Abstract
The objective of study was to evaluate the aging-associated changes, contractile characteristics of mesenteric lymphatic vessels (MLV), and lymph flow in vivo in male 9- and 24-mo-old Fischer-344 rats. Lymphatic diameter, contraction amplitude, contraction frequency, and fractional pump flow, lymph flow velocity, wall shear stress, and minute active wall shear stress load were determined in MLV in vivo before and after Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) application at 100 μM. The active pumping of the aged rat MLV in vivo was found to be severely depleted, predominantly through the aging-associated decrease in lymphatic contractile frequency. Such changes correlate with enlargement of aged MLV, which experienced much lower minute active shear stress load than adult vessels. At the same time, pumping in aged MLV in vivo may be rapidly increased back to levels of adult vessels predominantly through the increase in contraction frequency induced by nitric oxide (NO) elimination. Findings support the idea that in aged tissues surrounding the aged MLV, the additional source of some yet unlinked lymphatic contraction-stimulatory metabolites is counterbalanced or blocked by NO release. The comparative analysis of the control data obtained from experiments with both adult and aged MLV in vivo and from isolated vessel-based studies clearly demonstrated that ex vivo isolated lymphatic vessels exhibit identical contractile characteristics to lymphatic vessels in vivo.
Keywords: mesenteric lymphatic vessels, lymph flow velocity, wall shear stress, nitric oxide, aging
the mesenteric lymphatic network provides route for up to 90% of the daily formed lymph (4) and therefore is vital for water/lipid adsorption and maintenance of homeostasis of the body. The mesenteric lymphatic compartments operate under the conditions of potential exposure to the pathogens consumed with food and thus are permanently involved in the immune defense of the body against biologically aggressive environment. Each of the body functions mentioned above are affected by aging; however, the lymphatic-related component of the aging-induced dysfunctions of the body is greatly understudied. For the gastrointestinal tract, the potential aging-associated alterations of mesenteric lymph flow, as well as the corresponding changes in active mesenteric lymph pumping, are basically unknown, and therefore they are mainly ignored in clinical practice.
Mesenteric lymph flow in vivo was and is a subject of investigation by several groups (3, 5, 7, 14, 16, 42, 43) including its observations (17) in rats of Fischer-344 (F-344) strain, which were used in the current study. However, very few reports have demonstrated reduced lymph flow in aged animals in vivo and those that did were done by measuring lymph flow from cannulated thoracic duct in dogs (8) and main mesenteric lymph duct in Sprague-Dawley rats (26). In particular, it was reported (10) that aging significantly decreased lymph flow from the main mesenteric lymph duct in Sprague-Dawley rats by ∼60% between ages of 3 and 22 mo. Although these studies provided the first quantifications of the aging-associated reductions of the total lymph flow in vivo, including the diminished total lymph fluid outcome from the mesenteric lymphatic network, the detailed evaluation of the status of aged lymph flow has never been performed in vivo. Recently, we performed series of experiments to evaluate the aging-associated alterations of the contractility of the isolated rat MLV (30). These data demonstrated a severe weakening of the lymphatic pump in aged MLV including diminished lymphatic contraction amplitude and contraction frequency and, as a result, a depleted lymphatic pump flow. Additionally, it was shown that the nonspecific nitric oxide synthase (NOS) blockade was able to improve, to some degree, contractility and pumping of isolated aged rat MLV (30).
In vivo the total lymph flow through lymphatic vessels is a complicated combination of several factors and can be described as a combination of the intrinsic flow (i.e., spontaneous lymphatic vessels contractions generated flow) and the extrinsic flow (i.e., a cumulative result of the action of extra-lymphatic lymph-driving forces; Ref. 19). Therefore, in vivo characterization of the aging-associated alterations of mesenteric lymph flow is able to provide detailed information on the overall status of this lymphatic network in the aged body. Additionally, as an outcome of these current studies, we aimed to compare for the first time the lymph transport function of the isolated adult and aged MLV (30) with the transport function of the same type vessels situated in their natural environment to evaluate the aging-associated changes of lymph flow in MLV surrounded by aged mesenteric tissues.
METHODS
Animals and Surgery
For the current studies, we used Fischer-344 (F-344) male rats, a commonly used rat strain in aging-related research (29, 37) (animals were obtained from aged rat colony maintained by the National Institute of Aging). The animals ranged in age groups from adulthood to aged (9- and 24-mo old). All animal procedures for the current studies were reviewed and approved by the Texas A&M Health Science Center Institutional Animal Care and Use Committee on the Temple, Tx, campus.
To visualize MLV, rats were anesthetized with a solution containing a combination of fentanyl/droperidol (0.3 ml/kg im) and diazepam (2.5 mg/kg im). A 4-cm long midline abdominal incision was made through the skin, underlying fascia, and muscle layers. A small loop of intestine, 6–7 cm in length, was exteriorized through the incision. A section of the mesentery containing lymphatic vessels was positioned and secured in the observation chamber within the field of view of the intravital microscope. Throughout the duration of the experiment, the animal was located on a heated board; its heart rate and arterial blood oxygenation were monitored using a Nonin IPX1 pulse oximeter (Nonin Medical, Plymouth, MN). The exteriorized part of mesentery was constantly suffused (flow exchange rate 0.6 ml/min) with prewarmed 38°C albumin-physiological salt solution (APSS; in mM: 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.2 NaH2PO4, 5.0 dextrose, 2.0 sodium pyruvate, 0.02 EDTA, 3.0 MOPS, and 10 g/l BSA) with pH adjusted to 7.36 at 38°C. Mesenteric lymphatic vessels (MLV) suitable for observation (i.e., located clear of adipose tissue to allow monitoring of the lymphatic diameter changes and white blood cell flow within vessels) were identified and monitored as described below. Due to the large accumulation of adipose tissue by MLV in both adult and aged animals, only those portions of mesenteric collecting lymphatic vessels, which are located closer to intestine and have been linked by their location as group III by Benoit (3), have been found clear of fat and monitored in this study. After completion of the experiment, the rat was euthanized with pentobarbital (120 mg/kg body wt ip). The average body weight of the animals used in this study was 420 ± 8 g in 9-mo-old rats (n = 6) and 412 ± 33 g in 24-mo-old rats (n = 8). It should be noted that for the F-344 NIH NIA rat strain, the majority of the weight gain occurs before 7 mo of age [Fig. 8 from Turturro et. al (37)]. Therefore, both 9- and 24-mo age groups are within ranges of weight slightly >400 g and are not significantly different.
Experimental Protocol
In animals of both ages, the initial data collection was performed under controlled conditions while mesenteric segment of interest was suffused only by APSS. One or two data sets (duration of 1–2 min each) were recorded within 10 min after stable contractility of the observed lymphatic vessel was a achieved. If more than one data set was recorded for control conditions, these data sets were averaged and presented in the results for diameter analysis as n = 1. After completion of the data collection during the APSS suffusion (control group), the APSS in the observation chamber was replaced with prewarmed 38°C APSS containing the NOS inhibitor Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME; Sigma N 5751) at 100 μM (2, 11, 28, 31, 36, 39, 40) that constantly suffused the exteriorized tissue afterwards. The effectiveness of NOS blockade in rat lymphatic vessels induced by application of l-NAME at this concentration has been numerously demonstrated in previous reports (6, 18, 22–24, 30). The data collection was then repeated in the presence of l-NAME within the first 15 min of its action. One or two data sets (duration of 1–2 min each) were recorded within first 5 min of the l-NAME application. If more than one data set was recorded for “l-NAME (5 min)” group, these data sets were averaged and presented in the results for diameter analysis as n = 1. One or two data sets (duration of 1–2 min each) were then recorded within the 5–15 min following the start of l-NAME application interval. If more than one data set was recorded for “l-NAME (15 min)” group, these data sets were averaged and presented in the results for diameter analysis as n = 1.
Data Collection, Analysis, and Statistics
Principles of the data collection and processing.
