Abstract
Sepsis is a systemic immune response to infection that may result in multiple organ failure and death. Polymicrobial infections remain a serious clinical problem, and in the hospital, sepsis is the number-one noncardiac killer. Although the central nervous system may be one of the first systems affected, relatively little effort has been made to determine the impact of sepsis on the brain. In this study, we used the cecal ligation and puncture (CLP) model to determine the extent to which sepsis alters sleep, the EEG, and brain temperature (Tbr) of rats. Sepsis increases the amount of time rats spend in non-rapid eye movement sleep (NREMS) during the dark period, but not during the light period. Rapid eye movements sleep (REMS) of septic rats is suppressed for about 24 h following CLP surgery, after which REMS increases during dark periods for at least three nights. The EEG is dramatically altered shortly after sepsis induction, as evidenced by reductions in slow-frequency components. Furthermore, sleep is fragmented, indicating that the quality of sleep is diminished. Effects on sleep, the EEG, and Tbr persist for at least 84 h after sepsis induction, the duration of our recording period. Immunohistochemical assays focused on brain stem mechanisms responsible for alterations in REMS, as little information is available concerning infection-induced suppression of this sleep stage. Our immunohistochemical data suggest that REMS suppression after sepsis onset may be mediated, in part, by the brain stem GABAergic system. This study demonstrates for the first time that sleep and EEG patterns are altered during CLP-induced sepsis. These data suggest that the EEG may serve as a biomarker for sepsis onset. These data also contribute to our knowledge of potential mechanisms, whereby infections alter sleep and other central nervous system functions.
Keywords: thermoregulation, behavior, cytokines, circadian rhythms, cecal ligation and puncture
sepsis results from an amplified inflammatory response to an infectious process that leads to organ dysfunction or risk for secondary infection. As a clinical syndrome, sepsis is defined by the presence of infection and a systemic inflammatory response (7, 29). In spite of the ever-increasing sophistication of our antimicrobial armamentarium, sepsis continues to kill millions of people worldwide, and the diagnosis of sepsis still confers a 20–50% risk of mortality (1, 33). The National Center for Health Statistics now lists sepsis among the top 10 leading causes of death in the United States, and, in fact, sepsis/septic shock is the leading cause of death among patients in noncoronary hospital units (34, 48). For those who survive, the impact of severe sepsis does not end with hospital discharge. Mortality from all causes increases from 21% at admission to ∼75% five years after index admission for severe sepsis (60). Furthermore, survivors of sepsis often suffer from new, persistent, and dramatic cognitive impairment (25). These epidemiologic data demonstrate that sepsis must also be viewed as a long-term chronic condition and that severe sepsis remains a tremendous public health burden in terms of lives lost and cost. However, although sepsis is often associated with cognitive impairment and end-stage encephalopathy [e.g., (20)], relatively little research has focused on the impact of sepsis on central nervous system (CNS) processes.
Links between the immune system and the CNS have been identified and characterized during the last 30 yr. Infections impact the CNS, and sick individuals experience cognitive deficits, malaise, and lethargy. Infections also induce profound changes in sleep-wake behavior, appetite, social contact, and other usual daily activities (12), a constellation of symptoms referred to as sickness behavior. Sickness behavior, including changes in sleep, is viewed as an adaptive response of the host organism to infection that ultimately contributes to survival (22, 24).
To advance our understanding of the impact of traumatic infections on CNS processes, and specifically, sleep, in this study, we determined the extent to which sleep-wake behavior of rats is altered during CLP-induced sepsis. Because sepsis is a heterogeneous and complex pathology, in vivo models are critical tools and have become the mainstay of sepsis research [reviewed by Nemzek et al. (36)]. Two models frequently used to determine mechanisms and mediators of morbidity and mortality in response to sepsis are intraperitoneal administration of high doses of LPS and cecal ligation and puncture (CLP). However, bolus administration of LPS does not constitute an infection, as there is no replicating pathogen, whereas CLP mimics clinical conditions with respect to bowel perforation and subsequent peritonitis due to mixed intestinal flora. CLP is considered a clinically relevant sepsis model, in part, because it reproduces the dynamic changes in cardiovascular function observed in septic patients, recreates the progressive release of proinflammatory mediators (13, 14), and is sensitive to antibiotic treatment and fluid resuscitation (37). The CLP technique is relatively simple and yields reliable outcomes (46).
On the basis of previous studies of laboratory animals infected with select bacterial pathogens, we hypothesized that sepsis, as a polymicrobial infection, also would alter sleep. Specifically, we hypothesized that non-rapid eye movement sleep (NREMS) would increase during sepsis, whereas rapid eye movement sleep (REMS) would be suppressed. Moreover, to begin to elucidate potential mechanisms, whereby this infectious process alters sleep, we used immunohistochemistry to determine the impact of sepsis on the activity of brain stem nuclei known to be involved in the regulation of sleep. We now report that sleep of rats is altered for at least 4 days during the course of sepsis and that activity of brain stem nuclei involved in the regulation of some facets of sleep is altered in a manner demonstrating a potential role for the GABAergic system as a mediator of these responses.
METHODS
Animals
These experiments were performed on male Sprague-Dawley rats (300–350 g at surgery; Harlan, Indianapolis, IN). Before and after surgical manipulations, all rats were individually housed in standard cages and kept in temperature-controlled chambers at 21 ± 1°C. Lights were maintained on a 12:12-h light-dark cycle, and food and water were provided ad libitum. All procedures involving the use of animals were approved by the University of Michigan Committee on Care and Use of Animals in accordance with the U.S. Department of Agriculture Animal Welfare Act, and the Public Health Service policy on Humane Care and Use of Laboratory Animals.
Surgical Procedures
Two groups of rats were used in this study. Rats in group 1 (n = 37) were used to determine the impact of sepsis on sleep-wake behavior. These rats were subjected to two surgical procedures. First, a cranial surgery was used to implant instrumentation that allows subsequent determination of sleep-wake behavior. Second, an abdominal surgery was performed for the CLP procedure as a means of inducing sepsis. During the cranial surgery [procedures previously described by Imeri et al. (23)], three stainless-steel screws to serve as EEG-recording electrodes were implanted into the skull over frontal and parietal brain cortices. A calibrated 30-kOhm thermistor (Omega Engineering, Stamford, CT) was implanted between the dura mater and the skull over a parietal cortex to measure cortical brain temperature (Tbr). At the end of the surgery, the wound was treated with topical analgesic (L.M.X.4, lidocaine 4%; Ferndale IP, Ferndale, MI) and a triple antibiotic ointment (neomycin and polymixin B sulfate and bacitracin zinc, E. Fougera, Melville, NY). The animals were allowed 10 days of recovery after the surgical procedures. On the 5th postsurgical day, the rats were connected to the recording apparatus via a flexible tether for habituation to the recording environment.
