Abstract
Glucose uptake across the sarcolemma is regulated by a family of membrane proteins called glucose transporters (GLUTs), which includes GLUT4 (the major cardiac isoform) and GLUT12 (a novel, second insulin-sensitive isoform). Potential regional patterns in glucose transport across the cardiac chambers have not been examined; thus, we hypothesized that insulin-responsive GLUT4 and -12 protein and gene expression would be chamber specific in healthy subjects and during chronic heart failure (HF). Using a canine model of tachypacing-induced, progressive, chronic HF, total GLUT protein and messenger RNA in both ventricles and atria (free wall and appendage) were investigated by immunoblotting and real-time PCR. In controls, GLUT4, but not GLUT12, protein content was significantly higher in the atria compared with the ventricles, with the highest content in the right atrium (RA; P < 0.001). GLUT4 and GLUT12 mRNA levels were similar across the cardiac chambers. During chronic HF, GLUT4 and GLUT12 protein content was highest in the left ventricle (LV; by 2.5- and 4.2-fold, respectively, P < 0.01), with a concomitant increase in GLUT4 and GLUT12 mRNA (P < 0.001). GLUT4, but not GLUT12, protein content was decreased in RA during chronic HF (P = 0.001). In conclusion, GLUT4 protein was differentially expressed across the chambers in the healthy heart, and this regional pattern was reversed during HF. Our data suggest that LV was the primary site dependent on both GLUT4 and GLUT12 during chronic HF. In addition, the paradoxical decrease in GLUT4 content in RA may induce perturbations in atrial energy production during chronic HF.
Keywords: cardiac metabolism, GLUT4, GLUT12, polymerase chain reaction, canine model
heart failure (HF) is a complex syndrome that develops over months to years, and is characterized by structural, functional, and bioenergetic derangements of the heart, which progressively loses its ability to adequately pump blood and to meet the hemodynamic requirements of the body. Since the rate of glucose utilization in the heart is greater than in other tissues, impaired substrate utilization, as a result of reduced glucose uptake and/or oxidation, could be a major contributing pathophysiologic factor of contractile dysfunction in HF, and metabolic therapy has emerged as a promising new avenue for the treatment of HF (16). However, while it is usually well accepted that fatty acid utilization is decreased during nonischemic dilated cardiomyopathy, a switch of substrate selection toward glucose oxidation remains controversial, as several studies reported increased, unchanged, or decreased glucose uptake in the failing heart (12, 13, 16, 17, 20, 23, 26, 31). In particular, there is recent evidence suggesting that chronic HF may promote metabolic changes, such as insulin resistance resulting in decreased glucose uptake and utilization (1, 17, 37). However, the majority of these studies used experimental large animal models to evaluate myocardium metabolism after short-term (i.e., 2–4 wk) tachypacing, whereas cardiomyopathy develops over months to years in humans (18). Finally, the regulation of myocardial glucose transport during HF, which occurs by a family of membrane proteins called glucose transporters (GLUTs), is not well elucidated. Although GLUT4 is the major cardiac isoform and is responsible for insulin-stimulated glucose transport, there is new evidence that GLUT12 represents a novel, second insulin-sensitive GLUT (8, 22, 27, 32). However, its role is unknown in the heart.
The majority of the mentioned studies have solely investigated the global or left ventricular changes in cardiac energetics (6, 13, 17, 20, 26). Since HF is characterized by both global and local dyssynchronous contraction, one could argue that these regional alterations in contraction and flow distribution could induce regional changes in substrate metabolism, including glucose transport, which could, in turn, contribute to the overall cardiac functional impairment (13). In addition, although cellular energetic deficits have been reported in the atrial myocardium of subjects with chronic atrial fibrillation associated with HF, the role of glucose uptake and utilization in the atria has received little attention.
Despite the crucial role of glucose utilization in the heart and regional differences in myocardial structure and function (14, 29), the potential regional pattern in the regulation of glucose transport in the healthy myocardium and during chronic HF has not been investigated. Since glucose entry across the sarcolemma is regulated by GLUT, we hypothesized that 1) insulin-responsive GLUT4 and -12 protein expression will be chamber specific in the healthy and in the failing myocardium; 2) GLUT4 and -12 protein and mRNA expression will be altered during chronic HF. To test our hypothesis, we used our well-established canine model of chronic nonischemic dilated cardiomyopathy, which reproduces many aspects of chronic-progressive human HF.
MATERIALS AND METHODS
Canine model of chronic nonischemic cardiomyopathy.
