Abstract
Fetal hypoxia leads to progressive cardiac remodeling in rat offspring. The present study tested the hypothesis that maternal hypoxia results in reprogramming of matrix metalloproteinase (MMP) expression patterns and fibrillar collagen matrix in the developing heart. Pregnant rats were treated with normoxia or hypoxia (10.5% O2) from day 15 to 21 of gestation. Hearts were isolated from 21-day fetuses (E21) and postnatal day 7 pups (PD7). Maternal hypoxia caused a decrease in the body weight of both E21 and PD7. The heart-to-body weight ratio was increased in E21 but not in PD7. Left ventricular myocardium wall thickness and cardiomyocyte proliferation were significantly decreased in both fetal and neonatal hearts. Hypoxia had no effect on fibrillar collagen content in the fetal heart, but significantly increased the collagen content in the neonatal heart. Western blotting revealed that maternal hypoxia significantly increased collagen I, but not collagen III, levels in the neonatal heart. Maternal hypoxia decreased MMP-1 but increased MMP-13 and membrane type (MT)1-MMP in the fetal heart. In the neonatal heart, MMP-1 and MMP-13 were significantly increased. Active MMP-2 and MMP-9 levels and activities were not altered in either fetal or neonatal hearts. Hypoxia significantly increased tissue inhibitors of metalloproteinase (TIMP)-3 and TIMP-4 in both fetal and neonatal hearts. In contrast, TIMP-1 and TIMP-2 were not affected. The results demonstrate that in utero hypoxia reprograms the expression patterns of MMPs and TIMPs and causes cardiac tissue remodeling with the increased collagen deposition in the developing heart.
Keywords: tissue inhibitor of metalloproteinases, collagen deposition, cardiomyocyte proliferation, hypertrophy
substantial evidence has shown a clear association of adverse intrauterine environment with an increased incidence of cardiovascular disease and hypertension later in the life (4, 5, 14, 32), suggesting that the prenatal environment can change the postnatal physiology, namely programming. Programming is a result of adaptive alterations in gene expression patterns and phenotype in response to the in utero stresses, which modify the growth of specific organs during the critical period of development in early life (30). This may predispose the organism to a heightened susceptibility of cardiovascular disease in its adult life. Multiple stimuli have been identified as being capable of inducing fetal programming in animal models, including malnourishment, exposure to hypoxia, cocaine, nicotine, or glucocorticoid during the pregnancy (2, 14, 25, 32, 37, 48, 50). Recent animal studies have demonstrated that fetal hypoxia is linked to early changes in the developing cardiovascular system (6, 37, 39). In fact, physiological hypoxia is a normal part of fetal life for all vertebrates, and it plays an active role in vasculogenesis, angiogenesis, hematopoeisis, and chondrogenesis during the fetal development (39). The partial oxygen tension of embryo is below 10 mmHg, which is regarded as being hypoxic compared with normal adult tissues with the oxygen tension of 20–40 mmHg (45), indicating that the fetus is persistently hypoxic during the organ formation, growth, and maturation and that fetal tissues have a lower threshold to reach a state of oxygen insufficiency (38). Although a restricted oxygen supply is essential for intrauterine growth, excessive or severe hypoxia may compromise the normal fetal or neonatal development. The fetus may experience prolonged hypoxia under various conditions, such as pregnancy at high altitude, pregnancy with smoke, drug abuse, anemia, pulmonary disease, hypertension, etc. Fetal exposure to pathophysiological hypoxia results in the redistribution of blood flow to facilitate oxygen delivery to the vital organs, such as the brain and heart (41). In rodents, the heart is particularly vulnerable to stressors, such as hypoxia, during the late fetal development and early postnatal life when it undergoes rapid growth and maturation (30). The maturation process of cardiomyocytes in rats occurs over postnatal day 4 to 12, which is marked by binucleation and escape from the cell cycle (51). Nonetheless, little is known about cardiac remodeling and related genes expression patterns in the heart during the critical developmental stages of the fetus and neonate in response to fetal hypoxia.
It has been demonstrated that the timely breakdown and restructure of extracellular matrix (ECM) are critical for the normal fetal organ development (33). ECM is a complicated microenvironment including numerous matrix proteins (such as collagens), signaling molecules, proteases, and all of them contribute to the tissue remodeling process (40). The functional integrity of myocardium depends largely on the extracellular collagenous matrix (31). Aberrant amount, distribution, or organization of fibrillar collagens in the myocardium is associated with various pathophysiological changes in the heart (18). Many factors that participate in the cardiac tissue remodeling have been revealed, and matrix metalloproteinases (MMPs) are one of the most significant mediators in the ECM turnover. MMPs are a family of zinc-dependent proteases that consist of at least 25 different MMPs in vertebrate (12). Although the most studied MMPs in the heart are gelatinases, MMP-2 and MMP-9, that are capable of degrading type I and IV collagens, the major types of fibrillar collagens in the heart are type I and III collagens that are digested mainly by collagenase-1 (MMP-1) and collagenase-3 (MMP-13), respectively (40, 42, 44). In addition to the secreted MMPs, there is a unique subfamily of MMPs, namely membrane type(MT)-MMPs. MT-MMPs (MT1-MT6 MMPs) have been identified to be fully active enzymes once inserted into the cell membrane, rather than being secreted in the proenzyme form. Among the six types of MT-MMPs, MT1-MMP is highly expressed and is a primary enzyme that digests fibrillar collagens in the heart in addition to collagenases (10, 36, 40). Together with the four types of tissue inhibitors of metalloproteinases (TIMPs) identified, MMPs have been implicated in a variety of physiological and pathological processes in the cardiovascular system, ranging from fibrillar collagen digesting in the heart formation and growth to cardiac ischemia-reperfusion injury (23). Yet whether and to what extent MMPs and TIMPs expression patterns in the fetal heart are altered by in utero hypoxia remain to be elusive. Herein, we present evidence in a rat model that maternal hypoxia during gestation alters MMPs and TIMPs expression patterns in the developing heart and results in the abnormal cardiac growth pattern and cardiomyocyte proliferation with the aberrant content of fibrillar collagen network in fetal and neonatal hearts.
