Abstract
Low oxygen (O2) tension and mechanical deformation are stimuli for ATP release from erythrocytes. It has been shown previously that rabbit erythrocytes made less deformable with diamide, a thiol cross-linking agent, release less ATP in response to low O2 tension, suggesting a link between these two stimuli. In nonerythroid cells, activation of the Rho/Rho kinase signaling pathway has been reported to decrease cell deformability by altering Rho kinase-dependent cytoskeleton-protein interactions. We investigated the hypothesis that the Rho kinase inhibitor Y-27632 would increase erythrocyte deformability and thereby increase low O2 tension-induced ATP release from erythrocytes. Here we show that Y-27632 (1 μM) increases erythrocyte deformability (5%) and increases low O2 tension-induced ATP release (203%) from healthy human erythrocytes. In addition, we found that, when erythrocytes were made less deformable by incubation with diamide (100 μM), Y-27632 restored both deformability and low O2 tension-induced ATP release to levels similar to those measured in the absence of diamide. These findings suggest that the Rho kinase inhibitor Y-27632 is able to reverse the diamide-induced decrease in erythrocyte deformability and rescue low O2 tension-induced ATP release. These results further support a link between erythrocyte deformability and ATP release in response to low O2 tension.
Keywords: red blood cell, diamide, mastoparan 7, RhoA
the primary function of the erythrocyte is to supply oxygen (O2) to meet tissue needs. When exposed to low O2 tension, erythrocytes release both O2 and the vasodilator ATP, which binds to purinergic receptors on the vascular endothelium (4, 11). In skeletal muscle, this ATP induces the synthesis and release of endothelium-derived relaxing factors, which induce both a local and conducted vasodilation resulting in the appropriate distribution of perfusion to those regions of the tissue in need of O2 (10). It is well established that ATP released in response to both low O2 tension and mechanical deformation requires activation of the heterotrimeric G protein Gi (29). Although the mechanism by which Gi is activated by low O2 tension is not well understood, one possibility is that the conformational change in the membrane-bound hemoglobin molecule, which occurs as it desaturates, causes direct activation of the G protein (19). Such a stress-induced activation of G proteins has been observed in other cell types (15, 30). In a recent study, erythrocytes stiffened with diamide, a thiol cross-linking agent, released significantly less ATP in response to low O2 tension, supporting such an association (37). If decreases in deformability reduce low O2-mediated ATP release, we hypothesized that increases in deformability of the erythrocyte membrane could augment ATP release.
Erythrocyte deformability is determined by a variety of cellular properties, including membrane lipid and protein composition, cytoskeletal protein composition, and cytoplasmic viscosity (27). Recent studies in nonerythroid cells suggest that the Rho/Rho kinase signaling pathway can decrease deformability of cells by altering the properties of the actin cytoskeleton (1, 17, 18, 22). Rho kinase can be activated by the small GTP-binding protein RhoA, which has been identified in the human erythrocyte (5). However, for RhoA to activate Rho kinase, RhoA must first be geranylgeranylated, GTP bound, and become associated with the cell membrane (38). In nonerythroid cells, inhibition of Rho kinase has been shown to decrease the stiffness of the cell membrane (1, 17, 18, 22).
In the present study, we tested the hypothesis that the Rho kinase inhibitor Y-27632 increases erythrocyte deformability and augments the amount of ATP released in response to stimulation by low O2 tension.
METHODS
Isolation of erythrocytes.
Human blood was obtained by venipuncture and collected in a syringe containing heparin (500 U/30 ml). After collection, whole blood was centrifuged at 500 g at 4°C for 10 min. The plasma, buffy coat, and uppermost erythrocyte layer were removed by aspiration. The packed erythrocytes were resuspended and washed three times in buffer (in mM: 21.0 Tris, 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, 5.5 glucose, and 0.5% BSA fraction V, with pH adjusted to 7.4). Erythrocytes were prepared on the day of use. Blood was collected from 11 females and 9 males with an average age of 36 ± 3 yr (range 18–59 yr). The protocol for collection of human blood for these studies required informed consent and was approved by the Institutional Review Board of St. Louis University.
Erythrocyte membrane preparations.
