Abstract
Serotonin neurons of the caudal raphe facilitate ventilatory and sympathetic responses that develop following blood loss in conscious rats. Here, we tested whether serotonin projections to the caudal portion of the dorsomedial brain stem (including regions of the nucleus tractus solitarius that receive cardiovascular and chemosensory afferents) contribute to cardiorespiratory compensation following hemorrhage. Injections of the serotonin neurotoxin 5,7-dihydroxytryptamine produced >90% depletion of serotonin nerve terminals in the region of injection. Withdrawal of ∼21% of blood volume over 10 min produced a characteristic three-phase response that included 1) a normotensive compensatory phase, 2) rapid sympathetic withdrawal and hypotension, and 3) rapid blood pressure recovery accompanied by slower recovery of heart rate and sympathetic activity. A gradual tachypnea developed throughout hemorrhage, which quickly reversed with the advent of sympathetic withdrawal. Subsequently, breathing frequency and neural minute volume (determined by diaphragmatic electromyography) declined below baseline following termination of hemorrhage but gradually recovered over time. Lesioned rats showed attenuated sympathetic and ventilatory responses during early compensation and later recovery from hemorrhage. Both ventilatory and sympathetic responses to chemoreceptor activation with potassium cyanide injection were attenuated by the lesion. In contrast, the gain of sympathetic and heart rate baroreflex responses was greater, and low-frequency oscillations in blood pressure were reduced after lesion. Together, the data are consistent with the view that serotonin innervation of the caudal dorsomedial brain stem contributes to sympathetic compensation during hypovolemia, possibly through facilitation of peripheral chemoreflex responses.
Keywords: blood pressure; renal sympathetic nerve activity; nucleus tractus solitarius; 5,7-dihydroxytryptamine; tryptophan hydroxylase
progressive blood loss produces a multiphasic hemodynamic response characterized by an initial normotensive phase in which arterial blood pressure (BP) is maintained by a rise in sympathetic-mediated vascular resistance (15). After substantial blood loss, sympathetic compensation suddenly declines, resulting in a rapid and dramatic fall in BP (28, 39). With continued blood loss, sympathetic drive begins to slowly recover. If blood loss is stemmed, sympathetic activity rises following recovery of BP. Sympathetic activity also recovers if blood loss continues, although only up to a point (40). As hypovolemic shock ensues, the vasculature becomes unresponsive to adrenergic vasoconstrictors, resulting in vascular decompensation (20). This latter phase is very difficult to treat and marks the onset of severe and potentially irreversible shock.
The autonomic mechanisms that control the hemodynamic responses to blood loss are not well understood. We recently found that selective destruction of caudal hindbrain serotonin neurons accelerated the sympatholytic response to blood loss in conscious rats and significantly reduced the extent of sympathetic recovery following the hypotensive phase of hemorrhage (16). Metabolic acidosis was also exaggerated in serotonin-lesioned animals subjected to hemorrhage, despite their having elevated arterial blood oxygen levels and normal BP recovery. These data suggest that serotonin neurons of the caudal raphe promote cardiovascular responses that facilitate tissue oxygenation following blood loss, most likely by increasing cardiac output through a sympathetic-mediated increase in venous tone.
The mechanism by which serotonin facilitates sympathetic recovery following blood loss is not known. In our prior study, we observed that arterial Pco2 rose following the initial hypotensive phase of hemorrhage in parallel with recovery of sympathetic activity and ventilation in intact conscious animals, suggesting that central chemoreflex activation may contribute to cardiovascular compensation following blood loss. We also found that serotonin lesion impaired sympathetic and ventilatory responses to hyperoxic hypercapnia, indicating that caudal medullary serotonin neurons are necessary for normal responses to central chemoreceptor activation in conscious rats. The same lesion increased spontaneous baroreflex gain and reduced BP variability, indicating that serotonin neurons tonically suppress baroreflex sensitivity (16). Together, these findings suggest that caudal medullary serotonin neurons facilitate sympathetic recovery following blood loss by conveying important blood gas information to brain stem or spinal regions that regulate sympathetic and ventilatory responses to chemoreceptor activation, rather than by augmenting arterial baroreflex function.
It is not known which serotonergic projections mediate compensation during hemorrhage. The nucleus of the solitary tract (nTS) receives significant projections from serotonergic neurons of the caudal medullary raphe (29, 36). A high density of serotonergic projections target the medial and commissural subnuclei of the nTS where arterial baroreceptor and peripheral chemoreceptor afferents terminate (4). Therefore, we determined if selective destruction of serotonin nerve terminals in the dorsomedial brain stem (including regions of the nTS that receive cardiovascular and chemosensory afferents) attenuated recovery of renal sympathetic activity and ventilation following hemorrhage. We further determined whether loss of serotonin nerve terminals in this region altered heart rate (HR) and sympathetic baroreflex gain or peripheral chemoreflex activation, since both are presumed to be engaged during hypotensive hemorrhage (3, 15).
METHODS
Animals
Male Sprague Dawley rats (300–350 g; Harlan, Indianapolis, IN) were acclimated to the Comparative Medicine housing facility while given ad libitum access to food and water for at least 1 wk before surgery. The facility was maintained at a constant temperature of 22 ± 2°C with a light-dark cycle of 12:12 h. All experiments were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the United States National Institutes of Health.
Surgery
All rats were pretreated with the dopamine and norepinephrine reuptake blocker nomifensine (15 mg/kg in 20% β-cyclodextrin) at least 30 min before neurotoxin injection to prevent toxin-induced destruction of noradrenergic cells. Rats were anesthetized with pentobarbital sodium (65 mg/kg ip), intubated with an enodtracheal tube, and placed in a stereotaxic apparatus with the nose bar positioned 11 mm below a flat skull position. The back of the skull was exposed, and a small portion of the occipital bone was removed to view the dorsal surface of the brain stem at the level of calamus scriptorus. A glass micropipette injector with an outer diameter of ∼20 μm was filled with 5,7-dihydroxytryptamine (5,7-DHT; 5 mM in 0.1% ascorbic acid) or vehicle. Bilateral 100-nl injections of toxin or vehicle were made 0.5 mm ventral to calamus scriptorus at five rostrocaudal planes along the nTS: 1, 0.5, 0, −0.5, and −1 mm relative to calamus scriptorus. Injections were made ± 0.7 and 0.35 mm from the midline for the first and second of the two most rostral set of injections, respectively, and ± 0.25 mm from the midline for the caudal three sets of injections for a total of 10 injections. The neck muscle and skin were closed in separate layers.
Precisely 7 days later, all rats were reanesthetized with pentobarbital sodium (65 mg/kg ip) and implanted with bilateral femoral arterial catheters to enable direct measurement of arterial pressure and simultaneous arterial blood withdrawal as described previously (30). A third catheter was placed in the femoral vein. Catheters were externalized at the nape of the neck and secured with sutures. Following catheter implantation, a recording electrode with dual electrode leads was implanted to enable simultaneous recordings of renal sympathetic nerve activity (RSNA) and diaphragmatic electromyographic (dEMG) signals (16). Briefly, a renal sympathetic nerve fiber bundle was exposed and isolated through a left flank incision. The fiber bundle was placed on a bipolar electrode made from Teflon-coated stainless steel (bare diameter = 0.005 in.; A-M Systems, Everett, WA). The electrode connector was externalized at the nape of the neck with the vascular catheters. The preparation was embedded in a quick-drying, lightweight silicon (Kwik sil; World Precision Instruments), and incisions were closed in layers. A 2-cm lateral laparotomy was performed, and a second set of lead wires was tied into the abdominal side of the diaphragm using 6–0 nylon suture. The lead wires were coiled in the subcutaneous space, and the incisions were closed in layers. Animals were allowed to recover overnight before the experiment. Chemoreflex tests were performed 22–24 h after surgery. Spontaneous baroreflex assessments and hemorrhage studies were conducted the following day. Pharmacological arterial baroreflex studies were conducted on separate groups of similarly instrumented rats 22–24 h after surgery.
Data Acquisition
Arterial pressure, HR, RSNA, and dEMG were recorded on a Macintosh G4 Powerbook computer using PowerLab data acquisition software (Chart version 5.2.1; ADinstruments, Colorado Springs, CO). Arterial pressure was measured with a disposable pressure transducer (Transpac IV; Abbott Labs, North Chicago, IL) and a PowerLab bridge amplifier (ADinstruments). HR was calculated using peak-to-peak detection of the pulse pressure wave. Sympathetic activity and dEMG were sampled (4,000 Hz) and amplified (10–20,000×) with PowerLab Bioamplifiers (ADinstruments). Both RSNA and dEMG were filtered (1–1,000 Hz), rectified, and integrated over a 20-ms time constant. Background noise in the RSNA recordings was determined at the end of each experiment by measuring the signal remaining after ganglionic blockade (hexamethonium chloride, 30 mg/kg iv). Background noise was subtracted from nerve activity values to provide a measurement of RSNA. Background noise in the dEMG electrode recording was determined during expiration, when phrenic motor nerve activity was presumed to be absent. Only RSNA and dEMG data from animals with greater than a 2:1 signal-to-noise ratio were included in the data analysis. Integrated dEMG was smoothed using a triangular (Bartlett) window with a window width of 401 points. This process yielded defined cycles that were used as an index of phrenic motor nerve discharge. The value of the cycle nadir (which represented the average background dEMG) that occurred just before each cycle peak was subtracted from the subsequent cycle, and the resulting area under the curve (AUC) for each cycle was measured to obtain neural tidal volume (nTV). Breathing frequency (BF) was determined by peak-to-peak detection of the integrated dEMG signal and multiplied by the nTV to obtain neural minute volume (nMV).
