Abstract
The cellular receptor usage of numerous human enteroviruses can differ significantly between low-cell-culture-passaged clinical isolates and highly laboratory-passaged prototype strains. The prototype strain of coxsackievirus A21 (CVA21) displays a dual-receptor specificity as determined with a receptor complex consisting of decay-accelerating factor (DAF) and intercellular adhesion molecule 1 (ICAM-1). In this study, the cellular receptor interactions of low-cell-passage CVA21 clinical isolates with respect to their interactions with cell surface-expressed DAF and ICAM-1 were compared to those of the CVA21 prototype (Kuykendall) strain. Dual-receptor usage of DAF and ICAM-1 by CVA21 clinical isolates was confirmed by cell transfection and radiolabeled binding assays. The cellular attachment of clinical and prototype CVA21 strains to cells that coexpressed DAF and ICAM-1 was not additive compared to the viral binding to cells expressing one or other receptor. In fact, the binding data suggest there is an inhibition of CVA21 cellular attachment in environments where high-level coexpression of both DAF and ICAM-1 occurs. Antibody cross-linking of DAF rendered cells susceptible to lytic infection by the CVA21 clinical isolates. In a novel finding, three clinical isolates could, to various degrees, infect and lyse DAF-expressing cells in the absence of DAF-antibody cross-linking and ICAM-1 expression. Sequence analysis of the P1 region of clinical and prototype virus genomes identified a number of coding changes that may contribute to the observed enhanced DAF usage phenotype of the clinical CVA21 isolates. None of the amino acid changes was located in the previously postulated ICAM-1 footprint, a receptor-binding environment that was conserved on the capsid surface of all CVA21 clinical isolates. Taken together, the data suggest that community-circulating strains of CVA21 can infect target cells expressing either ICAM-1 or DAF alone and that such interactions extend tissue tropism and impact directly on viral pathogenesis.
The range and specificity of hosts targeted by viruses are largely determined by the expression of specific cellular receptors. Many viruses with different structures and host target cell ranges use multiple receptors to attach and enter susceptible target cells (9, 30, 32, 41, 43). Using more than one cellular receptor, therefore, may be of evolutionary advantage, enabling increased chances for such viruses to infect susceptible cells. In addition, it has been found that the cellular receptor usage of many viruses, notably human enteroviruses, differs significantly between clinical isolates and highly laboratory-passaged prototype strains. Notably clinical isolates of coxsackievirus B3 and some echoviruses exhibit differences in their interactions with cell surface-expressed decay accelerating factor (DAF) compared to their prototype strain (4, 38).
The prototype strain of coxsackievirus A21 (CVA21; Kuykendall) infects susceptible cells via interactions with a receptor complex consisting of intercellular adhesion molecule 1 (ICAM-1) and decay-accelerating factor (DAF) (33, 34). The N-terminal domain of ICAM-1 is responsible for CVA21 binding (34) and is also the cell attachment receptor for the major group human rhinoviruses (13). ICAM-1 functions as the internalization component of the CVA21 prototype strain receptor complex (33) due to its capacity to induce capsid conformational changes resulting in the formation of A particles (33), which is considered to be an essential prerequisite in the picornaviral infection process (12).
The second component of the CVA21 cellular receptor complex is DAF, a 70-kDa glycosylphosphatidylinositol-linked complement regulatory protein consisting of four extracellular short consensus repeats (SCRs) (25). Although the Kuykendall prototype strain of CVA21 requires the presence of ICAM-1 for cell entry, it uses DAF as a membrane sequestration receptor (34). DAF binding is a characteristic of many enteroviruses, including coxsackievirus B1 (CVB1), CVB3, CVB5, enterovirus 70, and numerous echoviruses (EVs) (2, 5, 15, 26, 32). The widespread usage of DAF as an attachment receptor may, however, be viewed as a pathogenic artifact having evolved as an adaptation to multiple passages in high-DAF-expressing cell cultures. In general, capsid interactions with DAF alone do not permit infection unless DAF is cross-linked by specific anti-DAF monoclonal antibodies (MAbs) (31). However, despite the inability of DAF interactions to mediate cell infection, clinical isolates of both CVB3 and EV11 possess a DAF-binding phenotype that has been postulated to increase virulence (4, 5, 19, 38).
The capacity of some viruses to use two or more receptors in a complex suggests that they may use the individual receptor components together in a manner that improves the likelihood of successful infection. This may be the case in the CVA21 interaction with DAF and ICAM-1, where both molecules are spatially closely associated when expressed simultaneously on a host cell (34). As noted above, the CVA21 prototype can use DAF in a different manner from a cell sequestration receptor to that of an internalization receptor if DAF is cross-linked on the cell surface (31). Cross-linked DAF-mediated CVA21 internalization demonstrates, however, slower kinetics than that mediated via ICAM-1 interactions, suggesting different mechanisms of viral internalization (31, 33). CVA21 lytic infection mediated by cross-linked DAF has been postulated to involve caveolae (31), most probably in the absence of a capsid conformational change since direct interactions with DAF alone are not sufficient to induce capsid conformational changes of numerous enteroviruses (27, 31). In further support of this new enteroviral internalization mechanism, EV type 1 (EV1) and the DAF-binding EV6 and EV11 have recently been shown to use caveola-dependent cell entry mechanisms (18, 37).
The DAF-binding footprint on the capsid surface of enteroviruses is difficult to locate, since many viruses bind to different epitopes on the DAF molecule. Using cryoelectron microscopy, the DAF-binding site on the surface of the EV7 virion was recently mapped as being close to the icosahedral twofold axes outside the capsid canyon capsid (14). Because of the structural similarities between the capsids of EV7 and CVB3 (11, 22), it was suggested that the DAF-binding footprint was similarly located in the twofold depression on the CVB3 capsid (14). For EV11, however, studies using bioselection of non-DAF binding mutants reported that the DAF binding epitopes on EV11 were located in a region close to the fivefold axes (38). Nevertheless, both studies support the notion that DAF binding occurs on the capsid surface and not in the capsid canyon. Furthermore, it has been suggested that DAF capsid interactions are normally involved in attachment of the virus rather than in initiating capsid conformational changes and subsequent uncoating (14, 38).