Washed erythrocytes, 3 ml, were added to an ice-cold hypotonic buffer [5 mM sodium phosphate (pH 7.5)-0.5 mM EGTA] supplemented with protease inhibitors (Complete Protease Inhibitor Cocktail Tablets; Roche) to lyse the cells (5). Lysed cells were centrifuged at 30,000 g for 20 min to separate the cytosolic proteins from the membrane proteins. The supernatant (cytosolic fraction) was saved for cytosol preparations, and the pellet was washed three times in the hypotonic buffer [5 mM NaPi (pH 7.5)-0.5 mM EGTA]. The membrane pellet was diluted in sample buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromphenol blue, and 0.125 M Tris·HCl, pH 6.8) and stored at −20°C.
Erythrocyte cytosol preparations.
The cytosolic fraction of the cell lysate was hemoglobin-depleted using a procedure modified from Boukharov and Cohen (5). In short, preswollen Anion Exchange Diethylaminoethyl Cellulose (DE52; Whatman) was prepared with 10× binding buffer [200 mM Tris·HCl (pH 7.5), 200 mM NaCl, and 5 mM EGTA] and then diluted with water to a 1× solution. A column was packed with the DE52 to 3–4 cm in height and washed one time with 1× binding buffer. The cytosol was loaded on the column (6 ml/1 ml DE52 matrix) and followed by three washes with 1 ml of 1× binding buffer to remove hemoglobin. Cytosolic bound proteins were eluted from the column using 3 ml of 0.4 M NaCl. The eluate was dialyzed overnight with 1 liter wash buffer (in mM: 21.0 Tris, 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, and 1.2 MgSO4) with two to three changes of buffer. Eluate was concentrated on Centricon-10 spin concentrator (Amicon) columns to the volume of packed erythroctyes before lysis (∼200–250 μl). The hemoglobin-depleted cytosol was diluted in sample buffer and stored at −20°C.
Western analysis.
Erythrocyte membrane and cytosol preparations were diluted in sample buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromphenol blue, and 0.125 M Tris·HCl, pH 6.8). Samples were boiled, loaded onto precast 4 to 20% polyacrylamide gels (Pierce), resolved by electrophoresis, and then transferred to polyvinylidene difluoride membranes. Membranes were then blocked in 0.1% Tween 20 in Starting Block and incubated overnight at 4°C with a rabbit polyclonal antibody against RhoA (1:2,000; Santa Cruz). Membranes were incubated with a secondary antibody, anti-rabbit (GE Healthcare Life Sciences) and visualized by enhanced chemiluminesence (Pierce). Following visualization, membranes were stripped with Restore Stripping Buffer (Pierce) and incubated with a primary monoclonal antibody against β-actin (Sigma), with secondary anti-mouse antibody (GE Healthcare Life Sciences) as a loading control.
Measurement of erythrocyte deformability.
Erythrocyte deformability was measured using the St. George's blood filtrometer (Carri-Med) (32–34). This device develops a calibrated pressure gradient across a vertically mounted 13-mm-diameter polycarbonate filter (Nucleopore) with 9.53 mm exposed surface diameter and average pore size of 5 μm. Proximal to the filter, the inlet tube was filled with either buffer (in mM: 21.0 Tris, 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, 5.5 glucose, and 0.5% BSA, pH adjusted to 7.4 at 37°C) alone or buffer containing erythrocytes diluted to a 10% hematocrit. For calibration, buffer was passed through the filter, and the time required for the fluid column to pass four fiber optic detectors was recorded digitally. The erythrocyte suspension was then passed through the calibrated filter for deformability measurements. The rate at which the erythrocyte suspension traversed the filter relative to the rate of the buffer alone was used to determine the red (blood) cell transit time (RCTT). The RCTT is dependent on the deformability of the erythrocytes, the hematocrit, and the size of the filter pores relative to the size of the erythrocytes studied. If average filter pore size and hematocrit are kept constant, then RCTT is an index of the degree of deformability of the erythrocytes. Under these conditions, a decrease in the RCTT indicates an increase in erythrocyte deformability and is unitless. Measurements of erythrocyte deformability were made after a 30-min incubation with the Rho kinase inhibitor Y-27632 (1 μM) or its vehicle, saline. In separate experiments, erythrocytes were incubated for 30 min with diamide (100 μM), a thiol cross-linking agent, or vehicle (saline), and deformability was measured. Diamide-exposed erythrocytes were subsequently incubated for 30 min with Y-27632 (1 μM) or saline, and deformability was measured again.
