Abstract
Environmental triggers of dilated cardiomyopathy are poorly understood. Acute exposure to acrolein, a ubiquitous aldehyde pollutant, impairs cardiac function and cardioprotective responses in mice. Here, we tested the hypothesis that chronic oral exposure to acrolein induces inflammation and cardiomyopathy. C57BL/6 mice were gavage-fed acrolein (1 mg/kg) or water (vehicle) daily for 48 days. The dose was chosen based on estimates of human daily unsaturated aldehyde consumption. Compared with vehicle-fed mice, acrolein-fed mice exhibited significant (P < 0.05) left ventricular (LV) dilatation (LV end-diastolic volume 36 ± 8 vs. 17 ± 5 μl), contractile dysfunction (dP/dtmax 4,697 ± 1,498 vs. 7,016 ± 1,757 mmHg/s), and impaired relaxation (tau 15.4 ± 4.3 vs. 10.4 ± 2.2 ms). Histological and biochemical evaluation revealed myocardial oxidative stress (membrane-localized protein-4-hydroxy-trans-2-nonenal adducts) and nitrative stress (increased protein-nitrotyrosine) and varying degrees of plasma and myocardial protein-acrolein adduct formation indicative of physical translocation of ingested acrolein to the heart. Acrolein also induced myocyte hypertrophy (∼2.2-fold increased myocyte area, P < 0.05), increased apoptosis (∼7.5-fold), and disrupted endothelial nitric oxide synthase in the heart. DNA binding studies, immunohistochemistry, and PCR revealed significant (P < 0.05) activation of nuclear factor-κB in acrolein-exposed hearts, along with upregulated gene expression of proinflammatory cytokines tumor necrosis factor-α and interleukin-1β. Long-term oral exposure to acrolein, at an amount within the range of human unsaturated aldehyde intake, induces a phenotype of dilated cardiomyopathy in the mouse. Human exposure to acrolein may have analogous effects and raise consideration of an environmental, aldehyde-mediated basis for heart failure.
Keywords: acrolein, oxidative stress, cardiomyopathy, environmental pollution
idiopathic dilated cardiomyopathy (DCM) is the underlying diagnosis in approximately one-third of cases of heart failure (HF) (15). While often attributed to remote infectious, metabolic, or toxic injury to the heart, in most circumstances the etiological factors responsible for DCM are difficult to identify. Epidemiological studies have established that pollution exposure is associated with increased mortality from several cardiovascular diseases, including HF (3, 5, 28). The biological mechanisms proposed to explain these adverse effects have included pollutant-induced alterations in autonomic tone, the elaboration of proinflammatory and prooxidant mediators, and the physical translocation of soluble constituents of pollutants into the circulation that have direct effects on the heart and vasculature. Theoretically, all of these broad mechanisms can unfavorably impact pathogenetic alterations and/or modifiers of DCM and HF (16). Nonetheless, little is known about the potential environmental triggers of DCM and the specific effects induced by individual constituents of the pollutant mix.
Aldehydes are ubiquitous pollutants in air and water generated by burning fossil fuels (10). They are also readily found in food and are natural products of lipid peroxidation and glucose oxidation (10). More than 300 different aldehydes have been identified in various foods, and at least 36 are present in water, often at levels exceeding maximal recommended concentrations (2, 10). Unsaturated aldehydes are highly reactive; form adducts with cell thiols and amine groups in sugars, phospholipids, proteins, and DNA bases (9, 25); and provoke oxidative stress and proinflammatory responses in tissue (30, 38). Nonetheless, the in vivo cardiovascular effects of exposure to aldehyde pollutants are not well defined.
Because toxicological profiles of environmental aldehyde mixtures are difficult to determine, we have previously focused on the cardiac effects of acrolein, a prototypical reactive α,β-unsaturated aldehyde classified by the Environmental Protection Agency (EPA) as a high-priority air and water toxic (7). These studies demonstrated that acute exposure to acrolein at concentrations documented in human disease, or doses approximating human oral total aldehyde intake, impaired cardiac function and intrinsic cardioprotective responses in mice (19, 42). However, the cardiac effects of long-term acrolein exposure, an issue with greater implications for public health, remain unknown. Notably, the abundance of acrolein and other aldehydes derived endogenously from lipid peroxidation (and their protein-aldehyde adducts) are known to be elevated in the failing heart (14, 33, 40, 41). In the current study, we evaluated whether long-term oral exposure to acrolein would engender inflammation, oxidant stress, and cardiomyopathy.
METHODS
Eight-week-old male C57BL/6 mice weighing ∼20 g were used. All animal studies were performed in compliance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals [Department of Health and Human Services Publication No. (NIH) 85-23, revised 1996] and were approved by the University of Louisville Institutional Animal Care and Use Committee.
Acrolein dosage and administration.
Acrolein was prepared daily from the acid hydrolysis of diethyl acetal acrolein as previously described (19, 42) and used within 4 h. In our previous study, we estimated the maximal human daily unsaturated aldehyde consumption to be 5 mg·kg−1·day−1 and maximal acrolein exposure to be 0.1–0.2 mg·kg−1·day−1 (42). Based on these estimates, and with the intent of using acrolein as a representative unsaturated aldehyde, we tested the chronic effects of 1 mg·kg−1·day−1 acrolein, representing a 5- to 10-fold greater dose than the expected human acrolein intake but only 20% of the expected overall unsaturated aldehyde intake. Animals were gavage-fed acrolein (in 200 μl water, n = 15) or the same volume of water (vehicle, n = 18) daily for 48 days.
Echocardiography.
M-mode, two-dimensional, and Doppler echocardiography in mice were performed under tribromoethanol sedation (0.25 mg/g ip) using a Philips Sonos 5500 machine and 15-MHz linear array transducer as previously described (14, 41). The two echocardiographers performing the study were blinded as to the assigned experimental group of each mouse. Measured parameters included end-diastolic (ED) and end-systolic (ES) diameter (D), end-diastolic anterior and posterior wall thickness (AWT and PWT, respectively), and the ejection time (ET) and heart rate as determined from the aortic Doppler trace. Left ventricular (LV) systolic function was indexed by the fractional shortening [FS = (EDD − ESD)/EDD] and the mean velocity of circumferential fiber shortening (Vcf = FS/ET) (34, 35). LV hypertrophy and/or wall thinning was assessed by the relative wall thickness [RWT = (AWT + PWT)/LVEDD]. Echocardiographic imaging was performed at baseline and after 48 days of acrolein feeding.
