Abstract
Sleep and feeding rhythms are highly coordinated across the circadian cycle, but the brain sites responsible for this coordination are unknown. We examined the role of neuropeptide Y (NPY) receptor-expressing neurons in the mediobasal hypothalamus (MBH) in this process by injecting the targeted toxin, NPY-saporin (NPY-SAP), into the arcuate nucleus (Arc). NPY-SAP-lesioned rats were initially hyperphagic, became obese, exhibited sustained disruption of circadian feeding patterns, and had abnormal circadian distribution of sleep-wake patterns. Total amounts of rapid eye movement sleep (REMS) and non-REMS (NREMS) were not altered by NPY-SAP lesions, but a peak amount of REMS was permanently displaced to the dark period, and circadian variation in NREMS was eliminated. The phase reversal of REMS to the dark period by the lesion suggests that REMS timing is independently linked to the function of MBH NPY receptor-expressing neurons and is not dependent on NREMS pattern, which was altered but not phase reversed by the lesion. Sleep-wake patterns were altered in controls by restricting feeding to the light period, but were not altered in NPY-SAP rats by restricting feeding to either the light or dark period, indicating that disturbed sleep-wake patterns in lesioned rats were not secondary to changes in food intake. Sleep abnormalities persisted even after hyperphagia abated during the static phase of the lesion. Results suggest that the MBH is required for the essential task of integrating sleep-wake and feeding rhythms, a function that allows animals to accommodate changeable patterns of food availability. NPY receptor-expressing neurons are key components of this integrative function.
Keywords: arcuate nucleus, NPY-saporin, obesity, rapid eye movement sleep
feeding and vigilance (sleep-wake) states are rhythmically expressed across the circadian cycle, and their rhythms are highly integrated (3, 64, 75). Integration of sleep-wake and feeding rhythms is of fundamental importance for survival. Indeed, a wakeful state is an intrinsic requirement for feeding and foraging behavior, and, in a changeable environment, only close communication between these particular rhythms would permit an animal to respond adaptively and opportunistically to changing patterns of food availability. Although the suprachiasmatic nucleus of the hypothalamus (SCN) has an acknowledged role in establishing and maintaining circadian rhythms (47, 63, 83), including sleep-wake rhythms (15), entrainment of circadian food-anticipatory rhythms does not require the SCN (79, 82), supporting the possibility that integration of sleep-wake and feeding also occurs outside the SCN. Furthermore, integration of physiological signals required to derive rhythms compatible with homeostatic requirements is likely to occur outside the SCN (6, 48, 75), as this arrangement would provide maximum flexibility for responding to varying challenges.
Surprisingly few experiments have attempted to determine sites responsible for integration of sleep and feeding rhythms. However, a large body of work has revealed that the mediobasal hypothalamic area (MBH), defined here as including the arcuate (Arc) and ventromedial nuclei, is a critical site for detection and integration of signals governing metabolism and feeding (6, 27, 29, 75). Circadian oscillators capable of sustaining their rhythms ex vivo for up to 3 days have been demonstrated in the MBH (1, 21). In some of these, the phase of the oscillation was altered by restricted feeding (21). Furthermore, expression of the clock gene, Per1, has been shown to be altered in the Arc and dorsomedial nuclei of the hypothalamus in association with entrainment to a restricted feeding schedule (42). The anatomical connections of the MBH, including its reciprocal connectivity with the SCN (95, 96, 98), are consistent with an integrative function related to rhythm generation. Furthermore, MBH lesions produced by various methods result in hyperphagia and disruption of the diurnal patterning of both feeding (29, 44) and sleep (54, 59). Finally, a very early study, and one of the few studies that has examined sites of integration of feeding and sleep-wake, found that rats with lesions in this hypothalamic area exhibited associated changes in sleep events and meal parameters (13).
In this experiment, we investigated the interactions of sleep-wake and feeding rhythms in rats given bilateral injections of a targeted toxin, neuropeptide Y (NPY) conjugated to saporin (SAP), a ribosomal disaggregating agent (5, 35). This conjugate (NPY-SAP) targets NPY receptor-expressing neurons for destruction by binding to internalizing NPY receptors. Our injections were directed at the Arc, where targeted neurons include (among others) pro-opiomelanocortin neurons and NPY and Agouti gene-related peptide (AGRP) coexpressing neurons, which are involved in both feeding and sleep (25, 31, 34). We found that NPY receptor-expressing neurons in the MBH are required for integration of sleep-wake and feeding rhythms. We conclude that this integration is based on food-related and metabolic signals detected by these neurons.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats purchased from Simonsen Laboratories (Gilroy, CA) were used in these experiments. They were housed individually in suspended wire mesh cages or in sleep monitoring chambers (described below) in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Rats were maintained on a 12:12-h light-dark (0700–1900) cycle with ad libitum access to standard pelleted rodent chow (F6 Rodent diet; Harlan Teklad, Madison,WI) and tap water, except as noted. All experimental procedures were approved by Washington State University Institutional Animal Care and Use Committee, which conforms to NIH Guidelines.
Surgical instrumentation and injection of SAP conjugates.
For stereotaxic microinjections, rats were anesthetized by using 1.0 ml/kg body wt of a ketamine-xylazine-acepromazine cocktail (5 ml ketamine HCl, 100 mg/ml; Fort Dodge Animal Health, Fort Dodge, IA; 2.5 ml xylazine, 20 mg/ml, Vedco, St. Joseph, MO; 1 ml acepromazine, 10 mg/ml Vedco, and 1.5 ml 0.9% saline solution). Injection parameters, modified from previous work using NPY-SAP to lesion Arc neurons (35), were used to deliver NPY-SAP and control solution. NPY-SAP (24 ng/50 nl; Advanced Targeting Systems, Carlsbad, CA) was dissolved in 0.1 M PBS (pH 7.4) and injected bilaterally into two sites per side into the Arc. Controls were injected at the same sites with an equivalent amount of blank-SAP (B-SAP; SAP conjugated to a “blank peptide” with no known function or receptor). Rostral injection sites were 2.5 mm caudal, ± 0.4 mm lateral to bregma and 8.3 mm ventral to dura mater. Caudal sites were 3.5 mm caudal, ±0.4 mm lateral to bregma, and 8.5 mm ventral to dura mater. Solutions were delivered through a pulled glass capillary pipette (30-mm tip diameter) using a Picospritzer (Parker; Cleveland, OH). Solutions were injected slowly over a 5-min period, and their movement through the injection pipette was monitored microscopically. Following the injection, the rats were instrumented for sleep recordings. An electrode assembly for recording the cortical EEG and electromyogram (EMG) was also implanted as described previously (50).
Experimental schedules.
The same 12 rats (NPY-SAP, n = 6 and B-SAP, n = 6) were utilized for all sleep and feeding experiments and were tested concurrently. Three-day feeding baselines were taken before and after surgery. One week after surgery, rats were placed in isolated sleep chambers (50) for 30 days while being monitored for sleep and feeding patterns. They were returned to sleep chambers again for 1 wk beginning 92 days after first entering the sleep chambers. While in the sleep chambers, rats were given continuous ad libitum access to food except during days 12–15, when food was available only during the dark period, and days 20–25, when food was available only during the light period.
We also assessed sensitivity to the direct suppressive effect of ambient light on feeding with 2 days of dark-dark (continuous dark) exposure on day 48, after the animals were removed from the sleep chambers and returned to their home cages. Rats were given 1 day of adaptation to dark-dark, and food intake was then measured during the subsequent 12 h of subjective day and 12 h of subjective night.
Measurement of vigilance states [rapid eye movement sleep (REMS), non-REMS (NREMS) and wake].
For sleep studies, rats were individually housed in Plexiglas cages that were placed in an environmental chamber (4 cages per chamber) with sound attenuation. Temperature and illumination conditions (13-watt full spectrum light, 780 lumens) were consistent across chambers. Rats with different treatments were interspersed among three different chambers. Rats were allowed to adapt to the chambers for 3 days prior to data collection. The EEG and EMG signals, recorded through a wire harness connected to a commutator (Plastics One, Roanoke VA) were amplified (Grass-Telefactor, West Warwick, RI) and recorded onto a computer at 128 Hz using SleepSign for Animals software (Kissei America). The recordings were analyzed in 8-s epochs, and each epoch was assigned to a vigilance state (wake, NREMS, or REMS) based on standard EEG and EMG criteria embedded in the program. Scored records were reviewed by eye to ensure proper assignment of vigilance states. We scored 22 h of the 24-h recording, because in many instances general animal husbandry, which would disturb the animals, occurred in the last hour of the light period. When food was removed or returned it was done within 15 min of either side of the transition from light to dark (or vice versa).
After the final sleep recording, blood glucose was measured on separate days under both nonfasted and 2-h fasted conditions by using a hand-held glucose meter (Abbott Diabetes Care, Alameda, CA).
Immunohistochemistry.
Rats were euthanized by deep isoflurane (Webster Veterinary Supply, Devens, MA) anesthesia. After transcardial perfusion and fixation in 4% formalin in PBS, brains were removed for immunohistochemical processing. For immunohistochemistry, brains were sectioned coronally into three parallel sets (30-μm thickness) and processed using standard avidin-biotin-peroxidase techniques. NPY-Y1 receptor density and cell bodies expressing α-melanocyte stimulating hormone (α-MSH) in Arc were detected using rabbit anti-NPY-Y1 (1:4,000; Immunostar) and sheep anti-α-MSH (1:10,000; Millipore). Due to the localization of NPY-Y1 on cell membranes and the fact that its expression density is not uniform across cells, the NPY-Y1 immunostaining was not used for quantification. Instead α-MSH neurons, which are known to express NPY receptors, were used for this purpose. In sections immunoreacted for α-MSH, immunopositive cell bodies were counted in the Arc in all sections between −2.16 and −3.17 mm caudal to bregma (58) to assess the effect of the NPY-SAP lesion within the Arc.
