Abstract
The Bcl2/adenovirus E1B 19-kDa interacting protein 3 (Bnip3) is an atypical BH3-only protein that is associated with mitochondrial dysfunction and cell death. Bnip3 is also a potent inducer of mitochondrial autophagy, and in this study we have investigated the mechanisms by which Bnip3 induces autophagy in cardiac myocytes. We found that Bnip3 induced mitochondrial translocation of dynamin-related protein 1 (Drp1), a protein involved in mitochondrial fission in adult myocytes. Drp1-mediated mitochondrial fission correlated with increased autophagy, and inhibition of Drp1 reduced Bnip3-mediated autophagy. Overexpression of Drp1K38E, a dominant negative of Drp1, or mitofusin 1 prevented mitochondrial fission and autophagy by Bnip3. Also, inhibition of mitochondrial fission or autophagy resulted in increased death of myocytes overexpressing Bnip3. Moreover, Bnip3 promoted translocation of the E3 ubiquitin ligase Parkin to mitochondria, which was prevented in the presence of a Drp1 inhibitor. Interestingly, induction of autophagy by Bnip3 was reduced in Parkin-deficient myocytes. Thus our data suggest that induction of autophagy in response to Bnip3 is a protective response activated by the cell that involves Drp1-mediated mitochondrial fission and recruitment of Parkin.
Keywords: mitochondria, dynamin-related protein 1, mitofusin 1, Bcl-2/adenovirus E1B 19-kDa interacting protein 3
bcl-2 family proteins are important regulators of the mitochondria-mediated cell death pathway (11). More recently, they have been implicated as regulators of autophagy (22, 27). Autophagy is a process involved in the degradation of long-lived proteins and organelles and plays an important role in cellular homeostasis and survival (21). In the heart, autophagy is essential in clearing dysfunctional mitochondria and a disruption in this process leads to rapid accumulation of dysfunctional mitochondria and decline in cardiac function (23). The Bcl-2/adenovirus E1B 19-kDa interacting protein 3 (Bnip3) is an atypical BH3-only protein that has been reported to induce cell death in cardiac myocytes by perturbing mitochondrial function (19, 29, 35). Bnip3 is expressed in the adult myocardium under normal conditions and is upregulated in failing hearts (12, 29). Bnip3 is also a potent inducer of autophagy in cells (12, 28). However, the mechanism by which Bnip3 mediates autophagy and its functional role in cardiac myocytes are unclear. Bnip3-mediated autophagy has been reported to both protect against and contribute to Bnip3-mediated cell death (1, 3, 12).
Mitochondria are dynamic organelles that are constantly undergoing fission and fusion to adapt to changes in the cellular environment. These processes are regulated by several different GTPases. Mitofusin 1 and 2 (Mfn1 and Mfn2) and optic atrophy 1 (Opa1) coordinate fusion of the outer and inner mitochondrial membranes, respectively (5, 6). Dynamin-related protein 1 (Drp1) is a cytoplasmic protein that translocates to mitochondria where it interacts with fission protein 1 (Fis 1) to promote fission (32, 37). The functional importance of mitochondrial dynamics in the myocardium is just beginning to be explored, and a few recent studies have demonstrated its importance for normal heart function. For instance, knockdown of the Drosophila fusion proteins MARF or Opa1 led to development of cardiomyopathy in flies (8). In addition, hearts in Drp1−/− embryos appeared structurally normal but myocytes isolated from Drp1−/− embryos had reduced contractility (36). Drp1 has also been implicated in cell death, and it was recently reported that inhibition of Drp1 reduced myocardial ischemia-reperfusion injury (26). Thus mitochondrial fission and fusion are clearly important processes involved in regulating both cell death and survival in the myocardium. We (12, 19) previously found that Bnip3 induces fragmentation of the mitochondrial network, but it is unclear whether it is part of the cell death process or an adaptation in response to Bnip3 activation.
In this study, we have investigated the mechanism by which Bnip3 promotes autophagy. We report that induction of mitochondrial autophagy in response to Bnip3 is a protective process and involves Drp1-mediated mitochondrial fission and recruitment Parkin to the mitochondria.
MATERIALS AND METHODS
Cardiac myocyte isolation and cell culture.
All animal protocols were in accordance with institutional guidelines and approved by the Institutional Animal Care and Use Committee of the University of California, San Diego. Adult cardiac myocytes were isolated from 250- to 300-g male Sprague-Dawley rats or 8- to 10-wk-old male C57BL/J mice as previously described (4, 18). Park2 knockout mice were obtained from Jackson Laboratories and have been described previously (9). Atrial derived HL-1 mouse myocytes (7) were cultured on gelatin-fibronectin-coated cell culture dishes in Claycomb medium supplemented with 10% FBS, 0.1 mM norepinephrine, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg /ml streptomycin.