METHODS
Experimental animals.
Time-dated pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI) and were randomly divided into two groups: normoxic control and hypoxic treatment of 10.5% oxygen from gestational day 15 to day 21, as described previously (50). Hearts were obtained from day 21 fetal and day 7 neonatal rats of mixed sex. The sample size was four or five pups per group. For Western immunoblots, hearts were flash frozen in liquid nitrogen and stored at −80°C until analysis. For the tissue slide preparation, hearts were fixed in 10% buffered formalin and embedded in paraffin. All procedures and protocols used in the present study were approved by the Institutional Animal Care and Use Committee and followed the guidelines by National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Myocardial morphometry.
Transverse sections of 5 μm prepared from the middle portion of each heart were mounted and stained with hematoxylin and eosin. Sections were viewed at 20× or 40× magnification. The images were digitized and analyzed by the Image-Pro Plus image analysis software. The left ventricular wall thickness was determined in the anterior wall, posterior wall, septal wall, and free wall.
Collagen measurement.
Fibrillar collagen structure and composition in fetal and neonatal hearts were examined by scanning electron microscopy (SEM), as described previously (35). In brief, the fetal and neonatal hearts were fixed in 2.5% glutaraldehyde and stored at 4°C until processing. Hearts were immersed in 10% NaOH for 2–4 days at room temperature, and then rinsed in distilled water for several times until the heart became transparent. Hearts were then treated with 1% aqueous solution of tannic acid for 2 to 3 h, rinsed in distilled water for overnight, and postfixed in 1% aqueous solution of OsO4 for 1 to 2 h. The specimens were dehydrated in a series of increasing ethanol concentrations (50%, 80%, and 100%) and further dehydrated by critical point drying at 31°C for 5–10 min. The samples were then mounted on a specimen holder for drying overnight in a desiccator, coated with gold, and examined with a Philips XL-20 SEM. High-resolution digital images were acquired directly to a computer. The collagen deposition in the heart was also determined by collagen staining using picrosirius red that binds specifically to collagens. Paraffin sections were first dewaxed, then further deparaffinized by xylene, and rehydrated sequentially in ethanol. Rehydrated sections were hematoxylin stained and washed for 10 min in water. The sections were stained in 0.1% picrosirius red for 1 h followed by washing in two changes of acidified water (5 ml glacial acetic acid in 1 l of water). Slides were then dehydrated again in increasing concentrations of ethanol up to 100%, followed by washing in xylene and mounting. Sections were viewed at 20× or 40× magnification, and the images were digitized and analyzed by the Image-Pro Plus image analysis software. Soluble collagen content was determined using the QuickZyme collagen assay kit (QuickZyme Biosciences) according to the instructions of the manufacturer. In brief, hearts were homogenized in 0.5 M acetic acid and pepsin (1:10 weight/tissue wet weight) and incubated overnight at 4°C. The samples were then centrifuged (10 min at 14,000 rpm), the supernatant was collected, and total protein was quantified. Samples (200 μg) and the collagen standard were added to assigned wells in a 96-well plate, and the dilution buffer was added to each well to a final volume of 140 μl/well. The Sirius Red dye solution was added to each well, and the plate was sealed and incubated on ice with gentle shaking for 20 min. The plate was then centrifuged at 3,000 g at 4°C. The collagen fiber that binds to Sirius Red dye forms a pellet at the bottom of the well. The pellets were washed three times with the washing buffer. The detection buffer was added to the pellets and mixed thoroughly, and the signal was read at 540 nm. A standard curve was generated using the collagen standard provided by the kit and collagen content (in μg) per well was determined. All steps were performed on ice to avoid degradation of the collagen fibers.
Ki-67 staining.
The application of the nuclear protein, Ki-67, was used to determine the cell proliferation of cardiomyocytes, as described previously (49). Hearts were fixed in 10% neutral buffered formalin and embedded in paraffin. Immunohistochemical detection of proliferation marker Ki-67 was performed using BD Pharmingen anti-Ig horseradish peroxidase detection kit. In brief, transverse slices of hearts were first deparaffinized in xylene and rehydrated with a series of decreased concentrations of alcohol (100%, 95%, 90%, 75%). To block the endogenous peroxidase activity, the slices were incubated with 0.3% H2O2 for 10 min. Nonspecific binding sites were blocked for 1 h at room temperature in a Tris-buffered saline solution containing 5% bovine serum albumin. The slices were then incubated with mouse monoclonal antibody against Ki-67 (1:50; Abcam, Cambridge, MA) overnight at 4°C. The slices were rinsed three times in phosphate-buffered saline for 5 min each time, followed by incubation with biotinylated goat anti-mouse IgG (1:50; BD Pharmingen) for 60 min at room temperature. The samples were then exposed to streptravidin-horseradish peroxidase and reacted with diaminobenzidine substrate solution according to the manufacturer's recommendations. The slices were viewed with a Zeiss microscope, and images were captured with an attached SPOT digital camera imaging system.
Western blot analysis.
Hearts were homogenized in a lysis buffer containing 150 mM NaCl, 50 mM Tris·HCl, 10 mM EDTA, 0.1% Tween-20, 1% Triton, 0.1% β-mercaptoethanol, 0.1 mM PMSF, 5 μg/ml leupeptin, and 5 μg/ml aprotinin (pH 7.4) and allowed to incubate for 1 h on ice. Homogenates were then centrifuged at 4°C for 10 min at 10,000 g, and supernatants were collected. Protein concentrations were measured using a protein assay kit (Bio-Rad, Hercules, CA). Samples with equal amounts of protein were loaded onto 10% polyacrylamide gel with 0.1% SDS and separated by electrophoresis at 100 V for 90 min. Proteins were then transferred onto nitrocellulose membranes. Nonspecific binding sites was blocked for 1 h at room temperature in a Tris-buffered saline solution containing 5% dry milk. The membranes were then probed with primary antibodies against collagen I, III, MMP-2 (Santa Cruz Biotechnology, Santa Cruz, CA; 1:300 dilution), MMP-1 (Calbiochem, San Diego, CA; 1:1,000 dilution), MMP-9, TIMP-1, TIMP-2, TIMP-3 (Millipore, Temecular, CA; 1:1,000 dilution), and TIMP-4 (Abcam). To assure equal loading, band intensities were normalized to β2-microglobulin (B2M) determined by its antibody (Abcam). After washing, membranes were incubated with secondary horseradish peroxidase-conjugated antibodies. Proteins were visualized with enhanced chemiluminescence reagents, and blots were exposed to Hyperfilm. The results were analyzed with the Kodak ID image analysis software.
