Abstract
We have previously demonstrated that platelet-activating factor (PAF)-induced increases in microvessel permeability were associated with endothelial gap formation and that the magnitude of peak endothelial intracellular Ca2+ concentration ([Ca2+]i) and nitric oxide (NO) production at the single vessel level determines the degree of the permeability increase. This study aimed to examine whether the magnitudes of PAF-induced peak endothelial [Ca2+]i, NO production, and gap formation are correlated at the individual endothelial cell level in intact rat mesenteric venules. Endothelial gaps were quantified by the accumulation of fluorescent microspheres at endothelial clefts using confocal imaging. Endothelial [Ca2+]i was measured on fura-2- or fluo-4-loaded vessels, and 4,5-diaminofluorescein (DAF-2) was used for NO measurements. The results showed that increases in endothelial [Ca2+]i, NO production, and gap formation occurred in all endothelial cells when vessels were exposed to PAF but manifested a spatial heterogeneity in magnitudes among cells in each vessel. PAF-induced peak endothelial [Ca2+]i preceded the peak NO production by 0.6 min at the cellular level, and the magnitudes of NO production and gap formation linearly correlated with that of the peak endothelial [Ca2+]i in each cell, suggesting that the initial levels of endothelial [Ca2+]i determine downstream NO production and gap formation. These results provide direct evidence from intact venules that inflammatory mediator-induced increases in microvessel permeability are associated with the generalized formation of endothelial gaps around all endothelial cells. The spatial differences in the molecular signaling that were initiated by the heterogeneous endothelial Ca2+ response contribute to the heterogeneity in permeability increases along the microvessel wall during inflammation.
Keywords: microvessel permeability, endothelial Ca2+ imaging, nitric oxide imaging, endothelial junctions, intracellular Ca2+ concentration
increased microvessel permeability to fluid and macromolecules is a hallmark of inflammation, resulting in edema formation and organ dysfunctions. A better understanding of the mechanisms that regulate microvessel permeability will benefit the development of potential therapeutic strategies for inflammation-related diseases.
Gap formation between endothelial cells has been indicated as the main transport pathway responsible for the increased permeability to fluid and macromolecules during inflammation (3, 6, 15, 17, 18, 22, 24, 29). Our previous study (17) demonstrated that the dynamic changes in endothelial gaps upon platelet-activating factor (PAF) stimulation closely correlated with the time course of increases in microvessel hydraulic conductivity (Lp), suggesting that the opening and closing of endothelial gaps contribute to PAF-induced transient increases in microvessel Lp.
It has been demonstrated that inflammatory mediator-induced Ca2+ influx into endothelial cells is essential for increases in microvessel permeability (7, 11, 12, 20) and that the magnitude of the Ca2+influx determines the degree of permeability increases (11, 12, 14). In addition, PAF-induced Ca2+/calmodulin-dependent endothelial nitric oxide (NO) synthase (eNOS) activation and NO production have been shown to play important roles in the regulation of microvessel permeability (27, 40, 44). Blockade of NO production by a nonspecific NOS inhibitor or a specific eNOS inhibitor, caveolin-1 scaffolding domain, attenuated PAF-induced increases in microvessel permeability (27, 41, 44). An increase of extracellular Ca2+ concentration that potentiated PAF-induced Ca2+ influx augmented NO production, resulting in an enhanced Lp increase (41). These studies demonstrated a magnitude correlation between PAF-induced increases in endothelial intracellular Ca2+ concentration ([Ca2+]i), NO production, and microvessel permeability at the single vessel level. At the individual endothelial cell level, previous studies (23, 26, 42) in venular microvessels demonstrated that endothelial [Ca2+]i does not uniformly increase in response to proinflammatory agents or a Ca2+ ionophore. The ionomycin-induced macromolecule leakages were most prominent at sites where the averaged increase in endothelial [Ca2+]i from a group of cells was the largest (26).
So far, the variations of inflammatory mediator-induced NO production and endothelial gap formation at the individual endothelial cell level have not been investigated. The temporal and spatial relationship between inflammatory mediator-induced increases in endothelial [Ca2+]i and NO production at the cellular level remains unknown. The present study aimed to investigate the temporal and spatial correlation between inflammatory mediator-induced increases in endothelial [Ca2+]i, NO production, and endothelial gap formation at the individual cell level in intact microvessels. Both conventional and confocal fluorescence imaging were used in this study. Endothelial [Ca2+]i was measured in either fura-2- or fluo-4-loaded vessels, and 4,5-diaminofluorescein (DAF-2) was used for NO measurements. Endothelial gaps were quantified by the accumulation of fluorescent microspheres (FMs) at endothelial clefts in individually perfused intact venules, and PAF was selected as a representative inflammatory mediator.