Adenoviral infections and transient transfections.
Myocytes were incubated with the indicated adenovirus for 3 h in M199 plus 2% heat-inactivated FBS. After infection, the cells were rinsed and then incubated in regular cell culture media. For silencing experiments, myocytes were incubated with 5 μM small interfering (si)RNA (Dharmacon) specific for Drp1 (no .E-088074-00) or nontargeting control (no. D-001910-10-05). After 48 h, the cells were rinsed and immediately incubated with Ad-β-gal or Ad-Bnip3. HL-1 cells were transfected using the FuGENE6 transfection reagent (Roche Applied Science) for 24 h, according to the manufacturer's instructions. For experiments aimed at determining mitochondrial fragmentation, HL-1 cells were transfected with empty vector, or pcDNA-Bnip3 (29), plus Myc-Mfn1 (5) or YFP-Drp1K38E (13), and GFP-LC3 (15) or Mito-DsRed2. For experiments assessing autophagy, HL-1 cells were transfected with empty vector, or pcDNA-Bnip3 plus Myc-Mfn1 or YFP-Drp1K38E, and mcherry-LC3 (12) or Mito-DsRed2.
Isolation of mitochondria.
Cardiac myocytes infected with Ad-β-gal or Ad-Bnip3 for 24 h were harvested in isolation buffer containing 250 mM sucrose, 5 mM KH2PO4, 2 mM MgCl2, 10 mM MOPS, 1 mM EGTA, 0.1% fatty acid free BSA, and protease inhibitors (Roche Applied Science). Cells were homogenized with a glass tissue grinder using a motor-driven homogenizer and centrifuged at 600 g for 5 min. The pellet was discarded, and the supernatant was centrifuged at 7,000 g for 10 min. The resultant supernatant was centrifuged at 20,000 g for 30 min to obtain cytosol. The mitochondrial pellet was resuspended in isolation buffer, centrifuged at 7,000 g for 10 min, and finally resuspended in the isolation buffer without BSA.
Fluorescence microscopy analysis.
To label autophagosomes, myocytes were infected with Ad-β-gal or Ad-Bnip3 plus Ad-GFP-LC3, and HL-1 cells were transfected with the indicated plasmid constructs plus GFP-LC3 or mcherry-LC3. To assess mitochondrial fragmentation, HL-1 cells were transfected with the indicated plasmids plus Mito-DsRed2 to label mitochondria. Mitochondrial morphology in live HL-1 cells was examined at 24 h posttransfection at ×63 magnification, and cells containing >50% of sphere-shaped mitochondria were classified as positive for activation of mitochondrial fission. Cells were examined using a Carl Zeiss AxioObserver Z1 equipped with a motorized z-stage and ApoTome for optical sectioning. Autophagy was measured as previously described (28, 30). For mitochondrial autophagy experiments, myocytes infected with Ad-β-gal or Ad-Bnip3 with Ad-GFP-LC3 were fixed in 4% formaldehyde (Ted Pella) in PBS (pH7.4), permeabilized with 0.2% Triton X-100 in PBS, and then blocked in 5% goat serum. The cells were incubated with anti-TOM20 and then with goat anti-rabbit Alexa-594 secondary antibody. For Drp1 translocation experiments, adult myocytes infected with Ad-β-gal or Ad-Bnip3 were fixed, permeabilized, and then blocked in the same conditions as above. The cells were incubated with anti-Drp1 (1:100) and anti-TOM 20 (Santa Cruz Biotechnology; 1:100) and then with goat anti-mouse Alexa-488 and goat anti-rabbit Alexa594 secondary antibodies (Invitrogen; 1:200).
Transmission electron microscopy.
Adult myocytes were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, postfixed in 1% osmium tetroxide, and then treated with 0.5% tannic acid, 1% sodium sulfate, cleared in 2-hydroxypropyl methacrylate, and embedded in LX112 (Ladd Research, Williston, VT). Sections were mounted on copper slot grids coated with parlodion and stained with uranyl acetate and lead citrate for examination on a Philips CM100 electron microscope (FEI, Hillsbrough, OR).
Cell death assay.
Cell death was assessed by measuring increased plasma membrane permeability to YoPro-1 (Invitrogen) as previously described (12). After infection with indicated adenoviruses for 24 h, live cells were stained with YoPro-1 for 5 min at 37°C and then examined by fluorescence microscopy.
Western blotting.
