Abstract
Herpes simplex virus type 1 (HSV-1) is a human pathogen of the alphaherpesvirus family which infects and spreads in the nervous system. Glycoproteins play a key role in the process of assembly and maturation of herpesviruses, which is essential for neuroinvasion and transneuronal spread. Glycoprotein B (gB) is a main component of the HSV-1 envelope and is necessary for the production of infectious particles. The cytoplasmic domain of gB, the longest one among HSV-1 glycoproteins, contains several highly conserved peptide sequences homologous to motifs involved in intracellular sorting. To determine the specific roles of these motifs in processing, subcellular localization, and the capacity of HSV-1 gB to complement a gB-null virus, we generated truncated or point mutated forms of a green fluorescent protein (GFP)-tagged gB. GFP-gB with a deletion in the acidic cluster DGDADEDDL (amino acids [aa] 896 to 904) behaved the same as the parental form. Deletion or disruption of the YTQV motif (aa 889 to 892) abolished internalization and reduced complementation by 60%. Disruption of the LL motif (aa 871 to 872) impaired the return of the protein to the trans-Golgi network (TGN) while enhancing its recycling to the plasma membrane. Truncations from residue E 857 abolished transport and processing of the truncated proteins, which had null complementation activity, through the Golgi complex. Altogether, our results favor a model in which HSV-1 gets its final envelope in the TGN, and they suggest that endocytosis, albeit not necessary, might play a role in infectivity.
Herpes simplex virus type 1 (HSV-1) is a human alphaherpesvirus which generally causes benign infections of the skin and mucosa. The virus also infects neurons of the sensory ganglia, where it establishes latency. HSV-1 periodically reactivates to cause peripheral recurrences and occasionally spreads transneuronally to cause severe central nervous system infections. Although the mechanisms of neuroinvasion and transneuronal spread of alphaherpesviruses are not fully understood, they seem to be linked to the process of assembly and maturation of virions in infected cells, in which glycoproteins play a major role (33, 56).
How HSV-1 particles are assembled and enveloped in infected cells is not completely known. The current view is that after primary envelopment at the inner nuclear membrane, capsids are de-enveloped through their exit from the nucleus and acquire their final envelope at the trans-Golgi network (TGN), where mature glycoproteins are incorporated (reviewed in reference 32). In support of this model, it has been shown that retention of glycoprotein D (gD) or gH in the endoplasmic reticulum (ER) of infected cells prevents their insertion into virions (6, 52, 61). Thus, proper intracellular transport of glycoproteins is necessary for successful incorporation into virions.
gB, one of the most conserved envelope glycoproteins among herpesviruses, is essential at many steps of the viral replication cycle. In vitro, gB is essential for attachment and entry of the virus into host cells, fusion, and cell-to-cell spread (42, 53), and its role in viral egress has been suggested (40). In vivo, HSV-1 gB is a potent inducer of the immune response (4, 20, 43, 47, 49, 50) and has also been involved in neuroinvasion (18, 34, 51, 62).
HSV-1 gB is a 904-amino-acid (aa) type I membrane glycoprotein composed of a 696-aa ectodomain, a 69-aa transmembrane domain, and a 109-aa carboxy-terminal domain. The cytoplasmic domain of gB is the longest among HSV-1 glycoproteins, which suggests that it might play a role during infection. Point or deletion mutations of the carboxy terminus of HSV-1 gB have been associated with syncytium formation (1), altered intracellular transport, and impaired assembly and egress of infectious viral particles (1, 7, 8, 10, 41). The cytosolic domains of several herpesviruses proteins contain motifs involved in their intracellular transport and subcellular localization. In particular, putative protein endocytosis motifs are present in all analyzed herpesviruses gBs (5, 57). Alignment of the HSV-1 gB amino acid sequence with that of its homologs reveals the presence of peptide sequences reminiscent of these motifs. To define their role, we used a deletion and point mutation approach to delineate domains involved in the intracellular transport of the protein and in the production of infectious particles. Members of our laboratory previously showed that a chimeric GFP-gB protein, containing the enhanced green fluorescent protein (EGFP) fused to the ectodomain of HSV-1 gB, is processed and localized in infected cells like native gB (44, 45). We constructed GFP-gB mutant forms and studied their processing, cellular localization, and capacity to complement a defective gB-null HSV-1.
MATERIALS AND METHODS
Cells and viruses.
The gB-null K082 virus (10) was propagated on the gB-expressing D6 cell line as described previously (44). Both the K082 virus and the D6 cell line were kindly provided by P. Desai and S. Person (Johns Hopkins University, Baltimore, Md.). The D6 cell line was grown in Dulbecco's modified essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 0.5 mg of G418 (Geneticin)/ml. Vero cells were grown in minimum essential medium supplemented with 5% FCS. Immunofluorescence assays were performed with Cos-7 cells, grown in DMEM plus 10% FCS. Cell culture reagents were all from Invitrogen (Cergy Pontoise, France).
Antibodies.
A rabbit polyclonal anti-gB antibody (R69) was generously provided by R. J. Eisenberg and G. H. Cohen (University of Pennsylvania, Philadelphia). A mouse monoclonal anti-GFP antibody was purchased from Roche (Mannheim, Germany). A sheep polyclonal anti- TGN 46 antibody was kindly provided by S. Ponnambalam (University of Leeds, Leeds, United Kingdom) or was purchased from Serotec (Oxford, United Kingdom). Cy3-coupled anti-rabbit immunoglobulin G (IgG), Cy3-coupled anti-mouse IgG, and Cy5-coupled anti-sheep IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, Pa.). Goat anti-mouse IgG peroxidase conjugate was from Sigma (Lyon, France).
Plasmids and constructions.
Plasmid pGFP-gB encodes HSV-1 gB fused to EGFP at its amino-terminal domain (44). Truncated forms of pGFP-gB were generated by PCR. Primers were designed to replace a native codon with a stop codon and to include the appropriate restriction sites. The primer used from the 5′ end for all constructions was 5′-TGAGAATTCCGTTACGTCATGCGGCTG-3′. The specific primers used from the 3′ end are summarized in Table 1. The resulting PCR products were cloned into the NheI and BamHI restriction sites in the gB cytoplasmic domain-encoding region of pGFP-gB. Site-directed mutations were generated by overlap extension PCR mutagenesis. Primers were designed to overlap and contain the desired mutations (see Table 1 for primer details). These primers were used separately with external primers (5′-TGAGAATTCCGTTACGTCATGCGGCTG-3′ in the 5′ direction and 5′-ACTGGATCCCAACCGGAGCAT-3′ in the 3′ direction) to amplify two DNA fragments flanking the desired mutation site within the pGFP-gB vector. The two amplified fragments containing an overlapping region of homology were mixed together, denatured, and amplified by PCR using the external primers. The final fragment was cloned into the NheI and BamHI restriction sites of pGFP-gB. Each mutation or truncation was verified by sequencing of the relevant region of the HSV-1 gB gene by the dideoxy chain termination method.
TABLE 1.