ATP measurements.
ATP release from the erythrocytes was measured using a quantitative luciferin-luciferase assay (4). Briefly, a 200-μl sample of the erythrocyte suspension (0.04% hematocrit) was injected in a cuvette containing 100 μl of firefly tail extract (10 mg/ml; Sigma) and 100 μl of a solution of d-luciferin (0.5 mg/ml; Research Products International). The light emitted from the reaction of ATP with the firefly tail extract was measured using a luminometer (TD 20/20; Turner Designs). To determine the ATP levels, the peak light emitted was compared with an ATP standard curve generated on the day of the experiment. ATP values were normalized to an erythrocyte count of 4 × 108.
Exposure of erythrocytes to low O2 tension in the presence and absence of pharmacological agents.
Washed erythrocytes were diluted to 20% hematocrit with a bicarbonate-based buffer (in mM: 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, 11.0 glucose, 21.4 NaHCO3, and 0.5% BSA, pH 7.4), incubated at 37°C, and equilibrated with 15% O2-6% CO2-balance nitrogen in a thin-film tonometer (model 237; Instrumentation Laboratories) (6). Either 1 μM of the selective Rho kinase activity inhibitor Y-27632 or its vehicle (saline) was added to the erythrocyte suspension, which was allowed to equilibrate for an additional 30 min. In separate experiments, erythrocytes were first incubated for 30 min with diamide (100 μM) or its vehicle (saline), followed by a 30-min incubation with either 1 μM Y-27632 or saline at which time ATP release from the erythrocytes was measured. The erythrocyte suspension was then equilibrated with 0% O2-6% CO2-balance nitrogen for 10 min, and ATP release was measured again. The Po2, Pco2, and pH of the erythrocyte suspension were determined at the time of ATP measurement using a blood gas analyzer (Nova Biomedical; Stat Profile pHOx).
Measurement of ATP release in response to mastoparan 7, a direct activator of the heterotrimeric G protein, Gi.
Washed erythrocytes diluted to a 20% hematocrit were equilibrated with 15% O2-6% CO2-balance nitrogen in a thin-film tonometer at 37°C with Y-27632 (1 μM) or its vehicle (saline). Thirty minutes after Y-27632 or saline was added to the erythrocyte suspension, ATP levels were measured. After baseline readings were obtained, a general activator of the heterotrimeric G protein, Gi, mastoparan 7 (Mas 7, 10 μM; GenScript), was added, and ATP release was measured after 5, 10, and 15 min. For each experiment, the maximal response to Mas 7 is reported.
Measurement of total ATP.
In all experiments in which ATP was measured, total intracellular levels of ATP were also determined to establish that Y-27632 did not alter ATP synthesis. A known number of erythrocytes (1% hematocrit) were lysed in distilled water at room temperature. This suspension was diluted 1:400 in wash buffer, and ATP was measured using the luciferin-luciferase assay. ATP values were normalized to ATP concentration per erythrocyte.
Measurement of hemoglobin.
Extracellular hemoglobin was measured at the completion of all ATP experiments to ensure levels of ATP measured were not a result of cell lysis. Erythrocyte suspensions were centrifuged at 500 g for 10 min at 4°C. The amount of hemoglobin present in the supernatant was measured using a spectrophotometer (Spectronic 20; Milton Roy) at 405 nm. Samples in which an increase in hemoglobin was detected were not included in the results.
Data analysis.
Statistical significance among experiments was determined using either an ANOVA or the Student's t-test, as appropriate. In the case of ANOVA, in the event that the F-ratio indicated that a change had occurred, a Fisher's least-significant difference test was performed to identify individual differences between groups. Results are reported as means ± SE.
RESULTS
Distribution of RhoA in erythrocytes.
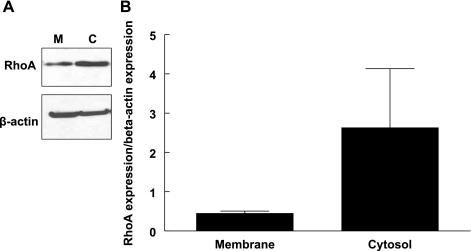
The distribution of RhoA between the membrane and cytosol of unstimulated erythrocytes was used as a measure of basal RhoA activation as described previously (5). In these samples, the amount of RhoA identified in the cytosol was fivefold greater than that measured in the membrane fraction (Fig. 1). This suggests that, in unstimulated erythrocytes, a smaller portion of RhoA is present in the active form.