Protocols
Protocol 1: Peripheral chemoreflex test.
On the day of the experiment, one of the subject's arterial catheters was connected to the BP transducer through saline-filled polyethylene (PE) tubing via an overhead swivel system while the rat rested unrestrained in its home cage. The RSNA and dEMG leads were connected to the appropriate amplifiers via lead wires extending from the same swivel. Rats were allowed to rest, unrestrained in their home cage for at least 1 h after connection to the recording instruments. A separate length of PE tubing filled with the appropriate dose of potassium chloride (KCN) and a 150-μl volume of isotonic saline was connected to the venous catheter via the overhead swivel. Ten to 15 min after connecting the catheter, drug was injected over 5 s. After 10 min, the tubing containing the KCN was exchanged for tubing containing a new dose of drug, and the rat was allowed to rest. Doses (3, 30, and 100 μg/kg) of KCN were given in random order, at 40- to 50-min intervals while all variables were measured continuously. These doses were chosen based on prior studies demonstrating their efficacy in producing reliable chemoreflex responses that were dependent on the integrity of the carotid body (8, 9).
Protocol 2: Hypotensive hemorrhage.
Approximately 22–24 h after chemoreflex testing, the same rats were connected to a withdrawal pump and an arterial pressure transducer through an overhead swivel system. After 1 h of habituation, arterial blood was withdrawn at a rate of 3.2 ml·min−1·kg−1 for 6 min, after which the withdrawal rate was adjusted to 0.52 ml/min for an additional 4 min. Data acquisition was begun 15 min before the experiment and continued throughout hemorrhage and for an additional 50 min following termination of hemorrhage. At the end of the experiment, rats were given hexamethonium chloride (30 mg/kg iv) for determination of background noise in the sympathetic recording electrode. Animals were then rapidly anesthetized with pentobarbital sodium (65 mg/kg iv) and perfused transcardially with 90 ml of sodium nitrite (50 mM) in 0.1 mM PBS followed by 90 ml of 4% formaldehyde. The brains were removed and postfixed in 4% formaldehyde for 1 h. The brain stem was blocked and postfixed for an additional hour and subsequently dehydrated in 30% sucrose. Brain stems were cut in 40-μm sections and collected serially into six wells. Sections were saved in cryoprotectant (30% sucrose + 30% ethylene glycol in PBS) and stored at −20°C for later immunohistochemical analysis.
Protocol 3: Baroreflex control of HR and RSNA.
Separate groups of rats were prepared and connected to the recording instrumentation as described above. After a least 1 h of habituation, while the animals remained unrestrained in their home cages, BP, HR, and RSNA were measured during a variable infusion of sodium nitroprusside (1 mg/ml; 3–20 μl/s iv) to produce a ramp decrease in BP of 50–60 mmHg over 3 min. The rats were allowed to rest for at least 1 h, after which a variable-rate infusion of phenylephrine (1 mg/ml; 3–13 μl/min iv) was given to produce a ramp increase in BP to 160 mmHg over 3 min. At the end of the experiment, rats were given hexamethonium chloride (30 mg/kg iv) for determination of background noise in the sympathetic recording. Rats were then anesthetized with pentobarbital sodium (65 mg/kg iv) and perfused transcardially to enable collection of brain tissue.
Immunohistochemistry
Immunohistochemical verification of serotonin lesion and lack of off-target neurotoxicity were assessed in the brains of lesioned and vehicle-injected rats. The extent of the neurotoxic lesion was verified by detection and quantification of serotonin nerve terminal density. To obtain an accurate assessment of serotonin nerve terminal density, a composite high-resolution image was produced by combining deconvolved images taken at different focal planes throughout the thickness of the tissue (see Microscopy). In adjacent brain sections, tyrosine hydroxylase (TH) immunohistochemistry was performed, and the numbers of TH-positive cell bodies were counted in the A2 region of the nTS to verify that noradrenergic cells were not affected by the neurotoxin injection. In this case, the immunolabel was exposed with nickel ammonium sulfate-intensified 3,3′-diaminobenzadine tetrahydrochloride (DAB). The permanent label enabled cell counting without signal fade. In addition, tryptophan hydroxylase (TPH) immunohistochemistry with DAB label was used to identify serotonin cell bodies in the caudal medullary raphe. Numbers of TPH-positive cells were counted throughout the medullary raphe to determine if neurotoxin injection in the dorsomedial medullary region caused significant retrograde degeneration and destruction of serotonin cell bodies, which could compromise collateral projections outside the dorsomedial medulla. Possible collateral destruction was further assessed by examining serotonin nerve terminal density in the rostroventrolateral medulla (RVLM) using serotonin fluorescence immunohistochemistry and deconvolution as described above for the nTS.
Serotonin immunoreactivity was exposed by incubating brain sections in 6% donkey serum, 0.2% Triton X, and rabbit anti-serotonin antibody (1:200,000; Immunostar) for 48 h at 4°C. Sections were then washed and incubated in 6% donkey serum, 0.2%Triton X, and donkey anti-rabbit Dylight 649 (1:200; Jackson ImmunoResearch) for 2 h. Sections were rinsed in PBS, mounted on gel-coated slides, and immediately coverslipped with fluoromount (Sigma). Sections were stored at −4°C for precisely 14 days before microscopy.
To visualize TH-positive cells, brain sections were incubated in mouse anti-TH (1:1,000; Chemicon) overnight at room temperature. Sections were washed and incubated in the appropriate biotinylated goat IgG for 1 h and incubated with avidin-biotin complex as described above. The specificity of all primary antibodies was verified by observation of characteristic labeling of known serotonergic and adrenergic cell groups in the brain stem (2, 14). Omission of the primary antibodies completely prevented cell labeling. Some sections were double-labeled for serotonin and TH to qualitatively assess noradrenergic cell density and morphology in regions where neurotoxin was injected. In this case, the above protocol for serotonin immunofluorescence was followed except that mouse anti-TH antibody (1:2,500; Chemicon) was added to the primary incubation step and donkey anti-mouse Cy3 (1:200; Jackson ImmunoResearch) was added to the second incubation.
For TPH immunohistochemistry, brain sections were incubated in sheep anti-TPH primary antibody (1:1,000; Chemicon) overnight at room temperature. Sections were then incubated with biotinylated goat anti-sheep IgG (1:1,000; Vector) for 1 h at room temperature followed by incubation with avidin-biotin complex (Vector) for 50 min. Immunoreactivity was exposed with DAB to produce a black cytoplasmic label.
Microscopy
Serotonin nerve terminal density quantification.
Tissue from lesioned and control rats were processed in parallel in two separate cohorts. All images were acquired at the same exposure length, exactly 14 days after tissue processing. Serotonin immunoreactive fiber density in the nTS was determined unilaterally at the level of the medial and commissural subnuclei within ± 120 μm of five rostralcaudal planes 13.08, 13.32, 13.56, 13.80, and 14.08 caudal to bregma. Serotonin fiber density was also determined in the RVLM at three levels: 11.8, 12.2, and 12.8 mm caudal to bregma.
Immunoreactive fiber density was determined as described previously with modifications (16). Briefly, images were taken with an Olympus inverted IX81 epifluorescence microscope with a motor-driven stage and a ×10 objective. Measurements were taken from composite images of 30 1-μm deconvolved optical sections obtained from each tissue section. Deconvolution was performed with Vaytek Deconvolution version 7.0 software using the rapid nearest neighbor method within each image. Background intensity of the composite image, i.e., the intensity of unlabeled neuropil averaged from four different brains, was subtracted from each image. Threshold intensity was set at 1.5 times background and was held constant for each region measured. An area of interest was outlined, and the percent of the area of interest that was occupied by the threshold signal was determined as nerve terminal density using the selection tools in NIH Image J software.
TPH and TH cell profiles.