LV pressure-volume studies.
Closed-chest LV pressure-volume (P-V) studies were performed in adult C57/BL6 mice (n = 8/group) anesthetized with 80 μg/g ip pentobarbital and mechanically ventilated (155–160 breaths/min, tidal volume 15 μl/g) as previously described (19). Body temperature was maintained at 37°C using a heating pad and lamps. A Millar 1.4-Fr conductance catheter (SPR-839) was inserted in the LV via the carotid artery, and pressure and conductance signals were visualized on-line using the ARIA-1 system (Millar). A small (<1-cm) abdominal incision was made to gain access to the subdiaphragmatic inferior vena cava (IVC). After hemodynamic stabilization for 15 min, recordings of pressure and conductance were performed under steady-state conditions and during transient mechanical IVC occlusion [to vary load and allow determination of the end-systolic pressure-volume relation (ESPVR)]. Intravenous hypertonic saline (0.5–1 μl/g) was then given to determine parallel conductance, and LV volume (μl) was derived from the parallel conductance and ex vivo cuvette calibration with heparinized, warm blood. LV systolic function was indexed by dP/dtmax, stroke work (area bounded by the P-V loop), maximal power (peak value of the product of LV pressure and flow), and end-systolic elastance (Ees, the slope of the ESPVR) (19, 41). LV diastolic function was assessed by the LVEDP, dP/dtmin, and tau, the time constant of LV relaxation (ms) (19, 33, 41).
Immunohistological studies.
Formalin-fixed, paraffin-embedded short-axis LV sections (5 μm) were deparaffinized and rehydrated for histological and immunohistochemical staining using standard techniques as previously described (14, 34, 41). Hematoxylin and eosin- stained sections were used to evaluate cardiomyocyte cross-sectional area. In separate studies, immunostaining was performed for the activated p65 subunit of nuclear factor (NF)-κB using anti-p65 antibody (Chemicon) as described previously (31). Nuclear staining intensity was quantified with a MetaMorph 4.5 imaging system and software (Universal Imaging). Digital images were acquired from six fields at standard intervals in each of five short-axis sections from each group. The threshold for p65 staining was predetermined and held constant for all sections analyzed.
Immunohistochemical staining for protein-nitrotyrosine was performed to index peroxynitrite generation in the heart. Deparaffinized and rehydrated tissue sections were incubated for 20 min with 10 mmol/l citric acid (pH 6.0) and then treated with enzymatic antigen retrieval to recover antigenicity. Nonspecific binding was blocked with 5% normal goat serum and 0.05% saponin (Sigma) in PBS (pH 7.4) for 30 min, followed by incubation with monoclonal anti-nitrotyrosine antibody (1:200; Santa Cruz Biotechnology) in PBS with 1% BSA and 0.05% saponin for 1 h at 37°C. Tissue sections were then incubated for 30 min at room temperature with Alexa fluor-555 anti-mouse IgG (1:500) secondary antibody (Invitrogen), which labeled nitrotyrosinated protein residues red, and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen), which labels nuclei blue. Images were made with a ×40 objective lens at 12 different locations in each tissue section. Mean fluorescence intensity was evaluated using MetaMorph software in 12 images/heart. Sections treated with peroxynitrite (1 mmol/l) were used as positive controls.
Western blotting.
Total protein extraction, SDS-PAGE Western blotting, and immunodetection using electrochemiluminescence protocols (Amersham Biosciences) were performed as previously described (19). IgG-purified polyclonal 1:2,000 anti-KLH-acrolein primary antibody and horseradish peroxidase-linked secondary antibody were used to evaluate protein-acrolein adducts (19). Protein adducts with 4-hydroxy-trans-2-nonenal (HNE) in the membrane fraction (isolated using differential centrifugation) were probed using both dot blots and Western blotting. Polyclonal anti-KLH-HNE primary antibody was used as previously described (34). For dot blots, protein (1.0 μg) was loaded in the wells of a Bio-Dot apparatus (Bio-Rad) and microfiltered through nitrocellulose membranes under vacuum. Primary antibodies for the detection of endothelial nitric oxide (NO) synthase (eNOS), phospho-eNOS-Ser1177, inhibitor of κBα (IκBα), and α-tubulin were obtained from Santa Cruz Biotechnology.
For immunoblot analysis of the monomeric and dimeric forms of eNOS, equal amounts of total protein lysates were subjected to low-temperature SDS-PAGE (LT-PAGE) (43). Briefly, the gel running buffer, 6% SDS-containing polyacrylamide gels, and the gel assembly were equilibrated to 4°C before running the samples. The samples were mixed with SDS containing gel-loading buffer and were not heated. The temperature of the gels was maintained below 10°C during electrophoresis by immersing the gel tanks in ice. Following LT-PAGE, the gels were transferred, and the blots were probed with anti-eNOS antibody and the corresponding secondary antibody. The intensity of the immunoreactive bands was quantified by ImageQuant TL software.
Electrophoretic mobility shift assay.
NF-κB DNA binding activity was quantified by electrophoretic mobility shift assay (EMSA). Nuclear protein extraction from frozen myocardium, the EMSA protocol, autoradiography, and densitometry were all performed as previously described (14). 32P-labeled consensus double-stranded oligonucleotides (sense, 5′-AGTTGAGGGGACTTTCCCAGGC-3′) containing the NF-κB binding site were used as probes. Specificity of NF-κB DNA binding activity was confirmed in competition studies using cold consensus or mutant oligonucleotides.
Real-time PCR and mRNA quantitation.
Total RNA isolation from LV tissue, cDNA synthesis, and quantitative real-time PCR were performed as previously described (14). mRNA transcripts for atrial natriuretic factor (ANF), tumor necrosis factor-α (TNF-α), and interleukin (IL)-1β were determined and normalized to glyceraldehyde-3-phosphate dehydrogenase expression using primer pairs previously described (14).
