Abstract
Systemic lupus erythematosus (SLE) is a risk factor for hypertension. Previously, we demonstrated that an established mouse model of SLE (female NZBWF1 mice) develops hypertension with renal inflammation and oxidative stress, both characteristics known as contributing mechanisms to the development of salt-sensitive hypertension. On the basis of this model, we hypothesized that blood pressure in SLE mice would be salt-sensitive. Thirty-week-old female SLE and control mice (NZW/LacJ) were fed 8% high-salt (HS) diet or normal diet (0.4% salt) for 4 wk. Plasma levels of double-stranded DNA (dsDNA) autoantibodies, a marker of SLE disease activity, were increased in SLE mice compared with controls (472 ± 148 vs. 57 ± 17 U/ml × 1,000, P < 0.001). HS did not alter dsDNA autoantibody levels in SLE or control mice. Mean arterial pressure was increased in SLE mice compared with controls (132 ± 3 vs. 118 ± 2 mmHg, P < 0.001) and was not significantly altered by the HS diet in either group. Similarly, albuminuria was higher in SLE mice compared with controls (10.7 ± 9.0 vs. 0.3 ± 0.1 mg/day) but was not significantly increased in SLE or control mice fed a HS diet. In summary, blood pressure during SLE is not salt-sensitive, and the HS diet did not adversely affect SLE disease activity or significantly augment albuminuria. These data suggest that renal inflammation and oxidative stress, characteristics common to both SLE and models of salt-sensitive hypertension, may have diverging mechanistic roles in the development of hypertension.
Keywords: inflammation, systemic lupus erythematosus, high salt diet, immune, autoimmune
inflammation or immune system activation is now well recognized as an important factor in the development of essential hypertension. Salt-sensitive hypertension accounts for a large percentage of patients with essential hypertension, including an estimated 26 million in the United States (32). Recent work from experimental animal models of salt-sensitive hypertension demonstrates an important role for immune system activation in the kidney as a contributing mechanism. For example, renal tubulointerstitial and vascular inflammation is associated with salt-sensitive models of hypertension in rats (20), and more recent evidence shows that immunosuppressive therapy prevents the development of hypertension in Dahl salt-sensitive rats, in part, by reducing T lymphocytes in the kidney (6, 13).
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease that predominantly affects young women during reproductive years. The prevalence of hypertension is normally very low in young women (2–14%) of this age group (16a). This is not the case in patients with SLE, in which the prevalence often ranges from over 30% to as high as 74%, depending on the cohort (1, 4, 18, 26, 28, 29). While the mechanisms that promote hypertension in this population are not clear, we recently demonstrated that anti-inflammatory treatments in an established mouse model of SLE (female NZBWF1 mice) with hypertension reduces blood pressure, renal inflammation, and oxidative stress (30, 31). On the basis of the well-known immune/inflammatory contributions to renal disease and hypertension during SLE and to the development of salt-sensitive hypertension in rats, the purpose of the present study was to test the hypothesis that blood pressure during the chronic autoimmune disease SLE is salt-sensitive. Using the NZBWF1 model of SLE, we tested the hypothesis by assessing the relationship between salt intake and blood pressure (pressure natriuresis).
MATERIALS AND METHODS
Animals.
Female NZBWF1 (SLE) and NZW/LacJ (control) mice were obtained from Jackson Laboratories (Bar Harbor, ME) at 3–5 wk of age. Mice were raised on normal rodent chow (0.4% NaCl) until 30 wk of age. At 30 wk, mice with no evidence of renal injury (urinary albumin <100 mg/dl by dipstick) were included in the study. At this age, less than 8% of the animals have albuminuria. Animals were randomly divided into four groups receiving either a normal salt (NS) diet of 0.4% NaCl or a high salt (HS) diet of 8% NaCl (Harlan, Madison, WI) for 4 wk. The experimental groups were as follows: Control/NS, Control/HS, SLE/NS, and SLE/HS.
Mice were placed in metabolic cages weekly to collect a 24-h urine sample for assessment of albumin. The animals had access to food and water ad libitum and food intake was monitored in a subset of mice. Animals were maintained on a 12:12-h light-dark cycle in temperature-controlled rooms. At the end of the experiment (34 wk), a blood sample was collected via the arterial catheter in anesthetized mice followed by death. All studies were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Autoantibody production.
The presence of plasma anti-double-stranded DNA (dsDNA) antibodies, a clinical hallmark of SLE, was measured at 34 wk via ELISA, as previously described by our laboratory (30, 31). Only NZBWF1 mice with positive plasma anti-dsDNA autoantibodies were included in these studies. Mice with autoantibody levels greater than 1 SD higher than the mean antibody level from control mice were considered positive as previously described (30).