Five adult beagle dogs (1–2 yr old) were chronically instrumented with modified Prevail 8086 pacemakers (Medtronic, Minneapolis, MN) with the pacing lead (model 4092; Medtronic) placed in the right ventricular apex. Following recovery from the pacemaker implant, pacing was performed at 180 beats/min for 2 wk, 200 beats/min for 6 wk, and 180 beats/min for 8 wk, to achieve and maintain stable left ventricular dysfunction. Serial echocardiographic measurements of the left ventricle (LV) and atrium were performed at baseline and at the end of the pacing period (with the pacemaker briefly deactivated for data acquisition to record under normal sinus rhythm conditions). Traditional two-dimensional and M-mode images were obtained on sedated dogs (0.5 mg/kg butorphonol im) by using a GE Vivid-7 echocardiograph system and a 3-MHz sector transducer. To measure atrial contractility, two-dimensional parasternal long-axis views were obtained to measure left atrial dimension and to calculate fractional area change (fractional area change = area end-systole − area end-diastole/area end-systole). Cardiac troponin C and troponin I were previously measured to rule out ischemia in this model (18). Ten age-matched beagle dogs served as controls for this study.
All procedures were approved by the Ohio State University Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Measurement of GLUT protein expression in crude membranes by Western immunoblot analysis.
Collected samples from healthy and failing canine myocardium, which had been flash frozen at the time of euthanasia and stored at −80°C, included: LV, right ventricle (RV), left atrial free wall (LAFW), right atrial free wall (RAFW), left atrial appendage (LAA), and right atrial appendage (RAA). Crude membranes were obtained from these samples, as previously described (10, 11). Briefly, frozen tissue samples (30 to 60 mg) were homogenized in 4 ml of homogenizing buffer of 210 mM sucrose, 40 mM NaCl, 2 mM ethylene glycol-bis [2-aminoethylether] N,N,N′,N′-tetraacetic acid, 30 mM HEPES (pH, 7.4), and protease inhibitor (Sigma, St. Louis, MO) by use of a polytron fitted with a generator. The homogenate was mixed with 3 ml of 58.3 mM sodium pyrophosphate and 1.17 mM KCl and stored on ice for 15 min. Crude membranes were then recovered by centrifugation at 100,000 g for 90 min at 4°C. Pellets were resuspended in 90 μl/mg of initial cardiac muscle with 10 mM Tris·HCl and 1 mM EDTA (pH, 7.4). Next, 16% SDS was added at 0.33 × total volume, samples were centrifuged at 3,000 g for 25 min, and the supernatant was stored at −80°C. Equal amounts of protein were subjected to SDS polyacrylamide gel electrophoresis, electrophoretically transferred to a polyvinylidene fluoride membrane with subsequent immunoblotting. Membrane proteins were incubated with a rabbit polyclonal antibody directed against the last 12 COOH-terminal amino acids of GLUT4 (AbD Serotec, Raleigh, NC) or with a rabbit polyclonal antibody directed against the 16 COOH-terminal amino acids of human GLUT12 protein (generated in Dr. Rogers' laboratory and validated for the detection of canine GLUT12 protein) (27), with subsequent appropriate secondary antibodies conjugated to horseradish peroxidase (GE Healthcare, Buckinghamshire, UK) as previously described (10, 11, 27, 36). Protein bands were visualized by enhanced chemiluminescence (KPL, Gaithersburg, MD) and quantified using Gel-Pro Analyzer blot scanning and analysis system (Media Cybernetics, Bethesda, MD). LV and/or the matched sample sites of a control dog were used as an internal control for control and HF samples, respectively; and the densities of the bands were expressed as relative units (%). Equal protein loading was confirmed by measuring calsequestrin protein expression on each membrane (Abcam, Cambridge, MA), since it has been previously shown that its expression remains constant throughout the regions of the heart of healthy subjects and during HF (2, 7). To confirm the specificity of our GLUT12 antibody, we also performed competitive binding of our antibody to the peptide to which the antibody was raised (data not shown).
RNA isolation and quantitative real-time PCR.
mRNA was analyzed in the different cardiac regions of control and HF dogs with the exception of the LAA, which was not available for the majority of the affected dogs. Following RNA isolation (Trizol reagent; Invitrogen, Carlsbad, CA) and quantification (Nanodrop; Thermo Scientific, DE), DNA contamination was removed using DNase I amplification grade (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. DNase-treated RNA was then converted to cDNA using Superscript II Reverse Transcriptase [with oligo(dT) as primer], an engineered version of the M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA), according to the manufacturer's recommendations.
Quantitative real-time PCR was performed to measure GLUT4 and GLUT12 gene expression in the healthy and failing myocardium, by using relative quantification. GLUT primers and potential housekeeping primers were identified using previously used canine sequences and from identified gene sequences (GenBank) (see Table 1) (3, 12).The stability of four candidate housekeeping genes (β-2-microglobulin, glyceraldehyde-3-phosphate dehydrogenase, ribosomal protein 5, and ribosomal protein 19) across the different cardiac regions was analyzed with the GeNorm software (PrimerDesign), and the ribosomal protein 19 (RPS19) gene was selected as a normalization control. The amplification was performed using SYBR Green Real-Time PCR Master Mix containing AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA). Each reaction contained 2× reaction buffer (SYBR Green I Dye, Amplitaq Gold DNA Polymerase, dNTPs with dUTP, passive reference, and optimized buffer components), forward and reverse primers (0.5 mM), 0.5 μg of cDNA, and DNase- RNase-free water up to 20 μl. Samples were run in triplicate in a Stratagene (La Jolla, CA) Mx3000P QPCR System in 96-well MicroAmp optical plates (Applied Biosystems). The PCR program used consisted of one cycle of 15 min at 95°C, followed by 40 cycles of 20 s at 95°C, 30 s at 56°C, and 10 s at 72°C. A melting curve was then performed to ensure product purity and the absence of primer dimers. Relative quantification was performed using RPS19 as the housekeeping gene, and data were normalized using an efficiency calculation (21) and were subsequently expressed as percentage of the control LV site.
