-glucose conditions. We recently observed a similar lack of hypoxia-induced increase in VEGF, HO-1, and erythropoietin mRNA levels in kidneys from 4-wk streptozotocin-diabetic rats (unpublished results). The lack of activated hypoxic gene response is further supported by the normal or near-normal hematocrits in several hypertensive models, including SHR, 2K, 1C, Dahl salt-sensitive hypertension, and ANG II-induced hypertension, which all have renal hypoxia. Rosenberger and colleagues showed that antioxidant treatment with tempol to hypoxic kidneys paradoxically increased HIF-1α expression, although tempol reduced tissue hypoxia determined by pimonidazole staining (96). In the absence of an adequate hypoxic gene response to maintain sufficient Po2 in the kidney, the result will be altered salt handling, sustained arterial hypertension, fibrosis, and oxidative stress (113). A number of hypoxia-inducing mechanisms have been identified in the tubule, among them increased metabolic demand, insufficient peritubular capillary perfusion due to imbalances in vasoactive substances, and constriction of efferent arterioles due to increased ANG II signaling (9, 107). In addition, ANG II-induced oxidative stress via NADPH oxidase activation will further aggravate hypoxia. Oxidative stress results in inefficient mitochondria respiration, endothelial damage, and loss of peritubular capillaries. The result is accelerated hypoxia in the tubulointerstitium (110). Hypoxia will also stimulate regulatory pathways for cellular proliferation and differentiation, and it is a powerful stimulus for activation of the immune system to induce differentiation of immature dendritic cells and proliferation of lymphocytes (89). Furthermore, hypoxia-induced HIF activation stimulates target genes, such as VEGF (89). Interestingly, Rudnicki et al. (97) reported that downregulated VEGF-A predicts progression of proteinuria, renal function, and degree of tubular atrophy and interstitial fibrosis in patients with stable or progressive proteinuric glomerulopathy. It should be noted that downregulation of VEGF-A occurred even though the HIF system was activated.
It has been reported that suppression of HIF-1α in the renal medulla of uninephrectomized Sprague-Dawley rats results in salt-sensitive hypertension via dysregulated TNa. Similarly, reduced HIF-1α levels in the renal medulla result in salt-sensitive hypertension also in normal rats (66). Ohtomo et al. (81) reported that induction of HIF-1α with the hypoxia mimetic cobalt chloride in an obese, hypertensive type 2 diabetic rat model protected kidney function via improvements of proteinuria and histological kidney injury. These improvements were associated with reduced generation of profibrotic factors, such as connective tissue growth factor and transforming growth factor-β (53, 54, 81). Uninephrectomized Sprague-Dawley rats transfected with a decoy oligonucleotide inhibiting HIF-1α activity developed hypertension as a consequence of reduced natriuretic response and reduced medullary RBF when challenged with a high-Na+ diet (66). Taken together, HIF-regulated genes are important determinants of arterial blood pressure in the long term. By regulating Na+ excretion and renomedullary function, HIF-mediated gene activation may act as an antihypertensive pathway. Concomitantly, chronic hypoxia, with or without proper counteracting accumulation of HIF, may induce progressive kidney injury.
Hemodynamic regulation, kidney function, and oxygen.
In rats, neonatal hyperoxic exposure during days 3–10 results in potentiated microvascular ANG II-induced contraction and impaired carbachol-induced vasodilation during adulthood (134). These impairments, in conjunction with the reduced nephron number and microvascular rarefaction in the rats exposed to hyperoxia, resulted in significantly elevated arterial blood pressure, implying that hyperoxia interferes with neonatal programming of adult cardiovascular and kidney function.
The majority of data regarding the specific role of O2 in regulating microvascular tone and kidney function originates from studies using experimental models of chronic hypoxia induced by either hypobaric or normobaric hypoxia. The most commonly used experimental models of hypertension involve administration of exogenous ANG II or induction of excessive endogenous ANG II production (2K, 1C or nephrectomy by ligation of the renal artery), and thus introduce a major confounding factor, since ANG II and the subsequent induction of oxidative stress also impair microvascular and kidney function. However, rats exposed to hypobaric hypoxia for 24 days develop severe arterial hypertension (79). The mechanism responsible for the development of hypertension in their model appears to be reduced NO generation, manifested as reduced urinary excretion of NO metabolites. The hypertension could be prevented by exogenous l-arginine. It is well known that increased O2− scavenges NO, which contributes to the vascular remodeling normally occurring in hypertension (118), but O2− will also enhance the myogenic response of the afferent arteriole (91).
