Abstract
A chronic increase in the concentration of sodium chloride in the cerebrospinal fluid (CSF) (↑CSF [NaCl]) appears to be critically important for the development of salt-dependent hypertension. In agreement with this concept, increasing CSF [NaCl] chronically by intracerebroventricular (icv) infusion of NaCl-rich artificial CSF (aCSF-HiNaCl) in rats produces hypertension by the same mechanisms (i.e., aldosterone-ouabain pathway in the brain) as that produced by dietary sodium in salt-sensitive strains. We first demonstrate here that icv aCSF-HiNaCl for 10 days also causes hypertension in wild-type (WT) mice. We then used both WT and gene-targeted mice to explore the mechanisms. In WT mice with a ouabain-sensitive Na,K-ATPase α2-isoform (α2S/S), mean arterial pressure rose by ∼25 mmHg within 2 days of starting aCSF-HiNaCl (0.6 nmol Na/min) and remained elevated throughout the study. Ouabain (171 pmol/day icv) increased blood pressure to a similar extent. aCSF-HiNaCl or ouabain given at the same rates subcutaneously instead of intracerebroventricularly had no effect on blood pressure. The pressor response to icv aCSF-HiNaCl was abolished by an anti-ouabain antibody given intracerebroventricularly but not subcutaneously, indicating that it is mediated by an endogenous ouabain-like substance in the brain. We compared the effects of icv aCSF-HiNaCl or icv ouabain on blood pressure in α2S/S versus knockout/knockin mice with a ouabain-resistant endogenous α2-subunit (α2R/R). In α2R/R, there was no pressor response to icv aCSF-HiNaCl in contrast to WT mice. The α2R/R genotype also lacked a pressor response to icv ouabain. These data demonstrate that chronic ↑CSF [NaCl] causes hypertension in mice and that the blood pressure response is mediated by the ouabain-like substance in the brain, specifically by its binding to the α2-isoform of the Na,K-ATPase.
Keywords: sodium, potassium-adenosine 5′-triphosphatase, gene-targeted mice, knockout/knockin, ouabain-like substance, intracerebroventricular infusion, central nervous system, cerebrospinal fluid
previous studies have suggested that the level of sodium in the cerebrospinal fluid (CSF) is a critical factor in the pressor response to dietary sodium in salt-dependent hypertension. Huang et al. (12) showed that in Dahl salt-sensitive (Dahl-S) and spontaneously hypertensive rats (SHRs), a high-salt diet increases the sodium concentration in the CSF (↑CSF [Na]) before the salt-induced increase in blood pressure. In addition, ↑CSF [Na] does not occur in response to a high-salt diet in the normotensive control strains for Dahl-S and SHRs, Dahl salt-resistant, and Wistar-Kyoto rats (12). Furthermore, both high-salt diet in Dahl-S and SHRs and direct intracerebroventricular (icv) infusion of sodium raise CSF [Na] to a similar extent (12–14) and cause hypertension by the same cascade of systems in the brain. Both activate the sympathetic nervous system and increase blood pressure by first stimulating brain mineralocorticoid receptors and then elevating the level of an endogenous ouabain-like substance (OLS) in the brain, thereby enhancing the activity of the central renin-angiotensin system (RAS) (6–16).
Therefore, an area of active investigation is the further delineation of the mechanisms whereby an increase in the concentration of sodium chloride in the CSF (↑CSF [NaCl]) raises blood pressure. One critical issue is which brain Na,K-ATPase isoform mediates the effects of the OLS during ↑CSF [NaCl]. Ouabain and OLS bind to the α (catalytic)-subunit of the Na,K-ATPase (21, 22), and there are three isoforms of the α-subunit in the brain (α1, α2, and α3). These isoforms are encoded by separate genes, have unique primary amino acid sequences, and have different tissue distributions. The α1-subunit is expressed in all brain cell types, whereas the α2- and α3-isoforms are much more limited in distribution (4, 22, 27, 29). The α2-isoform exists predominantly in glia but is also expressed in some neurons, whereas the α3-subunit protein is specific to neurons. It is not known which of these isoforms in the brain mediates the effects of the OLS during ↑CSF [NaCl].
In the present study, we first demonstrated that chronic ↑CSF [NaCl] raises blood pressure in mice and that the hypertensive response is mediated by the OLS in the brain. Upon confirmation of the latter concept, it followed that one or more of the three receptors for the OLS in the central nervous system (CNS) (Na,K-ATPase α1-, α2-, or α3-isoforms) were involved. We subsequently tested the effects of the OLS α2-isoform interaction directly by using mice whose endogenous α2-subunit has been genetically engineered by Cre/Lox gene targeting to have two amino acid substitutions (Leu111Arg and Asn122Asp) (2). The endogenous α2-isoform in mice that are homozygous for these substitutions (α2R/R) is highly resistant to ouabain binding but is otherwise fully expressed and functional (2). The ensuing data are consistent with a complete mediation of the hypertensive response to ↑CSF [NaCl] by the interaction of the OLS with the Na,K-ATPase α2-isoform in the brain, and they thereby implicate this isoform in the genesis of salt-induced hypertension.