Effects of arc-directed NPY-SAP injections on SAP distribution, orexin neurons, and corticosterone secretion.
Three independent cohorts of rats, injected with B-SAP or NPY-SAP as described for the sleep study, were utilized to further characterize effects of the Arc-NPY-SAP injection on surrounding tissue. In one group, the diffusion and cellular uptake of the injected SAP conjugates by neurons in the injection site was assessed using anti-SAP immunohistochemistry to detect SAP. Rats were injected with NPY-SAP or B-SAP, as described above. Four hours later they were euthanized and perfused. Brain sections throughout the hypothalamus were stained with anti-SAP (Advanced Targeting Systems) and evaluated microscopically throughout the hypothalamus from the SCN to the premammillary area.
A second group was used to evaluate the potential destruction by NPY-SAP of orexin neurons, which have been implicated in integration of sleep and feeding (41, 72–74). To specifically evaluate the potential direct effect of the NPY-SAP on hypothalamic orexin neurons, neurons expressing the orexin gene were quantified at two hypothalamic levels using in situ hybridization. Rats previously injected into the Arc with NPY-SAP or B-SAP, as described above, were euthanized and perfused ∼3 mo later. Brains were removed, postfixed overnight in 4% paraformaldehyde/PBS (4°C), and cryoprotected in a series of sucrose solutions (10–25%) for a total of 20 h. Hypothalamic sections were cut (20-μm thick) and every fifth section was mounted on slides, dried, and stored in desiccated slide boxes at −80°C until being processed for in situ hybridization using a protocol modified from our previous reports (36, 37). An antisense riboprobe of mouse prepro-orexin transcribed in the presence of 33P-UTP (Perkin-Elmer, Indianapolis, IN) was used for detecting orexin mRNA signals. Slides were dipped in K5 emulsion (Polysciences, Warrington, PA) after hybridization at 50°C for 16 h and placed in light-tight boxes containing desiccant and stored at 4°C for 40 days. The slides were then developed and counterstained in 0.5% cresyl violet in acetate buffer, dehydrated, and coverslipped with dibutyl phthalate and xylene (EM Sciences, Fort Washington, PA). Hybridization controls, using the sense probe, showed no hybridization signal.
Orexin neurons in the lateral hypothalamic area (LHA) and perifornical nucleus at two hypothalamic levels (−3.3 to −3.1 mm and −3.6 to −3.4 mm caudal to bregma) were counted (2 sections for each level, bilaterally) using cluster analysis. The Arc and dorsomedial hypothalamic nucleus were used as anatomic landmarks. A cluster of silver grains of orexin mRNA signals > 10 μm in diameter (usually ∼10–20 μm), containing > 20 silver grains and showing a clear neuron-like shape, was considered as one positive cell. The background of silver grains expression was < 5 in a 10 × 10-μm area outside of LHA or perifornical nucleus.
In a third group, we evaluated the integrity of the hypothalamo-pituitary-adrenocortical axis by testing the corticosterone response to glucoprivation, a stimulus known to elicit this response (67). To assess potential damage to the hypothalamo-pituitary-adrenocortical axis by the NPY-SAP injections, we challenged rats with systemic 2-deoxy-d-glucose (2DG; 250 mg/kg sc), an antiglycolytic agent known to stimulate corticosterone secretion. One month after Arc injection of NPY-SAP or B-SAP (as described above, n = 4 and 5, respectively), rats were habituated to opaque Plexiglas chambers for remote blood sampling and implanted with intra-atrial catheters constructed from Silastic tubing (inside diameter, 0.64 mm; outside diameter, 1.19 mm; Dow Corning, Midland, MI). Catheters were filled with polyvinylpyrrolidone solution (40,000 molecular weight; Sigma-Aldrich), 11 g polyvinylpyrrolidone in 20 ml 0.9% saline containing 1,000 U/ml heparin (Elkins-Sinn, Cherry Hill, NJ), and 2 mg/ml gentamicin (Schering-Plough Animal Health, Kenilworth, NJ). After recovery from surgery, rats were placed in the blood collection chambers and 2DG or saline was injected at time 0. Blood samples were collected by remote intravenous sampling 0, 30, 60, 90, and 120 min later. Plasma was collected and stored at −80°C for determination of corticosterone concentration by radioimmunoassay of duplicate aliquots using RIA kits obtained from Diagnostic Products (catalog no. TKRC-1; Los Angeles, CA). The lower limit of sensitivity for corticosterone was 20 ng/ml. 2DG and saline tests were separated by at least 1 wk.
Data analysis.
Data were statistically analyzed with SigmaStat software. Food intake and body weight were analyzed by a two-way repeated-measures ANOVA. For sleep recordings, the percentages of time spent in the various vigilance states were collected in 2-h blocks and then plotted against time. These results were analyzed by a two-way repeated-measures ANOVA (time vs. SAP treatment). Sleep data were also summed by period (light or dark), expressed as minute per 2 h, and analyzed by a two-way ANOVA (dark-light vs. SAP treatment). Finally, total time spent in the various vigilance states over 22 h was summed and analyzed by two-tailed t-tests (B-SAP vs. NPY-SAP). To analyze the effect of limited access to food on sleep states, the results within each SAP lesion (B-SAP or NPY-SAP) were analyzed separately with a 2 × 4 ANOVA (food access vs. light-dark period). In all ANOVA, post hoc testing was done by the Holm-Sidak test. P < 0.05 was considered significant. Cosinor analysis (51) of wake, NREMS, and REMS was performed using free software provided by the Circadian Rhythm Laboratory, University of South Carolina, Walterboro, SC (http://www.circadian.org/softwar.html).
RESULTS
Lesion analysis: in situ hybridization and corticosterone responses.
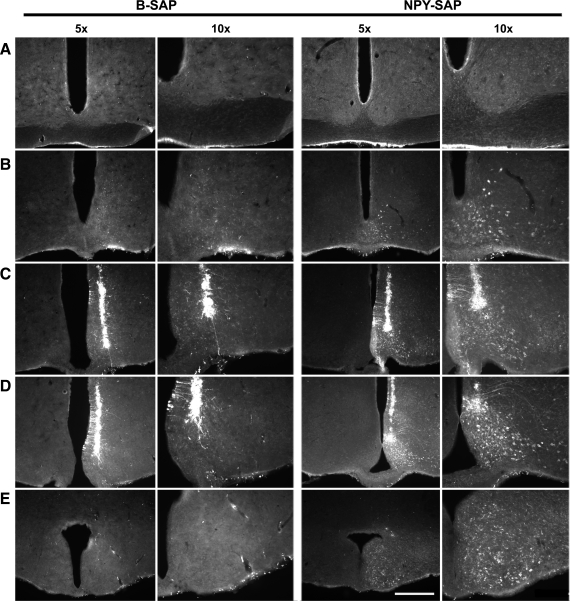
Cells in the MBH and along the microinjection pipette tract that internalized SAP after unilateral injections (50 nl) of B-SAP or NPY-SAP into the Arc at −2.5 and −3.5 μm caudal to bregma are shown for two rats (rats nos. 82 and 77, respectively) in Fig. 1. Both B-SAP and NPY-SAP were internalized by some neurons along the cannula tract where damage to cell membranes enabled entry of the conjugate into the cell. However, in the NPY-SAP rats, SAP-immunoreactivity (-ir) is also visible in neurons within the diffusion radius of the injection, where the conjugate was presumably internalized by NPY receptor-expressing neurons. In the NPY-SAP rats, the SAP-ir was present primarily within the Arc and extended from the retrochiasmatic area caudally to the premammillary area and was coextensive with NPY/AGRP and α-MSH cell bodies, which are known to express NPY receptors. The presence of SAP-ir in the ventromedial nuclei was minimal, and there was no evidence of internalization of the SAP conjugates in the SCN.
Fig. 1.
Distribution of saporin-immunoreactivity (SAP-ir) after injection of blank-SAP (B-SAP) or neuropeptide Y-SAP (NPY-SAP) into the arcuate nucleus (Arc). Injections (50 nl) were made at 2 rostrocaudal levels in the Arc. Rats were euthanized 4 h later. Distribution of SAP-ir is shown at 2 magnifications at each of 5 rostrocaudal levels for a representative rat from each treatment group between the SCN (A) and the mammillary recess of the 3rd ventricle (E). Micropipette tracts are present in C and D. SAP-ir is present along the tracts in both B-SAP and NPY-SAP rats where cells were damaged by pipette penetration. Internalization of SAP into cell bodies within the diffusion radius of the injected SAP conjugates is also present in the NPY-SAP rats, due to active internalization at NPY receptor binding sites. The diffusion radius, marked in this way, is concentrated in the Arc of the NPY-SAP rats, extends throughout the rostrocaudal extent of the Arc, and does not include the SCN. The calibration bar equals 0.5 mm and 1 mm for the 2 magnification levels (5X and 10X, respectively).
Results from the independent cohorts of NPY-SAP and B-SAP rats tested for orexin expression provide evidence that the lesion did not extend into the lateral hypothalamus. Cluster analysis of hybridization signals showed that rats given NPY-SAP injections into the ARC (n = 5) did not differ from B-SAP-injected controls (n = 3) in numbers of orexin neurons in the lateral and perifornical hypothalamus (21.4 ± 2.7 and 24.8 ± 2.2 neurons/per side, respectively; P > 0.3). Since orexin neurons are known to possess NPY receptors (18) and would have been targeted by NPY-SAP, the quantified data suggest that the NPY-SAP lesion did not extend into these areas.