Apoptosis quantitation.
Myocardial apoptosis was assessed by using the DeadEnd Fluorometric terminal deoxytransferase-mediated dUTP nick-end labeling (TUNEL) assay kit from Promega, which catalytically incorporates fluorescein-12-dUTP at the 3′-ends of fragmented DNA in apoptotic cells using recombinant terminal deoxynucleotidyl transferase (rTdT). Deparaffinized and rehydrated tissue sections were treated with Proteinase K (20 μg/ml) for 15 min at 37°C and then fixed with 4% methanol-free formaldehyde solution in PBS. All subsequent steps were performed following the manufacturer's instructions. All sections were counterstained with DAPI to label nuclei. Cardiomyocytes were identified by staining with anti-troponin I antibody (Santa Cruz Biotechnology) followed by Alexa Fluor 555-conjugated secondary antibody (Invitrogen). TUNEL-positive nuclei (cyan staining) were visualized directly by confocal microscopy (Zeiss LSM510) with nuclear staining confirmed by z-axis sections. Images were taken with a ×63 objective lens at six different locations in each tissue section, and nine sections per heart were evaluated to determine the overall apoptotic rate (total 54 fields/heart). DNase (10 U/ml)-treated sections were used as positive controls. Sections without rTdT treatment were considered as negative controls.
Statistical analysis.
Continuous variables are presented as means ± SD. Two-group comparisons were performed using an unpaired t-test. A P value <0.05 was considered significant.
RESULTS
Chronic acrolein consumption induces LV remodeling and dysfunction.
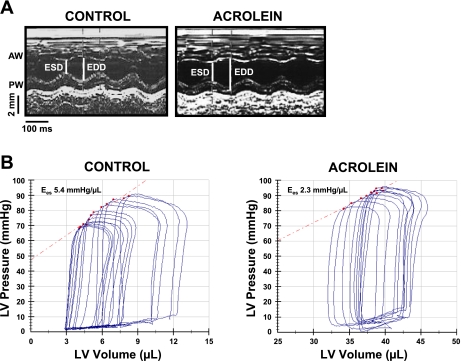
Mice gavage-fed acrolein at 1 mg·kg−1·day−1 for 48 days displayed no overt abnormalities or distress and no significant mortality. Body weight was similar between vehicle-fed and acrolein-fed animals after 48 days (control 26.1 ± 2.3 g; acrolein 26.0 ± 2.0 g). Baseline echocardiographic variables (before the start of feeding) were similar between the two groups. M-mode echocardiographic images obtained after the 48-day feeding period are shown in Fig. 1A. The acrolein-exposed mouse exhibited increased LV size and decreased FS compared with control. Group echocardiographic data (Table 1) indicate that acrolein exposure induced LV dilatation (increased EDD and ESD), LV systolic dysfunction (reduced FS and Vcf), and wall thinning (decreased RWT) consistent with a phenotype of DCM. While these changes were not severe (generally between ∼8 and 20% change), they were highly consistent and statistically significant. To evaluate LV function more precisely, P-V analysis was performed. Figure 1B shows representative P-V loops from control and acrolein-exposed mice during IVC occlusion, together with the corresponding ESPVRs. Consistent with the echocardiographic results, acrolein exposure induced LV dilatation with increased end-diastolic volume and end-systolic volume and depressed LV systolic function as indicated by the smaller Ees. Group data (Table 2) demonstrated consistent LV enlargement and more profound reductions in systolic function with diminished dP/dtmax, maximal power, Ees, and stroke work. Also evident was impairment of LV relaxation with decreased dP/dtmin and increased tau. Hence, chronic acrolein exposure induced pathological remodeling and LV dysfunction.
Fig. 1.
Chronic acrolein exposure depresses left ventricular (LV) function. A: M-mode echocardiograms from two mice, one acrolein-fed and the other vehicle-fed (control). AW and PW, anterior and posterior wall, respectively; ESD and EDD, end-systolic and end-diastolic diameter, respectively. B: LV pressure-volume loops and the corresponding end-systolic pressure-volume relations in representative control and acrolein-fed mice. Ees, end-systolic elastance.
Table 1.
Echocardiography in control and acrolein-exposed mice
| Control (n = 16) | Acrolein (n = 14) | P Value | |
|---|---|---|---|
| HR, beats/min | 469 ± 60 | 481 ± 62 | 0.585 |
| LVEDD, mm | 3.7 ± 0.1 | 4.0 ± 0.2* | <0.001 |
| LVESD, mm | 2.1 ± 0.2 | 2.5 ± 0.2* | <0.001 |
| FS,% | 43 ± 4 | 36 ± 3* | <0.001 |
| ET, ms | 51 ± 4 | 52 ± 7 | 0.771 |
| Vcf., circ/s | 8.5 ± 1.1 | 7.1 ± 1.0* | 0.0022 |
| AWT, mm | 0.78 ± 0.04 | 0.74 ± 0.06 | 0.060 |
| PWT, mm | 0.79 ± 0.03 | 0.76 ± 0.03* | 0.0089 |
| RWT | 0.42 ± 0.02 | 0.38 ± 0.02* | <0.001 |
Values are means ± SD; n, no. of mice. HR, heart rate; LV, left ventricular; EDD, end-diastolic diameter; ESD, end-systolic diameter; FS, fractional shortening; ET, ejection time; Vcf., velocity of circumferential fiber shortening; AWT and PWT, anterior and posterior wall thickness at end-diastole, espectively; RWT, relative wall thickness.
Statistical significance.
Table 2.
