Abstract
The incretin and food intake suppressive effects of intraperitoneally administered glucagon-like peptide-1 (GLP-1) involve activation of GLP-1 receptors (GLP-1R) expressed on vagal afferent fiber terminals. Central nervous system processing of GLP-1R-driven vagal afferents results in satiation signaling and enhanced insulin secretion from pancreatic-projecting vagal efferents. As the vast majority of endogenous GLP-1 is released from intestinal l-cells following ingestion, it stands to reason that paracrine GLP-1 signaling, activating adjacent GLP-1R expressed on vagal afferent fibers of gastrointestinal origin, contributes to glycemic and food intake control. However, systemic GLP-1R-mediated control of glycemia is currently attributed to endocrine action involving GLP-1R expressed in the hepatoportal bed on terminals of the common hepatic branch of the vagus (CHB). Here, we examine the hypothesis that activation of GLP-1R expressed on the CHB is not required for GLP-1's glycemic and intake suppressive effects, but rather paracrine signaling on non-CHB vagal afferents is required to mediate GLP-1's effects. Selective CHB ablation (CHBX), complete subdiaphragmatic vagal deafferentation (SDA), and surgical control rats received an oral glucose tolerance test (2.0 g glucose/kg) 10 min after an intraperitoneal injection of the GLP-1R antagonist, exendin-(9–39) (Ex-9; 0.5 mg/kg) or vehicle. CHBX and control rats showed comparable increases in blood glucose following blockade of GLP-1R by Ex-9, whereas SDA rats failed to show a GLP-1R-mediated incretin response. Furthermore, GLP-1(7–36) (0.5 mg/kg ip) produced a comparable suppression of 1-h 25% glucose intake in both CHBX and control rats, whereas intake suppression in SDA rats was blunted. These findings support the hypothesis that systemic GLP-1R mediation of glycemic control and food intake suppression involves paracrine-like signaling on GLP-1R expressed on vagal afferent fibers of gastrointestinal origin but does not require the CHB.
Keywords: blood glucose, incretin, satiety, vagotomy
the incidence of type 2 diabetes mellitus (T2DM) and obesity has risen dramatically over the last two decades. Current estimates show that more than two-thirds of US citizens are either obese or overweight and >24 million have T2DM (14a, 36). With common physiological impairments existing between T2DM and obesity, several reports have focused attention on the physiological and pharmacological merits of the glucagon-like peptide-1 (GLP-1) system in offering promising treatment options for T2DM (21, 28) and, in some cases, for obesity as well (19, 21, 25). GLP-1 is an incretin hormone released principally from two sources in the body in response to food ingestion: L-cells in the small intestine and proglucagon neurons in the nucleus tractus solitarii of the hindbrain. GLP-1-based pharmaceuticals increase insulin production, reduce hyperglycemia, and reduce food intake and body weight in humans and animal models (7, 12, 19–20, 41, 46). Both the incretin and food intake inhibitory effects of endogenous GLP-1 and exogenous GLP-1 ligands require activation of GLP-1 receptors (GLP-1R) (for review see Refs. 19 and 21). In the periphery, GLP-1Rs are expressed on vagal afferent fibers of gastrointestinal and hepatoportal origin, as well as on pancreatic β-cells. As the vast majority of endogenous intestinally derived GLP-1 is rapidly degraded by the dipeptidyl-peptidase-4 enzyme and similar endopeptidases, the circulating levels of GLP-1 are minimal (21). Thus, it would seem logical that paracrine-like signaling, resulting from food ingestion-driven GLP-1 release from intestinal L-cells acting on adjacent GLP-1R expressed on the peripheral terminals of vagal afferent fibers, would be an essential process in the mediation of endogenous GLP-1 effects (for review see Refs. 19 and 21). However, previous research has overlooked this site of action and instead suggested that GLP-1Rs within the hepatoportal region are the primary population responsible for GLP-1's effects (24, 32–33, 51). Given that specific branches of the vagus nerve differentially innervate multiple organs within the peritoneal cavity (53), it remains unclear whether the primary site of GLP-1R-mediated glycemic and food intake control are vagal afferents innervating the hepatoportal bed [innervated almost solely by the common hepatic branch of the vagus (CHB) and perhaps a small percentage by the celiac branch (10, 53)] or other vagal afferent branches that innervate the gastrointestinal tract (innervated by the CHB, gastric, and celiac branches).
Recent findings have shown that the incretin and food intake inhibitory effects of endogenous GLP-1 and exogenous GLP-1R agonists are mediated, at least in part, by a neural pathway involving subdiaphragmatic vagal afferents and efferents (1, 21, 23, 44, 50). However, the mechanism[s] through which vagal afferent GLP-1 signaling contributes to glycemic and food intake control remains poorly understood. It is possible that once secreted, GLP-1 activates GLP-1R-bearing vagal afferent neurons both in a paracrine fashion (local to the site of release in the small intestine) and in an endocrine fashion at GLP-1Rs expressed in the portal vein, liver, and/or upper gastrointestinal tract (44, 51). Subsequent to either paracrine or endocrine action by GLP-1, GLP-1R-mediated vagal afferent signals are processed by central nercous system neurons, which results in neuroendocrine, behavioral, and physiological responses that improve glycemic control and reduce feeding. One key neuroendocrine response to vagal afferent activation by GLP-1 is the subsequent vagal efferent neural transmission to the pancreatic β-cell, resulting in insulin secretion (8, 21, 31).