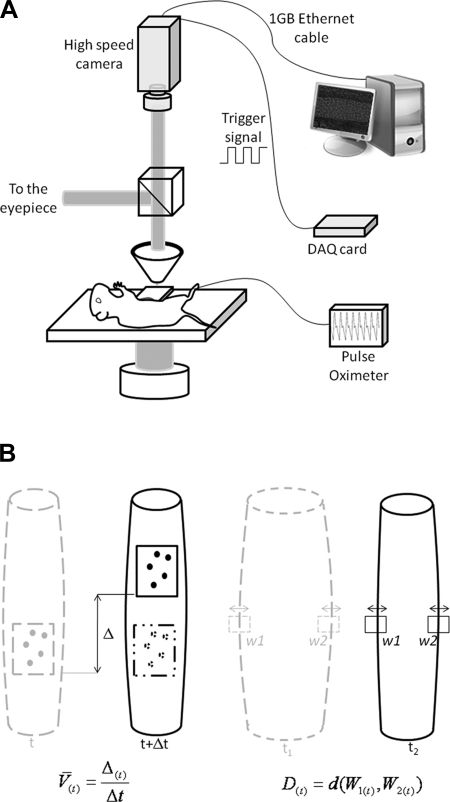
The preparation board was placed on the stage of an intravital microscope equipped with a high speed camera (Phantom V5.2; Vision Research, Wayne, NJ) triggered using a signal generated by a data acquisition board (PCI 6010; National Instruments, Austin, TX). The camera has an internal memory of 6 gigabytes and was set to acquire images at 500 frames per second, which for images of 512 pixels × 512 pixels × 10 bits (≈307 μm × 307 μm) would be enough for acquiring 37.5 s of continuous imaging. Giving the low contraction frequency of the aged control MLV, we needed much longer recording periods to acquire multiple contraction cycles so we triggered the camera to capture images at 500 frames per second for 16-ms intervals and then set it to remain idle for 34 ms. This pattern was repeated throughout the duration of the recording that, for the current study, ranged between 1 and 2 min. Every set of 8 images (16-ms interval) was used to measure 1 velocity and diameter value resulting in a time series of 20 data points per second (1). The camera was adjusted to align the vessel with the vertical direction of the CCD array. To minimize the use of the camera's internal memory and extend the recording time, the camera's field of view in the horizontal direction was limited to the diameter of the vessel plus an additional margin to make sure the vessel stays within the recording area in case of gut motion. The other direction was always set at 307.2 μm (512 pixels) throughout all the experiments. Figure 1 shows a schematic of the imaging setup.
Fig. 1.
A: schematic of the imaging setup showing the animal board with an observation chamber under the objective lens. Data from the high-speed camera were downloaded to the processing computer at the end of each acquisition cycle. B: schematic of the principles of the data processing techniques used. Left: cell tracking principle used to measure white blood cell velocity. The Δ corresponds to the displacement of the cells between 2 frames and Δt is the time period separating the 2 frames. Right: diameter tracking technique where two windows with selected sites of vessel walls were tracked over time and the distance (d) between the windows (W1 and W2) at any acquired time point (t) defines the diameter D. Details of the approach are provided in the methods.
To measure the diameter of the lymphatic vessel under investigation, the vessel walls were tracked throughout the recording using a custom designed automated, correlation-based, algorithm (1, 13). Figure 1B shows a schematic of this process where two windows (W1 and W2) around the vessel walls were selected by the user and the algorithm tracked the movement of these windows throughout the recording. To track the movement of these windows, the template windows in a frame were correlated to search windows in a subsequent frame and the maximum correlation location indicated the new position of the wall. The template window is centered on the current location of the vessel wall while the search window is a larger window centered on the same coordinates in a subsequent frame. The diameter of the vessel is simply the separation between these two windows [d(W1,W2)]. Similarly, the cells inside the vessel lumen were tracked to get velocity readings. The diameter tracking windows were used to set the spatial limits for the cell tracking algorithm that subtracts consecutive frames to eliminate all static features and enhance the contrast of the moving cells within the vessel walls. Then, the displacement of these cells between consecutive frames was calculated using a correlation tracking approach between interrogation windows placed within the vessel wall in consecutive frames. For every 16-ms interval (8 frames), four different values of velocity were generated that were then used in a filtering process to select a single, most reliable, value.
Assuming a Poiseuille flow, and using 1.5 cP as the dynamic viscosity (μ) of lymph (9, 21), we calculated shear stress (τ), using Eq. 1, knowing the average cell velocity (V̇) and the radius of the wall (r) (14):
| (1) |
Lymphatic diameter analysis.
We used the continuous diameter tracings to define systole and diastole in reference to the lymphatic contractile cycle (5, 20, 25, 41). The end-diastolic and end-systolic points in the diameter tracings were recorded for the control conditions and after the application of l-NAME. From the lymphatic end-diastolic and end-systolic diameters, the following lymph pump parameters were calculated: 1) normalized contraction amplitude (nAMP), the difference between the end-diastolic diameter (EDD) and end-systolic diameters (ESD) normalized to end-diastolic diameter, nAMP = (EDD − ESD)/EDD; 2) contraction frequency (FREQ), the number of contractions per minute; 3) ejection fraction (EF), the fraction of end-diastolic volume ejected during a single phasic lymphatic contraction, EF = (EDD2 − ESD2)/EDD2; 4) fractional pump flow (FPF), an index of minute lymph pump flow (minute pumping), FPF = EF × FREQ; and 5) amplitude-frequency product (AFP), AFP = nAMP × FREQ, was calculated as an additional index of minute pumping (12) to strengthen conclusions on observed changes in active lymph flow in selected age groups between different experimental conditions.
Lymph flow analysis.
Using the algorithm described above, we determined diastolic lymph flow velocity, maximal systolic lymph flow velocity, calculated diastolic wall shear stress, and maximal systolic wall shear stress. Additionally, we calculated the rate of changes of the phasic contraction-generated (i.e., active) wall shear stress as the difference between maximal systolic wall shear stress and diastolic wall shear stress divided by the duration of systole (time difference between end-diastolic and the next end-systolic diameter points). Analogous to the AFP (12), we calculated active shear-frequency product (ASFP), which we used as an index of the active minute wall shear stress “load” generated by phasic contractions, which the lymphatic endothelial cells experience per minute. ASFP was calculated as a difference between maximal systolic wall shear stress and diastolic wall shear stress times the contraction frequency.
In this study, we used a high-speed diameter and cell flow monitoring technique (14), which was modified as described above (1). Our current data processing approach operates optimally when ∼10 moving cells are present in the field of view at any moment. However, high levels of the intestinal motility together with high mesenteric fat accumulation in both adult and aged animal groups worsen data collection conditions compared with the young animals previously used in similar type of experiments (14) making it more challenging to measure flow continuously for long periods of time. To avoid recurring fast displacements of vessel wall out of the field of view during the course of experimental data collection, and shadowing of this field by moving fat tissues, we fasted all animals overnight. After fasting, MLV in both 9- and 24-mo-old fasted animals contained single flowing white blood cells within the lymphatic vessels with numerous prolonged periods of the absence of cells. During such periods, the determination of the lymph flow velocity and wall shear stress was not possible even though we observed continuous phasic contractions of the lymphatic vessels. As a result, for lymph flow analysis we implemented the manual selection of the contractile cycles during which flow of cells within lymphatic vessels was detected. Subsequently, values of lymph flow velocities, wall shear stresses, and calculations of active wall shear stress/dt were performed using data obtained from pairs of a diastole period and its subsequent systole, when cells were detected during both consecutive contractile phases. Therefore, in the results, the n number for the data corresponding to the characteristics of lymph flow depicts the number of selected single contraction cycles of “diastole-systole” with n = 38 of suitable contractile cycles during each experimental condition for 9-mo-old animals, and n = 40 of suitable contractile cycles during each experimental condition for 24-mo-old animals.