On days 10–12 after the cranial surgery, a 48-h recording period was used to obtain baseline data for each animal (see Experimental Protocol). After this baseline recording period, the abdominal surgery was performed (46). Some of the rats underwent CLP surgery, whereas others had sham surgery. Animals were deeply anesthetized, a 1-cm midline incision was made on the abdomen, and the cecum was exposed. The distal 1 cm of the cecum was ligated with a 4–0 Prolenesuture (Ethicon, Johnson & Johnson, Langhorne, PA) and perforated twice through-and-through with a 21-gauge needle. A small amount of stool was extruded to ensure that the wound was patent. The cecum was placed back into the abdominal cavity, and the abdomen was closed using silk sutures (4–0; Ethicon Johnson & Johnson, Langhorne, PA). Animals subjected to sham surgery underwent an identical procedure; however, the cecum was exposed but not ligated or punctured and then replaced. Two hours and 8 h after surgery (and twice daily for the rest of the protocol), each animal (SHAM, CLP) received a subcutaneous injection of a broad-spectrum antibiotic (Imipenem, 0.5 mg/rat) in 1 ml of lactated Ringers/D5W. As such, each rat received 10 ml of fluids during the postsurgical period.
Rats in group 2 (n = 38) were used to determine the impact of sepsis on activity of the cholinergic and GABAergic systems in selected brain stem nuclei. These animals did not receive a cranial implant, but were subjected to either CLP or sham surgeries, as described previously.
Experimental Protocol
Two separate experiments were conducted in this study. Animals in experiment 1 were used to determine the impact of sepsis on sleep-wake activity of rats. After recovery from the cranial surgery and adaptation to the recording apparatus, two 24-h undisturbed baseline recordings were obtained from these animals. After this 48-h baseline recording period, rats underwent abdominal surgery (CLP, n = 20; SHAM, n = 17) during the first 6 h of the light period. Recordings began at the beginning of the subsequent dark period and continued for as long as 96 h. The rats were undisturbed during these recordings, with the exception of administration of antibiotics twice daily.
Experiment 2 was a post hoc experiment, the design of which was guided by results of experiment 1. Rats in this experiment (SHAM, n = 12; CLP, n = 26) were used to obtain brains for immunohistochemical assessment of the impact of sepsis on selected neurotransmitter systems (see later). After determining the impact of sepsis on the sleep of rats (experiment 1), experimental time points that corresponded to periods during which differences in sleep-wake behavior were revealed were selected. The experimental time points selected for death were the midpoints of the light periods 30, 54, and 78 h postsurgery.
Recording Apparatus and Determination of Sleep-Wake Behavior
Signal conditioning and determination of sleep-wake behavior from rats have been previously described (23). Briefly, signals from the EEG electrodes and thermistors were fed into amplifiers (Colbourn Instruments, Lehigh Valley, PA; models S75–01 and S71–20, respectively). Motor activity was detected using an infrared sensor housed in an observation unit (BioBserve, Bonn, Germany). Movements detected by the infrared sensor were converted to a voltage output, the magnitude of which was directly related to the magnitude of movements detected. All the signals were subjected to analog-to-digital conversion (A/D board: PCI:3033E; National Instruments, Austin, TX) with 16-bit precision at a sampling rate of 128 Hz for EEG, 1 Hz for Tbr and 8 Hz for motor activity. The digitized EEG waveform, Tbr samples and integrated values for motor activity were stored as binary files until further processing. Arousal states were assigned with a resolution of 12 s by visual scoring of EEG, body movements, and Tbr, using custom software (ICELUS, M. Opp, University of Michigan). The EEG signal was subjected to fast Fourier transformation (FFT), yielding power spectra between 0.5 and 30 Hz in 0.5-Hz frequency bins. Arousal state was classified as previously described as NREMS, REMS, or wakefulness on the basis of state-dependent changes in multiple parameters, including the EEG, offline FFT values, body movements, and Tbr (23). Any 12-s epoch containing movement artifacts or electrical noise was tagged and excluded from subsequent spectral analyses.
Animal Perfusion and Tissue Sectioning
All rats in experiment 2 were transcardially perfused under pentobarbital sodium anesthesia (Nembutal; 1.5 ml ip of 50 mg/ml). Approximately 75 ml of room-temperature saline was perfused transcardially followed by 1,000 ml of a 3% paraformaldehyde solution and 250 ml of a 10% sucrose solution at 4°C. The brains were postfixed overnight in a 30% sucrose solution at 4°C. Brains were frozen and stored at −80°C before sectioning. Coronal brain stem sections (25 μm) were cut serially on a sliding microtome with a freezing stage and stored in a cryoprotectant solution (24% vol/vol glycerin, 29% vol/vol ethylene glycol, 0.1 M sodium phosphate dibasic) at −20°C until assay. In contrast to tissue from SHAM control animals, brain tissue from septic rats was difficult to section, presumably as a result of the infection.
Immunostaining
Immunohistochemistry was performed on a series of adjacent free-floating frozen brain sections, focusing on the cholinergic laterodorsal tegmental/pedunculopontine nuclei (LDT/PPT) and the GABAergic ventrolateral periaqueductal grey (vlPAG) of rats. All brain sections were processed using a double-labeling immunoperoxidase procedure to detect the immediate early gene product c-Fos and either choline acetyltransferase (ChAT) or glutamic acid decarboxylase-67 (GAD67). All reagents used were obtained from Vector Laboratories, unless otherwise stated. Tissue was sequentially incubated in rabbit anti-c-Fos polyclonal primary antibody (1:1,500; Abcam, Cambridge, MA), biotinylated goat anti-rabbit IgG secondary antibody (1:300) and ABC reagent (Vectastain Elite ABC kit). DAB (3,3′-diaminobenzidine) chromagen substrate (DAB substrate kit for peroxidase) was used to visualize c-Fos+ nuclei. Double labeling for ChAT was performed using successive incubations in sheep anti-ChAT primary antibody (1:18,000; Abcam), biotinylated rabbit anti-sheep IgG secondary antibody (1:200), and ABC reagent. Double-labeling for GAD67 was achieved by serial incubations in mouse anti-GAD67 monoclonal primary antibody (1:2,000; Chemicon International, Temecula, CA), rat adsorbed biotinylated horse anti-mouse IgG (1:200), and ABC reagent. Silver gray chromagen (Vector SG substrate kit for peroxidase) was used to visualize ChAT and GAD67 immunoreactivity in the cytoplasm. Negative controls were included in each assay by processing tissue without the respective primary antibodies. At the conclusion of the immunostaining procedure, sections were transferred to glass microscope slides (Superfrost Plus, Fisherbrand; Fisher Scientific, Waltham, MA), dehydrated through a series of graded alcohols (50%, 70%, 95%, and 100%), incubated in xylene and coverslipped with Permount mounting medium (Fisher Scientific).
Cell Counts in LDT/PPT and vlPAG
Coronal brain stem sections were taken from −6.72 to −9.23 mm relative to bregma to include LDT, PPT, and vlPAG. To assess the impact of sepsis on the cholinergic system, ChAT+, c-Fos+ and c-Fos+/ChAT+ dual-labeled cells were counted from a 1 in 200-μm series (1 in 8 series). GABAergic activation during sepsis was determined by counting GAD67+, c-Fos+, and c-Fos+/GAD67+ cells a 1 in 400-μm series (1 in 16 series). These series of sections were selected on the basis of previous studies by Maloney (31,32).