To date, systemic GLP-1R-mediated control of glycemia has been attributed principally to either activation of GLP-1R-expressing vagal afferent nerve terminals in the hepatic portal bed (24, 32–33, 51) or GLP-1R expressed on pancreatic β-cells (for a review see Ref. 21). These conclusions rest on the observation that intraportal infusion of a GLP-1R antagonist attenuated the incretin-mediated control of blood glucose levels following intragastric glucose infusion in anesthetized rodents, whereas delivery of the same dose of a GLP-1R antagonist to the jugular vein did not alter the plasma glucose or insulin response following intragastric glucose infusion (51). This report, however, did not directly test the necessity of vagal afferents innervating the hepatoportal region (i.e., the CHB) to the mediation of GLP-1's glycemic response. Moreover, given that jugular infusion of a ligand would not provide adequate exposure to vagal afferent terminals innervating the gastrointestinal tract (37), it would stand to reason that a focused examination of the physiological role of GLP-1R signaling on gastrointestinal-innervating vagal afferent fibers vs. hepatoportal-innervating vagal afferent fibers in glycemic control is needed.
To address the necessity of the GLP-1R expressed on the CHB to the mediation of GLP-1's glycemic and intake suppressive effects we performed selective surgical ablation of the CHB of the vagus (CHBX). CHBX eliminates any potential GLP-1R mediation from vagal afferent fibers of hepatoportal origin [innervating the portal vein, liver, and a portion of the duodenum (38, 53)], while leaving intact GLP-1R signaling of vagal afferent fibers innervating the stomach and a large portion of the small intestine (duodenum, jejunum, and ileum) that remain innervated by intact celiac and gastric branches of the vagus. These experiments, therefore, were designed to distinguish between the roles of gastrointestinal (paracrine)- and hepatoportal (endocrine)-expressed GLP-1R in mediating the incretin and intake inhibitory effects of endogenous and exogenous GLP-1. As a positive control, the necessity of all subdiaphragmatic vagal afferent nerve branches (including afferent fibers of the CHB, celiac, and gastric vagal branches) to glycemic and food intake control was examined by using a unique surgical procedure known as subdiaphragmatic vagal deafferentation (SDA). SDA selectively eliminates all vagal afferent fibers innervating the peritoneal cavity, but leaves intact a large percentage (∼50%) of the vagal efferent fibers innervating the peritoneal cavity, as well as approximately half of the afferent fibers and all of the efferent fibers innervating the thoracic cavity (34). Analysis of GLP-1's effects on glycemic and food intake control in rats that have undergone CHBX or SDA will allow for determination of the necessity of GLP-1R expressed on the CHB of the vagus in mediating peripheral GLP-1's incretin and food intake suppressive effects.
MATERIALS AND METHODS
Animals and Drugs
Adult male Sprague-Dawley rats (Charles River Laboratories), housed individually in hanging wire-bottom metal cages under a 12:12-h light-dark cycle (lights on 0800), had ad libitum access to rodent chow (Purina 5001; St. Louis, MO) and water except where noted. The GLP-1R antagonist, exendin-(9–39) (Ex-9), and GLP-1(7–36) (American Peptide, Sunnyvale, CA) were dissolved in sterile 0.9% NaCl. All procedures conformed to and have received approval by the institutional standards of The University of Pennsylvania Animal Care and Use Committee.
Surgeries
Selective surgical CHBX of the vagus.
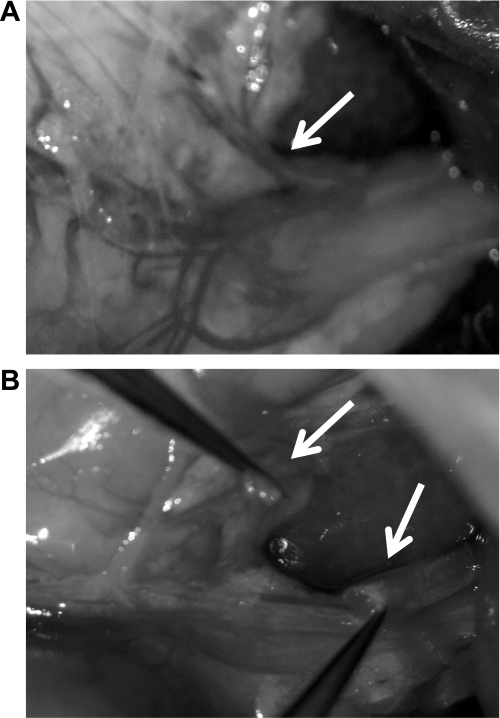
As described previously (15), under ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) anesthesia and analgesia (2 mg/kg Metacam), overnight food-deprived rats received a midline laparotomy, their stomachs and livers were carefully manipulated to expose the CHB of the vagus. For CHBX rats, the CHB was then selectively surgically ablated by cauterization. For sham-operated controls, the stomach and liver were manipulated and visualized as occurred for CHBX rats, but the CHB was left intact. Animals were allowed a 7-day minimum recovery period prior to behavioral and physiological test training. At the conclusion of all experimental testing, CHBX rats were again anesthetized, and the success of the CHBX surgery was confirmed by visual verification of ablation (Fig. 1). To our knowledge there are also no established functional behavioral or physiological tests that can be conducted to confirm CHBX; however, CHBX can accurately be confirmed visually (Fig. 1). Histological verification via the injection of a retrograde neuronal tracer, which is transported to cell columns of the dorsal motor nucleus of the vagus (DMV; as described below for the SDA procedure), is not possible for the CHBX because there are very few motor fibers in this branch that would allow retrograde labeling in the DMV (15, 39).