Comparative Analysis of the Parameters of the Contractile Activity of the Rat MLV In Vivo vs. Those Parameters of the Isolated Rat MLV in Control Conditions and After Nonspecific NOS Blockade Induced by 100 Mm l-NAME in Adult and Aged Groups
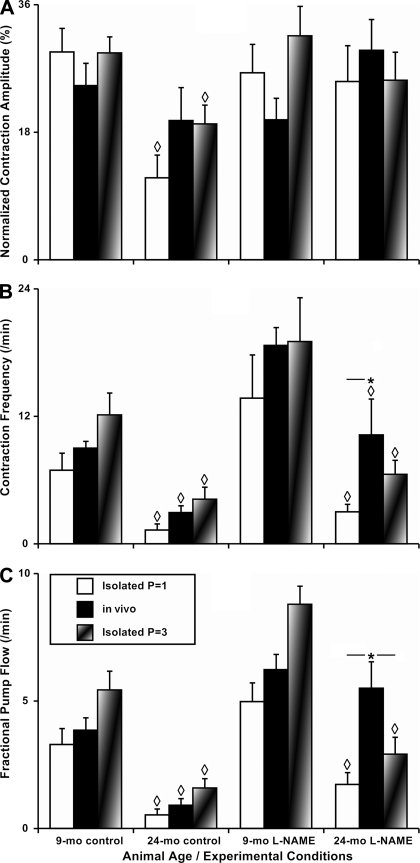
To strengthen our conclusions on the aging-associated alterations of the active lymphatic pumping in rat MLV and to evaluate potential influence on mesenteric lymph pump by aged tissue environment, we performed comparative analysis of our data obtained from current in vivo studies and data obtained from our previous isolated vessel-based experiments performed using similar lymphatic vessels from the similar age groups (30). We analyzed three main parameters of the contractile activity of rat MLV, contraction amplitude, contraction frequency, and FPF. These parameters, the basic contractile characteristics of the active lymph pump, were measured or calculated during both the in vivo and the isolated vessel-based experiments and were used to evaluate contractile inotropy (contraction amplitude), contractile chronotropy (contraction frequency), and the minute productivity of the active lymph pump (FPF). The contraction frequency and FPF were calculated in the current study and in our previous work (30) similarly. Therefore, we pooled these data together to run statistical tests for the combined in vivo plus isolated vessels data set. To compare the values of the contraction amplitude, we normalized the contraction amplitude values from isolated vessel-based experiments (30) to the raw end-diastolic diameter as we did in the current study. The normalization of the contraction amplitude to the passive lymphatic diameter, typical for isolated vessel-based studies, is not available for the in vivo-based data sets. Since isolated vessels in the previous study (30) were pretreated by l-NAME in the same concentration of 100 μM for 15 min, we combined control and l-NAME (15 min) treatment data from the current in vivo study with control and l-NAME treatment data from the isolated vessel studies obtained at levels of transmural pressure of 1 and 3 cm H2O. These levels of mesenteric intralymphatic pressure are the closest to the resting mesenteric end-diastolic lymphatic pressure of ∼2 cm H2O recorded in rat MLV in vivo (Fig. 7 from Ref. 5).
Fig. 7.
Comparison of the parameters of the contractile activity of the adult 9- and 24-mo rat MLV in vivo vs. those parameters of the isolated rat MLV under control conditions and after nonspecific NOS blockade induced by 100 μM of l-NAME. A–C: contractile activity parameters as noted on the vertical axis. Control: parameters of contractility of MLV in vivo or in isolated MLV at intravascular pressures of 1 cm H2O (Isolated P = 1) or at 3 cm H2O (Isolated P = 3). Significant differences (P ≤ 0.05) between active lymph pump parameters: ◊P ≤ 0.05, 9-mo control vs. 24-mo control and 9-mo l-NAME vs. 24-mo l-NAME; *P ≤ 0.05, MLV in vivo vs. isolated MLV (for such comparisons the horizontal line adjacent to * demonstrates pairs of the contractile parameters where the significant differences were observed).
Statistical differences were determined by two-way ANOVA, regression analysis, and paired Student's t-test (JMP software version 9.0.0. for Windows) as appropriate and considered significant at P ≤ 0.05. Only one lymphatic vessel was monitored in each animal.
RESULTS
Contractile Behavior of the MLV and Lymph Flow in Adult and Aged Rats Under Control Conditions
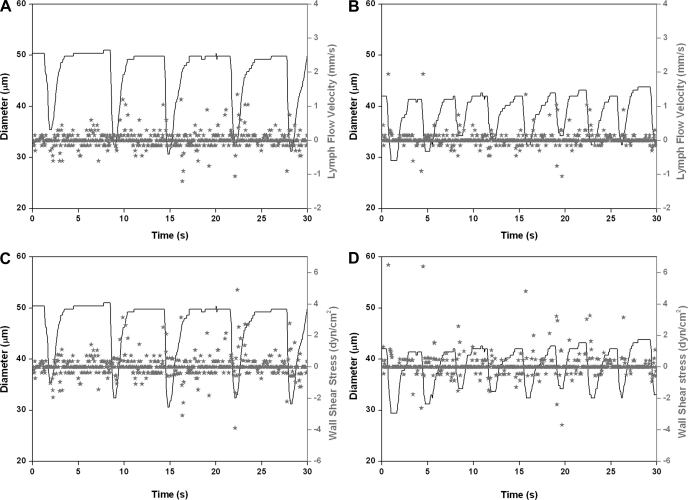
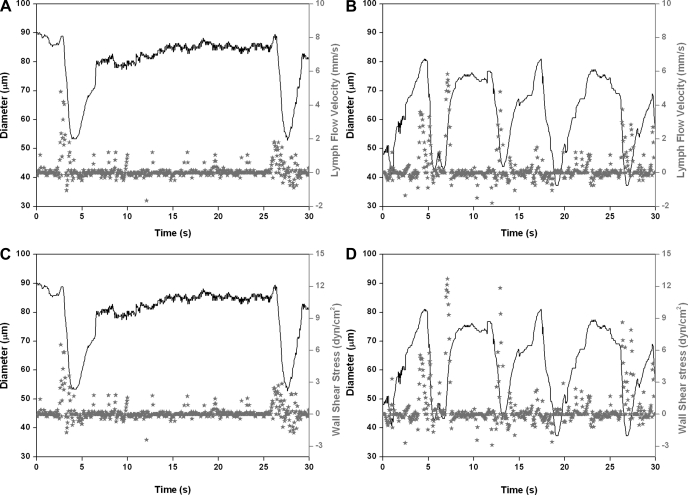
In this study, we performed, for the first time, a detailed evaluation of the contractile activity of MLV and lymph flow in vivo in 9- and 24-mo-old F-344 rats. In the first part of this study, we investigated, in detail, the contractile characteristics of MLV and lymph flow in adult (9-mo; n = 6) and aged animals (24-mo; n = 8) under control conditions. Figure 2, A and C (9-mo rat), and Fig. 3, A and C (24-mo rat), demonstrate representative diameter tracings overlapped with corresponding lymph flow velocity data points (Figs. 2A and 3A) or with wall shear stress data points (Figs. 2C and 3C), under control conditions.
Fig. 2.
Representative diameter tracings of mesenteric lymphatic vessels (MLV) in vivo obtained in 9-mo rat overlapped with corresponding lymph flow velocity data points (A and B), or with wall shear stress data points (C and D) recorded under control conditions (A and C), and after nonspecific nitric oxide synthase (NOS) blockade induced by 100 μM of Nω-nitro-l-arginine methyl ester (l-NAME; B and D).
Fig. 3.
Representative diameter tracings of MLV in vivo obtained in 24-mo rat overlapped with corresponding lymph flow velocity data points (A and B), or with wall shear stress data points (C and D) recorded under control conditions (A and C), and after nonspecific NOS blockade induced by 100 μM of l-NAME (B and D).