Cells were lysed in ice-cold buffer containing 50 mM Tris·HCl pH 7.4, 150 mM NaCl, 12 mM Na-deoxycholate, and 1% Triton X-100 plus complete protease inhibitor cocktail (Roche Applied Science) and then cleared by centrifugation at 20,000 g for 20 min. Proteins were separated by SDS-PAGE under reduced conditions, transferred to nitrocellulose, and incubated with an antibody. Although Bnip3 forms a homodimer when overexpressed in cells, it is sensitive to reduction by DTT (18). Therefore, all Western blots of Bnip3 represent the monomer. Antibodies used in the study were as follows: Mfn1 (Santa Cruz Biotechnology; 1:200 dilution), Mfn2 (Sigma-Aldrich; 1:1,000), Opa1 (BD Transduction Laboratory; 1:1,000), Drp1 (BD Transduction Laboratory; 1:1,000), Parkin (Cell Signaling Technology; 1:1,000), Bnip3 (Sigma-Aldrich, 1:1,000), cyclooxygenase IV (MitoScience; 1:1,000), actin (Sigma-Aldrich; 1:1,000), and c-myc (Sigma-Aldrich; 1:1,000). Blots were quantified and analyzed using Quantity One software (Bio-Rad). All Western blot experiments were repeated at least three times.
Statistical analysis.
All values are expressed as means ± SE. Statistical analyses were performed using ANOVA followed by Tukey's post hoc test for multiple comparisons to identify statistical significance between groups. P < 0.05 was considered significant.
RESULTS
Bnip3 induces translocation of Drp-1 to mitochondria.
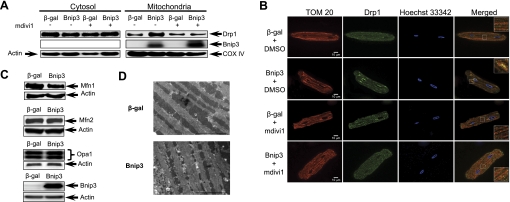
We and others (12, 19, 20) have previously reported that Bnip3 can cause changes in mitochondrial morphology in cell lines. To investigate the effect of Bnip3 on mitochondrial dynamics in cardiac myocytes, we examined changes in proteins involved in fission and fusion. We found that Drp1 was primarily localized to the cytosol in control cells and that Bnip3 promoted translocation of Drp1 to mitochondria in cardiac myocytes (Fig. 1, A and B). Treatment of cells with 40 μM mdivi1, a pharmacological inhibitor of Drp1 (33), prevented translocation of Drp1 to mitochondria in cells overexpressing Bnip3 (Fig. 1, A and B). We also investigated whether Bnip3 had an effect on proteins regulating mitochondrial fusion. Western blot analysis of proteins involved in mitochondrial fusion showed that Bnip3 caused a reduction in Mfn1 levels, but Mfn2 and Opa1 levels were unaltered (Fig. 1C). The effect of Bnip3 on mitochondrial morphology in cardiac myocytes was also verified by ultrastructural analysis. Electron micrographs confirm that mitochondria were smaller in myocytes overexpressing Bnip3 (Fig. 1D).
Fig. 1.
Bcl2/adenovirus E1B 19-kDa interacting protein 3 (Bnip3) promotes translocation of dynamin-related protein 1 (Drp1) to mitochondria in adult cardiac myocytes. Freshly isolated myocytes were infected with 50 multiplicity of infection (MOI) Ad-β-gal or Ad-Bnip3, and after 24 h, the cells were analyzed by Western blotting or fluorescence microscopy. A: Bnip3 induced translocation of Drp1 to mitochondria, which was prevented in the presence of 40 μmol/l mdivi1. Western blot represent 3 independent experiments. B: cells were fixed and stained with anti-TOM 20 (red) to label mitochondria, anti-Drp1 (green), and Hoechst 33342 (blue) to label nuclei. Representative images show cellular localization of Drp1 in the presence or absence of mdivi1. C: representative Western blots of mitochondrial fusion proteins mitofusin (Mfn)1, Mfn2, and optic atrophy 1 (Opa1) in cardiac myocytes infected with β-gal or Bnip3 for 24 h. D: ultrastructural analysis of mitochondria in adult rat cardiac myocytes overexpressing β-gal or Bnip3. COX IV, cytochrome c oxidase subunit IV. Scale bar = 5 μm.
Drp1 is required for induction of autophagy and mitophagy in adult myocytes by Bnip3.