MATERIALS AND METHODS
Animal preparation.
Experiments were performed in venular microvessels in rat mesenteries with diameters ranging between 35 and 50 μm. Female Sprague-Dawley rats (2–3 mo old, 220–250 g, Hilltop Laboratory Animal, Scottdale, PA) were anesthetized with pentobarbital sodium (65 mg/kg body wt) administered subcutaneously. A midline surgical incision (1.5–2 cm) was made in the abdominal wall, and the mesentery was gently removed from the abdominal cavity and spread over a glass coverslip attached to an animal tray for fluorescence imaging experiments. The upper surface of the mesentery was continuously superfused with mammalian Ringer solution at 37°C. Each experiment was performed on 1 microvessel/animal to avoid any potential effect of the applied inflammatory mediator on subsequent vessel experiments. All procedures and animal use were approved by the Animal Care and Use Committee of West Virginia University.
Conventional fluorescence imaging of endothelial [Ca2+]i and NO production in intact venules.
Conventional fluorescence imaging was conducted on a Nikon Diaphot 300 microscope equipped with a 12-bit digital, cooled, charge-couple device camera (ORCA, Hamamatsu), a filter changer (Lambda 10-2, Sutter Instruments, Novato, CA), and a single vessel perfusion rig. Fura-2 AM and DAF-2 diacetate (DA) were used for endothelial [Ca2+]i and NO measurements, respectively. The excitation wavelengths for fura-2 were selected by two interference filters (Oriel, 340 ± 5 and 380 ± 5 nm), and the emission was separated with a dichroic mirror (DM400) and an interference filter (Oriel, 500 ± 20 nm). The excitation wavelength for Ca2+ imaging alternated between 340 and 380 nm, and images were acquired with 0.25-s exposure at each wavelength. The DAF-2 excitation wavelength was selected by an interference filter (480/40 nm), and emission was separated by a dichroic mirror (505 nm) and a band-pass barrier (535/50 nM). To minimize DAF-2 photobleaching, a neutral density filter (0.5 neutral density) was positioned in front of the interference filter, and the exposure time was minimized to 0.12 s at 1-min intervals. MetaFluor software (Universal Imaging) was used for image acquisition and data analyses. Details have been described elsewhere (42, 43).
The experimental procedures of using fura-2 AM to measure endothelial [Ca2+]i (data shown in Fig. 2) have been previously described (42). To evaluate the correlation of endothelial [Ca2+]i with NO at the cellular level (data shown in Fig. 5), each vessel was cannulated and perfused by a micropipette containing both fura-2 AM (10 μM) and DAF-2 DA (5 μM) for 45 min at a perfusion pressure of 50 cmH2O. Before image acquisition, fura-2 AM was removed from the perfusate by recannulating the vessel with another micropipette, whereas DAF-2 DA was present throughout the experimental period. Detailed evaluations of NO measurements with continuous DAF-2 DA perfusion have been previously described (41). Fura-2 and DAF-2 images were alternatively collected from the same group of endothelial cells in the same focal plane under control conditions and after the application of PAF (10 nM) to the perfusate. Each image was positioned at least 100 μm away from the vessel cannulation site. No fluorescence cross-talk between fura-2 and DAF-2 was observed during image acquisition. Quantitative analysis of [Ca2+]i and NO at the individual endothelial cell level was conducted using manually selected regions of interest (ROIs) along the vessel wall. Each ROI covers the area of one individual endothelial cell, as indicated by the fluorescence outline. The basal NO production rate was calculated from the slope of the fluorescence intensity (FI) increase during albumin-Ringer perfusion after DAF-2 loading reached steady state. PAF-induced NO production was determined by the net changes in DAF-2 FI (FIDAF) after stimulation. The rate of FIDAF change was derived by first-differential conversion of cumulative FIDAF values over time. Details have been previously described (41).