The tubular transport of electrolytes requires energy, which is manifested in relatively high kidney QO2. At the cellular level, reduced O2 availability can reduce ATP production (38), which will affect all energy-requiring processes, including tubular transport. Using mpkCCD-c 14 cells, Husted et al. (38) demonstrated a direct regulation of collecting duct epithelial Na+ channels (ENaC) by O2 (38). Reducing O2 levels resulted in reduced ENaC protein levels, as well as reduced ENaC-mediated Na+ transport across the monolayer of the cultured cells. The O2 effects were independent of corticosteroids, and completely opposite to those described in previous reports showing hypoxia-stimulated ENaC expression in rat alveolar epithelial cells (85). Coincidentally, reduced dietary Na+ intake is known to increase medullary Po2 in both rats and humans (87, 112). Thus, the authors speculate that altering the medullary Po2 might be a mechanism for regulating tubular Na+ reabsorption and, therefore, might be important for maintaining Na+ balance and arterial blood pressure. The reduced renomedullary Po2 in the hypertensive kidney would reduce ENaC-mediated Na+ reabsorption and, therefore, be a mechanism to counteract the increased blood pressure. This is a speculation that remains to be solidified by experimental data, especially in the view of the very low Po2 required to induce the changes in tubular ENaC levels. However, Gomez et al. (33) showed that increased intrarenal hypoxia and reduced tubular response to furosemide predicted poor tubular function in poststenotic kidneys. The exact mechanism remains to be determined, but it might involve oxidative stress or hypoxia-induced alterations in the furosemide-sensitive Na+-K+-2Cl− transporter.
Link between renal hypoxia and fibrosis.
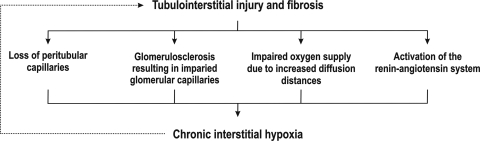
While the body can adapt to short-term hypoxia through induction of HIF-1α expression, chronic hypoxia may induce fibrogenesis. Functional renal impairment correlates with the degree of tubulointerstitial damage, and Nangaku (75, 76) proposed that the initial event, resulting in kidney failure, takes place mainly in the tubulointerstitium (Fig. 3). By exposing renal tubular cells to chronic hypoxia or the hypoxia mimetic cobalt chloride, the author showed that HIF activation can induce fibrosis (71). Tubular cells can transform into myofibroblasts, a process commonly referred to as epithelial-mesenchymal transdifferentiation (78). These myofibroblasts act as regulators of renal fibrogenesis and can induce production of collagen I and α-smooth muscle actin expression (71). In addition, transformed cells display increased migratory capacity compared with normal tubular cells. Epithelial-mesenchymal transdifferentiation was confirmed in vivo in a rat model of chronic kidney ischemia in which the left renal artery was ligated. Thus, Nangaku (75, 76) proposed chronic tubulointerstitial hypoxia as a final common pathway to ESRD, suggesting that hypoxia-induced tubulointerstitial damage causes interstitial fibrosis and loss of peritubular capillaries, whereupon fibrosis impairs O2 supply to tubular and interstitial cells, inducing apoptosis and epithelial-mesenchymal transdifferentiation. It is suggested that this constitutes a vicious cycle that accelerates the development of kidney damage (69, 75, 76, 93). The suggested hypothesis has further support from the study by Higgins et al. (37), who reported epithelial-mesenchymal transdifferentiation and epithelial cell migration as a result of HIF activation in primary renal epithelial cells and proximal tubules from mouse kidneys subjected to unilateral ureteral obstruction. Furthermore, inactivation of HIF-1α inhibited tubulointerstitial fibrosis, collagen deposition, and decreased inflammatory cell infiltration.
Fig. 3.
Schematic view of the vicious cycle relating tubulointerstitial fibrosis to chronic hypoxia, which creates a self-sustaining mechanism that accelerates the pathological process ending in end-stage renal disease. [Redrawn from the hypothesis presented by Nangaku (75, 76).]
Treatment Regimens Reducing Oxidative Stress and Hypoxia
Considerable therapeutic effort has been made to prevent or slow progression of hypertension-induced renal disease, and current treatments directed to inhibit ANG II signaling and reduce oxidative stress are indeed renoprotective (42). There is currently no direct evidence for a renoprotective role of the improved renal Po2 per se when ANG II signaling is inhibited, but this is an area that warrants further attention. Furthermore, treatment with antioxidants has been beneficial for kidney function in a wide range of animal models of experimental hypertension (119). Feeding pregnant SHR and their offspring an antioxidant-enriched diet for 24 wk, Zhan et al. (135) demonstrated delayed onset and reduced severity of hypertension. These reports suggest that not only conventional pharmaceutical interventions, but also modified diet and supplementation with potent antioxidants, may attenuate oxidative stress, be renoprotective, and reduce the severity of hypertension.