Corticosterone responses to 2DG were also similar in NPY-SAP and B-SAP groups. The values at time 0 were 172.4 ± 11.1 and 118.2 ± 24.7 ng/ml, respectively (P > 0.1). The peak response to 2DG occurred at either 60 (B-SAP) or 90 min (NPY-SAP) and was similar in magnitude for both groups. Peak responses to 2DG were 305.3 ± 39.7 and 306.3 ± 60.2 ng/ml above time 0 baseline for NPY-SAP and B-SAP groups, respectively (P < 0.05). Responses above baseline at the same time points after saline injection were and 34.1 ± 24.4 and 6.5 ± 40.2 ng/ml (P > 0.05), respectively. The presence of a corticosterone response indicates that the cortocotropin releasing hormone pathway from the paraventricular hypothalamus to the median eminence was not destroyed by this lesion.
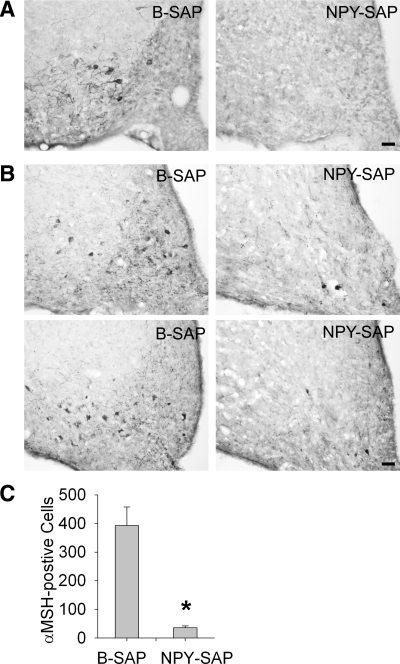
Compared with controls, the NPY-SAP-treated animals used in the sleep and feeding studies were found to have greatly reduced numbers of α-MSH immunopositive cell bodies in the Arc (Fig. 2, A and B), as shown previously (4). NPY-Y1 receptor immunoreactivity was also greatly reduced in the Arc (Fig. 2C), but cells expressing this receptor were not quantified. In all NPY-SAP rats, the ventral third ventricle was enlarged, indicating loss of tissue volume due to the lesion. The lesion appeared to be concentrated in the Arc in all rats, but there was varying involvement of tissue in the immediate surround. For example, in some NPY-SAP rats, the lesion extended rostrally into the retrochiasmatic nucleus. Caudally, the damaged tissue appeared to extend into the ventrolateral subnucleus of the ventromedial nucleus of the hypothalamus in several rats. Thus, while it is clear that the Arc was lesioned in all rats used in the study and was the only structure consistently damaged bilaterally in all the NPY-SAP rats, the individual differences in the lesion boundaries make it difficult to exclude completely the involvement of some surrounding MBH tissue in the effects we report.
Fig. 2.
Effects of NPY-SAP injections on NPY-receptor-expressing neurons in the Arc. A: coronal brain sections showing NPY-Y1 receptor immunoreactivity in the Arc at ∼2.6 mm caudal to bregma. B: coronal brain sections showing α-melanocyte stimulating hormone (α-MSH) immunoreactivity in Arc at 2.2 (top) and 3.0 mm (bottom) caudal to bregma. C: mean number of α-MSH immunopositive cell bodies in Arc of B-SAP and NPY-SAP rats. *P < 0.001. Calibration bar in A and B = 50 μm.
Effects of arc NPY-SAP injections on food intake.
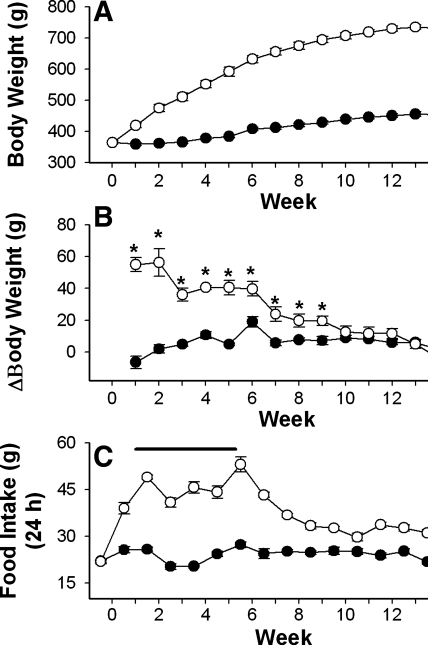
Similar to a previous report (5), we found that NPY-SAP lesion of Arc neurons caused hyperphagia and rapid weight gain for a number of weeks (6–7 wk for these rats), referred to as the dynamic phase of the lesion, followed by a phase of normophagia with near-normal rates of weight gain but sustained obesity, referred to as the static phase of the lesion (Fig. 3, A–C). During the final sleep measurements, conducted 14 wk after the Arc injections, rats were in the static phase of the lesion and their total daily food intake was similar to B-SAP controls. While in the dynamic phase, body weight change per week (means ± SE) in NPY-SAP rats ranged from 56.3 ± 8.7 g for week 1 to 40.5 ± 4.5 g for week 4. Subsequently, the rats remained obese, but their rate of weight gain gradually declined to 11.2 ± 4.0 g/wk for week 14. In comparison, B-SAP rats gained 1.8 ± 2.7 g during week 1 in the chambers, 4.8 ± 0.7 g during week 4 and 3.0 ± 2.7 g during week 14. Terminal weights after the 15 wk of the experiment were 455 ± 6 g for B-SAP rats and 742 ± 6 g for NPY-SAP rats. Although obese, the lesioned rats did not appear to be diabetic, as their nonfasted and 2-h fasted blood glucose levels did not differ from B-SAP rats, and values from both groups were in the normal range, as reported previously (5). In B-SAP rats, nonfasted and 2-h fasted blood glucose levels were 76 ± 4 and 77 ± 5 mg/dl. For NPY-SAP rats, glucose levels were 81 ± 3 and 78 ± 3 mg/dl, respectively.
Fig. 3.
Feeding and body weight in rats injected into the Arc with NPY-SAP or B-SAP. Weekly body weights in grams (A); weekly change (Δ) in body weight (B); mean 24-h food intake in NPY-SAP (○) or B-SAP (●) rats (C). In B, the change in body weight is calculated as the difference from the previous week's mean weight for each rat. The time periods during which rats were in sleep chambers are indicated by the heavy line in C. Data are expressed in grams (means ± SE) and surgery is week 0. Body weights and food intakes differed significantly between NPY-SAP and B-SAP groups beginning 1 week postinjection and for all subsequent measures (*P < 0.05). Changes (Δ) in body weight were significantly different until 9 wk postinjection (*P < 0.05), after which time the NPY-SAP rats transitioned from the dynamic to the static phase of growth (B).
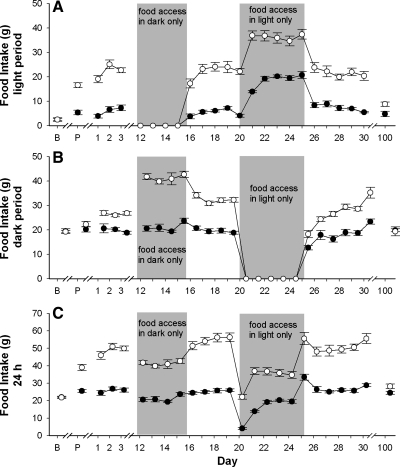
NPY-SAP lesions disrupted the light-dark distribution of food intake. During the dynamic phase, NPY-SAP rats ate nearly identical amounts in the light and dark periods during ad libitum food access (Fig. 4, A and B). Total daily average intake, calculated over 3 days prior to restricted feeding experiments was 25.8 ± 1.2 g for B-SAP and 49.0 ± 1.8 g for NPY-SAP rats. When food was restricted to the dark or light period, intake of both NPY-SAP rats and B-SAP rats was significantly reduced compared with their average total 24 h ad libitum intake prior to restriction (P < 0.001). Total daily intakes for these three conditions are shown in Fig. 3C. During 4 days of dark-only food access, NPY-SAP, and B-SAP rats ate 73.7 ± 2.2% and 81.8 ± 4.1% of their total 24 h ad libitum intake in light-dark, respectively. During 5 days of light-only food access, intakes were 64.5 ± 1.9% and 72.4 ± 3.1% of 24 h ad libitum intake during light-dark. The fact that rats of both groups ate less when food availability was limited to a 12-h period probably reflects a ceiling effect for feeding during the shortened access period. The % reduction was similar for both groups. Presumably, if the 12-h schedules were maintained over a longer time period, the rats would have adjusted their intakes to be equivalent to their intake on ad libitum feeding schedules.
Fig. 4.
Daily food intake across the experimental period in Arc-injected NPY-SAP and B-SAP rats. Food intake in grams (mean ± SE) for the 12-h light period (A), the 12-h dark period (B), and the total 24-h period (C) for B-SAP (●) or NPY-SAP (○) rats. x-Axis: B, baseline; P, postsurgery. Numbering begins with the introduction of the rats into the sleep chambers. Gray boxes, periods when food access was restricted to the dark period (4 days) or the light period (5 days). At all other times, food was available ad libitum. For chamber days 1–30, light period, dark period, and total food intake differed significantly between groups (P < 0.05). On day 100, intake during the light and total 24-h intake differed significantly between groups (P < 0.05), but intake during the dark did not differ.
Dark-dark exposure.
The results of dark-dark exposure showed that light had a suppressive effect on feeding in both groups of rats (P < 0.05 for both groups). On the normal light-dark schedule on the day preceding the dark-dark (continuous dark) condition, intake of B-SAP rats during the light period was 22 ± 2% of total daily intake. During the dark-dark condition, feeding during the subjective light period increased to 38 ± 4% of total daily intake in B-SAPs. On the day preceding the dark-dark condition, NPY-SAP rats consumed 35 ± 2% of their total daily intake in the light period. During the dark-dark condition, they increased their intake during the subjective light period to 46 ± 3% of total daily intake during dark-dark. For half of the NPY-SAP rats, this increase resulted in intakes that were greater during subjective light than during subjective dark. Thus, when the suppressive effect of light is taken into account, NPY-SAP rats lack the usual elevation of food intake in the dark (or subjective dark) period. These results provide evidence that NPY-SAP injections into the MBH did not abolish sensitivity to light.