Oligonucleotides used to generate HSV-1 gB mutations
| Constructiona | Position of truncation or description of mutation | Oligonucleotide (5′ → 3′) |
|---|---|---|
| Truncations | ||
| Δ896 | From aa 896 to stop codon | ATCGGATCCTCATTTGTTGGGAACTTGGGT |
| Δ889 | From aa 889 to stop codon | TATGGATCCTCAGTTGGTGTTGCGGCGCTT |
| Δ871 | From aa 871 to stop codon | TATGGATCCTCACGCGCTCGTGCCCTTCTT |
| Δ857 | From aa 857 to stop codon | TATGGATCCTCACATGGCCGACACCAGGGC |
| Δ849 | From aa 849 to stop codon | TATGGATCCTCACCGTATCATCTCCCTGGC |
| Point mutations | ||
| Y889A | Y889 to A | CGCCGCAACACCAACGCCACCCAAGTTCCCAAC (5′) |
| GTTGGGAACTTGGGTGGCGTTGGTGTTGCG (3′) | ||
| LL871AA | L871 and L872 to AA | GGCACGAGCGCGGCGGCCAGCGCCAAGGTC (5′) |
| GACCTTGGCGCTGGCCGCCGCGCTCGTGCC (3′) |
Single-letter amino acid abbreviations are used. Numbers represent amino acid positions within HSV-1 gB, including its signal peptide.
Transient transfections.
Cos-7 cells plated on glass coverslips in 35-mm-diameter dishes were transiently transfected at subconfluence with 1 μg of plasmid pGFP-gB or a truncated or point mutated gB-encoding plasmid by use of FuGENE 6 (Roche). Twenty-four hours after transfection, the cellular localization of the EGFP fusion proteins was detected with a conventional Zeiss Axiophot fluorescence microscope, with standard fluorescein isothiocyanate excitation-emission filter sets.
Immunofluorescence surface staining.
Twenty-four hours after transfection as described above, cells were fixed with freshly prepared 4% (wt/vol) paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 20 min at room temperature. Cells were washed three times with PBS and then incubated with the anti-gB antibody at a dilution of 1/300 in 10% donkey serum for 1 h at 37°C. Coverslips were washed again three times before cells were labeled with the Cy3-coupled anti-rabbit antibody for 1 h at 37°C. After three rinses with PBS and one with water, coverslips were mounted onto glass slides and then analyzed under the conventional Zeiss Axiophot fluorescence microscope.
Immunoblot analysis and endoglycosidase treatment.
Forty-eight hours after transfection, cells in 35-mm-diameter culture dishes were washed twice with PBS and lysed for 20 min on ice in a solution of 50 mM Tris-HCl (pH 8), 62.5 mM EDTA, 1% Nonidet P-40 (NP-40), and 0.4% sodium-deoxycholate supplemented with 15 μg of antipain-dihydrochloride/ml, 2.5 μg of aprotinin/ml, 2.5 μg of pepstatin/ml, 5 μg of chymostatin/ml, 2.5 μg of leupeptin/ml, and 100 μg of Pefabloc (protease inhibitors; Roche)/ml. Endoglycosidase H (endo H) and peptide N-glycosidase F (PNGase F) digestions were performed with about 1/10 of the cell lysates for 2 h at 37°C as recommended by the supplier (New England Biolabs). Samples were run on denaturing sodium dodecyl sulfate-10% polyacrylamide gels and blotted onto nitrocellulose membranes. Membranes were saturated with 5% nonfat milk in TBST (10 mM Tris-HCl [pH 8], 150 mM NaCl, 0.05% Tween 20) and then incubated for 1 h with anti-GFP antibody at a dilution of 1:1,000 in TBST plus 1% bovine serum albumin (BSA). The secondary antibody, goat anti-mouse IgG coupled to horseradish peroxidase, was diluted according to the supplier's instructions. The immunoblots were revealed by enhanced chemiluminescence (ECL; Amersham).
Fluorescence internalization assay.
Internalization assays were performed essentially as previously described (39). Cos-7 cells were transfected as described above. Twenty-four hours after transfection, cells were washed with PBS at room temperature and then with PBS at 4°C containing 0.2% (wt/vol) BSA. Cells were incubated with anti-gB antibody (1/300 dilution in PBS plus 10% donkey serum) or anti-GFP antibody (1/100 dilution in PBS plus 1% donkey serum) for 1 h at 4°C. Cells were washed twice with PBS at room temperature and once with DMEM plus 10% FCS at 37°C. The culture medium was added again, and cells were placed at 37°C for several time intervals. After incubation at 37°C, cells were fixed with methanol for 4 min at −20°C. Controls that were not allowed to internalize were washed three times with cold PBS-0.2% BSA after incubation with the primary antibody and were fixed immediately. Cells were washed three times after fixation with PBS-0.2% BSA and then were incubated for 1 h with anti-TGN 46 antibody (1/200 dilution in PBS with 10% donkey serum). After three washes with PBS-0.2% BSA, cells were stained with Cy5-coupled anti-sheep antibody (1/500 dilution) and Cy3-coupled anti-rabbit or anti-mouse antibody (1/800 dilution). Coverslips were washed three times with PBS-0.2% BSA and once with water and were mounted onto glass slides. Fluorescence was analyzed with a Bio-Rad MRC 1000 confocal microscope.
Recycling assay.
Cos-7 cells were transfected and surface stained at 4°C with an anti-GFP antibody as described for the internalization assay. Cells were washed twice with PBS-0.2% BSA and once with DMEM-10% FCS. Medium was added, and cells were then incubated at 37°C for 20 min, except for the antibody-binding controls, which were immediately fixed with methanol for 5 min at −20°C. After incubation at 37°C, cells were washed once with PBS. The remaining extracellular protein-bound anti-GFP antibody was stripped by treatment with an acid-glycine-saline solution at pH 3 (NaCl, 8 g/liter; KCl, 0.38 g/liter; MgCl2 · 6H2O, 0.10 g/liter; CaCl2 · 2H2O, 0.10 g/liter; glycine, 7.5 g/liter). Cells were washed three times with PBS-0.2% BSA. DMEM containing 10% FCS was added, and cells were incubated at 37°C for 30 min. Antibody-stripping controls were instead immediately fixed with freshly prepared 4% PFA for 20 min at room temperature. After the second incubation at 37°C, cells were fixed with freshly prepared 4% PFA for 20 min at room temperature. Cells (including antibody-binding and -stripping controls) were washed three times with PBS-0.2% BSA. Cells were then stained with the Cy3-coupled anti-mouse antibody (1/800 dilution). Coverslips were washed three times with PBS-0.2% BSA and once with water and were mounted onto glass slides. Fluorescence was analyzed with a Bio-Rad MRC 1000 confocal microscope.
Complementation assay.
Complementation assays were performed essentially as previously described (9). Briefly, 1.2 × 106 Vero cells were seeded in 60-mm-diameter culture dishes and were transfected the following day with 0.5 μg of pGFP-gB or a truncated or point mutated gB-encoding plasmid by use of Lipofectamine (Invitrogen). Twenty-four hours later, cells were infected with K082 virus at a multiplicity of infection of 1 PFU/cell. One hour after infection, the remaining extracellular virus was washed off by treatment with an acid-glycine-saline solution (9). Infected cell culture supernatants were harvested 24 h after infection and were titrated on D6 and Vero cells. The titer obtained from K082 virus complemented with GFP-gB was considered the reference value, and the complementing capacity of the truncated or point mutated protein was then calculated as a ratio with respect to this reference value.
RESULTS
Intracellular localization of truncated gB proteins in transfected cells.