Treatment with 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, e.g., statins, is common in hypertension. Statins lower serum cholesterol, reduce proteinuria, and improve renal function in patients with cardiovascular disease (101). However, in Ren2 rats with an overactivated ANG II system, statin treatment also reduced oxidative stress in kidneys and vasculature with subsequently reduced renal fibrosis (130). Furthermore, statins decreased ANG II-dependent ROS generation, NADPH oxidase activity, and expression of several NADPH-oxidase subunits in podocytes (130). Because statins normalize endothelium-dependent relaxation, even before affecting serum cholesterol levels, it was proposed that beneficial effects of statins are achieved through improved NO bioavailability and inhibition of O2− generation, which perhaps also act to ameliorate renal hypoxia (130).
Finally, directly targeting the chronic renal hypoxia can be a novel and beneficial treatment of hypertensive renal disease. Therefore, noninvasive methods to detect and evaluate the intrarenal O2 availability in affected patients are required. The BOLD-MRI technique measures the level of deoxyhemoglobin as a surrogate for tissue O2 availability. Using BOLD-MRI, Textor et al. (115) demonstrated that interventions to alter intrarenal QO2 can be monitored in patients with renal artery stenosis. Similarly, Gomez et al. (33) showed that furosemide-induced reductions in renal QO2 were related to impaired renal function in pigs with renovascular hypertension induced by unilateral renal artery stenosis. Animals with intact furosemide-induced suppression of QO2 were normotensive and had GFR comparable to controls. However, animals with reduced furosemide-induced QO2 suppression were hypertensive and had GFR of about 30% of control kidneys. Using computer tomography, the group also reported that pigs with preserved GFR and intact furosemide-induced QO2 suppression displayed a greater increase in tubular fluid concentrating ability compared with those with a suppressed furosemide-induced QO2 response. These studies demonstrate the usefulness of noninvasive imaging techniques to study the link between intrarenal O2 availability and hypertensive kidney damage and how this is related to elevated arterial blood pressure.
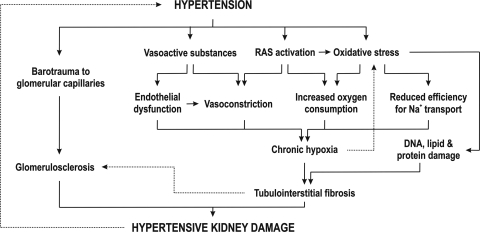
Renal hypoxia plays a crucial role for development of CKD and ESRD, and it potentially also participates in the onset and progression of hypertension, although this has to be further explored. Several pathways can induce excessive O2− generation, which is a main mechanism for inducing renal hypoxia. Oxidative stress precedes hypertension in several animal models of hypertension, and once initiated, it can become a self-sustaining ROS-generating system (Fig. 4). There is a close relationship between oxidative stress, renal hypoxia, and development and progression of hypertension and hypertensive renal disease, which highlights chronic renal hypoxia as a potential mechanism and a novel therapeutic target to protect the kidney against interstitial fibrosis and perhaps also to counteract hypertension.
Fig. 4.
Schematic presentation of the known mechanisms resulting in renal tissue hypoxia and kidney damage in hypertension. RAS, renin-angiotensin system.
Perspectives and Significance
Hypertension is closely associated with increased oxidative stress and reduced Po2 in the kidney, and with increased risk for development of progressive kidney dysfunction. Most clinical and experimental interventions that lower the arterial blood pressure also inhibit oxidative stress and restore kidney Po2, which might contribute to the renoprotection achieved by such interventions. However, there are some questions that still need to be resolved to advance the field: 1) Does the reduced kidney Po2 in hypertension contribute to maintaining or accelerating the hypertension? 2) Are kidneys protected from hypertensive damage by selective increases in renal O2 availability? 3) Why does not the reduced Po2 in the hypertensive kidneys result in activation of the HIF system? and 4) Is HIF activation protective against hypertensive kidney damage?