Cells with positive TPH immunoreactivity were counted in every sixth section, i.e., every 240 μm throughout the rostrocaudal extent of serotonin-rich nuclei known to project to the medial and commissural subnuclei of the nTS. These regions included the raphe magnus, the raphe obscurus, and the raphe pallidus (37). TH-positive cells were counted in every sixth section throughout the rostrocaudal extent of the A2 adrenergic nucleus. Profiles were generated by averaging cell numbers bilaterally approximately every 240 μm. Specific landmarks were used to identify the rostral poles of each nucleus, and cell numbers were counted every 240-μm section caudal to the first section showing the landmark.
Data Analysis
Chemoreflex responses.
The initial cardiovascular response to KCN injection was characterized by falls in HR and BP. Subsequent BP responses were heterogenous. In some cases, a biphasic BP response developed. Specifically, the BP fall that developed during the initial bradycardic response was followed by a prominent rise in pressure that occurred before HR recovery was complete (see Fig. 4A in results). The rise in BP occurred simultaneously with the burst of sympathetic activity within the first 6 s of the response. In some cases, there was no apparent pressor response during sympathetic activation. Instead, BP recovered slowly along with recovery of HR (see Fig. 4B). Both peak depressor and pressor responses to KCN were determined for each dose. The peak depressor response was determined as the difference in mean arterial pressure (MAP) between baseline (5 s average taken before injection) and during three cardiac cycles at the pressure nadir that developed during the bradycardic response. The peak pressor response was determined as the difference in MAP between the peak depressor response and MAP averaged over the two to three cardiac cycles containing the peak systolic response that developed immediately after the burst in sympathetic activity. If there was no apparent pressor response that coincided with the sympathetic burst, then the peak response was determined at the time point at which HR first began to recover. The HR chemoreflex response was determined as the change in HR between baseline (averaged over 5 s just before KCN injection) and the average HR between the nadir of HR after KCN injection through the peak pressor response. For those cases in which a pressor response did not develop within the first 10 s of injection, the HR response was averaged between the nadir of the HR and the first appearance of HR recovery.
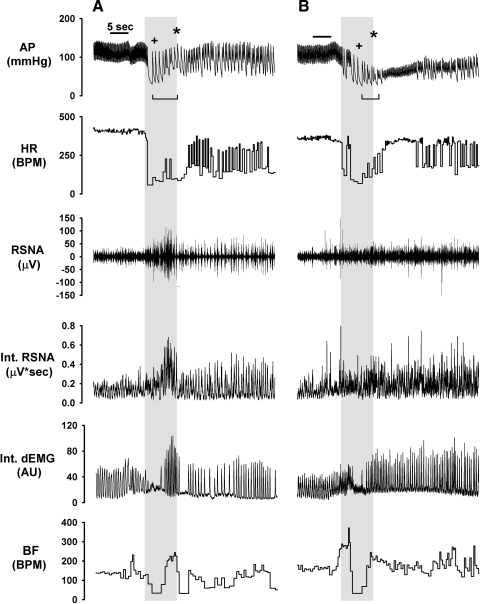
Fig. 4.
Examples of arterial pressure (AP), heart rate (HR), renal sympathetic nerve activity (RSNA), integrated RSNA (Int. RSNA), integrated diaphragmatic electromyographic activity (Int. dEMG), and breathing frequency (BF) during a 30 μg/kg dose of KCN in representative control (A) and lesioned (B) rats. Injection duration was 5 s. Timing of injections is indicated by horizontal bars above AP tracings. Gray shaded areas show 6-s period from start of bradycardic response during which peak sympathetic and ventilatory responses were determined. +Time point at which peak depressor response was calculated. *Point of peak pressor responses that coincided with the sympathetic burst. Rat shown in A had an obvious pressor peak. Rat in B showed no obvious pressor peak. In the latter case, the pressor peak was determined as the mean arterial pressure (MAP) when HR began to recover. Bracketed horizontal bar below AP tracing indicates duration over which HR response was averaged, i.e., from bradycardic nadir to peak pressor response (A) or to the start of HR recovery (B). BPM, beats/min; AU, arbitrary units.
The peak sympathetic response was determined by averaging integrated RSNA over the 3 s that immediately preceded the peak pressor response, or the first sign of HR recovery if an obvious pressor response was absent within the first 10 s. Peak sympathetic activity was normalized to baseline activity averaged over 5 s just before the injection.
The peak nTV was determined as the average AUC of the three highest-amplitude dEMG cycles that occurred within the 6 s that preceded the peak pressor response. The percent change was determined by normalizing the peak nTV to the average AUC of five dEMG cycles measured directly before KCN injection. BF was determined as the dEMG cycle frequency over the same intervals used for determination of nTV. BF and nTV were multiplied to obtain nMV. The peak nMV was normalized to baseline nMV to determine the percent increase at each dose. The amplitude of the dEMG cycles obtained from the same time period was used as a second index of tidal volume. The change in cycle amplitude was determined by normalizing the peak response to baseline cycle amplitude.
Two-way ANOVA with repeated measures were used to determine the effect of lesion and dose of KCN on respiratory and cardiovascular variables. Significant main effects of lesion or lesion-by-dose interactions were followed up with Newman-Keuls post hoc analyses to determine group differences at each dose. P values of <0.05 were considered significant for these and all other statistical analyses.
Hemorrhage
BF and nTV were determined throughout hemorrhage and recovery as described for chemoreflex analysis. The nTV was multiplied by the BF to obtain nMV. The nMV was normalized to baseline determined 5–10 s before hemorrhage. MAP, HR, and integrated RSNA, BF, and nMV were averaged across 30-s bins and compared between groups at 5-min intervals from the start of blood withdrawal through the 50-min posthemorrhage period. Two-way ANOVA with repeated measures were used to evaluate group differences over time. Significant main effects of lesion or interactions of lesion and time were followed up with Newman-Keuls post hoc analyses to determine group differences.
Arterial Baroreflex Sensitivity
HR or RSNA data obtained during phenylephrine and nitroprusside infusion were fit to a four-parameter logistical equation: HR = A/[1 + eB(MAP − C)] + D, where A is the range of HR or RSNA over the resulting sigmoidal curve, B is a gain parameter, C is MAP at the inflection point of the resulting curve, and D is the asymptotic minimum. The resulting parameters were used to determine maximal gain, (−B)(A)/4.56, as described by Kent et al. (12). Baseline RSNA is typically low in conscious, surgically recovered rats. Therefore, we also calculated baroreflex gain at baseline BP to determine if group differences in baroreflex control were present in resting rats. In this case, instantaneous gain (Y) over the range of MAP was determined by taking the first derivative of the individual curves. Gain at baseline BP, i.e., set-point gain, was determined for each animal by solving for Y at baseline BP in the best fit of the derivative curve according to the Gaussian expression Y= (k/s)e[(x−m)/s^2], where Y is instantaneous gain, x is the set-point BP, k is kurtosis, s is skewness, and m is MAP at midrange (MAP50). The resulting values for each variable were averaged across groups and compared between groups with Student's t-tests.
Spontaneous Baroreflex Sensitivity
Five-minute segments of BP and HR measurements recorded before hemorrhage were analyzed with Nevrokard SA-BRS software version 3.2.4 to determine baroreflex sensitivity by the sequence method (25). Gain was determined as the average slope of linear regressions obtained from a minimum of three sequences that satisfied the following constraints: three or more consecutive interbeat intervals (IBI) with variation in the same direction, >0.5 ms that correlated (r2 > 0.85) with systolic, diastolic, or mean arterial BP variations of >0.5 mmHg, with a three-beat delay. These parameters were chosen based on prior analyses demonstrating that they retrieved the most sequences with the highest gain in control rats (10). Cross-spectral analyses were performed on IBI and BP data using a 128-point fast-Fourier transformation with a smoothed Hamming window. Coherence between IBI and BP variability was determined as the square root of the ratio of the IBI and BP power spectra, with 50% overlap and zero padding of eight. Values were reported as the α-index in the low-frequency (LF, 0.06–0.6 Hz) and high-frequency (HF, 0.6–3.0) domains (27). In addition, BP variability was determined in the LF domain and in the time domain, the latter of which was determined as the standard deviation of systolic, mean, or diastolic BP during normal beats. HR variability in the HF and LF domains was also determined using the IBI power spectra criteria described above. HR variability was also determined in the HF and LF domains (17). Frequency domains were defined as described above for the α-indexes. Group means for each variable were compared by Student's t-test.
Tissue Analysis
Serotonin nerve terminal fiber density within the nTS and RVLM was compared between groups by two-way ANOVA with repeated measures over each level examined. The effect of neurotoxin injection on TH- and TPH-positive cell numbers was compared through the anatomical extent of the relevant nuclei by two-way ANOVA with repeated measures. Significant interactions and main effects were followed up with Newman-Keuls post hoc tests to determine at what level fiber density or cell number differed.