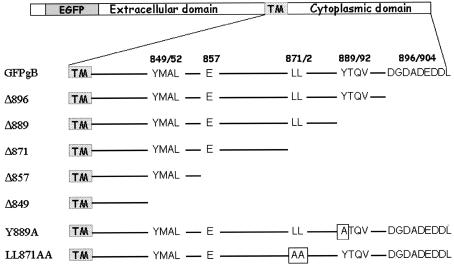
To analyze the function of the carboxy-terminal domain of gB, we constructed C-terminally truncated forms of a GFP-gB fusion protein, taking advantage of the fact that these proteins can be directly visualized in cells by fluorescence imaging. The cytosolic domain of gB contains putative intracellular transport motifs that are homologous to motifs found in other herpesviruses glycoproteins (5). Truncations were performed in order to successively eliminate these amino acid sequences (Fig. 1). By use of PCR, stop codons were inserted before a stretch of amino acid residues present at the carboxy terminus of the protein (Δ896), before the YTQV motif (Δ889), before the LL motif (Δ871), before a glutamate residue following the YMAL motif (Δ857), and before the YMAL motif (Δ849). The amplified products coding for these five truncated forms were cloned into the pGFP-gB parental plasmid, and the sequences were verified.
FIG. 1.
Representation of HSV-1 gB cytoplasmic domain. The top drawing depicts the EGFP-tagged gB. The line below depicts the cytoplasmic domain and the locations of highly conserved sorting motifs. The amino acid numbers bracketing the motifs are shown above. The bottom seven lines show the cytoplasmic domains of the mutated gB forms used in the study. Altered amino acids are shown in shaded boxes.
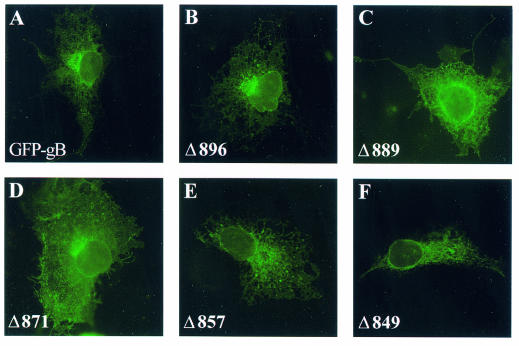
The subcellular localization of the truncated proteins in transfected Cos-7 cells was visualized by using the spontaneous fluorescence emitted by EGFP. For controls, images were compared to those obtained with the parental GFP-gB (Fig. 2). When observed by conventional fluorescence microscopy, the distribution of Δ896 was comparable to that of GFP-gB (compare Fig. 2A and B), i.e., the protein was present diffusely in the cytosol and also accumulated in the perinuclear region. The fluorescence observed in Δ889- and Δ871-expressing cells was markedly increased and continuous close to the cell surface, where it precisely lined the cell contours (Fig. 2C and D). In cells expressing Δ857 or Δ849 (Fig. 2E and F), GFP was only detected diffusely in the cytosol.
FIG. 2.
Comparative localization of GFP-gB and the truncated forms in transfected cells. Cos-7 cells were transfected with pGFP-gB or each of the truncated forms. Images show spontaneous GFP fluorescence 24 h after transfection. Fluorescence was visualized with a Zeiss Axiophot conventional microscope.
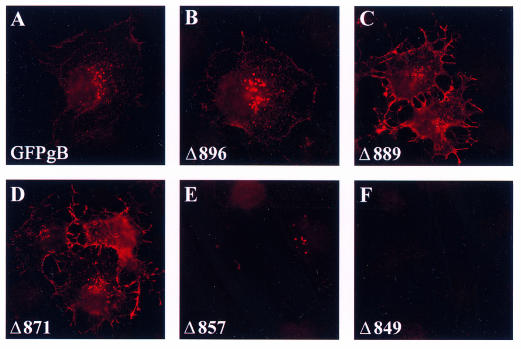
To determine whether the proteins reached the plasma membrane, we performed immunofluorescence experiments, using an anti-gB antibody in nonpermeabilized transfected cells. The Δ896 protein was detected at the cell surface in a punctate pattern, as previously described for parental GFP-gB (compare Fig. 3A and B) and for native gB (44). In cells expressing the truncated Δ889 and Δ871 proteins, the gB staining exhibited a continuous ringlike pattern at the cell membrane, suggesting that these deletion mutants accumulated at the cell surface (Fig. 3C and D). In contrast, Δ857 and Δ849 were not detected at the plasma membrane of any cell (Fig. 3E and F), suggesting that truncation from amino acid 857 prevented transport of the proteins to the cell surface.
FIG. 3.
Surface labeling of GFP-gB and the truncated forms in transfected cells. Cos-7 cells were fixed under nonpermeabilizing conditions 24 h after transfection with pGFP-gB or each of the truncated forms and were labeled with an anti-gB antibody and a Texas red-labeled secondary antibody. Fluorescence was visualized with a Zeiss Axiophot conventional microscope.
Posttranslational processing of truncated gB proteins.
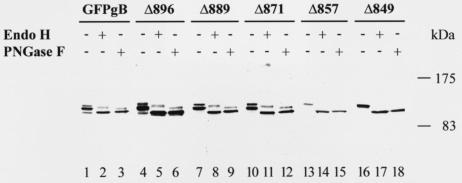
To further define the effects of truncations of the cytosolic domain on intracellular transport and maturation of gB, we analyzed the electrophoretic mobility of the truncated glycoproteins before and after endoglycosidase treatment. Lysates from Cos-7 cells expressing the parental GFP-gB or the truncated forms were treated with endo H or PNGase F and subsequently analyzed by immunoblotting with an anti-GFP antibody (Fig. 4). Before treatment, GFP-gB was detected essentially as a doublet migrating at about 140 kDa, with the lower and upper bands corresponding, respectively, to the high-mannose immature form and the mature form of the protein, which contains both high-mannose and complex-type oligosaccharides, since processing of gB is slow and incomplete (8, 35). The immature form displayed a high level of sensitivity to endo H (Fig. 4, lane 2), whereas the partial shift in mobility of the upper band reflects partial resistance to the enzyme, secondary to the acquisition of complex carbohydrates by the glycoprotein during its transit through the Golgi complex (45). For lysates containing the truncated Δ896, Δ889 and Δ871 proteins, two major bands were detected which displayed a mobility pattern identical to that of GFP-gB after endo H treatment, thus showing that these truncated forms are biochemically processed in the same way as the parental glycoprotein (Fig. 4, lanes 5, 8, and 11). In contrast, for lysates containing Δ857 and Δ849, only one major band was detected, which corresponded to the endo H-sensitive form of the proteins (Fig. 4, lanes 14 and 17). These results show that truncation from amino acid 857 impaired the transport of the resultant proteins through the Golgi complex and thus the late steps of their glycosylation.
FIG. 4.
Immunoblot analysis. Cos-7 cells were transfected with pGFP-gB or each of the truncated forms and were harvested 48 h later. Lysates were treated with endo H or PNGase F, as indicated above each lane, and subjected to denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunoblots were prepared and probed with an anti-GFP antibody.
Endocytosis of gB in transfected cells.
