Abstract
Maintenance of a 10% or greater reduced body weight results in decreases in the energy cost of low levels of physical activity beyond those attributable to the altered body weight. These changes in nonresting energy expenditure are due mainly to increased skeletal muscle work efficiency following weight loss and are reversed by the administration of the adipocyte-derived hormone leptin. We have also shown previously that the maintenance of a reduced weight is accompanied by a decrease in ratio of glycolytic (phosphofructokinase) to oxidative (cytochrome c oxidase) activity in vastus lateralis muscle that would suggest an increase in the relative expression of the myosin heavy chain I (MHC I) isoform. We performed analyses of vastus lateralis muscle needle biopsy samples to determine whether maintenance of an altered body weight was associated with changes in skeletal muscle metabolic properties as well as mRNA expression of different isoforms of the MHC and sarcoplasmic endoplasmic reticular Ca2+-dependent ATPase (SERCA) in subjects studied before weight loss and then again after losing 10% of their initial weight and receiving twice daily injections of either placebo or replacement leptin in a single blind crossover design. We found that the maintenance of a reduced body weight was associated with significant increases in the relative gene expression of MHC I mRNA that was reversed by the administration of leptin as well as an increase in the expression of SERCA2 that was not significantly affected by leptin. Leptin administration also resulted in a significant increase in the expression of the less MHC IIx isoform compared with subjects receiving placebo. These findings are consistent with the leptin-reversible increase in skeletal muscle chemomechanical work efficiency and decrease in the ratio of glycolytic/oxidative enzyme activities observed in subjects following dietary weight loss.
Keywords: energy metabolism, exercise, obesity
in humans, experimentally reducing adipose tissue mass lowers energy expenditure per unit of total metabolic mass by ∼15% below what is predicted on the basis of weight and body composition changes (35, 46). This reduction in energy expenditure is about 350 kcal/day below that predicted for body composition for a 70 kg male. The major effect of a 10% or greater weight loss on energy expenditure is a 30–40% reduction in the energy expended in physical activity above resting [nonresting energy expenditure (NREE)] (35, 46), which accounts for ∼80% of the variance in changes in 24-h energy expenditure following weight loss. We have found that the decline in NREE during maintenance of a reduced body weight is due largely to an ∼20% increase in skeletal muscle work efficiency [measured by indirect calorimetry during bicycle ergometry and by 31P-nuclear magnetic resonance (NMR) spectroscopy to quantify ATP consumption] at low workloads (46, 50). This increased skeletal muscle work efficiency, as well as the decline in 24-h energy expenditure, sympathetic nervous system tone, and circulating concentrations of bioactive thyroid hormones that characterize the weight-reduced state, are all reversed by administration of replacement doses of the adipocyte-derived hormone leptin (46).
Muscle fiber type is defined by the predominant myosin heavy chain (MHC) isoform expressed (51). Type I fibers (MHC I predominant) allow muscle to undergo prolonged aerobic contractions at a significantly lower energy cost than type II fibers (MHC II predominant) and, not surprisingly, are more evident in muscles of endurance athletes (15, 24, 25, 45). In contrast, type II fibers allow muscle to produce a higher force and power than type I fibers at a higher rate of ATP consumption and, not surprisingly, are more evident in leg muscles of sprinters and jumpers athletes (20). From an evolutionary standpoint one would predict that the beneficial effects of increasing the fraction of type 1 fibers, allowing increased energy efficiency and conservation of fat mass, would be particularly important during a period of undernutrition. Human muscle fibers are heterogenous, coexpressing multiple MHC isoforms, and therefore significant changes in the relative expression of different MHC isoforms within a muscle fiber may occur without necessarily affecting the fiber type.
MHC isoforms are the major determinant of contractile speed, force, and energy cost as reported in single muscle fibers and of muscle strength and endurance (9, 44). The differential efficiency of isoforms of the MHC, and their corresponding unique patterns of utilization of glucose vs. fat as fuel and of power generated per contraction (7, 30) provide a possible mechanism for the increased efficiency and utilization of free fatty acids as fuel following weight loss. Type I (MHC I isoform predominant, efficient, slow-twitch, mainly fatty acid oxidative) or type II (MHC II isoform predominant, less efficient but more powerful, fast-twitch, predominantly glycolytic) (5, 10) are coexpressed in muscle and are defined by the pH-responsive activation patterns of their myofibrillar ATPases. Type II MHC isoforms are subdivided into MHC IIa (more efficient and fatty acid oxidative) and MHC IIx (more powerful, less efficient, more glycolytic). The ATP consumption rate for MHC I predominant muscle fibers is ∼40% of that of MHC IIx and 65% of that of MHC IIa (16, 22, 26, 33, 44, 54). The respiratory quotient during low work levels, and the inorganic phosphate content of skeletal muscle, are significantly correlated with the relative proportion skeletal muscle fiber types (62, 66). Shifts in relative content of MHC isoforms, i.e., ratios of MHC I/MHC II and MHC IIa/MHC IIx, could account for the decline in NREE and increase in muscle work efficiency observed in weight-reduced subjects (50). We have recently shown that a significant fraction of the increased efficiency, as well as reliance on free fatty acids as fuel, during low level exercise are reflected in a decline in the ratio of glycolytic [phosphofructokinase, (PFK)] to fatty acid oxidative [cytochrome c oxidase (COX)] in weight-reduced subjects (23) which would be consistent with a greater relative expression of the MHC I isoform.
The sarcoplasmic reticulum Ca2+-ATPases (SERCAs) are a family of membrane-bound enzymes that drive free calcium ions from the cytosol into the sarcoplasmic reticulum by coupling ATP hydrolysis to the translocation of Ca2+ (41). SERCAs and MHCs contribute independently to muscle work efficiency (27). The SERCA2a isoform predominates in type I muscle fibers, while SERCA1 predominates in type II fibers and is uniquely able to uncouple ATP hydrolysis from Ca2+ transport, thereby releasing the enthalpy of ATP as heat (37, 41). We hypothesized that maintenance of a reduced body weight would induce leptin-reversible changes in activity of glycolytic and oxidative enzymes that were symptomatic of an overall increase in the relative expression of the more efficient MHC I and SERCA2 isoforms of skeletal muscle.
