Abstract
Cardiac ventricular myocytes possess an extensive t-tubular system that facilitates the propagation of membrane potential across the cell body. It is well established that ionic currents at the restricted t-tubular space may lead to significant changes in ion concentrations, which, in turn, may affect t-tubular membrane potential. In this study, we used the whole cell patch-clamp technique to study accumulation and depletion of t-tubular potassium by measuring inward rectifier potassium tail currents (IK1,tail), and inward rectifier potassium current (IK1) “inactivation”. At room temperatures and in the absence of Mg2+ ions in pipette solution, the amplitude of IK1,tail measured ∼10 min after the establishment of whole cell configuration was reduced by ∼18%, but declined nearly twofold in the presence of 1 mM cyanide. At ∼35°C IK1,tail was essentially preserved in intact cells, but its amplitude declined by ∼85% within 5 min of cell dialysis, even in the absence of cyanide. Intracellular Mg2+ ions played protective role at all temperatures. Decline of IK1,tail was accompanied by characteristic changes in its kinetics, as well as by changes in the kinetics of IK1 inactivation, a marker of depletion of t-tubular K+. The data point to remodeling of t tubules as the primary reason for the observed effects. Consistent with this, detubulation of myocytes using formamide-induced osmotic stress significantly reduced IK1,tail, as well as the inactivation of inward IK1. Overall, the data provide strong evidence that changes in t tubule volume/structure may occur on a short time scale in response to various types of stress.
Keywords: inward rectifier potassium channels, cardiac myocytes
cardiac ventricular myocytes possess an extensive system of t tubules, which penetrate the whole body of the cell. One of the suggested roles of t tubules is facilitation of the propagation of Ca2+ influx across the cell body, leading to a synchronous (7), and likely more efficient, contraction. The idea that t tubules underlie synchronous Ca2+ release is strongly supported by the experiments showing that in atrial or Purkinje cells, which lack or have significantly undeveloped t-tubular system, the rise of the Ca2+ concentration in the center of the cells is delayed compared with that at the outer sarcolemma (2, 9). Moreover, detubulation of ventricular myocytes also leads to slowing of the Ca2+ increase in the center of the cells (3, 31).
Although t tubules occupy only a small volume of the cell, up to few percents in ventricular myocytes (1, 26), nearly one-half of the cell membrane may reside in this structure (24). While the presence of various ion channels and pumps in t tubules has been long recognized, recent studies showed a highly uneven distribution of some of them (4). For example, experiments using detubulation of cardiac ventricular myocytes showed that the density of Ca2+ current, and likely the Ca2+ channels, in t tubules may be approximately sixfold higher than that in the outer sarcolemma (5).
The nature's implementation of t tubules, however, does not come without a price due to some specific features of these structures. In particular, ion fluxes in a highly restricted t-tubular space may lead to significant changes in the concentration of specific ions, which, in turn, may affect t-tubular, and thus whole cell (WC), membrane potential. For example, Clark et al. (8) showed that the outward flow of potassium ions through voltage-gated potassium channels during membrane depolarization leads to significant accumulation of t-tubular potassium, which can be easily revealed by large inward currents flowing through inward rectifier potassium (IK1) channels upon membrane repolarization close to resting membrane potential [so-called IK1 tail currents (IK1,tail)]. While it seems that this phenomenon is “difficult to miss,” closer inspection of the data in several studies involving isolated ventricular myocytes reveals that the inward tail currents are either not shown, or may not be present in the first place. One of the explanations would be that the t tubules may be affected during isolation procedure or in the course of electrophysiological measurements due to unknown type of stress. Support for this hypothesis comes from the findings that the organization and density of t tubules can be affected on a “long run” in various pathological conditions, including ischemic stress and in heart failure (21).
Results of our study show that IK1,tail are not a constant feature of the ventricular myocytes and can quickly change under a number of experimental conditions, such as intracellular perfusion with common intracellular solutions, or by metabolic poisoning with, for example, cyanide (CN). Importantly, we found that IK1,tail may be significantly reduced, despite the continuing presence of outward potassium currents (the source of t-tubular K+ accumulation) and IK1 channels (the carriers of tail current). The data strongly implicate t-tubule remodeling, including complete vesiculation of t tubules and loss of membrane capacitance (CM), and suggest a novel potential mechanism that may underlie a number of relevant clinical conditions involving metabolic stress. The data also highlight IK1,tail and IK1 “inactivation” (IK1,inact) as important and useful markers of t-tubular integrity.
METHODS
Animals.
All experiments involving mice were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (8th Edition, Committee for the Update of the Guide for the Care and Use of Laboratory Animals, National Research Council; The National Academic Press, Washington, DC) and protocols approved by the veterinary staff of the University Committee on Use and Care of Animals at the University of Michigan.
Solutions.
Solutions used are as follows (in mM): modified Tyrode: 137 NaCl, 5.4 KCl, 0.5 MgCl2, 0.16 NaH2PO4, 3 NaHCO3, 5 HEPES, 5 glucose, pH = 7.35 with NaOH; solution A: 117 NaCl, 5.4 KCl, 4 MgCl2, 0.16 NaH2PO4, 3 NaHCO3, 20 HEPES, 10 glucose, 0.1 μM EGTA, pH = 7.35 with NaOH; solution B: 25 ml solution A + 15 mg collagenase; solution C: 45 ml solution A + 225 mg bovine serum albumin + 62.5 mg taurine; and KINT: 140 KCl, 2 EGTA, 1 CaCl2, 10 HEPES, 5 K2ATP, pH = 7.3 with KOH.
Mg2+ ions were added to pipette KINT solution as MgCl2 salt. Total concentrations are indicated in the text.
Isolation of mouse ventricular cardiomyocytes.
Myocytes were isolated from the hearts of adult C57BL/6 mice (n = 24) of either sex anesthetized with Avertin (20 μl/g; intraperitoneal injection) using collagenase treatment essentially as described previously (19). Minor modifications included the use of 0.1 μM EGTA in the Ca2+-free solution and 0.6 mg/ml collagenase (Type 2; Worthington) supplemented with 50 μM Ca2+ (added 2 min after the beginning of recirculation of 25 ml total volume) for digestion. The temperature of perfusate was kept at ∼27–28°C. Right ventricular wall was removed before mincing and further trituration of the remaining tissue in solution gradually transiting (in six ∼5-min steps) from solution B to solution C at ∼30°C. The myocytes, usually optimally isolated at steps 4–6, were stored in respective solutions at room temperature (RT) and used in experiments within 1–6 h postisolation.
Patch-clamp measurements.
Ionic currents were recorded in WC configuration (13) using an Axopatch 200B amplifier, Digidata 1322A and pClamp 8.2 software (Molecular Devices). Patch pipettes were pulled on a horizontal puller (Sutter Instruments) from a hematocrit glass tubes (KIMBLE glass; no. 73813). Pipette tips were not fire-polished. Pipette resistance varied from 1 to 2 MΩ when filled with KINT solution. Pipette tips were coated with a mixture of mineral oil and paraffin (while hot) to reduce stray capacitance of the pipette.
In most experiments, series resistance compensation was implemented at 10-kHz filter setting in a “simplified” way, without using “prediction” mode of the amplifier and nullifying the CM transient. Specifically, the fast component of capacitative current (IC) in the cell-attached mode was compensated first. After breaking the membrane, series resistance compensation knob was adjusted until the IC was close to a “ringing” mode, indicating the maximum possible compensation. Uncompensated series resistance estimated using single-exponential approximation (Clampex function) was typically <1 MΩ (down to ∼0.4 MΩ).
In experiments focused on measurements of electrical parameters of t tubules (appendix; see Fig. 7), the currents were filtered at 100 kHz, and uncompensated series resistance calculated using single-exponential fit to IC was typically <0.5 MΩ. In all experiments, the limiting, and unavoidable, factor was the resistance to t tubules themselves (which is in series with the pipette resistance). For example, the time constant of t-tubular component was ∼200 μs, whereas the sarcolemmal time constant was much smaller, ∼40 μs (appendix).