Blood pressure.
At 34 wk of age, catheters were implanted into the left carotid artery, and animals were allowed 24 h to recover from surgery. Blood pressure was recorded for at least 2 h in conscious, freely moving mice for up to two consecutive days as previously described by our laboratory (30, 31).
Renal injury.
Urinary albumin excretion was evaluated as an index of renal injury in 24-h urine samples using a commercial ELISA, as previously published by our laboratory (30, 31). Urinary albumin was multiplied by the 24 h urine volume to calculate the excretion rate, as previously described.
Statistical analysis.
Data are presented as means ± SE. All statistical analyses were performed using Sigmastat 3.0 software (Systat, Richmond, CA). A two-way ANOVA was used to compare treatment and group effects. A Holm-Sidak post-hoc test was used for comparisons between multiple groups. Values were considered statistically different at P values < 0.05.
RESULTS
Disease activity.
Consistent with our previous data, plasma levels of the characteristic dsDNA autoantibodies are increased in SLE mice compared with controls (Fig. 1; 472 ± 113 vs. 58 ± 14 U/ml × 1,000, P < 0.001). The production of autoantibodies in high-salt fed SLE mice (525 ± 208 U/ml × 1000) or high-salt fed control mice (118 ± 32 U/ml × 1000) was not significantly changed.
Fig. 1.
Effect of high-salt diet on systemic lupus erythematosus (SLE) disease activity. Plasma levels of dsDNA autoantibodies (Units/ml × 1,000) were significantly increased in SLE animals compared with controls (n = 12–18). High-salt diet did not significantly affect autoantibody production in control or SLE animals. *P < 0.05 vs. corresponding control.
Salt intake.
Food intake was not different between control (3.9 ± 0.1 g/day) and SLE mice (3.6 ± 0.4 g/day) fed a normal-salt diet, as we previously reported (8, 25), and therefore, salt intake was the same between control (0.27 ± 0.01 mmol/day) and SLE (0.25 ± 0.03 mmol/day) mice on this diet. SLE mice fed a high-sodium diet ate more than control mice on a high-salt diet (4.4 ± 0.2 vs. 3.0 ± 0.1 g/day, P < 0.05) and, therefore, ingested significantly more salt than controls fed a high-salt diet (6.01 ± 0.32 mmol/day vs. 4.04 ± 0.13 mmol/day, P < 0.001).
Blood pressure.
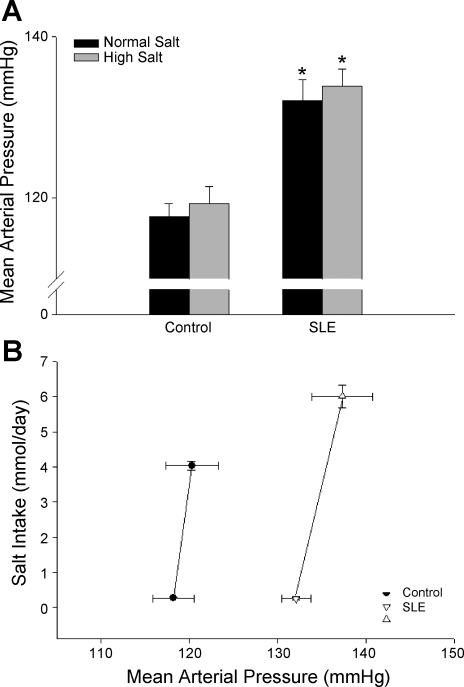
To assess the effect of salt intake on blood pressure during SLE, mean arterial pressure was measured in animals fed a normal- (0.4%) or high- (8%) salt diet (Fig. 2A). Consistent with our previous data, blood pressure was increased in SLE mice compared with controls (132 ± 3 vs. 118 ± 2 mmHg, P < 0.001). In high-salt fed mice, blood pressure was not significantly altered in either control or SLE mice (119 ± 2 and 134 ± 2 mmHg, respectively, P < 0.001). The data in Fig. 2B show blood pressure plotted vs. salt intake in a subset of the mice studied. These data show that the slope of the relationship between blood pressure and salt intake was not changed, thereby demonstrating that blood pressure during SLE is not salt-sensitive.
Fig. 2.
Effect of high-salt diet on blood pressure during SLE. A: mean arterial pressure (mmHg) was significantly increased in SLE animals compared with controls (n = 8–14). High-salt diet did not affect mean arterial pressure in control or SLE animals. *P < 0.05 vs. corresponding control. B: rightward parallel shift in the pressure natriuresis relationship (salt intake in mmol/day vs. mean arterial pressure in mmHg) was observed in a subset of animals (n = 4–8).