Gelatin zymography.
Hearts were homogenized in a lysis buffer containing 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 0.1% deoxycholic acid, 1% protease inhibitor, and 0.5% PMSF. Homogenates were then centrifuged at 4°C for 20 min at 15,000 g, and supernatants were collected. Protein concentrations were measured using a protein assay kit (Bio-Rad). Equal amounts of protein (60 μg) were loaded and separated by 10% Tris-glycine gel with 0.1% gelatin as substrate. The gel was renatured by renaturation buffer (Bio-Rad) for 1 h and then incubated with a development buffer (Bio-Rad) at 37°C for 48 h. The gel was stained with 0.5% Coomassie blue R-250 (Bio-Rad) for 1 h and then destained with destaining buffer (Bio-Rad) until the bands became clear. Data were analyzed with the Kodak ID image analysis software. Gelatinolytic activity was determined as clear zones or bands at the appropriate molecular weights. Mouse active MMP-9 (Chemicon, Temecular, CA) was used as a positive control.
Statistical analysis.
Data are expressed as means ± SE. Experimental number (n) represents the hearts of fetuses or neonates from different dams. Statistical significance (P < 0.05) was determined by ANOVA or Student's t-test, where appropriate.
RESULTS
Effect of hypoxia on fetal and neonatal heart weight and ventricular wall thickness.
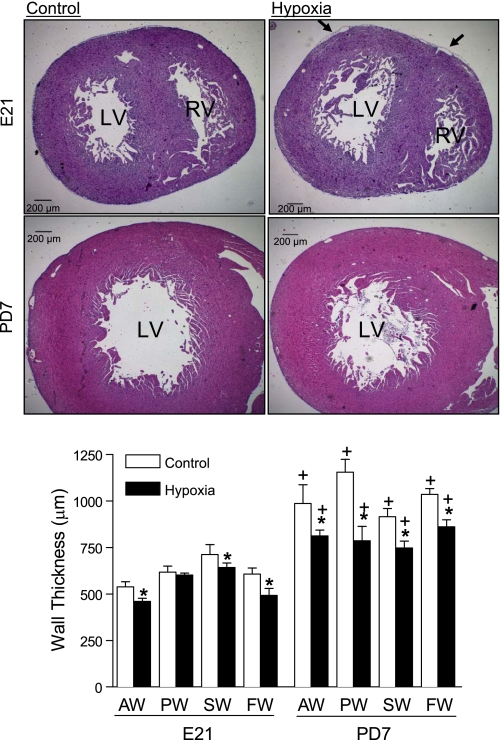
Maternal hypoxia significantly decreased fetal (3.9 ± 0.1 g vs. 3.2 ± 0.1 g; P < 0.05) and neonatal (15.0 ± 0.3 g vs. 9.2 ± 0.6 g; P < 0.05) body weight. There was no significant difference in fetal heart weight between the control and hypoxic animals (23.9 ± 0.9 mg vs. 22.6 ± 0.7 mg; P > 0.05). However, hypoxia significantly increased the heart-to-body weight ratio in fetal rats (6.1 ± 0.1 mg/g vs. 7.0 ± 0.2 mg/g; P < 0.05). Neonatal heart weight was significantly decreased in hypoxic animals (119.5 ± 3.6 mg vs. 75.8 ± 6.0 mg; P < 0.05), whereas the heart-to-body weight ratio in neonatal rats was not changed by hypoxia (8.0 ± 0.2 mg/g vs. 8.2 ± 0.5 mg/g; P > 0.05). To determine the effect of hypoxia on left ventricular wall thickness, hematoxylin and eosin-stained tissue slides were examined. As shown in Fig. 1, the thickness of the anterior wall, septal wall, and free wall was decreased in the fetal heart by hypoxia. As expected, the wall thickness of left ventricle was significantly increased in the neonatal heart, as compared with that of the fetal heart. However, fetal hypoxia resulted in significant decreases in the thickness of the anterior wall, posterior wall, septal wall, and free wall of left ventricle in the neonatal heart (Fig. 1). Additionally, the epicardial detachment from underling myocardium was seen in all samples of fetal hearts treated with maternal hypoxia (Fig. 1).
Fig. 1.
The effect of maternal hypoxia on ventricular morphology. Hearts were isolated from 21-day fetal (E21) and postnatal day 7 (PD7) rats in the control and hypoxic groups. Arrows show the detachment of epicardium in the hypoxic fetal heart. The left ventricular wall thickness was determined at anterior wall (AW), posterior wall (PW), septal wall (SW), and free wall (FW). LV, left ventricle; RV, right ventricle. Data are means ± SE. Data were analyzed by 2-way ANOVA. *P < 0.05, hypoxia vs. control; †P < 0.05, PD7 vs. E21; n = 3–5 per group.
Effect of hypoxia on fibrillar collagen structure and composition in fetal and neonatal hearts.