The major focus of the present study was to investigate the nature of the receptor usage of clinical CVA21 stains of virus obtained in routine diagnostic investigation of the community-based illnesses and that have had only minimal passage in cell cultures. In addition, we determined the nucleotide sequences of the P1 genomic region of the clinical CVA21 isolates, primarily to find out whether a previously identified ICAM-1 binding footprint defined for the prototype strain was conserved on the capsid surfaces. We also report the unexpected and novel finding that low-passage clinical CVA21 isolates can lytically infect DAF-expressing cells in the absence of either antibody cross-linking of DAF or ICAM-1 expression.
MATERIALS AND METHODS
Viruses and cells.
CVA21 prototype strain Kuykendall and the three clinical isolates (272101, 272598, and 275238) were obtained from Margery Kennett, Enterorespiratory Laboratory, Fairfield Hospital, Melbourne, Victoria, Australia. Isolate 272101 was obtained from a 26-year-old man infected with human immunodeficiency virus, isolate 275238 was from a 3-month-old baby deceased due to sudden infant death syndrome, and isolate 272598 was from an 8-year-old boy with an acute episode of croup. The clinical CVA21 isolates were passaged approximately three times in HeLa cells and/or human lung fibroblasts or HeLa-T cells and once in ICAM-1-expressing rhabdomyosarcoma (RD) cells (RD-ICAM-1) (34). The prototype strain of CVA21 was passaged approximately ten times in HeLa and/or human lung fibroblasts or HeLa-T cells and three to four times in RD-ICAM-1 cells.
HeLa cells were obtained from the American Type Culture Collection, Manassas, Va. Chinese Hamster Ovary (CHO) cells were obtained from Bruce Loveland, Austin Research Institute, Heidelberg, Victoria, Australia.
Antibodies.
The anti-ICAM-1 MAb WEHI specific for the first domain of ICAM-1 (1) was supplied by Andrew Boyd, Queensland Institute of Medical Research, Queensland, Australia. Anti-DAF MAb IA10 (immunoglobulin G2a [IgG2a]) recognizes the first SCR of DAF, VIIIA7 (IgG1) recognizes the third SCR and parts of the second SCR (16), IH4 (IgG1) recognizes the third SCR of DAF (7), while IIH6 (IgG1) recognizes the fourth SCR (16). MAbs IA10, VIIIA7, and IIH6 were gifts from Taroh Kinoshita, Department of Immunoregulation, Osaka University, Osaka, Japan. MAb IH4 was a gift from Bruce Loveland.
Viral purification and radiolabeled binding assays.
Confluent monolayers of RD-ICAM-1 cells in six-well tissue culture plates were inoculated with 500 μl of the appropriate strain of CVA21 [105 50% tissue culture infective dose(s)/ml] for 1 h at 37°C. Unbound virus was removed by three washes with methionine-cysteine-free Dulbecco modified Eagle medium (DMEM; ICN Biochemicals, Aurora, Ohio) and, after the addition of methionine-cysteine-free DMEM, cell monolayers were incubated for a further 2 h before the addition of 300 μCi of [35S]methionine-cysteine Trans-Label (ICN Radiochemicals, Irvine, Calif.). Infected monolayers were then incubated at 37°C in a 5% CO2 environment for 12 h. After three freeze-thaw cycles, viral lysates were purified in 5 to 30% sucrose gradients (32). Fractions were collected from the bottom of each tube and monitored by liquid scintillation counting on a 1450 Microbeta TRILUX (Wallac, Turku, Finland) to locate the 160S peak fractions to be used in radiolabeled virus-binding assays.
Radiolabeled virus-binding assays with HeLa cells were performed in 24-well tissue culture plates as described previously (32). Virus-binding assays of transfected CHO cells were performed with cell suspensions. Approximately 106 cells in 800 μl of DMEM containing 1% bovine serum albumin were incubated in the presence of 300 μl (∼105 cpm) of [35S]methionine-labeled virus for 2 h at room temperature. Cells were then washed four times with serum-free DMEM dissolved in 200 μl of 0.2 M NaOH-1% sodium dodecyl sulfate before the amount of [35S]methionine-labeled virus bound was determined by liquid scintillation counting (32). When required, cells were preincubated with 20 μg of anti-DAF or anti-ICAM-1 MAbs or phosphatidylinositol-specific phospholipase C (PI-PLC) (Sigma Chemicals, Sydney, New South Wales, Australia)/ml (i.e., 1.0 U/5 × 106 cells) (8) for 1 h at 37°C prior to the addition of radiolabeled virus.
Virus infectivity assays.
RD and RD-ICAM-1 cell monolayers in 96-well tissue culture plates were inoculated with 10-fold serial dilutions (100 μl/well in quadruplicate) of CVA21 in DMEM containing 1% fetal calf serum and then incubated at 37°C in a 5% CO2 environment for 48 h. Cell survival was quantitated by staining inoculated monolayers with 100 μl of a crystal violet-methanol solution (0.1% crystal violet, 20% methanol, 4.0% formaldehyde in phosphate-buffered saline [PBS])/well for 24 h. After a wash in distilled water, the relative absorbance of the stained cell monolayer was read on a multiscan enzyme-linked immunosorbent assay plate reader (Flow Laboratories, McLean, Va.) at 540 nm. Fifty percent endpoint titers were calculated by the method of Reed and Muench (28) by scoring wells as positive if the absorbance values were less than three standard deviations (SD) of the control no-virus wells.
When cell monolayer pretreatment with anti-receptor MAb was required, cells were incubated in the presence of MAb (20 μg/ml) for 1 h at 37°C. Cells monolayers were then inoculated with quadruplicate samples of the appropriate virus and incubated at 37°C in a 5% CO2 environment for 48 h before being stained as described above.