Stained sections were visualized using light microscopy. ChAT, GAD67 and c-Fos+ cells were counted using the Optical Fractionator work flow of Stereo Investigator version 7.0 (MicroBrightfield, Willison, VT). Contours were drawn at ×2 and ×4 magnification and were based on region outlines depicted in the Paxinos and Watson's rat brain atlas (44). Individual neurons were counted within contours at ×60 magnification by an individual blinded to the experimental manipulation of the animal. Counts were made bilaterally on sections through the LDT/PPT and the vlPAG. Sections between animals were matched as closely as possible, according to their position relative to bregma and the same number of sections per region/nuclei were selected for counting from each animal. Individual sections were counted, and numbers were summed to produce a single value for that animal based on brain region.
Statistical Analysis
Results are presented as means ± SE. Tests for statistical significance were performed using SPSS for Windows. Two types of statistical comparisons were made. To determine the impact of manipulations across time, analyses were restricted to within-group (SHAM, CLP) comparisons for time spent in each behavioral state, motor activity, Tbr, and delta power during NREMS. These within-group comparisons were made by means of a general linear model for repeated measures. The second comparison was used to determine whether the responses to CLP differed from those of control (SHAM) animals, i.e., these were between-group comparisons. For these analyses, difference scores were calculated for time spent in each behavioral state, Tbr, and delta power during NREMS. These difference scores were obtained by subtracting baseline values for each parameter from values obtained after CLP or SHAM surgery. Between-group differences were analyzed with a mixed model for repeated measures, using a first-order regression covariance structure where time was the repeated measure. Within- and between-group comparisons were performed on 6-h time blocks.
Within- and between-group analyses of dark-light differences in each behavioral state, motor activity, Tbr, and delta power during NREMS were performed by means of a one-way ANOVA, with manipulation (CLP, SHAM) as the independent variable. Post hoc comparisons, with Bonferroni correction, were performed within groups to evaluate differences between baseline and each experimental day.
The numbers of c-Fos+, ChAT+, and GAD67+ cells as a function of manipulation (CLP, SHAM) were analyzed by one-way ANOVA. For all analyses, an alpha level of P ≤ 0.05 was accepted as indicating statistically significant differences in values.
RESULTS
Data derived from this study are presented in two ways. First, comparisons within group (SHAM, CLP) demonstrate responses to respective surgical procedures across time. Second, comparisons between groups demonstrate the impact of sepsis on outcome measures compared with values obtained from control animals.
There were no significant differences among any of the biological parameters obtained during the preabdominal surgery baseline recording period. As such, the data for these two 24-h recording periods were averaged to provide a 24-h baseline profile of control values. The 24-h baseline profile is sequentially plotted in appropriate figures for 96 h (i.e., 4 days).
Sleep-Wake Behavior
During undisturbed baseline recordings, there were no differences in sleep-wake behavior between animals that were subsequently divided into the two manipulation groups. As a group, these rats exhibited normal diurnal variation in sleep-wake behavior, spending less time in wakefulness and more time in both NREMS and REMS during the light period than during the dark period of the 24-h light-dark cycle (% recording time NREMS: 41.7 ± 0.6 light period, 23.3 ± 0.7, dark period; % recording time REMS: 11.3 ± 0.2 light period, 5.1 ± 0.2 dark period; % recording time WAKE: 47.0 ± 0.7 light period, 71.6 ± 0.9 dark period).
There was no mortality in animals randomized into the SHAM group (n = 17). Of the n = 20 animals randomized into the CLP group, n = 2 (10%) died during sepsis. Corresponding baseline values were omitted from analyses and graphic representation for periods after rats had been euthanized. In this manner, the within-subjects nature of the protocol was maintained as baseline values, and post-CLP values always correspond across animals.
The impact of sepsis on NREMS, REMS, and wakefulness are summarized day-by-day across the postsurgical recording period to simplify data presentation.
Day 1.
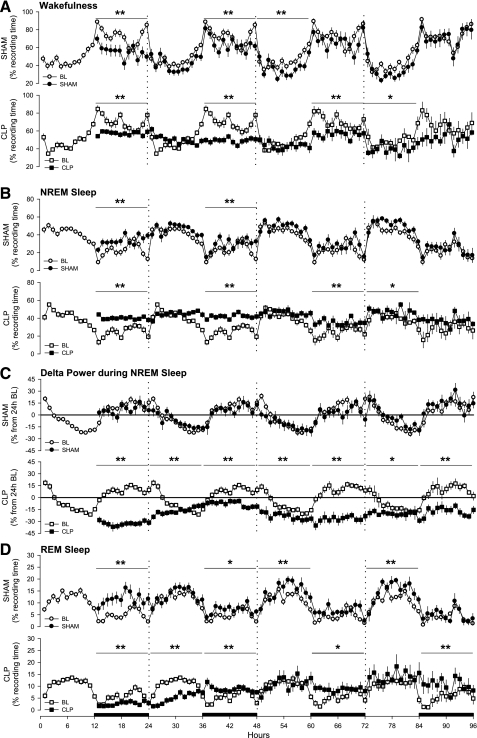
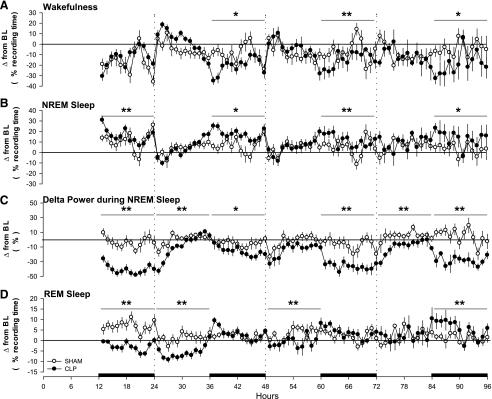
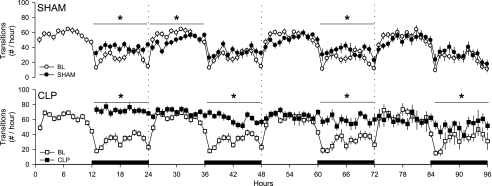
Arousal states were altered immediately after abdominal surgery in rats that had SHAM (control) surgery and in animals that had CLP surgery to induce sepsis (Figs. 1 and 2). During the initial postsurgery dark period, the amount of time spent in wakefulness was reduced, and NREMS increased in both groups with respect to baseline values (Fig. 1, A and B). The magnitude of the increase in NREMS was greater in CLP-septic rats than in control rats (Fig. 2B). Delta power during NREMS was altered only in CLP rats, which showed a marked reduction compared with baseline values (Fig. 1C and Fig. 2C). REMS was differentially affected in the two groups. REMS of control animals (SHAM) increased during the subsequent dark period following surgery (Fig. 1D), whereas REMS was almost absent in CLP-septic rats (Fig. 1D). Compared with baseline, sleep of rats in both groups was fragmented during the 12-h dark period of day 1, as indicated by an increase in the number of transitions from one behavioral state to another (Fig. 3).
Fig. 1.