Table 1.
Primer sequences used in real-time PCR
| Gene | Primer | Sequence 5′-3′ | Tm (°C) |
|---|---|---|---|
| GLUT4 | Forward | 5′-GCTTTGTGGCCTTCTTTGAG-3′ | 60°C |
| Reverse | 5′-CCTCCGCGATATACTGGAAA-3′ | ||
| GLUT12 | Forward | 5′-CTTTTTGTTGTTATGTTTATACCTGAGGAC-3′ | 60°C |
| Reverse | 5′-GGCACTAATTCTTCTTGGTGATGA-3′ | ||
| RPS19 | Forward | 5′-CCTTCCTCAAAAA/GTCTGGG-3′ | 59°C |
| Reverse | 5′-GTTCTCATCGTAGGGAGCAAG-3′ |
Tm, temperature; GLUT, glucose transporter; RPS19, ribosomal protein 19. Note: if a primer is located on two exons, the junctions are shown with a dividing forward slash (/).
Statistical analysis.
Values for the in vivo and in vitro variables were examined for normality (K-S test) and homogeneity of variances (Levene's test). Paired t-tests were performed for in vivo measurements, and one-way repeated-measure ANOVA, and t-test were performed for in vitro measurements as appropriate. Statistical significance was defined as P ≤ 0.05. All results are reported as means ± SE.
RESULTS
Animal model.
All animals completed the 4-mo period of RV tachypacing without any premature death or technical failure. Four months of RV tachypacing induced dilated cardiomyopathy, manifested by: 1) reduced LV systolic function, as evidenced by the decreased fractional shortening (P < 0.001); and 2) left ventricular dilatation, as evidenced by the increased ventricular internal diameter and volume (P < 0.05, Table 2). At the end of the pacing period, there was a tendency (P = 0.07) for the fractional area change of left atria, a measure of LA emptying volume, to be decreased in the HF group.
Table 2.
Echocardiographic parameters and hemodynamic parameters in dogs with tachypacing-induced, chronic-progressive heart failure
| Baseline | Chronic Heart Failure | |
|---|---|---|
| Left ventricle | ||
| IVSd, cm | 0.81 ± 0.04 | 0.62 ± 0.04† |
| IVSs, cm | 1.23 ± 0.11 | 0.75 ± 0.03† |
| LVPWd | 0.83 ± 0.01 | 0.66 ± 0.03* |
| LVPWs | 1.22 ± 0.03 | 0.92 ± 0.05* |
| LVIDd, cm | 2.83 ± 0.19 | 3.88 ± 0.43* |
| LVIDs, cm | 1.61 ± 0.15 | 3.19 ± 0.41† |
| EDV, ml | 31.35 ± 5.2 | 70.17 ± 17.91** |
| ESV, ml | 7.71 ± 1.8 | 47.03 ± 13.38* |
| FS, % | 43.46 ± 2.4 | 18.47 ± 1.6† |
| LV mass | 57.37 ± 7.7 | 70.09 ± 15.1 |
| Atria | ||
| LA area-ED, cm2 | 4.4 ± 0.38 | 6.2 ± 1.51 |
| LA area-ES, cm2 | 6.2 ± 0.48 | 8.1 ± 1.7 |
| LA FAC, % | 29.19 ± 0.91 | 25.21 ± 2.45 |
Values are means ± SE. IVSd, interventricular septal dimension-diastole; IVSs, interventricular septal dimension-systole; LVPWd, left ventricular posterior wall dimension-diastole; LVPWs, left ventricular posterior wall dimension-systole; LVID-d, left ventricular internal diameter-diastole; LVID-s, left ventricular internal diameter-systole; EDV, end-diastolic volume; ESV, end-systolic volume; FS, fractional shortening; LA, left atrium; ED, end-diastole; ES, end-systole; FAC, fractional area change.
P ≤ 0.05 versus baseline values.
P ≤ 0.01.
P = 0.052.
GLUT protein expression in the healthy and failing myocardium.