Fig. 1.
Representative images of the common hepatic branch of the vagus (CHB). A: an intact CHB prior to surgical ablation (arrows). B: visual verification of a surgically ablated CHB (arrows).
Complete SDA.
SDA consists of a transection of the left dorsal vagal afferent rootlets and dorsal (left) esophageal vagal trunks, resulting in complete SDA, while sparing approximately half of the abdominal vagal efferents. In contrast to neurotoxic lesion of unmyelinated vagal/nonvagal C-fibers by capsaicin, the SDA surgery eliminates all types (both A- and C-fibers) of vagal afferents. Furthermore, unlike complete subdiaphragmatic vagotomy, the SDA surgery leaves intact ∼50% of the vagal efferents innervating the peritoneal cavity. However, the SDA approach does not eliminate all of the limitations from the capsaicin or complete vagotomy approaches, as spinal afferents and ∼50% of the vagal afferents above the diaphragm remain intact. Furthermore, while SDA rats can be easily maintained on solid food, in contrast to complete subdiaphragmatic vagotomy, the eating rate of SDA rats has been reported as reduced relative to controls (5, 44).
The procedures for SDA are described in more detail elsewhere (34). As we have reported previously (23), in anesthetized rats, the procedure requires exposing and cutting the vagal afferent rootlets unilaterally (left) and then sectioning the contralateral (ventral) subdiaphragmatic vagal trunk. The sham procedure was similar, with the exception that the vagal rootlets and abdominal vagus were exposed but not manipulated further. SDA and sham rats were nursed with liquid diets (Test Diets AIN-76A) for ∼1 wk after surgery, and were then returned to ad libitum solid chow maintenance.
At the conclusion of all behavioral procedures, SDA surgery was verified functionally and histologically (5, 23). The functional test involved a within-subject repeated measures analysis of cholecystokinin (CCK)-induced food intake suppression, a response that depends on abdominal vagal afferent fibers (30, 47). Briefly, following 4-h food deprivation in the beginning of the dark cycle, rats were injected intraperitoneally with 3 μg/kg CCK-8 or saline according to a counterbalanced design. CCK-8 (American Peptides) reduced 30-min food intake by 43–72% in sham-operated rats. Utilizing an inclusion criteria used by others, SDA rats were included in the statistical analyses if CCK treatment resulted in a < 30% reduction of their food intake (5, 44). Histological verification involved examining the presence of retrograde labeling of vagal motor neurons in the DMV. Rats were injected intraperitoneally with 2 mg Fluoro-Gold (Fluorochrome) in 1 ml NaCl. Four days later they were anesthetized and intracardially perfused with PBS, followed by 4% formalin in PBS. An observer, blind to the rats' surgery and behavioral data, analyzed Fluoro-Gold labeling in the DMV on coronal sections. The inclusion criteria for SDA rats were that retrograde labeling be observed in the left DMV and that retrograde labeling in the right DMV be < 3% of the number in the left DMV. For sham rats, motor neurons were labeled bilaterally.
Glycemic Regulation
Effect of endogenous GLP-1R signaling on oral glucose tolerance test.
Rats' [CHBX (n = 7); control-CHB-sham (n = 7); SDA (n = 10); control-SDA-sham (n = 7)] food was withheld for a 6-h period (with ad libitum access to water) during the first half of the light cycle. Prior to testing, blood was collected from the tail tip, and glucose measured by a standard glucometer (Accucheck, Roche Diagnostics). Immediately following baseline blood collection, rats received an injection of the GLP-1R antagonist Ex-9 (0.5 mg/kg ip) or saline in a counterbalanced fashion and 20 min later, blood was again assessed for plasma glucose concentrations (time 0). Next, an oral glucose load was delivered to each rat (2.0 g/kg) via graduated burette of 25% glucose solution (oral glucose ingestion occurred within 3 min). Blood was again collected at 20, 40, 60, and 120 min post-glucose ingestion for analysis of blood glucose concentrations.
Feeding Behavior
Intake suppressive effects of GLP-1(7–36) in CHBX, SDA, and sham-operated control rats.