RESULTS
Histology
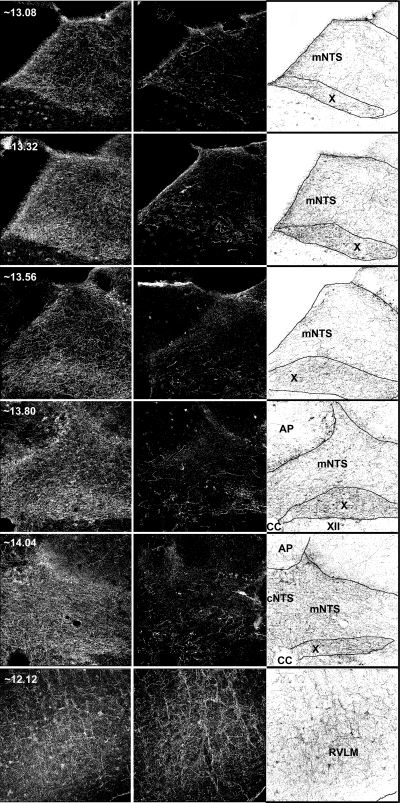
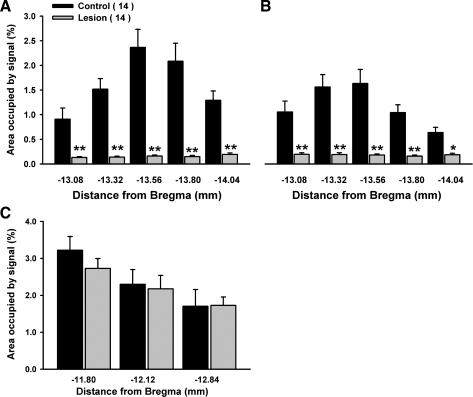
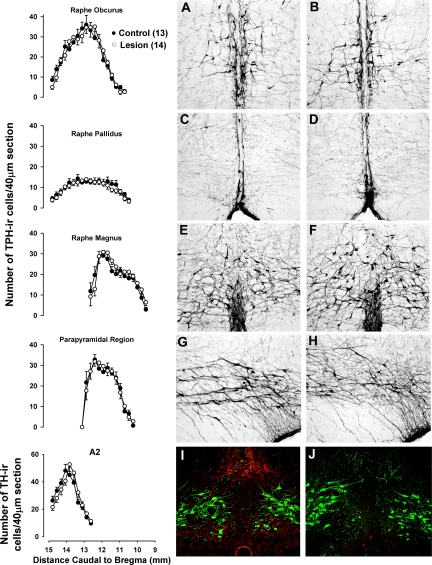
In intact animals, serotonin nerve terminal density was greatest in the dorsomedial regions of the medulla, particularly in the commissural and medial subnuclei of the nTS as well as the in the adjacent dorsal motor nucleus of the vagus (Fig. 1). Neurotoxin injection destroyed or damaged terminals in the dorsomedial medulla up to 1.2 mm rostral to the most rostral injection site (∼13.3 caudal to bregma). There was little serotonin innervation of the dorsal medulla rostral to this point in intact animals. Loss of serotonin immunoreactivity was substantial (>90% of detectable immunoreactivity) throughout the medial and commissural nTS regions (Fig. 2A), as well as in the underlying dorsal motor nucleus of the vagus (Fig. 2B). Nerve terminal loss extended ∼1.0 mm caudal to the most caudal injection site (∼15.40 mm caudal to bregma). Intact animals also demonstrated significant serotonin nerve terminal label throughout the ventrolateral regions of the medulla. These more ventral nerve terminals were spared by the dorsal targeting of the neurotoxin injection (Figs. 2C and 3). The numbers and morphology of TPH-immunoreactive cells in the raphe magnus, raphe pallidus, and raphe obscurus were not affected by the neurotoxin injection (Fig. 3, A–H). Likewise, the number and morphology of TH-immunoreactive cells within the nTS A2 region were not affected by the lesion (Fig. 3, I and J).
Fig. 1.
Serotonin immunoreactivity in five coronal planes of the nucleus of the solitary tract (nTS) (5 sets of images on top) and one coronal plane of the rostroventrolateral medulla (RVLM) (set of images on bottom) from brains of representative rats treated with vehicle (left) or 5,7-dihydroxytryptamine (middle) in the caudal dorsomedial brain stem. Approximate location of the section (in mm caudal to bregma) is indicated in the top left corner of the column of images on the left. Images in each row are from the same approximate location. Column of images on the right is the inverse of the column of images on the left showing the location of borders drawn for quantification of specific areas of interest: medial nTS (mNTS), dorsal motor nucleus of the vagus (X), area postrema (AP), commissural nTS (cNTS), and RVLM. CC, central canal.
Fig. 2.
Percent area of interest occupied by serotonin-immunoreactive fibers in the nTS (A), the dorsal motor nucleus of the vagus (B), or the RVLM (C) in each coronal plane. Data are group means ± SE. *P < 0.05 and **P < 0.01 vs. control.
Fig. 3.
Cell profiles of tryptophan hydroxylase (TPH)-immunoreactive (ir) neurons along the rostrocaudal extent of the raphe obscurus, the raphe pallidus, the raphe magnus, and the parapyramidal region (column on left, 4 graphs on top) as well as the cell profile of tyrosine hydroxylase (TH)-immunoreactive cells in the A2 region (bottom left). Also shown are representative images of TPH immunoreactivity in the raphe obscurus (A and B), the raphe pallidus (C and D), the raphe magnus (E and F), and the parapyramidal region (G and H) of rats treated with vehicle (A, C, E, and G) or 5,7-dihydroxytryptamine (B, D, F, and H). Representative images showing TH-immunoreactive cells (green) and serotonin fiber immunoreactivity (red) in the A2 region of rats treated with vehicle (I) or 5,7-dihyroxytryptamine (J). Data are group means ± SE.
Baroreflex and Chemoreflex
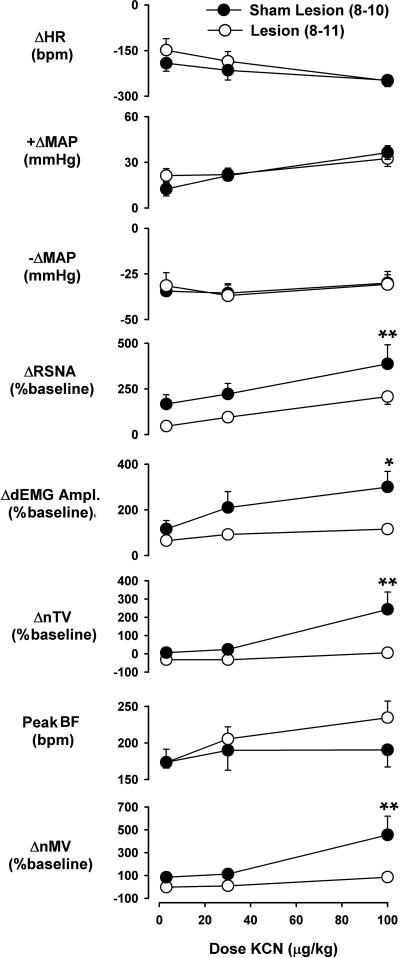
Baseline cardiovascular, sympathetic, and respiratory variables did not differ between rats before chemoreflex testing (Table 1). However, lesioned rats did have a slightly lower body weight (P < 0.05). Potassium cyanide injection produced a characteristic chemoreflex response with an immediate and dramatic bradycardic response that was accompanied by the onset of a brief apneic period lasting ∼2.5 s on average (Fig. 4A). Apnea was terminated by a dramatic increase in ventilation resulting from increases in both nTV and BF. This response was variable in duration but averaged ∼3 s at the highest dose. The peak ventilatory response was paralleled by a transient increase in sympathetic activity. Ventilation and sympathetic activity declined after the initial increase to levels near or above baseline where they remained for several seconds. The BP response was more variable. BP declined with the onset of bradycardia. At the highest dose, a transient pressor response often developed in parallel with the sympathoexcitatory response, which appeared to truncate the depressor effect observed during bradycardia. As a result, the depressor response did not appear to be dose dependent (Fig. 5). Neither HR nor BP responses were affected by lesion. However, the overall ANOVA showed a main effect of lesion on both RSNA (P < 0.05) and nMV (P < 0.01) because of an attenuation of the transient postapneic increase in sympathetic activity and ventilation in lesioned rats, particularly at the highest dose (Fig. 5). The overall ANOVA revealed a lesion-by-time interaction (P < 0.05) in the nTV response. Thus the greater nMV observed in control rats at the highest dose was due exclusively to increased nTV, since BF was not significantly affected by the lesion.
Table 1.
