Abstract
Histone methylation, a determinant of chromatin structure and gene transcription, was thought to be irreversible, but recent evidence suggests that lysine-specific demethylase-1 (LSD1, Kdm1a) induces demethylation of histone H3 lysine 4 (H3K4) or H3K9 and thereby alters gene transcription. We previously demonstrated a human LSD1 phenotype associated with salt-sensitive hypertension. To test the hypothesis that LSD1 plays a role in the regulation of blood pressure (BP) via vascular mechanisms and gene transcription, we measured BP and examined vascular function and endothelial nitric oxide (NO) synthase (eNOS) expression in thoracic aorta of male wild-type (WT) and heterozygous LSD1 knockout mice (LSD1+/−) fed either a liberal salt (HS; 4% NaCl) or restricted salt diet (LS; 0.08% NaCl). BP was higher in LSD1+/− than WT mice on the HS diet but not different between LSD1+/− and WT mice on the LS diet. Further examination of the mechanisms of this salt-sensitive hypertension in LSD1+/− mice on the HS diet demonstrated that plasma renin activity and plasma levels and urinary excretion of aldosterone were less in LSD1+/− than WT, suggesting suppressed renin-angiotensin-aldosterone system. In contrast, phenylephrine (Phe)-induced aortic contraction was greater in LSD1+/− than WT mice on the HS diet. Treatment of aortic rings with 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; a blocker of guanylate cyclase) enhanced Phe contraction in LSD1+/− compared with WT mice on the HS diet. Acetylcholine (Ach)-induced relaxation was less in LSD1+/− than WT mice on the HS diet. Endothelium removal or pretreatment with Nω-nitro-l-arginine methyl ester (blocker of NOS) or ODQ abolished Ach-induced relaxation in aorta of WT but had minimal effect in LSD1+/−. Vascular relaxation to sodium nitroprusside, an exogenous NO donor and guanylate cyclase activator, was decreased in LSD1+/− vs. WT mice on the HS diet. RT-PCR and Western blots revealed decreased eNOS mRNA expression and eNOS and guanylate cyclase protein in the heart and aorta of LSD1+/− compared with WT mice on HS diet. Thus, during the HS diet, LSD1 deficiency is associated with hypertension, enhanced vascular contraction, and reduced relaxation via NO-cGMP pathway. The data support a role for LSD1-mediated histone demethylation in the regulation of NOS/guanylate cyclase gene expression, vascular function, and BP during the HS diet.
Keywords: blood pressure; salt sensitivity; endothelium; nitric oxide; guanosine 3′,5′-cyclic monophosphate
genetic and environmental factors play an important role in the pathogenesis of cardiovascular disease such as hypertension (40). Epigenetic modification, a process involving all gene and chromatin modifications other than changes in the DNA sequence, could also affect the renin-angiotensin-aldosterone system (RAAS) and other hormonal and vascular control mechanisms of blood pressure (BP; Refs. 2, 21). DNA modification involves methylation, acetylation, and addition of other molecules to the DNA backbone (10). Histones are simple proteins rich in lysine (Lys) and arginine (Arg) that complex with and pack the DNA tightly into chromatin inside the nucleus. There are several types of histones including H1, H2A, H2B, H3, and H4 (41). Like DNA, histones can undergo modifications and become methylated, acetylated, or biotinylated, changes associated with alterations in chromatin structure and consequently gene transcription (33).
Histone H3 is one of the most actively and chemically modified histones, having multiple methylation sites in its Lys and Arg residues and various methylation states involving the addition of one, two, or three methyl groups (17, 35). Methylation of histone H3 can alter chromatin structure in a residue-dependent manner such that methylation at the H3 Lys 9 residue (H3K9) is associated with a repressive chromatin conformation in which histones are closely condensed and do not allow transcription factors to bind to their elements for transcription. In contrast, methylation at the H3K4 site is associated with an open chromatin structure, where transcription is activated (6, 18, 23, 37, 38, 43). While histone methylation was initially presumed irreversible, it has recently been found that the methylation state of histones is actively regulated not only by histone methyl transferases but also by demethylases (24, 33, 36). Specifically, it has been demonstrated that lysine-specific demethylase-1 (LSD1, also known as Kdm1a) induces demethylation of H3 (24, 36, 37). At baseline, LSD1 demonstrates specificity toward H3K4>>H3K9 methylation site. Following interaction with nuclear receptors such as hormone steroid receptors, LSD1 specificity is switched toward H3K9>>H3K4 methylation site (46). While histone demethylation was shown to cause epigenetic modifications and alterations in gene transcription, little is known regarding the role of LSD1 in the regulation of cardiovascular-renal function and BP.
We (45) have previously shown that polymorphic variants in the LSD1 gene are associated with salt-sensitive hypertension in humans. LSD-1 gene variants in the HyperPATH cohort hypertensive subjects of African-American descent demonstrated that minor allele carriers (rs587168) display BP salt sensitivity. In search of the potential mechanisms underlying the increases in BP, we found that the plasma renin activity and aldosterone levels were suppressed. These observations in humans suggested that these salt-sensitive increases in BP may not be due to phenotypic alterations in the RAAS and plasma volume and highlighted the possibility of interplay between LSD1 and altered vascular control mechanisms of BP during liberal salt intake (45).
The present study was designed to test the hypothesis that LSD1 plays a key role in the regulation of BP via mechanisms involving alterations in vascular function and gene expression. We used wild-type (WT, LSD1+/+) and heterozygous LSD1 knockout mice (HET, LSD1+/−) maintained on either a liberal salt or restricted salt diet to investigate whether 1) LSD1 deficiency is associated with increased BP and altered RAAS, 2) LSD1 deficiency is associated with altered vascular reactivity, and 3) the changes in vascular contraction/relaxation associated with LSD1 deficiency reflect alterations in the expression/activity of endothelial nitric oxide synthase (eNOS) and the nitric oxide (NO)-cGMP relaxation pathway.
MATERIALS AND METHODS
Animals.