All rats were habituated to experimental testing for 1 wk, consisting of overnight food deprivation (16 h; with ad libitum access to water), followed by an injection of saline (1 ml/kg ip) and 10 min later ad libitum access to 25% glucose solution (wt/vol) in a graduated burette. Injections occurred at 0900 (1 h into the light cycle). Immediately following glucose access, standard rodent chow was returned to the animal. Training/experimental testing sessions were separated by 48 h. For both the SDA and CHBX surgical procedure, separate surgical sham-operated control rats were tested with appropriate surgical experimental groups. For experimental testing, all rats [CHBX (n = 7); control-CHB-sham (n = 7); SDA (n = 11); control-SDA-sham (n = 10)] were food deprived overnight and received a counterbalanced injection of saline or GLP-1(7–36) (0.5 mg/kg ip) 10 min prior to ad libitum access to 25% glucose. Intakes of glucose were recorded to the nearest 0.1 ml every 10 min for 60 min.
Statistical Analysis
Data for each respective study were analyzed separately and expressed as means ± SE. For all experiments, comparisons between treatment means were analyzed by repeated-measures ANOVA (with drug treatment as within-subject and surgical group as between-subject variables). If appropriate, post hoc pairwise comparisons were made using Tukey's honestly significant difference test. As separate surgical sham-operated controls rats were generated and tested together with their appropriate surgical experimental groups (CHBX or SDA), no statistical comparisons were made between CHBX and SDA data. Statistical significance was defined as P values < 0.05.
RESULTS
Effect of Endogenous GLP-1R Signaling on Oral Glucose Tolerance Test
CHBX experiment.
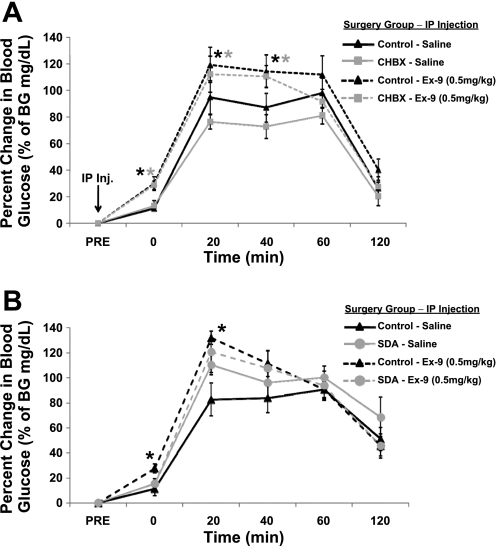
Following ingestion of an oral glucose load (2.0 g/kg), both control-CHB-sham rats and CHBX rats showed a similar profile of glycemic responses. Baseline blood glucose values were not significantly different between CHBX (84.0 ± 2.4 mg/dl) and control-CHB-sham (79.6 ± 1.5 mg/dl) rats. Two-way ANOVA for the overall area under the curve analysis (from time 0 min to time 120 min) revealed a significant main effect of drug, but did not reveal a significant effect of surgical group or group × drug interaction. A significant increase in blood glucose concentration was observed for both groups at 20, 40, and 60-min postingestion following intraperitoneal saline administration (Fig. 2A). Importantly, the GLP-1R antagonist Ex-9 (0.5 mg/kg) augmented this elevation in blood glucose at 20 and 40 min post-glucose ingestion and did so comparably for both surgical groups (nonsignificant group × drug interactions from 0 to 20 min). In addition, blood glucose levels were significantly elevated prior to glucose ingestion by intraperitoneal Ex-9 delivery alone (time 0) for both groups.
Fig. 2.
Percent change in blood glucose (mg/dl) following ingestion of an oral glucose load (2 g/kg; time 0) when combined with saline or exendin-(9–39) (Ex-9; 0.5 mg/kg ip) compared with pre-oral glucose tolerance test and pre-injection blood glucose levels. A: blockade of glucagon-like peptide-1 receptor (GLP-1R) by Ex-9 significantly increased blood glucose at times 0, 20, 40 min in CHB ablation (CHBX) and control rats. B: Ex-9 significantly increased blood glucose in control rats at time 0 and 20 min but failed to alter blood glucose values in subdiaphramatic vagal deafferentation (SDA) rats. *P < 0.05 from within-subject saline condition.
SDA experiment.
Baseline blood glucose values were comparable between SDA (68.1 ± 3.1 mg/dl) and control-SDA-sham (73.0 ± 4.1 mg/dl) rats. Two-way ANOVA for the overall area under the curve analysis (from time 0 min to time 120 min) revealed a significant main effect of surgical group but did not reveal a significant effect of drug or group × drug interaction. Both control-SDA-sham and SDA rats showed a significant increase in blood glucose following ingestion of a glucose load (Fig. 2B). There was a main effect of surgical group and a marginally significant effect for drug × group interaction (P = 0.066) from 0 and 20 min post-glucose ingestion. Following intraperitoneal Ex-9 delivery, the control-SDA-sham rats, but not the SDA rats, showed a significant increase in blood glucose concentration at times 0 and 20 min relative to vehicle-treated animals, thus supporting the requirement of vagal afferent mediation of endogenous GLP-1's incretin response.