Sepsis alters sleep and the EEG of rats. Depicted is the percentage of recording time spent in wakefulness (A), non-rapid eye movements sleep (NREMS) (B), EEG delta power (0.5–4.0 Hz) during NREMS (C), and rapid eye movements sleep (REMS) (D). Data were obtained during presurgical baseline recordings (BL, open symbols) and during 4 days after abdominal surgeries (solid symbols) from control rats (SHAM, ●) and septic rats (CLP, ■). Data are presented as hourly means ± SE. Values for EEG delta power during NREMS were normalized and expressed as a percentage from the 24-h average baseline values, depicted by the “zero” line. Statistical evaluations were performed on consecutive 6-h time blocks. Statistically significant changes relative to baseline values across time blocks are indicated as *P ≤ 0.05; **P ≤ 0.01. The black bars on the x-axis indicate the dark portion of the light-dark cycle.
Fig. 2.
The changes in sleep and the EEG of rats are not due to the surgical procedure, but are a direct response to sepsis. Hourly differences between presurgical baseline values and postsurgical values were calculated and are plotted for each group relative to baseline values (depicted by the zero lines). Depicted are group differences in time spent in wakefulness (A), NREMS (B), EEG delta power (0.5–4.0 Hz) during NREMS (C), and REMS (D) for control rats (SHAM, ○) and septic rats (CLP, ●). Data are presented as means ± SE. Statistical evaluations were performed on consecutive 6-h time blocks. Statistically significant differences between groups across time blocks are indicated as *P ≤ 0.05; **P ≤ 0.01. The black bars on the x-axis indicate the dark portion of the light-dark cycle.
Fig. 3.
Sepsis fragments sleep of rats. The number of transitions from one behavioral state to another were determined hourly from control rats (SHAM, ●) and septic rats (CLP, ■) during presurgical baseline recordings (open symbols) and during postsurgical recording days (solid symbols). Data are presented as means ± SE. Statistical evaluations were performed on consecutive 6-h time blocks. Statistically significant differences between baseline and postsurgical values across time blocks are indicated as *P ≤ 0.05. The black bars on the x-axis indicate the dark portion of the light-dark cycle.
Day 2.
Relative to baseline recordings, wakefulness was reduced and NREMS was increased in both groups, but this effect was limited to the dark period (Fig. 1, A and B) and was of greater magnitude in septic rats (Fig. 2, A and B). Delta power during NREMS was suppressed during the entire 24 h on day 2 in septic animals, but not in the control group (Fig. 1C). The suppression of REMS observed in septic rats during day 1 persisted during the 12 h of the light period of day 2, after which REMS of these animals increased during the dark period relative to baseline values (Fig. 1D). Time spent in REMS differed between groups during the light period, but not during the dark period (Fig. 2D). With respect to baseline values, sleep of septic rats (CLP) was fragmented during the dark period of the second postsurgical recording day (Fig. 3).
Day 3.
Both groups of rats spent less time awake during day 3, but the pattern differed between groups. The reduction in wakefulness of control rats was limited to the 12 h of the light period, whereas in septic animals wakefulness was reduced only during the dark period (Fig. 1A). Relative to baseline values, the magnitude of differences between the groups differed statistically only during the dark period (Fig. 2A). NREMS of septic rats (CLP) was increased for the entire 24-h period, although the majority of this effect was apparent during the dark period (Fig. 1B). Direct comparison between groups showed that the difference in the NREMS response to sepsis was limited to the 12 h of the dark period (Fig. 2B). Relative to baseline values, delta power during NREMS was reduced in septic animals during both the light and the dark period (Fig. 1C) and differed between groups during the dark period of day 3 (Fig. 2C). REMS was increased in the SHAM group during 12 h of the light period and in the CLP group during 12 h of the dark period (Fig. 1D). Sleep of control animals and septic rats was fragmented during the dark period of day 3 (Fig. 3).
Day 4.
No differences from baseline were observed in wakefulness, NREMS, or delta power during NREMS in control rats (Fig. 1, A–C). In contrast, septic rats still exhibited decreased wakefulness and increased NREMS during the light period (Fig. 1, A and B). Delta power during NREMS of septic rats remained decreased compared with baseline values across the 24-h period (Fig. 1C). REMS was increased with respect to baseline in both groups. However, in the control group, the increase in REMS was limited to the light period, whereas in septic rats, it was limited to the dark period (Fig. 1D). Direct comparison between groups indicated that the treatment differences were limited to the dark period for wakefulness, NREMS, and REMS (Fig. 2, A, B, and D), whereas the difference in delta power during NREMS was observed across the entire 24 h (Fig. 2C). In control animals, the number of transitions between states did not differ from baseline, whereas sleep was still fragmented during the dark period of septic rats (Fig. 3).
Brain Temperature
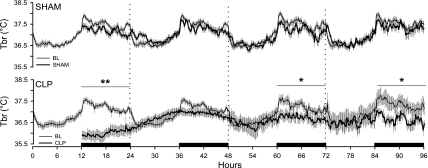
Brain temperature of control (SHAM) animals was not altered by the surgical procedure, although there were modest reductions in Tbr during the dark periods of the recording protocol that did not achieve statistical significance (Fig. 4). In contrast, there was a profound hypothermia manifest in CLP-septic rats during the 12 h dark period following surgery (Fig. 4). In these animals, there were trends for altered Tbr during day 2 that did not achieve statistical significance. During the dark periods of postsurgical days 3 and 4, Tbr of CLP-septic rats was still lower than during comparable baseline periods for these same animals (Fig. 4).
Fig. 4.
Sepsis alters thermoregulation of rats. Depicted are changes in brain temperature of rats during baseline (thin line) and during 4 days after abdominal surgery (thick line). Data were obtained from control rats (SHAM) and septic rats (CLP). Data are presented in 10-min intervals as means ± SE. Statistical evaluations were performed on consecutive 6-h time blocks. Statistical significance across time blocks is indicated as *P ≤ 0.05; **P ≤ 0.01. The black bars on the x-axis indicate the dark portion of the light-dark cycle.
Light-Dark Rhythms
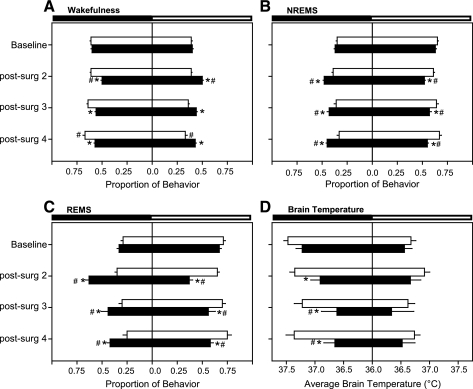
Visual inspection of data across the postsurgical recording period (Figs. 1 and 4) suggested that the normal diurnal rhythms of sleep-wake behavior and Tbr of CLP-septic animals were generally damped or flattened. To further analyze the impact of sepsis on these daily rhythms, the proportion of each behavioral state that was spent in the light or dark phase was calculated relative to values for that state across the entire 24-h period. For example, in healthy rats entrained to a 12:12-h light-dark cycle, a greater proportion of total daily NREMS or REMS occurs during the light phase, whereas a greater proportion of total wakefulness occurs during the dark phase. Similarly, average Tbr is greater during the dark phase than during the light phase of the light-dark cycle.