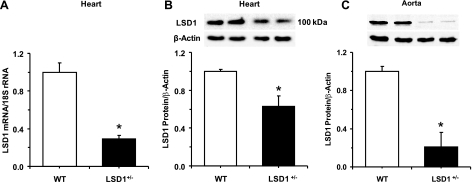
Adult male LSD1 heterozygous KO mice (LSD1+/−) and littermate WT mice were used. Briefly, LSD1+/− mice were generated by gene trap whereby the insertion of a β-geo cassette in intron 8 introduced a STOP codon and thereby led to a truncated LSD1 protein with no enzymatic demethylase activity. The genotypes were confirmed by PCR. The LSD1 homozygous KO (LSD1−/−) was embryonically lethal (∼ED6). In contrast, the heterozygous LSD1+/− mice were viable and fertile. RT-PCR analysis demonstrated significant decrease in LSD1 mRNA expression in the heart of LSD1+/− compared with WT mice. Also, Western blot analysis confirmed that LSD1 protein amount was significantly less in the heart and aorta of LSD1+/− compared with WT mice (Fig. 1). After birth, mice were housed in the animal facility in 12:12-h light-dark cycle at 22 ± 1°C ambient temperature and maintained on ad libitum regular Purina Rodent Chow (5053, 0.8% NaCl; Purina, St. Louis, MO) and tap water until time of initiation of controlled dietary salt intake. Adult mice from each genotype were placed on a liberal salt diet (4% NaCl) for 7 days (11, 28). In separate experiments, another group of mice from each genotype were placed on a restricted salt diet (0.08% NaCl) for 7 days. The initial LSD1+/− mice were created on a mixed background and have since been bred into mice with a CL57Bl/6 background for at least six generations, and the WT littermates with CL57Bl/6 background were used as controls. All experimental procedures followed the guidelines of and were approved by the Institutional Animal Care and Use Committee at Harvard Medical School.
Fig. 1.
RT-PCR of lysine-specific demethylase-1 (LSD1) mRNA expression in the heart (A) and Western blot analysis of LSD1 protein amount in the heart (B) and aorta (C) of WT and LSD1+/− mice. Data represent means ± SE (n = 4). Two representative gels for LSD1 and actin immunoreactive bands are presented for each genotype. *Measurements in LSD1+/− mice are significantly different (P < 0.05) from corresponding measurements in wild-type (WT) mice.
Systolic BP measurements.
Systolic BP was measured in conscious mice after reaching sodium balance on day 7 using tail-cuff plethysmography (BP Analyzer, model 179; IITC Life Science, Woodland Hills, CA) as previously described (29, 31). Mice were warmed at 30°C for 10 min and allowed to rest quietly before BP measurement. BP measurements were taken in the morning in a quiet room, and the mice were kept calm and handled by the same person. No sedation was used. Mice were acclimatized to the tail-cuff BP measurement procedure for ≥1 wk before the final measurements. Our previous tail-cuff BP measurements correlated with results obtained by telemetry (30).
Urine analysis.
On day 7, mice were placed in metabolic cages and a 24-h urine sample was collected for measurement of urinary aldosterone.
Plasma analysis.
Blood was collected in purple-top BD Microtainer tubes (EDTA). The plasma was separated by centrifugation, and aldosterone levels were determined in duplicates (200 μl each) using a solid-phase RIA kit (Diagnostic Products, Los Angeles, CA). Plasma renin activity was measured by radioimmunometric assay (DiaSorin, Stillwater, MN).
Tissue preparation.
In the morning of day 8, the mice were euthanized under isoflurane anesthesia, the thoracic cavity was opened, and the heart and thoracic aorta were rapidly excised. The aorta was placed in oxygenated Krebs solution, carefully dissected, cleaned of connective tissue under microscopic visualization, and cut into 2-mm-wide rings. Additional sections of the aorta and the heart were placed in liquid nitrogen immediately after collection in preparation for mRNA and protein analysis.
Isometric contraction.
Aortic segments were suspended between two tungsten wire hooks; one hook is fixed at the bottom of a tissue bath, and the other hook is connected to a Grass force transducer (FT03; Astro-Med, West Warwick, RI). Aortic segments were stretched under 0.5 g of resting tension and allowed to equilibrate for 45 min in a temperature controlled, water-jacketed tissue bath, filled with 50 ml Krebs solution continuously bubbled with 95% O2-5% CO2 at 37°C. The changes in isometric contraction were recorded on a Grass polygraph (model 7D; Astro-Med).
After tissue equilibration, a control contraction to 96 mM KCl was elicited. Once maximum KCl contraction was reached, the tissue was rinsed with Krebs three times, 10 min each. The control KCl-induced contraction followed by rinsing in Krebs was repeated twice. Aortic segments were stimulated with increasing concentrations of phenylephrine (Phe; 10−9 to 10−5 M), concentration-contraction curves were constructed, and the maximal Phe contraction was measured. The individual Phe concentration-response curves were further analyzed using a nonlinear regression curve (best-fit sigmoidal dose-response curve; Sigmaplot), and the effective Phe concentration that produced half the maximal contraction (ED50) was measured and presented as pED50 (−log M). In other experiments, the tissues were precontracted with Phe (10−5 M), increasing concentrations of acetylcholine (Ach; 10−9 to 10−5 M) were added, and the percent relaxation of Phe contraction was measured. We initially tested the Ach relaxation response in aortic segments precontracted with half-maximal Phe concentrations; however, the contractile response was small, making it difficult to discern and quantify the differences in the relaxation response to increasing concentrations of Ach. Therefore, the blood vessels were precontracted with 10−5 M Phe, and the relaxation experiments were performed using the same Phe concentration in the different experimental animals groups. Parallel contraction and relaxation experiments were performed in aortic rings pretreated with the NO synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 3 × 10−4 M) or the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10−5 M) for 10 min. Pretreatment with l-NAME for 10 min appeared to allow enough time for drug equilibration, as this protocol was sufficient to block Ach-induced relaxation. For endothelium intact vessels from both WT and LSD1+/− mice, extreme care was taken to avoid injury of the endothelium. To further test the role of the endothelium, Ach-induced relaxation was also measured in endothelium-denuded aortic rings. In these experiments, the endothelium was removed by rubbing the vessel interior five times around the tip of a forceps. Endothelium denudation was associated with abolishment of Ach relaxation in WT mice. We used the same procedure for endothelium denudation in LSD1+/− mice, and the same procedure abolished Ach-induced vascular relaxation in these mice. The relaxation of Phe-precontracted aortic rings in response to the exogenous NO donor and guanylate cyclase activator sodium nitroprusside (SNP) was also measured.