MATERIALS AND METHODS
A total of 10 obese subjects (4 males and 6 females) were studied at their maximum lifetime weight that they had maintained within a 2-kg range for at least 6 mo prior to enrollment (35, 42). Recruitment procedures and exclusion criteria for these studies, and data regarding the effects of weight changes on energy expenditure in some of these subjects, have been previously reported (35, 46, 47). All studies were approved by the respective Institutional Review Boards and are consistent with guiding principles for research involving humans (2). Written informed consent was obtained from all subjects prior to enrollment. Subject characteristics are presented in Table 1.
Table 1.
Subject clinical data
| Wtinitial | Wt−10%placebo | Wt−10%leptin | |
|---|---|---|---|
| Height, cm | 168.7 (2.6) | ||
| Body weight, kg | 126.3 (10.0) | 111.6 (9.2)* | 110.7 (9.3)* |
| Body mass index, kg/m2 | 43.8 (7.6) | 38.7 (7.2)* | 38.3 (7.3)* |
| Fat-free mass, kg | 70.0 (6.1) | 64.8 (6.0)* | 64.7 (5.8)* |
| Fat mass, kg | 56.0 (4.6) | 46.7 (4.3)* | 45.9 (4.3)* |
| Plasma leptin, ng/ml | 41.1 (8.4) | 32.8 (7.5) † | 50.3 (11.4) |
| Respiratory exchange ratio at rest | 0.85 (0.03) | 0.84 (0.05) | 0.86 (0.06) |
| Respiratory exchange ratio at 10 W power generated | 0.84 (0.03) | 0.83 (0.03)* | 0.84 (0.05) |
| Muscle efficiency at 10 W of power generated | 0.10 (0.06) | 0.13 (0.07) † | 0.11 (0.07) |
Values are means (SD); 10 subjects, 6 males, 4 females. Subject population was ethnically diverse consisting of 3 African-American, 1 Hispanic-American, 1 Pacific Islander-American, and 1 South Asian-American female, 1 African-American, and 3 Caucasian-American males.
P < 0.05 compared with Wtinitial,
P < 0.05 compared with Winitial, and Wt−10%leptin.
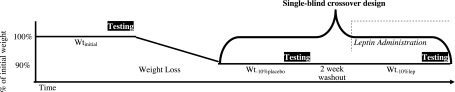
The research protocol is schematized in Fig. 1. Subjects were inpatients in the Clinical Research Center at Columbia Presbyterian Medical Center throughout this study. They were weighed daily at 6 AM and were instructed to consume all meals before midnight. As described previously (35, 49), subjects were fed a liquid formula diet [40% of calories as fat (corn oil), 45% as carbohydrate (glucose polymer), and 15% as protein (casein hydrolysate)], plus vitamin and mineral supplements, in quantities sufficient to maintain a stable weight (defined as a mean daily weight variation of < 10 g/day for at least 2 wk). This weight plateau is designated as Wtinitial.
Fig. 1.
Schematic of protocol. Studies, including assessment of circulating leptin (lep) concentrations, energy expenditure, body composition, and skeletal muscle biopsy, are identical at each testing period.
Following completion of studies (described below), at Wtinitial, subjects were provided 800 kcal of energy/day of the same liquid formula diet until they had lost ∼10% of Wtinitial. The duration of the weight loss phase ranged from 36 to 62 days. Once 10% weight loss had been achieved, intake was adjusted upward until subjects were again weight stable as described above. Subjects were then randomized to receive 5 wk of b.i.d. (8 AM and 8 PM) subcutaneous injections of saline (weight plateau is designated as Wt−10%placebo) or 5 wk of b.i.d. (8 AM and 8 PM) subcutaneous injections of recombinant human leptin (A-100, provided by Amgen, Thousand Oaks, CA and metreleptin provided by Amylin Pharmaceuticals, San Diego, CA) in doses that were calculated to achieve preinjection circulating leptin concentrations at 8 AM equal to those measured at Wtinitial (29, 48) (weight plateau is designated as Wt−10%leptin). Initial leptin doses were: 0.08 mg·kg fat mass−1·dose−1 in males, and 0.14 mg·kg fat mass−1·dose−1 in females (48). Circulating leptin concentrations at 8 AM were measured weekly in subjects receiving leptin, and dosages were adjusted until circulating leptin concentrations were similar to those measured at 8 AM at Wtinitial. Following completion of studies at Wt−10%placebo or Wt−10%leptin, subjects underwent a 2-wk washout period during which they received no injections. They were then crossed over to receive either leptin or placebo injections for an additional 5 wk during which time the same measurements performed at Wtinitial and the previous weight loss study period (Wt−10%placebo or Wt−10%leptin) were repeated. The order of testing was preserved between study periods. Subjects were unaware of whether they were receiving leptin or placebo and remained on a diet isocaloric to that initially demonstrated necessary to maintain a 10% reduced body weight throughout the leptin or placebo arms of the study.
Studies
Subjects underwent the following studies at each study period.
Body composition.
Fat-free mass and fat mass were determined by dual energy X-ray absorptiometry (43).
In vivo skeletal muscle fuel utilization and efficiency.
In vivo skeletal muscle fuel utilization and efficiency were determined by graded cycle ergometry (52). After a 10-min period of accommodation, the subjects pedaled at 60 rpm against graded resistance to generate 10 W, 25 W, and 50 W of power in successive 4-min intervals using a Sensormedics 880S bicycle and ergometer with electrical braking (53). Oxygen uptake, carbon dioxide production, and the respiratory exchange ratio were measured continuously (21) using a Sensormedics VMAX 29 metabolic cart (53). Rates of oxygen consumption were converted to kilocalorie based on the respiratory exchange ratio (40). Steady-state values were recorded at 0 W (rest), 10 W, 25 W, and 50 W. Generating 50 W of power is below the anaerobic threshold for even the most sedentary subjects. Below the anaerobic threshold, steady-state oxygen uptake and carbon dioxide production are easily attained within 2–3 min of cycling (63).