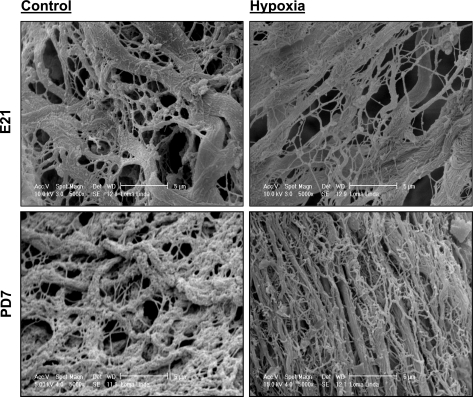
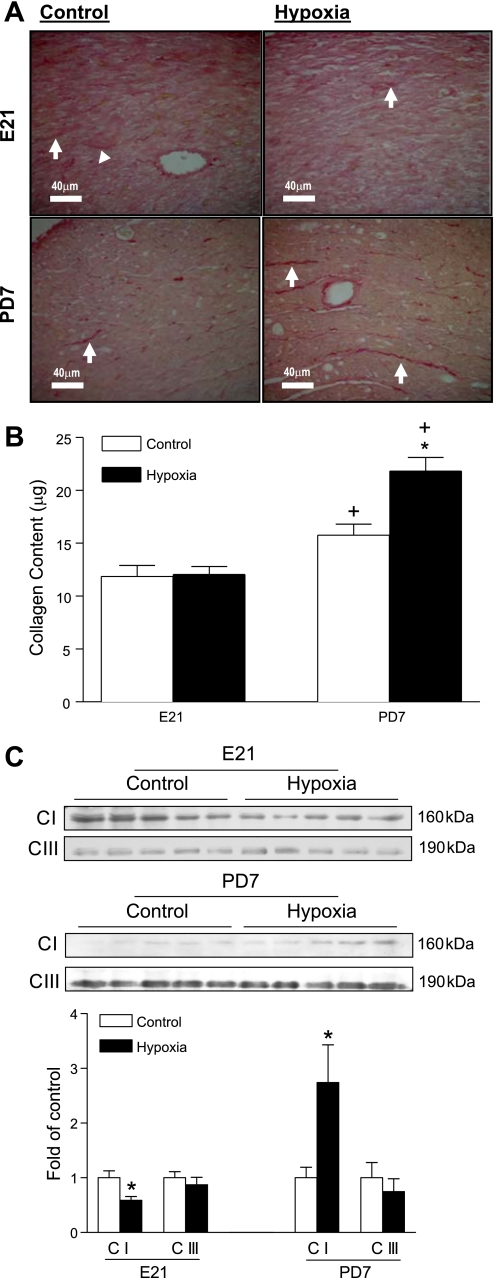
Fibrillar collagen structure and composition were assessed by scanning electron microscope in the heart from fetal and neonatal rats. As shown in Fig. 2, fibrillar collagen fibers were cross-linked randomly to form a complicated matrix network in both fetal and neonatal hearts. Maternal hypoxia did not change collagen matrix in the fetal heart, but increased fibrillar collagen weave matrix in the neonatal heart (Fig. 2). The collagen content and distribution in the left ventricle were examined further by the collagen staining using picrosirius red. As shown in Fig. 3A, collagen forms the fiber bundles in the interstitial space. Maternal hypoxia did not change the total collagen content in the fetal heart but increased it in the neonatal heart (Fig. 3A). The soluble collagen content in the heart was determined using a collagen assay kit. Figure 3B shows that maternal hypoxia had no significant effect on soluble collagen content in the fetal heart. The collagen content in the heart showed a development-dependent increase from the fetus to the neonate, and maternal hypoxia resulted in a significantly greater increase in fibrillar collagens in the neonatal heart (Fig. 3B). The expression of major types of collagens in the heart, collagen I and collagen III, were determined by Western blots (Fig. 3C). Maternal hypoxia caused a reduction in collagen I in the fetal heart but a significant increase in collagen I in the neonatal heart. In contrast, collagen III levels were not significantly altered in either fetal or neonatal hearts.
Fig. 2.
The effect of maternal hypoxia on fibrillar collagen structure and composition. Hearts were isolated from E21 and PD7 rats in the control and hypoxic groups. Fibrillar collagen structure and composition were examined by scanning electron microscope. Hypoxia increased the collagen matrix in PD7 but not E21 hearts. The images were representatives of the heart samples from 4 control E21, 4 hypoxic E21, 5 control PD7, and 6 hypoxic PD7 rats.
Fig. 3.
The effect of maternal hypoxia on the fibrillar collagen content. Hearts were isolated from E21 and PD7 rats in the control (C) and hypoxic (H) groups. The collagen content in tissue sections (A) and the soluble fraction of collagen content in the heart (B) were determined by picrosirius red staining. Arrows show the collagen deposition in the anterior wall of left ventricle. Protein levels of collagen I (C I) and collagen III (C III) were determined by Western blots (C). Data are means ± SE. Data in B were analyzed by 2-way ANOVA. Data in C were analyzed by t-test. *P < 0.05, hypoxia vs. control; †P < 0.05, PD7 vs. E21; n = 5 per group.
Effect of hypoxia on cell proliferation in fetal and neonatal hearts.
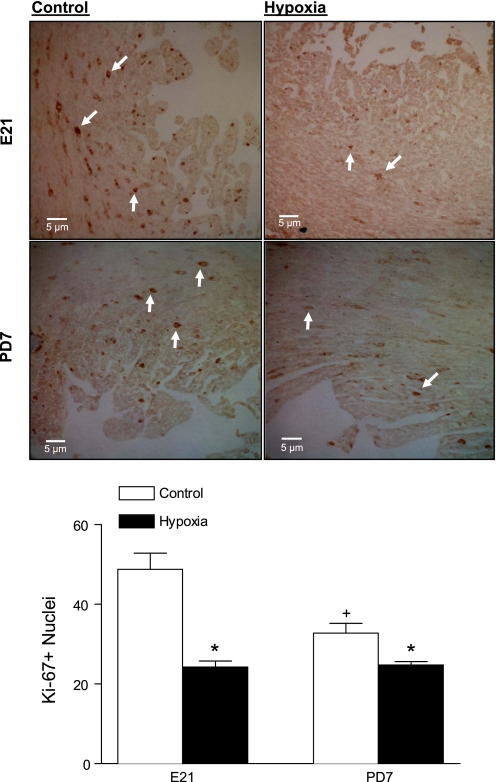
Cardiomyocyte proliferation in the fetal and neonatal hearts was determined by examining the immunostaining of the nuclear protein, Ki-67. The Ki-67 expression occurs throughout all phases of the cell cycle, except for the G0 phase. As shown in Fig. 4, the immunostaining of Ki-67 revealed the dark brown dots within the cells. Maternal hypoxia significantly decreased Ki-67 positive nuclei in both the fetal and neonatal hearts, suggesting a reduced proliferative activity of cardiomyocytes (Fig. 4). Additionally, cardiomyocyte proliferation showed a development-dependent decrease from the fetal to the neonatal heart (Fig. 4).