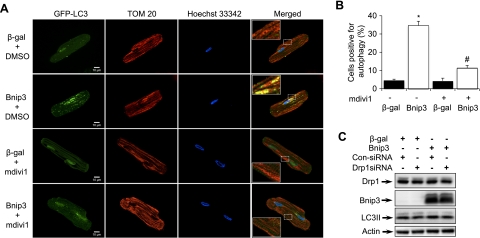
Previous studies from our laboratory and others (1, 3, 12, 30) have demonstrated that Bnip3 is a potent inducer of autophagy. To determine if Bnip3-mediated Drp1 activation was associated with induction of autophagy, myocytes were infected with Ad-β-gal or Ad-Bnip3 plus Ad-GFP-LC3 in the presence of vehicle or mdivi1. Fluorescence microscopy analysis showed that Bnip3 overexpression increased autophagy in myocytes, whereas inhibition of Drp1 with mdivi1 significantly reduced autophagy compared with β-gal (Fig. 2, A and B). Furthermore, Bnip3-mediated autophagy resulted in removal of mitochondria via autophagy as shown by colocalization between autophagosomes and mitochondria (Fig. 2A). Similarly, a decrease in Drp1 using siRNA resulted in reduced induction of autophagy in cells overexpressing Bnip3 as measured by a reduction in LC3II levels (Fig. 2C). This suggests that Drp1 plays an important role in Bnip3-induced autophagy.
Fig. 2.
Drp1 is required for induction of autophagy by Bnip3. Adult cardiac myocytes were infected with 50 MOI Ad-β-gal or Ad-Bnip3 plus GFP-LC3 for 24 h in the presence of DMSO or 40 μmol/l mdivi1. A: representative images of myocytes stained with anti-TOM 20 (red) to label mitochondria. Numerous autophagosomes (green) and mitochondria (red) colocalize (yellow) in myocytes overexpressing Bnip3. Although there were low levels of autophagy in the presence of mdivi1, there was no colocalization between autophagosomes and mitochondria. B: graph shows the percentage of cells positive for autophagosomes as analyzed by fluorescence microscopy (*P < 0.05 vs. β-gal; #P < 0.05 vs. Bnip3; n = 3). Data are means ± SE. C: downregulation of Drp1 using small interfering (si)RNA results in reduced induction of autophagy by Bnip3.
Mitochondrial fission is required for Bnip3-mediated autophagy.
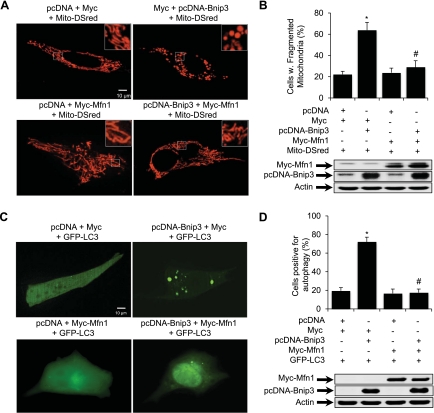
Mitochondria make up ∼35% of the cell volume in adult cardiac myocytes and are highly organized and compacted between contractile filaments. Due to the limitation in the spatial resolution, fluorescence microscopy is not always able to distinguish changes in mitochondrial morphology when they are packed too near each other. Therefore, we utilized HL-1 myocytes, whose mitochondria show dramatic morphologic changes in response to simulated myocardial ischemia-reperfusion (12, 26), to further explore if mitochondrial fission is required for induction of autophagy by Bnip3. To investigate whether restoring Mfn1 levels would prevent Bnip3-mediated autophagy, HL-1 myocytes were transfected with vector or Bnip3 with or without Mfn1. Analysis of mitochondrial morphology in live HL-1 cells showed that overexpression of Mfn1 promoted an increase in elongated fused mitochondria compared with control cells (Fig. 3A, bottom left). In contrast, Bnip3 alone disrupted the tubular network and the mitochondria formed sphere-like structures (Fig. 3A, top right). Disruption of the mitochondrial network by Bnip3 was prevented in cells overexpressing Mfn1 (Fig. 3A, bottom right). Quantitation showed that coexpression of Bnip3 and Mfn1 significantly reduced Bnip3-mediated mitochondrial fission (Fig. 3B). To investigate whether Mfn1 overexpression also inhibited Bnip3-induced autophagy, we examined HL-1 cells for the presence of autophagosomes. Analysis of cells for GFP-LC3-positive autophagosomes showed that Bnip3 alone increased autophagy (Fig. 3C, top right), whereas the coexpression of Mfn1 significantly prevented induction of autophagy (Fig. 3C, bottom right). Quantitation showed that enhanced levels of Mfn1 significantly reduced Bnip3-mediated autophagy (Fig. 3D).
Fig. 3.