Skeletal muscle work efficiency was calculated as: work done (kcal/min)/[energy expended (kcal/min) − resting energy expenditure (kcal/min)]. This calculation represents the slope of the line relating work performed above energy expenditure at rest (0 work performed) to energy expended during exercise (21, 63). The lack of horizontal movement in stationary bicycling minimizes the effects of weight loss on work performed. Whatever decreased work there is from lifting the weight-reduced leg up is theoretically matched by the decreased assist from the weight of the lighter leg coming down. In fact, we have previously shown that there are similar effects of weight loss on skeletal muscle work efficiency (increased) and fuel utilization (decreased glucose utilization) in subjects studied following weight loss with and without the addition of dead weight to the legs to replace lost weight (50).
Biochemical analyses of skeletal muscle properties.
Muscle needle biopsies (∼200–300 mg) were obtained under local anesthesia with 1% xylocaine from subjects in the postabsorptive state. Enzymatic activity levels of creatine kinase, COX, hexokinase, glycogen phosphorylase, PFK, GAPDH, and 2-hydroxyacyl CoA dehydrogenase (HADH), citrate synthase (CS; an indicator of mitochondrial mass), and carnitine palmitoyl transferase-1 were determined spectrophotometrically (14, 55, 56). The capacity of skeletal muscle to oxidize fatty acids is reflected in the ratio of HADH/CS and the activity of the mitochondrial respiratory chain is reflected in the ratio of COX/CS (64). The ratios of PFK/COX and PFK/HADH activity in muscle samples have been shown to provide estimates of the relative glycolytic/fatty acid oxidative capacity of muscle in vivo (36, 64), as well as with the relative proportions of fast versus slow-twitch fiber types (31).
RNA analyses.
Total RNA was extracted from preweighed frozen muscle samples using the TRI Reagent according to the manufacturer's protocol (Molecular Research Center, Cincinnati, OH). Extracted RNA was precipitated from the aqueous phase with isopropanol, and, after washing with ethanol, the pellet was dried and suspended in nuclease-free water. The RNA concentration was determined by optical density at 260 nm (using the conversion factor of 40 μg/ml per unit of optical density of 260 nm). The RNA samples were stored frozen at −80°C for subsequent analyses for specific gene expression using an end point RT-PCR approach. Prior to cDNA synthesis, RNA integrity was checked by electrophoresis of 500 ng total RNA on 1% agarose gel stained with Gelgreen stain (Biotium) and was found to be high-quality RNA based on intact 28S and 18S bands. One microgram of total RNA was reverse transcribed into cDNA for each sample using the SuperScript II RT from Invitrogen and a mix of oligo(dT) (100 ng/reaction) and random primers (200 ng/reaction) in a 20-μl total reaction volume at 45°C for 50 min, according to the provided protocol. At the end of the reverse transcription reaction, the tubes were heated at 90°C for 5 min to stop the reaction and were then stored at −80°C until used in the PCR reactions for specific mRNA analyses.
Specific PCR primers to amplify target mRNA sequences were designed using PrimerSelect software (Lasergene, DNAStar) and the reference mRNA sequence from NCBI GenBank (see Table 2 for primer information). The forward and reverse primers were designed on different exons separated by large introns so that the GDNA product will separate from the cDNA PCR product. Primers were purchased from Operon Biotechnologies (Huntsville, AL). For each target, The PCR reactions were carried out using (0.1 to 1 μl cDNA) cDNA (corresponding to 5 to 50 ng of total RNA) in the presence of 2 mM MgCl2 by using standard PCR buffer (Bioline, Taunton, MA), 0.2 mM dNTP, 1 μM specific primer set, and 0.75 unit of DNA polymerase (Bioline) in 25 μl total volume. For each primer set, PCR conditions (cDNA dilution and PCR cycle number) were set to optimal conditions so that the target mRNA product yields were in the linear range of the semilog plot when the yield is expressed as a function of the number of PCR cycles, and, for a given condition, target mRNA PCR yields were tightly correlated to input cDNA (8).
Table 2.
PCR primers used in RT-PCR to amplify mRNA in skeletal muscle
| Target mRNA | PCR Primers, 5′ →3′ | PCR Product Size, bp | GenBank Accession No. |
|---|---|---|---|
| Actin | Fwd: GCGGGCATTCACGAGACCA | 214 | NM_001100 |
| Rev: CGCCGATCCACACCGAGTATT | |||
| Atrogin | Fwd: AAGGGCAGCTGGATTGGAAGAAGATG | 198 | NM_058229 |
| Rev: TGAACAAGTTGATAAAGTCCTGGGGTGAAA | NM_148177 | ||
| Myostatin | Fwd: CTACAACGGAAACAATCATTACCA | 243 | NM_005259 |
| Rev: GTTTCAGAGATCGGATTCCAGTAT | |||
| IGF-I | Fwd: AGTGCTGCTTTTGTGATTTCTT | 251 | NM_000618 |
| Rev: CAATACATCTCCAGCCTCCTTA | |||
| SERCA 1 | Fwd: GCGAGGCCACCGAGACAGCACT | 213 | NM_173201 |
| Rev: CCACAGCAGCCCGGGAAGATTT | NM_004320 | ||
| SERCA 2 | Fwd: ATGTATATCTTTTGTTTTGGCTTGGTTTGA | 219 | NM_170665 |
| Rev: GCTTTAATCCGCTGCACACTCTTTC | NM_001681 | ||
| MHC I | Fwd: GGTGCGGGAGCTGGAGAATG | 404 | NM_000257 |
| Rev: GGGGCTTTGCTGGCACCTC | |||
| MHC IIa | Fwd: GGGTACGGGAGCTGGAAGGAGAGG | 426 | NM_017534 |
| Rev: TTACAGAGGGAAATGACCAAAGATG | |||
| MHC IIx | Fwd: CAGGACACCAGCGCCCATCT | 524 | NM_005963 |
| Rev: TTTCTTTGGTCACCTTTCAGCAGTT | |||
| PGC1α | Fwd: CACCAGCCAACACTCAGCTAAGTTAT | 222 | NM_013261 |
| Rev: GGTGTGAGGAGGGTCATCGTTTGT | |||
| PPARδ | Fwd: GCCAGTACTGCCGCTTCCAGA | 221 | NM_006238 |
| Rev: GCGGGCCTTCTTTTTGGTCAT |
SERCA, sarcoplasmic reticulum Ca2+-ATPase; MCH, myosin heavy chain; PPAR, peroxisome proliferator-activated receptor.