GRANTS
The work from our laboratory presented in this review was supported by the Swedish Research Council (Grants 9940, 72XD-15043, 10840, and 14X-2553), the Swedish Diabetes Association, the Swedish Society for Medical Research, the Lars Hierta Foundation, the Fredrik and Ingrid Thuring Foundation, the Magnus Bergvall Foundation, and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants K99/R00/DK077858.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Adler S, Huang H. Impaired regulation of renal oxygen consumption in spontaneously hypertensive rats. J Am Soc Nephrol 13: 1788–1794, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Adler S, Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol 287: F907–F913, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Aukland K, Krog J. Renal oxygen tension. Nature 188: 671, 1960 [DOI] [PubMed] [Google Scholar]
- 4. Beltowski J. Hypoxia in the renal medulla: implications for hydrogen sulfide signaling. J Pharmacol Exp Ther 334: 358–363, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature 435: 502–506, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bleyer AJ, Chen R, D'Agostino RB, Jr, Appel RG. Clinical correlates of hypertensive end-stage renal disease. Am J Kidney Dis 31: 28–34, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Blum M, Yachnin T, Wollman Y, Chernihovsky T, Peer G, Grosskopf I, Kaplan E, Silverberg D, Cabili S, Iaina A. Low nitric oxide production in patients with chronic renal failure. Nephron Exp Nephrol 79: 265–268, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Brazy PC, Klotman PE. Increased oxidative metabolism in renal tubules from spontaneously hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 257: F818–F825, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Brezis M, Greenfeld Z, Shina A, Rosen S. Angiotensin II augments medullary hypoxia and predisposes to acute renal failure. Eur J Clin Invest 20: 199–207, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Brezis M, Heyman SN, Epstein FH. Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1063–F1068, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Brodwall EK, Laake H. The relation between oxygen consumption and transport of sodium in the human kidney. Scand J Clin Lab Invest 16: 281–286, 1964 [PubMed] [Google Scholar]
- 12. Carriere A, Carmona MC, Fernandez Y, Rigoulet M, Wenger RH, Penicaud L, Casteilla L. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect. J Biol Chem 279: 40462–40469, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab 3: 277–287, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol 285: R117–R124, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 39: 269–274, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Chen HH, Chen TW, Lin H. Pravastatin attenuates carboplatin-induced nephrotoxicity in rodents via peroxisome proliferator-activated receptor alpha-regulated heme oxygenase-1. Mol Pharmacol 78: 36–45, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Chen S, Ge Y, Si J, Rifai A, Dworkin LD, Gong R. Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int 74: 1128–1138, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Chen Y, Gill PS, Welch WJ. Oxygen availability limits renal NADPH-dependent superoxide production. Am J Physiol Renal Physiol 289: F749–F753, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Chou SY, Porush JG, Faubert PF. Renal medullary circulation: hormonal control. Kidney Int 37: 1–13, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Davies KJ. Protein damage and degradation by oxygen radicals. I. General aspects. J Biol Chem 262: 9895–9901, 1987 [PubMed] [Google Scholar]
- 21. deCavanagh EM, Toblli JE, Ferder L, Piotrkowski B, Stella I, Inserra F. Renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan but not by amlodipine. Am J Physiol Regul Integr Comp Physiol 290: R1616–R1625, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Deng A, Miracle CM, Suarez JM, Lortie M, Satriano J, Thomson SC, Munger KA, Blantz RC. Oxygen consumption in the kidney: effects of nitric oxide synthase isoforms and angiotensin II. Kidney Int 68: 723–730, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature 415: 96–99, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Fenton H. Oxidation of tartaric acid in presence of iron. J Chem Soc 65: 899–911, 1894 [Google Scholar]
- 27. Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res 88: 44–51, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 18: 2644–2648, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Forde P, Scribner AW, Dial R, Loscalzo J, Trolliet MR. Prevention of hypertension and renal dysfunction in Dahl rats by alpha-tocopherol. J Cardiovasc Pharmacol 42: 82–88, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Fridovich Superoxide dismutases I. Annu Rev Biochem 44: 147–159, 1975 [DOI] [PubMed] [Google Scholar]