Fig. 1.
Western immunoblot showing the distribution of RhoA in membrane (M) and cytosol (C) of healthy human erythrocytes (A). Membrane and cytosolic fractions of healthy human erythrocytes were incubated with a RhoA polyclonal antibody generated against an internal region of RhoA. Twenty-five micrograms of total protein were loaded per lane, and values are reported as means ± SE, n = 3 experiments (B).
Effect of Y-27632, a Rho kinase inhibitor, on erythrocyte deformability.
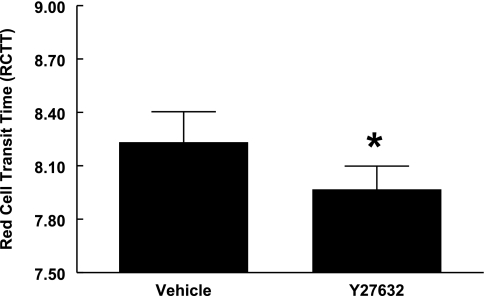
At the concentration of 1 μM, reported to be selective for inhibition of Rho kinase activity (16), a 30-min incubation with Y-27632 decreased RCTT (Fig. 2). This result indicates that incubation with Y-27632 increased erythrocyte deformability.
Fig. 2.
Effect of Y-27632 on erythrocyte deformability. Deformability of healthy human erythrocytes was determined in the absence and presence of Y-27632. Healthy human erythrocyte deformability was measured before and 30 min after incubation with Y-27632 or its vehicle, saline. Deformability values taken before incubation with either vehicle or Y-27632, 8.35 ± 0.16 and 8.39 ± 0.06 red cell transit time (RCTT), respectively, were not different between the two groups. A decrease in RCTT reflects an increase in erythrocyte deformability. Values are reported as means ± SE. *Different from baseline (P < 0.05), n = 9.
Effect of Y-27632 on low O2 tension-induced ATP release from erythrocytes.
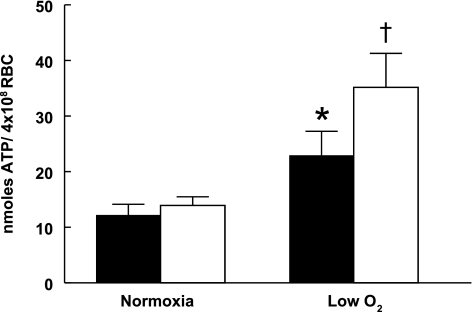
Erythrocytes were incubated with Y-27632 or saline for 30 min under normoxic conditions (Po2 = 107 ± 1 mmHg, Pco2 = 36 ± 0.4 mmHg, and pH = 7.37 ± 0.02) and then subjected to low O2 tension (Po2 = 11 ± 1 mmHg, Pco2 = 36 ± 0.4 mmHg, and pH = 7.37 ± 0.02). In the absence of Y-27632, exposure to low O2 tension increased erythrocyte ATP release (Fig. 3). Importantly, in the presence of Y-27632 (1 μM), low O2 tension-induced ATP release from erythrocytes was significantly greater compared with ATP release in the absence of Y-27632 (Fig. 3). Y-27632 had no effect on total erythrocyte ATP levels (Table 1).
Fig. 3.
Effect of Y-27632 on low O2 tension-induced ATP release from erythrocytes [red blood cells (RBC)]. Healthy human erythrocytes were equilibrated with a gas mixture containing 15% O2-6% CO2-balance nitrogen (Po2 = 107 ± 1 mmHg) in the absence (filled bars) or presence (open bars) of Y-27632. After a 30-min incubation period, baseline erythrocyte ATP release was established (normoxia). The gas mixture was changed to 0% O2-6% CO2-balance nitrogen (Po2 = 11 ± 1 mmHg), and ATP release from erythrocytes was measured (low O2). During the course of the experiment, temperature (37°C), pH (7.37 ± 0.02), and Pco2 (36 ± 0.4) were held constant. Values are reported as means ± SE. *Different from baseline (P < 0.05). †Different from all other values (P < 0.01), n = 13.