Mfn1 overexpression prevents Bnip3-mediated mitochondrial fission and autophagy. HL-1 cells were transiently transfected with vector or Bnip3 with or without Mfn1 for 24 h. Mito-DsRed or GFP-LC3 were included to label mitochondria or autophagosomes, respectively. A: representative images of mitochondrial morphology in live cells. B: quantitation of mitochondrial fission (*P < 0.05 vs. vector; #P < 0.05 vs. Bnip3; n = 3). Expression of Bnip3 and Mfn1 in HL-1 cells was verified by Western blot analysis 24 h posttransfection. C: representative images of autophagy in live cells. D: quantitation of autophagy (*P < 0.05 vs. vector; #P < 0.05 vs. Bnip3; n = 3). Expression of Bnip3 and Mfn1 in HL-1 cells was verified by Western blot analysis 24 h posttransfection. Data are means ± SE.
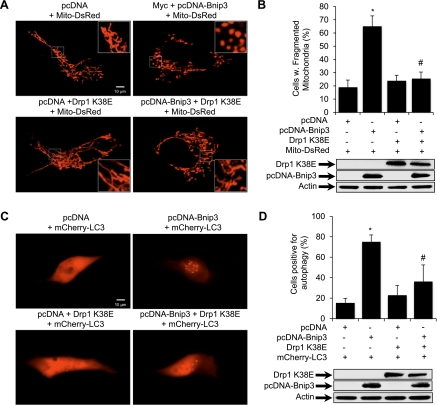
To further investigate if Drp1 is required to induce Bnip3-mediated mitochondrial fission and subsequent autophagy, HL-1 cells were transfected with Bnip3 and Drp1K38E, a dominant negative Drp1 mutant (13). Similar to Mfn1, overexpression of Drp1K38E resulted in the appearance of fused mitochondria (Fig. 4A, bottom left). Also, Bnip3 alone induced mitochondrial fission (Fig. 4A, top right), whereas overexpression of Drp1K38E inhibited Bnip3-mediated mitochondrial fission in HL-1 cells (Fig. 4A, bottom right). Quantitation showed that coexpression of Bnip3 and Drp1K38E significantly reduced Bnip3-mediated mitochondrial fission (Fig. 4B). We also analyzed the effect of Drp1K38E on autophagy and found that overexpression of Drp1K38E inhibited Bnip3-mediated autophagy (Fig. 4C, bottom right). Quantitation confirmed that the presence of Drp1K38E significantly reduced autophagy induced by Bnip3 overexpression (Fig. 4D). Drp1K38E alone had no effect on basal level of autophagy compared with vector control. Collectively, these findings suggest that mitochondrial fission is a prerequisite for Bnip3-mediated autophagy.
Fig. 4.
Drp1K38E prevents Bnip3-mediated mitochondrial fragmentation and autophagy. HL-1 cells were transiently transfected with vector or Bnip3 with or without YFP-Drp1K38E for 24 h. Mito-DsRed or mCherry-LC3 were included to label mitochondria or autophagosomes, respectively. A: representative images of mitochondrial morphology in live cells. B: quantitation of mitochondrial fission (*P < 0.05 vs. vector; #P < 0.05 vs. Bnip3; n = 3). Expression of Bnip3 and Drp1K38E in HL-1 cells was verified by Western blot analysis 24 h posttransfection. C: representative images of autophagy in live cells. D: quantitation of autophagy (*P < 0.05 vs. vector; #P < 0.05 vs. Bnip3; n = 3). Expression of Bnip3 and Drp1K38E in HL-1 cells was verified by Western blot analysis 24 h posttransfection. Data are means ± SE.
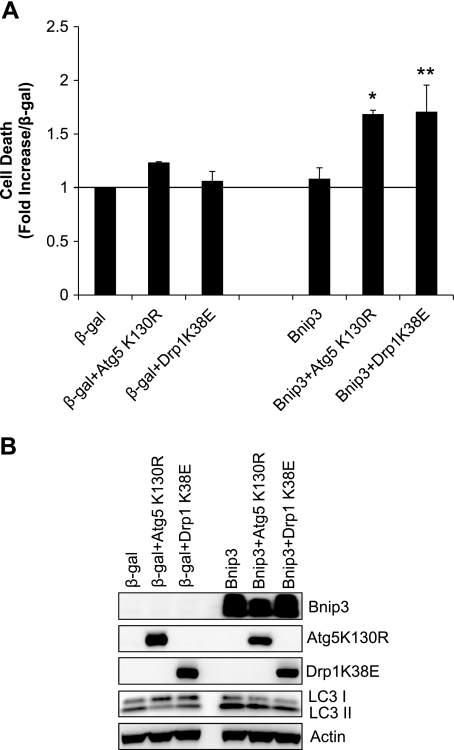
Inhibition of mitochondrial fission or autophagy results in cell death.