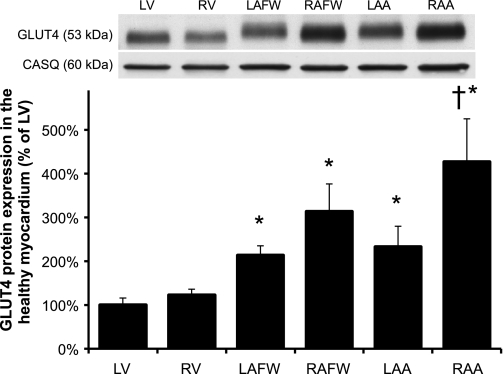
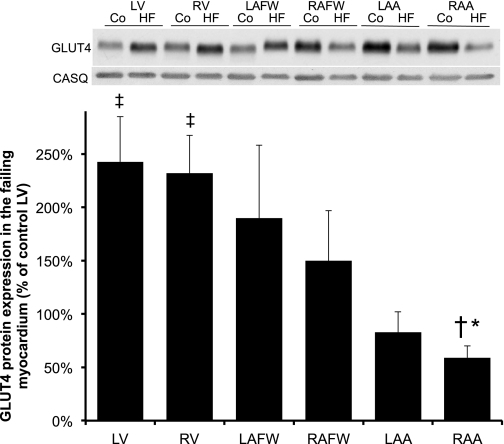
In healthy dogs (n = 10), total GLUT4 protein content was significantly higher in the atria compared with both ventricles, with the highest content present in the RA (increased by ∼3- and ∼4-fold in the RAFW and RAA, respectively, compared with the LV, P < 0.05, Figure 1). During HF, there was a statistically significant interaction between regional and treatment factors (P < 0.001, Fig. 2). The regional pattern of GLUT4 expression observed in the healthy myocardium was reversed during chronic HF, with a significant (P < 0.001) increase in total GLUT4 protein content observed in both failing ventricles compared with the control LV (P < 0.01), as well as to their matched control chambers (by 2.5- and 1.7-fold in LV and RV, P < 0.001 and P = 0.005, respectively, data not shown). In contrast, GLUT4 protein expression was decreased in the RA of the failing myocardium, with the lowest values of all cardiac regions seen in the RAA (decrease by two- and fourfold in RAFW and RAA when compared with their matched control site). In addition, there is a trend toward a reduction of total GLUT4 protein content in the LAA of the failing myocardium when compared with the control LV (P = 0.054).
Fig. 1.
Differential regional expression of glucose transporter-4 (GLUT4) protein in healthy myocardium. Top: Western blot of cardiac crude membranes. Top and bottom bands showed a representative immunoblot for GLUT4 and calsequestrin (CASQ) from the same membrane, respectively. Bottom: mean ± SE of total GLUT4 content of the myocardium of healthy dogs (n = 10). Density of the bands was expressed as % of each control left ventricle (LV) site. RV, right ventricle; LAFW, left atrial free wall; RAFW, right atrial free wall; LAA, left atrial appendage; RAA, right atrial appendage. *P ≤ 0.05 vs. LV; †P ≤ 0.05 vs. RV values.
Fig. 2.
Chronic-progressive heart failure (HF) induced alteration in GLUT4 protein expression throughout all cardiac regions. Top: Western blot of cardiac muscles from control (Co) and HF subjects. Top and bottom bands showed a representative immunoblot for GLUT4 (53 kDa) and its corresponding CASQ (60 kDa), respectively. Bottom: mean ± SE of total GLUT4 protein content in dogs with chronic-progressive HF (n = 5). Values represent the content normalized to each respective cardiac chamber. *P ≤ 0.05 vs. failing LV; ‡P ≤ 0.05 vs. control LV; †P ≤ 0.05 vs. failing RV.
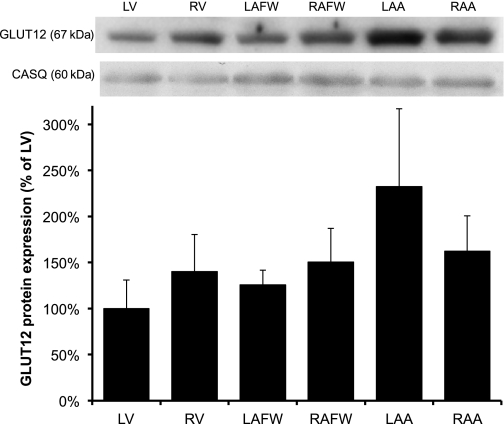
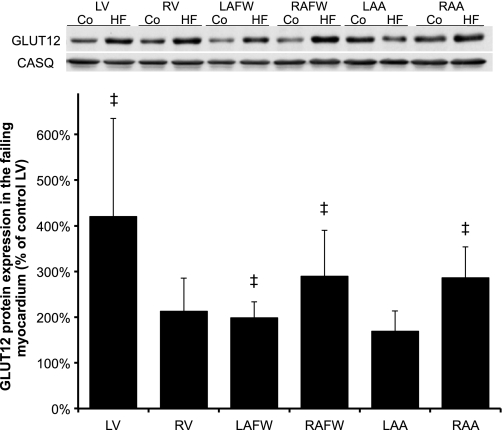
GLUT12 protein expression was similar across the cardiac chambers in the control group, although there was a tendency for total GLUT12 protein content to be lower in LV (P = 0.07) compared with the atria (Fig. 3). We observed a significant (P < 0.001) change during chronic HF, such as GLUT12 protein expression was primarily increased in the failing LV (by 4.2-fold, P < 0.001), as well as in both atria during HF compared with the control LV (between 2- and 2.9-fold in LAFW, RAFW, and RAA, respectively, P < 0.05, Fig. 4).