Intake Suppressive Effects of GLP-1(7–36) in CHBX, SDA, and Sham-Operated Control Rats
CHBX experiments.
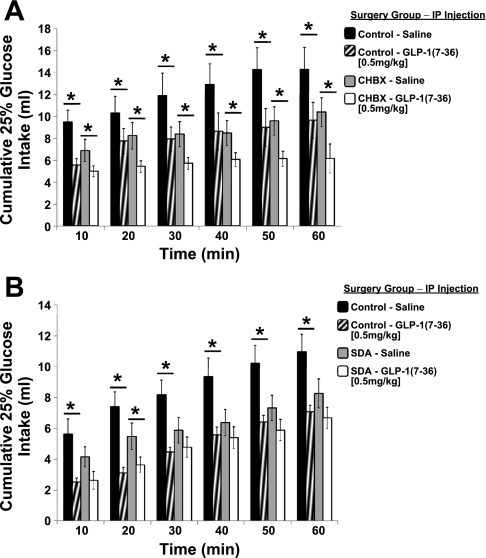
Intake of 25% glucose following intraperitoneal GLP-1(7–36) (0.5 mg/kg) was significantly suppressed in the neurologically intact control-CHB-sham rats at all time points compared with intake following intraperitoneal saline administration (Fig. 3A). Likewise, GLP-1(7–36) significantly suppressed glucose intake compared with vehicle in CHBX rats to an equivalent magnitude as that observed for the control-CHB-sham rats. There was a nonsignificant trend for reduced baseline glucose intake (following ip saline treatment) in the CHBX rats compared with the control-CHB-sham rats. The main effect of group and the group × drug interaction were not significant.
Fig. 3.
Cumulative 25% glucose intake following intraperitoneal injection of GLP-1(7–36) or saline. A: GLP-1 produced a comparable magnitude of glucose intake suppression at all time points for CHBX and control rats. B: GLP-1 significantly suppressed glucose intake of control rats at all time points, but failed to significantly suppress glucose intake of SDA rats except at 20 min. *P < 0.05 from within-subject saline condition.
SDA experiment.
To confirm that vagal afferent signaling is required for the mediation of the intake suppressive effects of intraperitoneal GLP-1(7–36), glucose intake was evaluated in separate experimental trials in SDA rats and neurologically intact control-SDA-sham rats. There was a main effect of drug with control rats showing suppression in glucose intake at all time points following intraperitoneal GLP-1(7–36) delivery (Fig. 3B). However, in SDA rats, GLP-1(7–36) only suppressed glucose intake at 20 min. At all other time points, GLP-1 failed to significantly suppress glucose intake in SDA rats, indicating the requirement of vagal afferent signaling in mediating the intake suppressive effects of systemic GLP-1. The group × drug interaction was marginally significant (P = 0.058); however, the main effect of group was not significant. There was also a marginally significant reduction in baseline glucose intake (following ip saline treatment) in the SDA rats compared with the control-SDA-sham rats following intraperitoneal saline administration (P = 0.059).
DISCUSSION
While abundant research demonstrates that the incretin and food intake inhibitory effects of systemic GLP-1 are mediated in part by vagal afferent signaling (1, 14, 21–23, 44, 50), the specific vagal branch(es) mediating these effects remain unclear. As GLP-1R are likely expressed on dendritic terminals of all subdiaphragmatic branches of the vagus nerve that differentially innervate the organs within the peritoneal cavity (16, 53), current experiments examined the role of gastrointestinal (paracrine-like GLP-1 signaling) vs. hepatoportal (endocrine-like GLP-1 signaling) expressing-GLP-1R in mediating the incretin and intake inhibitory effects of GLP-1. Here, we show that complete SDA, but not CHBX, attenuated the glycemic and food intake suppressive effects of endogenous and pharmacological systemic GLP-1. This suggests that these responses involve activation of GLP-1R expressed on dendritic vagal afferent fiber terminals, but do not require the dendritic terminals of the CHB of the vagus. An equivalent increase in blood glucose concentrations was observed in CHBX and control rats following intraperitoneal delivery of the GLP-1R antagonist Ex-9. Likewise, an equivalent suppression of food intake by intraperitoneal delivered GLP-1(7–36) was observed in CHBX rats and neurologically intact control rats. The collective results, together with the fact that intraperitoneal injections are absorbed through the visceral peritoneum (the membrane covering most abdominal organs), the mesentery and/or the omentum prior to entering into the portal vein (29), and the fact that high concentrations of intraperitoneal injected peptides are found in intestinal lymph (27) support the hypothesis that glycemic and food intake control by intestinally derived GLP-1 is mediated primarily by paracrine-like signaling on adjacent GLP-1R expressed on the celiac and/or gastric branches of the vagus nerve that innervate the small intestine and does not require mediation by the CHB.