Table 1.
Total intracellular ATP values of vehicle (saline)-treated and Y-27632-treated erythrocytes
| Total ATP Measurements, mM |
||
|---|---|---|
| Vehicle | Y-27632 | |
| Low O2 ATP release | 1.39 ± 1.4 | 1.32 ± 1.3 |
| Mastoparan 7 ATP release | 1.40 ± 1.4 | 1.40 ± 1.4 |
Values are means ± S.E.
Effect of Y-27632 on Mas 7-induced ATP release from erythrocytes.
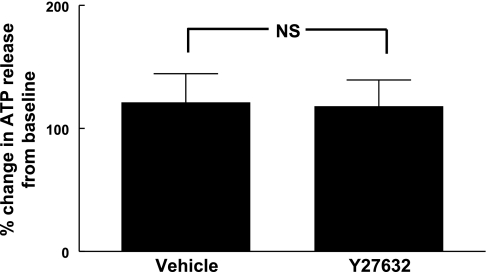
To ensure that the low O2 tension-induced increase in ATP release in the presence of Y-27632 was not the result of its direct activation of Gi, we determined ATP release in response to the direct Gi activator Mas 7 (10 μM). Mas 7-induced ATP release was unaltered by the presence of Y-27632 (Fig. 4).
Fig. 4.
Effect of Y-27632 on mastoparan 7-induced ATP release from erythrocytes. Healthy human erythrocytes were incubated with vehicle (saline) or 1 μM Y-27632 for 30 min. Mastoparan 7 (10 μM) was added to the erythrocyte suspension. Baseline ATP values for vehicle and Y-27632 were 24.8 ± 4.0 and 27.6 ± 6.4 nmol/4 × 108 erythrocytes, respectively, and ATP values after mastoparan 7 stimulation for vehicle and Y-27632 were 53.2 ± 7.2 and 60.0 ± 16.0 nmol/4 × 108 erythrocytes, respectively. While each stimulated value was significantly different (P < 0.01) from its baseline value, there was no significant difference between the two groups. NS, not significant. The peak ATP value is reported as percent change from baseline ± SE, n = 5.
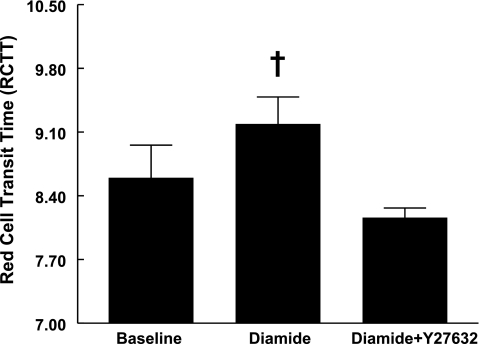
Effect of diamide and Y-27632 on erythrocyte deformability.
Erythrocytes were incubated with diamide (100 μM) or saline for 30 min. Consistent with previous reports (37), diamide significantly increased RCTT (Fig. 5), indicating a decrease in deformability. Diamide-stiffened erythrocytes were then treated with Y-27632 (1 μM) for 30 min. In the presence of Y-27632, RCTT decreased to a value not different from erythrocytes not treated with diamide. These results indicate that Y-27632 increases deformability after the erythrocytes are stiffened by diamide.
Fig. 5.
Effect of diamide in the absence and presence of Y-27632 on erythrocyte deformability. Healthy human erythrocyte deformability was measured before and after 30 min incubation with 100 μM diamide. Diamide-stiffened erythrocytes were then treated for an additional 30 min with 1 μM Y-27632. Values are reported as means ± SE. †Different from all other values (P < 0.01), n = 5.
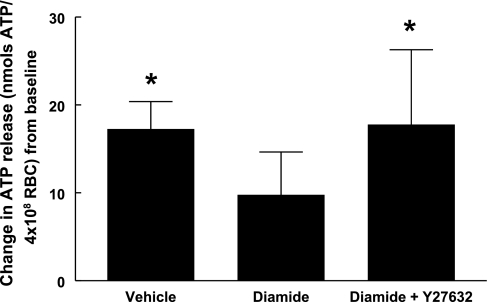
Effect of diamide and Y-27632 on low O2 tension-induced ATP release from erythrocytes.