Cell transfection.
CHO and RD cells were transfected to express ICAM-1 and/or DAF as described previously (34). Briefly, 500-μl aliquots of cells (5 × 106 to 1 × 107 cells/ml) were resuspended in electroporation buffer (20 mM HEPES, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2PO4, 6 mM glucose [pH 7.05]) and mixed with 75 μg of pEF-BOS (21) encoding DAF or ICAM-1 and 5 μg of pcDNA.neo in electroporation cuvettes (Bio-Rad, Richmond, Calif.). Cells were pulsed at 300 V and 250 μF with a Bio-Rad gene pulser, seeded into tissue culture flasks, and incubated at 37°C for 48 h until the formation of confluent monolayers. Receptor-expressing transfected cells were selected in DMEM containing G418 (400 μg/ml) and further enriched by fluorescence-activated cell sorting with the appropriate anti-receptor MAbs.
Flow cytometry.
DAF and ICAM-1 surface expression on transfected cells was analyzed by flow cytometry. Briefly, 106 dispersed cells were incubated on ice with the appropriate MAbs (5 μg/ml in PBS) for 20 min. Cells were then washed with PBS, pelleted at 1,000 × g for 5 min, resuspended in 100 μl of R-phycoerythrin-conjugated F(ab′)2 fragment of goat anti-mouse immunoglobulin diluted in PBS (Dako A/S), and incubated on ice for 20 min. Cells were washed and pelleted as described above, resuspended in PBS, and analyzed for DAF and ICAM-1 expression with a FACStar analyzer (Becton Dickinson, Sydney, Australia).
Viral RNA sequence analysis.
CVA21 isolates were propagated in confluent monolayers of RD-ICAM-1 cells. Viral cell lysates were precleared by low-speed centrifugation, and virions in the supernatant were pelleted by ultracentrifugation in an SW41 Ti rotor for 3 h at 40 000 rpm at 4°C. Viral pellets were resuspended in TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 7.5]), RNA was isolated from each strain with Trizol Ls reagent (Gibco-BRL Life Technologies), and the P1 regions of the genomes were amplified by a previously described long-distance strategy (17). The nucleotide sequences of the CVA21 P1 region were determined from purified PCR amplicons by using a primer-walking strategy and by using an ABI Prism BigDye terminator cycle sequencing ready-reaction kit (PE Biosystems) (17) according to the manufacturer's instructions. Nucleotide sequence alignments were generated by using the CLUSTAL X program (39).
Nucleotide sequence accession numbers.
The nucleotide sequences of the P1 region of CVA21 clinical isolates 272101, 275238, and 272598 described in the present study have been submitted to GenBank under accession numbers AY319942, AY319943, and AY319944, respectively.
RESULTS
Clinical strains of CVA21 bind to DAF and ICAM-1.
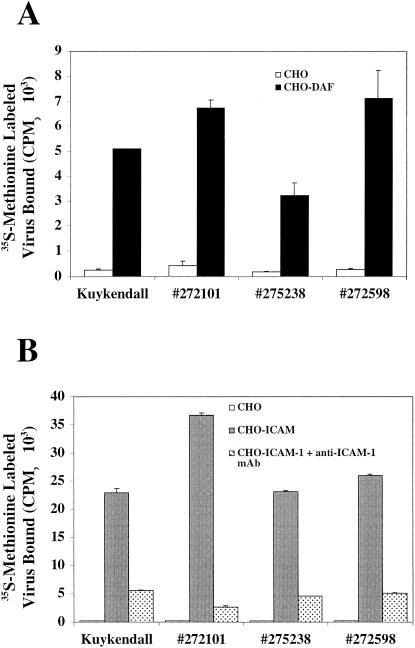
To determine whether clinical isolates of CVA21 bind to DAF and ICAM-1 in a manner that is either similar to or different from that of the prototype Kuykendall strain, CHO cells stably transfected to express either DAF or ICAM-1 (33) were used in radiolabeled virus-binding assays. No significant binding to CHO cells in the absence of DAF or ICAM-1 was observed for any of the CVA21 isolates (Fig. 1). All of the CVA21 strains bound to CHO cells expressing DAF (Fig. 1A), an interaction previously demonstrated for the prototype CVA21 Kuykendall strain (33). As expected, all clinical CVA21 isolates also bound to ICAM-1 expressed on the surface of CHO cells (Fig. 1B). Confirmation of the specificity of the CVA21-ICAM-1 interaction was verified by the action of an anti-ICAM-1 domain 1-specific MAb, which completely abolished virus binding to ICAM-1 (Fig. 1B). Overall, these results confirm that clinical isolates of CVA21 bind to two separate cellular receptors, DAF and ICAM-1, in a manner similar to that of the prototype strain.
FIG. 1.
Binding of [35S]methionine-labeled CVA21 prototype (Kuykendall) and three CVA21 clinical isolates (272101, 275238, and 272598) to DAF-expressing CHO cells (A) and ICAM-1-expressing CHO cells (B) in the presence or absence of an anti-ICAM-1 MAb. The levels of [35S]methionine-labeled virus bound were determined by liquid scintillation counting. The results are expressed as the means of triplicate samples plus the SD.