The findings of Rüttimann et al. (44) indicate that while vagal afferent signaling is required to mediate intraperitoneal GLP-1's reduction of spontaneous meal size, vagal transmission is not required to mediate GLP-1's reduction of meal size following portal vein administration (44). The present data are consistent with these findings and reveal that in the absence of vagal afferent mediation by the CHB of the vagus (i.e., following CHBX), intraperitoneal GLP-1-induced suppression of food intake and endogenous GLP-1 control of glycemia is equivalent to that of a neurologically intact rat. A conflicting prior report from Vahl et al. (51) shows that delivery of a GLP-1 antagonist to the portal vein impairs glucose tolerance. Based on this finding, the authors' concluded that the GLP-1Rs mediating GLP-1's incretin effects were expressed within the hepatoportal regions (presumably on the CHB) (51). However, this report did not directly test the necessity of the CHB of the vagus in mediating the responses. Nonetheless, when considering previous findings in combination with the present results, we conclude that while GLP-1R expressed within the hepatoportal region can mediate GLP-1-induced incretin signaling, CHB GLP-1R are not required for normal GLP-1 glycemic or food intake control. Following exogenous administration of either dipeptidyl-peptidase-4 inhibitors (e.g., sitagliptin) or long-acting GLP-1R agonists (e.g., exendin-4 or liraglutide), however, the mediating vagal afferent fibers may differ, as the available pool of circulating GLP-1-ligands would be enhanced in quantity as well as duration of action, thus expanding the numbers of distinct GLP-1R populations that potentially contribute to incretin and food intake control. Accordingly, it is interesting to note that exogenous GLP-1 was able to significantly reduce glucose intake in SDA rats, but only at one early time point (20 min). Therefore, we cannot eliminate the possibility that at least with respect to short-term food intake control, a percentage of the intake inhibitory effects may be due to direct ligand action in the central nercous system. Such an effect has been previously demonstrated for exogenous doses of GLP-1 and GLP-1R agonists (23, 44). Thus, future research is needed to further test the necessity of specific subbranches of the vagus nerve, as well as distinct GLP-1R-expressing central nercous system nuclei in mediating the intake and glycemic effects of exogenous GLP-1-based pharmaceuticals.
Results of two reports using electrophysiological methods have recorded from specific vagal branches mediating GLP-1 neuronal transmission and complement our conclusions. Bucinskaite et al. (14) have shown that intravenous administration of GLP-1 excites afferent nerves of the ventral gastric branch of the vagus; an effect blocked by the GLP-1R antagonist, Ex-9. Nakabayashi et al. (31) have shown that intraportal infusion of a truncated form of GLP-1 was able to excite afferent nerves of the CHB, while intraportal infusion of full-length GLP-1 showed no effect on CHB afferent activity. Whether truncated GLP-1 is physiologically relevant to glycemic and food intake control remains unclear; however, these latter data are congruent with our findings in that the CHB does not mediate GLP-1-induced vagal activation. While we did not directly examine the effects of truncated GLP-1 to glycemic and food intake control, the current set of data do not support a role for CHB mediation of endogenously released GLP-1, either in full or truncated form, following ingestion of glucose.
The subdiaphragmatic vagal afferent fibers include myelinated (A-type) or unmyelinated (C-type) fibers (11, 40). Previous research has utilized the C-fiber specific neurotoxin capsaicin to chemically destroy the unmyelinated afferent fibers in an attempt to examine the role of vagal afferent mediation of GLP-1's physiological effects (4, 22, 50). Unfortunately, this approach has limitations as it also destroys nonvagal unmyelinated sensory neurons (17) and spares A-fibers, which have also been shown to mediate numerous within-meal satiation signaling (42, 52). The use of total subdiaphragmatic vagotomy has also been employed to examine vagal-mediated responses to systemic GLP-1 (1). Total subdiaphragmatic vagotomy destroys vagal efferents in addition to afferents and, therefore, leads to disturbances in gastrointestinal motility and secretion (26). Furthermore, by eliminating 100% of subdiaphragmatic vagal efferents, total subdiaphragmatic vagotomy complicates the interpretation of data, as both vagal afferents and efferents are involved in meal-induced release of endogenous GLP-1 (43). While we cannot rule out the possibility that the SDA approach disrupts some vagal efferent mediated endogenous GLP-1 secretion and subsequent signaling, sparing ∼50% of the efferent fibers below the diaphragm should reduce the incidence of side effects compared with the total subdiaphragmatic vagotomy as discussed in materials and methods. Nonetheless, it is clear from the glycemic findings of the SDA and control rats following vehicle injections that intact vagal afferent signaling is required for the normal regulation of glycemia. Indeed, in response to a glucose load, the action of endogenous incretin hormones (i.e., GLP-1) on vagal afferent fibers that trigger insulin secretion via vagovagal reflexes (thus reducing blood glucose levels) would be eliminated and result in the observed higher plasma glucose levels. Such observations further support the specific role of vagal afferent signaling in glycemic regulation.