Effects of NPY-SAP on sleep patterns.
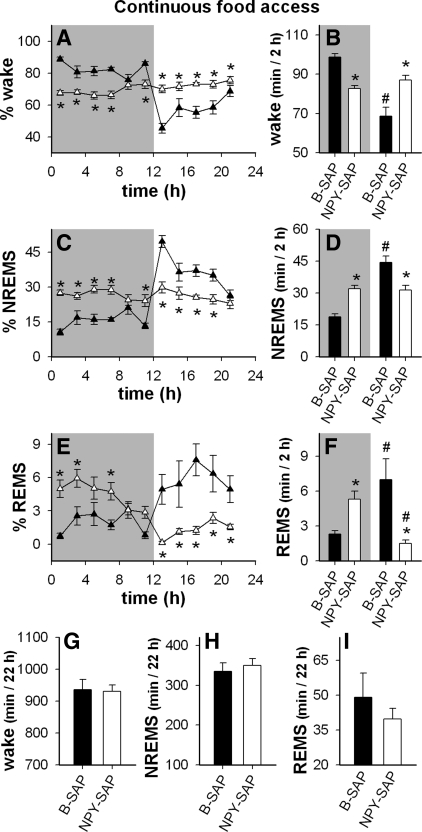
During the first few weeks after the NPY-SAP lesion (i.e., during the dynamic phase of the lesion), sleep patterns were disrupted in the lesioned rats, but their total sleep time was normal (Fig. 5, A–I). Circadian variation in NREMS was lost in the lesioned rats (Fig. 5C). Instead of occurring predominantly during the light period, NREMS was distributed equally across light and dark (Fig. 5D). In addition, the normal pattern of REMS was inverted in the NPY-SAP rats, such that REMS occurred mainly during the dark, rather than during the light (Fig. 5, E and F).
Fig. 5.
Distribution of vigilance states with ad libitum access to food in NPY-SAP (▲ and bars) and B-SAP (▴ and bars) rats. Results are averaged across 2 days (days 4 and 6) of sleep recording. A–F: shading indicates dark period; nonshaded regions indicate light period. A, C, and E: %time (mean ± SE) spent in each vigilance state per 2-h block (*P < 0.05, NPY-SAP vs. corresponding B-SAP value). B, D, and F: the average time spent in each vigilance state during dark vs. light periods, expressed as minute per 2 h (*P < 0.05, NPY-SAP vs. B-SAP for the same dark or light period; #P < 0.05 vs. the same group in the dark period). G, H, and I: total time in the indicated vigilance states during the 22 h of recording. Results show that during ad libitum feeding, the circadian variation in non-rapid eye movement sleep (NREMS) was lost and REMS concentration shifted from the light to the dark period in NPY-SAP rats, but total NREMS and REMS did not differ between groups.
Effects of restricted feeding on sleep patterns.
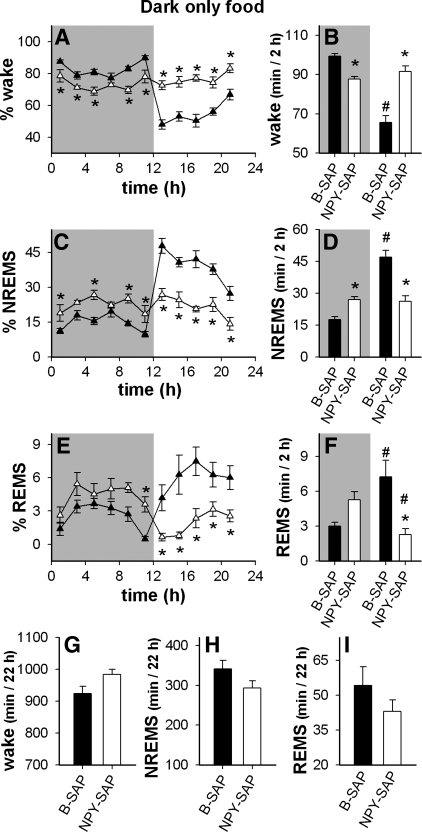
Next we addressed the question of whether the loss of circadian distribution of sleep patterns in the NPY-SAP rats was secondary to their altered feeding patterns. We hypothesized that the voracious appetite caused by the Arc lesion may have driven the rats to eat without regard to the light-dark cycle, thereby altering the circadian distribution of sleep-wake. To test this hypothesis, we subjected NPY-SAP and B-SAP rats to two restricted feeding paradigms described above. First, food intake was restricted to the dark period. We expected that if the lesioned rats had access to food only in the dark period when rats normally eat, they would revert to their normal circadian patterns of sleep-wake. Sleep patterns expressed in 2-h time blocks during dark period food restriction are shown for the two groups in Fig. 6. The results comparing light period to dark period within each treatment group are shown in Fig. 7 for the B-SAP and in Fig. 8 for the NPY-SAP group. As expected, in B-SAP controls, restricting food access to the dark period, when normal rats eat 75–80% of their caloric intake, had minimal effects on their eating and sleeping patterns (Figs. 4 and 6, respectively). However, in NPY-SAP rats (Fig. 6), the abnormal distribution of wake, the lack of circadian variation in NREMS and the REMS reversal persisted as during ad libitum feeding, in spite of the restricted feeding schedule. Thus, imposing a normal nocturnal feeding schedule did not normalize the sleep-wake patterns in the lesioned rats, indicating that their sleep-wake abnormalities were not secondary to their increased light period food intake.
Fig. 6.
Distribution of vigilance states when access to food was restricted to the dark period in NPY-SAP (▵ and white bars) and B-SAP (▴ and black bars) rats. Data were obtained 3 days after restricted access began. A–F: shaded regions indicate dark period. A, C, and E: % time in each vigilance state in 2-h blocks (*P < 0.05, NPY-SAP vs. corresponding B-SAP value). B, D, and F: amount of time spent in each vigilance state in the dark period vs. light period expressed as minute per 2 h (mean ± SE) (*P < 0.05, NPY-SAP vs. B-SAP within the same dark or light period; #P < 0.05 vs. the same group in the dark period). G, H, and I: total time in the indicated vigilance states during the 22 h of recording. Restricting food access to the dark period did not significantly alter the distribution NREMS or REMS or wake in either B-SAP or NPY-SAP rats, compared with the distribution during ad libitum feeding for the same group (see Fig. 5).
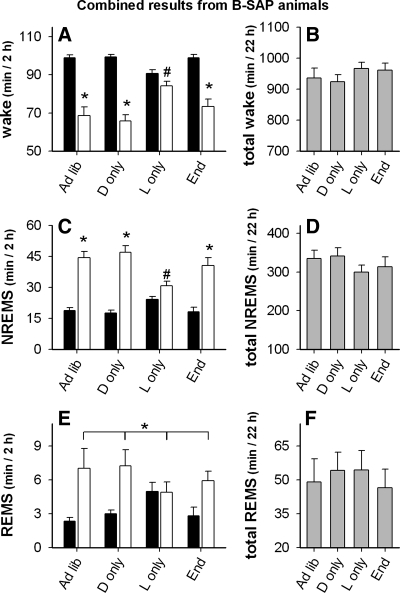
Fig. 7.
Effect of ad libitum (ad lib) and light- and dark-restricted feeding on wake, NREMS, and REMS in Arc B-SAP injected rats. (Con: ad libitum access; D only: dark period food only; L only: light period food only; End: ad libitum access, 104 days after Arc injection). Black bars are from dark period, white bars are from light period, gray bars are the sum of values from both dark and light periods. A and B: wake (*P < 0.001 vs. adjacent filled bar; #P < 0.01 vs. all other white bars). C and D: NREMS (*P < 0.001 vs. adjacent black bar; #P < 0.01 vs. all other white bars). E and F: REMS (*P < 0.001, white vs. adjacent black bars, 2-way ANOVA main effect). There were no differences detected between total values shown in B, D, and F.
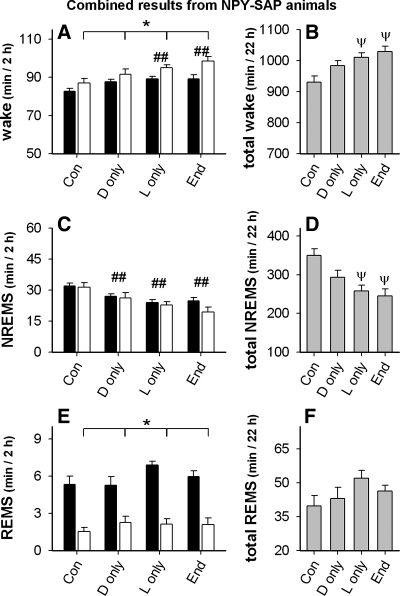
Fig. 8.
Effect of ad libitum and light- and dark-restricted feeding on wake, NREMS, and REMS in Arc NPY-SAP injected rats. (Con: ad libitum access; D only: dark period food only ; L only: light period food only; End: ad libitum access, 104 days after Arc injection). Black bars are from dark period, white bars are from light period, gray bars are sum of values from both dark and light periods. A: wake: *P < 0.001, main effect in the 2-way ANOVA, white bars vs. filled bars; ##P < 0.05, combined value from both bars vs. combined value of both bars from control (Con) day. B: total wake: ψP < 0.01 vs. control day. C: NREMS: ##P < 0.01, combined value from both bars vs. combined value of both bars on control day. D: total NREMS: ψP < 0.01 vs. control day. E: REMS: *P < 0.001, white bars vs. black bars, main effect in 2-way ANOVA. F: total REMS, no significant differences.