Despite all precautions, the estimated voltage errors at the peak of transient outward currents (up to 30–40 nA) are significant (up to 30–40 mV) but unavoidable. This, however, is essentially irrelevant for this study, since outward K+ currents were only used to produce t-tubular K+ accumulation. Voltage errors during the flow of IK1 and IK1,tail (few nA) were accordingly significantly smaller (but unavoidable again).
K+ currents were filtered at 2 kHz. Outward K+ currents and IK1,tail were sampled at 0.5-ms and IK1 at 4-ms intervals. In many experiments, a change of sampling rate was implemented during the voltage protocol. Undersampling (against Nyquist rate) did not affect the data in any significant way because of high signal-to-noise ratio of the currents. Applied voltages were not corrected for liquid junction potentials. Temperature of the perfusion solution was manipulated using inline heater (Warner Instruments). In a separate test, the exact temperature in the presumable location of the cell was confirmed directly in the flow chamber using a small insulated thermocouple probe (K-08113–28, Cole Parmer).
Measurements of CM.
In this study, total CM was routinely measured using Clampex built-in algorithm employing monoexponential fit to IC in response to change in membrane voltage (ΔVM) = 5 mV depolarizing voltage step from a holding potential of −75 mV. In some later experiments, studying detail kinetics of IC, total CM was also measured using integration of IC to estimate the total charge movement, Q, and to calculate CM according to CM ΔVM = Q relation. Since IC is clearly not a monoexponential process (see appendix), especially when recorded in normal ventricular myocytes with maximally engaged series resistance compensation of the patch-clamp amplifier (i.e., at higher frequency response), it may seem that the later technique is more appropriate than that employed in Clampex. However, due to the nature of the fitting algorithm, both approaches provide indistinguishable (P > 0.99) estimates of total CM. Therefore, for the sake of consistency, all CM values presented in this study (with the exception of CM data in appendix) were obtained using Clampex algorithm.
Measurements of IK1,tail and IK1,inact.
The accumulation/depletion of K+ is a time-dependent process reflecting a balance between complex K+ currents and diffusion of K+ from (or into) t tubules to (or out of) the extracellular space. Therefore, the following considerations were taken into account for practical purposes.
As shown in Fig. 1 at test pulses ∼400–500 ms, the tail current amplitudes reach a quasi-steady-state level, and, therefore, this time point was chosen in this study to avoid complications related to kinetics issues. Also, since the time constant of tail currents (which reflects diffusion rates of K+) is ∼50–80 ms (8), the contribution of the fast component of the transient outward K+ current (Ito,fast) to K+ accumulation is essentially eliminated at this (∼400 ms) time.
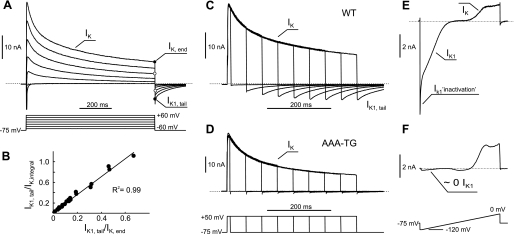
Fig. 1.
Major consequences of t-tubular K+ accumulation/depletion. A: activation of voltage-gated K+ channels by membrane depolarization leads to K+ accumulation in t tubules and appearance of tail currents upon repolarization to near resting membrane potential (VM). Larger outward K+ currents (IK) at the end of depolarizing voltage steps (IK,end) are associated with larger tail currents (IK1,tail), which flow through inward rectifier potassium current (IK1) channels. B: IK1,tail at the end of 400-ms test pulse (as in C) can be normalized to both amplitude of the outward IK, IK,end, and the integral of the outward IK (IK,integral), since both measures strongly correlate. The data are from 9 control and 15 detubulated myocytes. The units for Y-axis are in s−1. Absolute values (positive) are shown. C: at constant magnitude of depolarization step, the amplitude of tail currents increases as the duration of the step becomes larger. D: tail currents are essentially absent in ventricular myocytes from transgenic mice lacking IK1 (AAA-TG). E: membrane hyperpolarization from near-resting VM results in large inward IK1, which display prominent time-dependent “inactivation” (IK1,inactivation), likely due to K+ depletion in t tubules. Note: voltage ramp to 0 mV is preceded by 100-ms step to −120 mV (not clearly visible). F: IK1 are essentially absent in transgenic mice expressing dominant-negative Kir2.1 subunits (AAA-TG mice; Ref. 22). WT, wild type.
The choice of normalization procedures (below) is based on the fact that, at steady state, the K+ accumulation is largely determined, not by the integral of outward K+ current, but by its amplitude [i.e., essentially by K+ current at end of pulse (IK,end)].
IK1,tail were normalized to the amplitude of the outward current measured at the end of 400-ms depolarization step to +50 mV, IK,end. In practice, decline of IK1,tail was fit using two-exponential function, A1e(−t/τ1) + A2e(−t/τ2) + C, and the ratio IK1,tailN = (A1 + A2)/IK,end calculated, where t is time, τ1 is the time constant of the slow component, τ2 is time constant of fast component, A1 and A2 are amplitudes of individual exponential components, C is constant, and IK1,tailN is normalized IK1,tail.
The Δt ∼5 ms of current trace was excluded from the fit to avoid contribution from IC values, and therefore, the A1 and A2 are underestimated by e(−Δt/τ). With measured time constants and relative contributions of different components, the total amplitude of time-dependent part of IK1,tailN (A = A1 + A2) at time zero (beginning of repolarization) is underestimated by ≤15%.
IK1 was measured in response to voltage steps from Hp = −75 mV holding potential to various membrane potentials. The time course of IK1,inact was fit either with singe-exponential function (during 100-ms voltage step in early experiments) or with double-exponential function (during 400- to 500-ms voltage steps), as indicated in the results. The amplitude of IK1,inact was measured at Δt ∼ 8.5 ms after the beginning of voltage step. It follows from the measured time constants (e.g., at −120 mV) that the amplitude of inactivation at time zero may be underestimated by up to 40%. Therefore, the data were recalculated back to zero time and then normalized to the peak amplitude of IK1 as
or
Measurements and analysis of time-dependence of IK1,tail and IK1,inact.
Since the high-quality recordings in the WC configuration cannot be started immediately after breaking the membrane (e.g., due to necessary adjustments for series resistance compensation, measurements of CM, etc.), the earliest measurements of the currents were performed, on average, 1.7 ± 0.3 min after the establishment of WC. The data were also averaged over few recordings, taking additional ∼60 s.
Since the decline of measured currents in the WC configuration at high temperatures is quite quick compared with the time for necessary adjustments, the following strategy was implemented (see also Fig. 4A) in experiments involving 35°C. First, the WC configuration was established at RT, and initial recordings were performed just before the temperature change. Second, the temperature of the solution was changed to 35°C (over <1 min), and the next recordings were performed ∼5 min after the first ones. Therefore, the actual time during which the cell was exposed to high temperatures was significantly <5 min, and thus the effects are highly likely to be underestimated.
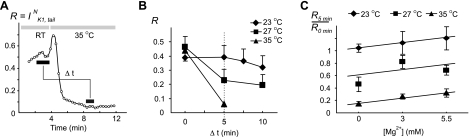
Fig. 4.
Effects of cell dialysis on IK1,tail. A: cell dialysis at 35°C leads to quick and significant decrease in the amplitude of IK1,tail (IK1,tailN). Because of the rapidity of the effect, ventricular myocytes were first voltage-clamped in the whole cell configuration at room temperature (RT), and the temperature was then increased to 35°C. Initial increase in IK1,tailN upon temperature step is likely due, at least in part, to temperature-dependent increase in IK1 (Table 1). Mg2+ ions were omitted in the pipette solution. B: experiments similar to that in A were carried out at other temperatures. At RT, IK1,tailN declined only by ∼18% after 10 min of cell dialysis. See methods for detailed description of the meaning of Δt. C: inclusion of Mg2+ ions in the pipette solution had a protective effect on IK1,tailN at all temperatures. In B and C, n = 5–7.
Detubulation of cardiac myocytes.