Amplifications were carried out in a Stratagene Robocycler with an initial denaturing step of 3 min at 96°C, followed by 24–27 cycles of denaturing 1 min at 96°C, annealing 1 min at 59°C, and extending 1 min at 72°C, and a final extension step of 3 min at 72°C. PCR products were separated on a 2.5% agarose gel by electrophoresis and stained with ethidium bromide. The ultraviolet light-induced fluorescence of stained DNA bands was captured by a digital camera, and the band intensities were quantified by densitometry with ImageQuant software (GE Healthcare) on digitized images and were reported as arbitrary scan units as reported previously (11). In this approach, each specific mRNA signal is expressed in arbitrary units per ng of total RNA.
Calculations and Statistical Analyses
Within-group comparisons (groups defined as the same subjects at Wtinitial, Wt−10%, or Wt10%) were made by repeated-measures ANOVA. Before analyses were performed, normality of data distributions was confirmed by Wilk-Shapiro testing. Statistical significance was prospectively defined as Pα < 0.05. Data were analyzed using Statistica 6.0 software (60).
RESULTS
Subject Characteristics
Weight reduction was associated with a significant decrease in body mass index, fat mass, and fat-free mass and in circulating leptin concentrations (Table 1). We have previously reported that leptin administration to weight-reduced subjects results in a significant further loss of fat mass due to an increase in 24-h energy expenditure (46). While we observed a tendency to lose weight following leptin administration in the present study, differences in weight and body composition between subjects at Wt−10%leptin and Wt−10%placebo did not reach statistical significance.
Bicycle Ergometry and Muscle Biochemical Analyses
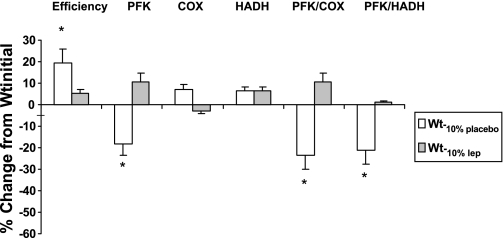
Clinically, at Wt-10%placebo there was a significant increase in greater skeletal muscle chemomechanical work efficiency and a significantly lower respiratory exchange ratio (indicating greater use of free fatty acids as fuel) at low levels of exercise by bicycle ergometry (Table 1, Fig. 2). Muscle efficiency and fuel utilization were largely normalized to Wtinitial values following leptin repletion but differences between Wt−10%leptin and Wt−10%placebo did not reach statistical significance. Biochemically, there was a significant decrease in the activity of the glycolytic enzyme, PFK, and in the ratios of glycolytic to fatty acid oxidative enzyme activity, whether expressed as the ratio of PFK to COX or PFK to HADH in muscle biopsy samples in subjects at Wt−10%placebo compared with both leptin replete states (Wtinitial and Wt−10%leptin) (Table 3).
Fig. 2.
Effects of weight loss and replacement administration of leptin on skeletal muscle work efficiency (calories expended per minute per watt of power generated during bicycle ergometry) and activity of glycolytic [phosphofructokinase (PFK)] and fatty acid oxidative [cytochrome c oxidase (COX) and hydroxyacyl CoA dehydrogenase (HADH)] in the vastus lateralis muscle of 10 subjects. Shown is the change from the initial value at the initial weight prior to weight loss in response to maintaining 10% body weight reduction with or without leptin treatment for ∼5 wk. *P < 0.05 compared with 0 and to Wt−10%leptin.
Table 3.
In vitro analyses of muscle biopsies of vastus lateralis
| Wtinitial | Wt−10%placebo | Wt−10%leptin | |
|---|---|---|---|
| Enzyme activity in 8 obese subjects (4 males, 4 females) | |||
| PFK, μM•min−1•g−1 | 47.9 (5.6) | 40.4 (5.0)* | 54.0 (4.9) |
| CK, μM•min−1•g−1 | 445.3 (28.5) | 434.8 (41.6) | 445.1 (29.2) |
| GAPDH, μM•min−1•g−1 | 455.8 (30.3) | 427.1 (49.6) | 465.6 (28.1) |
| CS, μM•min−1•g−1 | 7.7 (0.4) | 8.0 (0.6) | 6.9 (0.4) |
| HADH, μM•min−1•g−1 | 12.6 (1.2) | 12.8 (0.9) | 11.8 (0.4) |
| COX, μM•min−1•g−1 | 4.1 (0.4) | 4.5 (0.3) | 3.5 (0.5)† |
| Hexokinase, μM•min−1•g−1 | 2.1 (0.2) | 2.5 (0.2) | 2.2 (0.2) |
| Glycogen phosphorylase, μM•min−1•g−1 | 33.4 (3.1) | 34.9 (3.2) | 32.4 (2.2) |
| COX/CS | 0.5 (0.1) | 0.6 (0.1) | 0.5 (0.1) |
| HADH/CS | 1.6 (0.1) | 1.6 (0.1) | 1.7 (0.1) |
| PFK/COX | 13.2 (2.8) | 9.5 (1.5)* | 17.3 (2.3) |
| PFK/HADH | 5.1 (1) | 3.3 (0.4)* | 4.6 (0.4) |
| mRNA expression in 10 obese subjects (6 males, 4 females) in arbitrary units (AU)/ng RNA | |||
| Actin | 83.1 (4.8) | 84.0 (9.9) | 81.7 (9.8) |
| Atrogin | 25.6 (3.0) | 21.7 (3.3) | 19.3 (2.1) |
| Myostatin | 72.2 (13.5) | 61.1 (13.1) | 70.3 (13.1) |
| PGC1-α | 111.9 (8.4) | 104.5 (11.1) | 101.3 (12.0) |
| PPAR-δ | 20.4 (3.3) | 21.4 (3.3) | 20.9 (3.6) |
| IGF-I | 48.1 (5.5) | 46.4 (6.81) | 51.5 (5.3) |
| MHC I | 68.9 (7.4) | 86.0 (12.1)* | 75.4 (12.9) |
| MHC IIa | 90.5 (12.1) | 90.9 (11.3) | 99.8 (10.6) |
| MHC IIx | 78.7 (12.8) | 76.4 (11.9) | 89.4 (9.5)† |
| SERCA1 | 168.1 (19.1) | 157.6 (21.2) | 165.3 (19.6) |
| SERCA2 | 99.6 (18.3) | 131.8 (17.9)* | 115.6 (17.9) |
| MHC I/MHC IIa | 0.99 (0.22) | 1.00 (0.21) | 0.85 (0.18)‡ |
| MHC I/MHC IIx | 0.94 (0.17) | 1.13 (0.21)* | 0.98 (0.26) |
| MHC IIa/MHC IIx | 1.59 (0.38) | 1.36 (0.21) | 0.66 (0.08)*‡ |
| SERCA1/SERCA2 | 2.43 (0.53) | 1.44 (0.22) | 1.91 (0.40) |
Values are means ± SE. Weight loss and leptin effects on skeletal muscle. PFK, phosphofructokinase; CK, creatine kinase; CS, citrate synthase; HADH, hydroxyacyl CoA dehydrogenase; COX, cytochrome c oxidase.