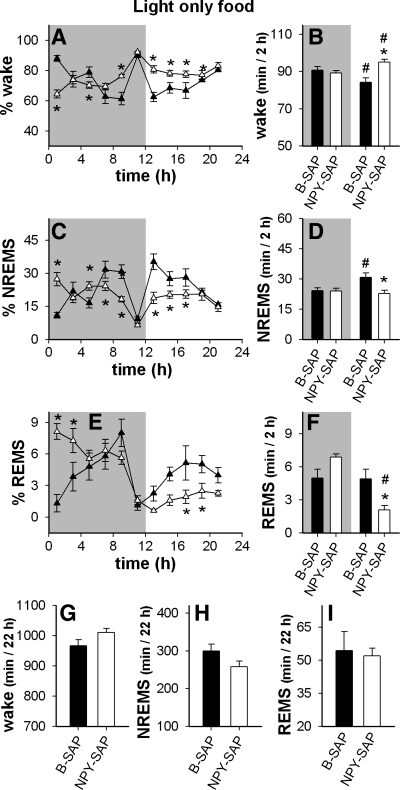
We then restricted food access to the light period, when normal rats sleep more and eat less than in the dark period, as shown in Fig. 9 (for within group comparisons see Figs. 7 and 8). Sleep patterns during light period restricted feeding are shown in 2-h blocks in these figures. Based on previous work (64), we expected that this feeding schedule would alter the circadian distributions of sleep-wake of B-SAP controls, but if NPY-SAP animals were unresponsive to photic or food entrainment signals, this manipulation would have no effect on their sleep-wake patterns. As expected, light period restricted feeding altered sleep patterns in B-SAP controls by reducing differences in the amount of wake, NREMS, and REMS between light and dark periods. In contrast, restricting feeding to the light phase did not alter sleep rhythms in NPY-SAP rats compared with patterns during ad libitum access to food (compare Fig. 9 to Fig. 5; also see Fig. 8), indicating that the ability of metabolic cues to alter sleep-wake rhythms was impaired by this lesion. Interestingly, light-period restricted feeding was associated with a significant increase in amount of wake (and a drop in NREMS) in the 2-h block just prior to light onset in both B-SAP and NPY-SAP groups (Fig. 9), suggesting that the failure of NPY-SAP rats to exhibit circadian patterning of either feeding or sleep was not caused by lack of sensitivity to light or inability to anticipate scheduled food availability.
Fig. 9.
Distribution of vigilance states when food was restricted to the light period in NPY-SAP (▵ and white bars) and B-SAP (▴ and black bars) rats. Results were obtained 3 days after onset of the feeding schedule. In A–F, shaded regions indicate dark period. A, C, and E: % time in each vigilance state in 2-h blocks (*P < 0.05, NPY-SAP vs. corresponding B-SAP value). B, D, and F: average amount of time spent in each vigilance state in the dark and light periods expressed as minute per 2 h (*P < 0.05, white bar vs. black bar within the same dark or light period; #P < 0.05 vs. the same bar in the dark period). G, H, and I: total time in the indicated vigilance states during the 22 h of recording. Light period restricted feeding significantly altered the distribution of sleep-wake in B-SAP controls compared with the distribution under ad libitum conditions, but had no effect on the distribution of sleep-wake in the NPY-SAP rats (see Fig. 5).
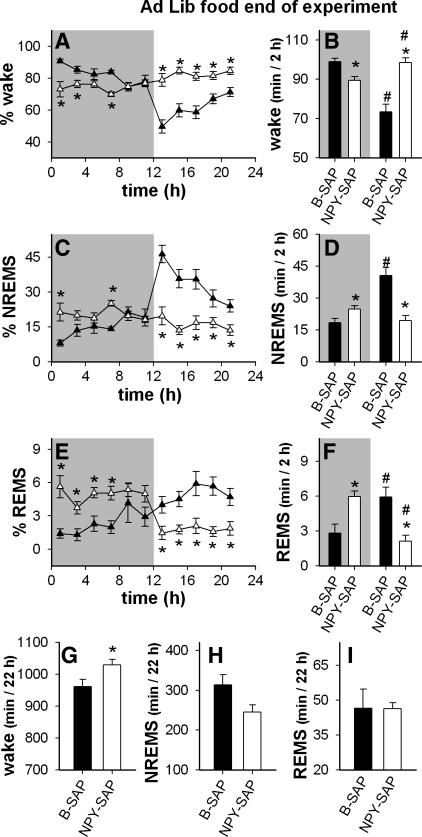
The pattern of feeding and weight gain over time after the Arc NPY-SAP injection was similar to the classic pattern described in rats with other types of lesions in the mediobasal hypothalamic region, consisting of a dynamic phase of hyperphagia and rapid weight gain, followed by a static phase during which daily food intake and rate of weight gain are reduced to levels more similar to control values (22). Sleep experiments described above were conducted during the dynamic phase of weight gain. Fourteen weeks after Arc injections, NPY-SAP lesioned rats were in the static phase of weight gain. Their 24-h food intake was close to the level of controls (28.2 ± 1.3 g compared with 24.5 ± 0.9 g, respectively), although the light period eating of NPY-SAP rats was still elevated compared with B-SAPs (32 ± 4% and 19 ± 4% of total daily intake, respectively, P < 0.05). Nevertheless, in NPY-SAP-treated rats, food intake was predominantly nocturnal during this time, as in normal rats. This suggested that the sleep patterns of the NPY-SAP rats may have also reestablished a more normal circadian distribution during the static phase. Therefore, we conducted a final sleep recording session during the static phase of weight gain while the animals had ad libitum access to food. The results showed that despite the change in their feeding patterns, the NPY-SAP rats continued to exhibit the same altered sleep patterns evident soon after the lesion, except that total sleep was somewhat reduced at this time (Fig. 10; see Fig. 8 for within-group comparisons). These findings strengthen the hypothesis that changes in sleep in NPY-SAP rats during the dynamic phase were not secondary to the lesion-induced hyperphagia.
Fig. 10.
Distribution of vigilance states under ad libitum feeding conditions 104 days after Arc injections in NPY-SAP (▵ and white bars) and B-SAP (▴ and black bars) rats. In A–F, shaded regions indicate the dark period. A, C, and E: % time in each vigilance state in 2-h blocks (*P < 0.05, NPY-SAP vs. corresponding B-SAP value). B, D, and F: average amount of time spent in each vigilance state in the dark period vs. light period expressed as minute per 2 h (*P < 0.05, white bar vs. black bar under same condition, #P < 0.05 vs. the same colored bar in the dark period). G, H, and I: total time in the indicated vigilance states during the 22 h of recording (*P < 0.05 vs. adjacent black bar). The sleep-wake changes apparent soon after Arc injections (see Fig. 4) persisted in NPY-SAP rats even after attenuation of lesion-induced hyperphagia.
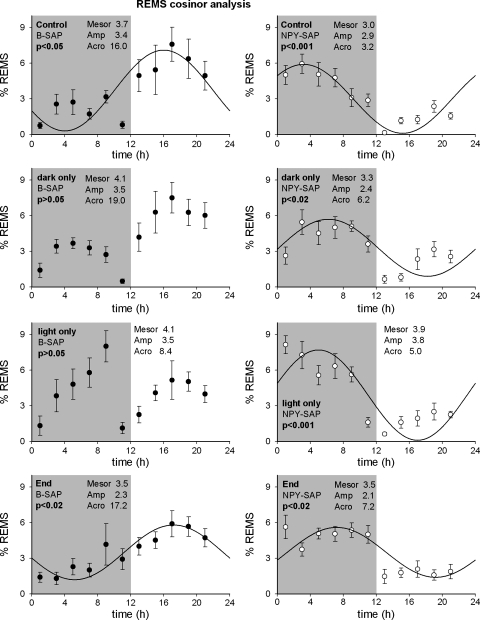
Although cosinor analysis is sometimes used to estimate circadian rhythmicity (52), we did not analyze a sufficient number of days to establish valid circadian rhythms of vigilance states. However, we subjected NREMS, REMS, and wake to cosinor analysis as an additional means of estimating and comparing their circadian distribution during the measurement period. Cosinor analysis for REMS distribution is shown in Fig. 11 for both groups (results for NREMS and wake not shown). The results of cosinor analysis are consistent with those we report using other measures. Consistent with previous results in normal rats (30), NREMS, REMS and wake showed circadian distributions that fit a sine wave with a period of 24 h (P < 0.02) in B-SAP rats when food was available ad libitum. However, food restriction altered the cosinor fit in B-SAPs. The cosinor fit of REMS for 24 h was not significant in B-SAP rats during food restriction. In addition, the fit was not significant for either NREMS or for wake during light period food restriction. In NPY-SAP rats, circadian periodicity was lost for NREMS and wake (P > 0.05), while REMS still fit the circadian wave form (P < 0.05). However, there was a 10.4-h difference in the acrophase of the REMS rhythm between B-SAP and NPY-SAP rats, indicating a shift or inversion in the phase of REMS in the lesioned rats. It is important to note that the reversal of REMS was not accompanied by a reversal of NREMS or by changes in the total amount of REMS. These results suggest that the photoperiod placement of REM and its normal relationship to NREMS pattern in rats requires NPY-receptor expressing neurons in the Arc.
Fig. 11.
Cosinor analysis of REMS distribution in animals with B-SAP (left graphs) and NPY-SAP (right graphs) lesions under different food restriction paradigms (control: ad libitum access; dark only: access in dark period only; light only: access in light period only; End: ad libitum access 104 days after lesion). Parameters from each fit (Mesor; Amp, amplitude; and Acro, acrophase) are indicated on each graph. In B-SAP animals the sine wave fit was significant when the animals had ad libitum access to food at both the beginning and end of the experiment. Restricting access to the light period resulted in a nonsignificant fit. The fit was also nonsignificant in the dark period, but the values from the fit are close to those obtained when the animals had ad libitum access to food. In the NPY-SAP animals, the fit was always significant regardless of when food was available, and the acrophase was always in the opposite time period from the values obtained from B-SAP animals that had ad libitum access to food.