Detubulation of isolated cardiac myocytes was achieved using formamide-induced osmotic shock, essentially as described in Kawai et al. (17) with the following modification. Specifically, solution C (see Solutions) was used for both storage and detubulation (washout of formamide) steps. Qualitatively, the efficiency of detubulation was confirmed by confocal imaging (Olympus FV-500, Microscopy Laboratory, University of Michigan) of cardiac myocytes labeled with membrane-specific dye di-4-ANNEPS.
In this paper, the word “detubulation” primarily refers to the procedure described above, as well as to the corresponding end results. The effects of formamide-induced osmotic shock are strong, and the resulting vesiculation of t tubules would also be called detubulation. However, quantitatively, detubulation is clearly not an all-or-none phenomenon. Some t tubules may not be affected at all, while others may be completely vesiculated, such that even small molecules like di-4-ANNEPS dye may lose access to them. In the intermediate states, the lumen of t tubules may be wide enough to allow for small-molecule diffusion (thus allowing for membrane labeling), yet it may have electrical resistance significantly large for the t tubule to be considered essentially electrically disconnected from the outer sarcolemmal, leading to reduction in apparent (measured) CM. Therefore, since membrane labeling and measurements of CM cannot be quantitatively compared, except for the extreme case of complete t-tubule vesiculation, the former experiments were only performed on cells detubulated using formamide treatment. With this in mind, the interpretation of the data should not lead to confusion.
Statistics.
Data are presented as means ± SE. Statistical significance was estimated using a two-tailed t-test, either nonpaired (e.g., as in experiments with detubulation) or paired (e.g., as in experiments with CN application). Throughout the paper, asterisks *, **, and *** represent P <0.05, <0.01, and <0.001, respectively.
RESULTS
Effects of accumulation/depletion of t-tubular K+.
The data in Fig. 1 highlight some major consequences of the presumable accumulation and depletion of K+ in t tubules of ventricular myocytes (similar results were observed in numerous experiments). As it has been shown by Clark et al. (8), outward K+ currents flowing on prolonged membrane depolarization lead to appearance of large, slowly decaying inward (tail) currents (τ ∼50–80 ms in Fig. 1A) upon repolarization to near resting membrane potential (Fig. 1A). The amplitude of tail currents is proportional to the amplitude of the outward currents of the same duration. Alternatively, with a constant depolarizing step, the amplitude of tail currents increases with the pulse duration and reaches maximum in ∼0.4–0.5 s (Fig. 1C). The data in Fig. 1B show that IK1,tail measured at the end of 400-ms test pulse can be normalized either to the IK,end (adopted in this study), or to the integral of the outward current.
Clark et al. (8) have performed a detailed analysis of the above phenomenon and provided unambiguous proof that 1) outward-flowing K+ currents (IK) lead to significant accumulation of K+ in t tubules; and 2) inward tail currents are carried exclusively by IK1 channels (IK1,tail), thus confirming their presence in t tubules. In further support of the latter, transgenic ventricular myocytes lacking IK1 (22) do not display any measurable IK1,tail (Fig. 1D). Clark et al. (8) have also shown not only that IK1 channels may serve as a tool to detect K+ accumulation, but that the inward flow of K+ through IK1 channels contributes significantly to the rate of clearance of accumulated K+. Consistent with the above, data in Fig. 1E show that IK1 at far negative membrane potentials display significant inactivation, likely reflecting depletion of t-tubular K+.
It follows from the above that the magnitude of IK1,tail is determined in a large degree by three independent parameters: 1) t-tubule structure (volume, internal constrictions, vesiculation, etc.); and the densities of t-tubular 2) outward K+ currents (e.g., Ito,fast, Ito,slow, etc.), which are the source of t-tubular K+ accumulation, and 3) IK1, which is a sensor of t-tubular K+ accumulation. Therefore, to isolate the effects originating from t tubules themselves, in the case of IK1,tail, the data should be normalized at least to the amplitude of outward IK,end (see Fig. 3B; methods).
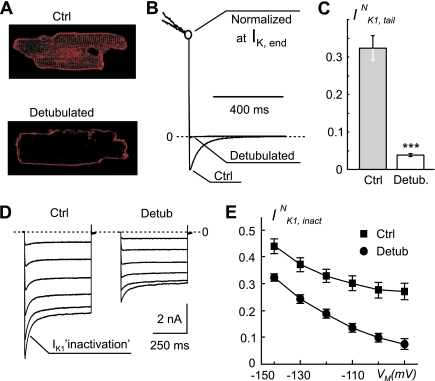
Fig. 3.
Effects of detubulated (Detub) on IK1,tail and IK1,inactivation. A: confocal images of control (Ctrl) and detubulated ventricular myocytes labeled with membrane specific dye di-4-ANNEPS. Detubulated leads to a loss of intramyocyte membrane labeling due to vesiculation of t tubules. B: current traces from randomly selected control and detubulated ventricular myocytes are normalized at the end of 400-ms voltage step to +50 mV to highlight significant decrease in the amplitude of IK1,tail in detubulated cells upon membrane repolarization to −75 mV. C: detubulated myocytes display nearly 8.5-fold reduction in IK1,tailN. n = 21 and n = 17 for control and detubulated, respectively. ***P < 0.001. D: representative records of IK1 measured in response to 500-ms voltage steps from −75-mV holding potential to potentials between −90 mV and −140 mV in control and detubulated myocytes. Prominent inactivation of IK1 in control myocytes is significantly decreased at all VM in detubulated cells. In addition, detubulated leads to significant decrease in total amplitude of IK1 (see results for detail). E: current traces in D were fit using two exponential function, and IK1,inactivation, IK1,inactN, was calculated as a ratio of the amplitude of time-dependent component of IK1 and the peak amplitude of IK1 at different VM. Detubulated leads to significant reduction in IK1,inactN at all VM. In D and E, n = 9 and n = 15 for control and detubulated, respectively.
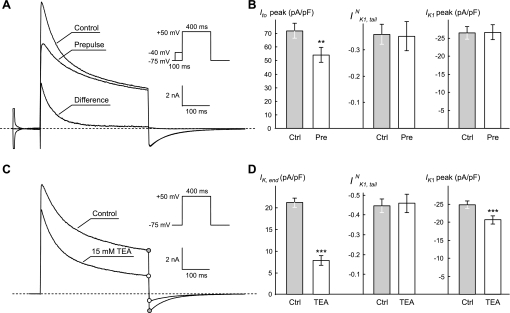
The data in Fig. 2 provide further support for the idea that the origin of the IK1,tail is the t-tubular accumulation of K+, which, under conditions adopted in this study, is largely dependent on the amplitude of IK,end. First, significant reduction of the amplitude of Ito,fast using 100-ms prepulse to −40 mV does not affect IK,end and IK1,tail. It is clear though that, in other experimental conditions (e.g., fast pulsing rates) or pathological situations (fast reentrant arrhythmia), the contribution of fast inactivating currents like Ito may be significant. Second, while application of 15 mM tetraethylammonium (TEA) suppresses both IK,end and IK1,tail by ∼64% (nearly ∼3-fold), the normalized tail currents (IK1,tailN) are essentially unchanged. It should be noted, however, that the TEA at this concentration also partially blocks IK1: ∼17% at −120 mV. The latter should be taken into account, since IK1 is a sensor of t-tubular K+ accumulation. Therefore, if secondary normalization (correction) of IK1,tail to the amplitude of IK1 is performed, the double-normalized IK1,tail would then be increased by the same 17%. Clearly, the effect of TEA on IK,end and thus on t-tubular K+ accumulation is dramatic in magnitude compared with its effect on double-normalized IK1,tail, thus further justifying the use of IK,end as a measure of K+ accumulation in the current experimental settings.
Fig. 2.