Pressure-volume parameters in control and acrolein-exposed mice
| Control (n = 8) | Acrolein (n = 8) | P Value | |
|---|---|---|---|
| HR, beats/min | 501 ± 63 | 451 ± 45 | 0.073 |
| LVEDV, μl | 17 ± 5 | 36 ± 8* | <0.001 |
| LVESV, μl | 8 ± 2 | 29 ± 7* | <0.001 |
| LVPSP, mmHg | 94 ± 9 | 80 ± 14* | 0.028 |
| LVEDP, mmHg | 7 ± 3 | 11 ± 6 | 0.134 |
| SW, mmHg · μl | 601 ± 214 | 378 ± 153* | 0.025 |
| dP/dtmax, mmHg/s | 7,016 ± 1,757 | 4,697 ± 1,498* | 0.010 |
| Maximal power, mW | 3.45 ± 1.61 | 1.98 ± 0.81* | 0.029 |
| Ees, mmHg/μl | 4.93 ± 1.16 | 3.35 ± 0.98* | 0.049 |
| dP/dtmin, mmHg/s | −8,002 ± 1,995 | −5,291 ± 1,957* | 0.013 |
| Tau, ms | 10.4 ± 2.2 | 15.4 ± 4.3* | 0.0094 |
Values are means ± SD; n, no. of mice. EDV, end-diastolic volume; ESV, end-systolic volume; PSP, peak systolic pressure; EDP, end-diastolic pressure; SW, stroke work; dP/dtmax and dP/dtmin, maximal and minimal rate of change in LV pressure, respectively; Ees, end-systolic elastance; tau, time constant of LV relaxation.
Statistical significance.
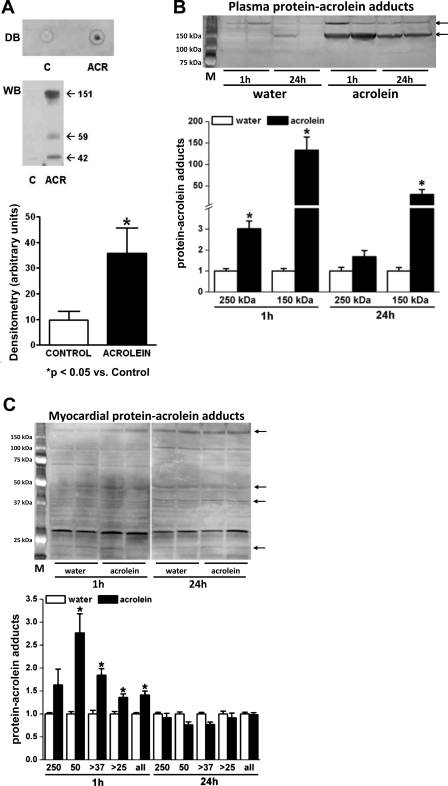
Chronic acrolein exposure generates myocardial oxidative stress and protein-acrolein adducts.
α,β-Unsaturated aldehydes are products of lipid-peroxidation and as such are sensitive markers of oxidative stress (8, 37, 40). Moreover, reactive aldehydes can induce cellular toxicity by adducting with cysteine, histidine, and lysine residues on proteins (9, 37). To index oxidative stress in the hearts of control and acrolein-exposed mice, we measured protein-HNE adducts. The abundance of protein-HNE adducts in total heart homogenates did not change in acrolein-exposed mice (data not shown). However, examination of the membrane fraction of the cardiac homogenates revealed robust augmentation of protein-HNE adducts as assessed by dot blot and Western blotting (Fig. 2A), indicating membrane-localized oxidative stress. We next determined whether acrolein-exposed mice exhibited greater formation of acrolein-protein adducts in serum and heart tissue. Hearts harvested from mice chronically fed acrolein did not exhibit appreciable increases in the abundance of protein-acrolein adducts over control (data not shown). Because these results were not striking, we further examined the abundance of plasma and myocardial acrolein adducts 1 and 24 h after a single oral dose. Plasma protein-acrolein adducts (∼150 kDa) increased markedly at both time points with the highest levels seen at 1 h (Fig. 2B), suggesting that ingested acrolein reaches the blood. Myocardial protein-acrolein adducts, involving proteins of varying molecular weight, were more modestly increased at 1 h but returned to baseline by 24 h (Fig. 2C), approximating the adduct levels observed in the hearts from chronically fed mice. These results suggest that, following oral exposure, sufficient acrolein translocates via the circulation to the heart to modify proteins. However, these adducts accumulate transiently and are then metabolically removed or degraded. Presumably, adduct formation is less pronounced after chronic exposure because of the metabolic disposition of extant tissue adducts.
Fig. 2.
Chronic acrolein exposure induces cardiac oxidative stress and protein modification. A: representative dot blot (DB) and Western blot (WB) performed on the membrane fractions of cardiac homogenates derived from acrolein (ACR)-fed and vehicle-fed [control (C)] mice and corresponding WB densitometry. B and C: WB and densitometry for protein-acrolein adducts in plasma (B) and myocardium (C) from mice fed a single dose of acrolein (1 mg/kg) or water 1 and 24 h after exposure. Augmented protein bands at different molecular weights are indicated by the arrows. M, molecular weight markers. *P < 0.05 vs. control; n = 4 mice/group.
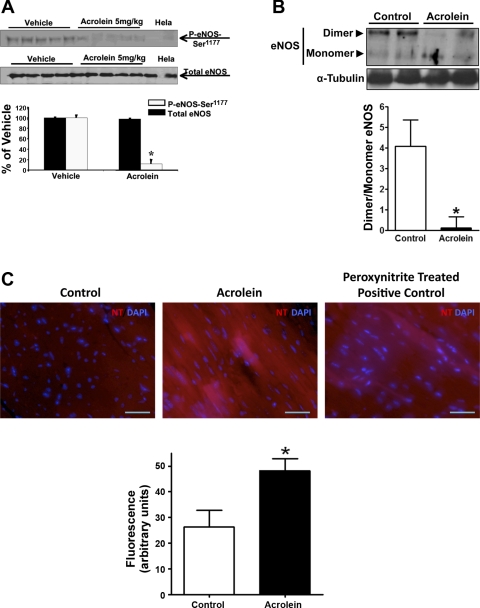
Chronic acrolein exposure disrupts myocardial eNOS function and induces nitrative stress.