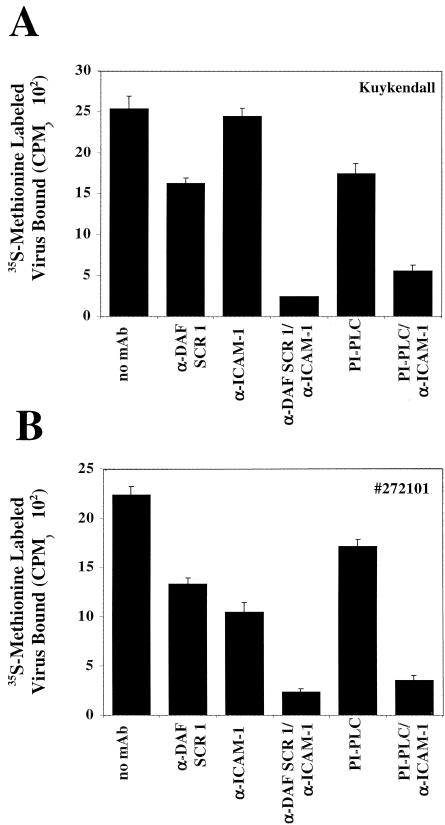
To further characterize the interaction of clinical isolates of CVA21 with DAF/ICAM-1, CVA21 virus-binding assays were undertaken on HeLa cells ubiquitously coexpressing DAF and ICAM-1. CVA21 binding was assessed by MAb blockade of individual receptors or MAb blockade in combination (Fig. 2). The cellular attachment of the prototype Kuykendall strain (34) (Fig. 2A) was compared to that of clinical CVA21 isolate 272101 (Fig. 2B), and both exhibited high-level binding to HeLa cells in the absence of MAb receptor blockade (Fig. 2). Specific MAb blockade of DAF SCR 1 partially blocked virus binding; however, it was unable to completely abolish viral attachment due to interaction with ICAM-1. When access to ICAM-1 was inhibited by MAb blockade, virus binding was reduced, more so for the clinical isolate 272101(Fig. 2B) than the prototype, but not completely inhibited due to alternate viral attachment to DAF. The specificity of the clinical and prototype strains of CVA21 for the N-terminal domains of the DAF and ICAM-1 was demonstrated by the capacity of anti-DAF SCR 1 and anti-ICAM-1 domain 1 MAbs to inhibit virus attachment to the same degree as pretreating the cells with a combination of phosphatidylinositol-specific phospholipase C (which cleaves glycosylphosphatidylinositol-linked proteins) and an anti-ICAM-1 MAb. Taken together, these results confirm that clinical isolate 272101, like the prototype strain, binds to the first SCR of DAF and the N-terminal domain of ICAM-1.
FIG. 2.
Binding of [35S]methionine-labeled CVA21 prototype Kuykendall (A) and clinical isolate 272101 (B) to HeLa cells in the presence of an anti-DAF SCR 1 MAb, an anti-ICAM-1 domain 1 MAb, and/or PI-PLC treatment. The levels of [35S]methionine-labeled virus bound were determined by liquid scintillation counting. The results are expressed as the means of triplicate samples plus the SD.
CVA21 binding to DAF and ICAM-1 is not additive.
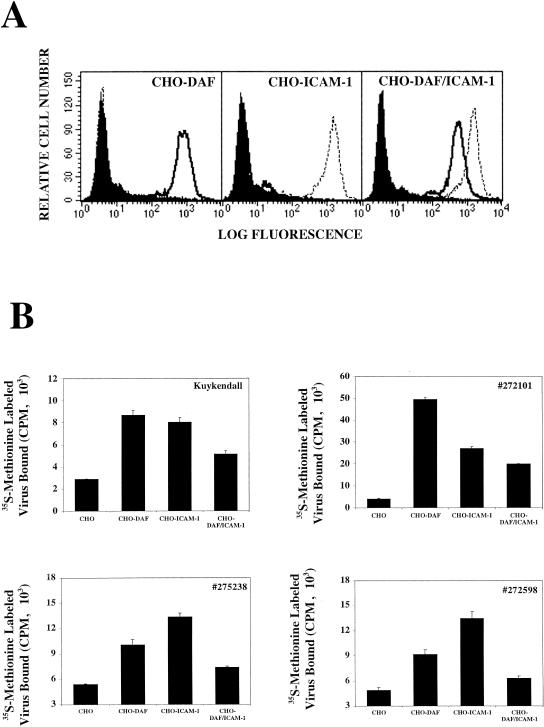
The capacity of the CVA21 clinical isolates to bind either DAF or ICAM-1 alone or in combination was assessed to determine whether the presence of both receptors on the surface of a host cell contributed to additive virion cell attachment. To address this question, CHO cells were transfected to express either DAF or ICAM-1 alone or in combination. Flow cytometric analysis revealed comparable levels of DAF or ICAM-1 expression on cells expressing either receptor alone or in combination (Fig. 3A). Minimal levels of background binding to CHO cells were observed for all CVA21 strains. Significant levels of binding to individually expressed DAF or ICAM-1 were exhibited by all CVA21 strains (Fig. 3B). Surprisingly, the amount of radiolabeled virus that bound to CHO cells coexpressing both DAF and ICAM-1 was significantly reduced compared to the amount bound when either of these receptors was expressed alone (Fig. 3B).
FIG. 3.
Binding of [35S]methionine-labeled CVA21 prototype (Kuykendall) and three CVA21 clinical isolates (272101, 275238, and 272598) to CHO cells expressing either DAF or ICAM-1 alone or in combination. (A) Flow cytometric analysis of surface DAF and ICAM-1 expression. Transfected CHO cells were incubated with either conjugate alone, anti-DAF MAb (IH4), or anti-ICAM-1 MAb (WEHI), and the specific binding was measured on a FACStar analyzer. The filled histograms represent the binding of the conjugate; the solid-line histograms represent the binding of the anti-DAF MAb, and the dotted-line histograms represent the binding of the anti-ICAM-1 MAb. (B) The levels of [35S]methionine-labeled virus bound were determined by liquid scintillation counting. The results are expressed as the means of triplicate samples plus the SD.
Clinical isolates of CVA21 can induce lytic infection of ICAM-1-negative cells via interactions with DAF.