Real-time RT-PCR and transcript analysis.
Total mRNA was extracted from the hearts using the RNeasy mini-kit (Qiagen Sciences, Germantown, MD) as previously described (29, 31). cDNA was synthesized from 1.5 μg RNA with the first-strand cDNA synthesis kit (GE Healthcare, Piscataway, NJ). PCR amplification reactions were performed in duplicate using the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) and the ΔΔCT method to determine mRNA levels as described previously(19). Gene expression was normalized to 18S rRNA levels. PCR amplification to detect eNOS and the housekeeping 18S rRNA was performed with TaqMan gene expression assays (proprietary primers and probes designed and synthesized by Applied Biosystems). Data are presented as fold increase relative to the measurements in WT mice.
Western blots and protein analysis.
Protein was extracted by homogenizing the heart or the aorta with RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA) as previously described (29, 31). Protein extracts (40 μg) were combined with an equal volume of 2× Laemmli loading buffer, boiled for 5 min, and size fractionated by electrophoresis on 7.5% SDS-polyacrylamide gels. Proteins were transferred from the gel to a nitrocellulose membrane by electroblotting. Membranes were incubated in 5% nonfat dried milk in TBS-Tween (USB, Cleveland, OH) for 1 h and then overnight at 4°C with rabbit anti-LSD1 antibody (1:1,000; AbCam, Cambridge, MA), mouse anti-eNOS antibody (1:2,500; BD Biosciences, San Diego, CA), or rat anti-soluble guanylate cyclase antibody (1:1,000; Cayman Chemical, Ann Arbor, MI). Membranes were washed, incubated with peroxidase-conjugated secondary antibody, and analyzed using enhanced chemiluminescence (Perkin-Elmer Life Sciences, Boston, MA). The blots were subsequently reprobed for β-actin (1:2,000), and the results were normalized to β-actin to correct for loading. Data are presented as fold increase relative to the measurements in WT mice.
Solutions and drugs.
Krebs solution contained the following (in mM): 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 NaH2PO4, 11.5 dextrose, 2.5 CaCl2, and 1.2 MgCl2, at pH 7.4, and bubbled with 95% O2-5% CO2. Ninety-six millimoles of KCl were prepared as Krebs solution with equimolar substitution of NaCl with KCl. Stock solutions of Phe, Ach, SNP, and l-NAME (10−1 M; Sigma, St. Louis, MO) were prepared in distilled water. Stock solution of ODQ (10−1 M) was prepared in DMSO. Final concentration of DMSO in experimental solution was <0.1%. All other chemicals were of reagent grade or better.
Statistical analysis.
The data were analyzed using ANOVA and are presented as means ± SE. For analysis of the RT-PCR data and determination of mRNA levels, the ΔΔCT method was used (19). The averages and SD were determined for all the technical replicates CT. With the use of the average technical replicate CT, ΔCT was calculated for each group (CT of target gene − CT of control gene 18S rRNA). After the average CT was determined for each group, ΔΔCT values (CT of test group − CT of control WT group) were calculated and presented as fold increase relative to the measurements in WT mice 2−(ΔΔCT). Scheffe's F-test was used for comparison of multiple means. Student's t-test or nonparametric tests for unpaired data were used as appropriate for comparison of two means. Differences were considered statistically significant if P < 0.05. All studies were accomplished with the individual performing the study blinded as to the genotype of the animal and the treatment group from which the tissues were obtained.
RESULTS
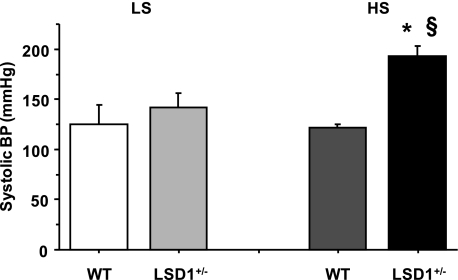
In mice on a liberal dietary salt intake, body weights were not significantly different between WT (31.6 ± 0.6g) and LSD1+/− mice (32.2 ± 3.3 g). Similar to the mice on a liberal salt diet, there was no difference in body weight between WT and LSD1+/− mice on a restricted salt diet. However, systolic BP was significantly greater in LSD1+/− mice on a liberal salt diet compared with WT mice on a liberal salt diet or LSD1+/− mice on a restricted salt diet (Fig. 2). In contrast, systolic BP was not significantly different between WT and LSD1+/− mice on a restricted salt diet (Fig. 2). Also, systolic BP was not significantly different between WT mice on a liberal salt diet and WT mice on a restricted salt diet (Fig. 2).
Fig. 2.
Systolic blood pressure (BP) in WT and LSD1+/− mice on restricted salt (LS) and liberal salt (HS) diets. Data represent means ± SE (n = 6). *Measurements in LSD1+/− mice are significantly different (P < 0.05) from corresponding measurements in WT. §Measurements in LSD1+/− mice on HS diet are significantly different (P < 0.05) from corresponding measurements in LSD1+/− mice on LS diet.
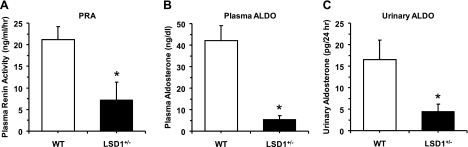
To test whether the increased BP in LSD1+/− mice on a liberal salt diet involve changes in the RAAS, we measured plasma renin activity and both plasma and urinary aldosterone. Importantly, both plasma renin activity and the aldosterone levels in plasma and 24-h urine were significantly less in LSD1+/− compared with WT mice (Fig. 3). We should note that the plasma used for analysis of the RAAS was collected while the animals were under isoflurane anesthesia, and these anesthetics may cause activation of RAAS and increase plasma renin activity and aldosterone levels (14), and therefore the results should be interpreted with caution. However, the same procedure for collecting plasma was used in both WT and LSD1+/− mice.
Fig. 3.
Plasma renin activity (PRA; A) and plasma levels (B) and 24-h urinary excretion of aldosterone (ALSO; C) in WT and LSD1+/− mice on HS diet. Data represent means ± SE (n = 4). *Measurements in LSD1+/− mice are significantly different (P < 0.05) from corresponding measurements in WT.
