Abstract
The combination of increasing blood flow and amino acid (AA) availability provides an anabolic stimulus to the skeletal muscle of healthy young adults by optimizing both AA delivery and utilization. However, aging is associated with a blunted response to anabolic stimuli and may involve impairments in endothelial function. We investigated whether age-related differences exist in the muscle protein anabolic response to AAs between younger (30 ± 2 yr) and older (67 ± 2 yr) adults when macrovascular and microvascular leg blood flow were similarly increased with the nitric oxide (NO) donor, sodium nitroprusside (SNP). Regardless of age, SNP+AA induced similar increases above baseline (P ≤ 0.05) in macrovascular flow (4.3 vs. 4.4 ml·min−1·100 ml leg−1 measured using indocyanine green dye dilution), microvascular flow (1.4 vs. 0.8 video intensity/s measured using contrast-enhanced ultrasound), phenylalanine net balance (59 vs. 68 nmol·min−1·100 ml·leg−1), fractional synthetic rate (0.02 vs. 0.02%/h), and model-derived muscle protein synthesis (62 vs. 49 nmol·min−1·100 ml·leg−1) in both younger vs. older individuals, respectively. Provision of AAs during NO-induced local skeletal muscle hyperemia stimulates skeletal muscle protein metabolism in older adults to a similar extent as in younger adults. Our results suggest that the aging vasculature is responsive to exogenous NO and that there is no age-related difference per se in AA-induced anabolism under such hyperemic conditions.
Keywords: microvascular blood flow, aging, nitric oxide
with advancing age, the stimulatory effect of amino acids on skeletal muscle protein synthesis is diminished, a phenomenon often termed anabolic resistance. Although the etiology of age-related anabolic resistance is uncertain, its hallmark characteristics of reduced protein synthesis and/or elevated circulating amino acid concentrations (5, 6, 8, 9, 22, 23, 38) suggest two general, nonexclusive mechanisms: 1) a reduced capacity for protein synthesis (utilization defect) and 2) a reduced availability of amino acids to cells for protein synthesis (delivery defect).
Notably, an elevated circulating concentration of amino acids does not necessarily indicate greater delivery to skeletal muscle, as the amino acids must also diffuse from the capillaries into the interstitial fluid and be transported from the interstitial fluid into skeletal muscle before becoming available for protein synthesis. Recent studies suggest that insulin stimulation of microvascular blood flow and intracellular amino acid availability is essential for insulin stimulation of skeletal muscle protein synthesis, which is impaired with aging (12, 13, 22, 32), at least when blood amino acid concentrations are at “low” fasting levels. Although these studies are supportive of a delivery defect rather than a utilization defect as the primary culprit in age-related anabolic resistance to insulin (at least when circulating amino acid concentrations are low), the role of amino acid delivery is less clear. The few prior studies comparing younger and older subjects under basal conditions and during hyperaminoacidemia uniformly fail to find a deficit in skeletal muscle amino acid transport (9, 38, 39), even under hyperinsulinemic conditions (38). However, the same studies report decrements in anabolic sensitivity and blood flow in older subjects relative to the young. Such studies suggest a utilization defect is responsible for age-related resistance to the anabolic effects of amino acids, although they do not rule out a role for blood flow apart from amino acid delivery.
One potential component of an age-associated amino acid delivery impairment may be a reduction in nitric oxide (NO)-mediated vasodilation. Insulin causes endothelium-dependent vasodilation through activation of endothelial NO synthase (eNOS) (25, 31), suggesting that age-related impairment of the hemodynamic response to insulin is due to reduced production of and/or responsiveness to NO. Thus, a reduced capacity for NO synthesis may occur with advancing age, leading to diminished NO-induced capillary recruitment and impaired metabolic regulation. Supportive of this possibility, administration of the NO donor sodium nitroprusside was recently shown to restore insulin stimulation of muscle protein synthesis in older adults under fasting conditions (32).
In the current study, we postulated that a utilization defect rather than an amino acid delivery defect is primarily responsible for age-related anabolic resistance to amino acids. Accordingly, we hypothesized that, compared with younger control subjects, older individuals would exhibit a lower anabolic response to amino acids when sodium nitroprusside (SNP) was concomitantly administered to stimulate microvascular blood flow in both younger and older subjects. To address the possibility that amino acid delivery was compromised in older subjects at steps distal to capillary delivery (i.e., transcapillary diffusion and inward transport), we also measured interstitial amino acid levels and phenylalanine transport into skeletal muscle (Fig. 1). Contrary to our hypothesis, we found that younger and older individuals responded similarly to amino acid infusion during concomitant vasodilator infusion, with no evidence of anabolic resistance to amino acids in the older subjects under these hyperemic conditions. Taken together, with previous studies which failed to find a deficit in skeletal muscle amino acid availability during hyperaminoacidemia, these findings suggest an important role for blood flow in anabolic sensitivity.
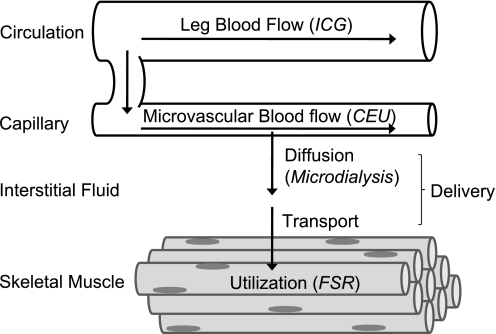
Fig. 1.
Simplified schematic illustrating amino acid flux from blood circulation into skeletal muscle tissue. Outflux of amino acids from tissue into circulation is not shown. Techniques used in the various physiological compartments are indicated in parentheses: ICG denotes indocyanine green, CEU denotes contrast-enhanced ultrasound, and FSR denotes fractional synthetic rate.
METHODS
Ethics statement.
The study was approved by The University of Texas Medical Branch (UTMB) Institutional Review Board and complied with the Declaration of Helsinki. Written informed consent was obtained from all subjects after explaining the procedures and risks.
Subjects.
Healthy, untrained younger [30 ± 2 yr, 5 female, 2 male] and older [67 ± 2 yr (means ± SE), 4 female, 3 male] individuals [30 ± 2% body fat for younger and older subjects combined] were studied before (BASAL) and during an infusion of SNP and AA (Premasol 10%; Baxter, Deerfield, IL) (SNP+AA). Volunteers were screened at the UTMB Institute for Translational Sciences-Clinical Research Center to determine study eligibility. Exclusion criteria included cardiac, liver, kidney, pulmonary, autoimmune, or vascular disease; hypocoagulation or hypercoagulation disorders, diabetes, cancer, obesity, anemia, infectious diseases, or an allergy to iodides, shellfish, or indocyanine green dye. Subjects taking anabolic steroids or corticosteroids in the past 6 mo were excluded, as were subjects unable to discontinue anti-inflammatory or prophylactic aspirin therapy (e.g., 14 days) or those engaged in regular aerobic or resistance exercise training. Subjects were instructed to continue all regular activities of daily living and maintain their usual diet during the week preceding the study. Finally, the older subjects underwent an ankle brachial index (ABI) test at the UTMB Vascular Lab to confirm the absence of peripheral vascular disease (ABI = the ratio of blood pressure in the ankle and blood pressure in the arm: 1.0–1.2 is normal).