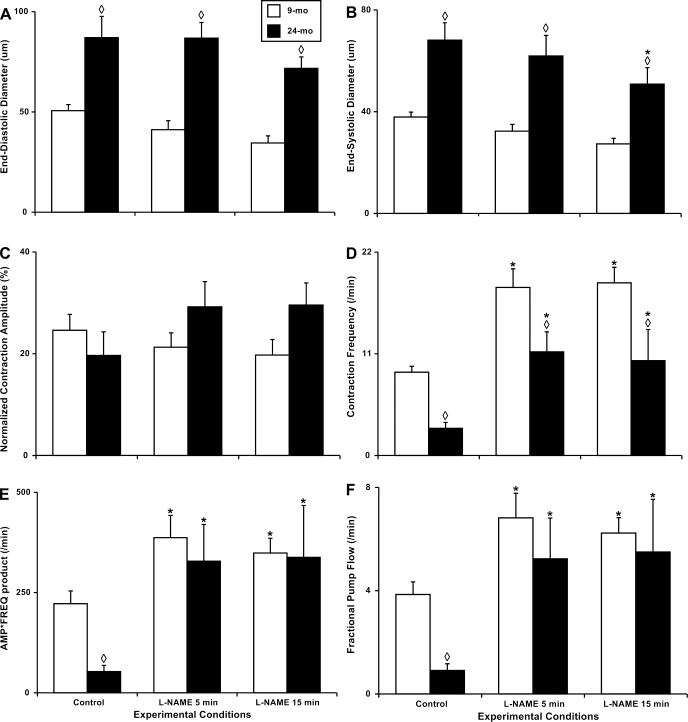
We found that in aged animals the lymphatic vessels diameters from the same location [group III by Benoit (3)] are significantly larger than in adult animals. The end-diastolic diameters and end-systolic diameters in 24-mo-old MLV were 71 and 79% greater than in their adult counterparts (Fig. 4, A and B). At the same time, we observed only minor, nonsignificant, lowering of the contraction amplitude in aged MLV vs. adult vessels under control conditions (20 and 25% of diameter changes during the contractions, respectively; Fig. 4C). The aging-associated negative chronotropy was observed in all aged MLV: we noted threefold decrease in their contraction frequency compared with adult MLV (Fig. 4D). As a result of the described aging-associated changes in lymphatic contractile force (contraction amplitude) and pacemaking (contraction frequency), the minute active lymphatic pumping was significantly lower in aged animals. Both indexes of the lymphatic pumping, AFP and FPF, were significantly diminished in the aged group with AFP 76% lower (∼4.2-fold lower) and FPF 77% lower (∼4.3-fold lower; Fig. 4, E and F) than the adult group.
Fig. 4.
Aging-associated alterations in the parameters of the contractile activity of the 9- and 24-mo rat mesenteric vessels in vivo under control conditions and after nonspecific NOS blockade induced by 100 μM of l-NAME for 5 and 15 min. A–F: contractile activity parameters as noted on the vertical axis. Significant differences between active lymph pump parameters: ◊P ≤ 0.05, 9-mo control vs. 24-mo control and 9-mo l-NAME vs. 24-mo l-NAME; *P ≤ 0.05, 9-mo control vs. 9-mo l-NAME conditions and 24-mo control vs. 24-mo l-NAME conditions.
Table 1 presents the raw data of parameters of the contractile activity of the rat MLV in vivo. All illustrative calculations of the percentage of change of contractile parameters between adult and aged MLV under control conditions described in the text section above are based on the data presented in this table in the lines corresponding to control.
Table 1.
Parameters of the contractile activity of the rat mesenteric lymphatic vessels in vivo in 9 and 24-mo-old animals
| Age/Treatment | End-Diastolic Diameter, μm | End-Systolic Diameter, μm | Contraction Amplitude, % | Contraction Frequency, min−1 | Amplitude-Frequency Product, min−1 | Fractional Pump Flow, min−1 |
|---|---|---|---|---|---|---|
| 9-mo Control | 51 ± 3 | 38 ± 2 | 25 ± 3 | 9.0 ± 0.6 | 222 ± 32 | 3.9 ± 0.5 |
| 9-mo l-NAME (5 min) | 41 ± 4 | 32 ± 3 | 21 ± 3 | 18.2 ± 2.0 | 387 ± 56 | 6.8 ± 1.0 |
| 9-mo l-NAME (15 min) | 34 ± 4 | 27 ± 2 | 20 ± 3 | 18.7 ± 1.7 | 348 ± 37 | 6.2 ± 0.6 |
| 24-mo Control | 87 ± 11 | 68 ± 7 | 20 ± 5 | 2.9 ± 0.6 | 53 ± 16 | 0.9 ± 0.3 |
| 24-mo l-NAME (5 min) | 87 ± 9 | 62 ± 8 | 29 ± 5 | 11.2 ± 2.2 | 328 ± 92 | 5.2 ± 0.6 |
| 24-mo l-NAME (15 min) | 72 ± 6 | 51 ± 6 | 30 ± 4 | 10.3 ± 3.4 | 338 ± 130 | 5.5 ± 1.0 |
Values are means ± SE; n = 6 (9 mo) and n = 8 (24 mo), where n represents the number of animals used for these studies. Control conditions and after administration of 100 μM Nω-nitro-l-arginine methyl ester (l-NAME) are shown.
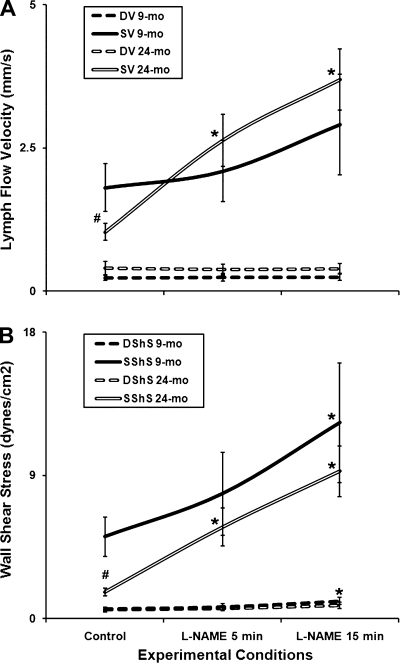
In addition, we analyzed the aging-associated differences in the characteristics of lymph flow in rat mesentery using selected single contraction cycles of “diastole-systole” with n = 38 suitable contractile cycles during each experimental condition for the 9-mo-old animals and n = 40 suitable contractile cycles during each experimental condition for the 24-mo-old animals. While the diastolic lymph flow velocity was slightly, but not significantly, higher in aged MLV, the maximal systolic lymph flow velocity was significantly (43%) lower in aged animals (Fig. 5A). Correspondingly, we did not find any aging-associated changes in calculated diastolic (resting) wall shear stress but during the phasic contractions the lymphatic endothelial cells in aged MLV experienced an approximately threefold reduction of the maximal systolic wall shear stress (Fig. 5B).
Fig. 5.
Aging-associated alterations in lymph flow velocity (A) and wall shear stress (B) in the 9- and 24-mo rat mesenteric vessels in vivo under control conditions and after nonspecific NOS blockade induced by 100 μM of l-NAME for 5 and 15 min. DV, diastolic lymph flow velocity; SV, systolic lymph flow velocity; D Sh S, diastolic wall shear stress; S Sh S, systolic wall shear stress; l-NAME 5 min and l-NAME 15 min, same parameters during the l-NAME (100 μM) administration within first 5 min and between 5 and 15 min, respectively. Significant differences between active lymph pump parameters: #P ≤ 0.05, 9-mo control vs. 24-mo control; *P ≤ 0.05, 9-mo control vs. 9-mo l-NAME conditions and 24-mo control vs. 24-mo l-NAME conditions.
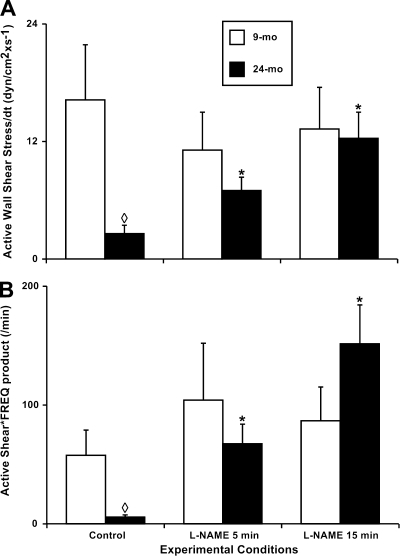
We also compared the rate of change of the phasic contraction-generated (i.e., active) wall shear stress in both adult and aged groups and found a dramatic ∼6.2-fold aging-associated decrease in 24-mo MLV compared with the 9-mo group (Fig. 6A). The phasic contraction-generated (active) minute wall shear stress “load” that the lymphatic endothelial cells experienced a minute, the ASFP, was ∼9.7-fold lower in aged MLV (Fig. 6B).
Fig. 6.
Aging-associated alterations in the indexes of the active wall shear stress namely, active wall shear stress/dt (A) and active shear * frequency product (B) in the 9- and 24-mo rat mesenteric vessels in vivo under control conditions and after nonspecific NOS blockade induced by 100 μM of l-NAME for 5 and 15 min. Significant differences between active lymph pump parameters: ◊P ≤ 0.05, 9-mo control vs. 24-mo control; *P ≤ 0.05, 24-mo control vs. 24-mo l-NAME conditions.