Effects of manipulation of outward IK. A: significant inactivation of the fast component of the transient outward IK (Ito,fast) using 100-ms prepulse from −75-mV holding potential to −40 mV (inset) does not affect IK1,tail recorded after 400-ms voltage step to +50 mV. B: quantification of the data from experiments in A. Peak Ito (Ito,peak) is reduced by ∼25%; P < 0.001, n = 5. **P < 0.01. C: application of 15 mM TEA (tetraethylammonium) leads to significant reduction of IK,end and proportional reduction of IK1,tail (denoted by circles). D: quantification of the data from experiments in C. The data show that nearly approximately threefold reduction in IK,end (P < 0.001) does not affect normalized IK1,tail (IK1,tailN). Note, that IK1 amplitude is decreased by ∼17% (P < 0.001) upon application of TEA; n = 7. IK1 amplitude density was measured at −120 mV. In solutions containing 15 mM TEA, NaCl concentration was reduced by 15 mM. ***P < 0.001. IK1,peak, peak IK1.
In this study, we have not performed detailed experiments to correlate the IK1,tail with other individual components of outward K+ currents. Also, in experiments below secondary, normalization to the amplitude of IK1 did not lead to further improvement of the analysis, suggesting no significant contribution of variation in IK1 density to the observed phenomena.
Detubulation decreases both IK1,tail and IK1,inact.
Removal of t tubules in cardiac myocytes by osmotic shock (3, 17) has been a useful tool in studying the membrane distribution of various ion channels (4). However, the effects of detubulation on IK1,tail and IK1,inact have never been studied. This is quite surprising for at least one reason: both IK1,tail and IK1,inact may serve as invaluable measures of the efficiency of detubulation procedure in individual myocytes, as opposed to simply relying on a suggestion that the procedure of detubulation is 100% efficient (see discussion).
As originally described by Kawai et al. (17), detubulation resulted in a significant reduction in t-tubular labeling with membrane-specific dyes, suggesting mechanical decoupling of t tubules from sarcolemmal membrane (Fig. 3A). Consistent with this, randomly selected (nonlabeled), presumably detubulated myocytes displayed significant reduction in both IK1,tail and IK1,inact (Fig. 3, B and D/E).
In contrast, the magnitude of IK1,inact does not depend on outward K+ currents, but rather on 1) t-tubules structure, and 2) the density of t-tubular IK1 itself. Accordingly, for the analysis of IK1,inact, the data were normalized to the peak amplitude of IK1 at test membrane potentials.
The data (Fig. 3C) show that detubulation leads to ∼8.5-fold reduction in IK1,tailN, as well as to significant reduction in normalized IK1,inact (IK1,inactN) (Fig. 3, D and E). It is important to note that, although the reduction in IK1,tailN was quite dramatic, yet it was not complete, and the reduction in IK1,inactN was disproportionally smaller, especially at far negative membrane potentials, further indicating incomplete detubulation in individual cells. The data are also consistent with the observation that some “detubulated” myocytes show measurable intracellular membrane labeling (not shown), in particular, closer to the outer sarcolemma. The average CM was also reduced by detubulation from 178.2 ± 5.6 to 110.1 ± 5.0 pF (P < 0.001), or by ∼38%. The decrease in CM is likely to be a minimum estimation, since incomplete vesiculation (detubulation) of individual t tubules would lead to significant increase in corresponding (t-tubular) time constant of IC (and thus decrease in its amplitude) to the extent it may not be reliably measured using the standard electrophysiological approach. In contrast, IK,end and IK1 densities were not affected by detubulation: 34.1 ± 2.6 vs. 33.2 ± 2.7 pA/pF and 33.0 ± 1.6 vs 32.6 ± 1.7 pA/pF, for IK,end and IK1 before and after detubulation, respectively. With regard to IK1, the data are consistent with the findings by Komukai et al. (18) in rat ventricular myocytes. It also follows directly from the data that IK1 is reduced by detubulation by the same magnitude as CM, since IK1 density is not affected.
Electrical parameters of t tubules.
Some important insights into the fine structure of t tubules can be inferred from the analysis of the IC recorded under conditions of maximally engaged series resistance compensation of the patch-clamp amplifier (i.e., at the fastest possible response time). The data can be analyzed and interpreted using the equivalent electrical circuit of ventricular myocyte under the WC conditions of the patch-clamp technique (see Fig. 7A, inset of appendix). T-tubular access resistance, Rt, should present itself as a second slower component of the IC. Detailed analytic and numerical analysis of IC is presented in the appendix. The data show that 31 ± 2% (n = 15) of the membrane is located in t tubules [t-tubular capacitance (Ct)], and that, in detubulated myocytes, this fraction is reduced approximately threefold (i.e., comprising in the end ∼10% of total CM). The true time constant of t-tubular component of IC (∼202 μs) is consistent with calculations using reasonable estimations of the average length (∼5 μm) and radius (∼100 nm) of individual t tubules. The data also provide the first experimental estimation of the Rt: ∼4.5 MΩ.
The data presented below show that both IK1,tail and IK1,inact can be affected by a number of conditions, which can collectively be considered as stress, thus strongly suggesting the involvement of t-tubular remodeling.
Effects of the temperature and Mg2+.
Original WC experiments were carried out at RT (∼21–23°C) using zero Mg2+ in the intracellular pipette solution. Under these conditions, there was no significant change in the amplitude of IK1,tail within 5 min, and only minor decline was observed at later times (Fig. 4B). However, at higher temperatures, there was a progressive increase in the rate of reduction of IK1,tail. Importantly, the decline of IK1,tail was not simply due to increase in the temperature, but rather due to a WC dialysis at high temperatures. This is supported by the fact that IK1,tail can be observed in myocytes incubated at RT for hours before establishing WC configuration. Incubating myocytes at ∼35°C before WC dialysis also did not affect the initial large-amplitude IK1,tail (not shown).
We have suggested that the absence of Mg2+ ions in the pipette solution may be one of the factors underlying the observed effects. Inclusion of Mg2+ indeed reduced the magnitude of IK1,tail decline (measured at 5 min after the beginning of cell dialysis) in a dose-dependent manner (Fig. 4B), although Mg2+ was clearly not the only or the major factor.
It might be argued that the observed decline of tail currents is due to inhibition of either IK (the source of K+ accumulation) or IK1 (the carrier of tail current) channels. The former scenario is essentially excluded, since the data are normalized to the IK,end. Also, measurements of IK1 show that its density is either not changed or even increased at high temperatures (Table 1). Therefore, the data strongly suggest the remodeling of t tubules as the underlying reason for the effect.
Table 1.
Effects of temperature on IK1, IK1 “inactivation”, and CM
| RT |
35°C |
|||||||
|---|---|---|---|---|---|---|---|---|
| [Mg] | 0 min | 5 min | % | <P | 0 min | 5 min | % | <P |
| IK1, pA/pF | ||||||||
| 0 | 35.6 ± 3.9 | 38.8 ± 5.0 | 7.7 | 0.05 | 39.8 ± 4.2 | 52.7 ± 6.2 | 31.5 | 0.01 |
| 5.5 | 30.1 ± 4.5 | 26.8 ± 3.0 | −9.2 | NS | 29.4 ± 1.5 | 35.4 ± 1.8 | 21.3 | 0.05 |
| IK1 “inactivation” (A/IK1) | ||||||||
| 0 | 0.30 ± 0.03 | 0.31 ± 0.03 | −3.7 | NS | 0.29 ± 0.02 | 0.07 ± 0.01 | −76.1 | 0.001 |
| 5.5 | 0.31 ± 0.04 | 0.32 ± 0.03 | −4.1 | NS | 0.39 ± 0.02 | 0.18 ± 0.02 | −54.4 | 0.001 |
| CM, pF | ||||||||
| 0 | 191 ± 25 | 190 ± 24 | −0.15 | NS | 149 ± 8 | 114 ± 6 | −23.5 | 0.001 |
| 5.5 | 171 ± 21 | 171 ± 18 | −0.24 | NS | 204 ± 26 | 159 ± 19 | −21.5 | 0.01 |
[Mg], Mg concentration; RT, room temperature; IK1, inward rectifier potassium current; A, amplitude; CM, membrane capacitance; NS, nonsignificant. %, an average percentage of change for individual cells (n = 5–7). P values were calculated using paired t-test.