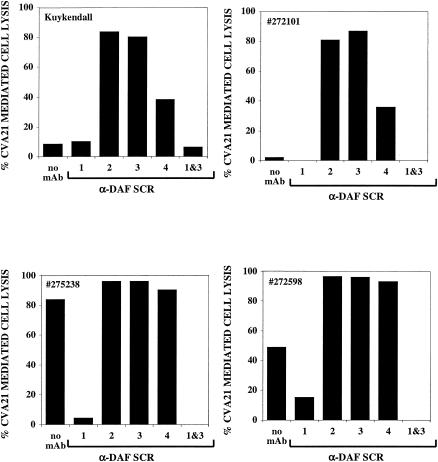
ICAM-1 is the major determinant for successful host cell entry of the CVA21 prototype strain (33). However, CVA21-mediated lytic infection of cells lacking ICAM-1 expression is possible in the presence of MAb cross-linked DAF (35). We investigated whether the clinical CVA21 isolates could lytically infect ICAM-1-negative cells via discrete interactions with cross-linked DAF. Monolayers of RD cells were either not treated or pretreated with specific MAbs directed against the individual SCRs 1, 2, 3, or 4 or a combination of anti-SCR 1 and SCR 3 of DAF prior to challenge with a single input multiplicity of CVA21. CVA21-mediated lytic infection was observed in cultures of RD cells pretreated with MAbs directed against DAF SCR 2, 3, and 4 (Fig. 4). Confirmation that, indeed, specific CVA21 capsid/DAF interactions mediated the lytic cell infection was supplied by findings that the addition of an anti-SCR 1 DAF MAb to cells pretreated with anti-SCR 3 DAF MAb completely blocked cell lysis.
FIG. 4.
Lytic infection of ICAM-1-negative RD cells by the CVA21 prototype (Kuykendall) and clinical isolates (272101, 275238, and 272598) in the presence of anti-DAF MAbs IA10 (SCR 1), VIIIA7 (SCR 2), IH4 (SCR 3), and IIH6 (SCR 4). Anti-DAF MAbs (20 μg/ml) were added to monolayers of RD cells cultured in 96-well plates. After incubation for 1 h at 37°C, the cells were challenged with ∼103 50% tissue culture infective dose(s) of the CVA21 isolates/well and incubated for 48 h at 37°C. Cell lysis was assessed by staining the cell monolayers with a crystal violet-methanol solution and then measuring the absorbance at 540 nm. The results are expressed as the mean percentage lysis of duplicate wells.
Interestingly, two of the clinical CVA21 isolates, 275238 and 272598, were capable of lytically infecting the ICAM-1-negative RD cells in the absence of cross-linking by anti-DAF MAbs (Fig. 4). In general, enterovirus binding to DAF is regarded as the sequestration of virions for interactions with additional internalizing receptors; consequently, there have been no conclusive reports to date demonstrating cell lytic infection mediated solely via interactions with DAF (15, 31, 38). The capacity of CVA21 clinical isolates 275238 and 2727598 to lytically infect cells in the absence of both antibody cross-linking of DAF and ICAM-1 is the first demonstration of such a receptor usage. The complete inhibition of cell lysis (Fig. 4) and significant reductions in progeny virus production (data not shown) by these strains of CVA21 after blockade with an anti-DAF SCR 1 MAb further confirms the integrity of this finding.
To continue the analysis of this novel DAF usage, clinical and prototype strains of CVA21 were titrated for lytic infectivity on monolayer cell cultures expressing DAF alone (RD) or in combination with ICAM-1 (RD-ICAM-1). All strains of CVA21 exhibited high levels of cell lytic activity in cells expressing both DAF and ICAM-1, whereas only isolates 275238 and 2727598 were observed in RD cells expressing DAF only to induce lytic titers comparable to those obtained in RD-ICAM-1 cells (data not shown). In fact, isolate 275238 yielded a lytic titer in RD cells ∼20-fold higher than in RD-ICAM-1 cells. Interestingly, at high virus input multiplicities, isolate 272101 also exhibited a significant level of lytic infection of RD cells expressing DAF only. The minimal but detectable level of lytic activity in RD cells by the prototype strain may be due a minority population of virions with an enhanced DAF usage phenotype (Fig. 4).
Analysis of the ICAM-1 binding footprint of CVA21 clinical isolates.
Since all strains of CVA21 examined herein exhibited a strong ICAM-1 attachment-internalization phenotype (Fig. 1), we investigated whether they possessed a conserved ICAM-1 binding footprint. The residues constituting the CVA21-ICAM-1 receptor binding footprint have previously been identified by using cryoelectron microscopy with purified ICAM-1 and prototype Kuykendall virions (44). Amino acid sequence analysis of the P1 coding regions of all CVA21 strains revealed the presence of a conserved ICAM-1 footprint that was identical to the previously published footprint except for a conservative coding change at VP2 168 of an Ala to Val (Fig. 5).
FIG. 5.
Multiple-sequence alignments of the VP1, VP2, and VP3 capsid proteins for the prototype CVA21 Kuykendall strain and clinical isolates 272101, 272598, and 275238. Amino acid changes in the clinical isolates relative to the Kuykendall strain are represented in boldface. Sequence alignments were generated by using the CLUSTAL X program (39). Individual amino acids that constitute the CVA21-ICAM-1 binding footprint are indicated by closed boxes (44). VP2 A168 is indicated by a black filled box.
In an attempt to explain the increased capacity of CVA21 clinical isolates to lytically infect DAF-expressing cells in the absence of ICAM-1 (Fig. 4) with respect to the prototype, we searched for amino acid differences outside the ICAM-1 binding footprint (Fig. 5). A number of amino acid changes were detected in the P1 region between the three CVA21 clinical isolates and the prototype strain (Fig. 5). No amino acid changes were detected between any of the CVA21 strains in the VP4 coding region (data not shown). In the VP3, VP2, and VP1 capsid proteins 13 identical changes in the same positions were detected in all clinical isolates with respect to the prototype strain. In addition, isolate 272101 exhibited a dissimilar change at position VP3 239 with an Ala compared to a Ser in the other two clinical isolates and possessed a further nine separate amino acid changes with respect to the prototype strain scattered throughout VP1, VP2, and VP3 (Fig. 5). At the amino acid level isolates 272598 and 272238 were identical, whereas at the nucleotide level a number of silent mutations between the two were detected (data not shown).