Baseline cardiorespiratory parameters measured before chemoreflex and hemorrhage testing
| Chemoreflex |
Hemorrhage |
|||
|---|---|---|---|---|
| Sham (9–10) | Lesion (8–12) | Sham (6–9) | Lesion (7–13) | |
| MAP, mmHg | 104 ± 3 | 110 ± 3 | 115 ± 2 | 116 ± 4 |
| HR, beats/min | 376 ± 17 | 385 ± 17 | 384 ± 12 | 418 ± 12 |
| RSNA, μV · s | 0.0516 ± 0.0096 | 0.0387 ± 0.0043 | 0.0344 ± 0.0032 | 0.0363 ± 0.005 |
| nTV, μV · s | 0.0586 ± 0.0096 | 0.0522 ± 0.0300 | 0.1209 ± 0.0376 | 0.1417 ± 0.0604 |
| BF, beats/min | 110 ± 13 | 106 ± 14 | 115 ± 9.1 | 123 ± 5 |
| Body wt, g | 348 ± 7 | 330 ± 8* | 340 ± 9 | 320 ± 8 |
Values are means ± SE determined within 10 min of the start of either experiment; no. of rats in parentheses.
MAP, mean arterial pressure; HR, heart rate; RSNA, integrated renal sympathetic nerve activity; nTV, neural tidal volume; BF, breathing frequency.
P < 0.05 vs. sham.
Fig. 5.
Change in heart rate (ΔHR), pressor response (+ΔMAP), depressor response (−ΔMAP), renal sympathetic nerve activity (ΔRSNA), dEMG cycle amplitude (ΔdEMG Ampl.), neural tidal volume (ΔnTV), peak breathing frequency (Peak BF), and change in neural minute volume (ΔnMV) in control and lesioned rats following injection of 3, 30, and 100 μg/kg KCN. Data are group means ± SE. *P < 0.05 and **P < 0.01 between groups. Group n is 10 for MAP and HR, 9 for RSNA, and 8 for all variables requiring dEMG in control group. Group n is 8 for variables requiring dEMG signal and 11 for all others in the lesion group.
Assessments of spontaneous baroreflex determined before hemorrhage (Table 2) revealed an increased gain in ascending sequences in lesioned rats when mean and diastolic BP were measured (P < 0.05 and P < 0.01, respectively). However, cross-spectral analysis of HR and BP variability did not differ between groups. BP variability assessed as the overall SD was reduced in lesioned rats (P < 0.01) as was systolic and mean BP variability in the LF domain (P < 0.01). The LF-to-HF ratio of HR variability was also lower in lesioned animals (P < 0.05) due primarily to more power in the HF domain (P < 0.01), although there was a strong tendency for reduced power in the LF domain (P = 0.075) among lesioned rats that likely contributed to the altered LF-to-HF ratio.
Table 2.
Spontaneous baroreflex gain, heart rate, and blood pressure variability in lesion and control rats
| Up Sequences, ms/mmHg | Down Sequences, ms/mmHg | All Sequences, ms/mmHg | Alpha, LF | Alpha, HF | LF power, mmHg2 | BP SDNN | LF RRI, nu | HF RRI, nu | LF-to-HF Ratio | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control (11) | ||||||||||
| SBP | 1.63 ± 0.16 | 1.70 ± 0.16 | 1.70 ± 0.13 | 0.76 ± 0.13 | 1.93 ± 0.26 | 16.8 ± 2.2 | 5.01 ± 0.43 | |||
| MBP | 2.00 ± 0.1 | 2.33 ± 0.14 | 2.17 ± 0.09 | 0.84 ± 0.14 | 2.29 ± 0.20 | 15.7 ± 2.9 | 4.22 ± 0.28 | 41.5 ± 4.8 | 39.2 ± 4.2 | 1.34 ± 0.27 |
| DBP | 1.88 ± 0.13 | 1.96 ± 0.13 | 1.93 ± 0.12 | 0.94 ± 0.17 | 2.07 ± 0.13 | 7.9 ± 0.8 | 3.97 ± 0.23 | |||
| Lesion (12) | ||||||||||
| SBP | 2.30 ± 0.27 | 2.20 ± 0.42 | 2.10 ± 0.26 | 0.69 ± 0.11 | 2.59 ± 0.60 | 8.4 ± 0.7** | 3.55 ± 0.20** | |||
| MBP | 2.59 ± 0.19* | 2.64 ± 0.28 | 2.56 ± 0.24 | 0.84 ± 0.12 | 3.43 ± 0.52 | 7.95 ± 0.8** | 3.33 ± 0.24** | 30.7 ± 3.4 | 54.2 ± 3.0** | 0.62 ± 0.10* |
| DBP | 2.32 ± 0.17** | 2.29 ± 0.19 | 2.62 ± 0.34 | 0.71 ± 0.11 | 2.99 ± 0.45 | 6.7 ± 0.6 | 3.11 ± 0.20** |
Values are group means ± SE determined for systolic (SBP), mean (MBP), and diastolic (DBP) blood pressure where applicable; no. of rats in parentheses. Spontaneous heart rate baroreflex gain was determined using the sequence method including up and down sequences as well as the average of all sequences that met baroreflex criteria outlined in text. Spontaneous heart rate baroreflex gain was also determined from the spectral method (correlation coefficient between heart rate interval and blood pressure) in the low (alpha LF)- and high (alpha HF)-frequency domains. Power of blood pressure variability in the low frequency domain (LF power) and in the time domain (BP SDNN) are shown as well as the heart rate variability in the LF and HF domains as well as the LF-to-HF ratio.
P < 0.05 and
P < 0.01 vs. control.
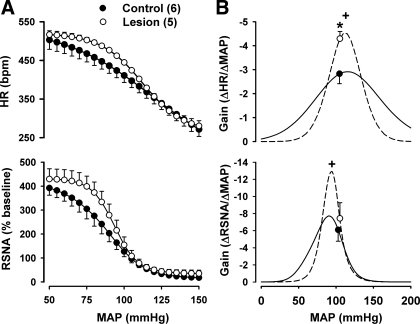
Baseline cardiovascular parameters did not differ in a separate cohort of lesioned and sham-lesioned rats subjected to evoked baroreflex responses (Table 3). Body weight was slightly lower in lesioned animals. However, the difference did not reach significance. Pharmacological manipulations of BP revealed significant differences in baroreflex control of both HR and sympathetic activity (Table 4). Maximal gain of both HR and RSNA reflex responses was greater in lesioned rats, as was gain at the set-point BP in the HR response (Fig. 6). HR range was also reduced in lesioned rats due, in part, to an elevated lower plateau.
Table 3.
Baseline cardiovascular parameters measured before pharmacological arterial baroreflex text
| Arterial Baroreflex |
||
|---|---|---|
| Sham (6) | Lesion (5) | |
| MAP, mmHg | 108 ± 5 | 105 ± 2 |
| HR, beats/min | 426 ± 13 | 442 ± 8 |
| RSNA, μV · s | 0.1173 ± 0.0839 | 0.0912 ± 0.006 |
| Body wt, g | 361 ± 11 | 334 ± 17 |
Data are means ± SE; no. of rats in parentheses.
Table 4.
Average HR and RSNA baroreflex parameters from curve fitting
| Range, beats/min | Maximal Gain, Δbeats/min/ΔmmHg | MAP50, mmHg | Lower Plateau, beats/min | Set-Point Gain, Δbeats/min/ΔmmHg | |
|---|---|---|---|---|---|
| Control HR (6) | 342 ± 31 | −3.1 ± 0.3 | 120 ± 7 | 189 ± 15 | −2.83 ± 0.41 |
| Lesion HR (5) | 255 ± 9** | −4.6 ± 0.3* | 112 ± 2 | 264 ± 16** | −4.29 ± 0.30* |
| Baseline, % | Δ%Baseline/ΔmmHg | Baseline, % | Baseline, % | Δ%Baseline/ΔmmHg | |
| Control RSNA (6) | 398 ± 30 | −9.1 ± 1.2 | 88 ± 4 | 14 ± 6 | −6.06 ± 1.35 |
| Lesion RSNA (5) | 396 ± 43 | −14.1 ± 2.6* | 93 ± 2 | 34 ± 14 | −7.40 ± 1.80 |
Values are group means ± SE; no. of rats in parentheses.
P < 0.05 and
P < 0.01 vs. control. MAP50 is mean arterial pressure at the midpoint of the curve. Set point gain is gain at baseline blood pressure.
Fig. 6.
A: baroreflex curves of HR and RSNA in control and lesioned rats. B: instantaneous gain of control and lesioned rats. *P < 0.05 between groups at set-point MAP. +P < 0.05 between groups at peak gain.