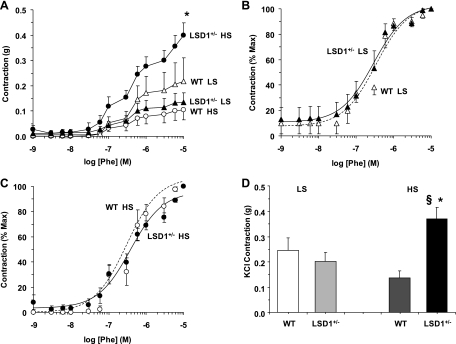
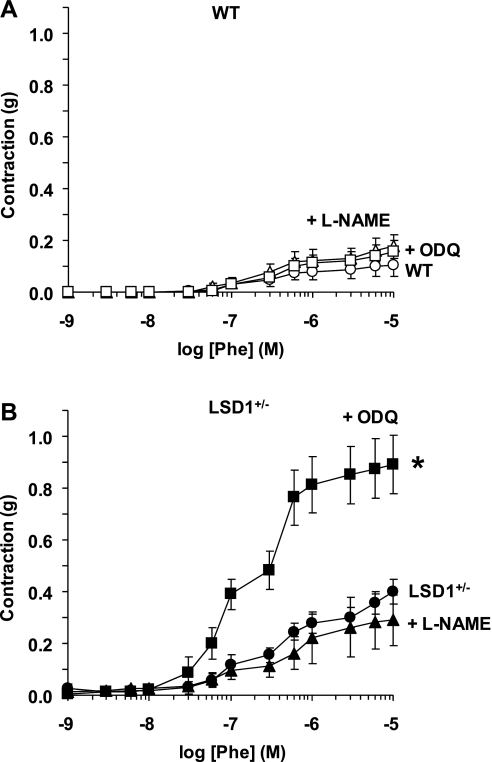
To test whether the increased BP in LSD1+/− mice on a liberal salt diet involves changes in vascular function, we measured vascular reactivity in isolated vessels of WT and LSD1+/− mice. In aortic segments of WT mice on a liberal salt diet, the α-adrenergic receptor agonist Phe caused concentration-dependent contraction that reached a maximum of 0.11 ± 0.04 g at 10−5 M. Phe (10−5 M)-induced contraction was maintained at steady state for ≥20 min. The maximum Phe contraction in LSD1+/− mice was significantly enhanced (P < 0.05) compared with WT mice on a liberal salt diet or LSD1+/− mice on a restricted salt diet (Fig. 4A). In comparison, Phe-induced aortic contraction was not significantly different amongst WT mice on either diet and LSD1+/− mice on a restricted salt diet (Fig. 4A). When the Phe-induced aortic contraction was presented as percentage of maximum and the Phe ED50 was calculated, Phe appeared to be equally potent in the aorta of LSD1+/− and WT mice regardless of the specific dietary salt intake (Fig. 4, B and C), with the Phe pED50 not significantly different among LSD1+/− restricted salt (6.16 ± 0.44), WT restricted salt (6.4 ± 0.11), LSD1+/− liberal salt 6.39 ± 0.12, and WT liberal salt (6.32 ± 0.18). The similarity in the Phe pED50 suggests similar sensitivity of the α-adrenergic receptors to Phe in the aorta of LSD1+/− and WT mice that is not effected by dietary salt or genotype. Measurement of aortic contraction to 96 mM KCl, a membrane depolarization-dependent and receptor-independent stimulant of Ca2+ influx from the extracellular space, produced similar results: enhanced contraction in LSD1+/− mice on a liberal salt diet compared with WT mice on the same diet or LSD1+/− mice on a restricted salt diet (Fig. 4D). In comparison, no significant difference was observed in KCl-induced contraction in aortic segments of LSD1+/− compared with WT mice on a restricted salt diet, supporting that the observed changes in vascular function in LSD1+/− mice are dependent on the liberal salt diet. Therefore, further mechanistic experiments were performed to determine the potential cellular mechanisms underlying the increased vascular reactivity in LSD1+/− mice on a liberal salt diet.
Fig. 4.
Phe- and KCl-induced contraction in aortic rings of WT and LSD1+/− mice on LS and HS diets. Aortic rings from each genotype and treatment group were stimulated with increasing concentrations of Phe, the contractile response was measured and presented in grams (A) or as %maximum Phe contraction in mice on LS (B) and HS diet (C). In other experiments, aortic rings were stimulated with high 96 mM KCl depolarizing solution and the contractile response was measured and presented in grams (D). Data represent means ± SE (n = 6 to 8).*Measurements in LSD1+/− mice on HS intake are significantly different (P < 0.05) from corresponding measurements in WT mice on HS intake. §Measurements in LSD1+/− mice on HS intake diet are significantly different (P < 0.05) from corresponding measurements in LSD1+/− mice on LS diet.
In aortic segments of WT and LSD1+/− mice on a liberal salt diet, pretreatment with the NOS inhibitor l-NAME did not cause any significant enhancement of Phe contraction (Fig. 5). Also, in aortic segments of WT mice, pretreatment with the guanylate cyclase inhibitor ODQ did not significantly alter Phe contraction (Fig. 5A). In contrast, pretreatment of aorta of LSD1+/− mice with ODQ was associated with dramatic and significant enhancement of Phe contraction (Fig. 5B). Phe-induced contraction was slightly but not significantly enhanced in endothelium-denuded (max 0.20 ± 0.11g) compared with intact vessels of WT mice on a liberal salt diet (max 0.11 ± 0.04g) and was not significantly different in endothelium-denuded (max 0.40 ± 0.08) compared with intact vessels of LSD1+/− mice on a liberal salt diet (max 0.40 ± 0.05).
Fig. 5.
Effect of nitric oxide synthase (NOS) and guanylate cyclase blockade on Phe-induced contraction in aortic rings of WT and LSD1+/− mice on HS intake. Aortic rings of WT (A) and LSD1+/− mice (B) were either nontreated (circles) or pretreated with the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 3 × 10−4 M; ▴) or the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10−5M) for 10 min (■). Tissues were stimulated with increasing concentrations of Phe, and the contractile response was measured and presented in grams. Data represent means ± SE (n = 4 to 8). *Measurements in ODQ-treated aortic segments are significantly different (P < 0.05) from corresponding measurements in nontreated segments.