DISCUSSION
Productive cell infection by the prototype strain of CVA21 is mediated by discrete interactions with surface-expressed DAF and ICAM-1 (33). In this relationship DAF functions to sequester CVA21 to the cell surface for subsequent interactions with ICAM-1 that induce capsid conformational changes and cell entry (34). However, the question as to whether multiple in vitro cell passages contribute to this pattern of receptor usage or not is a subject of much contention. In particular, the DAF-binding phenotype was an area of much conjecture, considering that the phylogenetically related prototype group A coxsackieviruses A13, A15, A18, and A20, which also use ICAM-1 as a cell internalization receptor, do not bind to surface-expressed DAF (23). Investigations were thus undertaken to observe whether the DAF-binding phenotype of the CVA21 prototype strain Kuykendall was conserved in low in vitro-passaged clinical isolates of CVA21 or was simply an artifact of multiple passage in cell cultures.
The radiolabeled virus-binding assays described here indicate that three clinical isolates of CVA21 showed receptor attachment patterns similar to that of the prototype CVA21 strain (Kuykendall), patterns characterized by the capacity to bind independently to either DAF or ICAM-1 (Fig. 1). The inability of a MAb blockade directed against either DAF or ICAM-1 to completely inhibit virus binding (Fig. 2) indicates that both receptors play an essential role in the attachment-infection process of clinical isolates of CVA21. Studies of CVA21 binding in environments of high-level coexpression of DAF and ICAM-1 demonstrate a reduced degree of virus binding compared to environments in which the two receptors are expressed individually (Fig. 3). These findings suggest that high-level coexpression of multiple receptors may indeed be inhibitory to optimal lytic infection. It is possible that the close proximity of DAF and ICAM-1, when coexpressed on a host cell surface (34), results in steric hindrance, causing a reduction in the availability of the receptor-binding sites. If this is the case, it can be reasoned that, whereas high-level expression of both receptors on a host cell does not necessarily correlate with an increased attachment level, an environment with dissimilar expression levels of the two different cellular receptors for the one virus may be potentially advantageous. Such an environment is likely to occur on the mucosal surface of the human enteric tract, where DAF expression is ubiquitous (25) and at significantly higher levels than ICAM-1, whose endogenous expression level is relatively low (42), awaiting induction by appropriate cytokines (40).
An unexpected finding of the present study was the capacity of low-passage clinical CVA21 isolates to utilize DAF interactions in a more functional role by of lytically infecting RD cells solely via DAF binding in the absence of antibody cross-linking (Fig. 4). A possible explanation for these novel findings is that the virus capsids of the clinical CVA21 isolates are able to cross-link DAF in a more substantial fashion than the prototype strain, thereby permitting virus internalization in a mechanism similar to the artificial cross-linking action of anti-DAF MAbs (Fig. 4). Similarly, differences in receptor usage have been observed between CVB3 prototypes and low-passage clinical isolates (4). More recently, variation in the utilization of different αv integrins has been reported between laboratory and field strains of foot-and-mouth disease virus, demonstrating that virus isolates can exhibit altered affinities for their cellular receptors (10).
Here we confirm that, in the absence of ICAM-1, MAb cross-linked DAF can serve as a functional internalization receptor for both prototype and clinical CVA21 strains (Fig. 4). It has previously been proposed that entry of CVA21 mediated by MAb cross-linked DAF occurs via caveolae, in contrast to the clathrin-coated pit entry route used during virus interaction with ICAM-1 (31). CVA21 entry via caveolae containing the cross-linked DAF hypothesis is supported by evidence indicating that MAb clustered DAF is endocytosed after recruitment into caveolae (20). A possible role for DAF interaction in caveola-mediated CVA21 entry has been confirmed by recent reports of cell internalization of a DAF binding strain of EV11 via lipid rafts and/or caveolae (37).
The widespread expression of DAF throughout the mammalian body (24) offers an adaptive advantage to viruses that display a higher affinity for DAF and can utilize this receptor for internalization. Such viruses may have an increased pathogenicity compared to other strains due to the expression of DAF on erythrocytes (16), offering DAF-binding viruses a readily available vehicle for travel throughout the human body. Interestingly, serial passage of coxsackievirus B3 and B5 isolates in polarized epithelial cells (where their natural internalization receptor, the coxsackie and adenovirus receptor [3], is located in tight cell-cell junctions and DAF on the apical surface) selected for DAF-binding variants, suggesting an important role for DAF infection of epithelial cell mucosal surfaces (36).
Genetic analysis of the P1 region of the genome coding for the capsid structural proteins detected a number of differences in the deduced amino acid sequences between clinical CVA21 isolates and the prototype strain. None of the observed coding changes mapped to the previously determined ICAM-1 footprint (44), and the differences were scattered throughout VP1, VP2, and VP3. Residues constituting the ICAM-1 footprint were conserved in both the prototype Kuykendall strain and all clinical isolates, except for amino acid 168 in VP2 (Fig. 5). At position 168 of VP2 the amino acid substitution (Val to Ala) is conservative and potentially of little significance in the conformation of the ICAM-1 binding site. Clinical isolate 272101, which exhibited significant lytic activity in RD cells expressing DAF only (although the lytic activity was not as great as that of the remaining clinical isolates), possessed 13 of 14 coding changes observed between all clinical isolates with respect to the prototype stain. The presence of a further nine additional changes in isolate 272101 compared to the other clinical isolates may have exerted some type of suppression on the enhanced DAF usage phenotype exhibited by isolates 275238 and 2727598. Repeated in vitro cell passage of the clinical CVA21 isolates possessing the elevated DAF usage phenotype in environments of both high DAF and ICAM-1 may exert pressure for the bioselection of virions with enhanced ICAM-1 usage at the cost of reducing functional DAF interactions. Generation of such populations of virions may yield the identification of key P1 amino acid changes responsible for the altered receptor usage phenotypes.