Prestudy testing.
Two weeks prior to conducting the metabolism study, subjects were admitted as outpatients to the CRC. Fasting glucose, total body fat, and leg lean and leg fat mass (dual energy X-ray absorptometry, or DEXA, Hologic, Natick, MA) were determined. Following the tests, subjects were fed and discharged.
Experimental protocol.
The experimental protocol is outlined in Fig. 2. Subjects reported to the CRC at noon the day before the study and were housed in standard hospital rooms for the remainder of the study. Each subject was fasted from 2200 until completion of the study the following day. Polyethylene catheters were inserted into the antecubital vein of both arms for infusion of stable isotopes, AAs, and Definity microbubbles (Bristol-Myers Squibb, New York, NY) on the morning of the study. Catheters were placed into the femoral artery (A) and vein (V) of one leg for AV sampling. The femoral A line was used for the infusion of SNP. Additionally, indocyanine green (ICG; Akorn, Buffalo Grove, IL) was infused into the femoral A using a port proximal from the SNP infusion for measurement of leg plasma flow at three time points, as previously described (4). Femoral A and V blood samples were obtained throughout the study for measurement of blood glucose, lactate (2300 STAT Plus; Yellow Springs Instruments, Yellow Springs, OH), and plasma insulin (Immulite 2000, Siemens, Deerfield, IL). Plasma and interstitial AA concentrations were measured by the Biomolecular Resource Facility and UTMB Protein Chemistry Lab using ion exchange HPLC (Hitachi L8800 Amino Acid Analyzer; Hitachi, Pleasanton, CA).
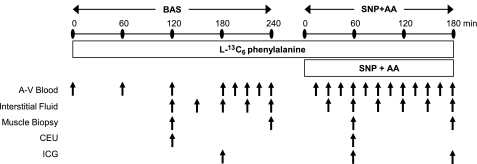
Fig. 2.
Experimental and stable isotope infusion protocol. After background blood samples were collected, a primed constant infusion of l-[ring-13C6] phenylalanine was maintained throughout the entire experiment. After a basal period of 240 min (BAS), all subjects received a primed constant infusion of sodium nitroprusside and amino acids (SNP+AA) for the following 180 min.
Anthropometric leg volume measurements were taken, and baseline blood samples were drawn for the analysis of background AA enrichment, and background absorbance. A primed (2 μmol/kg) continuous infusion of l-[ring-13C6] phenylalanine (0.08 μmol·kg−1·min−1) was started (time 0) and continued uninterrupted until study conclusion (Fig. 2). After a 240-min basal period (BASAL), the infusion of SNP was begun in the femoral artery (0.114 μg·kg−1·min−1) and a primed constant infusion of AAs was started in the peripheral vein of one arm (prime = 0.45 ml/kg; IR = 1.35 ml·kg−1·h−1). Both infusions continued for 180 min until the end of the study (SNP+AA). Subjects received less than one quarter of the manufacturer's recommended dose of SNP (0.5–10 μg·kg−1·min−1).
A total of four muscle biopsies (∼100–200 mg) were taken from the vastus lateralis, 10–15 cm above the knee, using a 5-mm Bergström needle, as previously described (3, 27). Two incisions were made ∼2 cm apart, and two biopsy samples were taken in opposite directions from each incision. All tissue was snap frozen in liquid nitrogen to abruptly stop all enzymatic reactions and stored in a −80°C freezer for later analysis. At the end of the study, all infusions were stopped, catheters removed, and the subjects were fed, monitored for 2 h, and discharged home with follow-up care instructions.
Protein abundance.
Western blot analyses were performed on skeletal muscle tissue to determine expression of key regulators involved in nitric oxide synthesis, cellular energy metabolism, and skeletal muscle protein anabolism. Briefly, blots loaded with 80 μg/protein were incubated overnight in the presence of rabbit primary antibody to mTOR (Ser2448 vs. total), AMPKα (Thr172 vs. total), eNOS, nNOS, or PGC-1α (Cell Signaling Technology, Beverly, MA). Following exposure to secondary anti-rabbit horeseradish peroxidase-linked antibody, proteins on the blots were detected using a ChemiDoc XRS system and quantified using Quantity One software (Bio-Rad, Hercules, CA). Phosphorylated protein content was divided by total protein content to control for small loading differences within each blot. Mouse control tissue was loaded on each gel and imaged to control for exposure differences between the blots.
Leg blood flow.
Leg plasma flow was determined utilizing the ICG dye-dilution technique and converted to blood flow (BF) using hematocrit as previously described (4). ICG concentrations were measured spectrophotometrically at λ=805 nm (Genesys 10vis; Thermo Fisher Scientific, Waltham, MA).
Analytical methods and calculations.
Glucose uptake (GU) across the leg was calculated as follows: GU = (CA − CV)·BF, where CA and CV are the blood glucose concentrations in the femoral artery and vein, respectively. BF is leg blood flow as calculated by ICG.
Phenylalanine enrichments and concentrations in arterial and venous blood samples were determined after the addition of an internal standard, deproteinization with sulfosalicylic acid, extraction with cation exchange chromatography, and tert-butyldimethylsilyl derivatization using GC-MS in electron impact mode (GC HP 5890, MSD HP 5989; Hewlett Packard, Palo Alto, CA) (4).
Muscle samples were weighed, and the proteins were precipitated with 450 μl of 10% sulfosalicylic acid. An internal standard containing phenylalanine was added to measure intracellular concentration. This experimental protocol utilized established phenylalanine kinetic measurements in skeletal muscle, as previously described (4, 27, 28). Assumptions for these techniques have been explained in detail elsewhere (4). The net balance (NB) for phenylalanine across the leg was calculated as follows: NB = (CA − CV)·BF, where CA and CV are the plasma AA concentrations in the femoral artery and vein, respectively. Mixed muscle fractional synthesis rate (FSR) is calculated by directly measuring the incorporation of l-[ring-13C6]-phenylalanine into protein, using the precursor-product model: FSR = [(EP2 − EP1)/(EM·t)]·60·100, where EP1 and EP2 are the enrichments of bound l-[ring-13C6]-phenylalanine in the first and second muscle biopsies, t is the time interval (min) between biopsies, and EM is the mean l-[ring-13C6]-phenylalanine enrichment in the muscle intracellular pool (2). Phenylalanine release from protein degradation (FMO) and phenylalanine uptake for protein synthesis (FOM) were calculated using a three-pool model as described elsewhere (37): FMO = {[CV·(EM − EV)/(EA − EM)] + CA}·[(EA/EM) − 1]·BF and FOM = FMO + NB, where EA, EA, and EM are the enrichments l-[ring-13C6]-phenylalanine in arterial blood, venous blood, and muscle intracellular pool, respectively.