We next examined whether acrolein disrupts eNOS function and promotes nitrative stress in the heart. As shown in Fig. 3A, a single oral dose of acrolein (5 mg/kg) profoundly suppressed eNOS phosphorylation at Ser1177, an indicator of eNOS activation (4), without affecting overall eNOS abundance in the heart. In contrast, chronic exposure to acrolein significantly diminished eNOS dimers and increased relative levels of eNOS monomers (Fig. 3B), suggestive of eNOS uncoupling (36). Uncoupling of eNOS would be expected to promote the generation of reactive oxygen species (ROS) and peroxynitrite (36, 39). Indeed, hearts from mice chronically fed acrolein exhibited significantly greater staining for protein nitrotyrosine, an index of peroxynitrite generation (Fig. 3C). These results indicate that chronic acrolein exposure disrupted and uncoupled eNOS and induced nitrative stress in the heart.
Fig. 3.
Acrolein increases myocardial nitrative stress. A: WB and quantitation for phospho (P)-endothelial nitric oxide synthase (eNOS)-Ser1177 and total eNOS performed on total cardiac homogenates from mice 24 h after a single oral dose of acrolein (5 mg/kg) or vehicle (n = 5/group). Hela, Hela cell lysate. B: WB and densitometry for eNOS dimer and monomer performed on cardiac homogenates derived from mice chronically fed acrolein or water for 48 days (n = 4–5/group). C: immunofluorescent stains for protein-nitrotyrosine (NT, red) with 4′,6-diamidino-2-phenylindole (DAPI) costain for nuclei (blue) in hearts harvested from acrolein-fed and control-fed mice as in B, along with fluorescence quantitation (control, n = 3; acrolein, n = 5). Peroxynitrite-treated sections were used as a positive control. *P < 0.05 vs. control.
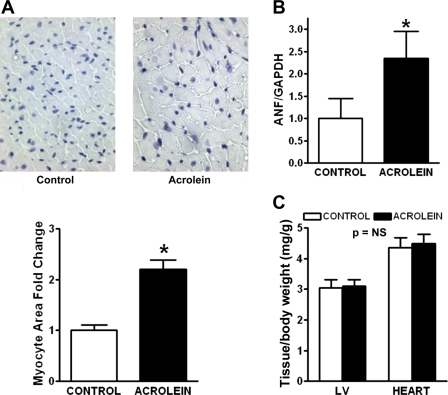
Chronic acrolein exposure induces myocyte hypertrophy and apoptosis.
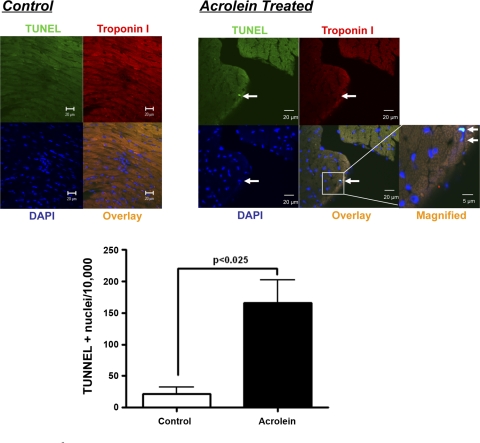
As shown in Fig. 4A, histological evaluation of acrolein-exposed hearts revealed myocyte hypertrophy, with a twofold increase in myocyte cross-sectional area compared with control hearts. There was no substantial difference in interstitial fibrosis (data not shown). Gene expression of the hypertrophic marker ANF was similarly augmented over twofold in acrolein-exposed hearts compared with control (Fig. 4B). Despite these observations, gravimetric analysis of the LV and whole heart did not reveal differences in LV or whole heart weight (normalized to body wt) between the groups. This suggested that the increase in myocyte size was offset by myocyte loss. Indeed, as shown in Fig. 5, we observed a greater frequency of TUNEL-positive nuclei in the hearts of acrolein-exposed mice; these were primarily in cardiomyocytes. Quantitation of the apoptotic rate revealed a more than sixfold increase in TUNEL-positive nuclei compared with control. Hence, chronic oral acrolein exposure induced prohypertrophic and proapoptotic effects in the heart.
Fig. 4.
Chronic acrolein exposure induces myocyte hypertrophy. A: representative histomicrographs of heart tissue from control and acrolein-fed mice demonstrating myocytes in cross section and corresponding quantitation of myocyte cross-sectional area. Also shown is the expression of the atrial natriuretic factor (ANF) gene in the heart by quantitative real-time PCR (B) and tissue gravimetric data (C) from the same experimental groups. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NS, not significant. *P < 0.05 vs. control.
Fig. 5.
Confocal microscopic images of terminal deoxytransferase-mediated dUTP nick-end labeling (TUNEL) staining in hearts from mice chronically fed acrolein or vehicle (control) and quantitation of TUNEL-positive nuclei. Myocytes were stained with anti-troponin I (red), and nuclei were stained with DAPI (blue). TUNEL-positive nuclei appear green-cyan on the overlaid images from the acrolein-exposed heart as shown in the magnified (zoom) image. Arrows denote a TUNEL-positive nucleus. Scale bar: standard magnification 20 μm; zoomed magnification 5 μm. n = 5/group.
Chronic acrolein exposure promotes myocardial inflammation.