Table 2 presents the raw data of the lymph flow characteristics in the rat MLV in vivo. All illustrative calculations of the percentage of change of parameters of lymph flow between adult and aged MLV under control conditions described in the text section above are based on the data presented in this table in the corresponding lines for control.
Table 2.
Characteristics of the lymph flow in the rat mesenteric lymphatic vessels in vivo in 9- and 24-mo old animals
| Age/Treatment | Diastolic Lymph Flow Velocity, mm/s | Maximal Systolic Lymph Flow Velocity, mm/s | Diastolic Wall Shear Stress, dyn/cm2 | Maximal Systolic Wall Shear Stress, dyn/cm2 | Active Wall Shear Stress/dt, dyn/cm2 × s−1 | Active Wall Shear stress-Frequency Product, min−1 |
|---|---|---|---|---|---|---|
| 9-mo Control | 0.23 ± 0.04 | 1.81 ± 0.42 | 0.61 ± 0.11 | 5.15 ± 1.24 | 16.23 ± 5.6 | 58 ± 21 |
| 9-mo l-NAME (5 min) | 0.24 ± 0.07 | 2.10 ± 0.53 | 0.74 ± 0.19 | 7.85 ± 2.61 | 11.12 ± 3.88 | 104 ± 48 |
| 9-mo l-NAME (15 min) | 0.25 ± 0.06 | 2.91 ± 0.87 | 1.11 ± 0.22 | 12.32 ± 3.75 | 13.26 ± 4.27 | 87 ± 28 |
| 24-mo Control | 0.41 ± 0.12 | 1.03 ± 0.15 | 0.59 ± 0.16 | 1.68 ± 0.23 | 2.59 ± 0.86 | 6 ± 2 |
| 24-mo l-NAME (5 min) | 0.38 ± 0.09 | 2.63 ± 0.46 | 0.64 ± 0.15 | 5.75 ± 1.19 | 6.99 ± 1.36 | 67 ± 17 |
| 24-mo l-NAME (15 min) | 0.39 ± 0.09 | 3.70 ± 0.53 | 0.85 ± 0.20 | 9.27 ± 1.59 | 12.32 ± 2.68 | 152 ± 33 |
Values are means ± SE; n = 38 (9 mo) and n = 40 (24 mo), where n represents the number of lymphatic contractile cycles analyzed. Control conditions and after administration of 100 μM l-NAME are shown.
Contractile Behavior of the MLV and Lymph Flow in Adult and Aged Rats After Local NOS Blockade Induced by Topical Administration of 100 μM l-NAME
Because of the importance of the NO molecule released by lymphatic endothelium for the regulation of lymphatic contractility and flow in adult (6, 18, 24, 28, 32, 33, 38) and aged (22, 23, 30) lymphatic vessels, in the current study we implemented in vivo the local NOS blockade induced by topical administration of 100 μM of l-NAME. We compared the contractile behavior of MLV and lymph flow in adult and aged groups before and after the l-NAME administration. Figure 2, B and D (9-mo rat), and Fig. 3, B and D (24-mo rat), demonstrate representative diameter tracings overlapped with corresponding lymph flow velocity data points (Figs. 2B and 3B) or with wall shear stress data points (Figs. 2D and D) after l-NAME administration.
We found that the NOS blockade with a duration of 15 min induced slight, but not significant, constriction in both adult and aged MLV (Fig. 4, A and B). Only end-systolic diameter in aged MLV was significantly decreased by 25% after 15 min of the l-NAME application. During these small changes in lymphatic diameters, after the NOS blockade in MLV of both aged groups, the difference between the contraction amplitude in adult and aged MLV was reversed by l-NAME from slightly negative (20% lower in aged group) to positive (50% higher in aged group; Fig. 4C). The greatest observed influence of the NOS blockade was its chronotropic effect. While in adult MLV the contraction frequency was significantly increased (∼2.1-fold) 15 min after the l-NAME application, in the aged MLV this increase in the lymphatic contraction frequency was >3.5-fold. The average contraction frequency of the aged MLV treated even only 5 min by l-NAME was 25% (although not statistically significant) higher than the contraction frequency in adult lymphatic vessels under control conditions (Fig. 4D). The main paradox of the influence of the NOS blockade in aged MLV was found when analyzing the indexes of their minute productivity, AFP and FPF, which increased in both age groups as consequence of chronotropic and inotropic influences of the l-NAME administration. In adult vessels, l-NAME application was able to increase significantly both of these indexes by 57 and 59%, respectively. In aged MLV, the influence of the NOS blockade was remarkably greater. Specifically, AFP was increased by 538%, and FPF by 511% compared with the control conditions after 15 min of the l-NAME administration (Fig. 4, E and F). Such influence of the elimination of NO on aged MLV was able to not only compensate for the observed ∼4.2-fold aging-associated depletion in minute productivity in the aged MLV lymph pump in vivo but, after only 5 min of the l-NAME application, was able to maintain this productivity at the same level as the l-NAME-treated adult lymphatic vessels.
Table 1 presents the raw data of parameters of the contractile activity of the rat MLV in vivo. All illustrative calculations of the percentage of change of contractile parameters between adult and aged MLV after the l-NAME administration described in text section above are based on the data presented in this table in the corresponding lines for l-NAME treatment.
Another important result is that the diastolic lymph flow velocity remained unchanged during the l-NAME application in both selected aged groups (Fig. 5A). At the same time, we observed a moderate, nonsignificant, increase in the maximal systolic lymph flow velocity in the adult group; in aged MLV the maximal systolic lymph flow velocity was more than tripled after 15 min of the l-NAME treatment (∼3.6-fold increase), thus being higher than in adult MLV under the same experimental conditions (Fig. 5A). Correspondingly, we did not find any aging-associated changes in calculated diastolic (resting) wall shear stress after the NOS blockade in both selected age groups while the maximal systolic wall shear stress was significantly increased in MLV of both selected ages (139% increase in adult and 452% increase in aged MLV; Fig. 5B). Although the absolute difference (roughly 7 dyn/cm2) is similar, the percentage as a reflection of the basal control level of the maximal systolic wall shear stress does matter. By underlying the degree of changes, we are underlying here the general tendency of lymphatic pumping in aged MLV to be increased dramatically after NO elimination towards values observed in adult animals at the same conditions.
Finally, for this portion of the study, we compared effects of the l-NAME treatment on the rate of change of the phasic contraction-generated (i.e., active) wall shear stress in both adult and aged groups. We found its dramatic ∼4.8-fold increase in 24-mo l-NAME-treated MLV compared with aged MLV under control conditions (Fig. 6A). Such changes after l-NAME administration nearly matched the rate of change in active wall shear stress for the NOS-blocked aged MLV vs. the NOS-blocked adult vessels, for which the rate did not change significantly from control during the l-NAME treatment. As a reflection of these changes the phasic contraction-generated (active) minute wall shear stress “load,” which the aged lymphatic endothelial cells experienced a minute after NOS blockade, showed the ASFP to increase by ∼25.3-fold compared with aged MLV in control conditions. However, the ASFP did not change significantly over the NOS blockade in adult MLV during the same period of time (Fig. 6B).
Table 2 presents the raw data of the lymph flow characteristics in the rat MLV in vivo. All illustrative calculations of the percentage of change of the parameters of lymph flow between adult and aged MLV after the l-NAME administration described in text section above are based on the data presented in this table in the corresponding lines for l-NAME treatment.
Comparative Analysis of the Parameters of the Contractile Activity of the Rat MLV In Vivo vs. Those Parameters of the Isolated Rat MLV Under Control Conditions and After the Nonspecific NOS Blockade Induced by l-NAME at 100 μM in the Adult and Aged Groups
As described in the methods, we performed comparative data analysis of the contractile parameters of rat MLV obtained both in vivo and in isolated vessels experiments. The results of this comparative analysis are presented in Fig. 7.