- 31. Friederich M, Olerud J, Fasching A, Liss P, Hansell P, Palm F. Uncoupling protein-2 in diabetic kidneys: increased protein expression correlates to increased non-transport related oxygen consumption. Adv Exp Med Biol 614: 37–43, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Goldblatt H, Lynch J, Hanzal R, Summerville W. Studies on experimental hypertension I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 59: 347–379, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gomez SI, Warner L, Haas JA, Bolterman RJ, Textor SC, Lerman LO, Romero JC. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Renal Physiol 297: F981–F986, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. He J, Whelton PK. Elevated systolic blood pressure as a risk factor for cardiovascular and renal disease. J Hypertens Suppl 17: S7–13, 1999 [PubMed] [Google Scholar]
- 36. Heaton GM, Wagenvoord RJ, Kemp A, Jr, Nicholls DG. Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur J Biochem 82: 515–521, 1978 [DOI] [PubMed] [Google Scholar]
- 37. Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Husted RF, Lu H, Sigmund RD, Stokes JB. Oxygen regulation of the epithelial Na channel in the collecting duct. Am J Physiol Renal Physiol 300: F412–F424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science 240: 1302–1309, 1988 [DOI] [PubMed] [Google Scholar]
- 40. Iversen BM, Sekse I, Ofstad J. Resetting of renal blood flow autoregulation in spontaneously hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 252: F480–F486, 1987 [DOI] [PubMed] [Google Scholar]
- 41. Johnson RJ, Segal MS, Srinivas T, Ejaz A, Mu W, Roncal C, Sanchez-Lozada LG, Gersch M, Rodriguez-Iturbe B, Kang DH, Acosta JH. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol 16: 1909–1919, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Kakinuma Y, Kawamura T, Bills T, Yoshioka T, Ichikawa I, Fogo A. Blood pressure-independent effect of angiotensin inhibition on vascular lesions of chronic renal failure. Kidney Int 42: 46–55, 1992 [DOI] [PubMed] [Google Scholar]
- 43. Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Katavetin P, Miyata T, Inagi R, Tanaka T, Sassa R, Ingelfinger JR, Fujita T, Nangaku M. High glucose blunts vascular endothelial growth factor response to hypoxia via the oxidative stress-regulated hypoxia-inducible factor/hypoxia-responsible element pathway. J Am Soc Nephrol 17: 1405–1413, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Kelly DJ, Hepper C, Wu LL, Cox AJ, Gilbert RE. Vascular endothelial growth factor expression and glomerular endothelial cell loss in the remnant kidney model. Nephrol Dial Transplant 18: 1286–1292, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Kitamura H, Shimizu A, Masuda Y, Ishizaki M, Sugisaki Y, Yamanaka N. Apoptosis in glomerular endothelial cells during the development of glomerulosclerosis in the remnant-kidney model. Exp Nephrol 6: 328–336, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 14: 2775–2782, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Kitiyakara C, Guzman NJ. Malignant hypertension and hypertensive emergencies. J Am Soc Nephrol 9: 133–142, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Knight JA. Free radicals: their history and current status in aging and disease. Ann Clin Lab Sci 28: 331–346, 1998 [PubMed] [Google Scholar]
- 50. Koivisto A, Matthias A, Bronnikov G, Nedergaard J. Kinetics of the inhibition of mitochondrial respiration by NO. FEBS Lett 417: 75–80, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Koivisto A, Pittner J, Froelich M, Persson AE. Oxygen-dependent inhibition of respiration in isolated renal tubules by nitric oxide. Kidney Int 55: 2368–2375, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest 112: 1831–1842, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kroening S, Neubauer E, Wessel J, Wiesener M, Goppelt-Struebe M. Hypoxia interferes with connective tissue growth factor (CTGF) gene expression in human proximal tubular cell lines. Nephrol Dial Transplant 24: 3319–3325, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Kroening S, Neubauer E, Wullich B, Aten J, Goppelt-Struebe M. Characterization of connective tissue growth factor expression in primary cultures of human tubular epithelial cells: modulation by hypoxia. Am J Physiol Renal Physiol 298: F796–F806, 2010 [DOI] [PubMed] [Google Scholar]
- 55. Kumar KV, Das UN. Are free radicals involved in the pathobiology of human essential hypertension? Free Radic Res Commun 19: 59–66, 1993 [DOI] [PubMed] [Google Scholar]
- 56. Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, Nagai T, Kaneko S, Komuro I. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation 114: 953–960, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 95: 588–593, 1997 [DOI] [PubMed] [Google Scholar]