Erythrocytes were incubated under normoxic conditions (Po2 = 127 ± 3 mmHg, Pco2 = 41 ± 0.6 mmHg, and pH = 7.32 ± 0.01) with diamide (100 μM) or saline for 30 min, followed by a 30-min incubation with Y-27632 (1 μM) or saline. Exposure to low O2 tension (Po2 = 14 ± 2 mmHg, Pco2 = 41 ± 0.6 mmHg, and pH = 7.32 ± 0.01) increased ATP release from erythrocytes incubated with saline vehicle (Fig. 6). In the presence of diamide alone, there was no significant increase in low O2 tension-induced ATP release from erythrocytes, consistent with previous findings from diamide-treated rabbit erythrocytes (37). However, when diamide-treated erythrocytes were incubated with Y-27632, low O2 tension-induced ATP release from erythrocytes was similar to that observed in the saline-treated erythrocytes.
Fig. 6.
Effect of diamide in the absence and presence of Y-27632 on low O2 tension-induced ATP release from erythrocytes. Healthy human erythrocytes were equilibrated under normoxic conditions in the presence of vehicle (saline), diamide, and diamide plus Y-27632. Baseline ATP release was not different among the vehicle-treated, diamide-treated, and diamide- plus Y-27632-treated erythrocytes (30.2 ± 3.6, 36.6 ± 4.40, and 33.0 ± 4.5 nmol/4 × 108 erythrocytes, respectively). Exposure to low O2 tension increased ATP release from vehicle-treated and diamide- plus Y-27632-treated erythrocytes (47.5 ± 3.7 and 50.7 ± 11.2 nmol/4 × 108 erythrocytes, respectively), but not from diamide-treated erythrocytes (46.4 ± 2.50 nmol/4 × 108 erythrocytes). During the course of the experiment, temperature (37°C), pH (7.32 ± 0.01), and Pco2 (41 ± 0.6 mmHg) were held constant. Values are reported as changes from baseline ± SE. *Different from baseline values (P < 0.05), n = 7.
DISCUSSION
In the present study, we tested the hypothesis that the selective Rho kinase inhibitor Y-27632 increases erythrocyte deformability and augments the amount of ATP released in response to stimulation with low O2 tension. Treatment of erythrocytes with Y-27632 increased erythrocyte deformability and increased low O2 tension-induced ATP release from erythrocytes (Figs. 2 and 3). To ensure that Y-27632 did not have nonspecific effects on other protein kinases known to be involved in the low O2 tension-induced ATP release pathway, the concentration of Y-27632 used (1 μM) was 10-fold lower than the concentration determined to be selective for inhibition of Rho kinase activity in other studies (9, 16).
Erythrocyte deformability is regulated, in part, by 1) physical properties of the cytoskeleton, 2) the interaction of the cytoskeleton with the membrane lipid bilayer, and 3) properties of the membrane lipids and proteins (27). Activation of the Rho/Rho kinase pathway has been shown to regulate the assembly of the actin cytoskeleton and promote cell contractility in a variety of nonerythroid cells through phosphorylation of downstream protein targets (13, 28). Those studies showed that activation of the Rho/Rho kinase signaling pathway is linked to an increase in stiffness of these cells. Although specific downstream targets of Rho kinase that may regulate erythrocyte deformability were not identified in the present study, previous reports indicate that Rho kinase phosphorylates several proteins involved in cross-linking of the spectrin cytoskeleton and the cell membrane. These include ezrin, radixin, moesin (13, 25), and adducin (2, 20). Disrupting any of these cytoskeleton-membrane associations could increase erythrocyte deformability consistent changes seen after treatment with Y-27632.
Diamide has been shown to decrease erythrocyte deformability by cross-linking the cytoskeleton through oxidizing the sulfhydryl groups on the spectrin molecule (24). Here we show that, even in the presence of this cytoskeleton cross-linking compound, the Rho kinase inhibitor Y-27632 increases both deformability and low O2 tension-induced ATP release from erythrocytes. These results suggest that, within the erythrocyte, Rho kinase may affect proteins outside the spectrin cytoskeleton or it may influence those associations that link the spectrin cytoskeleton to the erythrocyte cell membrane.