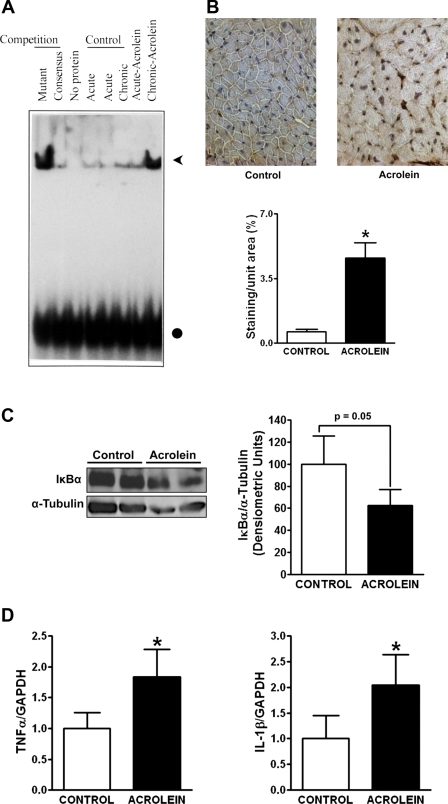
Reactive aldehydes are known to promote inflammation (30, 38), which is a hallmark of chronic HF (14, 22). NF-κB is a central transcriptional regulator of proinflammatory mediators such as TNF-α and IL-1β. To evaluate NF-κB activation, we performed EMSA using pooled cardiac tissue from animals with either acute (24 h after single dose of 1 mg/kg) or chronic oral acrolein exposure, along with appropriate controls. As seen in Fig. 6A, heart tissue from chronically exposed (but not acutely exposed) mice demonstrated robust activation of NF-κB. Figure 6B depicts activated NF-κB p65 subunit immunostaining and quantitation of nuclear immunoreactivity from control and acrolein-exposed hearts. Consistent with the DNA binding studies, the hearts from acrolein-exposed mice exhibited a robust (∼5-fold) increase in the nuclear localization of p65. Additionally, protein levels of IκBα (which binds cytoplasmic NF-κB thereby preventing its nuclear translocation) were decreased in hearts from acrolein-exposed mice (Fig. 6C). Moreover, in parallel with NF-κB activation, hearts from acrolein-exposed mice also exhibited significant (∼2-fold) upregulation of TNF-α and IL-1β mRNA expression compared with controls (Fig. 6D), which is indicative of sustained inflammation.
Fig. 6.
Chronic acrolein exposure induces inflammation in the heart. A: EMSA to determine nuclear factor (NF)-κB DNA binding activity of pooled myocardial nuclear protein extracts from control and acrolein-fed mice. Acute control and acrolein-fed mice were given a single dose of 1 mg/kg vehicle or acrolein, and tissue was harvested at 24 h. Chronic control and acrolein-fed mice were administered daily vehicle or acrolein (1 mg/kg) for 48 days, and tissue was harvested 24 h after the final dose. NF-κB DNA binding is indicated by the arrowhead. The circle indicates unbound oligonucleotide probe. B: representative immunohistochemical stains for the activated p65 subunit of NF-κB in hearts from control mice and mice chronically fed acrolein, together with quantitation of staining intensity by image analysis. Note the nuclear localization of p65 in the acrolein-exposed mouse heart. C: WB and densitometry for inhibitor of κBα (IκBα) in hearts from control mice and mice chronically fed acrolein as in A. D: myocardial gene expression of tumor necrosis factor (TNF) and interleukin (IL)-1β by real-time PCR in the same hearts as in B. *P < 0.05 vs. control.
DISCUSSION
In this study, we demonstrate for the first time that oral exposure to acrolein, a prototypical α,β-unsaturated aldehyde pollutant, at concentrations within the estimated range of human total unsaturated aldehyde exposure, induces a phenotype of DCM in the mouse. Specifically, 48 days of acrolein exposure induced: 1) LV dilatation, wall thinning, impairment of LV relaxation, and depressed contractility; 2) chronic membrane-localized oxidative stress associated with varying degrees of systemic and myocardial protein-acrolein adduct formation; 3) diminished levels and uncoupling of eNOS with associated myocardial nitrative stress; 4) myocyte hypertrophy and apoptosis without fibrosis; and 5) myocardial inflammation with activation of NF-κB and upregulation of TNF-α and IL-1β. The features of oxidant stress, hypertrophy, apoptosis, and inflammation are pathological hallmarks of the failing heart. Taken together, the results suggest that analogous environmental exposure to acrolein in humans can contribute to the development of DCM and/or exacerbate pathological remodeling in humans with preexisting disease. Our results further suggest the possibility that acrolein (and potentially other unsaturated aldehydes) can serve as a dietary xenobiotic mediator and/or modulator of cardiomyopathy.
Epidemiological data indicate that pollution exposure increases cardiovascular morbidity and mortality (3, 5, 28), with the most robust associations related to ischemic heart disease, dysrhythmias, HF, and cardiac arrest (28). A recent study of elderly survivors of acute myocardial infarction revealed that air pollution exposure increased both the risk of mortality and the risk for new-onset HF within four to five years (44). Because the development of new-onset HF following infarction is related to the progression of underlying LV remodeling over time (16), this suggests that exposure to one or a variety of constituent pollutants can exacerbate underlying structural remodeling. One proposed mechanism of pollution-related cardiovascular risk is the physical translocation of soluble pollutant constituents into the heart and vasculature via the circulation (5). However, little is known about the specific pathophysiological responses to individual constituents of source mixtures of environmental pollutants.
Acrolein is a ubiquitous aldehyde pollutant of considerable importance to public health (7). High levels of acrolein have been detected in several foods (ranging from 10 to 600 μg/kg), cigarette smoke (10–140 μg/cigarette), water samples, heated oils, automobile exhaust, coal, and industrial waste (10, 11, 42). Volatile aldehydes such as acrolein are important constituents of the vapor phase of urban air pollution and diesel exhaust and are considered hazardous air pollutants by the EPA (7, 29). Given the large number of environmental sources of acrolein and its potential for long-term toxicity, we sought to determine the effects of chronic acrolein exposure on the heart. In this study, we chose to examine the effects of ingested (as opposed to inhaled) acrolein because, in humans, even in smokers, the highest level of acrolein exposure is through food substances (42). Nevertheless, our findings that acrolein translocates to plasma and heart tissue following exposure (evidenced by the formation of adducts) and induces chronic changes in cardiac gene expression suggest the possibility that analogous exposure to acrolein in ambient air may, via physical transport in blood, produce similar responses. This is consistent with the high cardiovascular toxicity associated with the aldehyde-containing components of air pollution, diesel exhaust, and cigarette smoke (3, 18).