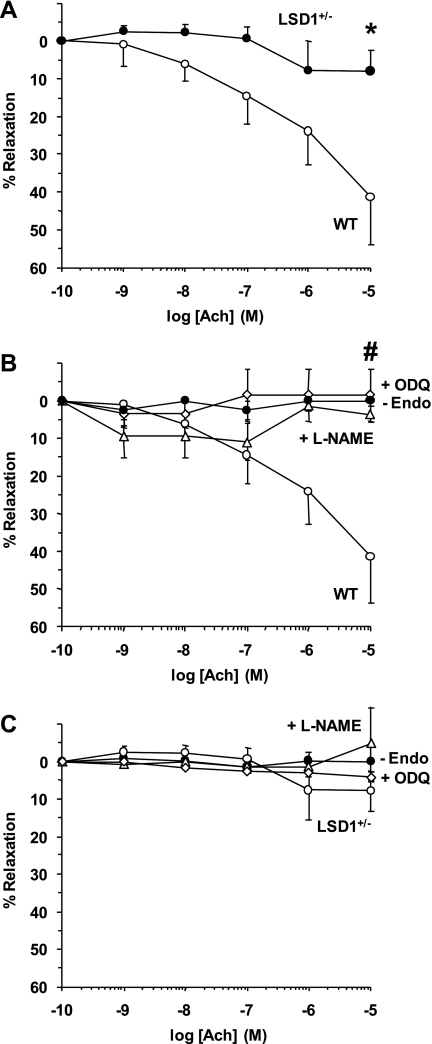
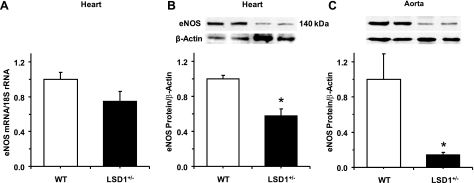
In aortic segments of WT mice precontracted with Phe (10−5 M), Ach caused concentration-dependent relaxation that reached 41.4 ± 12.4% at 10−5 M (Fig. 6). In contrast, Ach caused minimal relaxation in aortic segments of LSD1+/− mice (Fig. 6A). Pretreatment of aortic segments of WT mice with l-NAME or ODQ abolished Ach-induced relaxation (Fig. 6B). In contrast, in aortic segments of LSD1+/− mice, pretreatment with l-NAME or ODQ did not significantly alter the Ach response (Fig. 6C). Also, endothelium removal abolished Ach-induced relaxation in aorta of WT mice but did not significantly alter Ach relaxation in the aorta of LSD1+/− mice (Fig. 6). Consistent with the reduced vascular relaxation results, RT-PCR analysis revealed that cardiac eNOS mRNA expression was reduced in LSD1+/− compared with WT mice (Fig. 7A). Also, Western blot analysis revealed that eNOS protein amount was reduced in the heart and aorta of LSD-1+/− compared with WT mice (Fig. 7, B and C).
Fig. 6.
Ach-induced relaxation in aortic rings of WT and LSD1+/− mice on HS intake. Aortic rings of WT and LSD1+/− mice were precontracted with Phe (10−5 M), increasing concentrations of Ach were added and the %relaxation of Phe contraction was measured (A). In other experiments, Ach-induced relaxation of aortic segments of WT (B) and LSD1+/− mice (C) was compared in endothelium-intact aortic segments (○), endothelium-denuded segments (●), and aortic segments pretreated with l-NAME (3 × 10−4M; ▵) or ODQ (10−5M; ◊). Data represent means ± SE (n = 4 to 8). *Measurements in LSD1+/− mice are significantly different (P < 0.05) from corresponding measurements in WT mice. #Measurements in endothelium-denuded and l-NAME- or ODQ-treated aortic segments are significantly different (P < 0.05) from corresponding measurements in endothelium-intact nontreated segments.
Fig. 7.
RT-PCR of endothelial (e)NOS mRNA expression in the heart (A) and Western blot analysis of eNOS protein amount in the heart (B) and aorta (C) of WT and LSD1+/− mice on HS intake. Data represent means ± SE (n = 4). Two representative gels for eNOS and actin immunoreactive bands are presented for each genotype. *Measurements in LSD1+/− mice are significantly different (P < 0.05) from corresponding measurements in WT mice.
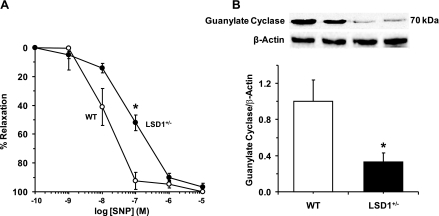
In aortic segments of WT mice precontracted with Phe (10−5 M), the exogenous NO donor and hence guanylate cyclase activator SNP caused concentration-dependent relaxation that reached a maximum at 10−5 M. In aortic segments of LSD1+/− mice, the SNP-induced concentration-relaxation curve demonstrated a rightward shift, and at 10−7 M concentration, SNP-induced relaxation was significantly reduced in LSD1+/− compared with WT mice on a liberal salt diet (Fig. 8A). SNP-induced relaxation was significantly blocked by ODQ, a known inhibitor of guanyalte cyclase activity. In WT mice, SNP (10−7 M)-induced relaxation was significantly reduced in ODQ-treated (7.9 ± 4.7%) compared with nontreated vessels (92.5 ± 5.90%). Also, in LSD1+/− mice, SNP (10−7 M)-induced relaxation was significantly reduced in ODQ-treated (19.2 ± 8.2%) compared with nontreated vessels (52.0 ± 5.4%). The significant blockade of SNP-induced vascular relaxation by ODQ supports the contention that it is largely due to increased guanylate cyclase activity. Also, Western blot analysis revealed a significant decrease in guanylate cyclase protein levels in the aorta of LSD1+/− compared with WT mice (Fig. 8B).
Fig. 8.
A: sodium nitroprusside (SNP)-induced relaxation in aortic rings of WT and LSD1+/− mice on HS intake. Aortic rings of WT and LSD1+/− mice were precontracted with Phe (10−5 M), increasing concentrations of SNP were added, and the %relaxation of Phe contraction was measured. Data represent means ± SE (n = 6 to 8). B: Western blot analysis of guanylate cyclase protein levels in aortic tissues from WT and LSD1+/− mice on HS intake. Data represent means ± SE (n = 4). Two representative gels for guanylate cyclase and actin immunoreactive bands are presented for each genotype. *Measurements in LSD1+/− mice are significantly different (P < 0.05) from corresponding measurements in WT mice.