The functional role of autophagy is unclear and has been reported to both contribute to and protect against cell death. To investigate the functional significance of enhanced autophagy in adult myocytes overexpressing Bnip3, we examined whether inhibiting mitochondrial fission or autophagy had an effect on cell viability. Interestingly, we found that adult myocytes were remarkably resistant to Bnip3-mediated cell death in our experiments but that inhibiting mitochondrial fission with Drp1K38E or autophagy with Atg5K130R resulted in significant cell death (Fig. 5A). Western blot analysis for LC3II confirmed that overexpression Drp1K38E or Atg5K130R reduced Bnip3-induced autophagy (Fig. 5B). This suggests that mitochondrial fission and subsequent autophagy are important protective processes induced by the cell in response to elevated levels of Bnip3.
Fig. 5.
Inhibition of mitochondrial fission or autophagy promotes cell death. Adult cardiac myocytes were infected with β-gal or Bnip3 plus Drp1K38E or Atg5K130R for 24 h. A: cell death was assessed at 24 h by measuring YoPro-1 uptake (n = 3; * P < 0.05 and **P < 0.05 vs. Bnip3). B: assessment of autophagy by Western blot analysis for LC3.
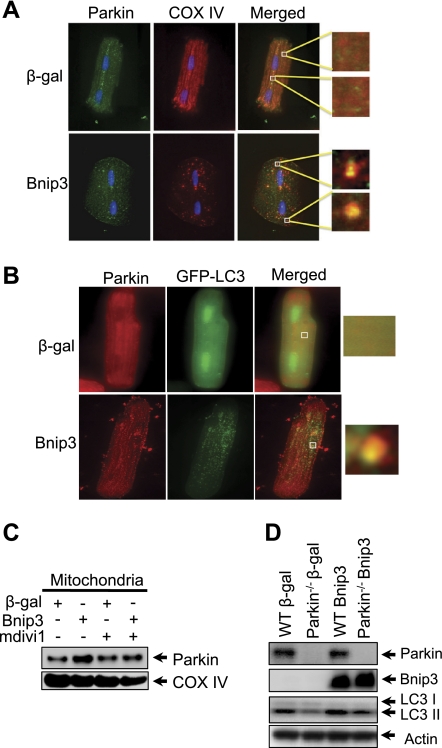
Bnip3-mediated autophagy involves translocation of Parkin to mitochondria.
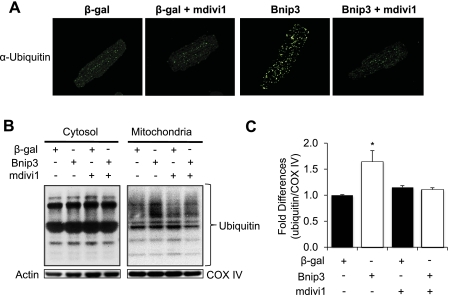
It was recently reported that mitochondria can be ubiquitinated by the E3 ligase Parkin, which promotes their removal by autophagosomes in HeLa cells (24). Thus we examined whether Bnip3-mediated mitochondrial autophagy involved Parkin in myocytes. We found that overexpression of Bnip3 promoted translocation of Parkin to mitochondria in myocytes (Fig. 6A). Parkin also colocalized with GFP-LC3-positive autophagosomes (Fig. 6B). The translocation of Parkin to mitochondria was confirmed by Western blotting (Fig. 6C). Interestingly, mitochondrial translocation of Parkin was inhibited in the presence of mdivi1. Moreover, to further examine the role of Parkin in Bnip3-mediated autophagy, we overexpressed Bnip3 in adult myocytes isolated from wild-type and Parkin−/− mouse hearts. We discovered that Parkin-deficient myocytes had reduced induction of autophagy in response to Bnip3 overexpression compared with WT myocytes (Fig. 6D), suggesting that Parkin plays an important role in the induction of autophagy. Since Parkin is an E3 ubiquitin ligase, we also examined whether translocation of Parkin correlated with increased ubiquitination of mitochondrial proteins. Immunofluorescence analysis showed that Bnip3 caused increased ubiquitination of proteins in myocytes (Fig. 7A). Moreover, Western analysis of cytosolic and mitochondrial fractions revealed that Bnip3 specifically increased ubiquitination of proteins in the mitochondria. Interestingly, the presence of the Drp1 inhibitor mdivi1 significantly reduced mitochondrial ubiquitination (Fig. 7, B and C).
Fig. 6.