P < 0.05 compared with Wtinitial;
P < 0.05 compared with Wt−10%placebo;
P < 0.01 compared with Wt−10%placebo.
Muscle Gene Expression
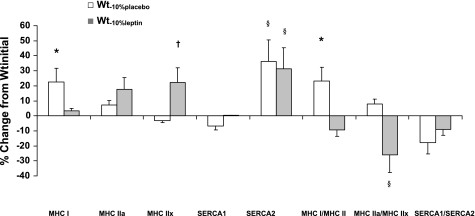
Subjects who were weight reduced without leptin repletion also demonstrated significantly increased expression of the more efficient MHC I isoform compared with the same subjects at Wtinitial or following weight loss but receiving leptin replacement (Wt−10%leptin) (Fig. 3, Table 3). Expression of the more efficient SERCA 2 isoform was significantly increased following weight loss but did not appear to be affected by leptin administration to weight-reduced subjects. No significant effects of weight loss or of leptin replacement on MHC IIa or IIx expression were noted. The ratio of MHCI/IIa (more efficient/less efficient) was significantly greater at Wt−10%placebo than at Wt−10%lep. The ratio of MHCI/IIx (more efficient/less efficient) was significantly greater at Wt−10%placebo than at Wtinitial. The ratio of MHC IIa/IIx expression (more efficient/less efficient) was significantly lower following leptin administration than in the same subjects at Wtinitial or Wt−10%placebo. The expression of the more efficient SERCA2 isoform was greater following weight loss and was not significantly affected by leptin repletion. Phenotype alterations increase in slow Type I MHC and SERCA2 mRNA expression occurred without any significant alterations in markers of muscle anabolic (IGF-I) or catabolic response (atrogin and myostatin) (Table 3). Furthermore, α-skeletal actin mRNA expression, which is fiber-type independent, remained unchanged in maintaining either a 10% weight reduction or in response to leptin treatment.
Fig. 3.
Effects of weight loss and replacement administration of leptin on (myosin heavy chain (MHC) mRNA isoform expression, as well as sarcoplasmic reticulum Ca2+-ATPase 1 (SERCA1) and -2 mRNA gene expression in the vastus lateralis muscle of 10 subjects. Shown is the change from the initial value at the initial weight prior to weight loss in response to maintaining 10% body weight reduction with or without leptin treatment for ∼5 wk. *P < 0.05 compared with 0 and to Wt−10%leptin, †P < 0.05 compared with Wt−10%placebo; §P < 0.05 compared with 0 and to Wt−10%placebo.
When data were analyzed by ANCOVA in which gender, somatotype, and weight plateau were each entered as dichotomous variables, no significant effects of gender or somatotype were noted.
DISCUSSION
Maintenance of a reduced or elevated body weight is associated with respective decreases and increases in 24-h energy expenditure and NREE (35, 49). These effects are predominantly due to changes in skeletal muscle work efficiency (1, 50) and are largely reversed by leptin repletion following weight loss (46). The major findings of the present study are: 1) maintenance of a reduced body weight is associated with a decreased activity of the glycolytic enzyme PFK, which is typical of more efficient skeletal muscle (23) and that is reversed by leptin repletion; and 2) maintenance of a reduced body weight is associated with changes in skeletal muscle gene expression, which are also consistent with greater skeletal muscle efficiency and some of which are reversed by leptin repletion. More specifically, the weight loss-associated increased expression of the more efficient MHC I isoform is reversed by leptin repletion, while the increased expression of the more efficient SERCA2 isoform does not appear to be affected by leptin. The observed effects of weight loss and of leptin repletion on skeletal muscle biochemistry and gene expression in vitro are all consistent with the changes that are observed in in vivo ergometry studies (23, 46, 50). We are not aware of any other studies that have reported these effects of either weight loss or leptin on skeletal muscle in humans. These novel findings are interesting with respect to muscle plasticity in the maintenance of a lower body weight and its implications to whole body physiology and energy metabolism.
As discussed in materials and methods, caloric intake at Wtinitial and following a 10% weight loss, was titrated to achieve a degree of weight stability that would be extremely difficult to sustain outside of a Clinical Research Center. This weight stability following weight loss is further demonstrated by the persistence of the resting respiratory exchange ratio at ∼0.85, which is the same value as the formula quotient of the liquid formula. The observation that leptin administration to weight-reduced subjects, which promotes a negative energy balance and further weight loss via increased energy expenditure on an isocaloric diet (46), does not result in a decrease in the respiratory exchange ratio due to increased oxidation of fatty acids (35) (Table 1) suggests that leptin administration affects muscle in a manner that blunts the increase in fatty acid oxidation that would normally occur during weight loss.
We (23) and others (32) have detected no changes in skeletal muscle fiber type following weight change when fiber types are qualitatively identified by histochemical staining for the predominant form of myofibrillar ATPase. This lack of change in skeletal muscle fiber type is not inconsistent with the results of this study. There is substantial evidence that changes in the exercise or hormonal milieu of muscle produce fiber-type specific changes in gene expression (4–6, 54, 58) without necessarily affecting the relative preponderance of the fiber types themselves. Direct assessment of changes in MHC isoform expression derived by high fidelity mRNA analyses is a more sensitive predictor of alterations in muscle function than assessment of changes in muscle fiber type. Since muscle fibers are classified by the predominant MHC isoform which is expressed, assessment of weight loss and leptin replacement effects on fiber type would be biased toward detecting changes in those fibers that are most mixed (closest to a 1:1 ratio of MHC I to II). Furthermore, the specificity of effects of weight loss and leptin replacement on MHC and SERCA is supported by the absence of an effect of these manipulations on levels of actin expression.