MATERIALS AND METHODS
Animal care and genotyping.
All studies were carried out in agreement with guidelines established by the Canadian Council on Animal Care and were approved by the University of Ottawa Animal Care Committee. Both α2R/R and wild-type (WT) mice were obtained in-house from a breeding colony. They were housed in group cages (except after implantation of an arterial catheter, telemeter or lateral brain ventricular cannula) in a temperature-controlled environment with a 12-h:12-h light-dark cycle, and standard chow and water were provided ad libitum.
Genotyping was performed as previously described (2), using DNA extracted from a small tail sample obtained at age 3 wk (2). An allelele-specific PCR reaction was performed using 5′-TCAGCTGTGGCTCCACGTGGG-3′ and 5′-GCATGGGGGATTGGGGGATTA-3′ as the forward and reverse primers. Forty PCR cycles were run, consisting of (45 s each): 94, 57, and 72°C for denaturing, annealing, and extension, respectively. These cycles were preceded by a 94°C denaturation period and were followed by extension at 72°C (5 min each). The PCR reaction produces ∼350 and 300 bp bands for the WT and α2R/R genotypes, respectively.
Blood pressure monitoring via telemetry.
The initial time courses for the blood pressure and heart rate effects of icv sodium and anti-ouabain antibody Fab fragments were established in telemetered mice. The minimum weight was set at 20 g for the study, and animals were 8–11 wk of age at the time of telemeter implantation. Two weeks before the commencement of icv infusions (on day −14), all animals received a TA11PA-C10 telemetry pressure transducer (Data Sciences International, St. Paul, MN) with the tubing implanted into the abdominal aorta under isoflurane anesthesia. The battery/transmitter was implanted in the peritoneal cavity. The mice were allowed to recover and the transmitter was activated on day −2 (day 0 being the day that icv infusions were started). Blood pressure monitoring was carried out continuously thereafter until the end of the study.
On day 0, a cannula was implanted into a lateral brain ventricle for icv infusions and was secured in place using dental acrylic, as previously described (5, 28). Stereotaxic coordinates for cannula implantation were 0.1 mm anterior, 1.0 mm lateral and 1.5 mm ventral to lambda. The cannula was subsequently connected to an Alzet model 1002 osmotic minipump that was implanted sc. The infusion rate for all icv fluids was 0.25 μl/h, including artificial CSF (aCSF) alone as a control (5), NaCl-rich aCSF (aCSF-HiNaCl), or aCSF-HiNaCl containing sheep anti-ouabain antibody Fab fragments (0.65 nmol/day; Digibind, obtained from Glaxo Smith Kline, Mississauga, ON, Canada). The base composition of aCSF (pH 7.0) was (in mM) as follows: 117 NaCl, 2.5 KCl, 0.65 NaH2PO4·2H2O, 2.27 NaH2PO4·7H2O, 0.5 Na2SO4, 2.14 MgCl2·6H2O, 1.0 CaCl2, and 27 NaHCO3 (14). The rates of Na+ infusion were 0.6 and 5 nmol/min for aCSF and aCSF-HiNa, respectively. aCSF was predetermined to have no effect on blood pressure or heart rate. The rate of icv sodium infusion for aCSF-HiNa was established a priori to increase blood pressure by ∼20–30 mmHg and was expected to produce a relatively small increase in CSF [Na] in the mouse since it is a 30% lower rate/g of icv sodium infusion than that which produces a 6–11 meq/l increase in CSF [Na] in the rat (13, 14).
Blood pressure monitoring via fluid-filled catheter.
After the time courses of the effects of aCSF-HiNaCl and anti-ouabain antibody Fab fragments were established via telemetry monitoring, blood pressure and heart rate were subsequently assessed via indwelling fluid-filled catheter at a time corresponding to the plateau in the pressor response in telemetered WT mice given aCSF-HiNaCl, using previously described procedures (28). Briefly, substances were administered icv via minipump as described above for telemetry studies, but no telemetry transmitter was implanted beforehand. Instead, a catheter made from antithrombogenic tubing (inner diameter, 0.025; and outer diameter, 0.040 in., Braintree Scientific, Braintree, MA) was filled with heparinized saline and implanted in a carotid artery 7 days after the start of the icv infusions under isoflurane anesthesia. The cannula was anchored with sutures and exteriorized at the back of the neck. Animals were allowed to recover for 26 h after surgery. The day following surgery, the catheter was connected to a pressure transducer that was in turn connected to a PowerLab/4SP data analysis system (AD Instruments, Grand Junction, CO). Blood pressure and heart rate were monitored in conscious, freely moving mice for at least 60 min, and the data were averaged over this period.