Microdialysis.
Three CMA 70 microdialysis probes (30 mm, 20-kDa cutoff; CMA Microdialysis, Solna, Sweden) were inserted percutaneously into the vastus lateralis muscle of one leg with an 18-gauge needle following 1% lidocaine administration ∼20 cm above the patella (9). Microdialysis probes were perfused at a rate of 5.0 μl/min using a CMA 102 microinfusion pump (CMA Microdialysis, Stockholm Sweden) with a solution consisting of Na+ (147 mM), K+ (4 mM), Ca2+ (2.3 mM), Cl− (156 mM), 40 g/l Dextran 70, and radioisotopes of phenylalanine (3H Phe, 0.108 μCi/ml) and leucine (14C Leu, 0.108 μCi/ml). Ethanol (5 mM) was included in the microdialysis perfusion medium to monitor skeletal muscle interstitial fluid flux in the area of the microdialysis probe. Probe recovery for phenylalanine and leucine was determined by the internal reference technique, as previously described (9, 24). In vivo recovery was calculated using the following formula: (Perfusatedpm − Dialysatedpm)/Perfusatedpm, where Perfusatedpm and Dialysatedpm are the disintegrations per minute of either 14C Leu or 3H Phe in the perfusate and dialysate, respectively.
Ethanol concentration in each perfusate and dialysate sample was measured according to the method described by Hickner et al. (15). Briefly, 150 μl of reagent mixture consisting of glycine-hydrazine buffer at pH 8.9 (74 μM Na4P2O7, 22 μM glycine, 60 μM hydrazine) and 0.54 μM NAD+ was added to a 96-well plate. Then 2 μl of sample was added, followed by 20 μl of enzyme (1.7 mg alcohol dehydrogenase in 1 ml ddH2O). Ethanol concentrations were measured in the perfusate and dialysate solutions by fluorometric-enzymatic assay (Flouraskan II, MTX Labs Systems, Vienna, VA), with the results expressed as the ethanol concentration in the inflowing perfusate: [ethanol]collected dialysate/[ethanol]infused perfusion medium = Cout/Cin, where Cout is the concentration of the dialysate and Cin is the concentration of the perfusate. Data are reported as the ethanol outflow/inflow (O/I) ratio, which is inversely related to local nutritive blood flow (17).
Skeletal muscle perfusion.
Contrast-enhanced ultrasound (CEU) imaging of the vastus lateralis muscle was performed in a transaxial plane ∼15–20 cm above the patella over the midportion of the vastus lateralis muscle using a P4–2-phased array transducer interfaced with the HDI-5000 ultrasound system (Philips ATL Ultrasound, Andover, MA). Power Doppler imaging was performed as previously described (9, 36). An intravenous infusion (3.5 ml/min for 8 min) of a suspension of octafluoropropane gas-filled albumin microbubbles (Definity) was given at 120 min of BASAL and again at 60 min SNP+AA. CEU was limited to two time points because the FDA mandates that only two vials of Definity can be infused per subject per day. Pulsing interval (PI) vs. video intensity (VI) data were curve fitted to the function, y = A (1-e−βt), where y is the video intensity at PI t, A is the plateau video intensity (an index of microvascular blood volume, MBV), and β is the rate of microvascular refilling (an indicator of microvascular flow velocity, MFV) (40). The product of MBV × MFV is an indicator of microvascular blood flow (MBF).
Statistical methods.
A repeated-measures analysis using restricted maximum likelihood estimation was used to obtain parameter estimates with the linear mixed model procedure in SPSS (SPSS, version 17; SPSS, Chicago, IL). These models allow for inclusion of data sets with small amounts of missing data. The mixed model tested the effects for age, time, and the (age × time) interaction. When the effect was significant (P < 0.05), we computed t-tests at each time point for descriptive purposes used only in the figures. All data are expressed as means ± SE.
RESULTS
Subject characteristics.
Subject characteristics are shown in Table 1. There were no differences between the groups in any measured variable other than age.
Table 1.
Subject Characteristics
| Younger (n = 7) | Older (n = 7) | |
|---|---|---|
| Age, yr | 30 ± 2 | 67 ± 2 |
| Height*, cm | 164 ± 2 | 164 ± 4 |
| Weight, kg | 70 ± 5 | 66 ± 5 |
| BMI, kg/m2 | 26 ± 2 | 24 ± 1 |
| Total lean mass, kg | 46 ± 3 | 45 ± 5 |
| Total fat mass, kg | 23 ± 4 | 19 ± 2 |
| Total fat mass, % | 31 ± 3 | 29 ± 4 |
| Skeletal Muscle eNOS, AU | 0.603 ± 0.081 | 0.607 ± 0.052 |
| Skeletal Muscle nNOS, AU | 1.148 ± 0.379 | 0.874 ± 0.133 |
| Skeletal Muscle PGC-1α, ΑU | 0.736 ± 0.125 | 0.685 ± 0.130 |
Values are expressed as means ± SE.
BMI, body mass index; eNOS, endothelial nitric oxide synthase; nNOS, nitric oxide synthase; AU, arbitrary units.
Significant difference between groups, P ≤ 0.05.
Plasma amino acids.
Baseline AA concentrations were similar between younger and older unless specified otherwise in Table 2. As expected, concentrations of most AAs increased during infusion of SNP+AA in both groups (effect of time, P < 0.05, Table 2). Plasma urea, glutamine, citrulline, valine, cystine, tyrosine, 1-methylhistidine, and 3-methylhistidine concentrations were significantly higher in older compared with younger (effect of age, P < 0.05).
Table 2.