- 59. Laycock SK, Vogel T, Forfia PR, Tuzman J, Xu X, Ochoa M, Thompson CI, Nasjletti A, Hintze TH. Role of nitric oxide in the control of renal oxygen consumption and the regulation of chemical work in the kidney. Circ Res 82: 1263–1271, 1998 [DOI] [PubMed] [Google Scholar]
- 60. Lee LK, Meyer TW, Pollock AS, Lovett DH. Endothelial cell injury initiates glomerular sclerosis in the rat remnant kidney. J Clin Invest 96: 953–964, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leong CL, Anderson WP, O'Connor PM, Evans RG. Evidence that renal arterial-venous oxygen shunting contributes to dynamic regulation of renal oxygenation. Am J Physiol Renal Physiol 292: F1726–F1733, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Leonhardt KO, Landes RR. Oxygen tension of the urine and renal structures. Preliminary report of clinical findings. N Engl J Med 269: 115–121, 1963 [DOI] [PubMed] [Google Scholar]
- 63. Levy MN, Imperial ES. Oxygen shunting in renal cortical and medullary capillaries. Am J Physiol 200: 159–162, 1961 [DOI] [PubMed] [Google Scholar]
- 64. Levy MN, Sauceda G. Diffusion of oxygen from arterial to venous segments of renal capillaires. Am J Physiol 196: 1336–1339, 1959 [DOI] [PubMed] [Google Scholar]
- 65. Li LP, Ji L, Santos EA, Dunkle E, Pierchala L, Prasad P. Effect of nitric oxide synthase inhibition on intrarenal oxygenation as evaluated by blood oxygenation level-dependent magnetic resonance imaging. Invest Radiol 44: 67–73, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li N, Chen L, Yi F, Xia M, Li PL. Salt-sensitive hypertension induced by decoy of transcription factor hypoxia-inducible factor-1alpha in the renal medulla. Circ Res 102: 1101–1108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lombardi D, Gordon KL, Polinsky P, Suga S, Schwartz SM, Johnson RJ. Salt-sensitive hypertension develops after short-term exposure to Angiotensin II. Hypertension 33: 1013–1019, 1999 [DOI] [PubMed] [Google Scholar]
- 68. Ma S, Ma L, Yang D, Luo Z, Hao X, Liu D, Zhu Z. Uncoupling protein 2 ablation exacerbates high-salt intake-induced vascular dysfunction. Am J Hypertens 23: 822–828, 2010 [DOI] [PubMed] [Google Scholar]
- 69. Mackensen-Haen S, Bader R, Grund KE, Bohle A. Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate. Clin Nephrol 15: 167–171, 1981 [PubMed] [Google Scholar]
- 70. Mak IT, Misra HP, Weglicki WB. Temporal relationship of free radical-induced lipid peroxidation and loss of latent enzyme activity in highly enriched hepatic lysosomes. J Biol Chem 258: 13733–13737, 1983 [PubMed] [Google Scholar]
- 71. Manotham K, Tanaka T, Matsumoto M, Ohse T, Inagi R, Miyata T, Kurokawa K, Fujita T, Ingelfinger JR, Nangaku M. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int 65: 871–880, 2004 [DOI] [PubMed] [Google Scholar]
- 72. Marcantoni C, Jafar TH, Oldrizzi L, Levey AS, Maschio G. The role of systemic hypertension in the progression of nondiabetic renal disease. Kidney Int Suppl 75: S44–S48, 2000 [PubMed] [Google Scholar]
- 73. Marklund SL, Holme E, Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta 126: 41–51, 1982 [DOI] [PubMed] [Google Scholar]
- 74. Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension 47: 238–244, 2006 [DOI] [PubMed] [Google Scholar]
- 75. Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 76. Nangaku M. Hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. Nephron Exp Nephrol 98: 8–12, 2004 [DOI] [PubMed] [Google Scholar]
- 77. Navar LG, Von Thun AM, Zou L, el-Dahr SS, Mitchell KD. Enhancement of intrarenal angiotensin II levels in 2 kidney 1 clip and angiotensin II induced hypertension. Blood Press Suppl 2: 88–92, 1995 [PubMed] [Google Scholar]
- 78. Ng YY, Huang TP, Yang WC, Chen ZP, Yang AH, Mu W, Nikolic-Paterson DJ, Atkins RC, Lan HY. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int 54: 864–876, 1998 [DOI] [PubMed] [Google Scholar]
- 79. Ni Z, Bemanian S, Kivlighn SD, Vaziri ND. Role of endothelin and nitric oxide imbalance in the pathogenesis of hypoxia-induced arterial hypertension. Kidney Int 54: 188–192, 1998 [DOI] [PubMed] [Google Scholar]
- 80. Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, Abe Y. Systemic and regional hemodynamic responses to tempol in angiotensin II-infused hypertensive rats. Hypertension 37: 77–83, 2001 [DOI] [PubMed] [Google Scholar]
- 81. Ohtomo S, Nangaku M, Izuhara Y, Takizawa S, Strihou CY, Miyata T. Cobalt ameliorates renal injury in an obese, hypertensive type 2 diabetes rat model. Nephrol Dial Transplant 23: 1166–1172, 2008 [DOI] [PubMed] [Google Scholar]
- 82. Palm F, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Angiotensin II type 2 receptors and nitric oxide sustain oxygenation in the clipped kidney of early Goldblatt hypertensive rats. Hypertension 51: 345–351, 2008 [DOI] [PubMed] [Google Scholar]