We found that in control groups of 9- and 24-mo-old MLV all three investigated contractile characteristics namely, contraction amplitude, contraction frequency, and FPF, obtained in isolated vessel-based experiments were not significantly different from those obtained in vivo (Fig. 7, A–C). Moreover, after l-NAME administration, as described in this study for the in vivo experiments and previously (30) for isolated vessels, all three parameters of contractile activity of the 9-mo-MLV were not significantly different between in vivo and isolated vessels data groups with the same degrees and directions of changes both in vivo and under isolated vessel conditions. The primary intriguing difference we found was in contractile chronotropy of the aged MLV in vivo after l-NAME administration compared with the isolated vessel experiments with the same aged MLV under the same experimental conditions (NOS blockade). The contraction frequency of the 24-mo MLV situated in vivo increased ∼3.5-fold compared with the frequency of the same vessels under control conditions (Table 1 and Fig. 7B). As a result of this positive l-NAME-induced chronotropy and the 50% (but nonsignificant) increase of the contraction amplitude, the minute pumping (FPF) of the l-NAME-treated aged MLV was ∼6.1-fold higher than in the same aged vessels under control conditions (Table 1 and Fig. 7C). In other words, the NOS blockade of aged MLV in vivo was able to not only compensate the aging-associated deficiency of minute pumping in aged vessels under control conditions but additionally enhanced their minute pumping all the way up to the levels of NOS-treated adult 9-mo MLV. In isolated aged MLV we observed much weaker, nonsignificant, positive chronotropy and a much smaller increase in pumping after l-NAME administration (30) (Fig. 7, B and C).
DISCUSSION
In this study, we performed, for the first time, a detailed evaluation of the parameters of the contractility of aged MLV in vivo and characterized lymph flow in the aged mesenteric lymphatic network. Results were compared with the same characteristics of lymphatic contractility and flow in adult MLV. We performed these evaluations under control conditions and after a NOS blockade of 100 μM l-NAME. This allowed us to make important conclusions about the comparative roles of the NO-dependent regulatory mechanisms, which control the lymphatic contractility and lymph flow in vivo in the adult and aged body. Importantly, we performed, for the first time, the direct detailed comparison of the characteristics of the lymphatic contractility in vivo and contractile characteristics of the isolated lymphatic vessels exteriorized from the same location for both adult and aged MLV under both control conditions and after NOS blockade.
Contractility of the MLV and Mesenteric Lymph Flow In Vivo in Adult Animals: a Comparison with Data Obtained in Isolated Vessel-Based Experiments
In this study, the usage of the adult animals for control group created additional opportunities to widen our basic knowledge of the normal lymphatic function when we performed a detailed characterization of the lymphatic contractile activity and lymph flow in the adult body, intact and under the conditions of disrupted synthesis of the NO molecule by topical l-NAME administration. Subsequent comparative analysis allowed us to make important conclusions on physiological relevance of the data obtained in isolated vessel-based experiments.
We found that the characteristics of the contractile activity and lymph flow in MLV in vivo in 9-mo-old F-344 rats under control conditions are similar to those reported in literature for young rats (3, 5, 7, 14, 16, 42, 43). In particular, our data matched the contractile characteristics of the mesenteric lymphangions located closer to the intestine, described as group III in Benoit's study (3). As an example, lymphangions studied by Benoit (3) contracted at resting conditions with a frequency of 7.3 ± 1.5 contractions/min, while in our current study contracted at 9.0 ± 0.6 contraction/min. Their average FPF, calculated from Table 2 in Benoit (3), was 4.16, while in our study it was 3.9 (Table 1). Interestingly, in general, the same parameters of the contractility of MLV were found in young Sprague-Dawley rats (3) and adult F-344 rats (current study). Such comparisons provide additional support to our earlier conclusions on the validity of the comparisons between adult F-344 and young Sprague-Dawley rats (23). These findings provide evidence that, in rats, the adult-like contractile activity of lymphatic vessels can be found in comparatively young ages, from 2 to 3 mo, while later this activity stays in a plateau-like state to at least the early adult ages (9-mo-old as in our studies).
Our analysis of the parameters of the mesenteric lymph flow in vivo in adult rats under control conditions demonstrated the same order of magnitude compared with literature data available for the rat mesenteric lymphatic network. In particular, the values of average diastolic lymph flow velocity of 0.23 mm/s and maximal systolic lymph flow velocity of 1.81 mm/s correlated to those observed in young Sprague-Dawley rats reported in several studies (7, 14–17) and in young F-344 rats (17). The average values of diastolic and maximal systolic wall shear stress, 0.61 and 5.15 dyn/cm2, respectively, were also found to be similar to the ranges determined in young Sprague-Dawley rats (14), which strengthen our general conclusions on the similar lymph contractility and flow patterns within young and adult rats of different strains. At the same time, we extended our analyses of lymphatic wall shear stress parameters and implemented, for the first time, the calculations of the rate of changes in active wall shear stress in MLV in vivo. Specifically, this parameter in average in adult animals was 16.23 dyn/cm2 × s−1. Newly implemented estimations of the active wall shear stress-frequency product allowed subsequent comparisons of the relative minute active wall shear stress load in contracting lymphatic vessels under different conditions within selected age groups.
In addition to the comparisons with the literature data obtained on mesenteric lymph flow in vivo, we performed a comparative analysis of the main contractile characteristics of the MLV namely; contraction amplitude, contraction frequency, and FPF, obtained in isolated vessel-based experiments and in vivo experiments. As described in methods and results, we pooled together the data from this study and renormalized contraction amplitude data previously published from isolated vessel-based studies (30). The characteristics of the contractile activity of the adult MLV under control “normal” conditions in vivo were not statistically different from those obtained during the isolated vessel-based experiments (done with the same vessels from similar animals under the same control conditions as in vivo; Fig. 7, A–C, left group of columns). These data attract additional attention by the fact that, after the analysis were performed, the averages of the contraction frequency and FPF in vivo were well positioned in the middle between the same parameters registered in isolated vessels at transmural pressures 1 and 3 cm H2O (Fig. 7). Therefore, it is reasonable to conclude that in our in vivo experiments the MLV were contracting at diastolic (resting) pressure of ∼2 cm H2O. This value matches the values for end-diastolic pressure of ∼2 cm H2O in MLV measured directly by the servo-null micropressure approach (Fig. 7 from Ref. 5). Moreover, comparative analysis of the contractile behavior of aged MLV (under control conditions) in vivo vs. the same type of isolated vessels demonstrated similar results for their aging-associated changes. The averages of contraction frequency and FPF in vivo also remained positioned between the same parameters registered in isolated vessels at transmural pressures 1 and 3 cm H2O as shown in Fig. 7, A–C, in the second to the left group of columns.
After performing this comparative analysis of the major contractile characteristics of the MLV in vivo and vessels isolated from the same location and from the same age/strain animals as those in vivo, we approached the important conclusion regarding methodology of the modern lymphatic research. Data analyses presented in this study confirmed the fact that carefully isolated and properly maintained ex vivo, lymphatic vessels can demonstrate nearly identical characteristics of those in vivo in terms of their contractility and therefore reflect their normal physiological function. In light of our present data, the recent statements that ex vivo studies ‘may not recapitulate “normal” functioning lymphatics’ (27), being initially not supported by any scientific analysis, now can be finally excluded from further considerations.
On the other hand, a careful analytical comparison of the influences of the NOS blockade in the aged MLV in vivo and in isolated vessel-based studies allowed us to determine the important differences (discussed below) between functioning of the lymphatic vessels per se and functioning of the same vessels under the additional influence of the aged tissue microenvironment.
Aging, Contractility of the MLV, and Mesenteric Lymph Flow In Vivo
As we mentioned above, there is little information available in literature dedicated to the aging-associated changes of lymphatic contractility and lymph flow. Therefore, the detailed evaluation of the status of lymphatic contractility and lymph flow in the aged body is an important initial task, which is able to provide additional, still mainly ignored knowledge on unknown but essential, elements of pathogenesis of many chronic disorders, which manifest or worsen with aging. While such evaluations of the aging of lymphatic functions are still in their infancy, we believe that the immediate discovery of aging-associated alterations of the regulatory mechanisms controlling lymph flow in the aged body is not possible before the completion of a careful descriptive characterization of the aging-altered parameters of lymphatic contractility and lymph flow started by this and similar studies.
The initial finding, which attracted our attention while comparing adult and aged MLV in vivo, was the fact that the aged lymphatic vessels from the same anatomical location within the mesenteric lymphatic network in rats of the same body weight have significantly greater resting lymphatic diameter. We consider this enlargement of the aged MLV as an indicator of the deep aging-associated remodeling of the lymphatic wall. With sparse literature data on sclerosis, enlargement, aneurism-like formations, muscle cell atrophy, and muscle layers' disorganization in aged lymphatic vessels (18, 34, 35), our current findings create a solid foundation for detailed follow-up studies on aging-associated alterations of the biomechanical properties of lymphatic wall.