We have previously estimated the maximal human acrolein exposure from food and water to be 0.1 mg·kg−1·day−1 (with an additional 0.1 mg·kg−1·day−1 from cigarette smoking) and the maximal human unsaturated aldehyde consumption to be 5 mg·kg−1·day−1 (42). In the current study, we evaluated the chronic effects of 1 mg·kg−1·day−1 acrolein, a dose fivefold lower than in our acute studies (42), representing a level 5- to 10-fold greater than maximal human acrolein consumption but only ∼20% of total estimated unsaturated aldehyde intake. We chose this intermediate dose given that the sensitivity to acrolein varies among experimental animals; compared with rabbits (LD50 7 mg/kg), mice are relatively less sensitive (LD50 40 mg/kg acrolein) (10). Human sensitivity to acrolein, however, has not been assessed. Whether different acrolein dosing regimens (e.g., lower but more frequent doses) would influence the results differently should be explored in future investigations.
Our results establish that environmental exposure to acrolein, via the oral route, induces a state of inflammation and oxidant stress in the heart, along with LV systolic dysfunction, myocyte hypertrophy, and apoptosis, all consistent with xenobiotic-mediated DCM. These effects are consistent with the known prooxidant and proinflammatory effects of α,β-unsaturated aldehydes, which have been shown to activate inflammatory genes and signaling (including NF-κB) (27, 30, 38) and promote monocyte adhesion to endothelial cells (13). Similarly, in our study, acrolein-exposed hearts exhibited NF-κB activation, proinflammatory cytokine (TNF-α, IL-1β) gene expression, and oxidative and nitrative stress. Furthermore, in our prior study (19), we have shown that oxidative stress is required for acrolein-induced contractile dysfunction, since such effects were prevented by the antioxidant N-acetylcysteine. These findings are of significance, since chronic inflammation and oxidant stress are hallmarks of HF and considered to be important mediators of pathological LV remodeling (12, 16, 22). Plasma TNF-α is an independent predictor of patient mortality in HF (6), and, in experimental models, TNF-α induces many aspects of HF, including contractile depression, hypertrophy, apoptosis, matrix metalloproteinase activation, and oxidative stress (14, 22). Similarly, systemic oxidant stress in human HF correlates with the degree of ventricular dysfunction (21). Signaling related to ROS has been strongly implicated in the induction of pathological cardiac hypertrophy, and ROS can also mediate apoptosis, alter calcium channels and calcium flux, and reduce myofilament calcium sensitivity (12, 20, 32). Moreover, in vivo treatment with ROS scavengers improves pathological LV remodeling (17).
The stimulus for inflammatory cytokines and oxidative stress in HF is generally thought to reflect a response to injury, hemodynamic abnormalities, neurohormonal activation, and alterations in tissue perfusion. Our data suggest that environmental triggers may also contribute to this process and thereby exacerbate the course and progression of HF, and that those with preexisting LV dysfunction may be especially sensitive to environmental acrolein exposure. Interestingly, epidemiological studies have established that human subjects with HF are more vulnerable to the adverse cardiovascular effects of pollution exposure (5). Moreover, similar to our results obtained with acrolein exposure, environmental carbon monoxide also induces pathological remodeling in hearts of normal rats (1), supporting the idea that pollutant exposure could also lead to adverse changes in the heart in the absence of underlying cardiomyopathy.
One underlying mechanism for acrolein-mediated cardiac remodeling may be related to the induced abnormalities in eNOS function. Alterations in eNOS coupling and NO synthesis can contribute substantially to pathological cardiac remodeling (24, 36, 39). When electron transfer from its reductase to oxidase domains is normally coupled, eNOS is generally cardioprotective and antihypertrophic (39). However, during pathological hypertrophy and HF, both eNOS downregulation and uncoupling can occur, thereby augmenting superoxide generation, diminishing NO bioavailability, and increasing peroxynitrite formation (12, 24, 36, 39). In our study, acute acrolein exposure suppressed eNOS activation, whereas chronic acrolein exposure decreased overall eNOS abundance and reduced the eNOS dimer-to-monomer ratio, consistent with eNOS uncoupling. The biological relevance of these changes was demonstrated by the approximately twofold increase in protein-nitrotyrosine levels in the heart, indicative of increased peroxynitrite generation. Hence, disruption of eNOS function may be in part responsible for increased free radicals and oxidant stress induced by acrolein.
The observed cardiomyopathic phenotype may have resulted from both direct and indirect effects of acrolein. We have demonstrated that oral acrolein exposure induces protein-acrolein adducts in both plasma and myocardium with adduct abundance decreasing in a time-dependent manner following exposure. This suggests that consumed acrolein physically circulates to remote sites such as the heart to directly disrupt protein function, thereby secondarily inducing cardiac injury and inflammation. In our previous studies, we demonstrated that acrolein primarily modifies sarcomeric, cytoskeletal, and mitochondrial proteins in the context of acute exposure (19, 42). The time dependence of adduct levels in the current study suggests ongoing metabolic disposition and turnover of protein-acrolein adducts both systemically and in the heart. This is consistent with prior studies that have demonstrated lability of aldehyde-adducted proteins and degradation by the proteasome and lysosomes in minutes to hours (23, 26). Long-term exposure and/or reduced metabolic capacity for aldehyde detoxification may therefore enhance the adverse effects of acrolein. In this regard, we have previously shown that aldose reductase, the main aldehyde-reducing enzyme in the heart, is significantly downregulated in HF (33). Hence, the cardiotoxic effects of environmental acrolein may be heightened in subjects with preexisting HF.
In summary, we have shown that long-term environmental exposure to acrolein, at an amount within the range of human unsaturated aldehyde intake, induces DCM in the mouse. Primary features included the induction of myocardial inflammation and oxidative/nitrative stress, which may represent responses to the formation of detrimental acrolein-protein adducts in the heart, together with myocyte hypertrophy and apoptosis. These results suggest that human exposure to acrolein can have analogous deleterious effects, especially in those with preexisting structural heart disease and/or reduced capacity for aldehyde detoxification. Moreover, our findings raise consideration of an underrecognized environmental basis for idiopathic DCM related to aldehyde constituents of natural food and the pollutant mix.
GRANTS
This work was supported by a Veterans Affairs Merit Award (S. D. Prabhu); National Institutes of Health Grants ES-11860 (A. Bhatnagar, S. D. Prabhu), HL-78825 (S. D. Prabhu, A. Bhatnagar), HL-99014 (S. D. Prabhu), HL-95593 (S. Srivastava), and RR-024489 (A. Bhatnagar, S. Srivastava, S. D. Prabhu); and an American Heart Association Scientist Development Grant (T. Hamid).