The leptin-responsive decline in the activity of PFK and the resultant decline in the ratio of glycolytic/oxidative enzyme ratios following weight loss are entirely consistent with the leptin-responsive increase in MHC I expression and the ratios of MHC I/MHC II and MHC IIa/ MHC IIx expression observed in weight-reduced subjects. A lower potential to oxidize glucose relative to fatty acids (as reflected in the lower PFK/COX and PFK/HADH ratios following weight loss) is characteristic of a great relative expression of the more efficient MHC I isoform (relative to MHC IIa or IIx) and MHC IIa (relative to MHC IIx) isoforms (5, 10). The increase in expression of the more efficient SERCA 2 isoform, which was not affected by leptin administration, would also contribute to the increased skeletal muscle work efficiency noted following weight loss.
Candidate signaling molecules that could account for these leptin-reversible changes in skeletal muscle physiology and biochemistry following weight loss are necessarily limited to leptin itself and molecules whose circulating concentrations are leptin-sensitive. Decreased circulating leptin concentrations following or during weight loss (34) may directly affect skeletal muscle, which expresses both the long and short leptin receptor isoforms (61). Direct effects of leptin on muscle could account for both the leptin-reversible decline in energy expenditure and utilization of glucose as fuel, since leptin directly stimulates thermogenesis (18), futile substrate cycling between lipogenesis and lipolysis (59), and glucose flux through the Krebs cycle in muscle (12, 13). However, central leptin administration also increases muscle thermogenesis (28), suggesting indirect effects of leptin on skeletal muscle.
Maintenance of a reduced body weight is associated with leptin-reversible declines in sympathetic nervous system tone and circulating concentrations of triiodothyronine (3, 46, 47), either or both of which may contribute to the observed leptin-mediated changes in muscle following weight loss. Declines in circulating concentrations of T3, working synergistically with decreased sympathetic nervous system tone, may constitute a mechanism by which the relative proportion of slow type I MHC versus fast type II MHC isoforms in muscle is increased following weight loss and reversed by leptin. Catecholamine administration induces increased expression of MHC II and decreased expression of MHC I in muscle (38, 39), while chemical sympathectomy in rats attenuates leptin-induced thermogenesis (17). There is a putative thyroid response element in the MHC I gene promoter, a mutation of which results in a loss of response of MHC I to T3 (65), and declines in circulating concentrations of bioactive thyroid hormones promote increased expression and protein content of MHC I and MHC IIa (10, 44), whereas MHC I gene expression is inhibited by T3 (19). Thyroid hormone may also affect and play a role in weight-loss induced changes in SERCA expression, which were not reversed by leptin-administration. The SERCA1 promoter gene contains multiple T3 response elements and the transcription rate of SERCA1 is increased fourfold in the presence of T3 in vitro (57). Hyperthyroidism induces an increase in SERCA1 expression accompanied by a decrease in SERCA 2, whereas hypothyroidism decreases both SERCA1 and SERCA2 expression and protein content (41, 57).
The meticulous maintenance of diet composition, fitness, and exercise patterns as well as the within-subjects design permitting each subject to serve as their own control significantly increase the sensitivity of this study to detect significant changes in skeletal muscle. However, there are a number of limitations to this study. First, the subject population is restricted to obese adults. While these results cannot be conclusively extrapolated to leaner populations, it should be noted that we have not detected significant differences in the metabolic responses to leptin administration or weight loss in previous studies of mixed populations of lean and obese subjects (35, 46, 47). Second, sample sizes were generally insufficient for assessment of protein content in addition to gene expression studies. In this regard, previous studies have reported that mRNA expression and protein content of different MHC and SERCA isoforms (1, 30) as well as PGC1α (6, 33) and peroxisome proliferator-activated receptor-δ (33, 45) vary similarly in response to exercise, weightlessness, hypothyroidism, and pulmonary disease. It is therefore likely that the maintenance of a reduced body weight is in fact associated with a leptin-sensitive increase in skeletal muscle MHC I protein content as well as in increase in SERCA 2 protein content that is not leptin sensitive. While most studies report concordance between the change in protein and mRNA levels; it has been reported that sometimes there is a discordance between some MHC mRNA and protein levels when determined at a single time point (4). This can be attributed to a dynamic state of transition whereby the mRNA change occurs faster than that of the protein. Clearly, further studies are needed which examine both lean and obese subjects and in which gene expression and protein content of muscle are both analyzed under different conditions.
Perspectives and Significance
These studies show that maintenance of an altered body weight is associated with in vivo and in vitro changes in skeletal muscle fuel utilization that are only evident at low levels of muscle work. Concordant changes are seen in vivo and in vitro and suggest possible mechanisms to account for the specific changes in skeletal muscle work efficiency that are present in individuals maintaining an altered body weight (50). They also suggest that interventions to decrease skeletal muscle work efficiency, either by exercise or pharmacotherapy, during and/or following weight reduction may circumvent some of the metabolic opposition to dynamic and sustained weight loss.
GRANTS
These studies were supported in part by National Institutes of Health Grants DK-64773, DK-26687, DK-37948, RR-00102, RR-00645, and ULR-024156.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K. M. B., D. R. J., F. H., R. L. G., D. G., K. H.P., E. L. S., R. L. L., and M. R. performed experiments; K. M. B., D. R. J., F. H., R. L. G., D. G., R. L. L., and M. R. analyzed data; K. M. B., F. H., R. L. G., D. G., R. L. L., and M. R. interpreted results of experiments; K. M. B., D. R. J., F. H., R. L. G., D. G., R. L. L., and M. R. edited and revised manuscript; K. M. B., D. R. J., F. H., R. L. G., D. G., K. H. P., E. L. S., R. L. L., and M. R. approved final version of manuscript; D. R. J., R. L. G., D. G., R. L. L., and M. R. conception and design of research; M. R. prepared figures; M. R. drafted manuscript.