Bnip3-mediated Parkin translocation to mitochondria is dependent on Drp1 activation. Myocytes were infected with Ad-β-gal or Ad-Bnip3 in the presence or absence of 40 μmol/l mdivi1 for 24 h. Representative images show that endogenous Parkin translocated to mitochondria in myocytes overexpressing Bnip3 (A) and that Parkin colocalized with GFP-LC3 positive autophagosomes (B). C: representative Western blot of mitochondrial fraction for Parkin. Bnip3-mediated translocation of Parkin was inhibited in the presence of the Drp1 inhibitor mdivi1. D: analysis of LC3 levels in adult wild-type (WT) and Parkin−/− mouse myocytes overexpressing β-gal or Bnip3 for 24 h.
Fig. 7.
Bnip3-mediated ubiquitination of mitochondrial proteins are dependent on Drp1 activation. A: representative images of myocytes stained with anti-ubiquitin (green). B: Bnip3 promoted an increase in ubiquitination of mitochondrial proteins that was abolished in the presence of mdivi1. C: quantitation of mitochondrial ubiquitination. (*P < 0.05 vs. β-gal; n = 3). Data are means ± SE.
DISCUSSION
Removal of mitochondria via autophagy is increasingly recognized as an important process in cells to maintain a healthy population of mitochondria. Reduced autophagy results in accumulation of dysfunctional mitochondria and has been linked to aging and development of heart failure (10). Recent studies (28, 30, 31) have demonstrated that mitochondrial autophagy is specifically activated in response to Bnip3 and its homologue Nix/Bnip3L and that this process is separate from activation of cell death. In this study, we discovered that induction of autophagy by Bnip3 requires Drp1-mediated mitochondrial fission and recruitment of Parkin to mitochondria. We also found that autophagy is a protective response activated by the cell in response to Bnip3.
Mitochondrial dynamics have been reported to play an important role in mitochondrial autophagy in other systems. For instance, inhibition of mitochondrial fission or induction of mitochondrial fusion inhibited nitric oxide-induced mitochondrial autophagy in primary neuronal cells (2). Similarly, autophagic degradation of mitochondria in yeast was reported to be dependent on fission mediated by the Drp1 homologue Dnm1 (25). Our findings demonstrate that Drp1-mediated mitochondrial fission is also required for mitochondrial autophagy by Bnip3 in adult cardiac myocytes. In addition, even modest reduction in Drp1 using siRNA caused a substantial reduction in Bnip3-induced autophagy, suggesting that Drp1 might be rate limiting in this pathway. It is unclear why mitochondrial fission is required before removal by autophagosomes. One possibility could be that mitochondria are too large to be engulfed by autophagosomes and fission will produce smaller fragments that can more easily be engulfed by the autophagosomes. Another possibility is that fission segregates dysfunctional mitochondria before removal by autophagy. For instance, Twig et al. (34) reported that mitochondrial fission produced two fragments with different ΔΨm. They also discovered that the mitochondrial fragment with low ΔΨm was more likely to be removed by autophagy, whereas the fragment with high ΔΨm had a higher probability of undergoing fusion. We (30) recently reported that Bnip3 can impair oxidative phosphorylation and ATP synthesis in cells in the absence of mitochondrial membrane permeabilization. The impairment in mitochondrial bioenergetics reduces the ΔΨm and may be the potential mechanism by which Bnip3 specifically targets mitochondria for degradation.
Parkin is an E3 ubiquitin ligase, and loss-of-function mutations in Parkin have been linked to Parkinson's disease (17). Recently, Parkin was identified to be an important regulator of mitochondrial autophagy. Narendra et al. (24) discovered that Parkin was rapidly recruited to dysfunctional mitochondria with low ΔΨm and that Parkin-mediated ubiquitination of mitochondria was required for removal by autophagosomes in neurons and HeLa cells. Parkin is highly expressed in many tissues, including the heart (17), but the functional role of Parkin in the myocardium is still unclear. Recently, one study reported that Parkin plays an important role in cardioprotection by ischemic preconditioning via induction of mitophagy (14). Our data demonstrate that Bnip3 overexpression induced translocation of Parkin to mitochondria, which correlated with increased ubiquitination of mitochondrial proteins in cardiac myocytes. We also found that Bnip3-mediated autophagy was reduced in Parkin null myocytes. Since Bnip3 specifically promotes mitochondrial autophagy, it suggests that Parkin plays an important role in this process. Numerous studies have found that Bnip3 causes a reduction in or loss of ΔΨm, which most likely promotes translocation of Parkin to those mitochondria. Although Parkin is an E3 ubiquitin ligase, it is possible that the increase in ubiquitinated mitochondrial proteins in our experiments is due to reduced removal of ubiquitinated proteins by the proteasome. This possibility needs to be explored. In addition, further investigation is needed to better understand how Parkin is involved in regulating mitochondrial autophagy in response to Bnip3.