During baseline recordings, there were no differences in the light-dark distribution of sleep-wake behavior or Tbr among rats subsequently randomized to the SHAM control group and those randomized to the CLP group (Fig. 5, A–D, baseline). Sham control surgeries had little effect on subsequent light-dark distributions of sleep-wake behavior or Tbr (Fig. 5), although there was a modest shift in the distribution of wakefulness of postsurgical day 4. In contrast, the light-dark distribution of sleep-wake behavior of rats subjected to CLP-sepsis was severely impacted (Fig. 5, A–C). On postsurgical days 2–4, the distribution of NREMS and REMS of septic rats shifted such that a greater proportion of these sleep phases occurred during the dark period (Fig. 5, B and C). Concurrently, the proportion of daily wakefulness exhibited by septic rats was greater during the light period compared with the distribution normally apparent (Fig. 5A). Average Tbr of septic rats across the 12-h dark period was reduced relative to control rats and to presurgical baseline values (Fig. 5D).
Fig. 5.
Sepsis alters the distribution of sleep and reduces the magnitude of diurnal changes in brain temperature. The proportion of time spent in wakefulness (A), NREMS (B), and REMS (C) is depicted for presurgical baseline values and for postsurgical days 2, 3, and 4. The proportionate behavior that occurs during the 12-h dark period, and the 12-h light period is shown for control rats (SHAM, open bars) and for septic rats (CLP, solid bars). Brain temperatures (D) are 12-h average temperatures during the dark period and the light period, and 36°C is arbitrarily defined as the “mid-point”. Within- and between-group statistical comparisons are presented *P ≤ 0.05 relative to control (SHAM) rats; #P ≤ 0.05 relative to presurgical baseline values.
Activity of Brain Stem Nuclei Involved in REMS Regulation
None of the rats subjected to sham surgery died prior to scheduled euthanization times. As such, n = 4 brains were obtained at each of the three experimental time points. In contrast, 6 out of 26 rats subjected to CLP surgery died before their scheduled death times, resulting in an n = 6 or 7 brains per time point. Because of the time- and labor-intensive nature of immunohistochemical assays and cell counting from multiple brain regions, a subset of n = 4 brains from the CLP-septic rats was randomly selected before tissue processing and included in this study.
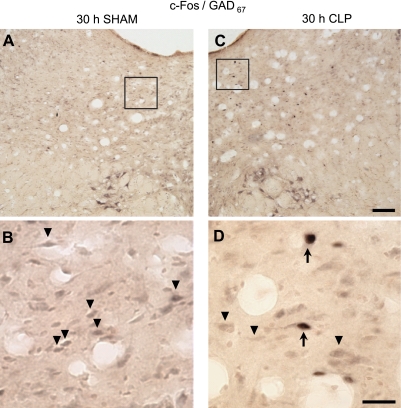
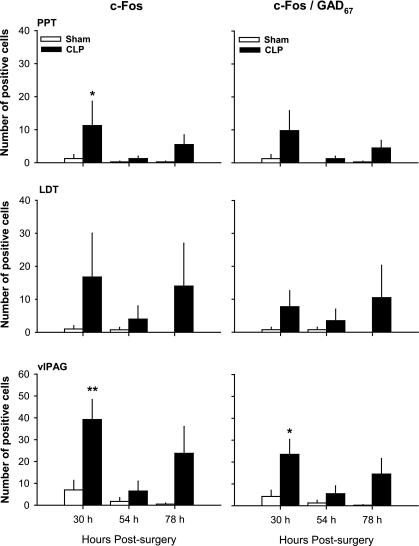
c-Fos+, ChAT+ and GAD67+ cells could be detected in tissue obtained from control (SHAM) and CLP-septic rats. Representative examples of GAD67-stained cells are given in Fig. 6. Although there was a tendency for the number of c-Fos+ cells to increase through the course of sepsis, statistical changes were revealed only in tissues taken from animals killed 30 h after sepsis onset. Thirty hours after surgery, the number of c-Fos+ cells increased statistically in PPT and vlPAG of septic rats compared with control rats (Fig. 7). Although there was variability across animals, most of the c-Fos+ neurons in PPT were GABAergic, being double stained for c-Fos/GAD67. However, the number of c-Fos+/GAD67+ cells did not differ statistically between CLP and control group in this nucleus (Fig. 7). In vlPAG, about 55% of the c-Fos+ neurons were GABAergic, and in this region, there was a statistical increase in the number of c-Fos+/GAD67+ double-stained cells in septic animals (Fig. 7). No c-Fos+/ChAT+ double-labeled neurons were found in tissue collected from any of the rats, irrespective of manipulation (data not shown).
Fig. 6.
Sepsis-induced alterations in activity of the brain stem GABAergic system are apparent in these representative tissue samples taken from a control rat (SHAM surgery; A and B) and a septic rat (CLP surgery; C and D). Both animals were killed 30 h after surgery. Sections contain the ventrolateral periaqueductal gray (vlPAG), and are approximately −7.04 mm from bregma (45). A and C: ×10 magnification, whereas the boxed insets (shown enlarged in B and D) are ×60 magnification. Examples of cells stained for glutamic acid decarboxylase-67 (GAD67) are indicated by black arrowheads, whereas examples of cells stained for both GAD67 and c-Fos are denoted by black arrows. Scale bars: C = 100 μm; D = 25 μm.
Fig. 7.
Sepsis alters activity of the brain stem GABAergic system. Presented are numbers of c-Fos+ neurons and c-Fos+/GAD67+ neurons in the pedunculopontine tegmental nucleus (PPT; top), laterodorsal tegmental nucleus (LDT; middle), and the ventrolateral periaqueductal grey (vlPAG; bottom) of rats after abdominal surgeries. Stereological counts were obtained from tissue in brains of rats killed at 30, 54, and 78 h after SHAM surgeries (open bars) or CLP surgeries (solid bars). Values are means ± SE from n = 4 animals per time point and condition. Statistically significant differences between groups are indicated as *P ≤ 0.05; **P ≤ 0.01.
DISCUSSION
Clinical reports suggest the brain may be one of the first organs affected by sepsis. For example, in a study designed to determine the effects of the intensive care unit (ICU) environment on sleep of patients, Freedman et al. (18) observed altered EEG patterns that preceded sepsis onset in patients who became septic during the recording period. Our present study demonstrates for the first time the impact of CLP-induced sepsis on EEG-defined sleep. Our results demonstrate that sleep and EEG patterns of laboratory rats are altered during sepsis. These sepsis-induced alterations in sleep include changes in the amount of sleep and in its diurnal distribution. In general, during the development of sepsis, the amount of time rats spend in NREMS increases during the dark (primarily active) period and is not dramatically altered during the light (primarily rest) period. NREMS during sepsis is fragmented and is associated with reductions in delta power during NREMS, suggesting that the quality of sleep is diminished. Furthermore, sepsis in rats may alter mechanisms of thermoregulation as septic animals exhibit damped diurnal rhythms of body temperature for at least 3 days after sepsis induction. We also demonstrate with an initial survey that the brain stem GABAergic system may contribute to the alterations in sleep during the early stages of this infectious challenge. Collectively, these results contribute to a modest yet growing literature that details the manner in which infections alter sleep and other CNS processes.