DISCUSSION
The present study demonstrates that in mice during liberal salt intake, LSD1 deficiency is associated with the following: 1) increased BP but suppressed RAAS activity; 2) enhanced vascular contraction and reduced endothelium-dependent relaxation; 3) reduced expression of cardiac and vascular eNOS; and 4) reduced vascular responsiveness to exogenous NO donors and activators of the cGMP-dependent relaxation pathway along with reduced guanylate cyclase protein levels. Importantly, the increased BP and vascular reactivity in LSD1-deficient mice are not seen when dietary salt intake is restricted, suggesting that salt intake has an epigenetic effect. Interestingly, the restricted salt intake is more likely to be at the level ingested by mice in their native environment.
BP was higher in LSD1+/− mice compared with WT mice on a liberal salt diet. RAAS plays an important role in the regulation of BP during changes in dietary salt intake. Angiotensin II stimulates the release of aldosterone from the adrenal cortex, which in turn activates mineralocorticoid receptor in the collecting ducts and increases sodium reabsorption and plasma volume (1, 8, 9, 12, 16, 22). Although a liberal salt intake may be temporarily associated with increased vascular volume (4), the volume overload results in inhibition of the RAAS, leading to increased salt and water excretion and restoration of vascular volume towards normal (8, 9, 22). Because of the prominent role of the RAAS in the regulation of BP, it was reasonable to hypothesize that the increased BP in LSD1+/− mice on a liberal salt diet may be due to activation of the RAAS. However, this is an unlikely primary mechanism since plasma renin activity and the plasma levels and urinary excretion of aldosterone were reduced in LSD1+/− compared with WT mice.
In addition to the hormonal mechanisms, BP can be regulated by cardiovascular mechanisms (3, 32). We (20, 27, 34, 42) have previously shown that a liberal salt diet alone in mice or rats is not associated with significant cardiac tissue damage. Also, in rats, a liberal salt diet alone is not associated with a significant increase in BP or vascular reactivity (7, 39). Consistent with our previous reports (7, 20, 27), we found that the BP and vascular reactivity were not significantly different between WT mice on restricted or liberal salt diets. This is likely because the vasoconstrictive effects of a liberal salt diet are normally counterbalanced by compensatory increases in NO production and vascular relaxation (7, 39). This is supported by reports that, in rats chronically treated with l-NAME or endothelin B receptor antagonist, either of which could inhibit NO production, a liberal salt diet is associated with increased BP and vasoconstriction (7). The observed lack of difference in BP in WT mice on liberal vs. restricted salt diets is consistent with our previous reports in Sprague-Dawley rats and WT mice on liberal sodium intakes (7, 39). On the other hand, the BP sensitivity to liberal salt intake in LSD1+/− mice suggests a potential interplay between LSD1 and the genes regulating the vascular control mechanisms of BP. The enhanced Phe contraction in the aorta of LSD1+/− compared with WT mice is consistent with altered vascular control mechanisms of BP in LSD1+/− mice on liberal dietary salt intake.
The enhanced Phe-induced aortic contraction in LSD1+/− mice on a liberal salt intake may not be due to enhanced sensitivity of the α-adrenergic receptors because the Phe ED50 was not different in the aorta of WT and LSD1+/− mice. Also, KCl-induced aortic contraction, a receptor-independent response involving Ca2+ entry from the extracellular space (15, 26), was greater in LSD1+/− than WT mice on a liberal salt diet. These results suggest that the enhanced vascular contraction in LSD1+/− mice could be due to activation of a common postreceptor contraction mechanism and/or inhibition of a common vascular relaxation mechanism.
Basal NO release from endothelial cells diffuses into vascular smooth muscle (VSM) where it activates guanylate cyclase, increases cGMP production, and inhibits VSM contraction (5, 13, 25). The lack of effect of l-NAME on Phe contraction in WT and LSD1+/− mice suggests that basal NOS activity and NO release may not differ between WT and LSD1+/− mice. Also, the lack of effect of the guanylate cyclase inhibitor ODQ on Phe contraction in the aorta of WT mice suggests little role of basal guanylate cyclase activity and cGMP production in the control of vascular tone in WT mice. In contrast, the dramatic enhancement of Phe by the guanylate cyclase inhibitor ODQ in aortic segments of LSD1+/− mice on liberal salt intake suggests enhanced basal guanylate cyclase activity and cGMP production.
Ach is known to stimulate endothelial eNOS activity and in turn activates the NO-cGMP pathway (5, 13). Stimulated eNOS activity appears to be reduced in LSD1+/− mice on liberal dietary salt intake because 1) Ach-induced relaxation was reduced in LSD1+/− compared with WT mice; 2) Ach-induced relaxation was blocked by endothelium removal or NOS or guanylate cyclase inhibitors in WT mice but was minimally affected in LSD1+/− mice; and 3) eNOS mRNA expression and protein amount were reduced in the heart and aorta of LSD1+/− compared with WT mice.
To further test the responsiveness of VSM to the NO-cGMP pathway, we measured aortic relaxation to the exogenous NO donor SNP. NO is known to activate guanylate cyclase and increase cGMP, which in turn activates protein kinase G and causes VSM relaxation (5, 13, 25). cGMP is also inactivated by endogenous phosphodiesterase-5 to GMP. The reduced responsiveness to SNP in LSD1+/− mice could involve one or more of several mechanisms including the following: 1) decreased vascular guanylate cyclase levels; 2) increased basal guanylate cyclase activity and consequently decreased responsiveness to further activation by exogenous NO donors; 3) increased phosphodiesterase-5 levels/activity; 4) decreased protein kinase G amount/activity; and 5) changes in vessel plasticity. Our observed reduction in guanylate cyclase levels, and exaggerated contractile response during guanylate cyclase inhibition in the aorta of LSD1+/− mice, are consistent with the first two possible mechanisms. Our findings are also in agreement with previous transcriptional target analysis studies that have identified LSD1 complexes that could potentially regulate the soluble guanylate cyclase signaling pathway (44). However, the present results do not exclude any of the other possible mechanisms outlined above. Future studies should further assess the relative contribution of the various components of the NO-cGMP-PKG pathway and the structural changes in the vessel architecture to the reduced vascular relaxation in the LSD1+/− mice.