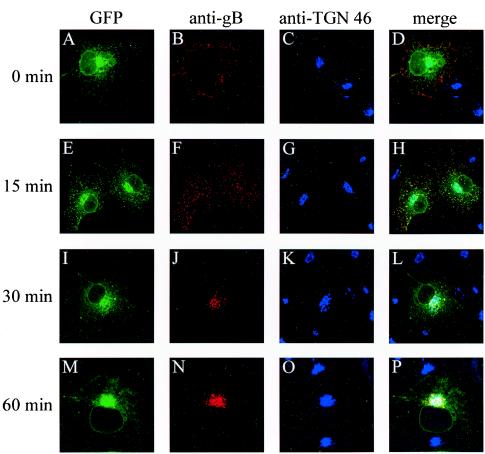
Several homologs of gB are internalized from the cell membrane to the Golgi complex. To investigate the endocytosis of HSV-1 gB, Cos-7 cells were transfected with pGFP-gB, incubated 24 h after transfection with an anti-gB antibody at 4°C for 1 h, and then shifted to 37°C to allow internalization of the gB-antibody complexes from the plasma membrane. At the same time, GFP fluorescence permitted us to visualize the level of expression and intracellular localization of the glycoprotein (Fig. 5A, E, I, and M). In cells that were not shifted to 37°C, referred to as time zero cells, GFP-gB was detected by the gB-specific antibody exclusively at the surfaces of cells (Fig. 5B). Fifteen minutes after the shift to 37°C, the Cy3-stained glycoprotein was detected in numerous intracellular vesicles dispersed inside the cell (Fig. 5F), and 30 min after the shift, these vesicles accumulated in a round compartment close to the nucleus (Fig. 5J). At 60 min, the whole amount of antibody-linked glycoprotein was concentrated in this compartment (Fig. 5N), which was identified as the TGN by dual labeling with an anti-TGN 46 antibody (46) revealed by Cy-5 (Fig. 5C, G, K, and O). These results demonstrate that HSV-1 gB present at the cell surface was internalized to concentrate in the TGN.
FIG. 5.
Internalization of GFP-gB. Cos-7 cells expressing GFP-gB were incubated at 4°C with an anti-gB antibody for 1 h and then returned to 37°C for 0 min (A to D), 15 min (E to H), 30 min (I to L), or 60 min (M to P). Cells were fixed in methanol and labeled with an anti-TGN 46 antibody. Images show spontaneous GFP fluorescence (A, E, I, and M) and indirect immunofluorescence from the anti-gB antibody and a Cy3-labeled secondary antibody (B, F, J, and N) or from the anti-TGN 46 antibody and Cy5-labeled secondary antibody (C, G, K, and O). (D, H, L, and P) Merged images. Fluorescence was visualized with a Bio-Rad MRC 1000 confocal microscope.
Endocytosis of truncated and point mutated forms of GFP-gB in transfected cells.
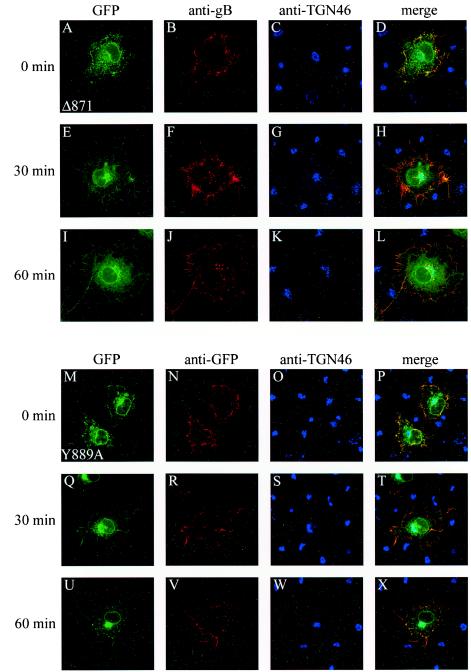
The process of internalization for several homologs of gB is dependent on motifs within the cytoplasmic domain. The increased accumulation at the cell membrane of Δ889 and Δ871 relative to that of GFP-gB and Δ896 suggested that truncations from amino acid 889 impair the internalization process. To investigate this hypothesis, we repeated the endocytosis assay described above, using Δ889 and Δ871. The two truncated proteins, which lack the LL and/or YTQV motifs, exhibited a localization pattern different from that of parental GFP-gB. At 0 min, in cells that were not shifted to 37°C, Cy3-stained Δ889 (not shown) and Δ871 (Fig. 6B) were detected more abundantly at the surfaces of cells, with a continuous pattern which lined the cell periphery. Thirty minutes after the shift, in contrast with GFP-gB, virtually the whole amount of antibody-linked glycoprotein remained at the cell surface (Fig. 6F). The same pattern was still observed at 60 min (Fig. 6J). These results indicate that deletions of the carboxy-terminal domain of gB from amino acid 889 prevent the internalization of the protein. In the same cells, GFP fluorescence revealed the presence of Δ889 or Δ871 in the TGN, as defined by the merge of GFP fluorescence with Cy5 labeling (Fig. 6D, H, and L). This shows that the proteins transit through the Golgi complex before reaching the cell surface.
FIG.6.
Internalization of Δ871 and Y889A. The same experiment as that for Fig. 5 was reproduced in Cos-7 cells expressing the truncated Δ871 protein or the mutated Y889A gB protein. Subcellular localization was examined by confocal microscopy as described in the legend for Fig. 5. (A to D and M to P) 0-min incubation at 37°C; (E to H and Q to T) 30-min incubation at 37°C; (I to L and U to X) 60-min incubation at 37°C. EGFP fluorescence of Δ871 and of Y889A is shown in panels A, E, and I and panels M, Q, and U, respectively; anti-gB staining of Δ871 and of Y889A is shown in panels B, F, and J and panels N, R, and V, respectively; and anti-TGN 46 staining of Δ871 and of Y889A is shown in panels C, G, and K and panels O, S, and W, respectively. (D, H, L, P, T, and X) Merged images.
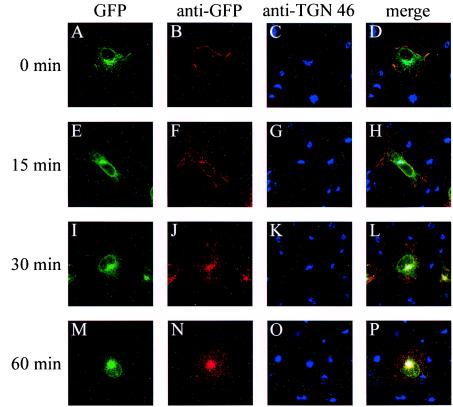
The peptide sequences that were deleted from Δ889 and Δ871 contain motifs homologous to the consensus endocytosis signals YXXΦ (where Y is a tyrosine, X is any amino acid, and Φ is a bulky hydrophobic amino acid) and LL (27, 31). To investigate the respective roles of tyrosine at position 889 (YTQV motif) and LL at positions 871 and 872 in internalization, we introduced point mutations in order to inactivate either of these motifs. By using PCR, two mutated GFP-gB constructs were produced, in which either tyrosine at position 889 was replaced with alanine (Y889A) or both leucine residues at positions 871 and 872 were replaced with alanine residues (LL871AA). Expression of the mutated glycoproteins was verified in transfected Cos-7 cells by fluorescence microscopy and Western blot analysis (not shown). The internalization assay was repeated as described above, using the mutated Y889A and LL871AA proteins. As shown in Fig. 6M to X, the replacement of tyrosine 889 with alanine conferred the same endocytosis pattern as deletion of YTQV or both YTQV and LL (Fig. 6A to L). The antibody-linked Y889A protein remained at the cell surface 30 and 60 min after the shift to 37°C, showing that Y889A failed to be internalized. In contrast, the replacement of LL871 with AA conferred a different pattern. At 0 min, the antibody stained only the glycoprotein present at the cell surface (Fig. 7B). As soon as 15 min after the shift to 37°C, vesicles containing antibody-stained LL871AA were present in cells (Fig. 7F), suggesting that endocytosis from the cell surface took place effectively. However, at 60 min, the merge of the fluorescence signals emitted by Cy3 and Cy5 suggested that while some of the LL871AA protein had reached the TGN (Fig. 7P), antibody-linked LL871AA was still present at the cell membrane and mostly numerous stained vesicles remained dispersed in cells at the latest time points analyzed (Fig. 7N).