DISCUSSION
NPY-SAP injections targeting the Arc disrupted the circadian distribution of both feeding and sleep. In the lesioned rats, NREMS and wake were distributed equally across the light and dark periods and REMS was phase reversed, occurring predominantly in the dark rather than the light period. The disrupted sleep patterns appeared to be permanent, at least up to 15 wk after the NPY-SAP injections. Food intake, which is predominantly nocturnal in normal rats, was also distributed almost equally across the light-dark cycle during the dynamic phase of the NPY-SAP lesion. Although a more normal feeding pattern was reestablished during the static phase, this pattern was lost upon removal of photic cues, revealing the persistence of disrupted circadian control of feeding in the NPY-SAP group. Disruptions in sleep-wake patterns in the NPY-SAP rats were not secondary to hyperphagia, as they persisted into the static phase when the lesioned rats were no longer hyperphagic. In addition, during the dynamic phase, when NPY-SAP rats were severely hyperphagic during both day and night, restricting food intake to the dark period did not normalize their sleep pattern. Nor did restriction of food intake to the light period alter the sleep-wake pattern in NPY-SAP rats, although light period food restriction did reduce day-night differences in NREMS in B-SAP controls, as shown previously in normal rats (69). Thus, the lesion produced an uncoupling of feeding and sleep-wake rhythms such that food availability no longer was able to influence the patterning of sleep-wake, suggesting that the integration of sleep and feeding rhythms requires the MBH. The Arc, which was targeted in our study and was the focus of the lesion effects, may be the site of integration. Alternatively, the NPY-receptor-expressing neurons in the MBH area may transmit critical information to other brain sites that may serve as rhythm generators (21, 41, 74, 75, 77).
The phase reversal of REMS observed in the NPY-SAP rats was not accompanied by a phase reversal of NREMS. REMS, and NREMS are known to be distinct states that can be differentially altered by various conditions; for example by drugs (24, 46, 53), by hormones (4, 79), by amino acid infusion (12), by selective gene knockouts (70), or by disease (33). Schedule of food availability is one such condition capable of differentially altering the distribution of REMS and NREMS, as shown by a previous study in which food and water were restricted to the light period for 29 days in normal rats (69). The prolonged restricted feeding schedule in that study caused reduced light-dark differences in NREMS and a reversal of REMS predominance to the dark period. Therefore, it is noteworthy that NPY-SAP lesions caused permanent changes in NREMS and REMS distribution that were similar to those produced by prolonged light period restricted feeding in normal rats but that persisted regardless of the feeding schedule. These data underscore the importance of feeding schedule (or metabolic state) in determining the circadian patterning of sleep states in normal rats. The results further suggest that the photoperiod placement of REM and its normal relationship to NREMS in rats requires NPY receptor-expressing neurons in the MBH.
The effects of the NPY-SAP lesion in the MBH on sleep-wake are clearly in agreement with the concept that the mechanisms that trigger wake, NREMS, and REMS are separable (4. 12, 24, 33, 46, 53, 69, 70, 79), as the lesion differentially affected the circadian distribution of these states. In addition, our findings concur with the proposal that these triggering mechanisms lie elsewhere than in the NPY-sensitive MBH neurons (59, 76, 91) since these sleep-wake states themselves were not abolished by the NPY-SAP lesion. Moreover, our findings indicate that these lesions did not alter the total amount of wake, NREMS, or REMS under any of the conditions we tested, suggesting that the mechanisms underlying homeostatic control of total sleep time are distinct from those controlling its circadian distribution, and further, that the homeostatic control of total sleep time does not require MBH NPY-sensitive neurons.
The SAP molecule, which comprises the toxic component of the NPY-SAP conjugate, gains entry into its target cell by agonist-driven receptor internalization subsequent to NPY receptor binding. All known rat NPY receptors, except the NPY-Y4 subtype, are internalizing receptors (56, 57, 94). Accordingly, we have shown previously that NPY-SAP binds to NPY receptors with greater affinity than NPY itself (5). We have also shown previously and in the present study that known NPY receptor-expressing neurons are profoundly reduced in number by NPY-SAP injections directed at the Arc. NPY-SAP is not retrogradely transported (5), and so produces a lesion of NPY receptor-expressing neurons that is limited to the diffusion radius of the injected solution. Therefore, it seems likely that the deficits reported in the present study can be attributed to loss of NPY, receptor-expressing neurons in the MBH. However, because NPY receptors are widely expressed in the MBH and are present on many neuronal phenotypes in addition to those evaluated here, we cannot claim that the lesions resulting from our Arc-directed injections were limited to NPY-receptor expressing neurons in the Arc. Nevertheless, our findings strongly suggest a role for the MBH (and very likely, the Arc itself) in the network of neurons involved in coordination of feeding and sleep-wake schedules.
Anatomical studies also provide evidence consistent with a role for the Arc and MBH in sleep and feeding patterns. Neural pathways have been identified connecting the MBH and SCN, providing a potential neural substrate for generation of day-night rhythms linked to metabolism, feeding, sleep-wake, and other homeostatic functions. These include substantial direct (45) and indirect (32) afferent input to the dorsal shell of the SCN and the peri-SCN region from the VMN and the Arc. Electrophysiological analysis of the Arc projections to the SCN and peri-SCN region (71) have indicated that > 80% of both SCN and peri-SCN cells are responsive to Arc stimulation and that some projections are excitatory and others inhibitory. Antidromic analysis indicated ∼13% of Arc cells contribute to the SCN/peri-SCN projection. Furthermore, neurons in the Arc are known to project to the dorsomedial hypothalamus (90), a proposed integrative site for circadian inputs from the SCN and metabolic cues arising from the Arc nucleus (75, 77). Thus, a reasonable interpretation of our findings is that an NPY-receptive population of neurons in the Arc importantly influences one or more of these sites to coordinate feeding with sleep and wake.
Specific peptidergic neurons within the Arc and Arc-SCN/peri-SCN projections appear to be components of an integrative mechanism for feeding and sleep-wake, including NPY receptor-expressing phenotypes that would be targeted by NPY-SAP. The Arc projections to the SCN shell or peri-SCN express pro-opiomelanocortin (28), galanin (2) and possibly NPY (8, 9), all of which are important for control of food intake. Some of these projections also are sensitive to estrogen (14), leptin (6), and ghrelin (98), which influence both feeding and sleep. The peptides NPY and ghrelin, which are normally elevated during food deprivation, increase feeding and suppress REMS in rats (86, 87, 88). NPY levels are elevated in the Arc by REMS deprivation (31). Obestatin, also present in the Arc, decreases feeding and increases REMS (89). In previous work, we found that Arc-directed injections of NPY-SAP abolished feeding responses to intraventricular administration of ghrelin and leptin (5). The diminished responsiveness of Arc NPY-SAP-injected rats to important food-related signals, coupled with data presented here, supports the hypothesis that NPY-receptor expressing neurons are crucial components of an MBH mechanism required for control of vigilance state by metabolic signals.
NPY-SAP lesions do not reproduce in their entirety the behavioral syndromes observed following lesions of the SCN or lesions of other types in the MBH. However, rats with NPY-SAP lesions do exhibit individual behavioral features that are components of both SCN and MBH lesion syndromes. These similarities and differences in lesion effects lead us to believe that neurons destroyed by NPY-SAP injections comprise a part of a central neural circuit for integration of feeding and sleep and that the NPY-SAP has provided a mechanism for more detailed delineation of circuit components. MSG lesions (46) and electrolytic lesions of the MBH (29) produce hyperphagia and obesity and increased light period feeding, as do NPY-SAP injections, but unlike NPY-SAP lesions, both MSG (54) and electrolytic lesions (13) increase total sleep, reduce wake, and eliminate day-night differences in NREMS and REMS. NPY-SAP lesion altered the normal nocturnal/diurnal distribution of both NREMS and REMS, but did not alter total REMS, NREMS, or wake time, and only the NPY-SAP lesion caused a phase reversal of REMS predominance. These three lesioning methods affect different cell populations (some in common and some unique to each technique) and thus it is not surprising that the behavioral syndromes produced by each approach would have aspects in common and aspects that are unique. While NPY-SAP selectively destroys specific populations of neurons, electrolytic lesions destroy all cell populations as well as fibers in passage, whereas neonatal MSG injections, although selective for cell bodies, also destroy circumventricular organs outside the MBH (55, 61), some of which have important roles in control of food and water intake (16, 17, 62, 65, 66, 68) and endocrine function (7, 19, 85).
Similarly, our NPY-SAP lesions only partially reproduce the effects of SCN lesions on feeding and sleep, suggesting that NPY-SAP damaged important reciprocal connections between Arc and SCN, but not the SCN itself. NPY-SAP rats remain sensitive to photic cues and appear to be able to anticipate light period food availability when placed on a restricted feeding. SCN lesions disrupt circadian rhythmicity of feeding (82, 92) and increase daytime feeding. However, in contrast to Arc NPY-SAP lesions, total daily intake of SCN lesioned rats does not differ from controls and lesioned rats do not become obese (84). SCN lesions alter the distribution of NREMS, REMS, and wake (28), as do NPY-SAP lesions, but the lesions differ importantly in their effects on REMS. In contrast to SCN lesions, which eliminate the circadian rhythm of both REM and NREMS (84, 92), NPY-SAP lesions did not eliminate rhythmic day-night differences in REMS, but caused them to phase reverse.
It is also significant that lesions in several structures outside the SCN produce effects on REMS and NREMS, supporting our contention that effects of our NPY-SAP injections were due to lesions localized predominantly in the Arc. Isolation of the SCN by knife cuts, unlike SCN lesions, has been reported to produce REMS phase reversal in a few rats (81). Lesions of the SCN (26; reviewed in Ref. 43) or ventral subperiventricular zone (38), knife-cut isolation of the SCN (81), MSG-induced destruction of the Arc (54), and MBH knife cuts (59, 97) all reduce day-night differences in NREMS. These sites are reciprocally interconnected (6, 71) and appear to form an afferent/efferent loop, the integrity of which may be required for control of day-night distribution of REMS and NREMS.