- 83. Palm F, Onozato M, Welch WJ, Wilcox CS. Blood pressure, blood flow, and oxygenation in the clipped kidney of chronic 2-kidney, 1-clip rats: effects of tempol and angiotensin blockade. Hypertension 55: 298–304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Park SK, Dadak AM, Haase VH, Fontana L, Giaccia AJ, Johnson RS. Hypoxia-induced gene expression occurs solely through the action of hypoxia-inducible factor 1alpha (HIF-1alpha): role of cytoplasmic trapping of HIF-2alpha. Mol Cell Biol 23: 4959–4971, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Planes C, Escoubet B, Blot-Chabaud M, Friedlander G, Farman N, Clerici C. Hypoxia downregulates expression and activity of epithelial sodium channels in rat alveolar epithelial cells. Am J Respir Cell Mol Biol 17: 508–518, 1997 [DOI] [PubMed] [Google Scholar]
- 86. Porkert M, Sher S, Reddy U, Cheema F, Niessner C, Kolm P, Jones DP, Hooper C, Taylor WR, Harrison D, Quyyumi AA. Tetrahydrobiopterin: a novel antihypertensive therapy. J Hum Hypertens 22: 401–407, 2008 [DOI] [PubMed] [Google Scholar]
- 87. Pruijm M, Hofmann L, Maillard M, Tremblay S, Glatz N, Wuerzner G, Burnier M, Vogt B. Effect of sodium loading/depletion on renal oxygenation in young normotensive and hypertensive men. Hypertension 55: 1116–1122, 2010 [DOI] [PubMed] [Google Scholar]
- 88. Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rama I, Bruene B, Torras J, Koehl R, Cruzado JM, Bestard O, Franquesa M, Lloberas N, Weigert A, Herrero-Fresneda I, Gulias O, Grinyo JM. Hypoxia stimulus: An adaptive immune response during dendritic cell maturation. Kidney Int 73: 816–825, 2008 [DOI] [PubMed] [Google Scholar]
- 90. Regalado M, Yang S, Wesson DE. Cigarette smoking is associated with augmented progression of renal insufficiency in severe essential hypertension. Am J Kidney Dis 35: 687–694, 2000 [DOI] [PubMed] [Google Scholar]
- 91. Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 298: H1769–H1775, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2- and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 93. Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2: 363–366, 1968 [DOI] [PubMed] [Google Scholar]
- 94. Roders MK, Glende EA, Recknagel RO. NADPH-dependent microsomal lipid peroxidation and the problem of pathological action at a distance. New data on induction of red cell damage. Biochem Pharmacol 27: 437–443, 1978 [DOI] [PubMed] [Google Scholar]
- 95. Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni Z, Wallace DC, Vaziri ND. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol 102: 255–260, 2007 [DOI] [PubMed] [Google Scholar]
- 96. Rosenberger C, Khamaisi M, Abassi Z, Shilo V, Weksler-Zangen S, Goldfarb M, Shina A, Zibertrest F, Eckardt KU, Rosen S, Heyman SN. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int 73: 34–42, 2008 [DOI] [PubMed] [Google Scholar]
- 97. Rudnicki M, Perco P, Enrich J, Eder S, Heininger D, Bernthaler A, Wiesinger M, Sarkozi R, Noppert SJ, Schramek H, Mayer B, Oberbauer R, Mayer G. Hypoxia response and VEGF-A expression in human proximal tubular epithelial cells in stable and progressive renal disease. Lab Invest 89: 337–346, 2009 [DOI] [PubMed] [Google Scholar]
- 98. Sadjadi J, Puttaparthi K, Welborn MB, 3rd, Rogers TE, Moe O, Clagett GP, Turnage RH, Levi M, Modrall JG. Upregulation of autocrine-paracrine renin-angiotensin systems in chronic renovascular hypertension. J Vasc Surg 36: 386–392, 2002 [DOI] [PubMed] [Google Scholar]
- 99. Samuni A, Krishna CM, Mitchell JB, Collins CR, Russo A. Superoxide reaction with nitroxides. Free Radic Res Commun 9: 241–249, 1990 [DOI] [PubMed] [Google Scholar]
- 100. Samuni A, Mitchell JB, De Graff W, Krishna CM, Samuni U, Russo A. Nitroxide SOD-mimics: modes of action. Free Radic Res Commun 12–13: 187–194, 1991 [DOI] [PubMed] [Google Scholar]
- 101. Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 17: 2006–2016, 2006 [DOI] [PubMed] [Google Scholar]
- 102. Schachinger H, Klarhofer M, Linder L, Drewe J, Scheffler K. Angiotensin II decreases the renal MRI blood oxygenation level-dependent signal. Hypertension 47: 1062–1066, 2006 [DOI] [PubMed] [Google Scholar]
- 103. Schmidt RJ, Baylis C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int 58: 1261–1266, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 32: 59–64, 1998 [DOI] [PubMed] [Google Scholar]
- 105. Schnackenberg CG, Wilcox CS. The SOD mimetic tempol restores vasodilation in afferent arterioles of experimental diabetes. Kidney Int 59: 1859–1864, 2001 [DOI] [PubMed] [Google Scholar]
- 106. Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension 33: 424–428, 1999 [DOI] [PubMed] [Google Scholar]
- 107. Schrier RW. Atlas of Diseases of the Kidney: Section 1. Disorders of Water, Electrolytes and Acid Base. Philadelphia, PA: Current Medicine, 1999 [Google Scholar]
- 108. Schurek HJ, Jost U, Baumgartl H, Bertram H, Heckmann U. Evidence for a preglomerular oxygen diffusion shunt in rat renal cortex. Am J Physiol Renal Fluid Electrolyte Physiol 259: F910–F915, 1990 [DOI] [PubMed] [Google Scholar]