Fig. 4.
The effect of maternal hypoxia on cardiomyocyte proliferation. Hearts were isolated from E21 and PD7 rats in the control and hypoxic groups. Cell proliferation was examined by the Ki-67 staining. Arrows show Ki-67 positive nuclei. Data are means ± SE. Data were analyzed by 2-way ANOVA. *P < 0.05, hypoxia vs. control; †P < 0.05, PD7 vs. E21; n = 4 per group.
Effect of hypoxia on MMPs and TIMPs in fetal and neonatal hearts.
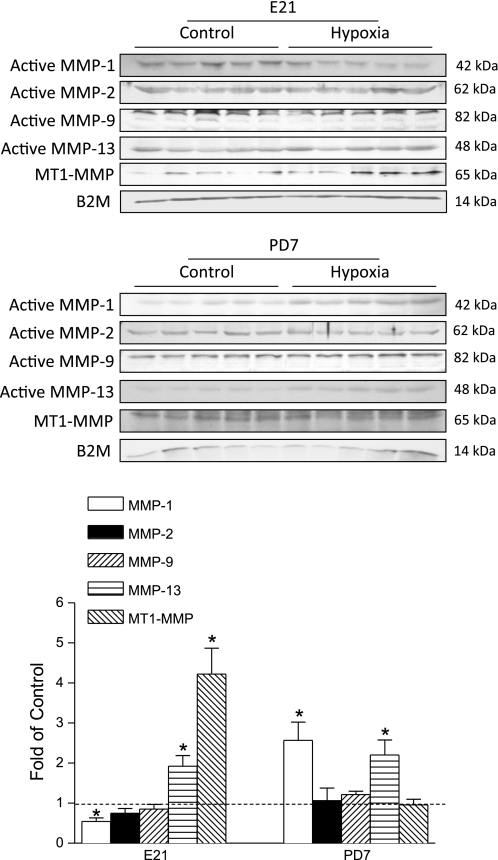
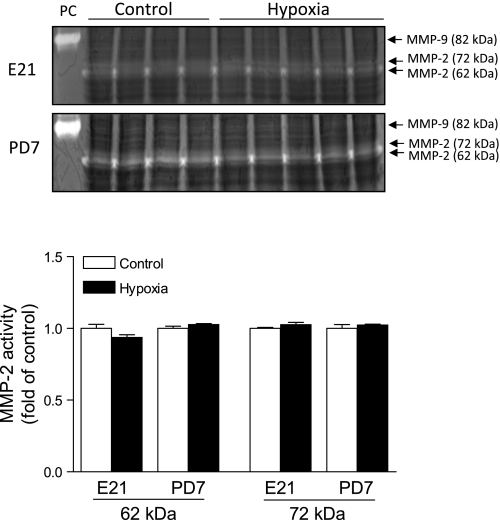
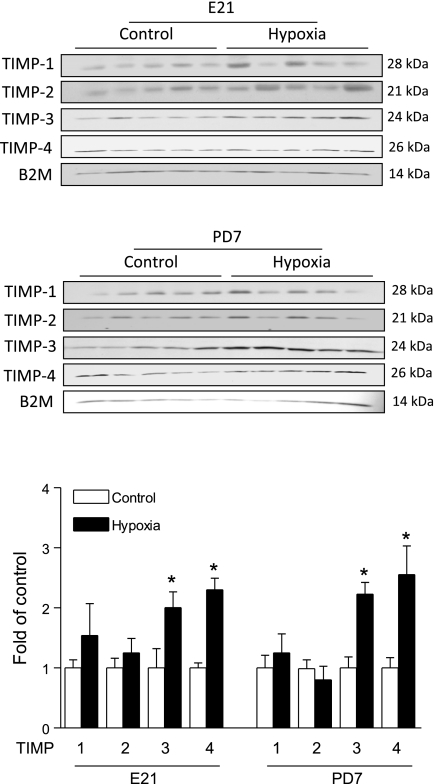
To elucidate the potential mechanisms underlying the hypoxia-induced cardiac remodeling, we determined the effect of maternal hypoxia on the expression of active MMP-1, MMP-2, MMP-9, MMP-13, MT1-MMP, and the expression of TIMP-1, TIMP-2, TIMP-3, TIMP-4 in fetal and neonatal hearts. As shown in Fig. 5, maternal hypoxia resulted in differential expression patterns of MMPs in the fetal and neonatal hearts. The MMP-1 expression was decreased in the fetal heart, but was increased in the neonatal heart. Although it may be debated whether rodents express MMP-1, previous studies clearly demonstrated the expression of MMP-1 in rat hearts (8, 25). MMP-13 was increased in both the fetal and neonatal hearts. MT1-MMP was increased only in the fetal heart. In contrast, the expression of active MMP-2 and MMP-9 were not altered in either fetal or neonatal hearts. Additionally, the proteolytic activities of MMP-2 and MMP-9 were measured with gelatin zymography. As shown in Fig. 6, there was a lack of activity of active MMP-9 at 82 kDa in fetal or neonatal hearts. MMP-2 activities at 62 kDa and 72 kDa were detected, but were not significantly altered by maternal hypoxia in either fetal or neonatal hearts. Although maternal hypoxia had no significant effect on TIMP-1 and TIMP-2 expressions, it significantly increased TIMP-3 and TIMP-4 levels in both the fetal and neonatal hearts (Fig. 7). Table 1 summarizes the relative changes of MMPs, TIMPs, and collagens in fetal and neonatal hearts in response to maternal hypoxia.
Fig. 5.
The effect of maternal hypoxia on matrix metalloproteinase (MMP) expression. Hearts were isolated from E21 and PD7 rats in the control and hypoxic groups. Active MMPs protein levels were determined by Western blots. MT, membrane type; B2M, β2-microglobulin. Data are means ± SE. Data were analyzed by t-test. *P < 0.05, hypoxia vs. control; n = 5 per group.