The pattern of changes in sleep, delta power during NREMS, and brain temperature suggests that CLP-induced sepsis impacts the circadian system. Visual inspection of the data indicates that these parameters do not exhibit diurnal variation, and essentially represent a “flat line” response to sepsis. As such, in rats the normal diurnal variation in sleep-wake behavior, EEG parameters, and brain temperature is not manifest during sepsis. These observations are consistent with other studies that describe similar effects on locomotor activity and body temperature of laboratory rodents in response to sepsis (13, 19). Sleep and body temperature are regulated by interactions between circadian timing systems and sleep-regulatory systems (17). The suprachiasmatic nucleus (SCN) of the hypothalamus is the primary circadian pacemaker that synchronizes many physiological processes and behaviors. The timekeeping apparatus of the SCN is a molecular mechanism that involves both transcriptional repressor genes, as well as transcriptional activators, which together create a feedback loop generating circadian oscillations (17). Cytokines such as IL-1 and TNF downregulate the expression of some of these clock genes (6, 11). Signaling receptors for IL-1 and TNF are distributed throughout the brain, including the hypothalamus (2, 5), and IL-1 signaling receptors are present in the SCN (6). As such, one possibility for the damped diurnal rhythmicity of the parameters reported in this study is the action of IL-1 and/or TNF, and possibly other inflammatory mediators, on neurons of the SCN. In addition to direct actions of cytokines on neurons in the SCN, the disruption to the normal diurnal rhythms for the parameters of interest in this study may also be due to endocrine responses to sepsis. CLP-induced sepsis alters rhythms of the hypothalamic-pituitary-adrenal axis (HPA) of rats (10), which is associated with increased immunoreactivity of corticotropin-releasing hormone in the hypothalamus (9). Cytokine actions in the brain are modulated via negative-feedback effects of corticosterone (56). Therefore, disruption of the normal rhythmicity of the HPA axis also may contribute to altered rhythms of sleep and brain temperature. Although few studies have demonstrated effects of sepsis on the SCN, data from our laboratory (unpublished) indicate that the number of c-Fos+ cells in the SCN increases during CLP-induced sepsis.
The impact of sepsis on NREMS of rats is most pronounced during the dark phase, whereas during the light phase the amount of time spent in NREMS does not appreciably differ from either presurgical baseline values or from values obtained from control rats subjected to sham surgeries. Rats are nocturnal animals, and the dark phase of the light-dark cycle is predominantly their active period. Although these data may be interpreted, such that sleep of rats during the light period represents something of a physiological ceiling, it is more likely that the relatively selective increases in NREMS during the dark period are due to the activation of overlapping systems that are involved in sleep regulation and in immune responses to infection. For example, there are diurnal rhythms of IL-1 and TNF mRNA and protein in healthy rat brain, with expression lower during the dark period than during the light period [reviewed by Opp (38)]. CLP-sepsis is characterized, in part, by increases in cytokines, notably IL-1, TNF, and IL-6 (13, 14, 19). Each of these cytokines is implicated in the regulation/modulation of NREMS. As such, sepsis-induced increases in sleep-regulatory cytokines during periods when their concentrations would normally be at a minimum are expected to increase NREMS. We have not measured cytokine concentrations in septic animals from samples taken during the dark period and are not aware of others, who may have done so. Therefore, although it is likely that sepsis-induced increases in cytokine concentrations during the dark phases are responsible for the increased NREMS observed during these time periods, such findings remain to be definitively demonstrated.
Administration of cytokines, bacterial LPS, or infection with bacterial, viral, or fungal pathogens is consistently reported to suppress REMS of laboratory animals [reviewed by Opp (38)]. This study indicates that sepsis also suppresses REMS of rats, but the effects may be more complex than those that occur in response to other types of infections or immune challenges. REMS of septic rats is suppressed for about 24 h following CLP surgery. After this time, REMS increases during the subsequent dark phases for at least three nights. On the basis of previous studies of immune challenge on sleep of laboratory animals, these increases in REMS of septic rats are unexpected. Mechanisms underlying the interaction between cytokines and the regulation of REMS are as yet not well understood. Of interest, recent studies of the bidirectional interactions between the CNS and peripheral inflammation have focused on the role of the cholinergic system (55). It is now known that macrophages and other nonneuronal cell types that produce cytokines also express ACh receptors (59). Central activation of ACh receptors, particularly muscarinic receptors, dose-dependently suppresses circulating IL-1 and TNF through mechanisms involving vagal nerve efferents and the α7 subunit of nicotinic receptors on macrophages and other cells (42, 43). Although subdiaphragmatic transection of the vagus nerve does not alter the amount of time rats spend in REMS (21, 40), it does alter the EEG (40), indicating that vagal afferents contribute to properties of the cortical EEG. In addition, vagal nerve stimulation attenuates stimulated cytokine production (47), improves survival of mice subjected to CLP (47), and increases the amount of time cats spend in REMS (57, 58). Although much remains to be learned, these intriguing studies demonstrate that interactions of the central cholinergic network and the vagus nerve not only contribute to the regulation of peripheral cytokine responses, but also to CNS processes, including sleep-wake behavior.
The neural substrates involved in REMS regulation have been extensively investigated for many years [reviewed by Jones (26) and McCarley (35)]. Cholinergic cell populations involved in REMS initiation are found in the LDT and PPT nuclei (31). GABAergic neurons located in the vlPAG project to REMS-regulating brain stem nuclei, including LDT/PPT. These vlPAG GABAergic neurons have been suggested to play a role in REMS regulation because lesions of the vlPAG increase REMS (30). As such, activation of these GABAergic neurons is expected to suppress REMS. Our immunohistochemical analyses indicate that the number of c-Fos+/GAD67+ neurons in vlPAG increases 30 h after CLP surgeries, but not after control surgeries. These results suggest that the GABAergic system in vlPAG is activated early in the development of sepsis. Additional studies are necessary to determine whether activation of central cholinergic inflammatory pathways and circulating cytokines play roles that are complementary to activation of brain stem GABAergic systems in the alterations in REMS of rats observed in these experiments.
Of the sleep-associated parameters quantified in this study, sepsis had the greatest impact on the EEG. The impact of sepsis on the EEG is evidenced by changes in delta power during NREMS, which was dramatically reduced in septic animals irrespective of whether the amount of time spent in NREMS differed from baseline control values. That is, delta power during NREMS is reduced during periods when NREMS duration is essentially normal and during periods when NREMS duration is increased. Because delta power during NREMS is widely regarded as an indicator of the depth or quality of sleep (8), these data suggest that sleep of septic animals is qualitatively poor compared with their baseline sleep prior to infection, or relative to the sleep of SHAM-operated control rats. The dissociation between the nocturnal increase in NREMS duration and the prolonged reduction in delta power during NREMS of septic animals could be interpreted as an attempt to compensate for poor quality sleep by spending more time in NREMS. Alternatively, cytokine actions on the EEG could reflect direct neural effects that are independent of the quality or quantity of sleep. The mechanisms through which cytokines influence delta power during NREMS are complex and not fully understood. Early studies (28, 39, 49, 52) demonstrate that cytokines alter EEG parameters and that these effects depend on the dose, time, and route of administration. For example, central administration of TNF enhances delta power during NREM sleep (50, 51), whereas intraperitoneal administration increases NREM sleep duration without a concomitant increase of EEG delta power (15, 16, 27, 51). IL-1 increases EEG delta power at doses too low to alter NREMS duration (39, 52). Moreover, both IL-1 and TNF enhance EEG delta power in a dose-dependent manner when locally applied to the surface of the cerebral cortex but do not alter NREM sleep duration under these conditions (51, 61, 62). Of importance to this present study, some doses of IL-1 administered directly into rat brain reduce EEG delta power, even when NREMS duration is enhanced (28, 39). These findings of the impact of IL-1 on EEG power spectra in rats are reminiscent of the suppressed NREMS delta power during periods of enhanced NREMS duration of septic rats in this study.