A possible explanation for the reduced DAF usage of isolate 272101 has been provided by a study in which bioselected EV11 variants that had lost their DAF binding phenotype were found to possess specific amino acid changes in the BC loop of VP1 and in the puff region of VP2 (38). Our sequence analysis revealed the presence of such unique differences in the BC loop of VP1 and the puff region of VP2 of isolate 272101 but not in same capsid region of the other CVA21 clinical isolates (Fig. 5). Although not shown to be conclusive in the area of virus attachment and cell entry, these observed 13-amino-acid changes between all clinical isolates and the prototype may potentially play a role in the development of the enhanced DAF usage phenotype. However, whereas not addressed in the present study, the involvement of additional changes located at other regions of the viral genome (e.g., 5′-untranslated region) in mediating cell lytic infection cannot be discarded.
A significant difference, however, between the DAF-EV7, DAF-CVB3 interaction and the DAF-CVA21 interaction is that DAF SCRs 2, 3, or 4 are involved in EV and CVB binding (6, 32), whereas DAF SCR 1 is involved in CVA21 attachment (33). Given the overall structural similarities between the EV7, CVB3, and CVA21 capsids, we propose that the involvement of the N-terminal domains of two separate receptors with their own separate binding sites on the CVA21 capsid (i.e., DAF and ICAM-1) may occur at any stage during infection. However, the involvement of SCRs 2 to 4 of DAF in EV7/CVB3 binding (5, 6) suggests that interactions with additional receptors, such as CAR in the case of CVB3 (3), may occur after those of DAF in order to minimize the interference with access to the specific DAF-binding epitopes on the virus capsid (14). Of some interest may be the detection of a slight difference in the migration of VP1 of the prototype strain relative to the clinical isolates able to lytically infect ICAM-1-negative RD cells (data not shown). Similarly, a variant of a CVB3 prototype (CB3-RD), generated after serial passage in RD cells, exhibited an altered VP1 mobility to the prototype and correlated with an altered receptor specificity toward DAF compared to the parental strain (29).
Taken together, the results in the present study indicate that the overall binding capability of clinical isolates to their cellular receptors has been conserved with respect to the prototype strain. However, there appear to be some discrete differences in the capacity of clinical CVA21 isolates to utilize these receptors. Similar to CVB3 field strains, the clinical CVA21 isolates possess a phenotype that facilitates the increased use of DAF in cell lytic infection, most probably as a result of passage in humans. The capacity of CVA21 to utilize both DAF and ICAM-1 for attachment and/or infection of host cells suggests the conservation of an advantageous phenotype, allowing individual and/or multiple receptor usage, thereby extending the tissue tropism of the virus and significantly increasing the chances of productive infection.
REFERENCES
- 1.Berendt, A. R., A. McDowell, A. G. Craig, P. A. Bates, M. J. E. Sternberg, K. Marsh, C. I. Newbold, and N. Hogg. 1992. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps but is distinct from the LFA-1 binding site. Cell 68:71-81. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson, J. M., M. Chan, K. R. Solomon, N. F. St. John, H. Lin, and R. W. Finberg. 1994. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 91:6245-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. F. Modlin, W. Weiland-Alter, J. A. Cunningham, R. L. Crowell, and R. W. Finberg. 1997. Clinical coxsackievirus B isolates differ from laboratory strains in their interaction with two cell surface receptors. J. Infect. Dis. 175:697-700. [DOI] [PubMed] [Google Scholar]
- 5.Bergelson, J. M., J. G. Mohanty, R. L. Crowell, N. F. St. John, D. M. Lublin, and R. W. Crowell. 1995. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55). J. Virol. 69:1903-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson, N. A., R. Kaufman, D. M. Lublin, T. Ward, P. A. Pipkin, P. D. Minor, D. J. Evans, and J. W. Almond. 1995. Characterization of the echovirus 7 receptor: domains of CD55 critical for virus binding. J. Virol. 69:54-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyne, K. E., E. S. Hall, M. A. Thompson, M. A. Arce, T. Kinoshoita, T. Fujita, D. J. Anstee, W. Rosse, and D. M. Lublin. 1992. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J. Immunol. 149:2906-2913. [PubMed] [Google Scholar]
- 8.Davitz, M. A., M. G. Low, and V. Nussenzweig. 1986. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PI-PLC). Selective modification of a complement regulatory protein. J. Exp. Med. 163:1150-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dragic, T., V. Litwin, G. P. Allaway, S. E. Martin, Y. Huang, K. A. Nagashima, C. Cayanon, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 10.Duque, H., and B. Baxt. 2003. Foot-and-mouth disease virus receptors: comparison of bovine αV integrin utilization by type A and O viruses. J. Virol. 77:2500-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filman, D. J., M. W. Wien, J. A. Cunningham, J. M. Bergelson, and J. M. Hogle. 1998. Structure determination of echovirus 1. Acta Crystallogr. D Biol. Crystallogr. 54:1261-1272. [DOI] [PubMed] [Google Scholar]
- 12.Fricks, C. E., and J. M. Hogle. 1990. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J. Virol. 64:1934-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greve, J. M., G. Davis, A. M. Meyer, C. P. Forte, S. C. Yost, C. W. Marlor, M. E. Kamarck, and A. McClelland. 1989. The major human rhinovirus receptor is ICAM-1. Cell 56:839-847. [DOI] [PubMed] [Google Scholar]