Fig. 6.
The effect of maternal hypoxia on the activity of MMP-2 and MMP-9. Hearts were isolated from E21 and PD7 rats in the control and hypoxic groups. The activities of MMP-2 and MMP-9 were determined by gelatin zymography. Clear bands indicate positive MMP activity. MMP-2 activities are indicated by clear bands associated with molecular mass of 62 kDa and 72 kDa. No clear bands associated with active MMP-9 at 82 kDa were present in the hearts. PC, positive control of mouse active MMP-9 at 82 kDa. Bottom: the mean values of densitometric analysis of MMP-2 activities in fetal and neonatal hearts. Data are means ± SE. Data were analyzed by t-test; n = 4 to 5 per group.
Fig. 7.
The effect of maternal hypoxia on tissue inhibitors of metalloproteinase (TIMP) levels. Hearts were isolated from E21 and PD7 rats in the control and hypoxic groups. TIMP protein levels were determined by Western blots. Data are means ± SE. Data were analyzed by t-test. *P < 0.05, hypoxia vs. control; n = 5 per group.
Table 1.
Maternal hypoxia-induced changes in the expression of MMPs, TIMPs, and collagens
| MMPs |
TIMPs |
Collagens |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 9 | 13 | MT1 | 1 | 2 | 3 | 4 | I | III | |
| E21 | ↓ | — | — | ↑ | ↑ | — | — | ↑ | ↑ | ↓ | — |
| PD7 | ↑ | — | — | ↑ | — | — | — | ↑ | ↑ | ↑ | — |
Hearts were isolated from 21-day fetal (E21) and postnatal day 7 (PD7) rats in the control and hypoxic groups, and expression levels of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), and collagens were determined by Western blot. MT, membrane type. ↑: increase by hypoxia; ↓: decrease by hypoxia; —: no change by hypoxia.
DISCUSSION
The present study demonstrates in a rat model that maternal chronic hypoxia causes remodeling of the developing heart in the fetus and neonate by decreasing cardiomyocyte proliferation and increasing the collagen deposition. Previous studies demonstrated that maternal hypoxia increased hypoxia-inducible factor 1α (HIF-1α) protein levels in rodent fetal hearts (3, 39), indicating tissue hypoxia of the fetal heart in response to maternal hypoxia. In the present study, the finding that maternal hypoxia significantly decreased immunostaining of the proliferation marker Ki-67 in fetal and neonatal hearts is intriguing. Although Ki-67 is a nonspecific cell proliferation marker and may stain both cardiomyocytes and cardiac fibroblasts, the previous finding that hypoxia promoted cardiac fibroblast proliferation in rodents and humans (1, 22) suggests that the maternal hypoxia-mediated decrease in the Ki-67 staining in fetal and neonatal hearts in the present study is primarily due to the reduced cardiomyocyte proliferation. In rat heart development, the transition of proliferative and hyperplasic growth of mononucleated cells to hypertrophic growth of binucleated cells and terminal differentiation of cardiomyocytes take place within the first 2 wk after birth (30). Consistent with the present finding, previous studies demonstrated an increase in the percentage and cell size of binuleated myocytes in fetal rat heart in response to maternal hypoxia (3), an early morphologic indicator of cardiomyocyte hypertrophy (9). Taken together, these studies suggest that fetal hypoxia causes a premature exit of the cell cycle in the fetal heart leading to fewer but larger cardiomyocytes in offspring. Indeed, enlarged myocytes have been demonstrated in the heart of adult offspring rats that had been exposed to hypoxia before birth (28, 47). The finding of maternal hypoxia-mediated remodeling of the heart during its critical developmental stages of the fetus and neonate suggests programming of aberrant heart function. Indeed, the functional impact of remodeling on postnatal development in the adult heart has been demonstrated in an essentially same animal model of maternal hypoxia, in which reduced MMP-2 and enhanced collagen accumulation were found along with left ventricular hypertrophy and stiffening, diastolic dysfunction, and increased ischemic injury in 4- and 7-mo-old offspring (47). Similar findings that maternal hypoxia causes fetal programming of ischemia-sensitive phenotype in the adult heart have been demonstrated in previous studies from our laboratory (28, 29, 37, 48, 50).
Whereas the mechanisms underlying the hypoxia-mediated reduction of myocyte proliferation in the fetal heart remain elusive, the present finding of the epicardial detachment in the hypoxic fetus provides a possible mechanism by which hypoxia inhibits proliferative and hyperplasic growth of the fetal heart. During the fetal development, the epicardium provides the precursor cells that give rise to various cell types in the heart, and it also supplies multiple growth factors to stimulate cardiac myocyte proliferation, including fibroblast growth factor (FGF)-2, Wingless-type MMTV integration site-9b, FGF-9, and other epicardially derived factors (34, 39). The detachment of epicardium from myocardium is likely to reduce the availability of mitogenic factors and growth factors to the myocardium and subsequently to inhibit myocardial proliferation (39). Additionally, the present study demonstrates that fetal hypoxia increases TIMP-3 and TIMP-4 expression levels in both fetal and neonatal hearts, suggesting another possible mechanism in the hypoxia-mediated downregulation of myocyte proliferation. In addition to the roles in modulating MMPs, it has been demonstrated that both TIMP-3 and TIMP-4 play a key role in inhibiting cardiomyocyte proliferation in rat hearts possibly in a MMP-independent and receptor-mediated manner (13, 16, 17, 42).