Fig. 3.
GLUT12 protein expression was similar across cardiac regions in the healthy myocardium. Top: Western blot of cardiac crude membranes. Bottom: mean ± SE of total GLUT12 content of the myocardium in healthy dogs (n = 6). Values represent the content normalized to LV.
Fig. 4.
Chronic HF selectively induced differential expression of GLUT12 protein content across the cardiac chambers. Top: Western blot of cardiac crude membranes in healthy and failing myocardium. Bottom: mean ± SE of total GLUT12 protein content of the myocardium during chronic-progressive HF (n = 5). Values represent the content normalized to each respective control cardiac chamber. ‡P ≤ 0.05 vs. control LV.
We found no statistical differences in calsequestrin protein content across cardiac regions in the control and HF groups, confirming equal protein loading (data not shown).
GLUT gene expression in the healthy and failing myocardium.
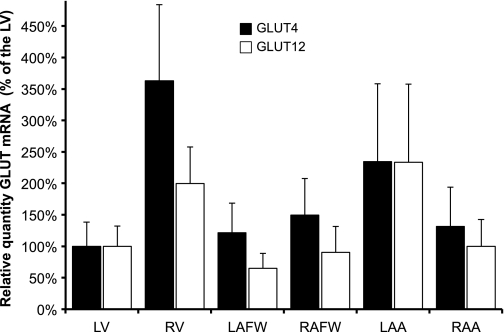
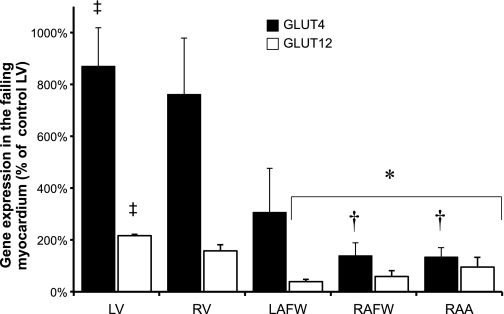
Real-time PCR demonstrated no statistical difference in GLUT4 and GLUT12 mRNA between the various cardiac regions in control dogs (Fig. 5). In contrast, GLUT4 and GLUT12 mRNA were significantly increased in the failing LV (by 8- and 2-fold, respectively, compared with the control LV, P = 0.002) (Fig. 6).
Fig. 5.
GLUT4 and GLUT12 gene expression was similar throughout all cardiac regions of the healthy canine myocardium. Mean ± SE of the relative quantity of GLUT4 (black bar) and GLUT12 (white bar) mRNA, with RPS19 as the housekeeping gene, in the myocardium of 6 healthy dogs (n = 3–6 per site). All samples compared with the control LV site.
Fig. 6.
Chronic HF induced increase in GLUT4 and GLUT12 gene expression in the LV compared with other cardiac regions. Mean ± SE of the relative quantity of GLUT4 and GLUT12 mRNA, with RPS19 as the housekeeping gene, in the failing myocardium (n = 3–4). All samples are compared with the LV control site of the healthy myocardium. *P ≤ 0.05 vs. failing LV; †P ≤ 0.05 vs. failing RV; ‡P ≤ 0.05 vs. control LV.
DISCUSSION
Our data demonstrated that 1) GLUT4, but not GLUT12, protein expression follows a regional pattern in healthy myocardium: GLUT4 protein expression was significantly higher in the atria compared with the ventricles, with the highest content present in the RA; 2) the regional pattern of GLUT4 protein expression observed in the healthy myocardium was reversed in dogs with advanced dilated cardiomyopathy: GLUT4 protein expression was increased in both ventricles and decreased in the RA of the failing myocardium; 3) GLUT12 protein expression was primarily increased in the failing LV; 4) GLUT4 and GLUT12 mRNA was increased in the failing LV.
GLUT4 expression in the healthy myocardium.
Although several investigators have suggested regional differences in myocardial structure and function, potential regional differences related to glucose metabolism have been infrequently investigated (13, 14, 29). Because of its hydrophilic nature, glucose is unable to pass the lipid bilayer of the plasma membrane by simple diffusion, thus the rate-limiting step for cardiac glucose utilization is glucose uptake, which is preceded and induced by translocation of GLUTs from an intracellular site to the cell surface (28). The translocation of GLUT4 protein, the major isoform, from an intracellular pool to the cell surface (active site) is required for full stimulation of glucose uptake and is largely regulated by insulin- and contraction/calcium-dependent processes, although these downstream signaling pathways are not well elucidated (5, 10, 28). Our data demonstrated a regional expression of GLUT4 protein across the cardiac regions of the healthy canine myocardium, and, surprisingly, GLUT4 protein expression was higher in the atria compared with the ventricles, with the highest values seen in the RA. It was recently demonstrated that inositol 1,4,5-triphosphate (IP3) receptor (abundantly expressed in the atria) is required for GLUT4 translocation and glucose uptake in cardiac myocytes (5). Thus, one could argue that the regional pattern of GLUT4 observed in the healthy myocardium could be related to the well-known difference in contraction/calcium signaling pathways between the ventricles and the atria, since the highest GLUT4 content was seen in the atria, which have higher inositol IP3 content and the ability to contract at a faster rate than the ventricles, especially the RA, the site of the pacemaker of the heart (35). Thus, in the present study, the regional difference in GLUT content may provide novel insights into the connection between calcium-dependent signaling pathways and GLUT4 translocation. Finally, this regional pattern at the protein level was not correlated with changes in mRNA levels across the cardiac chambers, suggesting that these regional changes occurred by a posttranscriptional mechanism in the healthy myocardium.