A further support for the involvement of t tubules comes from the analysis of CM and IK1,inact at two different temperatures: RT (virtually no effects on IK1,tail) and 35°C (maximum effect). Results are summarized in Table 1. In this set of experiments, the time course of IK1,inact at −120 mV was fit using single-exponential approximation (100-ms voltage step), and the amplitude of IK1,inact was normalized to the peak IK1 amplitude. The data show that, at RT, and independent of Mg2+ concentration, neither CM nor IK1,inact was affected. In contrast, cell dialysis at 35°C led to ∼75% reduction in the magnitude of IK1,inact, accompanied by ∼25% decrease in CM, similar to that observed during detubulation. At 35°C, Mg2+ ions provided some protection against the action of high temperature, similar to that found for IK1,tail (Fig. 4C).
Effects of metabolic inhibition.
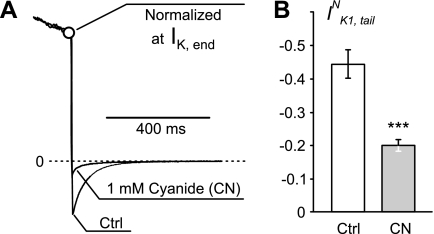
Mg2+ ions are known to be an important factor in the regulation of integrity and function of mitochondria (10), so it might well be the case that the beneficial effect of these ions was mediated through preservation of mitochondria failing under the WC dialysis. Increased temperature would likely lead to an increased load on mitochondria as well. Therefore, the rationale for further experiments was to test whether other general stress inducers with clear relation to mitochondrial function would have similar effects on tail currents and IK1,inact. Consistent with the above hypotheses, we found that application of CN, a well-known blocker of oxidative phosphorylation, led to significant and characteristic changes in both IK1,tail and IK1,inact, strongly pointing to remodeling of t tubules.
Figure 5A shows that, even at RT application of 1 mM, CN leads to more than twofold reduction in the amplitude of IK1,tailN at 10 min after the beginning of WC measurements. Again, this reduction was not due to inhibition of IK1 (Table 2; both total amplitude and the density of IK1 increased), but rather due to t-tubule remodeling. Modest reduction in IK1,tail, compared with that produced by formamide-induced detubulation or cell dialysis at 35°C, is consistent with smaller reduction in CM (∼7.3%; not statistically significant), suggesting vesiculation of some t tubules. An increased level of t-tubule constrictions is surely expected as well, although this may not be reflected in changes of apparent CM.
Fig. 5.
Effects of metabolic inhibition by cyanide (CN) IK1,tail. A: representative example of the effect of CN, a blocker of oxidative phosphorylation. Current traces were recorded from the same ventricular myocyte before and after 10-min application of 1 mM CN. The data are normalized at the end of 400-ms voltage step to +50 mV to highlight significant decrease in the amplitude of IK1,tail upon membrane repolarization to −75 mV. B: application of CN at RT results in more than twofold decrease of IK1,tail (IK1,tailN). In B, n = 16. ***P < 0.001.
Table 2.
Effects of cyanide on IK1 and IK,end
| 0 min | 10 min | %Change | P | |
|---|---|---|---|---|
| IK1 | ||||
| nA | 5.71 ± 0.38 | 6.43 ± 0.40 | 13.5 ± 3.5 | <0.05 |
| pA/pF | 33.6 ± 2.0 | 41.1 ± 2.5 | 22.7 ± 2.9 | <0.05 |
| IK,end | ||||
| nA | 3.79 ± 0.40 | 3.93 ± 0.44 | 3.28 ± 3.45 | NS |
| pA/pF | 21.4 ± 1.4 | 23.9 ± 1.6 | 11.9 ± 3.6 | <0.05 |
IK,end, K+ current at end of pulse. %Change, average %change for individual cells (n = 16). P values are calculated using paired t-test.
We also performed experiments where the myocytes were incubated with 1 mM CN at RT or at 36°C for 0.1–4 h before cell rupture. Consistent with the hypothesis, IK1,tailN were reduced to 0.34 ± 0.05 (n = 8) at RT and to 0.20 ± 0.04 (n = 9) at 36°C compared with 0.44 ± 0.04 (n = 16), the value obtained from cells continuously incubated at RT in the absence of CN. Clearly, longer exposure times were required for the effects of CN to be observed, consistent with more protective intracellular environment of intact myocytes compared with those used in WC dialysis experiments.
Since metabolic stress is associated with increases in intracellular Mg2+, it seems the protective role of these ions described in the previous section is consistent with significantly longer times necessary to affect IK1,tail in intact myocytes.
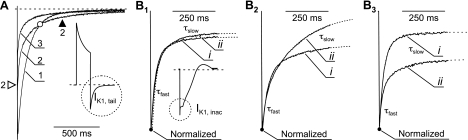
Details of IK1,tail inhibition during CN poisoning and the kinetics of IK1,tail itself are rather informative. In particular, in most cases the kinetics of IK1,tail can be best described by two (exponential) components, fast and slow, and in many cells the data show clear relations between them during CN application. It is likely that the slow component reflects diffusion of K+ from deep t tubules (e.g., axial t tubules), and the fast component reflects diffusion of K+ from transverse t tubules, proximal to the outside space. Figure 6A shows a representative example of so-called crossover effect of IK1,tail. Despite the reduction in the amplitude of IK1,tail, at some time during inhibition the late current is actually increasing. Quantitatively, this can be explained, for example, by increasing the time constant of slow component. Indeed, after ∼10 min in CN, the slow time constant was increased by ∼61%, from 83 ± 5 to 129 ± 15 ms (P < 0.01), while the fast time constant was not changed (20.3 ± 2.5 vs. 22.3 ± 2.1 ms; P > 0.05). However, not every cell displayed as clear “crossover” phenomenon as in the Fig. 6A, and of 16 analyzed cells, 3 displayed increase in the time constant of slow component. The amplitude fraction of slow component (Rslow)
where Afast and Aslow, the amplitudes of fast and slow components, respectively, varied widely from 1 (in 2 of 16 cells fast component could not be determined) to ∼0.2, and from 1 to ∼0.3 before and after CN application, respectively. While the averaged Rslow was not significantly affected (0.56 ± 0.05 and 0.49 ± 0.03, respectively; P > 0.2), there was a strong correlation (coefficient of determination, r2, ∼0.7) between the initial Rslow and the magnitude of its change (Rslowbefore − Rslowafter). Specifically, in cells with large initial Rslow, this fraction was reduced (e.g., from 0.83 to 0.68), and, alternatively, in cells with small initial Rslow, this fraction was increased (e.g., from 0.21 to 0.31). The data can be interpreted in the following way. Small Rslow values would be indicative of either 1) minimally or 2) maximally constricted t tubules (up until complete vesiculation of the deep distal part). In the first case, diffusion of K+ is fast in all t tubules. In the second case, strong constriction or complete vesiculation of deep t tubules essentially excludes them from accumulation/depletion process, leaving proximal t tubules as major players (fast diffusion). Therefore, during stress, myocytes with initially minimal t tubules constrictions (small Rslow) would first progress to an intermediate state of moderate t-tubular constrictions (large Rslow). Alternatively, myocytes with initially moderate t-tubular constrictions (large Rslow) would progress to a state where most of deep t tubules are effectively disconnected (small Rslow).
Fig. 6.
Effects of CN on the kinetics of IK1,tail and IK1,inactivation. A: time dependence of changes in the kinetics of IK1,tail (inset; IK1,tailN) during application of 1 mM CN from a selected experiment. Current recordings were sequentially taken before (1), during (2), and 10 min after (3) application of CN. The data highlight a so-called “crossover” phenomenon (○). At some point during inhibition of tail currents, the total amplitude of IK1,tailN is decreased (2; ▵), while the late current is increased (2; ▴). B: selected examples of changes of inactivation of IK1 before (i) and after (ii) exposure to CN for 10 min. Dotted lines represent the two-exponential fits to the data. B1: no significant change in both fast (τfast) and slow components (τslow). B2: significant increase in the amplitude of τslow. B3: significant decrease in the amplitude of τfast.