Blood pressures were measured in this way in mice receiving icv aCSF, aCSF-HiNaCl (the same rates as in telemetry studies to compare the two blood pressure monitoring methods), or ouabain (171 pmol/day). The icv ouabain dose used was determined in preliminary studies to increase blood pressure to a similar extent as that produced by icv aCSF-HiNaCl. Separate groups of WT mice also received the following treatments: subcutaneous (sc) aCSF-HiNaCl, icv aCSF-HiNaCl + sc sheep anti-ouabain antibody Fab fragments (0.65 nmol/day), icv aCSF-HiNaCl + icv nonspecific sheep IgG Fab fragments, the same dose as that used for icv specific anti-ouabain antibody (0.65 nmol/day; Rockland Immunologicals, Gilbertsville, PA) or sc ouabain. All sc doses were the same as the corresponding icv doses in order to distinguish between central and peripheral effects. Nonspecific sheep IgG Fab fragments were dialyzed overnight at 4°C in aCSF before icv infusion to remove small molecules (e.g., Na+ azide added by the manufacturer), as previously described (28). After dialysis, the protein concentration in the preparation was quantified by microprotein assay (1).
Measurement of CSF [Na].
To assess the change in CSF [Na] in the current model of hypertension, separate groups of WT mice were randomized to receive either icv aCSF-HiNa as above or no treatment. On day 8 of icv aCSF-HiNaCl infusion, 5–10 μl of CSF were collected from the cisterna magna as previously described (17) under isoflurane anesthesia and was diluted 1:3 with distilled water before assay. CSF was similarly collected in age-matched untreated controls. A sodium-sensitive microelectrode (Microelectrodes, Bedford, NH) was used to measure sodium levels in the diluted samples. A set of standards ranging from 126–174 mM [Na] in aCSF was diluted and similarly assessed as a reference.
Statistics.
Repeated comparisons of blood pressure and heart rate data (telemetry studies) between groups (e.g., icv aCSF vs. icv aCSF-HiNaCl) were carried out using a one-way ANOVA for repeated measures. When the ANOVA indicated significant differences between groups, post hoc comparisons were made by group t-tests. For single comparisons of blood pressure and heart rate between groups (measured via indwelling fluid-filled catheter), a two-way ANOVA was used. CSF [Na] in WT mice receiving icv aCSF-HiNaCl was compared with that in WT controls by one-tailed t-test.
RESULTS
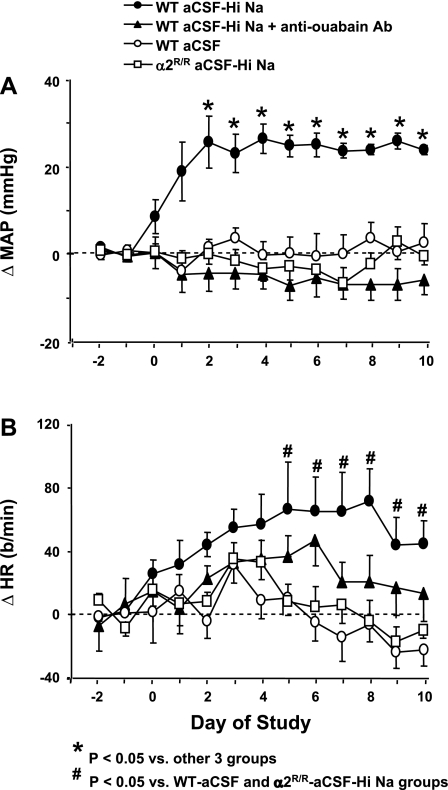
We first assessed the effects of a 10-day icv infusion of NaCl-rich aCSF (aCSF-HiNaCl) on blood pressure. In WT mice, icv infusion of aCSF-HiNaCl significantly increased 24-h mean arterial pressure (MAP) within 2 days (Fig. 1A). Thereafter, MAP reached a plateau that was 22–29 mmHg above baseline (P < 0.05 vs. baseline, Fig. 1A). Systolic and diastolic blood pressures during both the day and night showed similar responses to the 24-h MAP values (data not shown).
Fig. 1.
Twenty-four hour blood pressure and heart rate (HR) data obtained via telemetry before and after the start of intracerebroventricular (icv) infusions in wild-type (WT) mice or mice that are homozygous for 2-point mutations encoding a ouabain-resistant Na,K-ATPase α2-isoform (α2R/R). Infusions of various substances started on day 0 and continued until the end of the study on day 10. Values represent means ± SE of the changes from the baseline mean value in mean arterial pressure (ΔMAP; A) and ΔHR (B). Each value shown was in turn obtained from 24 MAP or HR measurements recorded hourly. aCSF, artificial cerebrospinal fluid (rate of Na+ infusion, 0.6 nmol/min); aCSF-HiNaCl, NaCl-rich aCSF (rate of Na+ infusion, 5.0 nmol Na+/min); aCSF-HiNaCl + anti-ouabain Ab, aCSF-HiNaCl + an anti-ouabain antibody (0.65 nmol/day). Baseline MAP and HR (combined mean of day −1 and day −2 values) for α2R/R mice given aCSF-HiNa were 125 ± 5 and 546 ± 10; n = 10. The respective baseline mean MAP and HR for aCSF, aCSF-HiNaCl, and aCSF-HiNaCl + Ab treatment groups in WT mice were [in mmHg and beats/min (b/min), respectively] 115 ± 3 and 553 ± 20, n = 8; 117 ± 6 and 548 ± 20, n = 5; and 120 ± 4 and 592 ± 15, n = 9. *P < 0.05 vs. other 3 groups. #P < 0.05 vs. WT-aCSF and α2R/R-aCSF-HiNa groups.