- 109. Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 42: 31–38, 2003 [DOI] [PubMed] [Google Scholar]
- 111. Silva GB, Garvin JL. Angiotensin II-dependent hypertension increases Na transport-related oxygen consumption by the thick ascending limb. Hypertension 52: 1091–1098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stillman IE, Brezis M, Heyman SN, Epstein FH, Spokes K, Rosen S. Effects of salt depletion on the kidney: changes in medullary oxygenation and thick ascending limb size. J Am Soc Nephrol 4: 1538–1545, 1994 [DOI] [PubMed] [Google Scholar]
- 113. Tanaka T, Nangaku M. The role of hypoxia, increased oxygen consumption, and hypoxia-inducible factor-1 alpha in progression of chronic kidney disease. Curr Opin Nephrol Hypertens 19: 43–50, 2010 [DOI] [PubMed] [Google Scholar]
- 114. Taylor NE, Maier KG, Roman RJ, Cowley AW., Jr NO synthase uncoupling in the kidney of Dahl S rats: role of dihydrobiopterin. Hypertension 48: 1066–1071, 2006 [DOI] [PubMed] [Google Scholar]
- 115. Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, Grande JP, Gomez SI, Romero JC. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol 19: 780–788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tigerstedt R, Bergman P. Niere und Kreislauf. Skand Arch Physiol 8: 223–271, 1989 [Google Scholar]
- 117. Touyz RM. Oxidative stress and vascular damage in hypertension. Curr Hypertens Rep 2: 98–105, 2000 [DOI] [PubMed] [Google Scholar]
- 118. Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res 34: 5–14, 2011 [DOI] [PubMed] [Google Scholar]
- 119. Tylicki L, Rutkowski B, Horl WH. Antioxidants: a possible role in kidney protection. Kidney Blood Press Res 26: 303–314, 2003 [DOI] [PubMed] [Google Scholar]
- 120. Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108: 1253–1258, 2003 [DOI] [PubMed] [Google Scholar]
- 121. Vaziri ND, Ni Z, Wang XQ, Oveisi F, Zhou XJ. Downregulation of nitric oxide synthase in chronic renal insufficiency: role of excess PTH. Am J Physiol Renal Physiol 274: F642–F649, 1998 [DOI] [PubMed] [Google Scholar]
- 122. Weidemann A, Bernhardt WM, Klanke B, Daniel C, Buchholz B, Campean V, Amann K, Warnecke C, Wiesener MS, Eckardt KU, Willam C. HIF activation protects from acute kidney injury. J Am Soc Nephrol 19: 486–494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Nephron Po2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int 59: 230–237, 2001 [DOI] [PubMed] [Google Scholar]
- 124. Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Renal oxygenation defects in the spontaneously hypertensive rat: role of AT1 receptors. Kidney Int 63: 202–208, 2003 [DOI] [PubMed] [Google Scholar]
- 125. Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol 288: H22–H28, 2005 [DOI] [PubMed] [Google Scholar]
- 126. Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill PS, Aslam S, Wang X, Ji H, Sandberg K, Jose P, Wilcox CS. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension 48: 934–941, 2006 [DOI] [PubMed] [Google Scholar]
- 127. Welch WJ, Mendonca M, Aslam S, Wilcox CS. Roles of oxidative stress and AT1 receptors in renal hemodynamics and oxygenation in the postclipped 2K,1C kidney. Hypertension 41: 692–696, 2003 [DOI] [PubMed] [Google Scholar]
- 128. Welch WJ, Tojo A, Wilcox CS. Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol Renal Physiol 278: F769–F776, 2000 [DOI] [PubMed] [Google Scholar]
- 129. Welch WJ, Wilcox CS. AT1 receptor antagonist combats oxidative stress and restores nitric oxide signaling in the SHR. Kidney Int 59: 1257–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 130. Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi PR, Hayden MR, Rehmer N, De Marco VG, Andresen BT, Wei Y, Ferrario C, Sowers JR. Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension 51: 474–480, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, Bauersachs J. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of Thioredoxin 2. Hypertension 54: 338–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA 93: 6770–6774, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yamamizu K, Shinozaki K, Ayajiki K, Gemba M, Okamura T. Oral administration of both tetrahydrobiopterin and l-arginine prevents endothelial dysfunction in rats with chronic renal failure. J Cardiovasc Pharmacol 49: 131–139, 2007 [DOI] [PubMed] [Google Scholar]
- 134. Yzydorczyk C, Comte B, Cambonie G, Lavoie JC, Germain N, Ting Shun Y, Wolff J, Deschepper C, Touyz RM, Lelievre-Pegorier M, Nuyt AM. Neonatal oxygen exposure in rats leads to cardiovascular and renal alterations in adulthood. Hypertension 52: 889–895, 2008 [DOI] [PubMed] [Google Scholar]
- 135. Zhan CD, Sindhu RK, Vaziri ND. Up-regulation of kidney NAD(P)H oxidase and calcineurin in SHR: reversal by lifelong antioxidant supplementation. Kidney Int 65: 219–227, 2004 [DOI] [PubMed] [Google Scholar]