Previous studies have identified a potential link between low O2 tension-induced ATP release and erythrocyte deformability (19, 37). In one study, treatment of erythrocytes with diamide resulted in a decrease in erythrocyte deformability and a decrease in low O2 tension-induced ATP release (37). Our finding that Y-27632 increases erythrocyte deformability and increases low O2 tension-induced ATP release provides further support for a link between erythrocyte deformability and low O2 tension-induced ATP release.
Deformation-induced ATP release is impaired in erythrocytes from patients with pulmonary hypertension, a condition in which erythrocytes were found to be less deformable (36). It has also been shown that the Rho/Rho kinase signaling pathway is increased in animal models of pulmonary hypertension (8). Similar findings have been reported in type 2 diabetes. Erythrocytes from patients with type 2 diabetes do not release ATP in response to low O2 (35), and their erythrocytes have been reported to be less deformable (14, 26). An increase in expression and activation of the Rho/Rho kinase pathway has been observed in a variety of nonerythroid cells obtained from humans and animal models of type 2 diabetes (3, 7, 12, 21, 31). Therefore, it is possible that the increased expression or activity of Rho/Rho kinase could have adverse affects on erythrocyte deformability and low O2 tension-induced ATP release in pulmonary hypertension and type 2 diabetes.
As stated previously, the mechanism by which low O2 tension activates Gi has not been fully established. There have been reports suggesting Gi is activated mechanically by local membrane deformations induced by the change in hemoglobin conformation as it desaturates (19). However, Rho kinase could also be affecting other currently unidentified aspects of the initiation mechanism. Diamide-induced erythrocyte stiffening is reported to result from the formation of disulfide bonds that lead to cross-linking of the spectrin cytoskeleton (37). However, diamide is an oxidizing agent that has the potential for additional effects on the signal pathway for ATP release from erythrocytes. Importantly, the concentration of diamide used in this study has been shown to have no effect on: 1) total intracellular ATP levels, 2) ATP release in response to direct activation of Gi with Mas 7, or 3) receptor-mediated ATP release (37).
Diamide can affect the binding of O2 to hemoglobin. However, studies reporting this effect looked at much higher concentrations of diamide (2–5 mM) than those used in the present study (23). At a concentration of 100 μM, diamide was shown to have no affect on hemoglobin O2 saturation (37).
In summary, we show here that Y-27632, an inhibitor of Rho kinase, increased erythrocyte deformability. In addition, the increased deformability was associated with an increase in low O2 tension-induced ATP release from the erythrocytes. These results support the hypothesis that Rho kinase is a regulatory component of erythrocyte deformability. Thus decreased Rho kinase activation may be an important mechanism for the regulation of low O2 tension-induced ATP release from human erythrocytes.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-89094 and HL-64180.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Jo Schreiweis for excellent technical assistance.
REFERENCES
- 1. An SS, Pennella CM, Gonnabathula A, Chen J, Wang N, Gaestel M, Hassoun PM, Fredberg JJ, Kayyali US. Hypoxia alters biophysical properties of endothelial cells via p38 MAPK- and Rho kinase-dependent pathways. Am J Physiol Cell Physiol 289: C521–C530, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Anong WA, Franco T, Chu H, Weis TL, Devlin EE, Bodine DM, An X, Mohandas N, Low PS. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood 114: 1904–1912, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arita R, Hata Y, Nakao S, Kita T, Miura M, Kawahara S, Zandi S, Almulki L, Tayyari F, Shimokawa H, Hafezi-Moghadam A, Ishibashi T. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes 58: 215–226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 1992 [DOI] [PubMed] [Google Scholar]
- 5. Boukharov AA, Cohen CM. Guanine nucleotide-dependent translocation of RhoA from cytosol to high affinity membrane binding sites in human erythrocytes. Biochem J 330: 1391–1398, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chalmers C, Bird BD, Whitwam JG. Evaluation of a new thin film tonometer. Br J Anaesth 46: 253–259, 1974 [DOI] [PubMed] [Google Scholar]