Renal injury.
Urinary albumin excretion was increased in SLE mice compared with controls (Fig. 3; 10.7 ± 9.0 vs. 0.3 ± 0.1 mg/day), as we previously reported (8, 30, 31). Although there was a tendency for elevated urinary albumin excretion, it was not statistically increased in SLE mice (19.7 ± 12.8 mg/day) or control mice (0.4 ± 0.1 mg/day) fed a high-salt diet.
Fig. 3.
Effect of high-salt diet on albuminuria during SLE: Urinary albumin excretion (mg/day) was increased in SLE animals compared with controls (n = 15–18). High-salt diet did not significantly increase albumin excretion in control or SLE animals.
DISCUSSION
In the present study, we examined whether increased salt intake affects blood pressure in a mouse model of SLE (pressure natriuresis). The major findings of this study are as follows: 1) SLE hypertension is not salt-sensitive; 2) high-salt intake does not significantly alter urinary albumin excretion in mice with SLE; and 3) a high-salt intake does not alter SLE activity, as assessed by the presence of circulating autoantibodies.
The potential for increased dietary salt to influence blood pressure has been recognized and studied for decades. Blood pressure is salt-sensitive in ∼26 million Americans (32), and many of the mechanisms that contribute to salt-sensitive hypertension have been reviewed (22, 27). Mattson and colleagues (6, 13) recently published a series of studies demonstrating an important role for the adaptive immune system in the development of salt-sensitive hypertension in rats. For example, chronic administration of mycophenolate mofetil (MMF) to Dahl salt-sensitive rats reduced blood pressure and renal injury and that this was associated with a decrease in renal T lymphocyte infiltration (6, 13). Similarly, Rodriguez-Iturbe's group (21) demonstrated that MMF prevented the salt-sensitive hypertension that occurs after chronic infusion with ANG II. That work showed that the prevention of salt sensitivity was accompanied by a decrease in interstitial infiltration of T cells and macrophages, and the reduction of inflammatory cells was associated with reduced oxidative stress (21). Therefore, renal tubulointerstitial inflammation and oxidative stress are important underlying mechanisms that contribute to salt-sensitive hypertension in the rat. Rodriguez-Iturbe has also recently advanced the concept that salt-sensitive hypertension may have autoimmune origins mediated, in part, by renal heat shock proteins (17). The notion that hypertension has autoimmune origins is not new, with several early reports showing increased serum levels of autoantibodies in patients and experimental animal models of essential hypertension (3, 7, 9).
The prevalence of hypertension in patients with the autoimmune disease SLE is very high (1, 4, 18, 26, 28, 29). Our recently published data show that treatment with etanercept to inhibit the biological activity of tumor necrosis factor alpha in NZBWF1 mice with SLE reduces blood pressure, oxidative stress, and renal inflammation (30). Given the significant evidence for renal inflammation and autoimmune reactivity in both humans and experimental models of salt-sensitive hypertension, we asked whether blood pressure in a mouse model of SLE with hypertension is salt-sensitive. On the basis of the similarities between the SLE model and established salt-sensitive models of hypertension, we anticipated that blood pressure would be salt-sensitive in the female NZBWF1 mice with SLE.
The hypertension observed in SLE mice fed normal chow is consistent with our published work (24, 30, 31), and the blood pressure values in control animals are comparable to those in a number of inbred mouse strains (23). A 4-wk administration of a high-salt diet did not significantly alter blood pressure in either experimental group, suggesting that the ability of the kidneys to handle a large salt load is not impaired. It is possible that a transient increase in blood pressure occurred early on in the experimental protocol; however, the physiological significance of such a change is difficult to know. Although blood pressure is not salt-sensitive, the possibility remains that blood pressure will become salt-sensitive as these mice progress toward end-stage renal disease and the loss of functional nephrons becomes severe. Therefore, the experimental design, excluding mice with preexisting renal injury is applicable to understanding the effect of dietary salt on blood pressure in patients with SLE who have not reached end-stage renal failure. The prevalence of end-stage renal failure in patients with SLE is typically low, ranging from less than 5% up to 22% depending on the cohort (14).