FIG. 7.
Effect of mutation of the LL motif on internalization. After transfection, LL871AA-expressing cells were incubated with an anti-GFP monoclonal antibody for 1 h and then returned to 37°C for different times. After methanol fixation and labeling with an anti-TGN 46 antibody, cells were incubated with Cy3- and Cy5-labeled secondary antibodies. Panels A to D, E to H, I to L, and M to P correspond to 0-, 15-, 30-, and 60-min incubation times, respectively, at 37°C. (A, E, I, and M) GFP fluorescence; (B, F, J, and N) anti-GFP immunofluorescence; (C, G, K, and O) TGN 46 localization; (D, H, L, and P) merged images.
Recycling of the parental and mutated gB proteins.
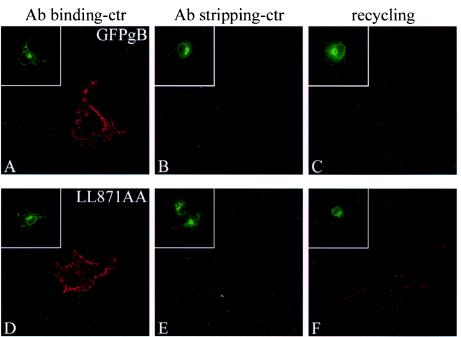
The increased presence of LL871AA in vesicles under the cell membrane revealed impaired transport due to the loss of the LL motif. Since more LL871AA protein was nevertheless present at the cell surface than was the case for parental gB, we hypothesized that once internalized, the mutated protein might recycle back to the cell surface. We thus set up a recycling assay based on surface labeling as performed in the endocytosis test. After surface staining with the anti-GFP antibody at 4°C, transfected cells were either immediately fixed and analyzed or were incubated for 20 min at 37°C to allow internalization. Subsequently, cells were treated with an acid-glycine solution to remove antibody linked to the cell surface. Therefore, only internalized proteins were protected from stripping. Cells were then incubated at 37°C for 30 min, fixed under nonpermeabilizing conditions, labeled with a Cy3-coupled secondary antibody, and examined under a fluorescence microscope. During this incubation, antibody-linked proteins which had been internalized during the first incubation at 37°C could return back to the plasma membrane. Only proteins which recycled to the cell surface were then detectable by the secondary antibody. As seen in Fig. 8C, almost no antibody-linked GFP-gB was detected at the cell surface after the second incubation for 30 min at 37°C. In contrast, whereas the antibody linked to LL871AA was efficiently stripped from the surface 0 min after the acid-glycine treatment (Fig. 8E), Cy3 staining revealed that antibody-linked LL871AA was again abundantly present at the cell membrane 30 min after the second incubation at 37°C (Fig. 8F). These results confirm that the LL motif is necessary in some step of the internalization process and that disruption of this motif led to partial recycling of the protein to the cell surface.
FIG. 8.
Recycling of internalized GFP-gB and LL871AA to the plasma membrane. After transfection, GFP-gB- or LL871AA-expressing cells were stained with an anti-GFP antibody at 4°C and then either immediately fixed (A and D) or incubated for 20 min at 37°C to allow internalization (B, C, E, and F). After incubation, cells were subjected to an acid-glycine treatment and then immediately fixed under nonpermeabilizing conditions (B and E) or placed again at 37°C for 30 min before fixation (C and F). Fixed cells were labeled with a Cy3-coupled secondary antibody and examined by confocal microscopy. Insets show the spontaneous GFP fluorescence emitted in transfected cells.
Complementation of a defective gB-null virus by truncated or point mutated gB.
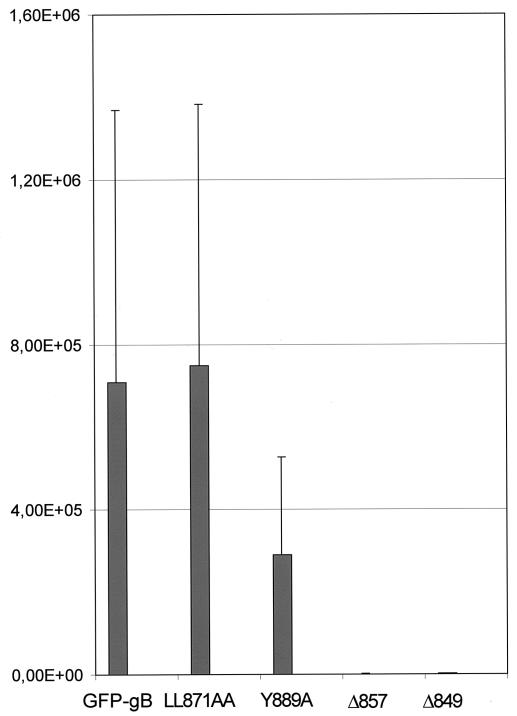
To investigate the effects of mutations in the carboxy-terminal domain of gB on its ability to rescue the infectivity of a gB-defective virus, we performed a previously reported complementation assay (8, 44, 60). Vero cells were transfected with the constructs expressing each of the truncated or point mutated forms of GFP-gB and then were infected the following day with the gB-null K082 virus. The supernatant was retrieved 24 h later and titrated on complementing D6 cells. As a control, results were compared with those obtained with parental GFP-gB, considered the reference value. The mutated protein Y889A showed reproducible reduced complementation in the range of 40 to 50% that of GFP-gB (Fig. 9). Identical results were obtained with the truncated proteins Δ871 and Δ889 in five independent experiments (not shown). Thus, disruption of the YTQV motif or its deletion similarly decreased the complementation capacity of the protein. In contrast, LL871AA had full complementation activity. Proteins truncated from amino acid 857 had no complementation activity (Fig. 9). These results confirm that complete maturation of gB in the Golgi complex is necessary for complementation activity. They also suggest that abolished internalization of gB from the cell surface to the TGN might affect the production of infectious particles.
FIG. 9.
Complementation of K082 virus by GFP-gB or the mutated Y889A, LL871AA, Δ857, or Δ849 protein. Vero cells were transfected with plasmids expressing GFP-gB or the mutated proteins and were infected 24 h later with K082 at a multiplicity of infection of 1. Extracellular virions were washed off at 1 h postinfection by treatment with an acid-glycine-saline solution, followed by three washes with PBS. Infected cell culture supernatants were harvested 24 h after infection and titrated on D6 and Vero cells. Titers shown are the averages from three independent experiments.
DISCUSSION
gB is the most highly conserved glycoprotein among herpesviruses (42) and is essential for the production of infectious particles. The envelope gB is involved in virus entry and (possibly) egress, fusion, cell-cell spread (8, 53), pathogenesis in vivo, and neuroinvasiveness (18, 34, 62). The C-terminal portion of HSV-1 gB is required for some of these functions, since mutations in this domain have been associated with altered intracellular transport, syncytium formation (8, 13, 17, 59), and neuropathogenicity (12, 19, 28). The cytoplasmic tails of cytomegalovirus (CMV), varicella-zoster virus (VZV), and pseudorabies virus (PRV) gB homologs and those of several other glycoproteins, such as HSV, PRV, and VZV gE, contain consensus peptide motifs involved both in intracellular transport of the protein and in endocytosis from the cell surface in transfected and infected cells (5). Overall, our results show that a peptide sequence located from residues 857 to 871 plays a role in ER-to-Golgi-complex transport of HSV-1 gB. They also show that the protein is internalized from the cell surface to accumulate in the TGN and that this process occurs in two steps. Disruption of the YTQV motif totally abolishes the first step, which mediates internalization from the cell surface to endosomes and diminishes the complementation capacity of the protein by a factor of two. Disruption of the LL motif impairs the second step, which mediates the retrograde transport from early or recycling endosomes to the TGN and has no effect on the complementation capacity of the protein. These results suggest that endocytosis, albeit dispensable, might play a role in the production of infectious HSV-1 particles.