- 14.He, Y., F. Lin, P. Chipman, C. M. Bator, T. S. Baker, M. Shoham, R. J. Kuhn, E. M. Medof, and M. G. Rossmann. 2002. Structure of decay-accelerating factor bound to echovirus 7: a virus receptor complex. Proc. Natl. Acad. Sci. USA 99:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karnauchow, T. M., S. Dawe, D. M. Lublin, and K. Dimock. 1998. Short consensus repeat domain 1 of decay-accelerating factor is required for enterovirus 70 binding. J. Virol. 72:9380-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinoshita, T., M. E. Medof, R. Silber, and V. Nussenzweig. 1985. Distribution of decay accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J. Exp. Med. 162:75-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindberg, A. M., C. Polacek, and S. Johansson. 1997. Amplification and cloning of complete enterovirus genomes by long distance PCR. J. Virol. Methods 65:191-199. [DOI] [PubMed] [Google Scholar]
- 18.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martino, T. A., M. Petric, M. Brown, K. Aitken, C. J. Gauntt, C. D. Richardson, L. H. Chow, and P. P. Liu. 1998. Cardiovirulent coxsackieviruses and the decay-accelerating factor (CD55) receptor. Virology 244:302-314. [DOI] [PubMed] [Google Scholar]
- 20.Mayor, S., K. G. Rothberg, and F. R. Maxfield. 1994. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science 264:1948-1951. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima, S., and S. Nagata. 1990. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 18:5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muckelbauer, J. K., I. Kremer, I. Minor, G. Diana, F. J. Dutko, J. Groarke, P. C. Pevear, and M. G. Rossman. 1995. The structure of coxsackievirus B3 at 3.5 Å resolution. Structure 3:653-667. [DOI] [PubMed] [Google Scholar]
- 23.Newcombe, N., E. S. Johansson, G. G. Au, A. M. Lindberg, R. D. Barry, and D. R. Shafren. 2003. Cellular receptor interations of C-cluster human group A coxsackieviruses. J. Gen. Virol. 84:3041-3050. [DOI] [PubMed]
- 24.Nicholson-Weller, A., J. P. March, C. E. Rosen, D. B. Spicer, and K. F. Austen. 1985. Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood 65:1237-1244. [PubMed] [Google Scholar]
- 25.Nicholson-Weller, A., and C. E. Wang. 1994. Structure and function of decay accelerating factor CD55. J. Lab. Clin. Med. 123:485-491. [PubMed] [Google Scholar]
- 26.Powell, R. M., V. Schmitt, T. Ward, I. Goodfellow, D. J. Evans, and J. W. Almond. 1998. Characterization of echoviruses that bind decay-accelerating factor (CD55): evidence that some haemegglutinating strains use more than one cellular receptor. J. Gen. Virol. 79:1707-1713. [DOI] [PubMed] [Google Scholar]
- 27.Powell, R. M., T. Ward, D. J. Evans, and J. W. Almond. 1997. Interaction between echovirus 7 and its receptor, decay-accelerating factor (CD55): evidence for a secondary cellular factor in A-particle formation. J. Virol. 71:9306-9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed, L. J., and H. A. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 29.Schmidtke, M., H.-C. Selinka, A. Heim, B. Jahn, M. Tonew, R. Kandolf, A. Stelzner, and R. Zell. 2000. Attachment of coxsackievirus B3 variants to various cell lines: mapping of phenotypic differences to capsid protein VP1. Virology 275:77-88. [DOI] [PubMed] [Google Scholar]
- 30.Schneider-Schaulies, J., L. M. Dunster, R. Schwartz-Albiez, G. Krohne, and V. ter Meulen. 1995. Physical association of moesin and CD46 as a receptor complex for measles virus. J. Virol. 69:2248-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shafren, D. R. 1998. Viral cell entry induced by cross-linked decay-accelerating factor. J. Virol. 72:9407-9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafren, D. R., R. C. Bates, M. V. Agrez, R. L. Herd, G. F. Burns, and R. D. Barry. 1995. Coxsackieviruses B1, B3 and B5 use decay accelerating factor as a receptor for cell attachment. J. Virol. 69:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shafren, D. R., D. J. Dorahy, S. J. Greive, G. F. Burns, and R. D. Barry. 1997. Mouse cells expressing human intercellular adhesion molecule-1 are susceptible to infection by coxsackievirus A21. J. Virol. 71:785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafren, D. R., D. J. Dorahy, R. A. Ingham, G. F. Burns, and R. D. Barry. 1997. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 71:4736-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafren, D. R., D. J. Dorahy, R. F. Thorne, T. Kinoshita, R. D. Barry, and G. F. Burns. 1998. Antibody binding to individual short consensus repeats of decay-accelerating factor enhances enterovirus cell attachment and infectivity. J. Immunol. 160:2318-2323. [PubMed] [Google Scholar]
- 36.Shieh, J. T. C., and J. M. Bergelson. 2002. Interaction with decay-accelerating factor facilitates coxsackievirus B infection of polarized epithelial cells. J. Virol. 76:9474-9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. K. Brown. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 76:9302-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stuart, A. D., T. A. McKee, P. A. Williams, C. Harley, S. Shen, D. I. Stuart, T. D. K. Brown, and S. M. Lea. 2002. Determination of the structure of a decay-accelerating factor-binding clinical isolate of echovirus 11 allows mapping of mutants with altered receptor requirements for infection. J. Virol. 76:7694-7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson, J. D., D. G. Higgins, and T. J. Gobson. 1994. Clustal_W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tosi, M. F., J. M. Stark, C. W. Smith, A. Hamedani, D. C. Gruenert, and M. D. Infeld. 1992. Induction of ICAM-1 expression on human airway epithelial cells by inflammatory cytokines: effects on neutrophil-epithelial cell adhesion. Am. J. Respir. Cell Mol. Biol. 7:214-221. [DOI] [PubMed] [Google Scholar]
- 41.Triantafilou, K., D. Fradelizi, K. Wilson, and M. Triantafilou. 2002. GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J. Virol. 76:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van de Stolpe, A., and P. T. van der Saag. 1996. Intercellular adhesion molecule-1. J. Mol. Med. 74:13-33. [DOI] [PubMed] [Google Scholar]
- 43.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 44.Xiao, C., C. M. Bator, V. D. Bowman, E. Reider, Y. He, B. Hebert, J. Bella, T. S. Baker, E. Wimmer, R. J. Kuhn, and M. G. Rossman. 2001. Interaction of coxsackievirus A21 with its cellular receptor, ICAM-1. J. Virol. 75:2444-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]