While our data show the necessity of non-CHB subdiaphragmatic vagal afferents for the satiating effect of GLP-1 administered intraperitoneally to mimic the putative action of the endogenous peptide, both CHBX and SDA rats demonstrate a slight reduction in the normal consumption of a glucose solution relative to intact controls. We can only speculate as to why this effect occurred. The presence of sweet taste receptors (e.g., T1R2/T1R3) expressed throughout the small intestine is widely documented (48–49, 54). It has been postulated that postgastric components involving sweet taste receptors mediate the acquisition of preferences for associated flavors and may play a role in the development of the rewarding response to ingested carbohydrate solutions (2–3, 13). Both the duodenum and a yet-to-be-identified postabsorptive detection site(s) for glucose levels are thought to be involved in generation of the reinforcing signal (3). It may well be that ablation of CHB or total vagal afferents using the SDA procedure eliminated the neural transmission responsible for these gastrointestinal-derived reinforcing signals for the ingested glucose, as the CHB innervates not only the hepatoportal bed, but also a percentage of the duodenum. Indeed, Novin et al., (35) has previously suggested that the positive reinforcing effect of glucose may depend, in part, on CHB signaling. While previous research has shown that vagal afferents are not required for flavor-nutrient preference conditioning to a complex starch solution (45), it is important to point out that the ingestate used in the current experiments was glucose. Whether glucose vs. starch differentially engage vagal-mediated signaling for flavor preferences remains to be determined. An additional hypothesis that could explain the differences in glucose intake following vehicle injections may relate to potential differential gastric emptying rates between vagotomized (either CHBX or SDA) rats and surgical controls (9, 18). Indeed, it has previously been demonstrated that selective vagotomies can accelerate gastric emptying of liquid meals, and that the hepatic and celiac branches in particular play an important role in mediating gastric emptying inhibitory signaling coming from the gastrointestinal tract (18). Thus, it is possible that with accelerated gastric emptying, the glucose absorption rate from the small intestine may also have been increased and subsequently detected by nonvagal mechanisms. Such an effect may have ultimately influenced the continued ingestion of glucose. However, such a hypothesis is speculation and requires further testing.
Perspectives and Significance
The current findings show that systemic GLP-1R mediation of glycemic and food intake suppression does not require the CHB. An equivalent increase in blood glucose concentration was observed in CHBX and control rats following intraperitoneal delivery of the GLP-1R antagonist Ex-9. Conversely, SDA rats with complete ablation of all subdiaphragmatic vagal afferents did not demonstrate an Ex-9-induced hyperglycemia. Likewise, an equivalent suppression of food intake by intraperitoneal delivered GLP-1 was observed in CHBX and neurologically intact control rats; whereas SDA rats showed an almost complete absence of intake suppression by intraperitoneal GLP-1. The collective results support a role for paracrine-like GLP-1 signaling on GLP-1R expressed on vagal afferent fibers innervating the gastrointestinal tract.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-085435 (to M. R. Hayes), DK-089752 (to S. E. Kanoski), and DK-021397 (to H. J. Grill), as well as External Research Grants from Merck, and Investigator Initiated Sponsor Proposal Grants 36266 and 38723 (to: M. R. Hayes and H. J. Grill).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.R.H., W.L., and H.J.G. conception and design of research; M.R.H., S.E.K., B.C.D.J., T.M.L., A.L.A., S.M.F., and M.A. performed experiments; M.R.H. and S.E.K. analyzed data; M.R.H., S.E.K., M.A., W.L., and H.J.G. interpreted results of experiments; M.R.H. prepared figures; M.R.H. and H.J.G. drafted manuscript; M.R.H., S.E.K., B.C.D.J., M.A., W.L., and H.J.G. edited and revised manuscript; M.R.H., S.E.K., B.C.D.J., T.M.L., A.L.A., S.M.F., M.A., W.L., and H.J.G. approved final version of manuscript.
ACKNOWLEDGEMENTS
We thank Anthony Balduzzi, Polly van den Berg, Kalina Eneva, Andrea Spaeth, and Shiru Zhao for technical assistance and Jimmy Valmer for a critical reading of the manuscript.