Hemorrhage
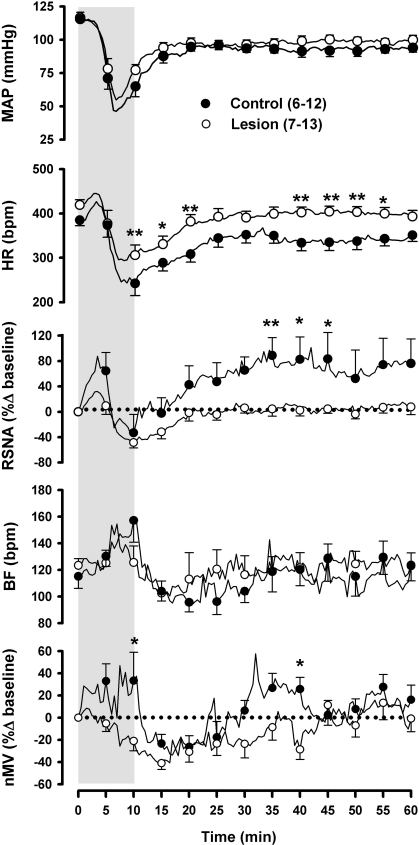
Baseline cardiorespiratory variables obtained before hemorrhage did not differ between groups (Table 1). The slightly lower body weight in lesioned animals observed before chemoreflex was no longer significant when measured before hemorrhage because of a slight increase in variability within groups. Intact rats showed a characteristic biphasic cardiovascular response to blood loss that included a short normotensive response that was paralleled by a slight tachycardia and robust sympathoexcitation (Fig. 7). This phase was followed by an abrupt decline in BP, HR, and RSNA after ∼9–12 ml/kg of blood loss. Following termination of hemorrhage, BP and HR recovered to levels slightly below baseline, with BP achieving a plateau before HR. RSNA recovered in parallel with HR and reached a plateau nearly 100% higher than baseline where it remained throughout the rest of the recording period. In control rats, BF increased progressively throughout hemorrhage. Neural minute ventilation increased at the onset of blood withdrawal but dropped briefly during the onset of hypotension. However, nMV quickly recovered and continued to increase until hemorrhage termination. Following termination of hemorrhage, both BF and nMV declined precipitously to levels below baseline. Both slowly returned to baseline within 30 min after the start of hemorrhage. Neural minute ventilation subsequently rose above baseline and remained elevated for 10–15 min before wavering around the baseline.
Fig. 7.
MAP, HR, RSNA, BF, and nMV in control and lesioned rats during hemorrhage (gray shaded box) and recovery. Data are group means ± SE. Group n are 12 for MAP and HR and 6 for RSNA and ventilatory variables in the control group. Group n are 13 for MAP and HR, 8 for RSNA, and 7 for ventilatory variables in the lesion group. *P < 0.05 and **P < 0.01 between groups.
The change in BP over the course of hemorrhage was similar in lesioned and sham-lesioned animals. However, the overall ANOVA revealed a main effect of lesion on HR (P < 0.05) due to higher HR at the BP nadir and throughout recovery in lesioned animals. Overall, lesioned rats had attenuated sympathetic (P < 0.05) and ventilatory responses (P < 0.05) to hemorrhage. Although group differences in sympathetic activation determined 5 min after the start hemorrhage were not significant, a t-test examining the maximal sympathetic response during the initial compensatory phase developed just after the start of hemorrhage revealed a significantly greater response in intact rats (87.6 ± 15.1 vs. 32.1 ± 8.7, P < 0.05 when corrected for repeated measures). Sympathetic withdrawal among lesioned rats was similar to controls in timing and extent. However, the subsequent recovery of sympathetic activity was reduced in the lesioned animals. Likewise, lesioned rats did not show the characteristic rise in nMV that was consistently observed 30 min after the start of hemorrhage in control animals.
DISCUSSION
The sympathetic compensatory responses that develop with severe blood loss are relatively well defined. They occur in two distinct stages: 1) during an initial phase in which rapid increases in sympathetic-mediated vascular resistance and HR commence with the advent of blood loss and 2) a later phase during which sympathetic activity and HR recover slowly following a transient sympathetic withdrawal that accompanies the hypotensive response to severe hemorrhage (16). Ventilatory responses to hemorrhage are less well characterized. Conscious male rabbits show a relatively stable BF during early blood loss that rapidly increases as BP drops (34). In the same model, nTV decreases as BF rises with hypotension. Little is known about the effects of hemorrhage on ventilation after termination of blood loss in the conscious animal. In general, the mechanisms that regulate early and secondary cardiorespiratory compensatory responses to blood loss are not well understood. The data herein suggest that normal serotonin innervation of the dorsomedial aspect of the caudal brain stem is critical for both. Specifically, we found that selective lesion of serotonin nerve terminals in the dorsomedial medulla attenuated compensatory sympathetic and ventilatory responses during active blood withdrawal as well during later compensation following the development of hypotension in the conscious rat.
The serotonin cells that facilitate compensatory responses to hemorrhage have yet to be identified. The dorsomedial aspect of the medulla targeted in these studies includes the nTS, which receives afferent input from arterial baroreceptors and peripheral chemoreceptors. In turn, the nTS receives serotonin-immunopositive projections from multiple sites, including the nodose ganglion, the dorsal raphe, and a subset of caudal raphe nuclei, i.e., the raphe obscurus, magnus, and pallidus (23, 29, 36, 38). In addition, serotonin-positive cell soma send projections to the commissural nTS from the area postrema and mediolateral portions of the nTS (4, 22). In a prior study, we found that both the initial and secondary sympathoexcitatory and ventilatory responses to blood loss were attenuated in rats subjected to selective destruction of serotonin cells within the caudal medullary raphe. The effect of the caudal raphe serotonin lesion on compensatory responses to blood loss was similar to that of animals subjected to the dorsomedial serotonin nerve terminal lesions in the current study (16). In our prior study, the caudal raphe lesions effectively reduced serotonin nerve terminal density in the commissural nTS by 50–60%. Together, the evidence suggests that serotonergic projections from the caudal raphe to the nTS or other regions within the dorsomedial medulla likely contribute to ventilatory and sympathetic responses to the early and secondary compensatory responses to blood loss.
Our prior studies also indicate that early sympathetic compensation during blood loss in the conscious rat is almost wholly dependent upon the integrity of sinoaortic nerves that carry arterial baroreceptor and peripheral chemoreceptor afferents (24). Thus, the near-complete absence of a rise in sympathetic activity or ventilation during active blood loss in rats subjected to dorsomedial medulla serotonin nerve terminal lesion suggests that serotonin projections here facilitate sympathetic responses to baroreceptor unloading, peripheral chemoreceptor activation, or both during hemorrhage. In the present study, however, lesioned rats showed evidence of increased baroreflex sensitivity. Both maximal and set-point HR baroreflex gain observed during evoked changes in BP were elevated in lesioned rats. Likewise, lesioned animals showed greater HR baroreflex gain during spontaneous elevations in BP, suggesting that a reduction in serotonin input to the dorsomedial medulla enhanced vagally mediated baroreflex control of HR. This view was further supported by evidence of increased HR variability in the HF domain among lesioned rats. Despite the preponderance of evidence that baseline vagal tone was elevated in lesioned rats, their baseline HR tended to be higher than controls. Indeed, during hemorrhage, lesioned animals had a higher HR at the nadir of the bradycardic response to blood loss and throughout recovery from hemorrhage. Interestingly, lesioned rats also showed an elevated lower plateau in the HR response to phenylephrine infusion when baroreceptor activation was maximal. Although it is not clear what might account for these effects, it is tempting to speculate that serotonin released in the dorsomedial medulla suppresses cardiac sympathetic drive through a mechanism that is, at least in part, independent of arterial baroreflex modulation.
Arterial baroreflex control of sympathetic activity was also increased in the lesioned rats, as demonstrated by an increase in maximal gain of sympathetic responses evoked by pharmacologically induced changes in BP. Lesioned rats also showed lower spontaneous BP variability in the LF domain compared with control rats. BP variability in the LF domain reflects the waxing and waning of sympathetic-dependent vasoconstriction as baroreceptors are alternately loaded and unloaded. Thus, reduced BP variability in the LF domain reflects an overall reduction in sympathetic-mediated vasomotion due either to reduced sympathetic activity overall or greater sympathetic control over BP oscillation around the set-point BP. However, baseline sympathetic activity did not appear to differ between groups, although such comparisons are difficult to make because of variations in anatomy and nerve-electrode preparations between animals. Lesion also had no effect on the set-point gain of evoked sympathetic baroreflex responses. Therefore, the origin of reduced LF oscillations in BP at rest in lesioned rats is not readily apparent. Our findings of increased maximal sympathetic gain, coupled with increased set-point gain in the HR baroreflex response, supports the contention that baroreflex sensitivity is increased, and thus BP is more vigorously defended in euvolemic animals in the absence of normal serotonin innervation of the dorsomedial medulla. Our findings that early and secondary compensatory responses to hemorrhage are attenuated in lesioned animals despite their having increased baroreflex responsiveness suggests the intriguing possibility that peripheral chemoreceptor activation may be a more important stimulus to elicit compensatory responses to blood loss than unloading of arterial baroreceptors.