The heart rate response was more variable than that for MAP, and heart rate increased over the course of the study only for WT mice treated with icv aCSF-HiNaCl (Fig. 1B). There were significant differences in the heart rate responses on days 5–10 (Fig. 1B) between WT treated with aCSF-HiNaCl versus two other groups: WT-aCSF and α2R/R-aCSF-HiNaCl.
Previous studies suggest that ↑CSF [Na] is a critical event in the genesis of the hypertension that develops in Dahl-S and SHRs after they are placed on a high-salt diet (12). We therefore further examined the mechanisms of the hypertension produced by ↑CSF [NaCl] in WT mice. The above pressor response to icv aCSF-HiNaCl in WT mice was abolished by icv anti-ouabain antibody Fab fragments (Fig. 1A), indicating that it is meditated in mice by an OLS.
We also compared the response to icv aCSF-HiNaCl in WT mice with a normal α2S/S versus gene-targeted mice that are homozygous for two point mutations encoding an endogenous α2-isoform that is highly resistant to ouabain (α2R/R). The endogenous α2-isoform that is expressed in α2R/R mice has ∼1,000-fold lower affinity for ouabain than that of α2S/S, but it is otherwise fully functional (e.g., it has no known abnormalities in the binding and transport of Na+ and K+ ions) (21). The α2R/R mice had no significant blood pressure response to icv aCSF-HiNaCl in contrast with the α2S/S genotype (Fig. 1A). Heart rate did not significantly change from baseline in α2R/R mice given aCSF-HiNaCl or WT given either aCSF or aCSF-HiNa + anti-ouabain Fab icv (Fig. 1B).
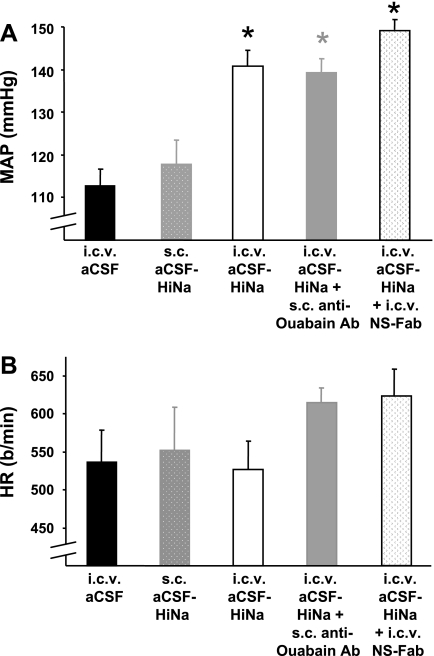
Once the above telemetry studies established that the pressor response to icv aCSF-HiNaCl reached a plateau within a few days of starting the infusion, subsequent studies focused on the values at a time point corresponding to this plateau. These studies were made with one-time blood pressure and heart rate measurements carried out via indwelling fluid-filled catheter on day 8 of icv and/or sc infusion. Two groups from telemetry studies (icv aCSF and aCSF-HiNaCl in WT mice) were repeated to compare daytime blood pressure measurements made via fluid-filled catheter and telemeter. Both blood pressure monitoring methods showed a significantly greater blood pressure value for icv aCSF-HiNaCl treatment than for aCSF on day 8 (Figs. 1A and 2A).
Fig. 2.
Effect of route of administration of NaCl-rich aCSF and/or anti-ouabain Ab on MAP (A) or HR (B) recorded after the indicated treatments in WT mice. Infusions [icv and/or subcutaneous (sc)] of substances were carried out for a period of 8 days as described in materials and methods, and blood pressures and HRs were measured on the last day of treatment via an indwelling fluid-filled catheter that had been implanted on the previous day; n = 5 to 6 in each group. Values represent means ± SE. *P < 0.05 vs. icv aCSF group. NS-Fab, nonspecific sheep IgG antibody Fab fragments (0.65 nmol/day).
One group of WT controls for these studies received sc aCSF-HiNaCl instead of icv. Blood pressure in this group was not significantly different from that of other WT controls treated with icv aCSF (Fig. 2A). Another control group received icv aCSF-HiNaCl + sc anti-ouabain Fab. The blood pressures were nearly identical between treatment groups receiving icv aCSF-HiNaCl given alone or in combination with sc Fab (Fig. 2A). In both of the latter groups receiving aCSF-HiNaCl, blood pressure was significantly greater than that for WT receiving aCSF. Therefore, the MAP response to aCSF-HiNaCl that was abolished when the anti-ouabain Fab fragments were given icv (Fig. 1A) was still present when the sc Fab fragments were given instead. There were no significant differences in heart rate among the above groups (Fig. 2B). The above findings support the concept that the present effects of icv aCSF-HiNaCl and anti-ouabain Fab fragments on blood pressure are due to central and not peripheral effects.