DISCLOSURES
There are no conflicts of interest to disclose.
REFERENCES
- 1. Andre L, Boissiere J, Reboul C, Perrier R, Zalvidea S, Meyer G, Thireau J, Tanguy S, Bideaux P, Hayot M, Boucher F, Obert P, Cazorla O, Richard S. Carbon monoxide pollution promotes cardiac remodeling and ventricular arrhythmia in healthy rats. Am J Respir Crit Care Med 181: 587–595, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Assembly of Life Sciences (U.S.) Committee on Aldehydes Formaldehyde and Other Aldehydes. Washington, DC: Natl Acad, 1981, p. ix, 340 p. [Google Scholar]
- 3. Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res 99: 692–705, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Boo YC, Kim HJ, Song H, Fulton D, Sessa W, Jo H. Coordinated regulation of endothelial nitric oxide synthase activity by phosphorylation and subcellular localization. Free Radic Biol Med 41: 144–153, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 103: 2055–2059, 2001 [DOI] [PubMed] [Google Scholar]
- 7. DeWoskin RS. and United States Environmental Protection Agency Toxicological Review of Acrolein (CAS No. 107-02-8) in Support of Summary Information on the Integrated Risk Information System (IRIS). Washington, DC: U.S. Environmental Protection Agency, 2003 [Google Scholar]
- 8. Eaton P, Li JM, Hearse DJ, Shattock MJ. Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat heart. Am J Physiol Heart Circ Physiol 276: H935–H943, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11: 81–128, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Feron VJ, Til HP, de Vrijer F, Woutersen RA, Cassee FR, van Bladeren PJ. Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat Res 259: 363–385, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol 144: 95–146, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115: 500–508, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Go YM, Halvey PJ, Hansen JM, Reed M, Pohl J, Jones DP. Reactive aldehyde modification of thioredoxin-1 activates early steps of inflammation and cell adhesion. Am J Pathol 171: 1670–1681, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation 119: 1386–1397, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 161: 996–1002, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Jessup M, Brozena S. Heart failure. N Engl J Med 348: 2007–2018, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res 87: 392–398, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Logue JM, Small MJ, Stern D, Maranche J, Robinson AL. Spatial variation in ambient air toxics concentrations and health risks between industrial-influenced, urban, and rural sites. J Air Waste Manag Assoc 60: 271–286, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Luo J, Hill BG, Gu Y, Cai J, Srivastava S, Bhatnagar A, Prabhu SD. Mechanisms of acrolein-induced myocardial dysfunction: implications for environmental and endogenous aldehyde exposure. Am J Physiol Heart Circ Physiol 293: H3673–H3684, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Luo J, Xuan YT, Gu Y, Prabhu SD. Prolonged oxidative stress inverts the cardiac force-frequency relation: role of altered calcium handling and myofilament calcium responsiveness. J Mol Cell Cardiol 40: 64–75, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Mak S, Lehotay DC, Yazdanpanah M, Azevedo ER, Liu PP, Newton GE. Unsaturated aldehydes including 4-OH-nonenal are elevated in patients with congestive heart failure. J Card Fail 6: 108–114, 2000 [PubMed] [Google Scholar]
- 22. Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 91: 988–998, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Marques C, Pereira P, Taylor A, Liang JN, Reddy VN, Szweda LI, Shang F. Ubiquitin-dependent lysosomal degradation of the HNE-modified proteins in lens epithelial cells. FASEB J 18: 1424–1426, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, Ketner EA, Majmudar M, Gabrielson K, Halushka MK, Mitchell JB, Biswal S, Channon KM, Wolin MS, Alp NJ, Paolocci N, Champion HC, Kass DA. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation 117: 2626–2636, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nath RG, Chung FL. Detection of exocyclic 1,N2-propanodeoxyguanosine adducts as common DNA lesions in rodents and humans. Proc Natl Acad Sci USA 91: 7491–7495, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okada K, Wangpoengtrakul C, Osawa T, Toyokuni S, Tanaka K, Uchida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem 274: 23787–23793, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid Redox Signal 1: 255–284, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 109: 71–77, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Pratt GC, Palmer K, Wu CY, Oliaei F, Hollerbach C, Fenske MJ. An assessment of air toxics in Minnesota. Environ Health Perspect 108: 815–825, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol 68: 1255–1267, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. J Biol Chem 277: 32063–32070, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol 34: 379–388, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Srivastava S, Chandrasekar B, Bhatnagar A, Prabhu SD. Lipid peroxidation-derived aldehydes and oxidative stress in the failing heart: role of aldose reductase. Am J Physiol Heart Circ Physiol 283: H2612–H2619, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Srivastava S, Chandrasekar B, Gu Y, Luo J, Hamid T, Hill BG, Prabhu SD. Downregulation of CuZn-superoxide dismutase contributes to beta-adrenergic receptor-mediated oxidative stress in the heart. Cardiovasc Res 74: 445–455, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Syed F, Diwan A, Hahn HS. Murine echocardiography: a practical approach for phenotyping genetically manipulated and surgically modeled mice. J Am Soc Echocardiogr 18: 982–990, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest 115: 1221–1231, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci USA 95: 4882–4887, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem 274: 2234–2242, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Umar S, van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem 333: 191–201, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation 112: 2812–2820, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, Ford R, Spinale FG, Riggs DW, Srivastava S, Bhatnagar A, Bolli R, Prabhu SD. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation 121: 1912–1925, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang GW, Guo Y, Vondriska TM, Zhang J, Zhang S, Tsai LL, Zong NC, Bolli R, Bhatnagar A, Prabhu SD. Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide-induced PKCepsilon signaling and cardioprotection. J Mol Cell Cardiol 44: 1016–1022, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297: H1829–H1836, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zanobetti A, Schwartz J. Particulate air pollution, progression, and survival after myocardial infarction. Environ Health Perspect 115: 769–775, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]