In the present study, the finding that maternal hypoxia causes an increased fibrillar collagen content in neonatal, but not fetal, hearts is intriguing and suggests a critical window in compensatory remodeling of the collagen matrix in the neonatal heart resulting from hypoxia-mediated premature exit of cell cycle in the fetal heart. In the heart, collagens are the primary extracellular proteins supporting the myocardium and determining the tissue stiffness. The present study demonstrates that hypoxia differentially regulates collagen I and collagen III expression in the developing heart. The increased collagen content found in the neonatal heart is mainly due to an increase in collagen I. During the postnatal development, collagen I represents more than 85% of the total collagen in the heart (7, 11). Unlike the finding in the neonatal heart, collagen I was decreased in the fetal heart by hypoxia. Although it remains unclear what is the major type of collagens in the fetal heart, collagen III is suggested to be one of the most important fibrillar collagens in the heart during the fetal development (21). The finding that hypoxia did not significantly affect collagen III levels in the fetal heart agrees with the lack of apparent changes in the total fibrillar collagen content in the fetal heart. Although the lack of changes in collagen III was also demonstrated in neonatal hearts in the present study, a previous study showed that maternal hypoxia increased the deposition of both collagen I and III in the heart of adult offspring (47), suggesting a continuous remodeling process in the heart during the postnatal development.
The present findings of decreased MMP-1 in the fetal heart and increased MMP-1 in the neonatal heart are somewhat surprising, given that MMP-1 as collagenase 1 is a primary enzyme that digests collagen I. This suggests a complex pattern of the interaction between collagens and MMPs in the developing heart. It is possible that fetal hypoxia caused an imbalance of collagen synthesis and degradation in the developing heart with the effect of synthesis predominant. Multiple pathways have been identified in the hypoxia-enhanced synthesis of collagen I, including reactive oxygen species, mitogen-activated protein kinase, and transforming growth factor-β1 (1, 19, 20). Although it is not known at present, the possibility that the collagen I and III genes have different HIF-1 promoter sites that may regulate differentially expressions of collagen I and III in the heart remains an intriguing area for the further investigation. The changes in MMP-1 levels in the same direction of those in collagen I observed in the hypoxic hearts may reflect a compensatory response to the synthesis of collagen I. On the other hand, hypoxia-mediated changes in MMP-1 expression levels may in turn lead to a compensatory response of collagen I synthesis that exceeds its degradation. Similar findings of the apparent paradoxical changes of collagens and MMPs in the same direction resulting from the hypoxia treatment were also obtained in mice (20), supporting the notion of feedback regulation of collagens on MMPs levels. It has been shown that the increased collagens can activate the discoidin domain receptor (DDR) and therefore upregulate MMPs (43, 46). In the heart, DDR 2 is primarily expressed in fibroblasts that are the major source of ECM components and MMPs (15, 24). Similarly, MMP-13 (collagenase 3) was significantly increased by hypoxia in both fetal and neonatal hearts, whereas collagen III remained unchanged. This further supports the notion that fibrillar collagens in the developing heart are regulated primarily through their synthesis rather than their degradation via collagenases. In addition to collagenases, MT1-MMP is another primary enzyme that digests fibrillar collagens in the heart (10, 36). The increased MT1-MMP levels in the hypoxic fetal heart may contribute to the decreased collagen I, and the lack of MT1-MMP increment in the neonatal heart may enhance the accumulation of collagen I synthesis resulting from fetal hypoxia. Although collagenases (MMP-1 and MMP-13) possess high substrate specificity for fibrillar collagens, MT1-MMP has been shown to degrade nonmatrix substrates including cytokines, bioactive peptides, and growth factors in the myocardium (40). It is possible that the elevated MT1-MMP also contributes to the reduced cardiomyocyte proliferation observed in the hypoxic fetal heart. The lack of effect of fetal hypoxia on MMP-2 and MMP-9 expression levels and activities and their endogenous inhibitors TIMP-2 and TIMP-1 in the heart indicates the minimal role of the gelatinases in hypoxia-mediated remodeling of fetal and neonatal hearts. Nonetheless, the active MMP-2 levels were found decreased in the heart of adult rats that had been exposed to hypoxia before birth (47), suggesting a continuous programming of MMPs expression patterns in the heart during the postnatal development.
The present study provides new insights in the maternal hypoxia-mediated heart remodeling during its critical developmental stages of the fetus and neonate and suggests a role of altered MMP-TIMP expressing patterns in the developing heart. Although it is difficult to demonstrate a true cause-effect relation of MMPs-TIMPs and cardiac remodeling in living animals, particularly in a pregnant animal model at present due to the lack of selective inhibitors and the difficulty of their use in a pregnant animal model, as well as the difficulty of transgenic approach in a rat model, the present findings provide important physiological information and basis for future more mechanistic investigations possibly using an approach of short interfering RNA in cultured organ/heart or cardiomyocytes. Given that hypoxia is one of the most important and clinically relevant stresses to the fetus, and that large epidemiological studies indicate a link between in utero adverse stimuli during gestation and an increased risk of heart disease in the adulthood, the possibility that fetal hypoxia may result in programming of a heightened vulnerability of remodeling and failing heart later in life provides a mechanistic understanding worthy of investigation in humans. Indeed, it has been shown in rats that antenatal hypoxia enhances collagen accumulation and left ventricular hypertrophy along with stiffening, diastolic dysfunction, and the increased heart susceptibility to ischemia-reperfusion injury in adult offspring (28, 29, 37, 47, 48, 50).