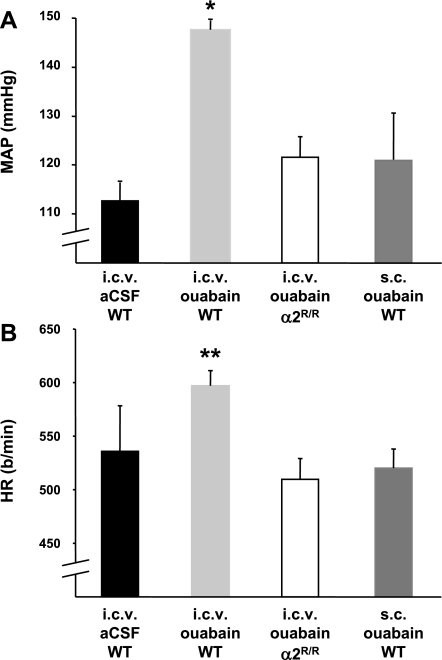
Previous studies have shown that chronic ouabain treatment administered peripherally in WT mice (10.3 nmol/day ip, based on a formula weight of 729 g/mol and an average mouse weight of 25 g) raises blood pressure (3). α2R/R mice are resistant to this treatment, i.e., they do not develop hypertension (3). In the present study, we first examined whether blood pressure can also be increased in WT mice when a low icv dose of ouabain is administered, as the effects of this treatment on blood pressure have not been previously tested in mice. The MAP in the WT genotype given icv ouabain (171 pmol/day icv) was ∼30 mmHg greater than that of WT mice treated with icv aCSF (Fig. 3A). Thus comparing sc versus icv administration of ouabain, there is a ∼60-fold difference in the dose that achieves a 20–30 mmHg increase in blood pressure. This suggests a central mechanism for the present icv ouabain treatment. Our data showing that there is no significant blood pressure elevation in WT mice when a regimen of 171 pmol/day of ouabain is given sc instead of icv (P = 0.32 vs. icv aCSF, Fig. 3A) support this idea.
Fig. 3.
Effect of ouabain administration on MAP (A) or HR (B) recorded after the indicated treatments. Infusions (icv and/or sc) of ouabain (171 pmol/day) were carried out for a period of 8 days as described in materials and methods, and blood pressures and HRs were measured on the last day of treatment; n = 5 to 6 in each group, except for icv ouabain (n = 8). Values represent means ± SE. *P < 0.05 vs. icv aCSF group. **P < 0.05 vs. α2R/R-icv ouabain and WT-sc ouabain groups.
In addition, the blood pressure response to icv ouabain in α2R/R mice was not significantly different from that of WT mice given icv aCSF (P = 0.14, Fig. 3A) but was significantly less than WT given icv ouabain (P < 0.001, Fig. 3A). Therefore, the pressor response to chronic icv ouabain treatment that was present in WT mice was absent in the α2R/R genotype. Taken together, the above findings indicate that it is the ouabain resistance of the α2-isoform in the brain of the α2R/R genotype that abolishes the blood pressure response to icv ouabain. Given the current results showing the brain-specific effects of icv aCSF-HiNaCl and icv anti-ouabain Fab, it follows that it is the resistance of the α2-subunit protein specifically in the brain that also prevents the pressor response to ↑CSF [NaCl].
To compare the current rise in CSF [Na] achieved in mice during icv infusion of NaCl-rich aCSF with previous increases measured in rats receiving similar treatment, CSF was collected from WT mice on day 8 of icv infusions of aCSF-HiNaCl. As a reference, CSF was also collected from age-matched mice receiving no treatment. In mice receiving icv aCSF-HiNaCl infusions, CSF [Na] was 164.7 ± 1.8 (mean ± SE, n = 6) versus 159.5 ± 1.8 meq/l (n = 5) in the unoperated controls (P < 0.05).
DISCUSSION
The current study demonstrates multiple findings that have not been previously reported. First, chronic icv ouabain (171 pmol/day) but not the same dose given sc increases blood pressure in WT mice. Since ouabain is a specific inhibitor of the Na,K-ATPase, this finding suggests that a chronic decrease in Na,ATPase activity in the brain raises blood pressure in WT mice, extending the range of species for a mechanism initially proposed in rats (25). Second, in contrast to α2S/S (WT) mice, in the α2R/R genotype, icv ouabain did not raise blood pressure above that of controls treated with icv aCSF. These findings demonstrate that the above pressor response to icv ouabain is mediated by the α2-isoform in the brain. Third, chronic icv infusion of NaCl-rich aCSF (aCSF-HiNaCl) causes hypertension in mice, and this pressor response was abolished by icv but not sc anti-ouabain antibody. This indicates that the pressor response to ↑CSF [NaCl] is mediated specifically by an OLS in the brain. Fourth, α2R/R mice did not develop hypertension in response to icv aCSF-HiNaCl, again in contrast to WT mice. These results demonstrate that the ouabain-binding site on the Na,K-ATPase α2-isoform is critical in the pressor response to ↑CSF [NaCl]. Thus the interaction of the OLS with the α2-isoform in the brain is essential for the hypertension caused by ↑CSF [NaCl].
We had previously shown that acute (60 min) icv infusions of NaCl-rich aCSF increase blood pressure in mice and that this response is mediated by the brain OLS (28). The current results extend those previous findings to demonstrate that chronically ↑CSF [NaCl] also causes a sustained increase in blood pressure via the OLS in the brain.