ACKNOWLEDGMENTS
We thank our research subjects and members of the nursing and nutrition staffs of the Irving Center for Clinical Research at Columbia Presbyterian Medical Center for dedicated help with their care. We also thank Anqi X. Qin (University of California Irvine) for excellent technical service in the RNA analyses.
REFERENCES
- 1.Amati F, Dube J, Shay C, Goodpaster B. Separate and combined effects of exercise training and weight loss on exercise efficiency and substrate oxidation. J Appl Physiol 105: 825–831, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Physiological Society Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Aronne L, Mackintosh R, Rosenbaum M, Leibel R, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol 38: R222–R225, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Bahi L, Garnier A, Fortin D, Serrurier B, Veksler V, Bigard A, Ventura-Clapier R. Differential effects of thyroid hormones on energy metabolism of rat slow- and fast-twitch muscles. J Cell Physiol 203: 589–598, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Baldwin K, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol 90: 345–357, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Baldwin K, Haddad F. Skeletal muscle plasticity: cellular and molecular responses to altered physical activity paradigms. Am J Phys Med Rehabil 81, Suppl 11: S40–S51, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Barclay C, Constable J, Gibbs C. Energetics of fast- and slow-twitch muscle fibers of the mouse. J Physiol 472: 61–80, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bickel C, Slade J, Haddad F, Adams G, Dudley G. Acute molecular responses of skeletal muscle to resistance exercise in able-bodied and spinal cord-injured subjects. J Appl Physiol 94: 2255–2262, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Bottinelli R, Reggiani C. Human skeletal muscle fibre: molecular and functional diversity. Prog Biophys Mol Biol 73: 195–262, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Caiozzo V, Baker M, Baldwin K. Novel transitions in MHC isoforms: separate and combined effects of thyroid hormone and mechanical unloading. J Appl Physiol 85: 2237–2248, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Caiozzo V, Haddad F, Lee S, Baker M, Paloski W, Baldwin K. Artificial gravity as a countermeasure to microgravity: a pilot study examining the effects on knee extensor and plantar flexor muscle groups. J Appl Physiol 107: 36–46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceddia R, WIlliam W, Curi R. Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: evidence for an effect of leptin on glucose uptake and decarboxylation. Int J Obes 23: 75–82, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Ceddia R, William W, Curi R. The response of skeletal muscle to leptin. Front Biosci 6: D90–D97, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Colberg S, Simoneau J, Thaete F, Kelley D. Skeletal muscle utilization of free fatty acids in women with visceral obesity. J Clin Invest 95: 1846–1853, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costill D, Daniels J, Evans W, Fink W, Krahenbuhl G, Saltin B. Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol 40: 149–154, 1976 [DOI] [PubMed] [Google Scholar]
- 16.Crow M, Kushmerick M. Chemical energetics of slow- and fast- twitch muscle of the mouse. J Gen Physiol 79: 147–166, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobbins R, Szczepaniak L, Zhang W, McGarry J. Chemical sympathectomy alters regulation of body weight during prolonged ICV leptin infusion. Am J Physiol Endocrinol Metab 284: E778–E787, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Dulloo A, Stock M, Solinas G, Boss O, Montani J, Seydoux J. Leptin directly stimulates thermogenesis in skeletal muscle. FEBS Lett 515: 109–113, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Edwards J, Bahl J, Fink I, Cheng S, Morkin E. Thyroid hormone influences beta myosin heavy chain (beta MHC) expression. Biochem Biophys Res Commun 199: 1482–1488, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Fitts R, Widrick J. Muscle mechanics: adaptations with exercise-training. Exerc Sport Sci Rev 24: 427–473, 1996 [PubMed] [Google Scholar]
- 21.Gaesser GA, Brooks GA. Muscular efficiency during steady-rate exercise: effects of speed and work rate. J Appl Physiol 38: 1132–1139, 1975 [DOI] [PubMed] [Google Scholar]
- 22.Gibbs CL, Gibson WR. Energy production of rat soleus muscle. Am J Physiol 223: 864–871, 1972 [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith R, Joanisse D, Gallagher D, Pavlovich K, Shamoon E, Leibel R, Rosenbaum M. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am J Physiol Regul Integr Comp Physiol 298: R79–R88, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gollnick P, Armstrong R, Saltin B, Saubert C, Sembrowich W, Shepherd R. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol 34: 107–111, 1973 [DOI] [PubMed] [Google Scholar]
- 25.Gollnick P, Armstrong R, Saubert C, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol 33: 312–319, 1972 [DOI] [PubMed] [Google Scholar]
- 26.Hans Y, Geiger P, Cody M, Macken R, Sieck G. ATP consumption rate per cross bridge depends on myosin heavy chain isoform. J Appl Physiol 94: 2188–2196, 2003 [DOI] [PubMed] [Google Scholar]
- 27.He H, Giordana F, Hilal-Dandan J, Choi DJ, Rockman H, McDonough P, Bluhm W, Meyer M, Sayen M, Swanson E, Dillmann W. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J Clin Invest 100: 380–389, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry B, Andrews Z, Rao A, Clarke I. Central leptin activates mitochondrial function and increases heat production in skeletal muscle. Endocrinology 152: 2609–2618, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 292: 1568–1575, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Houdijk H, Bobbert M, deHaan A. Evaluation of a Hill based muscle model for the energy cost and efficiency of muscular contraction. J Biomech 39: 536–543, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Jaworski A, Porter M, Holmback A, Downham D, Lexell J. Enzyme activities in the tibialis anterior muscle of young moderately active men and women: relationship with body composition, muscle cross-sectional area and fibre type composition. Acta Physiol Scand 176: 215–225, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Kempen KP, Saris WH, Kuipers H, Glatz JF, Van Der Vusse GJ. Skeletal muscle metabolic characteristics before and after energy restriction in human obesity: fibre type, enzymatic β-oxidative capacity and fatty acid-binding protein content. Eur J Clin Invest 28: 1030–1037, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Kushmerick M. Energetics of muscle contraction. In: Handbook of Physiology, edited by Adrian R, Geiger S. Philadelphia, PA: Saunders, 1983, p. 189–236 [Google Scholar]