Collectively, these previous studies demonstrate that the EEG is highly sensitive to cytokine actions. Furthermore, these previously reported data of cytokine actions on the EEG provide a potential mechanistic basis for the hypothesis that the brain is an early responder to septic insult. Data derived from this current study also support the hypothesis that the EEG may serve as a predictor of sepsis onset, an electroencephalographic biomarker. Although we cannot mimic all facets of the manner in which sepsis develops in patients in the ICU, our data do indicate that the EEG is altered within a few hours of the surgical manipulation to induce sepsis. The reduction in delta power during NREMS is not a byproduct of the surgical procedure per se, or anesthesia, because EEG delta power during NREMS of control rats subjected to sham surgeries is not altered. Furthermore, the suppression of EEG delta power during NREMS of septic rats does not normalize during the 3 days following the surgical manipulation, long after effects of anesthesia and the surgical incision are past. We can only conclude that polymicrobial infection in the periphery has dramatic effects on the cortical EEG of rats during the infection. Whether such changes in the EEG persist for periods longer than the 84 h recording postsurgery in this study remains to be determined.
Infection with bacteria, viruses, or fungus alters sleep of laboratory animals and humans [reviewed by Opp and Toth (41)]. For example, previous experimental studies have determined the impact of infection with selected bacterial pathogens (e.g., Staphylococcus aureus, Streptococcus pyogenes, and Escherichia coli) on sleep of laboratory rabbits. NREMS of rabbits inoculated intravenously with S. aureus (53), S. pyogenes, or E. coli (54) increases for periods ranging from 2 to 24 h, whereas REMS is suppressed for 2–28 h, depending on the pathogen. In addition to changes in the amount of time spent in NREMS or REMS, the quality of sleep in rabbits infected with these pathogens is diminished, as evidenced by reductions in delta power during NREMS. In these studies, positive blood cultures were evident in most rabbits inoculated with S. aureus, S. pyogenes, or E. coli, indicating septicemia. Therefore, laboratory rabbits made septic by intravenous inoculation of selected bacterial pathogens may exhibit alterations in sleep that are generally of shorter duration than those of rats made septic by CLP. In rats, infection following CLP results from a mixed population of enteric bacteria, with blood cultures positive for multiple bacterial species (36). Whether or not the difference between rabbits and rats in altered sleep during infection is attributable to species differences or to the polymicrobrial nature of sepsis in rats induced by CLP is not known.
As with any study in which an animal model is used to mimic the human condition, there are limitations to this study. From a technical standpoint, the time- and labor-intensive nature of immunohistochemical assessment of the impact of sepsis on multiple brain regions necessitated selecting relatively few time points during the development of sepsis at which to euthanize animals. Ongoing studies of rats and mice aim to further elucidate mechanisms and mediators responsible for the impact of sepsis on the brain. In addition, physical characteristics of brains from septic rats are altered, such that the tissue is difficult to section. Importantly, from a clinical perspective, because of the heterogeneous nature of sepsis in the ICU, we are unable to mimic all aspects of sepsis onset in patients using this model. Nevertheless, this present study advances our knowledge of how sleep of laboratory animals is altered during infection by focusing on a polymicrobial infection that mimics many of the clinical facets of sepsis in patients.
Perspectives and Significance
This study demonstrates that CLP-induced sepsis dramatically affects mechanisms involved in the regulation of sleep, EEG, and brain temperature. These conclusions are made on the basis of sepsis-induced alterations in the amount of time spent in each behavioral state, the severe fragmentation of the sleep-wake cycle, and the damping of diurnal rhythms of sleep and brain temperature. Limited clinical data suggest that the EEG is altered prior to sepsis onset (18). We cannot mimic the onset of sepsis as might occur in the ICU, but the marked reduction in delta power during NREMS of septic rats supports the notion that sepsis alters the EEG in a manner that may serve as an indicator of pending sepsis onset. Of significance, early intervention is still the most effective management strategy for improving sepsis outcomes. Monitoring in the ICU perhaps could be used as a method to detect alterations in the EEG that may be characteristic of impending sepsis. Such a “warning” that sepsis is eminent could prove to be a valuable tool in sepsis management. Finally, although the protocol used in this study does not allow us to determine the complete duration of sepsis-induced alterations in CNS processes, observations from clinical studies suggest there are long-term consequences of sepsis on cognitive function. For example, patients surviving severe sepsis suffer from new, persistent, and dramatic cognitive impairment or global loss of cognitive function after discharge (25). Preclinical data indicate that CLP sepsis alters cognitive function of rats as assessed by an inhibitory avoidance task for at least 30 days (3, 4). Collectively, these clinical observations and data obtained in this present study indicate that additional effort is warranted to elucidate mechanisms underlying the complex relationships between traumatic infection and multiple facets of CNS function.
GRANTS
These studies were supported by the National Institutes of Health (HL080972) and the Department of Anesthesiology at the University of Michigan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The technical assistance of Jamie Bowmar, Jill Priestly, Sara Waugh, Cailin Wilson, and Amrita George is greatly appreciated.
Present address for A. M. Ingiosi, R. M. Raymond, Jr., and M. R. Opp: Department of Anesthesiology and Pain Medicine, University of Washington Medical School, Seattle, WA 98104.