Based on recent studies by us and others, evidence is emerging that elevated levels of Bnip3 lead to activation of both autophagy (1, 3, 12, 16, 28) and apoptosis (19, 29, 35) and that these are two completely separate processes. It is also becoming clear that autophagy is a protective process that is activated by the cell in response to Bnip3. We previously reported that Bnip3-mediated autophagy was independent of activation of the mitochondrial cell death pathway and that removal of mitochondria by autophagy was essential for cellular survival (12, 30). In addition, Bellot et al. (3) found that Bnip3-mediated autophagy during hypoxia was a survival mechanism that promoted tumor progression. Interestingly, we found that downregulation of Bnip3 using siRNA during hypoxia had little effect on general autophagy in cardiac myocytes (data not shown). Thus it is possible that there exists a redundancy in the cell and that other BH3-only proteins such as Bad (22) and Nix (3) can compensate for loss of Bnip3. Also, since Bnip3 specifically induces mitophagy in myocytes (28), it is possible that silencing of Bnip3 during hypoxia only reduces mitophagy without any effect on general autophagy. It is surprising that elevated levels of Bnip3 result in the activation of two opposing pathways in cells. However, it is quite possible that the cell is inducing autophagy as a repair mechanism to prevent accidental cell death. The ability to repair itself and prevent unnecessary death is especially important in a postmitotic cell such as a myocyte that cannot be easily replaced. Once the level of damage is too overwhelming and the cell is beyond rescue, apoptosis will become the dominant pathway and the cell will die.
In summary, adult cardiac myocytes induce mitochondrial autophagy in response to elevated levels of Bnip3 and we discovered that this process involves Drp1-mediated mitochondrial fission and recruitment of Parkin to mitochondria. Most importantly, the induction of autophagy is a protective response activated by the cell in response to Bnip3. Many studies have implicated autophagy as a target for cardioprotection and our study suggest that induction of autophagy represents a potential therapeutic target for prevention of Bnip3-mediated cell death in the heart. However, further studies are necessary to gain increased understanding into how mitochondria are targeted for removal by autophagy.
GRANTS
This work was supported by a Scientist Development Grant from the American Heart Association and by National Heart, Lung, and Blood Institute Grants R01-HL-087023 and R01-HL-101217.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, Gibson SB. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 4: 195–204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25: 3900–3911, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29: 2570–2581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carreira RS, Lee Y, Ghochani M, Gustafsson AB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy 6: 462–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101: 15927–15932, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95: 2979–2984, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dorn GW, Clark CF, II, Eschenbacher WH, Kang MY, Engelhard JT, Warner SJ, Matkovich SJ, Jowdy CC. MARF and Opa1 control mitochondrial and cardiac function in Drosophila. Circ Res 108: 12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem 278: 43628–43635, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Gottlieb RA, Gustafsson AB. Mitochondrial turnover in the heart. Biochim Biophys Acta 1813: 1295–1301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gustafsson ÅB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol 292: C45–C51, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ 14: 146–157, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol 14: 340–345, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by parkin and p62/SQSTM1. PLos One 6: e20975, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720–5728, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanzawa T, Zhang L, Xiao L, Germano IM, Kondo Y, Kondo S. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene 24: 980–991, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the Parkin gene cause autosomal recessive juvenile Parkinsonism. Nature 392: 605–608, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol 295: H2025–H2031, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kubli DA, Ycaza JE, Gustafsson AB. Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem J 405: 407–415, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Landes T, Emorine LJ, Courilleau D, Rojo M, Belenguer P, Arnaune-Pelloquin L. The BH3-only Bnip3 binds to the dynamin Opa1 to promote mitochondrial fragmentation and apoptosis by distinct mechanisms. EMBO Rep 11: 459–465, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 120: 159–162, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 26: 2527–2539, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13: 619–624, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183: 795–803, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nowikovsky K, Reipert S, Devenish RJ, Schweyen RJ. Mdm38 protein depletion causes loss of mitochondrial K+/H+ exchange activity, osmotic swelling and mitophagy. Cell Death Differ 14: 1647–1656, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122: 927–939, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Quinsay MN, Thomas RL, Lee Y, Gustafsson AB. Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophagy 6: 17–24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res 91: 226–231, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Rikka S, Quinsay MN, Thomas RL, Kubli DA, Zhang X, Murphy AN, Gustafsson AB. Bnip3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell Death Differ 18: 721–731, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, Ney PA. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci USA 104: 19500–19505, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanaka A, Youle RJ. A chemical inhibitor of DRP1 uncouples mitochondrial fission and apoptosis. Mol Cell 29: 409–410, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vande VC, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg AH. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol 20: 5454–5468, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol 186: 805–816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol 23: 5409–5420, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]