Detailed analysis of a related phenomenon of IK1,inact also revealed a number of important relationships (Fig. 6B). Within a time frame of 400 ms, IK1,inact can be best described by two-exponential function. Similar to IK1,tail, the time constant of slow component of IK1,inact was increased from 94 ± 9 to 129 ± 9 ms (P < 0.01) during CN poisoning, although the time constant of fast component was not significantly affected (21 ± 1 and 20 ± 1 ms, before and after CN application, respectively; P = 0.53). However, in contrast to the experiments with detubulation (Fig. 3), the total amplitude of IK1,inact was not affected. Instead, we found a shift in the contribution of individual components to IK1,inact. Specifically, the amplitude fraction of fast component of IK1,inact [Rfast = Afast/IK1(−120 mV)], was reduced by ∼25%, from 0.27 ± 0.02 to 0.20 ± 0.02 (P < 0.001), and thus the relative amplitude of slow component was equally increased. It should be noted that individual myocytes displayed both reductions and increases in the relative contribution of fast and slow components with average effect, as described above. This was not due to uncertainty in data fits or experimental error, but rather a reflection of a complex nature of t-tubule remodeling in individual myocytes. Since both IK1,tail and IK1,inact have their origin in t tubules, the same reasoning for the mechanism of redistribution of different components of IK1,tail (above) can be applied to IK1,inact.
DISCUSSION
T tubules are considered a constant feature of normal adult cardiac myocytes. It has been shown, however, that the t-tubular system can be significantly affected during several pathological conditions, including various types of heart failure (see Ref. 21 for review). Reduction in t-tubule density (15), disorganization (20), or even dilation (16) of t tubules was described. Unfortunately, with regard to the mechanisms underlying t-tubule remodeling in adult myocardium, most of the published work is in a great degree descriptive or correlative. It has been shown, for example, that suppression of junctophilin, a protein involved in the formation of the junctional complex between t tubules and sarcoplasmic reticulum (28) in cultured mouse ventricular myocytes leads to disorganization of t-tubular system (29). Structural proteins involved in t-tubule organization surely play a significant role in their remodeling (especially during development), but they are unlikely to be the sole cause of a general phenomenon in adult myocardium. Finding at least one of the common processes underlying t-tubule remodeling may prove to be difficult, since, during complex pathological conditions, numerous molecular pathways are being affected.
Results of our work suggest a hypothesis that t-tubule remodeling in adult myocardium during various types of stress may be a result of their mechanical reorganization likely mediated by metabolically stressed mitochondria. Here is a brief logic behind the hypothesis.
Osmotic shock (Fig. 3), cell dialysis with common pipette solutions at high temperatures (Fig. 4), and specific metabolic stress using CN (Fig. 5) all lead to quick changes in specific markers of t-tubule integrity (IK1,tail and IK1,inact).
The effects of osmotic shock are clearly mediated by mechanical (osmotic forces).
Similarly, mitochondrial swelling is a common consequence of various experimental conditions in which Mg2+ ions play essential protective role (10).
Cyanide poisoning (and generally most of the metabolic disturbances) has been shown to also lead to enlargement of mitochondria in the heart (27).
Therefore, swelling of mitochondria (under a variety of conditions) would likely lead to squeezing the lumen of a nearby t tubule (or even its complete vesiculation), thus affecting K+ accumulation/depletion and corresponding K+ currents.
Clearly, there are likely numerous other stresses (e.g., oxidative) that may lead to changes in mitochondrial volume, and thus the described phenomena should be taken into account accordingly.
A number of previous findings are consistent with the “mitochondrial” hypothesis. Mitochondria are relatively large organelles, some spanning the whole sarcomere length. Numerous ultrastructural studies show that mitochondrial wall is in a very close opposition to t tubules (e.g., Ref. 25). In fact, tight contacts were identified between mitochondrial outer membranes and t-tubular membranes, or between mitochondrial outer membranes and junctional sarcoplasmic reticulum, which is tightly associated with t tubules (14). Cross section of t tubules is clearly not a perfect circle, and t tubules may appear significantly squeezed or distorted in various ways, as observed in electron microscopy images (1). Also, frequent constrictions were observed in confocal microscopy studies (25). Savio-Galimberti et al. (25) speculated that these constrictions may be associated with dyadic junctions, although we are tempted to suggest that opposing mitochondria is at the origin of this phenomenon. In fact, it is quite hard to imagine how mitochondria may not be involved in this kind of mechanical effects, knowing their ability to respond quickly to various metabolic challenges by volume changes.
Unfortunately, direct observation of mitochondria-dependent squeezing of t tubules in intact cardiac myocytes is challenging, if not possible at all. In particular, optical microscopy does not currently allow for assessment of dynamic changes in t tubules, as well as for high spatial and temporal resolution of small changes in the size of mitochondrion (which should be on the order of t-tubule diameter). In this regard, electrophysiological (indirect) approach employing measurements of CM and t-tubular K+ currents as effectors and sensors of K+ accumulation/depletion (and thus the state of t tubules) is very useful. Conclusions derived from electrophysiological data, however, need to be based on the best possible interpretation of the results. In particular, even mild squeezing of t-tubular lumen would surely lead to changes in K+ accumulation/depletion, but virtually no change in measured CM. Alternatively, severe but yet incomplete constrictions would lead to a decrease in measured CM, but will not prevent t-tubular membrane labeling with di-4-ANNEPS dye.
IK1,tail and IK1,inact are two other independent (of CM) measures of t-tubular system integrity. Surprisingly, a literature survey revealed that tail currents were not observed in a number of studies employing similar control experimental conditions (11, 30, 32). It follows from our results that prolonged intracellular perfusion, combined with potentially higher RTs (see Fig. 4A), would be a reasonable explanation of a lack of tail currents in some studies. In fact, in WC experiments performed at high temperatures (Fig. 4A) tail currents would likely be missed if the experimenter would spend more than few minutes to adjust the equipment for recordings.
We have first confirmed that tail currents (Fig. 1) do exist (12), that they are exclusively due to K+ accumulation in t tubules, and are carried by t-tubular IK1 channels (Fig. 3; Ref. 8). As expected, detubulation led to manifold (>8×) reduction in IK1,tailN (Fig. 3), consistent with manifold (>2.8×) reduction in t-tubular membrane fraction. Tail currents may also be reduced due to 1) inhibition of a specific outward K+ current (or currents) responsible for K+ accumulation, or 2) inhibition of IK1 (the carrier of tail currents), or 3) even by expansion of t tubules (leading to reduced K+ accumulation). None of these options, however, seems viable, since the densities of both 1) IK,end and 2) IK1 were not affected, while 3) CM was significantly reduced by detubulation. The data on the t-tubular density of IK1 are similar to that reported in rat by Komukai et al. (18). However, it was found in the above study that steady-state outward K+ current is highly concentrated in rat t tubules (i.e., reduced by detubulation), which is in contrast to our finding that, in mouse, IK,end (the total current) is not affected by detubulation.
It also should be noted that the magnitude of the effects of t-tubular accumulation is likely to be different in the hearts of different animals, including larger mammals. First of all, the composition and the properties of the outward K+ currents (channels) are clearly different (see Ref. 23 for review) as may be their membrane distribution (largely unknown). Secondly, t-tubular structure may be quite different as well. For example, confocal microscopy studies showed that, in rabbit ventricular myocytes, the average diameter of t tubules is twofold larger compared with that in rat ventricular myocytes (25).
IK1,inact is not dependent on outward K+ currents, and thus the interpretation of relevant results is simplified. In the first approximation, one would suggest that the magnitude of decrease in IK1,tailN and in IK1,inactN should be comparable, since both primarily reflect the diffusional properties of K+ in t tubules. Nevertheless, the effect of detubulation on IK1,inact was generally significantly smaller, especially at far negative membrane potentials (Fig. 3, D and E). There may be several interpretations of this disagreement with several important conclusions. For example, the t-tubular location of voltage-gated K+ channels responsible for K+ accumulation may be different from that of t-tubular IK1 channels. Also, the effects of detubulation of IK1,inact may be “blunted” by less adequate voltage control in t tubules due to significantly larger IK1 compared with IK1,tail. This would also be consistent with smaller decrease in IK1,inact at far negative membrane potentials, where the IK1 is very large (up to ∼10 nA).