Various mutations of the HSV-1 gB cytoplasmic domain have been shown to impair the processing and ER-to-Golgi-complex transport of the glycoprotein (9). For instance, large deletions of the carboxy-terminal domain (48) or linker insertion mutagenesis (10) slowed the ER-to-Golgi-complex transport of truncated gB. The sequence of the C-terminal domain of gB predicts two alpha-helical domains (13, 37). We show that a gB polypeptide that is truncated from amino acid 857, in alpha-helical domain I, is retained in the ER, whereas truncations in alpha-helical domain II up to residue 871 do not impair transport and full glycosylation through the Golgi complex. The maturation of Δ857 through the Golgi complex was completely inhibited, since Western blotting showed the absence of a mature form of the protein 48 h after transfection, as shown by the complete sensitivity of the detected form to endo H. Moreover, Δ857 did not reach the cell surface. Therefore, the 14-aa region encompassing residues 857 to 871 should contain a domain that is essential for transport of the protein to the Golgi complex. Surprisingly, in a previous study truncations of the cytoplasmic tail of the HSV-2 gB homolog did not prevent transport to the Golgi complex or to the cell surface (13). In that study, gB-2 polypeptides containing truncations in homologous domains were detected at the cell surface. Whether this discrepancy is due to differences in the assays is not clear. However, for VSV gG, a diacidic signal was identified that was required for export from the ER (36). In addition, specific ER-to-Golgi-complex transport signal sequences have been identified in the cytoplasmic tail of VZV gB (24). These include a 12-aa sequence containing a consensus YXXΦ motif followed by two acidic residues in the pattern EXXE. Deletion of this whole region or alanine substitution of the first glutamate residue decreased similarly the amount of VZV gB transported to the Golgi complex by >95%. In contrast, replacement of the tyrosine residue of the YXXΦ motif only decreased this transport by 50%. These results strongly suggested that the glutamate residue present at the carboxyl end of this peptide sequence has a key role in functioning of the signal. In HSV-1 gB, a highly similar peptide sequence is present which contains the YMAL motif followed by EXXE. Our results showing that removal of the acidic peptide EXXE from glutamate 857 had the same effect as that previously shown for VZV gB further favor the role of this motif in ER-to-Golgi-complex transport.
HSV-1 gB polypeptides truncated from residue 857, which did not go through the Golgi complex, were unable to complement the K082 gB-null virus. Fan et al. showed similarly that gB-2 truncated from the homolog acidic peptide or from the YMAL sequence had drastically reduced or abolished complementation capacity (13). In PRV gB, deletion of the two C-terminal alpha-helical domains prevented incorporation of the protein in virions (37). Altogether, these results are consistent with previous reports showing that HSV-1 mutants expressing either ER-retained gD or ER-restricted gH contain no detectable gD or gH, respectively (6, 61), and favor a model in which HSV-1 acquires its envelope in a post-ER compartment, presumably the TGN (52).
The alpha-helical domain II of HSV-1 gB contains putative endocytosis motifs, i.e., the LL motif and a YTQV motif similar to the consensus signal YXXΦ, followed by an acidic cluster, DGDADEDD. Proteins which contain similar motifs accumulate in the Golgi apparatus, both in transfected and infected cells (22, 23). Our results showed that at the steady state, gB exhibited a light punctate pattern of cell surface staining but was mainly concentrated in the TGN when expressed in Cos-7 cells. This localization was in part due to endocytosis from the cell surface, which required <30 min to be complete. Removal of the C-terminal acidic peptide did not modify endocytosis. In CMV gB, a C-terminal acidic cluster was shown to participate in the early endocytic-recycling pathway of the protein. However, this function relied in part on a phosphorylatable serine residue present in the acidic motif and varied according to the cell types analyzed, since dephosphorylation impaired the internalization of the protein from the surface in MDCK cells but not in HF cells (15, 57). In addition, the acidic motif was shown to be a key element in vectorial sorting of CMV gB in polarized cells (58). It is noticeable that the acidic cluster present in HSV-1 gB contains a glycine residue in place of the phosphorylatable serine residue present in CMV and PRV gB.
In gB, the redundancy of endocytosis motifs raises the issue of the respective role of each of these signals. Our results show that endocytosis of HSV-1 gB depends principally on the YTQV sequence, since either removal or disruption of this signal abolished gB internalization. The tyrosine residue was essential for the functioning of the signal, since replacement of this amino acid with an alanine completely inhibited internalization at the latest time points analyzed, as shown for PRV, VZV, and CMV gB (14, 23, 57), VZV and PRV gE (38, 55, 63), and VZV gH (39). Our hypothesis is that, as demonstrated for several proteins with similar motifs (2, 21, 26, 27), the YTQV motif interacts with the adaptor protein complex AP-2 to mediate the first step of the process of internalization from the cell surface to early endosomes.
Another endocytosis motif, LL, is present at the N-terminal end of the second predicted alpha helix of HSV-1 gB. For VZV gB, the LL motif was reported to play no role in internalization, since gB containing a disrupted motif was reported to localize to the Golgi complex as did the native protein (23). Similarly, in HSV-2 gB, the LL motif did not affect the overall distribution of the protein (13). Our results differ from these previous reports. In HSV-1 gB, replacement of LL with AA did not abolish the process of internalization, since antibody-linked protein did reach the TGN. However, analysis at all time points of the endocytosis assay showed that LL871AA was present in small vesicular structures distributed throughout the cytoplasm. Thus, the first step of internalization took place effectively, but the subsequent return to the TGN was impaired.
Two independent pathways for the transport of proteins from early or recycling endosomes to the TGN have been demonstrated (30). The small mannose-6-phosphate receptor of 46 kDa (MPR46) and the human immunodeficiency virus Env glycoprotein reach the TGN via a pathway involving late endosomes. Transport from late endosomes to the TGN is mediated through the interaction of the protein TIP47 and a diaromatic motif present in the cytoplasmic tail of the proteins (3). A second pathway by which proteins are delivered directly from early or recycling endosomes to the TGN (29, 30) can also be used by MPR46. The LL motif in the MPR46 tail is involved in this pathway, probably through interaction with AP-1 complexes (54). Interestingly, the return of an LL/AA mutant of MPR46 from the plasma membrane or endosome pool to the TGN was impaired but not abolished, while recycling from the endosomes to the plasma membrane was enhanced (54). Our results, showing the presence of LL871AA at the TGN and increased recycling to the cell membrane together, favor the hypothesis that HSV-1 gB follows the second pathway.