The level of salt intake has an epigenetic effect on the vasculature, i.e., salt intake, an environmental factor, modifies vascular function. We (45) have previously documented that salt intake also modifies the level of LSD1 in WT mice. The present results suggest that LSD1 could be the molecular mediator of this epigenetic effect via modification of the expression of the genes regulating the NO-cGMP pathway. As βGeo cassette was inserted after exon 8 in LSD1 locus (total 20 exons), it is possible that it would generate a partial nonfunctional protein that could act as a dominant negative rather than true null but this may not alter our conclusions. In the case of a dominant negative nonfunctional LSD1 protein, the phenotype observed would be due to inhibition of LSD1 function, rather than to decreased LSD1 levels. LSD1 deficiency was associated with reduced eNOS expression, which could lead to decreased vascular relaxation and increased contraction. However, the LSD1-mediated mechanisms that lead to altered eNOS levels are unclear. An in silico search did not reveal any evidence for methylation changes at the eNOS gene locus. However, it is not known whether eNOS is a direct LSD1 target or the LSD1 effects are indirect. It is known that LSD1 can repress or activate gene transcription depending on whether it demethylates the H3K4 site (resulting in transcription repression) or the H3K9 site (resulting in transcription activation) (6, 18, 23, 37, 38, 43). In the present study, we documented that LSD1+/− mice, with decreased LSD1 levels, have decreased vascular eNOS mRNA levels and reduced eNOS and guanylate cyclase protein levels. We speculate that to the extent that LSD1 directly modifies the expression of these genes regulating the vascular NO-cGMP pathway, it likely does so by altering the methylation state at the H3K9 site rather than the H3K4 site.
An in silico search reveals that ∼45% of genes are potentially affected by LSD1. This is supported by the finding that complete removal of LSD1 in homozygous LSD1 knockout is associated with embryonic lethality. To test for other unrelated/distant pathway, we assessed inflammatory markers levels in these animals and found no significant change in the LSD1+/− vs. LSD1+/+ mice. Nevertheless, it is not unlikely that LSD1+/− mice would show some changes in a host of other signaling and contraction/relaxation mechanisms, and these certainly represent important experiments for further characterization of the LSD1-deficient phenotype in future studies. However, any such changes would not explain the increased BP that was observed only in the LSD1-deficient mice on a liberal salt diet, which is the focus of the present study.
The present study demonstrated an association between LSD1 deficiency and a vascular phenotype characterized by increased vascular contraction and decreased vascular relaxation and hypertension. Hypertension is a multifactorial disorder, and although the RAAS was not stimulated in this phenotype, other neuronal or hormonal factors may still be altered. Also, as in other hypertensive phenotypes, it is often difficult to assess a cause-and-effect relationship between the vascular changes and hypertension. Future studies could address the potential role of hypertension vs. LSD1 deficiency as the principle cause of the observed vascular effects by either studying prehypertensive animals (if such exist) and/or repeating the vascular studies in animals that have normalized BP for a month with a pharmacologic intervention.
Thus LSD1 deficiency in mice is associated with increased BP when salt intake is liberal but not when restricted, as well as suppressed RAAS during liberal salt intake. Our findings in mice are consistent with our previously observed salt-sensitive hypertension and suppressed RAAS in humans with LSD1 gene variants (45). In humans, the LSD1 variant affects renovascular constriction, a finding consistent with the increased vascular reactivity in LSD1 deficient mice on a liberal salt diet. Whether overexpression of polymorphic LSD1 variant is associated with changes in vascular reactivity is an interesting area for future investigation. The present data in rodents also suggest a role for LSD1 in the regulation of vasoreactivity and the NO-cGMP pathway when salt intake is liberal. Further studies are needed to elucidate whether the mechanisms responsible for the previously observed salt-sensitive phenotype in humans also relate to vascular dysfunction and whether alterations in the NO/cGMP pathway mediate this effect. Also, given the increased contraction to KCl (and Phe) in the LSD1+/− mice on a liberal salt diet, it would seem important to investigate contraction to activators of l-type calcium channels such as BayK 8644 and to examine the morphometry of the vessels for potential hypertrophic changes, and these represent important experiments for future investigation.
GRANTS
This work was supported by National Institutes of Health Grants HL-104032 (L. H. Pojoga), K23-HL-084236 (to J. S. Williams), HL-69208 (to G. H. Williams), GM-078458 (to Y. Shi) and HL-65998 and HL-98724 (to R. A. Khalil), The Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant HD-60702 (to R. A. Khalil), and American Heart Association Grant 0735609T (to L. H. Pojoga). L. H. Pojoga was the recipient of a New Investigator Award of the 2010 Council for High Blood Pressure Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the helpful technical assistance of Paul Loutraris.