REFERENCES
- 1. Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044: 127–131, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Ackroff K, Touzani K, Peets TK, Sclafani A. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol Behav 72: 691–703, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Ackroff K, Yiin YM, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav 99: 402–411, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahren B. Sensory nerves contribute to insulin secretion by glucagon-like peptide-1 in mice. Am J Physiol Regul Integr Comp Physiol 286: R269–R272, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 26: 11052–11060, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374: 1606–1616, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Balkan B, Li X. Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol Regul Integr Comp Physiol 279: R1449–R1454, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Berger T, Ceder L, Hamfelt A, Meurling S. Effect of highly selective vagotomy on gastric emptying. Scand J Gastroenterol 11: 829–832, 1976 [PubMed] [Google Scholar]
- 10. Berthoud HR, Kressel M, Neuhuber WL. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol (Berl) 186: 431–442, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Berthoud HR, Patterson LM, Willing AE, Mueller K, Neuhuber WL. Capsaicin-resistant vagal afferent fibers in the rat gastrointestinal tract: anatomical identification and functional integrity. Brain Res 746: 195–206, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 8: 436–447, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Bonacchi KB, Ackroff K, Touzani K, Bodnar RJ, Sclafani A. Opioid mediation of starch and sugar preference in the rat. Pharmacol Biochem Behav 96: 507–514, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bucinskaite V, Tolessa T, Pedersen J, Rydqvist B, Zerihun L, Holst JJ, Hellstrom PM. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil 21: 978–e78, 2009 [DOI] [PubMed] [Google Scholar]
- 14a.Data and Statistics Center for Disease Control and Prevention. Overweight and Obesity [ http://www.cdc.gov/obesity/data/index.html]
- 15. De Jonghe BC, Horn CC. Chemotherapy-induced pica and anorexia are reduced by common hepatic branch vagotomy in the rat. Am J Physiol Regul Integr Comp Physiol 294: R756–R765, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J Comp Neurol 428: 558–576, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Gamse R. Capsaicin and nociception in the rat and mouse. Possible role of substance. P Naunyn Schmiedebergs Arch Pharmacol 320: 205–216, 1982 [DOI] [PubMed] [Google Scholar]
- 18. Gleysteen JJ, Burdeshaw JA, Hallenbeck GA. Gastric emptying of liquids after different vagotomies and pyloroplasty. Surg Gynecol Obstet 142: 41–48, 1976 [PubMed] [Google Scholar]
- 19. Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav 100: 503–510, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149: 4059–4068, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol Gastrointest Liver Physiol 273: G920–G927, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Gremeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 115: 3554–3563, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knudsen LB. Liraglutide: the therapeutic promise from animal models. Int J Clin Pract Suppl 64: 4–11, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Kraly FS, Jerome C, Smith GP. Specific postoperative syndromes after total and selective vagotomies in the rat. Appetite 7: 1–17, 1986 [DOI] [PubMed] [Google Scholar]
- 27. Lo CM, Xu M, Yang Q, Zheng S, Carey KM, Tubb MR, Davidson WS, Liu M, Woods SC, Tso P. Effect of intraperitoneal and intravenous administration of cholecystokinin-8 and apolipoprotein AIV on intestinal lymphatic CCK-8 and apo AIV concentration. Am J Physiol Regul Integr Comp Physiol 296: R43–R50, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5: 262–269, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther 178: 562–564, 1971 [PubMed] [Google Scholar]
- 30. Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol Regul Integr Comp Physiol 272: R1245–R1251, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol Endocrinol Metab 271: E808–E813, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Nishizawa M, Nakabayashi H, Kawai K, Ito T, Kawakami S, Nakagawa A, Niijima A, Uchida K. The hepatic vagal reception of intraportal GLP-1 is via receptor different from the pancreatic GLP-1 receptor. J Auton Nerv Syst 80: 14–21, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Nishizawa M, Nakabayashi H, Uchida K, Nakagawa A, Niijima A. The hepatic vagal nerve is receptive to incretin hormone glucagon-like peptide-1, but not to glucose-dependent insulinotropic polypeptide, in the portal vein. J Auton Nerv Syst 61: 149–154, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Norgren R, Smith GP. A method for selective section of vagal afferent or efferent axons in the rat. Am J Physiol Regul Integr Comp Physiol 267: R1136–R1141, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Novin D, Robinson K, Culbreth LA, Tordoff MG. Is there a role for the liver in the control of food intake? Am J Clin Nutr 42: 1050–1062, 1985 [DOI] [PubMed] [Google Scholar]
- 36. Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology 132: 2087–2102, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Peters JH, McKay BM, Simasko SM, Ritter RC. Leptin-induced satiation mediated by abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol 288: R879–R884, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Phillips RJ, Baronowsky EA, Powley TL. Afferent innervation of gastrointestinal tract smooth muscle by the hepatic branch of the vagus. J Comp Neurol 384: 248–270, 1997 [PubMed] [Google Scholar]
- 39. Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol Regul Integr Comp Physiol 253: R361–R370, 1987 [DOI] [PubMed] [Google Scholar]
- 40. Prechtl JC, Powley TL. The fiber composition of the abdominal vagus of the rat. Anat Embryol (Berl) 181: 101–115, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 56: 8–15, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav 81: 249–273, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140: 1687–1694, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150: 1174–1181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav 78: 285–294, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol 293: R983–R987, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol Regul Integr Comp Physiol 249: R638–R641, 1985 [DOI] [PubMed] [Google Scholar]
- 48. Steinert RE, Beglinger C. Nutrient sensing in the gut: Interactions between chemosensory cells, visceral afferents and the secretion of satiation peptides. Physiol Behav. In press [DOI] [PubMed] [Google Scholar]
- 49. Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clin Nutr 30: 524–532, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146: 3748–3756, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148: 4965–4973, 2007 [DOI] [PubMed] [Google Scholar]
- 52. van de Wall EH, Duffy P, Ritter RC. CCK enhances response to gastric distension by acting on capsaicin insensitive vagal afferents. Am J Physiol Regul Integr Comp Physiol 289: R695–R703, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Wang FB, Powley TL. Vagal innervation of intestines: afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res 329: 221–230, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Young RL. Sensing via intestinal sweet taste pathways. Front Neurosci 5: 23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]