GLUT4 expression in the failing myocardium.
Dilated cardiomyopathy is the third cause of HF, and is a progressive disease characterized by ventricular dilation and functional impairment in the absence of coronary lesions and/or hypertension (25). We have previously demonstrated that our animal model of dilated cardiomyopathy induced by tachypacing has striking similarities to the disease in human beings, including progressive left ventricular dilatation and hypertrophy, progressive LV systolic dysfunction, and regional LV dyssynchrony, as well as decreased functional capacity (18, 30). We hypothesized that this regional dyssynchrony could affect the regulation of glucose transport and, to our knowledge, this is the first study to identify a regional heterogeneity of GLUT protein and gene expression in the failing canine myocardium. The upregulation of GLUT4 content in both ventricles (primarily in the LV combined with an upregulation of its transcript level) could account for the increased reliance of the myocardium on glucose uptake and oxidation as the primary energy substrate, as reported in animal models of pacing induced dilated cardiomyopathy and HF, as well as in humans (6, 12, 13, 26). Thus, this increased GLUT protein content in both ventricles could provide an adaptive metabolic profile to fully support the increased energetic demand secondary to the increase in ventricular wall stress (dilatation) and muscle mass (hypertrophy) observed in our model of chronic HF. However, the regulation of glucose transport during HF, as it relates to GLUT, is still not well understood with several studies reporting controversial results (12, 18). In agreement with our findings, several studies have reported an increase in GLUT4 protein content in the failing LV, while other studies have found that GLUT4 protein expression was unchanged, as well as a paradoxical downregulation of GLUT4 mRNA in the failing myocardium in the face of increased glucose uptake (12, 24, 34). In addition, Nikolaidis et al. (17) reported a 31% reduction in GLUT4 translocation and subsequently decreased glucose uptake, which was attributed to myocardial insulin resistance in their canine model of pacing-induced end-stage HF. However, it should be noted that their animal model was paced for a shorter duration (35 days) and at a nonphysiologically high stimulation rate (240 beats/min). Therefore, we used a model that maintains LV dysfunction at stimulation rates that are closer to normal ranges for conscious dogs and that induced a progressive development of HF. In addition, we previously demonstrated that 16 wk of RV tachypacing induced irreversible systolic dysfunction (up to 7 wk after termination of pacing), which is in contrast with previous reports of canine tachypacing models (23, 30). Further experiments are required to determine the effect of the duration of HF on the regulation of myocardial glucose transport. In brief, our findings suggested that the switch in myocardial metabolic substrate preference toward glucose is present, as evidenced by the increase in GLUT4 protein content in the ventricles (mostly LV) of our canine model that emulates chronic progressive human dilated cardiomyopathy. However, it should be pointed out that myocardial glucose uptake was not directly measured in this study. In addition, GLUT protein content was measured in total cellular membranes, and thus plasma membrane-associated GLUT4 could not be differentiated from intracellular membrane-associated GLUT4.
One surprising finding from our study was the paradoxical decrease in the expression of GLUT4 protein in the RA during chronic HF (with the lowest content in RAA), which may induce perturbations in energetic production, and thus may play a role in the pathogenesis of atrial fibrillation, the most commonly sustained arrhythmia in adults, in particular for those with chronic HF (9). Indeed, using a canine model of tachycardia-induced HF, profound deficits in atrial bioenergetics characterized by depletion of ATP and creatine kinase were reported and correlated with the duration of induced atrial fibrillation (4), although the role of atrial glucose metabolism was not assessed in this study. In addition, glycolytic inhibition has been shown to selectively deprive ATP to sarcoplasmic reticulum calcium ATPase pump and to amplify the calcium handling abnormalities associated with early afterpotentials in the atria of old rats, suggesting a role for altered glucose oxidation in the pathogenesis of atrial fibrillation (19). In our model of chronic-progressive HF, we previously reported alterations in atrial function, as evidenced by the shortening of the atrial refractoriness, which suggested an enhanced propensity to develop atrial fibrillation (30). Therefore, one could argue that the paradoxical decrease in GLUT4 protein expression could provide a metabolic substrate for the development of atrial fibrillation. Overall, this study demonstrates a regional distribution of both protein and gene expression of GLUT4, a key metabolic regulator of glucose uptake, in the healthy and failing myocardium. Indeed, we observed a regional distribution of GLUT4 within the ventricles but also within the atria, with the highest GLUT4 protein content in the RAA of healthy dogs. Therefore, every attempt should be made to identify the location of a sample when acquiring heart tissue and comparisons regarding myocardial substrate metabolism should be made between similar regions.