Plasma amino acids
| Younger |
Older |
|||||
|---|---|---|---|---|---|---|
| Time | Basal | 60 min SNP+AA | 180 min SNP+AA | Basal | 60 min SNP+AA | 180 min SNP+AA |
| Taurine | 5.4 ± 0.2 | 6.4 ± 0.9 | 5.4 ± 1.1 | 5.9 ± 0.4 | 6.8 ± 0.6 | 6.3 ± 0.4 |
| Phosphoserine | 42.4 ± 5.9 | 57.3 ± 9.7 | 52.5 ± 7.0 | 44.3 ± 10.4 | 92.6 ± 36.1 | 58.5 ± 5.0 |
| Urea# | 1883.9 ± 835.9 | 3930.7 ± 1108.0 | 5058.3 ± 976.7 | 5075.5 ± 1025.5 | 5211.0 ± 1191.6 | 7192.1 ± 709.7 |
| Aspartic Acid** | 0.8 ± 0.8 | 24.5 ± 4.7 | 21.0 ± 3.6 | 1.0 ± 1.0 | 26.0 ± 3.1 | 29.6 ± 3.8 |
| Threonine** | 110.2 ± 10.2 | 210.6 ± 21.4 | 193.7 ± 14.3 | 130.0 ± 18.3 | 217.0 ± 20.2 | 210.6 ± 19.0 |
| Serine** | 88.9 ± 8.1 | 157.6 ± 15.4 | 141.9 ± 10.1 | 87.3 ± 6.4 | 147.5 ± 6.9 | 142.5 ± 9.1 |
| Glutamic Acid** | 66.6 ± 7.7 | 127.0 ± 15.7 | 116.2 ± 17.5 | 66.4 ± 8.7 | 150.3 ± 23.9 | 136.2 ± 18.1 |
| Glutamine# | 1118.3 ± 60.8 | 1243.5 ± 70.3 | 1143.1 ± 61.7 | 1416.9 ± 142.4 | 1315.3 ± 62.4 | 1220.2 ± 91.8 |
| Glycine* | 199.0 ± 16.6 | 292.3 ± 21.3 | 247.2 ± 18.3 | 226.3 ± 25.0 | 289.1 ± 16.0 | 256.2 ± 16.0 |
| Alanine** | 204.0 ± 12.5 | 374.3 ± 31.3 | 303.6 ± 26.4 | 232.2 ± 35.9 | 345.6 ± 37.9 | 330.9 ± 44.7 |
| Citrulline# | 28.1 ± 2.8 | 36.9 ± 3.2 | 40.3 ± 2.7 | 41.8 ± 7.6 | 47.8 ± 5.1 | 51.4 ± 4.5 |
| A Aminobutyric Acid##* | 9.6 ± 1.5 | 14.4 ± 1.4 | 17.8 ± 2.3 | 16.6 ± 2.2 | 19.3 ± 2.3 | 21.5 ± 1.8 |
| Valine#** | 149.0 ± 9.0 | 403.7 ± 38.7 | 428.8 ± 83.8 | 203.8 ± 37.1 | 457.8 ± 28.0 | 573.7 ± 32.5 |
| Methionine** | 17.3 ± 2.1 | 73.4 ± 8.9 | 71.2 ± 7.6 | 19.2 ± 3.0 | 75.2 ± 4.8 | 80.7 ± 7.0 |
| Cystine## | 8.8 ± 1.8 | 12.3 ± 2.2 | 10.0 ± 1.7 | 16.3 ± 4.2 | 17.1 ± 4.0 | 20.1 ± 2.8 |
| Isoleucine** | 38.0 ± 3.6 | 223.0 ± 26.9 | 260.4 ± 34.3 | 51.2 ± 10.9 | 249.0 ± 11.7 | 306.2 ± 22.6 |
| Leucine** | 108.2 ± 8.0 | 420.2 ± 49.2 | 494.4 ± 62.6 | 140.4 ± 22.9 | 473.0 ± 21.7 | 582.3 ± 41.8 |
| Tyrosine## | 35.8 ± 6.0 | 53.5 ± 8.5 | 45.2 ± 4.9 | 54.7 ± 7.0 | 63.3 ± 3.0 | 56.6 ± 5.4 |
| Phenylalanine** | 53.0 ± 3.2 | 117.4 ± 12.0 | 124.5 ± 13.4 | 63.2 ± 6.9 | 123.0 ± 5.2 | 139.6 ± 11.2 |
| NH3* | 63.6 ± 6.2 | 96.4 ± 7.5 | 90.5 ± 7.0 | 65.8 ± 6.8 | 96.8 ± 7.2 | 97.1 ± 5.4 |
| Ornithine** | 48.6 ± 6.2 | 90.1 ± 12.1 | 120.5 ± 22.6 | 59.4 ± 5.9 | 105.4 ± 7.9 | 147.5 ± 10.0 |
| Lysine** | 161.5 ± 15.8 | 395.2 ± 41.6 | 365.8 ± 36.5 | 183.5 ± 23.6 | 397.1 ± 12.6 | 386.7 ± 34.7 |
| 1-Methyl Histidine## | 10.6 ± 2.9 | 13.9 ± 4.1 | 11.3 ± 3.0 | 22.6 ± 4.1 | 22.3 ± 4.0 | 20.4 ± 3.4 |
| Histidine** | 63.4 ± 3.4 | 136.2 ± 12.0 | 127.1 ± 9.5 | 67.8 ± 7.7 | 130.6 ± 6.8 | 131.3 ± 10.1 |
| 3-Methyl Histidine## | 3.1 ± 0.2 | 3.2 ± 0.3 | 3.0 ± 0.3 | 5.1 ± 0.8 | 4.8 ± 0.7 | 3.8 ± 0.7 |
| Arginine** | 73.3 ± 9.5 | 299.3 ± 34.4 | 294.6 ± 30.5 | 84.4 ± 10.1 | 301.8 ± 9.7 | 313.1 ± 28.6 |
Values are expressed as means ± SE (μM). Underlined amino acids were included in the SNP+AA infusion.
Significant effect of age (P < 0.05).
Significant effect of age (P < 0.01).
Significant effect of time (P < 0.05).
Significant effect of time (P < 0.001).
Leg BF.
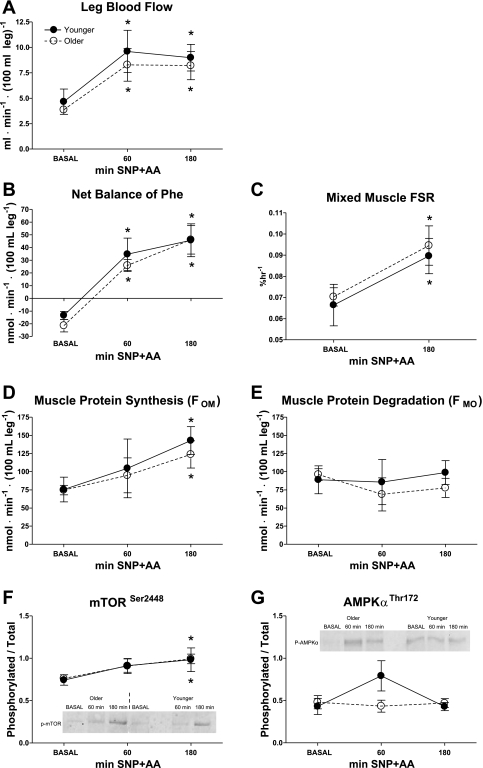
There was a sustained twofold increase in BF in both groups following SNP+AA (effect of time, P < 0.001) (Fig. 3A). There were no effects of age or age × time interactions.