Mutated gB which failed to be internalized had reduced but not abolished activity for complementation of a gB-null K082 virus. Truncation or disruption of the YTQV signal consistently decreased the titers of infectious particles to 40% that of the wild type. In a previous report, gB with a truncation of its C-terminal 28 amino acids was reported to behave the same as the wild-type (1). However, our results are similar to those obtained with a truncated PRV gB, which failed to be internalized and exhibited a 10-fold reduction in complementation activity compared with the native protein (37). Moreover, a similar modest but reproducible twofold reduction in titers of infectious viral particles was obtained by disturbing the normal TGN localization of human CMV gB (11). The role of membrane protein endocytosis during the virus replication cycle is not completely understood. Many herpesvirus gB homologs accumulate through endocytosis in the TGN, which is currently considered the site of final virus assembly. However, this process was shown to be dispensable for virus envelopment and production of infectious particles for CMV (25) and PRV (37), indicating that sufficient levels of glycoprotein can be transported to the site of virus assembly. Indeed, newly synthesized HSV-1 gB transits through the TGN on its way to the cell membrane and could then be incorporated into virions. This could be different in polarized cells, in which internalization has been proposed to target glycoproteins to specific cell sites (16). Further studies will be necessary to investigate the role of endocytosis in the proper localization of HSV-1 gB for incorporation into virions in polarized cells.
Acknowledgments
We thank S. Ponnambalam for the gift of the anti-TGN46 antibody and Roselyn J. Eisenberg and Gary H. Cohen for providing the anti-gB antibody. We are indebted to Prashant Desai and Stanley Person for the gift of the K082 virus and the D6 cells. We thank Serge Benichou for helpful discussions and advice. We are most grateful to Katy Janvier for generously sharing her research expertise. We thank all members of the Benichou and Benarous laboratories and especially Ricardo Madrid, Erwann Lerouzic, Clarisse Berlioz-Torrent, and Guillaume Blot for reagents and helpful suggestions. We thank Lilia Cantero Aguilar for technical assistance.
I. Beitia Ortiz de Zarate is a recipient of a Scientist Training Fellowship from the Department of Education, Universities and Research of the Basque Government. This work was financed partly by a grant from GlaxoWellcome (no. 963676).
REFERENCES
- 1.Baghian, A., L. Huang, S. Newman, S. Jayachandra, and K. G. Kousoulas. 1993. Truncation of the carboxy-terminal 28 amino acids of glycoprotein B specified by herpes simplex virus type 1 mutant amb1511-7 causes extensive cell fusion. J. Virol. 67:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blot, G., K. Janvier, S. Le Panse, R. Benarous, and C. Berlioz-Torrent. 2003. Targeting of the human immunodeficiency virus type 1 envelope to the trans-Golgi network through binding to TIP47 is required for env incorporation into virions and infectivity. J. Virol. 77:6931-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonneau, R. H., L. A. Salvucci, D. C. Johnson, and S. S. Tevethia. 1993. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology 195:62-70. [DOI] [PubMed] [Google Scholar]
- 5.Brideau, A. D., L. W. Enquist, and R. S. Tirabassi. 2000. The role of virion membrane protein endocytosis in the herpesvirus life cycle. J. Clin. Virol. 17:69-82. [DOI] [PubMed] [Google Scholar]
- 6.Browne, H., S. Bell, T. Minson, and D. W. Wilson. 1996. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J. Virol. 70:4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bzik, D. J., B. A. Fox, N. A. DeLuca, and S. Person. 1984. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology 137:185-190. [DOI] [PubMed] [Google Scholar]
- 8.Cai, W. H., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai, W. Z., S. Person, C. DebRoy, and B. H. Gu. 1988. Functional regions and structural features of the gB glycoprotein of herpes simplex virus type 1. An analysis of linker insertion mutants. J. Mol. Biol. 201:575-588. [DOI] [PubMed] [Google Scholar]
- 10.Cai, W. Z., S. Person, S. C. Warner, J. H. Zhou, and N. A. DeLuca. 1987. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J. Virol. 61:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crump, C. M., C. H. Hung, L. Thomas, L. Wan, and G. Thomas. 2003. Role of PACS-1 in trafficking of human cytomegalovirus glycoprotein B and virus production. J. Virol. 77:11105-11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel, J. P., E. P. Boyer, and J. L. Goodman. 1993. Two novel single amino acid syncytial mutations in the carboxy terminus of glycoprotein B of herpes simplex virus type 1 confer a unique pathogenic phenotype. Virology 192:112-120. [DOI] [PubMed] [Google Scholar]
- 13.Fan, Z., M. L. Grantham, M. S. Smith, E. S. Anderson, J. A. Cardelli, and M. I. Muggeridge. 2002. Truncation of herpes simplex virus type 2 glycoprotein B increases its cell surface expression and activity in cell-cell fusion, but these properties are unrelated. J. Virol. 76:9271-9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favoreel, H. W., G. Van Minnebruggen, H. J. Nauwynck, L. W. Enquist, and M. B. Pensaert. 2002. A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J. Virol. 76:6845-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fish, K. N., C. Soderberg-Naucler, and J. A. Nelson. 1998. Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of Ser900. J. Virol. 72:6657-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folsch, H., H. Ohno, J. S. Bonifacino, and I. Mellman. 1999. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99:189-198. [DOI] [PubMed] [Google Scholar]
- 17.Gage, P. J., M. Levine, and J. C. Glorioso. 1993. Syncytium-inducing mutations localize to two discrete regions within the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B. J. Virol. 67:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerdts, V., J. Beyer, B. Lomniczi, and T. C. Mettenleiter. 2000. Pseudorabies virus expressing bovine herpesvirus 1 glycoprotein B exhibits altered neurotropism and increased neurovirulence. J. Virol. 74:817-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman, J. L., and J. P. Engel. 1991. Altered pathogenesis in herpes simplex virus type 1 infection due to a syncytial mutation mapping to the carboxy terminus of glycoprotein B. J. Virol. 65:1770-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanke, T., F. L. Graham, K. L. Rosenthal, and D. C. Johnson. 1991. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J. Virol. 65:1177-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haucke, V., and P. De Camilli. 1999. AP-2 recruitment to synaptotagmin stimulated by tyrosine-based endocytic motifs. Science 285:1268-1271. [DOI] [PubMed] [Google Scholar]
- 22.Heineman, T. C., and S. L. Hall. 2002. Role of the varicella-zoster virus gB cytoplasmic domain in gB transport and viral egress. J. Virol. 76:591-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heineman, T. C., and S. L. Hall. 2001. VZV gB endocytosis and Golgi localization are mediated by YXXphi motifs in its cytoplasmic domain. Virology 285:42-49. [DOI] [PubMed] [Google Scholar]
- 24.Heineman, T. C., N. Krudwig, and S. L. Hall. 2000. Cytoplasmic domain signal sequences that mediate transport of varicella-zoster virus gB from the endoplasmic reticulum to the Golgi. J. Virol. 74:9421-9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvis, M. A., K. N. Fish, C. Soderberg-Naucler, D. N. Streblow, H. L. Meyers, G. Thomas, and J. A. Nelson. 2002. Retrieval of human cytomegalovirus glycoprotein B from cell surface is not required for virus envelopment in astrocytoma cells. J. Virol. 76:5147-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamiguchi, H., K. E. Long, M. Pendergast, A. W. Schaefer, I. Rapoport, T. Kirchhausen, and V. Lemmon. 1998. The neural cell adhesion molecule L1 interacts with the AP-2 adaptor and is endocytosed via the clathrin-mediated pathway. J. Neurosci. 18:5311-5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirchhausen, T., J. S. Bonifacino, and H. Riezman. 1997. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr. Opin. Cell Biol. 9:488-495. [DOI] [PubMed] [Google Scholar]