GLUT12 expression in the healthy and failing heart.
Although GLUT4 is the predominant insulin-responsive glucose transporter isoform, GLUT4 knockout mice are reported to not develop diabetes while insulin-stimulated glucose uptake into muscle was diminished but not eliminated, suggesting that another insulin-sensitive transport system was present (32). GLUT12 protein was identified in insulin-sensitive tissues such as fat and skeletal muscle, and GLUT4, -5, and -12 account for 98% of mRNA in human skeletal muscle (33). In addition, it has been recently shown that GLUT12 translocates to the cell surface after insulin stimulation in skeletal muscle, suggesting that it may provide redundancy to the dominant GLUT4 system (32). In addition, it was recently demonstrated that GLUT12 overexpression in transgenic mice improved whole body insulin sensitivity mediated by an increased glucose clearance rate under insulin-stimulated, but not basal, conditions in insulin-sensitive tissues (22). Although this isoform represents a novel second insulin-sensitive glucose transport system, its role has not been investigated under physiologic and pathophysiological conditions in the heart. In this study, we examined both GLUT12 gene and protein expression pattern in the myocardium of healthy subjects and during chronic HF. We demonstrated that, as for the insulin-sensitive transporter GLUT4, GLUT12 isoform is present in significant quantities in the healthy myocardium, although we did not observe any significant regional differences, in contrast to GLUT4. During HF, we observed a fourfold increase in GLUT12 protein content in the failing LV, along with a significant increase in its transcript level, suggesting that this change was, in part, transcriptionally regulated similarly to GLUT4. In addition, increases in GLUT12 content in both atria were modest during HF and were not accompanied by a significant increase in gene expression. This apparent discrepancy could be explained in part by regional differences in GLUT12 mRNA and protein degradation. Similarly, dissociation between gene and protein expression of enzymes involved in fatty acid metabolism has been reported in a rodent model of HF (15). Overall, our data suggest a functional role for GLUT12 during HF, which may work in concert with GLUT4 to augment insulin-stimulated glucose uptake and subsequently glucose oxidation in the face of increased ATP demand in the failing LV.
Perspectives and Significance
Over five million Americans suffer from chronic-progressive HF, which is characterized by global and regional dyssynchronous contraction, as well as by bioenergetic derangements. This study provides novel insights into the molecular regulation of myocardial glucose uptake, which was regionally dependent. In addition, using a canine model of chronic-progressive, dilated cardiomyopathy, our data suggested that a regional metabolic shift occurs such as the LV is the most dependent site on both insulin-responsive GLUT4- and GLUT12-mediated glucose transport. In addition, GLUT4 protein content was significantly decreased in the RA, which might provide a metabolic substrate favoring the development of atrial fibrillation, a common comorbidity during HF.
GRANTS
This study was supported by the National Institutes of Health Grants RR-023083 (to V. A. Lacombe) and HL-089836 (to C. A. Carnes), by the Australian National Health and Medical Research Project Grant 350424 (to S. Rogers), and the Diabetes Australia Research Trust (to S. Rogers).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Pacemakers and leads used in this study were donated by Medtronic, Minneapolis, MN.
REFERENCES
- 1. Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation 116: 434–448, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Babu GJ, Bhupathy P, Timofeyev V, Petrashevskaya NN, Reiser PJ, Chiamvimonvat N, Periasamy M. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci USA 104: 17867–17872, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brinkhof B, Spee B, Rothuizen J, Penning LC. Development and evaluation of canine reference genes for accurate quantification of gene expression. Anal Biochem 356: 36–43, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Cha YM, Dzeja PP, Shen WK, Jahangir A, Hart CY, Terzic A, Redfield MM. Failing atrial myocardium: energetic deficits accompany structural remodeling and electrical instability. Am J Physiol Heart Circ Physiol 284: H1313–H1320, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Contreras-Ferrat AE, Toro B, Bravo R, Parra V, Vásquez C, Ibarra C, Mears D, Chiong M, Jaimovich E, Klip A, Lavandero S. An inositol 1,4,5-triphosphate (IP3)-receptor pathway is required for insulin-stimulated glucose transporter 4 translocation and glucose uptake in cardiomyocytes. Endocrinology 151: 4665–4677, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Dávila-Román VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 40: 271–277, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Gergs U, Berndt T, Buskase J, Jones LR, Kirchhefer U, Müller FU, Schlüter KD, Schmitz W, Neumann J. On the role of junctin in cardiac Ca2+ handling, contractility, and heart failure. Am J Physiol Heart Circ Physiol 293: H728–H734, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schürmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab 282: E974–E976, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Kalifa J, Maixent JM, Chalvidan T, Dalmasso C, Colin D, Cozma D, Laurent P, Deharo JC, Djiane P, Cozzone P, Bernard M. Energetic metabolism during acute stretch-related atrial fibrillation. Mol Cell Biochem 317: 69–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lacombe VA, Hinchcliff KW, Devor ST. Effects of exercise and glucose administration on content of insulin-sensitive glucose transporter in equine skeletal muscle. Am J Vet Res 64: 1500–1506, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Lacombe VA, Hinchcliff KW, Kohn CW, Devor ST, Taylor LE. Feeding of meals of varying soluble carbohydrate diets affects muscle glycogen synthesis after exercise in horses. Am J Vet Res 65: 916–923, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Lei B, Lionetti V, Young ME, Chandler MP, d'Agostino C, Kang E, Altarejos M, Matsuo K, Hintze TH, Stanley WC, Recchia FA. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol 36: 567–576, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Lionetti V, Guiducci L, Simioniuc A, Aquaro GD, Simi C, De Marchi D, Burchielli S, Pratali L, Piacenti M, Lombardi M, Salvadori P, Pingitore A, Neglia D, Recchia FA. Mismatch between uniform increase in cardiac glucose uptake and regional contractile dysfunction in pacing-induced heart failure. Am J Physiol Heart Circ Physiol 293: H2747–H2756, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Lukas A, Antzelevitch C. Differences in the electrophysiological response of canine ventricular epicardium and endocardium to ischemia. Role of the transient outward current. Circulation 88: 2903–2915, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Morgan EE, Chandler MP, Young ME, McElfresh TA, Kung TA, Rennison JH, Tserng KY, Hoit BD, Stanley WC. Dissociation between gene and protein expression of metabolic enzymes in a rodent model of heart failure. Eur J Heart Fail 8: 687–693, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Neubauer S. The failing heart–an engine out of fuel. N Engl J Med 356: 1140–1151, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res 61: 297–306, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Nishijima Y, Feldman DS, Bonagura JD, Ozkanlar Y, Jenkins PJ, Lacombe VA, Abraham WT, Hamlin RL, Carnes CA. Canine nonischemic left ventricular dysfunction: a model of chronic human cardiomyopathy. J Card Fail 11: 638–644, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Ono N, Hayashi H, Kawase A, Lin SF, Li H, Weiss JN, Chen PS, Karagueuzian HS. Spontaneous atrial fibrillation initiated by triggered activity near the pulmonary veins in aged rats subjected to glycolytic inhibition. Am J Physiol Heart Circ Physiol 292: H639–H648, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-α in pacing-induced heart failure. Circulation 106: 606–612, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: 2002–2007, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Purcell SH, Aerni-Flessner LB, Willcockson AR, Diggs-Andrews KA, Fisher SJ, Moley KH. Improved insulin sensitivity by GLUT12 overexpression in mice. Diabetes 60: 1478–1482, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qanud K, Mamdani M, Pepe M, Khairallah RJ, Gravel J, Lei B, Gupte SA, Sharov VG, Sabbah HN, Stanley WC, Recchia FA. Reverse changes in cardiac substrate oxidation in dogs recovering from heart failure. Am J Physiol Heart Circ Physiol 295: H2098–H2105, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation 104: 2923–2931, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Recchia FA, Lionetti V. Animal models of dilated cardiomyopathy for translational research. Vet Res Commun 31: 35–41, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Recchia FA, McConnell PI, Bernstein RD, Vogel TR, Xu X, Hintze TH. Reduced nitric oxide production and altered myocardial metabolism during the decompensation of pacing-induced heart failure in the conscious dog. Cir Res 83: 969–979, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Rogers S, Macheda ML, Docherty SE, Carty MD, Henderson MA, Soeller WC, Gibbs EM, James DE, Best JD. Identification of a novel glucose transporter-like protein-GLUT-12. Am J Physiol Endocrinol Metab 282: E733–E738, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res 79: 249–258, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Sharma S, Razeghi P, Shakir A, Keneson BJ, 2nd, Clubb F, Taegtmeyer H. Regional heterogeneity in gene expression profiles: a transcript analysis in human and rat heart. Cardiology 100: 73–79, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Sridhar A, Nishijima Y, Terentyev D, Khan M, Terentyeva R, Hamlin RL, Nakayama T, Gyorke S, Cardounel AJ, Carnes CA. Chronic heart failure and the substrate for atrial fibrillation. Cardiovasc Res 84: 227–236, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Stuart CA, Howell Ma, Zhang Y, Yin D. Insulin-stimulated translocation of glucose transporter (GLUT) 12 parallels that of GLUT4 in normal muscle. J Clin Endocrinol Metab 94: 3535–3542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stuart CA, Yin D, Howell ME, Dykes RJ, Laffan JJ, Ferrando AA. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab 291: E1067–E1073, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol 555: 1–13, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walden AP, Dibb KM, Trafford AW. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol 46: 463–473, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Waller AP, Kohler K, Burns TA, Mudge MC, Belknap JK, Lacombe VA. Naturally occurring compensated insulin resistance selectively alters glucose transporters in visceral and subcutaneous adipose tissue without change in AS160 activation. Biochim Biophys Acta 1812: 1098–1103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Witteles RM, Tang WH, Jamali AH, Chu JW, Reaven GM, Fowler MB. Insulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic link. J Am Coll Cardiol 44: 78–81, 2004 [DOI] [PubMed] [Google Scholar]