- 7. Chang S, Hypolite JA, DiSanto ME. Increased basal phosphorylation of detrusor smooth muscle myosin in alloxan-induced diabetic rabbit is mediated by upregulation of Rho-kinase β and CPI-17. Am J Physiol Renal Physiol 290: F650–F656, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Connolly MJ, Aaronson PI. Key role of the RhoA/Rho kinase system in pulmonary hypertension. Pulm Pharmacol Ther 24: 1–14, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellsworth M. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc 36: 35–41, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Failli P, Alfarano C, Franchi-Micheli S, Mannucci E, Cerbai E, Mugelli A, Raimondi L. Losartan counteracts the hyper-reactivity to angiotensin II and ROCK1 over-activation in aortas isolated from streptozotocin-injected diabetic rats (Abstract). Cardiovasc Diabetol 8: 32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukata Y, Oshiro N, Kaibuchi K. Activation of moesin and adducin by Rho-kinase downstream of Rho. Biophys Chem 82: 139–147, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Garnier M, Attali JR, Valensi P, Delatour-Hanss E, Gaudey F, Koutsouris D. Erythrocyte deformability in diabetes and erythrocyte membrane lipid composition. Metabolism 39: 794–798, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Gudi SR, Clark CB, Frangos JA. Fluid flow rapidly activates G proteins in human endothelial cells. Involvement of G proteins in mechanochemical signal transduction. Circ Res 79: 834–839, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol 57: 976–983, 2000 [PubMed] [Google Scholar]
- 17. Ito S, Majumdar A, Kume H, Shimokata K, Naruse K, Lutchen KR, Stamenovic D, Suki B. Viscoelastic and dynamic nonlinear properties of airway smooth muscle tissue: roles of mechanical force and the cytoskeleton. Am J Physiol Lung Cell Mol Physiol 290: L1227–L1237, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95: 3479–3487, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Kolavennu V, Zeng L, Peng H, Wang Y, Danesh FR. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes 57: 714–723, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Lee JS, Panorchan P, Hale CM, Khatau SB, Kole TP, Tseng Y, Wirtz D. Ballistic intracellular nanorheology reveals ROCK-hard cytoplasmic stiffening response to fluid flow. J Cell Sci 119: 1760–1768, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Lotero LA, Jordan JA, Lopez RM, Garcia-Perez AI, Diez JC. Influence of oxidation and crosslinking on oxygen binding properties of mouse erythrocytes. Cell Biochem Funct 19: 89–95, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Maeda N, Kon K, Imaizumi K, Sekiya M, Shiga T. Alteration of rheological properties of human erythrocytes by crosslinking of membrane proteins. Biochim Biophys Acta 735: 104–112, 1983 [DOI] [PubMed] [Google Scholar]
- 25. Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140: 647–657, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McMillan DE, Utterback NG, Puma JL. Reduced erythrocyte deformability in diabetes. Diabetes 27: 895–901, 1978 [DOI] [PubMed] [Google Scholar]
- 27. Mohandas N, Chasis JA. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol 30: 171–192, 1993 [PubMed] [Google Scholar]
- 28. Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 290: C661–C668, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol Heart Circ Physiol 286: H940–H945, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Shaw A, Xu Q. Biomechanical stress-induced signaling in smooth muscle cells: an update. Curr Vasc Pharmacol 1: 41–58, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Soliman H, Craig GP, Nagareddy P, Yuen VG, Lin G, Kumar U, McNeill JH, Macleod KM. Role of inducible nitric oxide synthase in induction of RhoA expression in hearts from diabetic rats. Cardiovasc Res 79: 322–330, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275: H1726–H1732, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol Heart Circ Physiol 271: H2717–H2722, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281: C1158–C1164, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Sprague RS, Stephenson AH, Bowles EA, Stumpf MS, Lonigro AJ. Reduced expression of G(i) in erythrocytes of humans with type 2 diabetes is associated with impairment of both cAMP generation and ATP release. Diabetes 55: 3588–3593, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Sprague RS, Stephenson AH, Ellsworth ML, Keller C, Lonigro AJ. Impaired release of ATP from red blood cells of humans with primary pulmonary hypertension. Exp Biol Med (Maywood) 226: 434–439, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Sridharan M, Sprague RS, Adderley SP, Bowles EA, Ellsworth ML, Stephenson AH. Diamide decreases deformability of rabbit erythrocytes and attenuates low oxygen tension-induced ATP release. Exp Biol Med (Maywood) 235: 1142–1148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Werner N, Nickenig G, Laufs U. Pleiotropic effects of HMG-CoA reductase inhibitors. Basic Res Cardiol 97: 105–116, 2002 [DOI] [PubMed] [Google Scholar]