Furthermore, we found a severe negative chronotropic effect of aging on the contractility of MLV in vivo. Specifically, their contraction frequency was depleted while contraction amplitude of the aged vessels was only slightly diminished. Consequently, we observed a profound aging-associated decrease in the minute productivity of the aged MLV in vivo that we mainly linked not to the altered degree of contractile displacement of the lymphatic wall but to the diminished number of the contractile events in aged lymphatic vessels. At the same time, we believe that we discovered additional signs of sluggishness of the contractile events in aged MLV. Substantial reduction in the rate of change in development of phasic contraction-generated wall shear stress indicates the slower development of systolic contractile force in aged lymphatic wall with slower generation of the lymph-propelling local axial pressure gradient. Greatly diminished maximal systolic lymph flow velocity may also be considered as an additional sign of slower and weaker generation of the pressure wave inside aged lymphatic vessels. This aging-associated decrease of the phasic contraction-generated (active) wall shear stress correlates with profound depletion in contraction frequency of the aged MLV. These two overlapping events of lymphatic aging cumulatively induced dramatic depletion in minute active wall shear stress load (∼9.7-fold decrease in ASFP) to lymphatic endothelial cells, which is the first time has been described for aged lymphatic vessels. This aging-associated decrease in minute active wall shear stress load therefore creates a ground for disruption of the phasic contraction-generated shear/NO-dependent regulatory mechanisms of the lymphatic contractile events (6, 18, 22, 24, 30) in aged mesenteric lymphatic network. Currently, there are not enough data to determine the nature of the aging-associated negative chronotropy in the MLV, the existence of which was confirmed both in vivo as well as in isolated vessel-based experiments (Fig. 7). We propose that the negative chronotropy and the consequent depletion of the minute active wall shear stress load may occur due to the yet unknown aging-associated alterations in lymphatic pacemaking. At the same time, this decrease of minute active wall shear stress load will induce a reduction in the amount of phasically generated NO through depletion of already well-described mechanisms (6, 24). Further, the diminished phasic NO release in aged MLV will consequently intensify the slowing of the lymphatic contractile events by reducing the rate of lymphatic diastolic relaxation and diastolic lymphatic filling (24) and therefore will slow the aged lymphatic contractility even more. We observed this in our current studies. However, to date the complex detailed evaluation of the functional importance for this newly discovered phenomenon of the aging-associated reduction in the minute active wall shear stress load in aged MLV remains to be performed.
In addition, we want to underline here that the resting diastolic lymph flow velocity only elevated slightly while the corresponding resting diastolic wall shear stress remained unchanged (due to the vessel enlargement) in aged MLV.
Aging, Contractility of the MLV, and Mesenteric Lymph Flow In Vivo Under Conditions of the NOS Blockade
As we described in methods, in this study we implemented topical application of 100 μM of the nonselective NOS inhibitor l-NAME to eliminate phasic contraction-generated and steady flow-generated release of the NO in both adult and aged MLV. Such experimental conditions we created taking into account the importance of the NO molecule released by lymphatic endothelium for regulation of lymphatic contractility and flow in adult (6, 18, 24, 28, 32, 33, 38) and aged (22, 23, 30) lymphatic vessels. It is crucial to underline here that in both adult and aged mesenteric tissues the topical application of l-NAME did not induce any generalized effects, like increases in lymph formation. This was confirmed by the absence of any changes in resting diastolic lymph flow velocity during the l-NAME administration (Table 2 and Fig. 5A). The importance of the exclusion of the nonspecific effects of the NOS blockade while investigating the contractility of MLV was illustrated by the report of Galanzha et al. (16). In that study, the application of the nonselective NOS blocker Nω-nitro-l-arginine induced constriction in half of the observed rat MLV, while the other half of the vessels demonstrated dilation. This may be considered a result of post-Nω-nitro-l-arginine (l-NNA) increases in lymph formation in part of the animals and as a sign of corresponding increased volumetric load. Unfortunately, the separate evaluation of the diastolic and systolic lymph flow has not been done in that study (16), which creates difficulty for the interpretation of its inconsistent results.
In our study with low levels of lymph flow (fasted rats), we found that the topical l-NAME administration, without increase in diastolic lymph flow (Fig. 5), was able to induce changes in lymphatic tone, chronotropy and minute pumping of 9-mo-old MLV. However, constriction and increase of the contraction frequency during NO absence did not lead to decrease of the contraction amplitude as was found at low levels of flow for solely NO-modulated shear-dependent regulatory mechanisms of the thoracic duct contractility (23, 24). Potentially, in the MLV another mechanism prevents NOS-blockade-induced negative inotropy by maintaining contraction amplitude at the same level. This observation supports our previously expressed hypothesis on the potential existence of an additional yet unidentified shear-dependent, but NO-independent, mechanism for the regulation of lymphatic contractile strength in the MLV (30).
An intriguing consequence of the topical l-NAME administration in aged MLV in vivo is the profound differences depicted in the chronotropic response between in vivo and isolated aged vessels. As shown in Fig. 7B, the contraction frequency of in vivo l-NAME-treated MLV was significantly greater than that during similar treatment in isolated vessels at a transmural pressure of 1 cm H2O. Cumulatively, the minute productivity of the aged MLV in vivo was significantly greater than that in isolated vessels at transmural pressures of 1 and 3 cm H2O during the NOS blockade (Fig. 7C). At the same time, the l-NAME application in aged MLV in vivo was able not only to compensate the aging-associated deficit in minute pumping (compare FPF in vivo in Fig. 7C for the 24-mo l-NAME vs. 24-mo control) but also to increase pumping in aged vessels up to levels of pumping in adult MLV (compare FPF in vivo in Fig. 7C for the 24-mo l-NAME vs. 9-mo control). We conclude that MLV in adulthood possesses considerable contractile reserves such that even “chronically developed” alterations in muscle cell density and orientation in aged lymphatic wall (18) are not able to preclude the ability of the aged MLV to be rapidly (within 5 min) stimulated, under resting conditions, up to the levels of pumping in the adult MLV. At the same time the question remains to be answered, “will the rapid l-NAME (or any other drug)-induced stimulation maintain the levels of aged minute lymphatic pumping similar to the adult counterparts during the periods of the increased volumetric load (lymph formation) in aged body, and, if so, how long will it be maintained”? Moreover, the observations that l-NAME administration in vivo induced ∼2.5-fold greater increase of lymphatic pumping compared with isolated vessels (Fig. 7C) move us to the idea that in aged tissues surrounding the aged MLV, the additional source of some yet unidentified metabolites that stimulate lymphatic contractions and the effect of which may be counterbalanced or blocked by NO release. The post-l-NAME observed increases in diastolic wall shear stress (due to the vessels constriction, but not the diastolic lymph flow velocity, shown in Fig. 5) and minute active wall shear stress load (due to the increases in lymphatic contraction frequency shown in Fig. 6) in aged MLV cannot be linked to the described differences between in vivo and isolated vessels since the NO synthase function was blocked during both experimental conditions. As a matter of fact, we believe that a focus for future follow-up investigations on the nature of the aging-associated changes of pumping of the MLV to a large extent will relate to discovery of the mechanisms of potential interaction of the aged contractile lymphatic vessels and aged tissues surrounding them.
In conclusion, we performed, for the first time, a detailed evaluation of the parameters of the contractility and characterized the lymph flow in vivo in an aged mesenteric lymphatic network under control conditions and after nonselective blockade of 100 μM of the NOS by l-NAME. We found that active pumping of the rat MLV in vivo is severely depleted, predominantly through the aging-associated decrease in lymphatic contractile frequency. Such changes correlate with enlargement of aged mesenteric vessels, which experienced much lower minute active shear stress load than their adult counterparts. At the same time, pumping in aged MLV in vivo may be rapidly increased back to levels of adult vessels predominantly through the increase in contraction frequency induced by NO elimination. Such findings support the idea that in aged tissues surrounding the aged MLV, an additional source of some yet unidentified metabolites that stimulate lymphatic contractions and the effect of which may be counterbalanced or blocked by NO release. The comparative analysis of the control data obtained in experiments with both adult and aged MLV in vivo and in the isolated vessel-based studies clearly demonstrated that carefully isolated and properly maintained ex vivo lymphatic vessels can exhibit nearly identical characteristics to those in vivo for their contractility and thus reflects their normal physiological function.