In defining the anatomical substrates of the MBH NPY-SAP lesion, several structures in the surrounding hypothalamic areas can be ruled out as contributors to the findings we report. For example, it is not likely that the effects of the NPY-SAP lesion are due to damage to the median eminence. Although such damage is possible and even likely with NPY-SAP or other lesions directed at the Arc, previous work has shown that transection of the pituitary stalk does not alter either REMS or NREMS (59). Moreover, we show here that corticosterone response to glucoprivation is still present after Arc-directed NPY-SAP lesions, suggesting that the pathway to the median eminence from the paraventricular corticotropin releasing hormone neurons remains functional in the lesioned rats.
Various lines of investigation have compellingly indicated a role for orexin neurons as a link between homeostatic behaviors, including food intake, and sleep-wake states. These neurons are proposed to be crucial for coordination of sleep-wake with homeostatic behaviors (72–75, 77, 93), and they express NPY receptors (18), rendering them susceptible to NPY-SAP toxicity. For these reasons, it is important to ask whether the NPY-SAP lesion inadvertently damaged orexin neurons. However, this is unlikely for several reasons. First, these neurons are quite widely dispersed in lateral and perifornical hypothalamic areas (10, 60), where most would be outside the expected diffusion radius of our 50-nl Arc injection. Second, in an independent cohort of rats injected with NPY-SAP or B-SAP using the same protocol as for animals in the main feeding-sleep study, we showed that the numbers of orexin-expressing neurons did not differ between groups. Third, effects of NPY-SAP injection differ importantly from those produced by techniques such as orexin gene deletion (23), lateral hypothalamic injection of orexin-SAP (20) or siRNAs targeting the orexin gene (11). Such disruptions of orexin neuron function produce hypophagia, not hyperphagia, as observed in the Arc-injected NPY-SAP rats. In addition, these procedures commonly cause cataplexy, increased nocturnal incidence of both REM sleep and NREM, and significant increase in 24-h total NREM. Our lesioned rats had increased nighttime REM and NREM compared with controls, but overall total time spent in REM, NREM, and wake did not differ between groups, and cataplectic attacks were not observed. Therefore, our lesion may have disrupted the transfer of information between the MBH and orexin neurons, but we conclude that our results are not due to direct damage to orexin neurons themselves.
The hypothesis that crucial metabolic signals are no longer effective in influencing REMS pattern in NPY-SAP rats does not address the very interesting question of why REMS was shifted to the dark phase in these animals, as opposed to remaining in the light period or redistributing across the light-dark period. Perhaps there is a genetically determined default timing that favors nocturnality of REMS but the favored REMS timing is shifted to the light phase in nocturnal animals, such as the rat, by mechanisms also controlled within the Arc. Perhaps such a mechanism was disabled in our NPY-SAP rats. Shifts in nocturnality and diurnality of behavior and physiology in normal animals during seasonal changes in food availability, in genetically modified and brain-lesioned animals, as well as in human disease conditions, are well documented and have been discussed (4, 39, 40), but brain sites and mechanisms for such changes are not yet known.
Perspectives and Significance
While much previous work demonstrates that the MBH and NPY-sensitive neurons are critical for control of food intake and body adiposity (for a review, see Ref. 24), the uncoupling of feeding and sleep rhythms by the MBH NPY-SAP lesion that we report here is a novel finding. The disruptive consequences of this uncoupling for both feeding and sleep attests to the importance of food-related cues in controlling circadian distribution of sleep states and underline the fundamental importance of circadian rhythm integration for both adaptation to the environment and homeostatic efficiency. The availability of targeted neurotoxins, such as NPY-SAP, has made possible phenotype-selective lesions and in doing so, has provided an important tool for delineating functionally specific components of complex neural circuits. The present results indicate that this approach offers promise for further advances in understanding the organization of feeding and sleep. A fuller understanding of the MBH contribution to circadian rhythm integration may have implications for understanding mechanisms of sleep and feeding disorders that occur under certain work conditions and disease states (39, 40, 51).
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-040498 and DK-00345072 (to S. Ritter) and DK-067146 (to S. M. Simasko).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.F.W., S.M.S., and S.R. conception and design of research; M.F.W., S.M., A.-J.L., T.T.D., and E.M.R. performed experiments; M.F.W., S.M., A.-J.L., E.M.R., S.M.S., and S.R. analyzed data; M.F.W., S.M., A.-J.L., S.M.S., and S.R. interpreted results of experiments; M.F.W., S.M., A.-J.L., T.T.D., S.M.S., and S.R. prepared figures; M.F.W., S.M.S., and S.R. drafted manuscript; M.F.W., A.-J.L., S.M.S., and S.R. edited and revised manuscript; M.F.W., S.M., A.-J.L., T.T.D., E.M.R., S.M.S., and S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of S. Mukherjee: Department of Neurosurgery, Scott and White Hospital, Temple, TX 76503.
REFERENCES
- 1.Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci 22: 350–356, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res 916: 172–191, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Borbely AA. Effects of light on sleep and activity rhythms. Prog Neurobiol 10: 1–31, 1978 [DOI] [PubMed] [Google Scholar]
- 4.Bradbury MJ, Dement WC, Edgar DM. Effects of adrenalectomy and subsequent corticosterone replacement on rat sleep state and EEG power spectra. Am J Physiol Regul Integr Comp Physiol 275: R555–R565, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology 146: 1179–1191, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Buijs RM, Scheer FA, Kreier F, Yi C, Bos N, Goncharuk VD, Kalsbeek A. Organization of circadian functions: interaction with the body. Prog Brain Res 153: 341–360, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Buller KM. Role of circumventricular organs in pro-inflammatory cytokine-induced activation of the hypothalamic-pituitary-adrenal axis. Clin Exp Pharmacol Physiol 28: 581–589, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Card JP, Moore RY. Ventral lateral geniculate nucleus efferents to the rat suprachiasmatic nucleus exhibit avian pancreatic polypeptide-like immunoreactivity. J Comp Neurol 206: 390–396, 1982 [DOI] [PubMed] [Google Scholar]
- 9.Card JP, Moore RY. Neuropeptide Y localization in the rat suprachiasmatic nucleus and periventricular hypothalamus. Neurosci Lett 88: 241–246, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Chen CT, Dun SL, Kwok EH, Dun NJ, Chang JK. Orexin A-like immunoreactivity in the rat brain. Neurosci Lett 260: 161–164, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Chen L, McKenna JT, Bolortuya Y, Winston S, Thakkar MM, Basheer R, Brown RE, McCarley RW. Knockdown of orexin type 1 receptor in rat locus coeruleus increases REM sleep during the dark period. Eur J Neurosci 32: 1528–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danguir J, Nicolaidis S. Intravenous infusions of nutrients and sleep in the rat: an ischymetric sleep regulation hypothesis. Am J Physiol Endocrinol Metab 238: E307–E312, 1980 [DOI] [PubMed] [Google Scholar]
- 13.Danguir J, Nicolaidis S. Sleep and feeding patterns in the ventromedial hypothalamic lesioned rat. Physiol Behav 21: 769–777, 1978 [DOI] [PubMed] [Google Scholar]
- 14.De La Iglesia HO, Blaustein JD, Bittman EL. Oestrogen receptor-α-immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J Neuroendocrinol 11: 481–490, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Eastman CI, Mistlberger RE, Rechtschaffen A. Suprachiasmatic nuclei lesions eliminate circadian temperature and sleep rhythms in the rat. Physiol Behav 32: 357–368, 1984 [DOI] [PubMed] [Google Scholar]
- 16.Edwards GL, Ritter RC. Ablation of the area postrema causes exaggerated consumption of preferred foods in the rat. Brain Res 216: 265–276, 1981 [DOI] [PubMed] [Google Scholar]
- 17.Edwards GL, Ritter RC. Area postrema lesions increase drinking to angiotensin and extracellular dehydration. Physiol Behav 29: 943–947, 1982 [DOI] [PubMed] [Google Scholar]
- 18.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol 402: 442–459, 1998 [PubMed] [Google Scholar]
- 19.Ganong WF. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin Exp Pharmacol Physiol 27: 422–427, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci 21: 7273–7283, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guilding C, Hughes AT, Brown TM, Namvar S, Piggins HD. A riot of rhythms: neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol Brain 2: 28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallonquist JD, Brandes JS. Ventromedial hypothalamic lesions and weight gain in rats: absence of a static phase. Physiol Behav 27: 709–713, 1981 [DOI] [PubMed] [Google Scholar]
- 23.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30: 345–354, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Herrera-Solis A, Vasquez KG, Prospero-Garcia O. Acute and subchronic administration of anandamide or oleamide increases REM sleep in rats. Pharmacol Biochem Behav 95: 106–112, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Huhman KL, Albers HE. Neuropeptide Y microinjected into the suprachiasmatic region phase shifts circadian rhythms in constant darkness. Peptides 15: 1475–1478, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Ibuka N, Nihonmatsu I, Sekiguchi S. Sleep-wakefulness rhythms in mice after suprachiasmatic nucleus lesions. Waking Sleeping 4: 167–73, 1980 [PubMed] [Google Scholar]
- 27.Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol 493: 72–85, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Kineman RD, Kraeling RR, Crim JW, Leshin LS, Barb CR, Rampacek GB. Localization of proopiomelanocortin (POMC) immunoreactive neurons in the forebrain of the pig. Biol Reprod 40: 1119–1126, 1989 [DOI] [PubMed] [Google Scholar]