Indeed, blood loss has been shown to activate peripheral chemoreceptors. Hemorrhage sufficient to produce a local decrease in carotid body Po2 stimulates carotid sinus afferent discharge during the early hypotensive response to blood loss in anesthetized cat (3). The carotid body receives blood flow far beyond its metabolic need. Thus, even small reductions in blood flow can activate chemoreceptor afferents independent of changes in PaO2. Indeed, reduced delivery of well-oxygenated blood activates carotid chemoreceptor afferents in anesthetized cats, suggesting that prolonged loss of blood volume may contribute to extended activation of the carotid chemoreceptors independent of the oxygen tension in the arterial blood (18).
Loss of serotonin innervation of the dorsomedial medulla attenuated both the ventilatory and sympathetic responses to KCN injection, suggesting that peripheral chemoreflex function is facilitated by serotonin innervation of the dorsomedial medulla. In our conscious rat model, the sympathetic and ventilatory responses to KCN injection were similar in magnitude to those described by others using a comparable model and range of doses (9). However, we observed comparatively large bradycardic responses that were not affected by lesion. The bradycardic response to KCN injection appeared to be near maximal, even at the lowest dose tested, raising the possibility that the effect of serotonin lesion on the HR response may have been obscured by the large magnitude of the chronotropic effect of KCN injection. The profound bradycardic response to KCN also obscured sympathetic-induced pressor responses, which effectively prevented any observable effect of lesion on the BP response to KCN. Nevertheless, deficits in ventilatory and sympathetic chemoreflex responses coupled with attenuated ventilatory and sympathetic compensation during hemorrhage in the lesioned animals suggest that serotonin innervation of the dorsomedial medulla is required for appropriate peripheral chemoreflex responses to blood loss.
Our observations of higher HR during hemorrhage and recovery in lesioned animals seem counter to the view that an attenuated peripheral chemoreflex contributes to deficits in compensation during hemorrhage in these animals. Exposure to hypoxic gas normally elicits tachycardia, in contrast to KCN-induced chemical hypoxia, which elicits bradycardia. The origin of the tachycardic response to systemic hypoxia produced by hypoxic gas inhalation remains elusive but has been speculated to result from direct central nervous system hypoxia rather than from activation of peripheral chemoreceptors (6). The tachycardic effect of systemic hypoxia is attenuated and even reversed in the presence of increasing CO2 concentrations, whereas hypoxia restricted to the carotid body produces bradycardia in dogs (6, 13). In the conscious rat, exposure to hypoxic hypercapnia produces a robust bradycardia that is mediated by the arterial chemoreflex (41). Thus, selective stimulation of peripheral chemoreceptors elicits bradycardia rather than tachycardia. In our prior studies using the same model of hemorrhage as used in the current study, we found that arterial blood oxygen tension was increased and remained elevated following hemorrhage-induced hyperventilation. This effect likely minimized central nervous system hypoxia. We also observed evidence of metabolic acidosis within the first 10 min of hemorrhage and rising PaCO2 during recovery. Thus, it is entirely possible that a portion of the bradycardic response to moderate hemorrhage in the intact rat may be mediated by arterial chemoreceptor stimulation due to carotid body hypoperfusion and metabolic acidosis early in hemorrhage as well as secondary respiratory acidosis (elevated PaCO2) later during recovery. Thus, the higher HR that emerged during hemorrhage in lesioned rats in the present study may represent yet another manifestation of impaired peripheral chemoreflex function.
In contrast to our findings, the majority of evidence indicates that serotonin projections to the nTS do not influence ventilatory responses to peripheral chemoreceptor stimulation (26, 35). Instead, serotonin cells appear to be integral to the ventilatory responses induced by changes in pH and PaCO2 in the central nervous system. Hypercapnia or acidosis activates TPH-positive cells in primary cultures obtained from the rat caudal raphe, possibly through inhibition of acid-sensitive potassium TASK channels (5, 31, 35). In accord, the integrity of caudal raphe serotonin cells is necessary for normal ventilatory responses to hypercapnia in awake rats (5, 16). Thus, efforts to identify an influence of serotonin on peripheral chemoreflex function in prior studies may have been hindered by suppressed serotonin cell activity due to respiratory alkolosis following hypoxia-induced hyperventilation or alternatively by anesthesia, which can hyperpolarize TASK channel-expressing neurons (32).
The attenuated recovery of sympathetic activity following termination of hemorrhage in lesioned animals was paralleled by a slower recovery of nMV. We observed a similar attenuation of sympathetic and ventilatory recovery in rats subjected to lesion of caudal hindbrain serotonin cells in our prior study (16). We also noted that the caudal raphe serotonin cell lesions attenuated sympathetic and ventilatory responses to selective central chemoreflex stimulation with hyperoxic hypercapnia. In this prior study, we found that hemorrhage reduced arterial pH and increased lactic acid more in lesioned rats despite evidence that these animals had greater arterial blood oxygen saturation. As a result, we proposed that the slow increase in ventilation and sympathetic activity that developed with hemorrhage may have been due, at least in part, to a chemoreflex response to rising PaCO2 levels that developed during buffering of metabolic acidosis. We further concluded that serotonergic neurons in the caudal raphe were necessary for adequate stimulation of these reflex responses. Evidence from the present study is consistent with the possibility that activation of caudal hindbrain serotonin cells during secondary respiratory acidosis contributes to release of serotonin in the dorsomedial medulla, which in turn may facilitate peripheral chemoreflex responses during recovery from hemorrhage. Whether serotonin is released in the dorsomedial medulla during metabolic acidosis remains to be determined.
In preliminary studies, we found that, by 10–12 days after nTS injection of the neurotoxin, TPH-immunoreactive cell bodies in the caudal raphe began to degenerate. Because serotonergic cells of the caudal raphe send collateral axons to a wide range of targets, it is plausible that neurotoxin injection could lead to off-target effects (1, 19). Studies herein were conducted precisely 1 wk after neurotoxin injection when the morphology and number of caudal raphe serotonin cells were normal. Likewise, serotonin nerve terminal density was normal in the C1 region of the RVLM and surrounding areas containing important serotonergic targets that influence ventilatory rhythm generation, chemosensation, as well as sympathetic activity (7, 33). It is possible that neurotoxicity in dorsomedial medullary terminal fields of serotonin cells may have altered their activity and thus serotonin release in off-target areas before evidence of morphological changes. However, this prospect seems unlikely given that we observed similar deficits in sympathetic recovery following hemorrhage and more substantial deficits in the early compensatory response to hemorrhage among rats injected with neurotoxin in the dorsomedullary region compared with rats injected with neurotoxin specifically targeting the areas around the C1 region. In the former case, serotonin immunoreactivity was normal in the C1 region, whereas, in the later case, serotonin immunoreactivity was only 10% of normal. If the loss of serotonin release in the C1 region mediated these effects, it seems more likely that injections targeting the C1 region would have had more substantial effects than those targeting the dorsomedial medulla.
Although 5,7-DHT is relatively selective for serotonergic neurons, the neurotoxin can be taken up by noradrenergic nerve terminals that express the norepinephrine reuptake transporter (11). Adrenergic receptors expressed in the nTS have also been found to enhance peripheral chemoreflex responses to KCN injection in the rat (8). However, it is unlikely that our results were due to off-target effects on adrenergic neurons, since we did not observe differences in adrenergic cell number or morphology within the A2 region of the nTS. To date, we have only examined serotonergic and adrenergic innervation of the nTS after 5,7-DHT lesion. It is entirely possible that compensatory sprouting and reinnervation by neurons containing other neurotransmitters or altered receptor expression may have contributed to the observed deficits in autonomic function during hemorrhage or chemoreflex stimulation. This possibility remains to be explored.
Perspectives
The data herein suggest the intriguing possibility that peripheral chemoreflex activation may make a significant contribution to the early and secondary sympathetic compensatory responses that occur with progressive blood loss and that serotonin released in the nTS during hemorrhage facilitates this effect. Prior work has demonstrated that arterial baroreceptor activation inhibits the evoked response of chemosensitive neurons in the nTS, indicating an important reciprocal interaction between arterial baro- and peripheral chemoreceptor afferents within the dorsomedial medulla (21). Evidence from the present study suggests that serotonin released in the dorsomedial medulla inhibits arterial baroreflex gain, which, in turn, may permit a more robust response to peripheral chemoreceptor stimulation for a given BP. Our prior work indicates that serotonin cells in the caudal raphe are necessary for normal sympathetic and ventilatory responses to early and secondary compensatory responses to hemorrhage as well as for normal sympathetic and ventilatory responses to hypercapnia. Based on findings in the current study, we speculate that serotonin cells of the caudal raphe release serotonin in the dorsomedial medulla, particularly during metabolic acidosis or secondary respiratory acidosis when their activity may be increased. Serotonin released in the nTS may in turn facilitate peripheral chemoreflex activation of sympathetic activity and ventilation during the carotid body tissue hypoxia that accompanies hemorrhage. Modulation of these integrated reflex responses by serotonin may promote better oxygenation of blood and a more beneficial distribution of blood volume during hypovolemia.