GRANTS
This work was supported by the National Institutes of Health Grants HL-82779, HL-83966, HL-89012, and HD-031226 (all to L. Zhang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Agocha A, Lee HW, Eghbali-Webb M. Hypoxia regulates basal and induced DNA synthesis and collagen type I production in human cardiac fibroblasts: effects of transforming growth factor-beta1, thyroid hormone, angiotensin II and basic fibroblast growth factor. J Mol Cell Cardiol 29: 2233–2244, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Bae S, Gilbert RD, Ducsay CA, Zhang L. Prenatal cocaine exposure increases heart susceptibility to ischaemia-reperfusion injury in adult male but not female rats. J Physiol 565: 149–158, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol 285: H983–H990, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1: 1077–1081, 1986 [DOI] [PubMed] [Google Scholar]
- 5. Bateson P, Barker D, Clutton-Brock T, Deb D, D′Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature 430: 419–421, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Camm EJ, Hansell JA, Kane AD, Herrera EA, Lewis C, Wong S, Morrell NW, Giussani DA. Partial contributions of developmental hypoxia and undernutrition to prenatal alterations in somatic growth and cardiovascular structure and function. Am J Obstet Gynecol 495: e24–e34, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Carver W, Terracio L, Borg TK. Expression and accumulation of interstitial collagen in the neonatal rat heart. Anat Rec 236: 511–520, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Chen K, Li D, Zhang X, Hermonat PL, Mehta JL. Anoxia-reoxygenation stimulates collagen type-I and MMP-1 expression in cardiac fibroblasts: modulation by the PPAR-gamma ligand pioglitazone. J Cardiovasc Pharmacol 44: 682–687, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Corstius HB, Zimanyi MA, Maka N, Herath T, Thomas W, van der Laarse A, Wreford NG, Black MJ. Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr Res 57: 796–800, 2005 [DOI] [PubMed] [Google Scholar]
- 10. D′Armiento J. Matrix metalloproteinase disruption of the extracellular matrix and cardiac dysfunction. Trends Cardiovasc Med 12: 97–101, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Eghbali M, Weber KT. Collagen and the myocardium: fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol Cell Biochem 96: 1–14, 1990 [DOI] [PubMed] [Google Scholar]
- 12. Fanjul-Fernández M, Folgueras AR, Cabrera S, López-Otín C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim Biophys Acta 1803: 3–19, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Fedak PW, Smookler DS, Kassiri Z, Ohno N, Leco KJ, Verma S, Mickle DA, Watson KL, Hojilla CV, Cruz W, Weisel RD, Li RK, Khokha R. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation 110: 2401–2409, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359: 61–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Organization of fibroblasts in the heart. Dev Dyn 230: 787–794, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Hammoud L, Burger DE, Lu X, Feng Q. Tissue inhibitor of metalloproteinase-3 inhibits neonatal mouse cardiomyocyte proliferation via EGFR/JNK/SP-1 signaling. Am J Physiol Cell Physiol 296: C735–C745, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Hammoud L, Xiang F, Lu X, Brunner F, Leco K, Feng Q. Endothelial nitric oxide synthase promotes neonatal cardiomyocyte proliferation by inhibiting tissue inhibitor of metalloproteinase-3 expression. Cardiovasc Res 75: 359–368, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Heeneman S, Cleutjens JP, Faber BC, Creemers EE, van Suylen RJ, Lutgens E, Cleutjens KB, Daemen MJ. The dynamic extracellular matrix: intervention strategies during heart failure and atherosclerosis. J Pathol 200: 516–525, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle 7: 1128–1132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu CP, Dandapat A, Liu Y, Hermonat PL, Mehta JL. Blockade of hypoxia-reoxygenation-mediated collagen type I expression and MMP activity by overexpression of TGF-beta1 delivered by AAV in mouse cardiomyocytes. Am J Physiol Heart Circ Physiol 293: H1833–H1838, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Jackson M, Connell MG, Smith A. Development of the collagen network of the human fetal myocardium: an immunohistochemical study. Int J Cardiol 41: 77–86, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Kacimi R, Vessey DA, Honbo N, Karliner JS. Adult cardiac fibroblasts null for sphingosine kinase-1 exhibit growth dysregulation and an enhanced proinflammatory response. J Mol Cell Cardiol 43: 85–91, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res 85: 413–423, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Kania G, Blyszczuk P, Eriksson U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med 19: 247–252, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Kwak HB, Kim JH, Joshi K, Yeh A, Martinez DA, Lawler JM. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J 25: 1106–1117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lawrence J, Chen M, Xiong F, Xiao D, Zhang H, Buchholz JN, Zhang L. Foetal nicotine exposure causes PKCε gene repression by promoter methylation in rat hearts. Cardiovasc Res 89: 89–97, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leco KJ, Khokha R, Pavloff N, Hawkes SP, Edwards DR. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem 269: 9352–9360, 1994 [PubMed] [Google Scholar]
- 28. Li G, Bae S, Zhang L. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am J Physiol Heart Circ Physiol 286: H1712–H1719, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig 10: 265–274, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Louey S, Thornburg KL. The prenatal environment and later cardiovascular disease. Early Hum Dev 81: 745–751, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Marijianowski MM, van der Loos CM, Mohrschladt MF, Becker AE. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J Am Coll Cardiol 23: 1204–1208, 1994 [DOI] [PubMed] [Google Scholar]
- 32. McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85: 571–633, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem 274: 21491–21494, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Nesbitt T, Lemley A, Davis J, Yost MJ, Goodwin RL, Potts JD. Epicardial development in the rat: a new perspective. Microsc Microanal 12: 390–398, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Ohtani O, Ushiki T, Taguchi T, Kikuta A. Collagen fibrillar networks as skeletal frameworks: a demonstration by cell-maceration/scanning electron microscope method. Arch Histol Cytol 51: 249–261, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem 272: 2446–2451, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCε gene repression in rat hearts. Circ Res 107: 365–373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patterson AJ, Zhang L. Hypoxia and fetal heart development. Curr Mol Med 10: 653–666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ream M, Ray AM, Chandra R, Chikaraishi DM. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol 295: R583–R595, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87: 1285–1342, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Teitel D, Rudolph AM. Perinatal oxygen delivery and cardiac function. Adv Pediatr 32: 321–347, 1985 [PubMed] [Google Scholar]
- 42. Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: ‘embracing the MMP-independent-side of the family’. J Mol Cell Cardiol 48: 445–453, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell 1: 13–23, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol 13: 1637–1652, 1989 [DOI] [PubMed] [Google Scholar]
- 45. Webster WS, Abela D. The effect of hypoxia in development. Birth Defects Res C Embryo Today 81: 215–228, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, Li Y. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem 280: 548–555, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Xu Y, Williams SJ, O′Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J 20: 1251–1253, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Xue Q, Dasgupta C, Chen M, Zhang L. Foetal hypoxia increases cardiac AT2R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc Res 89: 300–308, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xue Q, Ducsay CA, Longo LD, Zhang L. Effect of long-term high-altitude hypoxia on fetal pulmonary vascular contractility. J Appl Physiol 104: 1786–1792, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J Pharmacol Exp Ther 330: 624–632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang L. Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig 12: 2–13, 2005 [DOI] [PubMed] [Google Scholar]