REFERENCES
- 1.Angus D, Linde-Zwirble W, Lidicker J, Clermont G, Carcillo J, Pinsky M. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Ban EM. Interleukin-1 receptors in the brain: characterization by quantitative in situ autoradiography. Immunomethods 5: 31–40, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F. Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med 33: 221–223, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F. Long-term cognitive impairment in sepsis survivors. Crit Care Med 33: 1671, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Bette M, Kaut O, Schafer MK, Weihe E. Constitutive expression of p55TNFR mRNA and mitogen-specific up-regulation of TNF alpha and p75TNFR mRNA in mouse brain. J Comp Neurol 465: 417–430, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Beynon AL, Coogan AN. Diurnal, age, and immune regulation of interleukin-1beta and interleukin-1 type 1 receptor in the mouse suprachiasmatic nucleus. Chronobiol Int 27: 1546–1563, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Bone RC. Toward an epidemiology and natural history of SIRS (Systemic Inflammatory Response Syndrome). JAMA 268: 3452–3455, 1992 [PubMed] [Google Scholar]
- 8.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms 14: 557–568, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Carlson DE, Chiu WC, Fiedler SM, Hoffman GE. Central neural distribution of immunoreactive Fos and CRH in relation to plasma ACTH and corticosterone during sepsis in the rat. Exp Neurol 205: 485–500, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson DE, Chiu WC, Scalea TM. Cecal ligation and puncture in rats interrupts the circadian rhythms of corticosterone and adrenocortical responsiveness to adrenocorticotrophic hormone. Crit Care Med 34: 1178–1184, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci USA 104: 12843–12848, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun 67: 6603–6610, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebong SJ, Call DR, Bolgos G, Newcomb DE, Granger JI, O'Reilly M, Remick DG. Immunopathologic responses to non-lethal sepsis. Shock 12: 118–126, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFα treatment. J Neurosci 17: 5949–5955, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang J, Wang Y, Krueger JM. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol Regul Integr Comp Physiol 274: R655–R660, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur J Neurosci 29: 1820–1829, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med 163: 451–457, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Gourine AV, Rudolph K, Tesfaigzi J, Kluger MJ. Role of hypothalamic interleukin-1β in fever induced by cecal ligation and puncture in rats. Am J Physiol Regul Integr Comp Physiol 275: R754–R761, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Hamed SA, Hamed EA, Abdella MM. Septic encephalopathy: relationship to serum and cerebrospinal fluid levels of adhesion molecules, lipid peroxides and S-100B protein. Neuropediatrics 40: 66–72, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Hansen MK, Krueger JM. Subdiaphragmatic vagotomy does not block sleep deprivation-induced sleep in rats. Physiol Behav 64: 361–365, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12: 123–137, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Imeri L, Bianchi S, Opp MR. Antagonism of corticotropin-releasing hormone alters serotonergic-induced changes in brain temperature, but not sleep, of rats. Am J Physiol Regul Integr Comp Physiol 289: R1116–R1123, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci 10: 199–210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304: 1787–1794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones BE. Paradoxical REM sleep promoting and permitting neuronal networks. Arch Ital Biol 142: 379–396, 2004 [PubMed] [Google Scholar]
- 27.Kubota T, Li N, Guan Z, Brown RA, Krueger JM. Intrapreoptic microinjection of TNF-alpha enhances non-REM sleep in rats. Brain Res 932: 37–44, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Lancel M, Mathias S, Faulhaber J, Schiffelholz T. Effect of interleukin-1β on EEG power density during sleep depends on circadian phase. Am J Physiol Regul Integr Comp Physiol 270: R830–R837, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31: 1250–1256, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature 441: 589–594, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Maloney KJ, Mainville L, Jones BE. c-Fos expression in GABAergic, serotonergic, and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci 20: 4669–4679, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maloney KJ, Mainville L, Jones BE. c-Fos expression in dopaminergic and GABAergic neurons of the ventral mesencephalic tegmentum after paradoxical sleep deprivation and recovery. Eur J Neurosci 15: 774–778, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Martin CM, Priestap F, Fisher H, Fowler RA, Heyland DK, Keenan SP, Longo CJ, Morrison T, Bentley D, Antman N. A prospective, observational registry of patients with severe sepsis: the Canadian Sepsis Treatment and Response Registry. Crit Care Med 37: 81–88, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546–1554, 2003 [DOI] [PubMed] [Google Scholar]
- 35.McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med 8: 302–330, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Nemzek JA, Hugunin KM, Opp MR. Modeling sepsis in the laboratory: merging sound science with animal well-being. Comp Med 58: 120–128, 2008 [PMC free article] [PubMed] [Google Scholar]
- 37.Newcomb D, Bolgos G, Green L, Remick DG. Antibiotic treatment influences outcome in murine sepsis: mediators of increased morbidity. Shock 10: 110–117, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Opp MR. Cytokines and sleep. Sleep Med Rev 9: 355–364, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Opp MR, Obál F, Krueger JM. Interleukin-1 alters rat sleep: temporal and dose-related effects. Am J Physiol Regul Integr Comp Physiol 260: R52–R58, 1991 [DOI] [PubMed] [Google Scholar]
- 40.Opp MR, Toth LA. Somnogenic and pyrogenic effects of interleukin-1β and lipopolysaccharide in intact and vagotomized rats. Life Sci 62: 923–936, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Opp MR, Toth LA. Neural-immune interactions in the regulation of sleep. Front Biosci 8: d768–d779, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al Abed Y, Tracey KJ. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci USA 103: 5219–5223, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, Tracey KJ. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun 23: 41–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 5th ed Burlington, MA: Elsevier Academic, 1986 [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 5th ed Burlington, MA: Elsevier Academic Press, 1986 [Google Scholar]
- 46.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4: 31–36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med 265: 663–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakr Y, Vincent JL, Ruokonen E, Pizzamiglio M, Installe E, Reinhart K, Moreno R. Sepsis and organ system failure are major determinants of post-intensive care unit mortality. J Crit Care 23: 475–483, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol Regul Integr Comp Physiol 253: R142–R149, 1987 [DOI] [PubMed] [Google Scholar]
- 50.Takahashi S, Fang J, Kapás L, Wang Y, Krueger JM. Inhibition of brain interleukin-1 attenuates sleep rebound after sleep deprivation in rabbits. Am J Physiol Regul Integr Comp Physiol 273: R677–R682, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Takahashi S, Kapás L, Seyer JM, Wang Y, Krueger JM. Inhibition of tumor necrosis factor attenuates physiological sleep in rabbits. NeuroReport 7: 642–646, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Tobler I, Borbély AA, Schwyzer M, Fontana A. Interleukin-1 derived from astrocytes enhances slow wave activity in sleep EEG of the rat. Eur J Pharmacol 104: 191–192, 1984 [DOI] [PubMed] [Google Scholar]
- 53.Toth LA, Krueger JM. Alterations of sleep in rabbits by Staphylococcus aureus infection. Infect Immun 56: 1785–1791, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toth LA, Krueger JM. Effects of microbial challenge on sleep in rabbits. FASEB J 3: 2062–2066, 1989 [DOI] [PubMed] [Google Scholar]
- 55.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117: 289–296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turnbull AV, Rivier C. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev 79: 1–71, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Valdes-Cruz A, Magdaleno-Madrigal VM, Martinez-Vargas D, Fernandez-Mas R, Almazan-Alvarado S. Long-term changes in sleep and electroencephalographic activity by chronic vagus nerve stimulation in cats. Prog Neuropsychopharmacol Biol Psychiatry 32: 828–834, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Valdes-Cruz A, Magdaleno-Madrigal VM, Martinez-Vargas D, Fernandez-Mas R, Almazan-Alvarado S, Martinez A, Fernandez-Guardiola A. Chronic stimulation of the cat vagus nerve: effect on sleep and behavior. Prog Neuropsychopharmacol Biol Psychiatry 26: 113–118, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Waldburger JM, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS. Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis Rheum 58: 3439–3449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med 31: 2316–2323, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Yasuda T, Yoshida H, Garcia-Garcia F, Kay D, Krueger JM. Interleukin-1beta has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity. Sleep 28: 177–184, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Yoshida H, Peterfi Z, Garcia-Garcia F, Kirkpatrick R, Yasuda T, Krueger JM. State-specific asymmetries in EEG slow wave activity induced by local application of TNFα. Brain Res 1009: 129–136, 2004 [DOI] [PubMed] [Google Scholar]