GRANTS
This work was supported by National Heart Lung and Blood Institute Grants HL-072354 and HL-076162 and by the American Heart Association, Mid-West affiliate 081559G.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Allen GV, Cechetto DF. Serotoninergic and nonserotoninergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cord. J Comp Neurol 350: 357–366, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong DM, Ross CA, Pickel VM, Joh TH, Reis DJ. Distribution of dopamine-, noradrenaline-, and adrenaline-containing cell bodies in the rat medulla oblongata: demonstrated by the immunocytochemical localization of catecholamine biosynthetic enzymes. J Comp Neurol 212: 173–187, 1982 [DOI] [PubMed] [Google Scholar]
- 3. Buerk DG, Nair PK, Whalen WJ. Two-cytochrome metabolic model for carotid body PtiO2 and chemosensitivity changes after hemorrhage. J Appl Physiol 67: 60–68, 1989 [DOI] [PubMed] [Google Scholar]
- 4. Calza L, Giardino L, Grimaldi R, Rigoli M, Steinbusch HW, Tiengo M. Presence of 5-HT-positive neurons in the medial nuclei of the solitary tract. Brain Res 347: 135–139, 1985 [DOI] [PubMed] [Google Scholar]
- 5. Dias MB, Nucci TB, Margatho LO, Antunes-Rodrigues J, Gargaglioni LH, Branco LG. Raphe magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J Appl Physiol 103: 1780–1788, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Downing SE, Remensnyder JP, Mitchell JH. Cardiovascular responses to hypoxic stimulation of the carotid bodies. Circ Res 10: 676–685, 1962 [DOI] [PubMed] [Google Scholar]
- 7. Guyenet PG, Haselton JR, Sun MK. Sympathoexcitatory neurons of the rostroventrolateral medulla and the origin of the sympathetic vasomotor tone. Prog Brain Res 81: 105–116, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Hayward LF. Evidence for alpha-2 adrenoreceptor modulation of arterial chemoreflexes in the caudal solitary nucleus of the rat. Am J Physiol Regul Integr Comp Physiol 281: R1464–R1473, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Hayward LF, Johnson AK, Felder RB. Arterial chemoreflex in conscious normotensive and hypertensive adult rats. Am J Physiol Heart Circ Physiol 276: H1215–H1222, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Henze M, Hart D, Samarel A, Barakat J, Eckert L, Scrogin K. Persistent alterations in heart rate variability, baroreflex sensitivity, and anxiety-like behaviors during development of heart failure in the rat. Am J Physiol Heart Circ Physiol 295: H29–H38, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Horn AS, Baumgarten HG, Schlosserberger HG. Inhibition of the uptake of 5-hydroxytryptamine, noradrenaline and dopamine into rat brain homogenates by various hydroxylated tryptamines. J Neurochem 21: 233–236, 1973 [DOI] [PubMed] [Google Scholar]
- 12. Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology 57: 295–310, 1972 [DOI] [PubMed] [Google Scholar]
- 13. Koehler RC, McDonald BW, Krasney JA. Influence of CO2 on cardiovascular response to hypoxia in conscious dogs. Am J Physiol Heart Circ Physiol 239: H545–H558, 1980 [DOI] [PubMed] [Google Scholar]
- 14. Kohler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience 7: 951–975, 1982 [DOI] [PubMed] [Google Scholar]
- 15. Korner PI, Oliver JR, Zhu JL, Gipps J, Hanneman F. Autonomic, hormonal, and local circulatory effects of hemorrhage in conscious rabbits. Am J Physiol Heart Circ Physiol 258: H229–H239, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Kung LH, Glasgow J, Ruszaj A, Gray T, Scrogin KE. Serotonin neurons of the caudal raphe nuclei contribute to sympathetic recovery following hypotensive hemorrhage. Am J Physiol Regul Integr Comp Physiol 298: R939–R953, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuwahara M, Yayou K, Ishii K, Hashimoto S, Tsubone H, Sugano S. Power spectral analysis of heart rate variability as a new method for assessing autonomic activity in the rat. J Electrocardiol 27: 333–337, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Lahiri S, Rumsey WL, Wilson DF, Iturriaga R. Contribution of in vivo microvascular Po2 in the cat carotid body chemotransduction. J Appl Physiol 75: 1035–1043, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Li YQ, Takada M, Shinonaga Y, Mizuno N. Collateral projections of single neurons in the nucleus raphe magnus to both the sensory trigeminal nuclei and spinal cord in the rat. Brain Res 602: 331–335, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Liu LM, Ward JA, Dubick MA. Hemorrhage-induced vascular hyporeactivity to norepinephrine in select vasculatures of rats and the roles of nitric oxide and endothelin. Shock 19: 208–214, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Mifflin SW. Inhibition of chemoreceptor inputs to nucleus of tractus solitarius neurons during baroreceptor stimulation. Am J Physiol Regul Integr Comp Physiol 265: R14–R20, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Newton BW, Maley B, Traurig H. The distribution of substance P, enkephalin, and serotonin immunoreactivities in the area postrema of the rat and cat. J Comp Neurol 234: 87–104, 1985 [DOI] [PubMed] [Google Scholar]
- 23. Nosjean A, Compoint C, Buisseret-Delmas C, Orer HS, Merahi N, Puizillout JJ, Laguzzi R. Serotonergic projections from the nodose ganglia to the nucleus tractus solitarius: an immunohistochemical and double labeling study in the rat. Neurosci Lett 114: 22–26, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Osei-Owusu P, Scrogin K. Role of the arterial baroreflex in 5-HT1A receptor agonist-mediated sympathoexcitation following hypotensive hemorrhage. Am J Physiol Regul Integr Comp Physiol 290: R1337–R1344, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Padley JR, Overstreet DH, Pilowsky PM, Goodchild AK. Impaired cardiac and sympathetic autonomic control in rats differing in acetylcholine receptor sensitivity. Am J Physiol Heart Circ Physiol 289: H1985–H1992, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Perez H, Ruiz S. Medullary responses to chemoreceptor activation are inhibited by locus coeruleus and nucleus raphe magnus. Neuroreport 6: 1373–1376, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Pinna GD, Maestri R, Raczak G, Rovere MT LA. Measuring baroreflex sensitivity from the gain function between arterial pressure and heart period. Clin Sci (Lond) 103: 81–88, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol Heart Circ Physiol 260: H305–H318, 1991 [DOI] [PubMed] [Google Scholar]
- 29. Schaffar N, Kessler JP, Bosler O, Jean A. Central serotonergic projections to the nucleus tractus solitarii: evidence from a double labeling study in the rat. Neuroscience 26: 951–958, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Scrogin KE. 5-HT1A receptor agonist 8-OH-DPAT acts in the hindbrain to reverse the sympatholytic response to severe hemorrhage. Am J Physiol Regul Integr Comp Physiol 284: R782–R791, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci 6: 1139–1140, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Sirois JE, Lei Q, Talley EM, Lynch C, III, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci 20: 6347–6354, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Botzinger complex. J Comp Neurol 455: 499–512, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Strittmatter RR, Schadt JC. Sex differences in the respiratory response to hemorrhage in the conscious, New Zealand white rabbit. Am J Physiol Regul Integr Comp Physiol 292: R1963–R1969, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol 566: 543–557, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thor KB, Helke CJ. Serotonin- and substance P- containing projections to the nucleus tractus solitarii of the rat. J Comp Neurol 265: 275–293, 1987 [DOI] [PubMed] [Google Scholar]
- 37. Thor KB, Helke CJ. Serotonin and substance P colocalization in medullary projections to the nucleus tractus solitarius: dual-colour immunohistochemistry combined with retrograde tracing. J Chem Neuroanat 2: 139–148, 1989 [PubMed] [Google Scholar]
- 38. Thor KB, Hill KM, Harrod C, Helke CJ. Immunohistochemical and biochemical analysis of serotonin and substance P colocalization in the nucleus tractus solitarii and associated afferent ganglia of the rat. Synapse 2: 225–231, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Tiniakov R, Osei-Owusu P, Scrogin KE. The 5-hydroxytryptamine1A receptor agonist, (+)-8-hydroxy-2-(di-n-propylamino)-tetralin, increases cardiac output and renal perfusion in rats subjected to hypovolemic shock. J Pharmacol Exp Ther 320: 811–818, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Tiniakov R, Scrogin KE. The spleen is required for 5-HT1A receptor agonist-mediated increases in mean circulatory filling pressure during hemorrhagic shock in the rat. Am J Physiol Regul Integr Comp Physiol 296: R1392–R1401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walker BR. Role of vasopressin in the cardiovascular response to hypoxia in the conscious rat. Am J Physiol Heart Circ Physiol 251: H1316–H1323, 1986 [DOI] [PubMed] [Google Scholar]