Fig. 3.
Leg blood flow (BF; A) was measured by ICG dye dilution in healthy younger and older adults during BASAL and again during SNP+AA at 60 and 180 min. Phenylalanine net balance (NB, B) was measured in healthy younger and older adults during BASAL and again during SNP+AA at 60 min and 180 min. No differences were found between younger and older. Values are expressed as ml·min−1·100 ml leg volume−1. Fractional synthesis rate (FSR; C) of mixed muscle was measured in healthy younger and older subjects during BASAL and during AA + SNP at 180 min. Muscle protein synthesis (FOM, D) and degradation (FMO, E) were calculated in healthy younger and older adults during BASAL and again during SNP+AA at 60 min and 180 min. Ratios of phosphorylated to total protein expression of mTOR (F), and AMPKα (G) in skeletal muscle of younger and older individuals. Student's t-tests were conducted for descriptive purposes only after general linear mixed model revealed a significant effect of time for mTOR (P < 0.05). Student's t-tests were conducted for descriptive purposes only after general linear mixed model revealed a significant effect of time (P < 0.01). *Significant difference from BASAL (Student's t-test, P < 0.05). No differences between younger and older. Lines connecting related data are for descriptive purposes only and do not imply linear changes with time.
NB of phenylalanine.
Phenylalanine NB was negative in both younger and older during BASAL and became positive (effect of time, P < 0.05) after 60 and 180 min of SNP+AA infusion (Fig. 3B). There were no effects of age or age × time interaction.
Mixed muscle FSR.
Mixed-muscle FSR increased similarly (effect of time, P < 0.05) in both younger and older by 180 min of SNP+AA (Fig. 3C). There were no effects of age or age × time interaction.
Model-derived FOM and FMO.
FOM increased in both younger and older during SNP+AA (Fig. 3D). There was a significant effect of time (P < 0.01) but not age or (age × time) interaction on FOM. FMO remained unchanged following SNP+AA infusion in younger and older (Fig. 3E). There were no effects of time, age or age × time interaction on FMO.
Protein abundance.
Phosphorylation of mTOR increased in both younger and older in response to SNP+AA (effect of time, P < 0.05, Fig. 3F). There was a marginal increase in AMPKα phosphorylation at 60 min in younger individuals (Student's t-test, P = 0.06, Fig. 3G), but there were no effects of time, age, or time × age interaction. Baseline expression of skeletal muscle eNOS, nNOS, and PGC-1α were similar between younger and older (Table 1).
Glucose and insulin.
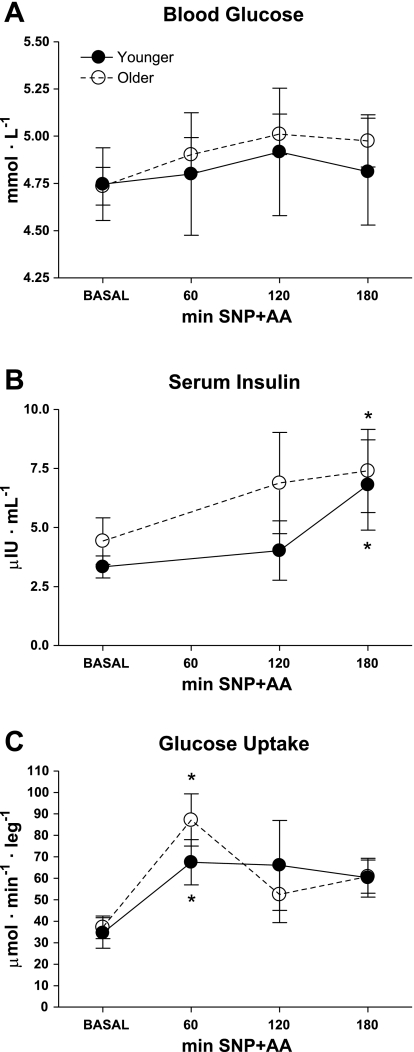
Mean blood glucose concentrations were similar between the groups (younger = 4.7 ± 0.2 and older = 4.7 ± 0.1 mmol/l) and remained at steady state throughout the experiment (Fig. 4A). Mean serum insulin concentrations were at steady-state during the BASAL period in both groups (younger = 3.3 ± 0.5 and older = 4.4 ± 1.0 μIU/ml) and gradually increased (effect of time, P < 0.05, Fig. 4B) in both younger and older by 180 min. Insulin concentrations returned to BASAL in both groups by 240 min (not shown). Glucose uptake was similar in both groups at BASAL (younger = 35 ± 7 and older = 37 ± 5 μmol·min−1·leg−1) (Fig. 4C). Glucose uptake peaked at 60 min SNP+AA in both younger and older (Student's t-test, P < 0.05). There were no age-related difference or responses in blood lactate or lactate release across the leg (data not shown). There were no effects of age or age × time interaction for insulin concentrations or glucose uptake.
Fig. 4.
Blood glucose (A) was measured in healthy younger and older adults during BASAL and again during SNP+AA at 60, 120, and 180 min. Serum insulin (B) was measured during BASAL and again during SNP+AA at 120 and 180 min. Glucose uptake (C) was calculated during BASAL and again during SNP+AA at 60 min, 120 min, and 180 min. Student's t-tests were conducted for descriptive purposes only after a general linear mixed model revealed a significant effect of time (P < 0.01). *Significant difference from BASAL. No differences between younger and older. Lines connecting related data are for descriptive purposes only and do not imply linear changes with time.
Microdialysis.
The microdialysis ethanol technique revealed no changes in interstitial flow within or between the groups at any time (Table 2). Similarly, there were no changes in probe recovery of d-[3H] phenylalanine and d-[14C] leucine.
Skeletal muscle perfusion.
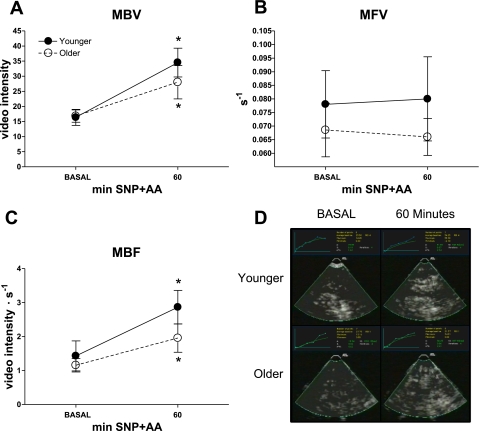
MBV, i.e., capillary recruitment, increased in younger and older following SNP+AA infusion (effect of time, P < 0.005) with no effect of age or age × time interaction (Fig. 5A). Infusion of SNP+AA did not alter MFV in either younger or older (Fig. 5B). MBF, the product of MBV and MFV, increased in younger and older following SNP+AA infusion (effect of time, P < 0.01), with no effect of age or age × time interaction (Fig. 5C). Representative screenshots are shown for younger and older subjects (Fig. 5D).