The protein expression of the α2-isoform was previously examined in the α2R/R mouse brain and was found to be similar to that of the α2S/S genotype (2). Likewise, the expression of the individual α1- and α3-subunit proteins is not different between α2R/R and α2S/S mice, nor is there any difference between the two genotypes in the aggregate α-subunit expression, as detected with an antibody that cross-reacts with all three isoforms (2). Therefore, the current effects of the α2R/R amino acid substitutions cannot be attributed to altered expression levels of Na,K-ATPase α-subunits.
In contrast to α-subunit protein expression, the binding of the specific Na,K-ATPase ligand ouabain does differ between α2S/S and α2R/R genotypes, as expected. Normally in WT rodents, the Na,K-ATPase α1-isoform is highly resistant to the binding of ouabain, and the two remaining isoforms (α2 and α3) are ouabain sensitive. However, in α2R/R mice, the α2-isoform is ouabain resistant by design. Thus, in tissues that only express the α1- and α2-isoforms [e.g., vascular smooth muscle (VSM) and the heart], there should be no detectable ouabain binding in α2R/R mice. Indeed, [3H]ouabain binding capacity is absent in these two tissues in the α2R/R genotype, even though the protein expression level of the α2-isoform is normal (2).
In contrast, the brain contains an abundance of an additional isoform (α3), which binds ouabain with high affinity. In the study of Dostanic et al. (2), specific [3H]ouabain binding in the brains of α2R/R mice was reduced from that in the α2S/S genotype by ∼29%. Presumably, in the α2R/R genotype, this reduction in ouabain binding reflects the ouabain resistance of the endogenous α2-isoform, and the residual ouabain binding arises from the α3-subunit.
Given that ∼71% of the WT ouabain binding capacity persists in the brains of α2R/R mice (2) and that the WT α3-subunit protein has a greater affinity for ouabain than the WT α2-subunit, it is surprising that the pressor responses to icv ouabain and aCSF-HiNaCl are absent in α2R/R mice. The present lack of blood pressure responses to icv aCSF-HiNaCl and icv ouabain in α2R/R mice suggests that the role of the brain α3-subunit, if any, in these responses is minimal relative to that of α2. The Na,K-ATPase α3-isoform in the brain may only be relevant at higher infusion rates of icv aCSF-HiNaCl/ouabain than were used here, or it may predominantly serve other functions besides the regulation of blood pressure.
Earlier we had hypothesized that the inhibition of the WT Na,K-ATPase α2-isoform by the OLS contributes to the normal pressor response to ↑CSF [NaCl] (5). If this were the case, then the inhibition of the α2-isoform via reduced α2 gene expression should either increase baseline blood pressure or should augment the pressor response to the Na,K-ATPase inhibitor OLS during ↑CSF [NaCl]. In support of this hypothesis, the heterozygous α2 gene knockout (homozygous α2 deletions produce neonatal lethality) enhances the acute pressor response to icv aCSF-HiNaCl, as well as to icv ouabain (5). However, from our previous study, it could not be concluded whether other isoforms also contributed to hypertensive response to ↑CSF [NaCl]. The current studies demonstrate that the α2-subunit alone in the brain is critically important in regulating the blood pressure response to ↑CSF [NaCl] and to ouabain/OLS in the brain.
In the present study, icv infusion of NaCl-rich aCSF in WT mice elevated CSF [Na] above normal levels found in unoperated WT mice by ∼5 meq/l. This is near the range of increases in CSF [Na] produced (6–11 meq/l) when salt-sensitive hypertensive rat strains, such Dahl-S and SHRs, are placed on a high-salt diet (12) or when Dahl-S or Wistar rats are given icv NaCl-rich aCSF (13, 14). It is also less than that generated by a high-salt diet in salt-sensitive hypertensive mice (unpublished observation). Therefore, the change in CSF [Na] that occurs with the current icv NaCl-rich aCSF infusion in WT mice does not exceed those that occur in other experimental models of salt-induced hypertension.
Although it is often assumed that Na+ is the ion that is critical for hypertension induced by oral salt intake, some studies performed in rat hypertensive models have demonstrated that Cl− plays an equally important, if not more important, role than Na+ (24, 30). There is also evidence that when salt is given centrally in rats, Cl− significantly contributes to the ensuing pressor response (26). However, the latter study was performed under anesthesia, which itself is known to affect sympathetic activity and blood pressure. To our knowledge, studies on the specific roles of Na+ versus Cl− in the pressor response to icv NaCl-rich aCSF have not been performed in conscious rats or mice. This is an understudied area that deserves further investigation.
In addition to being expressed in the brain, the Na,K-ATPase α2-subunit is expressed as well in VSM, where it is also critical for the regulation of blood pressure. Like the present icv administration of ouabain, peripheral (sc) ouabain treatment also raises blood pressure (3, 20, 31, 33) and the latter pressor response is absent in α2R/R mice (2). The latter effect is likely due to resistance of the α2-isoform in VSM to ouabain in the systemic circulation (2). In support of an effect of α2-isoform inhibition in VSM to produce vasoconstriction, arteries isolated from heterozygous α2 knockout mice have increased myogenic tone versus WT (32). However, in the current study, when the doses of ouabain and aCSF-HiNa that were effective icv were given sc instead, there was no significant effect on blood pressure. These results argue against a direct impact of the α2R/R mutations in VSM to block the present increases in blood pressure caused by icv aCSF-HiNaCl infusion.