REFERENCES
- 1. Adler GK, Williams GH. Aldosterone: villain or protector? Hypertension 50: 31–32, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res 100: 520–526, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cain AE, Khalil RA. Pathophysiology of essential hypertension: role of the pump, the vessel, and the kidney. Semin Nephrol 22: 3–16, 2002 [PubMed] [Google Scholar]
- 4. de Wardener HE, He FJ, MacGregor GA. Plasma sodium and hypertension. Kidney Int 66: 2454–2466, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Fleming I, Busse R. NO: the primary EDRF. J Mol Cell Cardiol 31: 5–14, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Garcia-Bassets I, Kwon YS, Telese F, Prefontaine GG, Hutt KR, Cheng CS, Ju BG, Ohgi KA, Wang J, Escoubet-Lozach L, Rose DW, Glass CK, Fu XD, Rosenfeld MG. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell 128: 505–518, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giardina JB, Green GM, Rinewalt AN, Granger JP, Khalil RA. Role of endothelin B receptors in enhancing endothelium-dependent nitric oxide-mediated vascular relaxation during high salt diet. Hypertension 37: 516–523, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Guyton AC. Blood pressure control–special role of the kidneys and body fluids. Science 252: 1813–1816, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Hall JE. Control of sodium excretion by angiotensin II: intrarenal mechanisms and blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 250: R960–R972, 1986 [DOI] [PubMed] [Google Scholar]
- 10. Hiltunen MO, Yla-Herttuala S. DNA methylation, smooth muscle cells, and atherogenesis. Arterioscler Thromb Vasc Biol 23: 1750–1753, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Holtzman EJ, Braley LM, Williams GH, Hollenberg NK. Kinetics of sodium homeostasis in rats: rapid excretion and equilibration rates. Am J Physiol Regul Integr Comp Physiol 254: R1001–R1006, 1988 [DOI] [PubMed] [Google Scholar]
- 12. Hurwitz S, Fisher ND, Ferri C, Hopkins PN, Williams GH, Hollenberg NK. Controlled analysis of blood pressure sensitivity to sodium intake: interactions with hypertension type. J Hypertens 21: 951–959, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol 53: 503–514, 2002 [PubMed] [Google Scholar]
- 14. Kataja J, Viinamaki O, Punnonen R, Kaukinen S. Renin-angiotensin-aldosterone system and plasma vasopressin in surgical patients anaesthetized with halothane or isoflurane. Eur J Anaesthesiol 5: 121–129, 1988 [PubMed] [Google Scholar]
- 15. Khalil RA, van Breemen C. Intracellular free calcium concentration/force relationship in rabbit inferior vena cava activated by norepinephrine and high K+. Pflügers Arch 416: 727–734, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Kosachunhanun N, Hunt SC, Hopkins PN, Williams RR, Jeunemaitre X, Corvol P, Ferri C, Mortensen RM, Hollenberg NK, Williams GH. Genetic determinants of nonmodulating hypertension. Hypertension 42: 901–908, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Lan F, Shi Y. Epigenetic regulation: methylation of histone and non-histone proteins. Sci China C Life Sci 52: 311–322, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Lee MG, Wynder C, Cooch N, Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature 437: 432–435, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, Williams GH, Adler GK. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension 39: 614–618, 2002 [PubMed] [Google Scholar]
- 21. Matouk CC, Marsden PA. Epigenetic regulation of vascular endothelial gene expression. Circ Res 102: 873–887, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AH, Gunther T, Buettner R, Schule R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437: 436–439, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem 79: 155–179 [DOI] [PubMed] [Google Scholar]
- 25. Murad Shattuck Lecture F. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med 355: 2003–2011, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca2+]i in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol 278: C834–C844, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Oestreicher EM, Martinez-Vasquez D, Stone JR, Jonasson L, Roubsanthisuk W, Mukasa K, Adler GK. Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-l-arginine methyl ester-induced myocardial injury. Circulation 108: 2517–2523, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Oliverio MI, Best CF, Smithies O, Coffman TM. Regulation of sodium balance and blood pressure by the AT(1A) receptor for angiotensin II. Hypertension 35: 550–554, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Pojoga LH, Adamova Z, Kumar A, Stennett AK, Romero JR, Adler GK, Williams GH, Khalil RA. Sensitivity of NOS-dependent vascular relaxation pathway to mineralocorticoid receptor blockade in caveolin-1-deficient mice. Am J Physiol Heart Circ Physiol 298: H1776–H1788, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pojoga LH, Romero JR, Yao TM, Loutraris P, Ricchiuti V, Coutinho P, Guo C, Lapointe N, Stone JR, Adler GK, Williams GH. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-l-arginine methyl ester and angiotensin II. Endocrinology 151: 1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pojoga LH, Yao TM, Sinha S, Ross RL, Lin JC, Raffetto JD, Adler GK, Williams GH, Khalil RA. Effect of dietary sodium on vasoconstriction and eNOS-mediated vascular relaxation in caveolin-1-deficient mice. Am J Physiol Heart Circ Physiol 294: H1258–H1265, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ponnuchamy B, Khalil RA. Cellular mediators of renal vascular dysfunction in hypertension. Am J Physiol Regul Integr Comp Physiol 296: R1001–R1018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pons D, de Vries FR, van den Elsen PJ, Heijmans BT, Quax PH, Jukema JW. Epigenetic histone acetylation modifiers in vascular remodelling: new targets for therapy in cardiovascular disease. Eur Heart J 30: 266–277, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology 141: 3871–3878, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Shi X, Hong T, Walter KL, Ewalt M, Michishita E, Hung T, Carney D, Pena P, Lan F, Kaadige MR, Lacoste N, Cayrou C, Davrazou F, Saha A, Cairns BR, Ayer DE, Kutateladze TG, Shi Y, Cote J, Chua KF, Gozani O. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 442: 96–99, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet 8: 829–833, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119: 941–953, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Shi YJ, Matson C, Lan F, Iwase S, Baba T, Shi Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell 19: 857–864, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Smith L, Payne JA, Sedeek MH, Granger JP, Khalil RA. Endothelin-induced increases in Ca2+ entry mechanisms of vascular contraction are enhanced during high-salt diet. Hypertension 41: 787–793, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Staessen JA, Wang J, Bianchi G, Birkenhager WH. Essential hypertension. Lancet 361: 1629–1641, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Talbert PB, Henikoff S. Histone variants–ancient wrap artists of the epigenome. Nat Rev Mol Cell Biol 11: 264–275 [DOI] [PubMed] [Google Scholar]
- 42. Turchin A, Guo CZ, Adler GK, Ricchiuti V, Kohane IS, Williams GH. Effect of acute aldosterone administration on gene expression profile in the heart. Endocrinology 147: 3183–3189, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Wang J, Scully K, Zhu X, Cai L, Zhang J, Prefontaine GG, Krones A, Ohgi KA, Zhu P, Garcia-Bassets I, Liu F, Taylor H, Lozach J, Jayes FL, Korach KS, Glass CK, Fu XD, Rosenfeld MG. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature 446: 882–887, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W, Liang J, Sun L, Yang X, Shi L, Li R, Li Y, Zhang Y, Li Q, Yi X, Shang Y. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell 138: 660–672, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Williams JSY, Adler GK, Khalil RA, Romero JR, Sinha S, Ponnuchamy B, Williams GH. Genetic alteration of a histone demethylase is associated with altered aldosterone and vascular responsiveness: an intermediate phenotype of human hypertension. Hypertension 52: E38, 2007 [Google Scholar]
- 46. Wissmann M, Yin N, Muller JM, Greschik H, Fodor BD, Jenuwein T, Vogler C, Schneider R, Gunther T, Buettner R, Metzger E, Schule R. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol 9: 347–353, 2007 [DOI] [PubMed] [Google Scholar]