Kinetics of both IK1,tailN and IK1,inactN under normal conditions and during various types of stress are quite informative. It is clear that no measurable processes (e.g., tail currents) in a whole system of t tubules of varying lengths and diameters can be perfectly described by single- or double-exponential function. A good two-exponential fit to most of the data, however, does point to at least two clear types of underlying t tubules and/or distribution of relevant K+ channels. In particular, both IK1,tailN and IK1,inactN are characterized by fast and slow components. Importantly, under normal conditions, the time constants match each other pretty well: ∼20–21 ms and ∼83–94 ms for fast and slow components, respectively. As shown by Clark et al. (8), the clearance of accumulated K+ is accelerated by inward IK1, so to compare the kinetics of IK1,tailN and IK1,inactN one should take into account the magnitude of IK1 in both cases. In this regard, both the amplitude of IK1,tail at −75 mV and IK1 at −120 mV are in a nanoampere range, thus justifying quantitative comparison and suggesting the common underlying t-tubular structure.
At this point, it seems reasonable to suggest that, for example, the slow components reflect K+ diffusion out of the deep axial compartment of t-tubule system, while fast components correspond to its radial (less deep) part. Further support for this scenario arrives from the analysis of the time course of kinetics change during CN-induced stress (Fig. 6). Specifically, we found that, during decline of the respective currents, the slow component becomes even slower, while no significant changes are observed in the “speed” of fast component. The hypothesis of swelling mitochondria squeezing the lumen of t tubules fits the observed phenomena nicely. On average, diffusion of K+ from deep (axial) t tubules should be significantly slowed (increased τslow) by creation of a “bottle-neck”, while the outer part would be still be exposed to the extracellular space (no change in τfast), where τfast and τslow are the time constants of corresponding components of tail currents. It may be argued that a bottle-neck phenomenon should lead to increased amplitude of the tail currents due to increased accumulation of t-tubular K+ during membrane depolarization (contrary to the data). The counterargument though is that the K+ accumulation itself may be reduced due to compromised membrane potential control (leading to reduced t-tubular K+ currents) in deep t tubules until the point when some t tubules are completely disconnected (detubulation). A strong bottle-neck effect would correspond to significantly reduced accumulation of K+ and very large τslow, which, in turn, would be largely determined by diffusion of K+ into the cell only through K+ channels (IK1) and relevant pumps/exchanges rather than by diffusion through t-tubular lumen to the outside solution. Without detailed modeling studies, however, it is premature to go beyond the above considerations aimed to assign some meaning to the observed components.
Some physiologically important conclusions can be further distilled from the results. In particular, the data in Fig. 6A show that, despite the decrease in the total amplitude of tail currents during metabolic stress, late currents may actually be significantly increased (so-called “crossover effect”). In intact myocytes firing action potentials, the voltage-clamp data in Fig. 6A can be interpreted in the following way: metabolic stress during repetitive firing may lead to abnormal accumulation of K+ in a subset of t tubules, followed by their depolarization, sufficient to activate voltage-dependent conductances, which, in turn, may lead to WC afterdepolarizations (delayed or early) and thus ultimately to cardiac arrhythmias. It seems that, under normal conditions, the effects of t-tubular K+ accumulation on action potential may be minor. In particular, Bu et al. (6) found that, in intact rat heart, optically measured action potential waveforms in t tubules and in the outer sarcolemma are indistinguishable. Specifics of experimental design in the above study, including the use of RT and low-stimulation frequency, might have contributed to not observing the expected phenomena, and, therefore, further studies are surely warranted.
In conclusion, the results of this study provide strong support for the significant and fast remodeling of cardiac t tubules in response to various types of stress.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-069052 (A. N. Lopatin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.C., F.W., and A.N.L. performed experiments; L.C., F.W., and A.N.L. analyzed data; L.C. and A.N.L. interpreted results of experiments; L.C. and A.N.L. prepared figures; L.C. and A.N.L. edited and revised manuscript; L.C., F.W., and A.N.L. approved final version of manuscript; A.N.L. conception and design of research; A.N.L. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Tiffany Yang, John Stephen, and Jean Park for excellent technical support during this study.
Present address of L. Cheng: 393 Xinyi Rd., Department of Pharmacology, Xinjiang Medical University, Urumqi, Xinjiang 830001, China (e-mail: lewisclf@gmail.com).
APPENDIX
Estimation of T-Tubular Fraction of CM
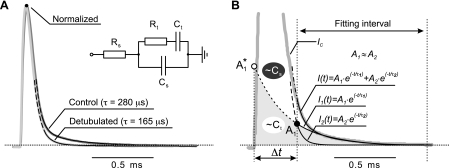
A simplified electrical circuit of ventricular myocyte in a WC configuration of patch-clamp technique, which accounts for the presence of t tubules, is shown in Fig. 7, inset. Rs is series resistance (primarily due to pipette resistance); Rt and Ct are t-tubular resistance and capacitance, respectively; and Cs is capacitance of outer sarcolemma. For the sake of simplicity, the intracellular resistance was assumed to be significantly smaller than Rs. T-tubular network is surely a complex structure with t tubules of varying length and radius, and thus the more sophisticated model may include distributed system of t tubules reflecting the available data. However, the limitations of current electrophysiological techniques are such that the precision of the measurements cannot match the proposed complexity of t-tubule system. Therefore, a simple scheme as in the Fig. 7A (inset) is the best practical electrical approximation of ventricular myocytes. It follows from this model that the IC should decay as a two-exponential process. Indeed, the data in Fig. 7A show that, in control ventricular myocytes with intact t tubules, IC displays a clear slow component preceded by a large-amplitude fast capacitative transient due to charging the outer sarcolemmal membrane. Consistent with t-tubular origin of the slow component, detubulation leads to its significant acceleration and decrease in amplitude.
Fig. 7.
Measurements of t-tubular electrical parameters. A: capacitative (gray colors) currents, IC, were recorded in response to 5-mV depolarizing step from a holding potential of −75 mV with fully engaged series resistance compensation (see methods). The currents were filtered at 100 kHz and sampled at 5 μs. 100 current traces were averaged, and late IC fit with two-exponential function (black lines), starting at a defined point in time as described in B and the text. A: IC were normalized at the peak amplitude for comparison purposes. Detub leads to significant decrease in slow component of IC. B: the starting point for fitting, Δt, varied in individual myocytes to fulfill the condition of similar amplitudes for both components (A1∼A2). Black solid lines represent the total fit, I(t), and individual components, I1(t) and I2(t), while dashed lines show corresponding extrapolations to earlier times. With this condition, both fitted components cross at ∼Δt (●). The slow component was extrapolated to zero time to estimate initial amplitude, A1* (○). The area corresponding to slow component (gray) is proportional to t-tubular membrane capacitance, Ct. The total area C under IC was calculated by integration of the current trace, and the sarcolemmal component, Cs, was calculated as C − Ct.
For quantification purpose IC were fit as described in Fig. 7B. Since IC are significantly distorted at early times by amplifier filtering (100 kHz) and because of very large amplitude of the fast component, only the late part of IC was fit. Details of the fitting procedure can be found in Fig. 7 legend.
The data show that, in control ventricular myocytes, the experimentally determined time constant of fast component (τ2) was approximately sixfold smaller (n = 15; P < 0.001) compared with that of the slow one (τ1): 226 ± 10 and 40 ± 2 μs, respectively. From the measured amplitudes and time constants of individual components of IC and the total integral of IC (which is invariant of filtering and any other potential distortions of the IC), the corresponding areas (integrals) of the two components can be calculated. Specifically, the measured fractional area of slow component (apparent fractional Ct) Cta = 0.46 ± 0.03 (n = 15). Cta is proportional, although not directly (see below), to the true value of t-tubular CM, Ct. Importantly, despite the time constants of two distinct components, which differ significantly (∼6 fold), it is not correct to assign all or even most of the integral of slow component to the t-tubular compartment (Ct) and vice versa. This is because t-tubular compartment (Rt, Ct; Fig. 7A, inset) is not in a parallel connection with sarcolemmal counterpart (e.g., Rs and Rt are connected serially).