To confirm that we were, in fact, achieving a high-salt intake, food consumption was measured in a subset of mice fed both a normal- and high-salt diet. Food intake was not different between SLE and control mice on a normal-salt diet, as we previously published (8, 25). Food intake was not altered by high salt in control animals but was significantly higher in SLE mice. Therefore, SLE mice ingested a larger amount of salt, ruling out the possibility that blood pressure was not elevated due to a failure to achieve high-salt intake. An 8% sodium chloride diet was used in this study because this is the amount used in early studies to genetically select for salt sensitivity in rats (19). The salt intake achieved far exceeds the typical salt intake of 9.6 g per day (164 mmol/day) that might be observed in a typical Western diet (5) in humans, and, therefore, represents a significant salt challenge to the mice. The fact that blood pressure in SLE mice is not altered by this high level of salt intake is an indication that SLE is not associated with salt-sensitive hypertension. In animals fed the high-salt diet, there was a tendency for increased autoantibody production. Although this was not statistically different, it is provocative to speculate that increased dietary salt could promote humoral immunity and that perhaps a longer exposure to high salt could accelerate disease progression.
Renal interstitial inflammation and oxidative stress are commonly associated with salt-sensitive hypertension (6, 15, 21). The finding that blood pressure in mice with SLE is not salt-sensitive, despite our previously published reports showing renal inflammation and oxidative stress (30), may be revealing about the mechanisms that contribute to hypertension during SLE. Either renal inflammation and oxidative stress promote hypertension through a pathway different from salt-sensitive models or, perhaps, they occur secondarily to the hypertension. While the latter cannot be definitively disproved, several factors argue against blood pressure-induced renal injury and inflammation in this model. First, SLE is an immune complex-mediated disease that is initiated by a loss of immune tolerance and the production of autoantibodies. Therefore, immune system activation and inflammation are well-known factors that drive the development of SLE. Second, published studies demonstrate that lowering blood pressure in the NZBWF1 mouse is not necessarily associated with reductions in renal injury and inflammation (11). Third, we have data published in abstract form showing that bilateral renal denervation in NZBWF1 mice reduces renal injury but has no effect on blood pressure (12). Taken together, these data suggest that the renal injury, oxidative stress, and inflammation in SLE mice are not secondary to hypertension.
The parallel rightward shift in the pressure natriuresis relationship is indicative of a renal vascular mechanism that promotes the hypertension (10) and, in support of this, we have unpublished data showing that renal vascular resistance is increased in SLE mice compared with controls. The reasons that renal inflammation and oxidative stress do not associate with salt-sensitive hypertension in NZWBF1 mice, whereas they do in other rodent models are not clear; however, blood pressure in inbred mice is not typically salt-sensitive unless experimentally induced. Therefore, species-specific differences may account for the disparate blood pressure responses to dietary salt, even though both SLE mice and Dahl salt-sensitive rats have renal inflammation and oxidative stress in common.
Because SLE is a disorder that predominantly affects young women, only female NZBWF1 mice were included in this study. Therefore, these experiments did not address the important possibility that mechanisms of salt-sensitive hypertension may be sex-specific, for which there is evidence to support. For example, a recent study by Nakano and Pollock (16) showed renal infusion of endothelin-1 into female rats, but not males, increased sodium excretion and urine flow via the ETA receptor, suggesting that a mechanism to explain why young females are typically protected against salt-sensitive hypertension is related to differences in endothelin-mediated regulation of renal medullary flow. Published data from our laboratory show that urinary endothelin production is increased in mice with SLE (31). The mechanistic contribution of renal endothelin to the control of blood pressure during SLE is not clear and whether the regulation of medullary flow by edothelin helps to protect against salt-sensitive hypertension during SLE remains to be tested.
Perspectives and Significance
The present study shows that blood pressure in an established mouse model of SLE with hypertension is not salt-sensitive. This is an important finding as it furthers our understanding about the mechanisms that contribute to the development of hypertension in this chronic inflammatory disease. The data also suggest that characteristics common to both SLE hypertension and salt-sensitive models of hypertension (i.e., tubulointerstitial inflammation and oxidative stress) exert their effects on blood pressure through different mechanisms. The rightward parallel shift in the pressure natriuresis relationship suggests that the renal inflammation and oxidative stress observed by us and others during SLE may contribute to the hypertension through a renal vascular mechanism. Determining the factors that promote renal vascular changes and the parallel shift in the pressure natriuresis relationship during SLE will be important for better understanding the underlying reasons for the prevalent hypertension in this patient population.