- 34.Leibel R, Chua S, Rosenbaum M. Obesity. In: The Metabolic and Molecular Bases of Inherited Disease, edited by Scriver C, Beaudet A, Sly W, Valle D. New York: McGraw-Hill, chapt. 157, 2001, p. 3965–4028 [Google Scholar]
- 35.Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Linossier M, Dormois D, Perrier C, Frey J, Geyssant A, Denis C. Enzyme adaptations of human skeletal muscle during bicycle short-sprint training and detraining. Acta Physiol Scand 161: 439–445, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Loukianov E, Ji Y, Baker D, Reed T, Babu J, Loukianova T, Greene A, Shull G, Periasamy M. Sarco(endo)plasmic reticulum Ca2+ ATPase isoforms and their role in muscle physiology and pathology. Ann NY Acad Sci 853: 281–289, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Lynch G, Hayes A, Campbell S, Williams D. Effects of β2-agonist administration and exercise on contractile activation of skeletal muscle fibers. J Appl Physiol 81: 1610–1618, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Lynch G, Ryall J. Role of β-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 88: 729–767, 2007 [DOI] [PubMed] [Google Scholar]
- 40.McArdle W, Katch F, Katch V. Exercise Physiology. Baltimore, MD: WIlliams & Wilkins, 1996, p. 101–116 [Google Scholar]
- 41.de Meis L, Arruda A, Carvalho D. Role of sarco/endoplasmic reticulum Ca2+-ATPase in thermogenesis. Biosci Rep 25: 181–190, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Najjar M, Rowland M. Anthropometric Reference Data and Prevalence of Overweight. Washington, D.C.: Department of Health and Human Services National Center for Health Statistics Series 11. No. 238, p 87–1688: 1987 [PubMed] [Google Scholar]
- 43.Pietrobelli A, Formica C, Wang Z, Heymsfield S. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol Endocrinol Metab 271: E941–E951, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Reggiani C, Potma E, Bottinelli R, Canepari M, Pellegrino M, Steinen G. Chemo-mechanical energy transduction in relation to myosin isoform composition in skeletal muscle fibres of the rat. J Physiol 15: 449–460, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricoy J, Encinas A, Cabello A, Madero S, Arenas J. Histochemical study of the vastus lateralis muscle fibre types of athletes. J Physiol Biochem 54: 41–47, 1998 [PubMed] [Google Scholar]
- 46.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel R. Low dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115: 3579–3586, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenbaum M, Hirsch J, Murphy E, Leibel R. The effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr 71: 1421–1432, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Rosenbaum M, Murphy E, Heymsfield S, Matthews D, Leibel R. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab 87: 2391–2394, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Rosenbaum M, Ravussin E, Matthews D, Gilker C, Ferraro R, Heymsfield S, Hirsch J, Leibel R. A comparative study of different means of assessing long-term energy expenditure in humans. Am J Physiol Regul Integr Comp Physiol 270: R496–R504, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau J, Heymsfield S, Joanisse D, Hirsch J, Murphy E, Matthews D, Segal K, Leibel R. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 285: R183–R192, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Saez L, Leinwand L. Characterization of diverse forms of myosin heavy chain expressed in adult human skeletal muscle. Nucleic Acids Res 14: 2951–2969, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Segal K, Presta E, Gutin B. Thermic effect of food during graded exercise in normal weight and obese men. Am J Clin Nutr 40: 995–1000, 1984 [DOI] [PubMed] [Google Scholar]
- 53. Sensormedics VMax Reference Manual. Yorba Linda, CA: Sensormedics, 1998 [Google Scholar]
- 54.Sieck G, Regnier M. Plasticity in skeletal, cardiac, and smooth muscle. J Appl Physiol 90: 1158–1164, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Simoneau J, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol Endocrinol Metab 257: E567–E572, 1989 [DOI] [PubMed] [Google Scholar]
- 56.Simoneau J, Colberg S, Thaete F, Kelley D. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J 9: 273–278, 1985 [PubMed] [Google Scholar]
- 57.Simonides W, Thelon M, VanderLinden C, Larsen P, Hardeveld C. Mechanism of thyroid-hormone regulated expression of SERCA genes in skeletal muscle: implications for thermogenesis. Biosci Rep 90: 139–154, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Simonides W, van Hardeveld C. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid 18: 205–216, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Solinas G, Summermatter S, Mainieri D, Gubler M, Pirola L, Wymann M, Rusconi S, Montani J, Seydoux J, Dulloo A. The direct effect of leptin on skeletal muscle thermogenesis is mediated by substrate cycling between de novo lipogenesis and lipid oxidation. FEBS Lett 577: 539–544, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Statsoft Statistica. Tulsa, OK: Statsoft, ver. 5, 1997 [Google Scholar]
- 61.Tartaglia L, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards G, Campfield L, Clark F, Deeds J, Muir C, Sanker S, Moriarity A, Moore K, Smutko J, Mays G, Wolf E, Monroe C, Tepper R. Identification and cloning of a leptin receptor, OB-r. Cell 83: 1263–1271, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Vandenborne K, Walter G, Ploutz-Snyder L, Staron R, Fry A, DeMeirler K, Dudley G, Leigh J. Energy-rich phosphates in slow and fast human skeletal muscle. Am J Physiol Cell Physiol 268: C869–C876, 1995 [DOI] [PubMed] [Google Scholar]
- 63.Wasserman K, Hansen J, Sue D, Casaburi R, Whipp B. Principles of Exercise Testing and Interpretation. Baltimore, MD: Lippincott Williams & Wilkins, 1999, p. 36 [Google Scholar]
- 64.Wiedermann F, Vielhaber S, Schroder R, Elger C, Kunz W. Evaluation of methods for the determination of mitochondrial respiratory chain enzyme activities in human skeletal muscle. Anal Biochem 279: 55–60, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Wright C, Haddad F, Qin A, Bodell P, Baldwin K. In vivo regulation of β-MHC gene in rodent heart: role of T3 and evidence for an upstream enhancer. Am J Physiol Cell Physiol 276: C883–C891, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Zurlo F, Nemeth P, Choksi R, Sesodia S, Ravussin E. Whole-body energy metabolism and skeletal muscle biochemical characteristics. Metabolism 43: 481–486, 1994 [DOI] [PubMed] [Google Scholar]