GRANTS
This work was supported in part by the National Institutes of Health (NIH RO1-AG-030578 and HL-094269) and by the Texas A&M Health Science Center College of Medicine and Department of Systems Biology and Translational Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Akl T, Rahbar E, Zawieja D, Gashev A, Moore J, Coté G. Fast imaging system and algorithm for monitoring microlymphatics. In: Optical Diagnostics and Sensing X: Toward Point-of-Care Diagnostics Proceedings of the SPIE, edited by Coté G. Bellingham, WA: SPIE, 2010, p. 75720K–75720K75726 [Google Scholar]
- 2. Arenas IA, Xu Y, Davidge ST. Age-associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by TNF-α antagonism. Am J Physiol Heart Circ Physiol 290: H1259–H1263, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Benoit JN. Relationships between lymphatic pump flow and total lymph flow in the small intestine. Am J Physiol Heart Circ Physiol 261: H1970–H1978, 1991 [DOI] [PubMed] [Google Scholar]
- 4. Benoit JN, Zawieja DC. Gastrointestinal lymphatics. In: Physiology of the Gastrointestinal Tract, edited by Johnson L. New York: Raven Press, 1994, p. 1669–1692 [Google Scholar]
- 5. Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989 [DOI] [PubMed] [Google Scholar]
- 6. Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol 297: H1319–H1328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brill GE, Galanzha EI, Ul'ianov SS, Tuchin VV, Stepanova TV, Solov'eva AV. [Functional organization of lymphatic microvessels of the rat mesentery.] Ross Fiziol Zh Im I M Sechenova 87: 600–607, 2001 [PubMed] [Google Scholar]
- 8. Bulekbaeva LE. [The volume rate of lymph flow in dogs in postnatal ontogeny.] Zh Evol Biokhim Fiziol 24: 599–600, 1988 [PubMed] [Google Scholar]
- 9. Burton-Opitz R, Nemser R. The viscosity of lymph. Am J Physiol 45: 25–29, 1917 [Google Scholar]
- 10. Chevalier S, Ferland G, Tuchweber B. Lymphatic absorption of retinol in young, mature, and old rats: influence of dietary restriction. FASEB J 10: 1085–1090, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Datte JY, Yapo PA, Offoumou MA. Nitric oxide effect on 5-hydroxytryptamine-induced vasoconstrictions of isolated smooth muscle. Pharmacol Rep 57: 113–120, 2005 [PubMed] [Google Scholar]
- 12. Davis MJ, Lane MM, Davis AM, Durtschi D, Zawieja DC, Muthuchamy M, Gashev AA. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol Heart Circ Physiol 295: H587–H597, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dixon JB, Gashev AA, Zawieja DC, Moore JE, Jr, Cote GL. Image correlation algorithm for measuring lymphocyte velocity and diameter changes in contracting microlymphatics. Ann Biomed Eng 35: 387–396, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13: 597–610, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Fedosov IV, Tuchin VV, Galanzha EI, Solov'eva AV, Stepanova TV. Recording of lymph flow dynamics in microvessels using correlation properties of scattered coherent radiation. Quantum Electronics 32: 970–974, 2002 [Google Scholar]
- 16. Galanzha EI, Brill GE, Solov'eva AV, Stepanova TV. [Nitric oxide in the lymphatic microvessel regulation.] Ross Fiziol Zh Im I M Sechenova 88: 983–989, 2002 [PubMed] [Google Scholar]
- 17. Galanzha EI, Tuchin VV, Zharov VP. In vivo integrated flow image cytometry and lymph/blood vessels dynamic microscopy. J Biomed Opt 10: 054018, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Gashev AA. Basic mechanisms controlling lymph transport in the mesenteric lymphatic net. Ann NY Acad Sci 1207, Suppl 1: E16–20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gashev AA. Lymphatic vessels: pressure- and flow-dependent regulatory reactions. Ann NY Acad Sci 1131: 100–109, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 11: 477–492, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Gashev AA, Orlov RS, Borisov AV, Kliuchin'ski T, Andreevskaya MV, Bubnova NA, Borisova RP, Andreev YA, Erofeev NP, Priklonskaya EG. [The mechanisms of lymphangion interaction in the process of the lymph movement.] Fiziol Zh SSSR Im I M Sechenova 76: 1489–1508, 1990 [PubMed] [Google Scholar]
- 22. Gashev AA, Zawieja DC. Hydrodynamic regulation of lymphatic transport and the impact of aging. Pathophysiology 17: 277–287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gasheva OY, Knippa K, Nepiushchikh ZV, Muthuchamy M, Gashev AA. Age-related alterations of active pumping mechanisms in rat thoracic duct. Microcirculation 14: 827–839, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575: 821–832, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Granger HJ, Kovalcheck S, Zweifach BW, Barnes GE. Quantitative analysis of active lymphatic pumping. In: Proceedings of the VII Summer Computer Simulation Conference. La Jolla, CA: Simulation Councils, 1977, p. 562–565 [Google Scholar]
- 26. Hollander D, Dadufalza V. Influence of aging on vitamin A transport into the lymphatic circulation. Exp Gerontol 25: 61–65, 1990 [DOI] [PubMed] [Google Scholar]
- 27. Kwon S, Sevick-Muraca EM. Noninvasive quantitative imaging of lymph function in mice. Lymphat Res Biol 5: 219–231, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol Regul Integr Comp Physiol 274: R790–R796, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Nadon NL. Maintaining aged rodents for biogerontology research. Lab Anim (NY) 33: 36–41, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation 18: 463–473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakaike R, Shimokawa H, Yasutake H, Sumimoto H, Ito A, Numaguchi K, Egashira K, Takeshige K, Takeshita A. Effects of l-arginine analogs on vasomotion of isolated porcine coronary arteries. Am J Physiol Heart Circ Physiol 268: H1966–H1972, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Ohhashi T, Mizuno R, Ikomi F, Kawai Y. Current topics of physiology and pharmacology in the lymphatic system. Pharmacol Ther 105: 165–188, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Ohhashi T, Yokoyama S. Nitric oxide and the lymphatic system. Jap J Physiol 44: 327–342, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Orlov RS, Borisov AV, Borisova RP. [Lymphatic Vessels. Structure and Mechanisms of Contractile Activity.] Leningrad, USSR: Nauka, 1983, p. 253 [Google Scholar]
- 35. Rabinovitz AJ, Saphir O. The thoracic duct; significance of age-related changes and of lipid in the wall. Circulation 31: 899–905, 1965 [DOI] [PubMed] [Google Scholar]
- 36. Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol 101: 746–752, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 54: B492–501, 1999 [DOI] [PubMed] [Google Scholar]
- 38. von der Weid PY, Zhao J, Van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol 280: H2707–H2716, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Wang A, Nishihashi T, Trandafir CC, Murakami S, Ji X, Shimizu Y, Kurahashi K. Involvement of endothelial cyclo-oxygenase metabolites in noradrenaline-induced contraction of rat coronary artery. Clin Exp Pharmacol Physiol 32: 628–632, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Watanabe S, Yashiro Y, Mizuno R, Ohhashi T. Involvement of NO and EDHF in flow-induced vasodilation in isolated hamster cremasteric arterioles. J Vasc Res 42: 137–147, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Zawieja DC, Greiner ST, Davis KL, Hinds WM, Granger HJ. Reactive oxygen metabolites inhibit spontaneous lymphatic contractions. Am J Physiol Heart Circ Physiol 260: H1935–H1943, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Zweifach B. Micropressure measurements in the terminal lymphatics. In: 7th Europ Conf Microcirculation, Aberdeen, Part II Bibl Anat, edited by Ditzel J, Lewis DH. Aberdeen, Scotland, no. 12, 1972, p. 361–365 [PubMed] [Google Scholar]
- 43. Zweifach B, Prather J. Micromanipulation of pressure in terminal lymphatics in the mesentery. Am J Physiol 228: 1326–1335, 1975 [DOI] [PubMed] [Google Scholar]