- 29.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav 87: 221–244, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Kitka T, Katai Z, Pap D, Molnar E, Adori C, Bagdy G. Small platform sleep deprivation selectively increases the average duration of rapid eye movement sleep episodes during sleep rebound. Behav Brain Res 205: 482–487, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Koban M, Le WW, Hoffman GE. Changes in hypothalamic corticotropin-releasing hormone, neuropeptide Y, and proopiomelanocortin gene expression during chronic rapid eye movement sleep deprivation of rats. Endocrinology 147: 421–431, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience 110: 73–92, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Krueger JM, Majde JA. Humoral links between sleep and the immune system: research issues. Ann NY Acad Sci 992: 9–20, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Leibowitz SF. Brain neuropeptide Y: an integrator of endocrine, metabolic and behavioral processes. Brain Res Bull 27: 333–337, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Li AJ, Dinh TT, Ritter S. Hyperphagia and obesity produced by arcuate injection of NPY-saporin do not require upregulation of lateral hypothalamic orexigenic peptide genes. Peptides 29: 1732–1739, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li AJ, Ritter S. Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur J Neurosci 19: 2147–2154, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Li AJ, Wang Q, Ritter S. Differential responsiveness of dopamine-β-hydroxylase gene expression to glucoprivation in different catecholamine cell groups. Endocrinology 147: 3428–3434, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci 21: 4864–4874, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res 106: 447–462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maywood ES, O'Neill J, Wong GK, Reddy AB, Hastings MH. Circadian timing in health and disease. Prog Brain Res 153: 253–269, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci 24: 10493–10501, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minana-Solis MC, Angeles-Castellanos M, Feillet C, Pevet P, Challet E, Escobar C. Differential effects of a restricted feeding schedule on clock-gene expression in the hypothalamus of the rat. Chronobiol Int 26: 808–820, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Brain Res Rev 49: 429–454, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Mistlberger RE, Antle MC. Neonatal monosodium glutamate alters circadian organization of feeding, food anticipatory activity and photic masking in the rat. Brain Res 842: 73–83, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol 389: 508–534, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Monti JM, Leopoldo M, Jantos H. The serotonin 5-HT7 receptor agonist LP-44 microinjected into the dorsal raphé nucleus suppresses REM sleep in the rat. Behav Brain Res 191: 184–189, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42: 201–206, 1972 [DOI] [PubMed] [Google Scholar]
- 48.Mrosovsky N. Beyond the suprachiasmatic nucleus. Chronobiol Int 20: 1–8, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Mrosovsky N, Hattar S. Diurnal mice (Mus musculus) and other examples of temporal niche switching. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191: 1011–1024, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Mukherjee S, Kazerooni M, Simasko SM. Dose-response study of chronic alcohol induced changes in sleep patterns in rats. Brain Res 1208: 120–127, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, Trinder J. Nature's clocks and human mood: the circadian system modulates reward motivation. Emotion 9: 705–716, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia 6: 305–323, 1979 [PubMed] [Google Scholar]
- 53.Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci 8: 520–550, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Olivo M, Kitahama K, Valatx JL, Jouvet M. Neonatal monosodium glutamate dosing alters the sleep-wake cycle of the mature rat. Neurosci Lett 67: 186–190, 1986 [DOI] [PubMed] [Google Scholar]
- 55.Olney JW. The toxic effects of glutamate and related compounds in the retina and the brain. Retina 2: 341–359, 1982 [PubMed] [Google Scholar]
- 56.Parker SL, Parker MS, Buschauer A, Balasubramaniam A. Ligand internalization by cloned neuropeptide Y Y5 receptors excludes Y2 and Y4 receptor-selective peptides. Eur J Pharmacol 474: 31–42, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Parker SL, Parker MS, Sah R, Balasubramaniam A, Sallee FR. Self-regulation of agonist activity at the Y receptors. Peptides 28: 203–213, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1997 [Google Scholar]
- 59.Peterfi Z, Makara GB, Obal F, Jr, Krueger JM. The anterolateral projections of the medial basal hypothalamus affect sleep. Am J Physiol Regul Integr Comp Physiol 296: R1228–R1238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price MT, Olney JW, Lowry OH, Buchsbaum S. Uptake of exogenous glutamate and aspartate by circumventricular organs but not other regions of brain. J Neurochem 36: 1774–1780, 1981 [DOI] [PubMed] [Google Scholar]
- 62.Reddy VM, Meharg SS, Ritter S. Dose-related stimulation of feeding by systemic injections of monosodium glutamate. Physiol Behav 38: 465–469, 1986 [DOI] [PubMed] [Google Scholar]
- 63.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 418: 935–941, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Richter CP. “Dark-active” rat transformed into “light-active” rat by destruction of 24-hr clock: function of 24-hr clock and synchronizers. Proc Natl Acad Sci USA 75: 6276–6280, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ritter RC, Edwards GL. Area postrema lesions cause overconsumption of palatable foods but not calories. Physiol Behav 32: 923–927, 1984 [DOI] [PubMed] [Google Scholar]
- 66.Ritter S, Stone SL. Area postrema lesions block feeding induced by systemic injections of monosodium glutamate. Physiol Behav 41: 21–24, 1987 [DOI] [PubMed] [Google Scholar]
- 67.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology 144: 1357–1367, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Rogulja I, Harding JW, Ritter S. Reduction of 125I-angiotensin II binding sites in rat brain following monosodium glutamate treatment. Brain Res 419: 333–335, 1987 [DOI] [PubMed] [Google Scholar]
- 69.Roky R, Kapas L, Taishi TP, Fang J, Krueger JM. Food restriction alters the diurnal distribution of sleep in rats. Physiol Behav 67: 697–703, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Romanowski CP, Fenzl T, Flachskamm C, Wurst W, Holsboer F, Deussing JM, Kimura M. Central deficiency of corticotropin-releasing hormone receptor type 1 (CRH-R1) abolishes effects of CRH on NREM but not on REM sleep in mice. Sleep 33: 427–436, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saeb-Parsy K, Lombardelli S, Khan FZ, McDowall K, Au-Yong IT, Dyball RE. Neural connections of hypothalamic neuroendocrine nuclei in the rat. J Neuroendocrinol 12: 635–648, 2000 [DOI] [PubMed] [Google Scholar]
- 72.Sakurai T. Orexin: a link between energy homeostasis and adaptive behaviour. Curr Opin Clin Nutr Metab Care 6: 353–360, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Sakurai T. Roles of orexins and orexin receptors in central regulation of feeding behavior and energy homeostasis. CNS Neurol Disord Drug Targets 5: 313–325, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Sakurai T. Roles of orexins in regulation of feeding and wakefulness. Neuroreport 13: 987–995, 2002 [DOI] [PubMed] [Google Scholar]
- 75.Saper CB. Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res 153: 243–252, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron 68: 1023–1042, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci 28: 152–157, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol 402: 435–441, 1998 [PubMed] [Google Scholar]
- 79.Sinton CM, Fitch TE, Gershenfeld HK. The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res 8: 197–203, 1999 [DOI] [PubMed] [Google Scholar]
- 80.Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms 17: 284–292, 2002 [DOI] [PubMed] [Google Scholar]
- 81.Stephan FK, Nunez AA. Elimination of circadian rhythms in drinking, activity, sleep, and temperature by isolation of the suprachiasmatic nuclei. Behav Biol 20: 1–61, 1977 [DOI] [PubMed] [Google Scholar]
- 82.Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol 25: 545–554, 1979 [DOI] [PubMed] [Google Scholar]
- 83.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69: 1583–1586, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stoynev AG, Ikonomov OC, Usunoff KG. Feeding pattern and light-dark variations in water intake and renal excretion after suprachiasmatic nuclei lesions in rats. Physiol Behav 29: 35–40, 1982 [DOI] [PubMed] [Google Scholar]
- 85.Summy-Long JY, Kadekaro M. Role of circumventricular organs (CVO) in neuroendocrine responses: interactions of CVO and the magnocellular neuroendocrine system in different reproductive states. Clin Exp Pharmacol Physiol 28: 590–601, 2001 [DOI] [PubMed] [Google Scholar]
- 86.Szentirmai E, Hajdu I, Obal F, Jr, Krueger JM. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res 1088: 131–140, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Szentirmai E, Kapas L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol Regul Integr Comp Physiol 292: R575–R585, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Szentirmai E, Krueger JM. Central administration of neuropeptide Y induces wakefulness in rats. Am J Physiol Regul Integr Comp Physiol 291: R473–R480, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Szentirmai Krueger JM E. Obestatin alters sleep in rats. Neurosci Lett 404: 222–226, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Brain Res Rev 27: 89–118, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Villablanca JR. Counterpointing the functional role of the forebrain and of the brainstem in the control of the sleep-waking system. J Sleep Res 13: 179–208, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Van den Pol AN, Powley T. A fine-grained anatomical analysis of the role of the rat suprachiasmatic nucleus in circadian rhythms of feeding and drinking. Brain Res 160: 307–326, 1979 [DOI] [PubMed] [Google Scholar]
- 93.Vanitallie TB. Sleep and energy balance: Interactive homeostatic systems. Metabolism 55: S30–S35, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Voisin T, Goumain M, Lorinet AM, Maoret JJ, Laburthe M. Functional and molecular properties of the human recombinant Y4 receptor: resistance to agonist-promoted desensitization. J Pharmacol Exp Ther 292: 638–646, 2000 [PubMed] [Google Scholar]
- 95.Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol 258: 230–252, 1987 [DOI] [PubMed] [Google Scholar]
- 96.Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol 258: 204–229, 1987 [DOI] [PubMed] [Google Scholar]
- 97.Yamaoka S. Participation of limbic-hypothalamic structures in circadian rhythm of slow wave sleep and paradoxical sleep in the rat. Brain Res 151: 255–268, 1978 [DOI] [PubMed] [Google Scholar]
- 98.Yi CX, van der Vliet J, Dai J, Yin G, Ru L, Buijs RM. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147: 283–294, 2006 [DOI] [PubMed] [Google Scholar]