Fig. 5.
Microvascular blood flow was measured by contrast-enhanced ultrasound (CEU). Microvascular blood volume (MBV; A), microvascular flow velocity (MFV; B), microvascular blood flow (MBF; C), and representative images captured during 10-s pulsing intervals (D) in healthy younger and older subjects during BASAL and SNP+AA at 60 min. Student's t-tests were conducted for descriptive purposes only after general linear mixed model revealed a significant effect of time for MBV (P < 0.005) and MBF (P < 0.01). *Significant difference from BASAL. Lines connecting related data are for descriptive purposes only and do not imply linear changes with time.
Skeletal muscle phenylalanine trafficking.
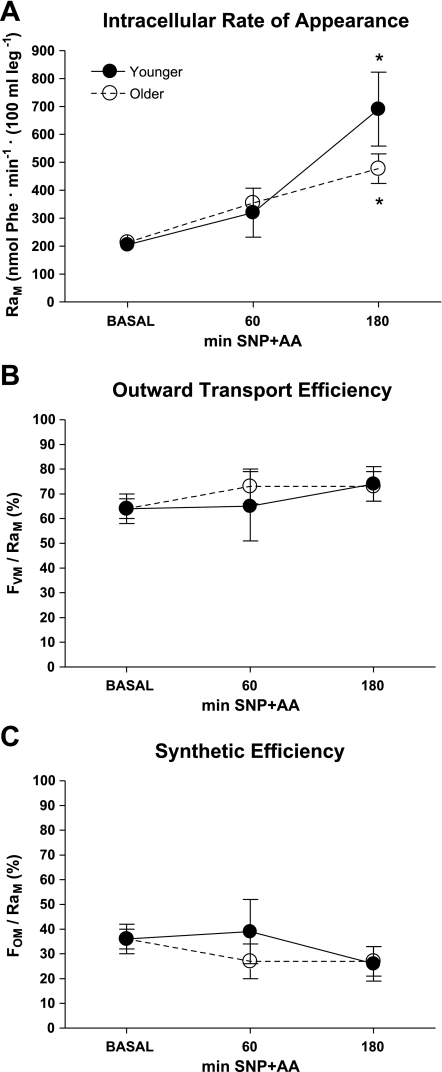
Intracellular rate of appearance (RaM) increased similarly (effect of time, P < 0.001) in younger and older individuals in response to SNP+AA (Fig. 6A). There were no changes in outward transport efficiency (FVM/RaM, Fig. 6B) or synthetic efficiency (FOM/RaM, Fig. 6C). There were no significant differences between younger and older individuals at any time.
Fig. 6.
Skeletal muscle phenylalanine trafficking. Intracellular rate of appearance (RaM; A), outward transport efficiency (FVM/RaM; B), and synthetic efficiency (FOM/RaM; C) were calculated. Student's t-tests were conducted for descriptive purposes only after general linear mixed model revealed a significant effect of time for RaM (P < 0.05). *Significant difference from BASAL. There were no significant differences between younger and older individuals at any time. Lines connecting related data are for descriptive purposes only and do not imply linear changes with time.
DISCUSSION
Our findings demonstrate, for the first time, that when AA provision is combined with the NO-donating vasodilator, SNP, skeletal muscle protein anabolism is equally and potently promoted in both younger and older individuals. Functional expansion of the microvasculature within skeletal muscle has the potential to positively impact AA transport and exchange by expansion of the endothelial surface area, similar to previously observed effects on muscle glucose transport (1, 14, 35). Our data demonstrate a pronounced, persistent increase in microvascular recruitment in response to an infusion of SNP along with supplemental AAs. This increase in muscle perfusion was due to opening of new capillary beds, since BFV did not change after the administration of SNP+AA. This study expands upon our knowledge that age-related anabolic resistance, accompanied by reduced hemodynamic responses following acute aerobic exercise (8) and during insulin stimulation (11, 12, 22), can be ameliorated by improving hemodynamic responses (12, 32). Although these studies are suggestive of age-related blunting in amino acid utilization, they have not ruled out the possibility that inadequate amino acid delivery plays a role in anabolic resistance. Here, we circumvented impairments in nutritive blood flow by using an exogenous NO donor, SNP, to achieve similar nutritive hyperemia in younger and older subjects. In this setting, in contrast to the period immediately following endurance type exercise (8), all measures of anabolic sensitivity and energy metabolism were similar between older and younger. In other words, when amino acid delivery was stimulated equally in both older and younger subjects, we were unable to show an age-related defect in amino acid utilization.
The NO signaling pathway is an important modulator of skeletal muscle blood flow during functional hyperemia (10, 18, 20, 30, 33). Adequate nutrient exchange promoted by augmented capillary flow is requisite during times of physical activity when oxygen and glucose demands are increased and removal of cellular byproducts must occur. Further, increased capillary flow is also desirable following meals at rest when increased AA availability may stimulate protein anabolism. Although we did not find any age-related differences in eNOS or nNOS protein content in leg skeletal muscle, previous investigations have shown that aging is associated with a diminished production of NO during both the rested state (16), as well as following exercise (26). Additionally, the relative contribution of NO to the induction of limb blood flow during exercise may be blunted by as much as 45% in older adults compared with younger adults (26). Those studies suggested impairment in the ability of some older individuals to promote vasodilation via NO production, but not necessarily due to diminished sensitivity to NO per se. Our finding that the microvascular response to an infusion of SNP+AA persists in older individuals indicates that signaling pathways downstream of NO remain largely intact with healthy aging, permitting a hemodynamic response that may facilitate the anabolic response to AAs, which was similar to that found in young. Our results suggest a possible role for NO donors or other strategies to increase NO levels in the management of age-related sarcopenia, and perhaps other muscle-wasting conditions.
Plasma availability of most AA was similar between older and younger. Plasma concentrations of 3-methyl histidine (3MH) and cystine were significantly higher in older at baseline compared with younger. While this could be indicative of age-related differences in skeletal muscle protein catabolism and antioxidant status, there was no correlation between 3MH concentrations and skeletal muscle protein degradation (FMO).
Because of the invasive nature of our studies and the inclusion criteria that were applied during subject recruitment, the older subjects in our studies were relatively healthy compared with the general age-matched population. We reason that this resulted in between-group comparisons where any age-related differences were less likely confounded by underlying medical conditions. Of course, more research is necessary to investigate whether similar anabolic responses can be induced in less healthy individuals with hemodynamic impairments in NO-mediated vasodilation (41). Resting skeletal muscle nutritive blood flow (12), insulin-induced blood flow (22), and eNOS expression (16) have been reported to be reduced with aging. However, our study suggests that such impairments at rest, as well as anabolic resistance in response to exogenous amino acids, are not necessary components of healthy aging. Furthermore, plasma insulin and glucose uptake across the leg increased to similar extents in both younger and older in response to SNP+AA. Both female and male volunteers were included in this study. While others recently reported age-related anabolic resistance as sex specific (29), we found no such sex effects among our small subject population.