Within the brain, the Na,K-ATPase α2-isoform is expressed predominantly in glia. When combined with the fact that the blood pressure effects of ouabain or OLS in the brain are mediated exclusively by the α2-isoform, the association of α2 expression with glia raises the question whether the central pressor effects of these glycosides are initiated by their binding to the α2-isoform in this cell type. Previous studies have shown that icv ouabain or Na-rich aCSF activates the RAS in the mouse brain (5, 28). Mediation of this effect via the inhibition of the α2-isoform by OLS/ouabain is suggested by the fact that the heterozygous α2 knockout also activates the RAS in the brain (5). Coincidentally, as much as 90% of the angiotensinogen in the brain is found in glia (23), and the upregulation of RAS proteins specifically in glia raise blood pressure (18, 19). The CNS pathways and mechanisms whereby ouabain or OLS activate the brain RAS to raise blood pressure are not known, but a starting point to addressing this conundrum may be to examine the cellular and/or electrophysiological responses of glia per se to ouabain and/or OLS.
Perspectives/significance.
Increases in CSF [NaCl] precede high blood pressure in rat models of hypertension that are induced by a high-salt diet. In rats, an ↑CSF [NaCl] caused by direct chronic icv infusion of NaCl-rich aCSF activates the same cascade of systems in the brain that leads to hypertension following a high-salt diet in salt-sensitive strains, supporting the concept that ↑CSF CSF [NaCl] is a critical event in the hypertensive response to a high-salt diet. A crucial step in this cascade is the binding of an endogenous OLS in the brain to the Na,K-ATPase, leading to elevated sympathetic activity and hypertension. The present results show that ↑CSF [NaCl] also causes hypertension in the mouse via an action of the OLS in the brain and that the interaction of the OLS specifically with the α2-isoform of the Na,K-ATPase in the CNS mediates this response. The α2-isoform thus may be a specific target for the development of novel antihypertensive therapy. Since the inhibition of the Na,K-ATPase α2-isoform by the OLS appears to be a critical step in the above cascade leading to salt-dependent hypertension, then overexpression of the α2-isoform (e.g., via gene therapy vectors) in the brain in animal models of this disease would represent a novel “test of concept” approach to the treatment of this form of hypertension.
GRANTS
This work was supported by Heart and Stroke Foundation of Ontario Grant-in-Aid NA-6324 (to J. W. Van Huysse) and by National Heart, Lung, and Blood Institute Grants R01-HL-28573 and R01-HL-66062 (to J. B. Lingrel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J. W. Van Huysse produced the conception and design of research, analyzed data, interpreted results of experiments, and drafted manuscript; J. W. Van Huysse, I. Dostanic, and J. B. Lingrel edited and revised manuscript; J. W. Van Huysse, I. Dostanic, and J. B. Lingrel approved final version of manuscript; and X. Hou and H. Wu performed experiments.
ACKNOWLEDGMENTS
We are very grateful to Dr. Frans Leenen for providing a critical review of the manuscript.
REFERENCES
- 1. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 2. Dostanic I, Lorenz JN, Schultz Jel J, Grupp IL, Neumann JC, Wani MA, Lingrel JB. The alpha2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem 278: 53026–53034, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB. The alpha2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 288: H477–H485, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Hieber V, Siegel GJ, Fink DJ, Beaty MW, Mata M. Differential expression of Na+K+-ATPase alpha isoforms in the central nervous system. Cell Mol Neurobiol 11: 253–262, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hou X, Theriault SF, Dostanic-Larson I, Moseley AE, Lingrel JB, Wu H, Dean S, Van Huysse JW. Enhanced pressor response to increased CSF sodium concentration and to central ANG I in heterozygous alpha2 Na+-K+-ATPase knockout mice. Am J Physiol Regul Integr Comp Physiol 296: R1427–R1438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang BS, Harmsen E, Yu H, Leenen FH. Brain ouabain-like activity and the sympathoexcitatory and pressor effects of central sodium in rats. Circ Res 71: 1059–1066, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Huang BS, Leenen FH. Brain “ouabain” and angiotensin II in salt-sensitive hypertension in spontaneously hypertensive rats. Hypertension 28: 1005–1012, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Huang BS, Leenen FH. Brain “ouabain” mediates the sympathoexcitatory and hypertensive effects of high sodium intake in Dahl salt-sensitive rats. Circ Res 74: 586–595, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Huang BS, Leenen FH. Blockade of brain “ouabain” prevents sympathoexcitatory and pressor responses to high sodium in SHR. Am J Physiol Heart Circ Physiol 271: H103–H108, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Huang BS, Leenen FH. Both brain angiotensin II and “ouabain” contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension 32: 1028–1033, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Huang BS, Leenen FH. Sympathoexcitatory and pressor responses to increased brain sodium