GRANTS
These studies were supported by American Heart Association Postdoctoral Fellowships 4350019 (to K. W. Mathis) and 2260874 (to M. Venegas-Pont), as well as National Institutes of Health Grants HL085907 (to M. J. Ryan), HL092284 (to M. J. Ryan), HL085907S1 (to M. J. Ryan), and HL051971 (UMMC-Physiology).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Al-Herz A, Ensworth S, Shojania K, Esdaile JM. Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol 30: 493–496, 2003 [PubMed] [Google Scholar]
- 3.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol 128: 1211–1216, 1982 [PubMed] [Google Scholar]
- 4.Budman DR, Steinberg AD. Hypertension and renal disease in systemic lupus erythematosus. Arch Intern Med 136: 1003–1007, 1976 [PubMed] [Google Scholar]
- 5.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 81: 341–354, 2005 [DOI] [PubMed] [Google Scholar]
- 6.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frostegard J, Wu R, Gillis-Haegerstrand C, Lemne C, de Faire U. Antibodies to endothelial cells in borderline hypertension. Circulation 98: 1092–1098, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Gilbert EL, Ryan MJ. High dietary fat promotes visceral obesity and impaired endothelial function in female mice with systemic lupus erythematosus. Gend Med 8: 150–155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudbrandsson T, Hansson L, Herlitz H, Lindholm L, Nilsson LA. Immunological changes in patients with previous malignant essential hypertension. Lancet 1: 406–408, 1981 [DOI] [PubMed] [Google Scholar]
- 10.Hall JE, Mizelle HL, Hildebrandt DA, Brands MW. Abnormal pressure natriuresis. A cause or a consequence of hypertension? Hypertension 15: 547–559, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Herlitz H, Svalander C, Tarkowski A, Westberg G. Effect of captopril on murine systemic lupus erythematosus disease. J Hypertens Suppl 6: S684–S686, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Mathis KW, Venegas-Pont M, Ray WH, Dwyer T, Ryan MJ. Renal denervation reduces albuminuria independently of arterial pressure in systemic lupus erythematosus. Hypertension 56: E152, 2010 [Google Scholar]
- 13.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Mojcik CF, Klippel JH. End-stage renal disease and systemic lupus erythematosus. Am J Med 101: 100–107, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Nagasu H, Satoh M, Kuwabara A, Yorimitsu D, Sakuta T, Tomita N, Kashihara N. Renal denervation reduces glomerular injury by suppressing NAD(P)H oxidase activity in Dahl salt-sensitive rats. Nephrol Dial Transplant 25: 2889–2898, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension 53: 324–330, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.National Center for Health Statistics Health, United States, 2007. With Chartbook on Trends in Health of Americans. Hyattsville, MD: National Center for Health Statistics, 2007 [PubMed] [Google Scholar]
- 17. Parra G, Quiroz Y, Salazar J, Bravo Y, Pons H, Chavez M, Johnson RJ, Rodriguez-Iturbe B. Experimental induction of salt-sensitive hypertension is associated with lymphocyte proliferative response to HSP70. Kidney Int Suppl S55–S59, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Petri M. Detection of coronary artery disease and the role of traditional risk factors in the Hopkins Lupus Cohort. Lupus 9: 170–175, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension 7: 340–349, 1985 [PubMed] [Google Scholar]
- 20.Rodriguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Iturbe B, Romero F, Johnson RJ. Pathophysiological mechanisms of salt-dependent hypertension. Am J Kidney Dis 50: 655–672, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Ryan MJ, Didion SP, Davis DR, Faraci FM, Sigmund CD. Endothelial dysfunction and blood pressure variability in selected inbred mouse strains. Arterioscler Thromb Vasc Biol 22: 42–48, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Ryan MJ, McLemore GR., Jr Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 292: R736–R742, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Ryan MJ, McLemore GR, Jr, Hendrix ST. Insulin resistance and obesity in a mouse model of systemic lupus erythematosus. Hypertension 48: 988–993 2006 [DOI] [PubMed] [Google Scholar]
- 26.Sabio JM, Vargas-Hitos JA, Navarrete-Navarrete N, Mediavilla JD, Jimenez-Jaimez J, Diaz-Chamorro A, Jimenez-Alonso J, Grupo Lupus Virgen de las Nieves. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol 38: 1026–1032, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Sanada H, Jones JE, Jose PA. Genetics of salt-sensitive hypertension. Curr Hypertens Rep 13: 55–66, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sella EM, Sato EI, Leite WA, Oliveira Filho JA, Barbieri A. Myocardial perfusion scintigraphy and coronary disease risk factors in systemic lupus erythematosus. Ann Rheum Dis 62: 1066–1070, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selzer F, Sutton-Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension 37: 1075–1082, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56: 643–649, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR, Jr, Jones AV, Reckelhoff JF, Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 296: R1282–R1289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001 [DOI] [PubMed] [Google Scholar]