Perspectives and Significance
In recent years, considerable scientific attention has focused on the age-associated metabolic responses to a myriad of combinations of exercise and nutrition (7, 9, 19, 21, 34). Often, these age-associated differences can be largely contributed to underlying comorbidities (i.e., frailty, obesity etc.) rather than age per se (19, 34). Although our study was not designed to elucidate the isolated effects of SNP or AA infusion on hemodynamics and skeletal muscle protein anabolism, the results obtained show that the aging vasculature is responsive to NO plus AAs, and that under hyperemic conditions, muscle protein anabolism responds similarly in healthy younger and older adults. These are important findings as previous studies have demonstrated that 1) the anabolic response to resistance exercise plus AAs is delayed in healthy older adults (7), 2) the blunted postexercise anabolic response is not due to diminished AA availability in healthy older adults (8), and 3) aerobic exercise can overcome the blunted anabolic and hemodynamic responses to nutrient stimuli in healthy older adults (12). To our knowledge, this is the first report showing that a combination of local NO-induced skeletal muscle hyperemia plus hyper-aminoacidemia, in the absence of exercise, stimulates a robust and similar net anabolic response in healthy younger and older adults. This investigation suggests that healthy aging vasculature is responsive to exogenous NO and that there is no age-related difference per se in AA-induced anabolism under these hyperemic conditions. Whether hyperemia can stimulate an anabolic response equally in younger and older adults under low AA conditions remains to be demonstrated.
GRANTS
This research was supported by grants from the National Institutes of Health/National Institute on Aging R01 AG21539 (to M. Sheffield-Moore) and the Claude D. Pepper Older Americans Independence Center grant P30 AG024832 (to J. Goodwin). Studies were conducted on the Clinical Research Center at the University of Texas Medical Branch at Galveston, funded by Grant M01 RR00073 from the National Center for Research Resources, National Institutes of Health, U.S. Public Health Service.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors. (Table 3)
Table 3.
Interstitial determinations
| Younger |
Older |
|||||
|---|---|---|---|---|---|---|
| Time | Basal | 60 min SNP+AA | 180 min SNP+AA | Basal | 60 min SNP+AA | 180 min SNP+AA |
| Probe Recoveries | ||||||
| 3H Phe outflow/inflow ratio | 0.26 ± 0.03 | 0.27 ± 0.05 | 0.29 ± 0.06 | 0.38 ± 0.04 | 0.39 ± 0.03 | 0.45 ± 0.06 |
| 14C Leu outflow/inflow ratio | 0.26 ± 0.04 | 0.26 ± 0.04 | 0.28 ± 0.06 | 0.35 ± 0.03 | 0.35 ± 0.03 | 0.37 ± 0.03 |
| Ethanol outflow/inflow ratio | 0.47 ± 0.02 | 0.48 ± 0.03 | 0.48 ± 0.10 | 0.47 ± 0.04 | 0.49 ± 0.03 | 0.49 ± 0.10 |
| Amino Acid Concentrations | ||||||
| Taurine | 6.5 ± 0.9 | 5.8 ± 0.6 | 5.8 ± 0.4 | 8.0 ± 1.0 | 7.5 ± 1.3 | 8.2 ± 1.3 |
| Phosphoserine | 26.9 ± 5.2 | 16.5 ± 4.1 | 13.3 ± 2.1 | 38.9 ± 7.6 | 25.7 ± 5.8 | 22.3 ± 4.4 |
| Urea | BD | BD | 1815.5 ± 307.8 | 2617.2 ± 576.3 | 5165.8 ± 3243.3 | 2306.9 ± 362.7 |
| Aspartic Acid | BD | 5.3 ± 1.3 | 10.3 ± 0.8 | BD | 10.7 ± 3.5 | 11.7 ± 1.6 |
| Threonine | 27.2 ± 3.6 | 39.2 ± 6.0 | 43.1 ± 6.2 | 33.2 ± 5.0 | 40.5 ± 6.9 | 51.8 ± 12.9 |
| Serine | 19.5 ± 1.6 | 29.1 ± 4.1 | 31.5 ± 3.9 | 21.1 ± 2.8 | 27.8 ± 3.8 | 33.4 ± 6.0 |
| Glutamic Acid | 11.0 ± 1.7 | 15.7 ± 5.2 | 12.0 ± 3.8 | 16.4 ± 5.9 | 24.6 ± 8.8 | 19.3 ± 5.4 |
| Glutamine | 438.6 ± 44.4 | 445.9 ± 56.0 | 439.6 ± 32.4 | 548.9 ± 73.7 | 459.8 ± 102.3 | 434.4 ± 119.7 |
| Glycine | 76.8 ± 10.6 | 94.2 ± 13.1 | 84.8 ± 9.4 | 96.4 ± 12.9 | 88.6 ± 12.4 | 91.1 ± 14.0 |
| Alanine | 88.3 ± 11.3 | 115.6 ± 13.2 | 116.8 ± 4.5 | 100.9 ± 23.4 | 121.8 ± 32.3 | 135.7 ± 28.3 |
| Citrulline | 8.5 ± 1.3 | 8.7 ± 1.6 | 9.7 ± 1.4 | 14.5 ± 2.8 | 13.5 ± 3.3 | 15.3 ± 2.6 |
| γ-Aminobutyric acid | 0.5 ± 0.5 | 1.4 ± 0.9 | 5.1 ± 1.4 | 3.6 ± 2.2 | 5.0 ± 2.9 | 5.1 ± 2.0 |
| Valine | 41.6 ± 4.2 | 78.5 ± 9.1 | 118.6 ± 14.4 | 54.5 ± 9.5 | 92.7 ± 19.7 | 133.0 ± 21.8 |
| Methionine | 6.6 ± 0.7 | 13.3 ± 2.1 | 20.0 ± 3.0 | 7.2 ± 0.8 | 14.1 ± 3.0 | 20.3 ± 5.1 |
| Cystine | 5.7 ± 0.8 | 6.0 ± 0.8 | 7.1 ± 1.3 | 9.6 ± 1.7 | 8.4 ± 1.6 | 10.9 ± 2.3 |
| Isoleucine | 9.9 ± 1.2 | 36.5 ± 6.2 | 57.9 ± 6.0 | 13.9 ± 3.2 | 43.3 ± 9.1 | 68.5 ± 12.6 |
| Leucine | 28.1 ± 3.1 | 76.0 ± 11.0 | 113.1 ± 14.6 | 39.9 ± 7.4 | 90.3 ± 17.6 | 128.7 ± 23.6 |
| Tyrosine | 8.3 ± 1.0 | 12.9 ± 2.6 | 9.6 ± 1.3 | 21.0 ± 4.9 | 10.1 ± 3.2 | 12.9 ± 6.4 |
| Phenylalanine | 14.8 ± 1.4 | 24.9 ± 2.6 | 32.9 ± 2.7 | 18.6 ± 3.6 | 28.7 ± 6.0 | 38.5 ± 7.2 |
| NH3 | 60.3 ± 4.9 | 60.4 ± 4.0 | 56.4 ± 2.2 | 57.6 ± 4.1 | 51.8 ± 6.5 | 82.2 ± 23.9 |
| Ornithine | 8.8 ± 1.3 | 16.0 ± 2.7 | 21.0 ± 0.7 | 13.2 ± 2.6 | 18.3 ± 3.7 | 31.6 ± 6.9 |
| Lysine | 42.7 ± 4.2 | 74.4 ± 11.5 | 77.8 ± 7.5 | 49.8 ± 8.4 | 77.0 ± 14.8 | 90.7 ± 16.0 |
| 1-Methyl Histidine | BD | 4.6 ± 0.4 | 5.1 ± 0.9 | 7.2 ± 0.9 | 6.6 ± 1.0 | 6.6 ± 1.1 |
| Histidine | 16.9 ± 1.6 | 26.5 ± 2.8 | 30.7 ± 4.3 | 16.3 ± 2.4 | 25.6 ± 4.7 | 28.3 ± 4.2 |
| 3-Methyl Histidine | BD | BD | BD | BD | BD | BD |
| Arginine | 18.0 ± 2.6 | 45.2 ± 7.1 | 54.2 ± 5.9 | 22.5 ± 3.9 | 47.5 ± 9.9 | 60.0 ± 10.6 |
Values are expressed as means ± SE (μM). Underlined amino acids were included in the SNP+AA infusion. Concentrations that fell below detectable limits are noted as BD.