The analytic solution (of the second-order differential equation) for the relevant time constants and amplitudes of the IC for the electrical scheme in Fig. 7A (inset) gives the following expressions.
Time constants, τ1 and τ2, can be determined as roots of the following quadratic equation:
| (A1) |
where
| (A2) |
and
| (A3) |
are true t-tubular and sarcolemmal CM values, respectively. It is clear that the time constants, τ1 and τ2, depend on all of the parameters of the scheme and do not represent the true t-tubular (RtCt) or sarcolemmal (RsCs) time constants, except in the limiting case when the difference between time constants is infinitely large. It is possible, however, to estimate the true underlying parameters (e.g., Ct and Rt) using measured time constants and corresponding amplitudes.
The amplitudes and time constants are linked by the following relations:
| (A4) |
This equation reflects the fact the total amplitude of IC is determined only by the current flowing through an uncharged capacitor, Cs.
| (A5) |
This equation states that the areas (integrals) Ai* τi of the corresponding components total the overall capacitance of the cell, CM = Ct + Cs.
From the above equations, the amplitudes of the individual components can also be calculated.
| (A6) |
and
| (A7) |
As in the case with time constants, the measured amplitudes (A1 and A2) are also functions of many parameters of the scheme and thus do not represent the amplitudes of true t-tubular or sarcolemmal components in isolation. The measured amplitudes, however, can be used to estimate the true underlying parameters (e.g., Ct and Rt).
The analytic solution of the problem using the above equations is impractical, however. Therefore, the equations were introduced into Microsoft Excel to numerically investigate the parametric space of the model. Specifically, the measured τ1, τ2, and Cta were used as target values in minimization procedures (Microsoft Solver) to estimate true Ct, Rt, and Rs.
Numerical analysis shows that with ∼6-fold difference between the time constants τ1 and τ2, the fractional area of the slow component (Cta) is ∼1.5-fold larger than that corresponding to true Ct, thus leading to a fraction of t-tubular membrane of 31 ± 2%.
Less intuitive, the measured time constants differ from those corresponding to t tubules and sarcolemma alone only by ∼12%: τt = RtCt ∼ 202 μs (vs. 226 μs) and τs = RsCs ∼ 45 μs (vs. 40 μs), respectively.
Rt and Rs were estimated at ∼4.5 and ∼0.4 MΩ, respectively. Estimation of Rs is very close to that measured experimentally during routine series resistance compensation in patch-clamp measurements. In contrast, Rt cannot be measured directly, and thus the corresponding value above represents the first practical estimation of this parameter in (mouse) ventricular myocytes.
Analysis similar to that above was also performed with detubulated myocytes. We found that detubulation was associated with ∼17% decrease in τ1 and ∼16% decrease in τ2, ultimately leading to ∼2.8-fold decrease in t-tubular fractional area (to ∼11% of total CM).
REFERENCES
- 1. Amsellem J, Delorme R, Souchier C, Ojeda C. Transverse-axial tubular system in guinea pig ventricular cardiomyocyte: 3D reconstruction, quantification and its possible role in K+ accumulation-depletion phenomenon in single cells. Biol Cell 85: 43–54, 1995 [PubMed] [Google Scholar]
- 2. Berlin JR. Spatiotemporal changes of Ca2+ during electrically evoked contractions in atrial and ventricular cells. Am J Physiol Heart Circ Physiol 269: H1165–H1170, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Brette F, Komukai K, Orchard CH. Validation of formamide as a detubulation agent in isolated rat cardiac cells. Am J Physiol Heart Circ Physiol 283: H1720–H1728, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Brette F, Orchard C. Resurgence of cardiac t-tubule research. Physiology (Bethesda) 22: 167–173, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Brette F, Salle L, Orchard CH. Differential modulation of L-type Ca2+ current by SR Ca2+ release at the T tubules and surface membrane of rat ventricular myocytes. Circ Res 95: e1–e7, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bu G, Adams H, Berbari EJ, Rubart M. Uniform action potential repolarization within the sarcolemma of in situ ventricular cardiomyocytes. Biophys J 96: 2532–2546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng H, Cannell MB, Lederer WJ. Propagation of excitation-contraction coupling into ventricular myocytes. Pflügers Arch 428: 415–417, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Clark RB, Tremblay A, Melnyk P, Allen BG, Giles WR, Fiset C. T-tubule localization of the inward-rectifier K+ channel in mouse ventricular myocytes: a role in K+ accumulation. J Physiol 537: 979–992, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cordeiro JM, Spitzer KW, Giles WR, Ershler PE, Cannell MB, Bridge JH. Location of the initiation site of calcium transients and sparks in rabbit heart Purkinje cells. J Physiol 531: 301–314, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dow DS, Walton KG, Fleischer S. Control of mitochondrial swelling by Mg2+. The relation of ion transport to structural changes. J Bioenerg 1: 247–271, 1971 [DOI] [PubMed] [Google Scholar]
- 11. DuBell WH, Lederer WJ, Rogers TB. K+ currents responsible for repolarization in mouse ventricle and their modulation by FK-506 and rapamycin. Am J Physiol Heart Circ Physiol 278: H886–H897, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Fiset C, Clark RB, Larsen TS, Giles WR. A rapidly activating sustained K+ current modulates repolarization and excitation-contraction coupling in adult mouse ventricle. J Physiol 504: 557–563, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 14. Hayashi T, Martone ME, Yu Z, Thor A, Doi M, Holst MJ, Ellisman MH, Hoshijima M. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. J Cell Sci 122: 1005–1013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res 49: 298–307, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Kaprielian RR, Stevenson S, Rothery SM, Cullen MJ, Severs NJ. Distinct patterns of dystrophin organization in myocyte sarcolemma and transverse tubules of normal and diseased human myocardium. Circulation 101: 2586–2594, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Kawai M, Hussain M, Orchard CH. Excitation-contraction coupling in rat ventricular myocytes after formamide-induced detubulation. Am J Physiol Heart Circ Physiol 277: H603–H609, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Komukai K, Brette F, Yamanushi TT, Orchard CH. K+ current distribution in rat sub-epicardial ventricular myocytes. Pflügers Arch 444: 532–538, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Lopatin AN, Shantz LM, Mackintosh CA, Nichols CG, Pegg AE. Modulation of potassium channels in the hearts of transgenic and mutant mice with altered polyamine biosynthesis. J Mol Cell Cardiol 32: 2007–2024, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, Sejersted OM. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol 574: 519–533, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Louch WE, Sejersted OM, Swift F. There goes the neighborhood: pathological alterations in T-tubule morphology and consequences for cardiomyocyte Ca2+ handling. J Biomed Biotechnol 2010: 503 906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McLerie M, Lopatin AN. Dominant-negative suppression of IK1 in the mouse heart leads to altered cardiac excitability. J Mol Cell Cardiol 35: 367–378, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev 85: 1205–1253, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Pasek M, Brette F, Nelson A, Pearce C, Qaiser A, Christe G, Orchard CH. Quantification of t-tubule area and protein distribution in rat cardiac ventricular myocytes. Prog Biophys Mol Biol 96: 244–257, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Savio-Galimberti E, Frank J, Inoue M, Goldhaber JI, Cannell MB, Bridge JH, Sachse FB. Novel features of the rabbit transverse tubular system revealed by quantitative analysis of three-dimensional reconstructions from confocal images. Biophys J 95: 2053–2062, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res 84: 266–275, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Suzuki T. Ultrastructural changes of heart muscle in cyanide poisoning. Tohoku J Exp Med 95: 271–287, 1968. 5707900 [Google Scholar]
- 28. Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell 6: 11–22, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res 107: 520–531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu H, Guo W, Nerbonne JM. Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J Gen Physiol 113: 661–678, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang Z, Pascarel C, Steele DS, Komukai K, Brette F, Orchard CH. Na+-Ca2+ exchange activity is localized in the T tubules of rat ventricular myocytes. Circ Res 91: 315–322, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Zhou J, Jeron A, London B, Han X, Koren G. Characterization of a slowly inactivating outward current in adult mouse ventricular myocytes. Circ Res 83: 806–814, 1998 [DOI] [PubMed] [Google Scholar]