and ouabain are mediated via brain ANG II. Am J Physiol Heart Circ Physiol 270: H275–H280, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on high salt diet. Am J Physiol Heart Circ Physiol 287: H1160–H1166, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Huang BS, Veerasingham SJ, Leenen FH. Brain “ouabain,” ANG II, and sympathoexcitation by chronic central sodium loading in rats. Am J Physiol Heart Circ Physiol 274: H1269–H1276, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Huang BS, Wang H, Leenen FH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs. -resistant rats. Am J Physiol Heart Circ Physiol 281: H1881–H1889, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Huang BS, White RA, Jeng AY, Leenen FH. Role of central nervous system aldosterone synthase and mineralocorticoid receptors in salt-induced hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 296: R994–R1000, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Huang BS, Ganten D, Leenen FH. Responses to central Na+ and ouabain are attenuated in transgenic rats deficient in brain angiotensinogen. Hypertension 37: 683–686, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Liu L, Duff K. A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse (Demonstration video). J Vis Exp 21: pii: 960, 2008. doi:10.3791/960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morimoto S, Cassell MD, Beltz TG, Johnson AK, Davisson RL, Sigmund CD. Elevated blood pressure in transgenic mice with brain-specific expression of human angiotensinogen driven by the glial fibrillary acidic protein promoter. Circ Res 89: 365–372, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Morimoto S, Cassell MD, Sigmund CD. Glia- and neuron-specific expression of the renin-angiotensin system in brain alters blood pressure, water intake, and salt preference. J Biol Chem 277: 33235–33241, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Pulina MV, Zulian A, Berra-Romani R, Beskina O, Mazzocco-Spezzia A, Baryshnikov SG, Papparella I, Hamlyn JM, Blaustein MP, Golovina VA. Upregulation of Na+ and Ca2+ transporters in arterial smooth muscle from ouabain-induced hypertensive rats. Am J Physiol Heart Circ Physiol 298: H263–H274, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Price EM, Rice DA, Lingrel JB. Structure-function studies of Na,K-ATPase. Site-directed mutagenesis of the border residues from the H1–H2 extracellular domain of the alpha subunit. J Biol Chem 265, 6638–6641, 1990 [PubMed] [Google Scholar]
- 22. Rhee HM, Hokin LE. Inhibition of ouabain-binding to (Na+ + K+)ATPase by antibody against the catalytic subunit but not by antibody against the glycoprotein subunit. Biochim Biophys Acta 558: 108–112, 1979 [DOI] [PubMed] [Google Scholar]
- 23. Schinke M, Baltatu O, Böhm M, Peters J, Rascher W, Bricca G, Lippoldt A, Ganten D, Bader M. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc Natl Acad Sci USA 96: 3975–3980, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidlin O, Tanaka M, Bollen AW, Yi SL, Morris RC., Jr Chloride-dominant salt sensitivity in the stroke-prone spontaneously hypertensive rat. Hypertension 45: 867–873, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Shah J, Jandhyala BS. Role of Na+,K+-ATPase in the centrally mediated hypotensive effects of potassium in anaesthetized rats. J Hypertens 9: 167–170, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Shah J, Jandhyala BS. Studies on the role(s) of cerebrospinal fluid osmolality and chloride ion in the centrally mediated pressor responses of sodium chloride. Clin Exp Hypertens A 13: 297–312, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta 988: 185–220, 1989 [DOI] [PubMed] [Google Scholar]
- 28. Van Huysse JW, Hou XH. Pressor response to CSF sodium in mice: mediation by a ouabain-like substance and renin-angiotensin system in the brain. Brain Res 1021: 219–223, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Watts AG, Sanchez-Watts G, Emanuel JR, Levenson R. Cell-specific expression of mRNAs encoding Na+,K+-ATPase-α- and β-subunit isoforms within the rat central nervous system. Proc Natl Acad Sci USA 88: 7425–7430, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whitescarver SA, Ott CE, Jackson BA, Guthrie GP, Jr, Kotchen TA. Salt-sensitive hypertension: contribution of chloride. Science 223: 1430–1432, 1984 [DOI] [PubMed] [Google Scholar]
- 31. Zhang J, Hamlyn JM, Karashima E, Raina H, Mauban JR, Izuka M, Berra-Romani R, Zulian A, Wier WG, Blaustein MP. Low-dose ouabain constricts small arteries from ouabain-hypertensive rats: implications for sustained elevation of vascular resistance. Am J Physiol Heart Circ Physiol 297: H1140–H1150, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang J, Lee MY, Cavalli M, Chen L, Berra-Romani R, Balke CW, Bianchi G, Ferrari P, Hamlyn JM, Iwamoto T, Lingrel JB, Matteson DR, Wier WG, Blaustein MP. Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J Physiol 569: 243–256, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zulian A, Baryshnikov SG, Linde CI, Hamlyn JM, Ferrari P, Golovina VA. Upregulation of Na+/Ca2+ exchanger and TRPC6 contributes to abnormal Ca2+ homeostasis in arterial smooth muscle cells from Milan hypertensive rats. Am J Physiol Heart Circ Physiol 299: H624–H633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]