ACKNOWLEDGMENTS
We thank the study participants, James Angel, Kishore Lakshman, Deandria J. Lavine, Linda M. Yang, the nurses of the Clinical Research Center, and the recruitment coordinators at the University of Texas Medical Branch Sealy Center on Aging.
REFERENCES
- 1.Baron AD, Tarshoby M, Hook G, Lazaridis EN, Cronin J, Johnson A, Steinberg HO. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes 49: 768–774, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Baumann PQ, Stirewalt WS, O'Rourke BD, Howard D, Nair KS. Precursor pools of protein synthesis: a stable isotope study in a swine model. Am J Physiol Endocrinol Metab 267: E203–E209, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Bergstrom J, Furst P, Nore LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol 36: 693–697, 1974 [DOI] [PubMed] [Google Scholar]
- 4.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab 268: E75–E84, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19: 422–424, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 294: E392–E400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 104: 1452–1461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durham WJ, Casperson SL, Dillon EL, Keske MA, Paddon-Jones D, Sanford AP, Hickner RC, Grady JJ, Sheffield-Moore M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J 24: 4117–4127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durham WJ, Casperson SL, Dillon EL, Keske MA, Paddon-Jones D, Sanford AP, Hickner RC, Grady JJ, Sheffield-Moore M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J 24: 4117–4127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald SM, Bashari H, Cox JA, Parkington HC, Evans RG. Contributions of endothelium-derived relaxing factors to control of hindlimb blood flow in the mouse in vivo. Am J Physiol Heart Circ Physiol 293: H1072–H1082, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia 52: 1889–1898, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 56: 1615–1622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab 291: E745–E754, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gudbjornsdottir S, Sjostrand M, Strindberg L, Lonnroth P. Decreased muscle capillary permeability surface area in type 2 diabetic subjects. J Clin Endocrinol Metab 90: 1078–1082, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Hickner RC, Ekelund U, Mellander S, Ungerstedt U, Henriksson J. Muscle blood flow in cats: comparison of microdialysis ethanol technique with direct measurement. J Appl Physiol 79: 638–647, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Hickner RC, Kemeny G, McIver K, Harrison K, Hostetler ME. Lower skeletal muscle nutritive blood flow in older women is related to eNOS protein content. J Gerontol A Biol Sci Med Sci 58: 20–25, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Hickner RC, Rosdahl H, Borg I, Ungerstedt U, Jorfeldt L, Henriksson J. The ethanol technique of monitoring local blood flow changes in rat skeletal muscle: implications for microdialysis. Acta Physiol Scand 146: 87–97, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Kalliokoski KK, Langberg H, Ryberg AK, Scheede-Bergdahl C, Doessing S, Kjaer A, Kjaer M, Boushel R. Nitric oxide and prostaglandins influence local skeletal muscle blood flow during exercise in humans: coupling between local substrate uptake and blood flow. Am J Physiol Regul Integr Comp Physiol 291: R803–R809, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol 106: 2040–2048, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Lee-Young RS, Ayala JE, Hunley CF, James FD, Bracy DP, Kang L, Wasserman DH. Endothelial nitric oxide synthase is central to skeletal muscle metabolic regulation and enzymatic signaling during exercise in vivo. Am J Physiol Regul Integr Comp Physiol 298: R1399–R1408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy C, Miller BF. Protein consumption following aerobic exercise increases whole-body protein turnover in older adults. Appl Physiol Nutr Metab 35: 583–590 [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 20: 768–769, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennie MJ. Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl Physiol Nutr Metab 34: 377–381, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Methods 40: 31–38, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin's vascular effects in humans. J Clin Invest 94: 2511–2515, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheffield-Moore M, Urban RJ, Wolf SE, Jiang J, Catlin DH, Herndon DN, Wolfe RR, Ferrando AA. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab 84: 2705–2711, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Sheffield-Moore M, Yeckel CW, Volpi E, Wolf SE, Morio B, Chinkes D, Paddon-Jones D, Wolfe RR. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab 287: E513–E522, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS One 3: e1875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 81: 209–237, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94: 1172–1179, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes 59: 2764–2771, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tschakovsky ME, Joyner MJ. Nitric oxide and muscle blood flow in exercise. Appl Physiol Nutr Metab 33: 151–161, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring) 19: 312–318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53: 1418–1423, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 290: E1191–E1197, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Volpi E, Ferrando AA, Yeckel CW, Tipton K, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 101: 2000–2007, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 85: 4481–4490, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286: 1206–1212, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97: 473–483, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Weil BR, Stauffer BL, Greiner JJ, DeSouza CA. Prehypertension is associated with impaired nitric oxide-mediated endothelium-dependent vasodilation in sedentary adults. Am J Hypertens 24: 976–981, 2011 [DOI] [PubMed] [Google Scholar]