- 28.Kostal, M., I. Bacik, J. Rajcani, and H. C. Kaerner. 1994. Replacement of glycoprotein B gene in the herpes simplex virus type 1 strain ANGpath DNA by that originating from nonpathogenic strain KOS reduces the pathogenicity of recombinant virus. Acta Virol. 38:77-88. [PubMed] [Google Scholar]
- 29.Mallard, F., C. Antony, D. Tenza, J. Salamero, B. Goud, and L. Johannes. 1998. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J. Cell Biol. 143:973-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallet, W. G., and F. R. Maxfield. 1999. Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J. Cell Biol. 146:345-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks, M. S., H. Ohno, T. Kirchhausen, and J. S. Bonifacino. 1997. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 7:124-128. [DOI] [PubMed] [Google Scholar]
- 32.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mettenleiter, T. C. 2003. Pathogenesis of neurotropic herpesviruses: role of viral glycoproteins in neuroinvasion and transneuronal spread. Virus Res. 92:197-206. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell, B. M., and J. G. Stevens. 1996. Neuroinvasive properties of herpes simplex virus type 1 glycoprotein variants are controlled by the immune response. J. Immunol. 156:246-255. [PubMed] [Google Scholar]
- 35.Navarro, D., I. Qadri, and L. Pereira. 1991. A mutation in the ectodomain of herpes simplex virus 1 glycoprotein B causes defective processing and retention in the endoplasmic reticulum. Virology 184:253-264. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura, N., S. Bannykh, S. Slabough, J. Matteson, Y. Altschuler, K. Hahn, and W. E. Balch. 1999. A di-acidic (DXE) code directs concentration of cargo during export from the endoplasmic reticulum. J. Biol. Chem. 274:15937-15946. [DOI] [PubMed] [Google Scholar]
- 37.Nixdorf, R., B. G. Klupp, A. Karger, and T. C. Mettenleiter. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J. Virol. 74:7137-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasieka, T. J., L. Maresova, and C. Grose. 2003. A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis. J. Virol. 77:4191-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peeters, B., N. de Wind, M. Hooisma, F. Wagenaar, A. Gielkens, and R. Moormann. 1992. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J. Virol. 66:894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pellett, P. E., K. G. Kousoulas, L. Pereira, and B. Roizman. 1985. Anatomy of the herpes simplex virus 1 strain F glycoprotein B gene: primary sequence and predicted protein structure of the wild type and of monoclonal antibody-resistant mutants. J. Virol. 53:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pereira, L. 1994. Function of glycoprotein B homologues of the family herpesviridae. Infect. Agents Dis. 3:9-28. [PubMed] [Google Scholar]
- 43.Pereira, L., M. Ali, K. Kousoulas, B. Huo, and T. Banks. 1989. Domain structure of herpes simplex virus 1 glycoprotein B: neutralizing epitopes map in regions of continuous and discontinuous residues. Virology 172:11-24. [DOI] [PubMed] [Google Scholar]
- 44.Potel, C., K. Kaelin, I. Gautier, P. Lebon, J. Coppey, and F. Rozenberg. 2002. Incorporation of green fluorescent protein into the essential envelope glycoprotein B of herpes simplex virus type 1. J. Virol. Methods 105:13-23. [DOI] [PubMed] [Google Scholar]
- 45.Potel, C., K. Kaelin, L. Danglot, A. Triller, C. Vannier, and F. Rozenberg. 2003. Herpes simplex virus type 1 glycoprotein B sorting in hippocampal neurons. J. Gen. Virol. 84:2613-2624. [DOI] [PubMed] [Google Scholar]
- 46.Prescott, A. R., J. M. Lucocq, J. James, J. M. Lister, and S. Ponnambalam. 1997. Distinct compartmentalization of TGN46 and beta 1,4-galactosyltransferase in HeLa cells. Eur. J. Cell Biol. 72:238-246. [PubMed] [Google Scholar]
- 47.Qadri, I., C. Gimeno, D. Navarro, and L. Pereira. 1991. Mutations in conformation-dependent domains of herpes simplex virus 1 glycoprotein B affect the antigenic properties, dimerization, and transport of the molecule. Virology 180:135-152. [DOI] [PubMed] [Google Scholar]
- 48.Raviprakash, K., L. Rasile, K. Ghosh, and H. P. Ghosh. 1990. Shortened cytoplasmic domain affects intracellular transport but not nuclear localization of a viral glycoprotein. J. Biol. Chem. 265:1777-1782. [PubMed] [Google Scholar]
- 49.Sanchez-Pescador, L., P. Paz, D. Navarro, L. Pereira, and S. Kohl. 1992. Epitopes of herpes simplex virus type 1 glycoprotein B that bind type-common neutralizing antibodies elicit type-specific antibody-dependent cellular cytotoxicity. J. Infect. Dis. 166:623-627. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Pescador, L., L. Pereira, E. D. Charlebois, and S. Kohl. 1993. Antibodies to epitopes of herpes simplex virus type 1 glycoprotein B (gB) in human sera: analysis of functional gB epitopes defined by inhibition of murine monoclonal antibodies. J. Infect. Dis. 168:844-853. [DOI] [PubMed] [Google Scholar]
- 51.Sivadon, V., P. Lebon, and F. Rozenberg. 1998. Variations of HSV-1 glycoprotein B in human herpes simplex encephalitis. J. Neurovirol. 4:106-114. [DOI] [PubMed] [Google Scholar]
- 52.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment→deenvelopment→reenvelopment pathway. J. Virol. 75:5697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spear, P. G. 1993. Membrane fusion induced by herpes simplex virus, p. 201-232. In J. Bentz (ed.), Viral fusion mechanisms. CRC Press Inc., Ann Arbor, Mich.
- 54.Tikkanen, R., S. Obermuller, K. Denzer, R. Pungitore, H. J. Geuze, K. von Figura, and S. Honing. 2000. The dileucine motif within the tail of MPR46 is required for sorting of the receptor in endosomes. Traffic 1:631-640. [DOI] [PubMed] [Google Scholar]
- 55.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomishima, M. J., G. A. Smith, and L. W. Enquist. 2001. Sorting and transport of alpha herpesviruses in axons. Traffic 2:429-436. [DOI] [PubMed] [Google Scholar]
- 57.Tugizov, S., E. Maidji, J. Xiao, and L. Pereira. 1999. An acidic cluster in the cytosolic domain of human cytomegalovirus glycoprotein B is a signal for endocytosis from the plasma membrane. J. Virol. 73:8677-8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tugizov, S., E. Maidji, J. Xiao, Z. Zheng, and L. Pereira. 1998. Human cytomegalovirus glycoprotein B contains autonomous determinants for vectorial targeting to apical membranes of polarized epithelial cells. J. Virol. 72:7374-7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walev, I., M. Lingen, M. Lazzaro, K. Weise, and D. Falke. 1994. Cyclosporin A resistance of herpes simplex virus-induced “fusion from within” as a phenotypical marker of mutations in the Syn 3 locus of the glycoprotein B gene. Virus Genes 8:83-86. [DOI] [PubMed] [Google Scholar]
- 60.Wanas, E., S. Efler, K. Ghosh, and H. P. Ghosh. 1999. Mutations in the conserved carboxy-terminal hydrophobic region of glycoprotein gB affect infectivity of herpes simplex virus. J. Gen. Virol. 80:3189-3198. [DOI] [PubMed] [Google Scholar]
- 61.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuhasz, S. A., and J. G. Stevens. 1993. Glycoprotein B is a specific determinant of herpes simplex virus type 1 neuroinvasiveness. J. Virol. 67:5948-5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu, Z., Y. Hao, M. D. Gershon, R. T. Ambron, and A. A. Gershon. 1996. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J. Virol. 70:6563-6575. [DOI] [PMC free article] [PubMed] [Google Scholar]