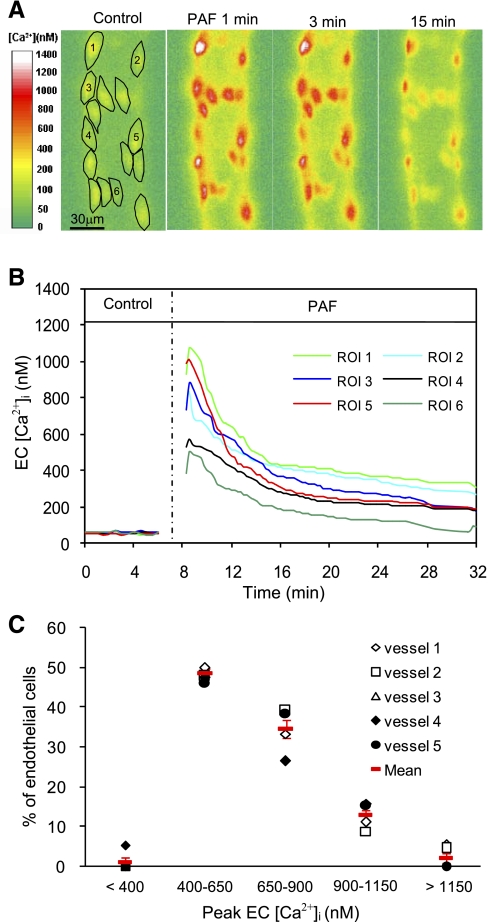
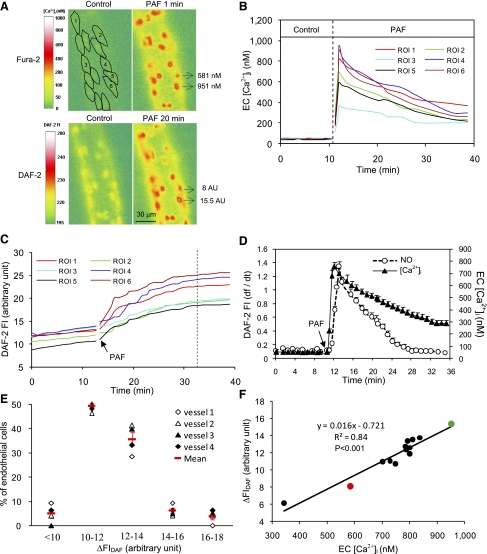
Fig. 2.
Variation of endothelial intracellular Ca2+ concentration ([Ca2+]i) responses to PAF among ECs of one vessel. A: fura-2 ratio images from one representative experiment showing the changes in endothelial [Ca2+]i at 1, 3, and 15 min after the start of PAF application in 15 regions of interest (ROIs). B: time course of PAF-induced changes in endothelial [Ca2+]i in 6 of the 15 ROIs. C: histogram showing the distribution of PAF-induced peak endothelial [Ca2+]i in 88 ECs of 5 vessels. Red symbols show means ± SE.
Fig. 5.
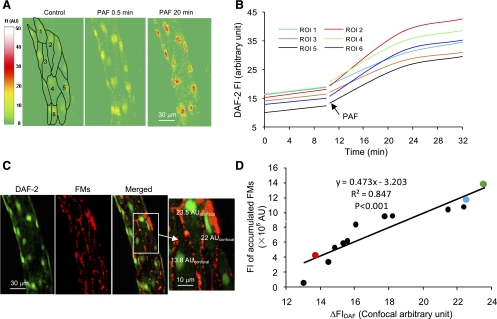
Spatial correlation between PAF-induced NO production and endothelial gap formation. A: DAF-2 confocal images from one representative experiment showing changes in FIDAF at the cellular level before and after the start of PAF perfusion at 0.5 and 20 min. Each image represents the projection of five consecutive sections from the bottom half of the vessel. B: time-dependent DAF-2 cumulative FI curve in 6 of 13 ECs. Images were collected for 20 min at 10-min intervals. C: confocal images of DAF-2 (green) and FMs (red) projected from the lower half of the same vessel in A. The three ECs in the magnified image illustrate that the cell had higher FM accumulation correlated with a larger magnitude of NO production, where ΔFIDAF was measured in confocal AU (AUconfocal). D: linear relationship between the magnitude of NO production and endothelial gap formation of 13 ECs of the representative vessel. The red, blue, and green circles represent the three ECs in the magnified image in C.
Correlative quantifications of endothelial [Ca2+]i and NO with endothelial gap formation.
Experiments were conducted on a Leica TCS SL confocal microscope attached to a single microvessel perfusion rig. A Leica ×20 objective (HC Plan APO, numerical aperture: 0.7) with ×3 electronic zoom was used for image acquisition. Stacks of images were obtained from the bottom half of each vessel by optical sectioning at successive x-y focal planes through the z-axis. Figure 1A shows the z-stack of confocal images in relation to the vessel orientation.
Fig. 1.
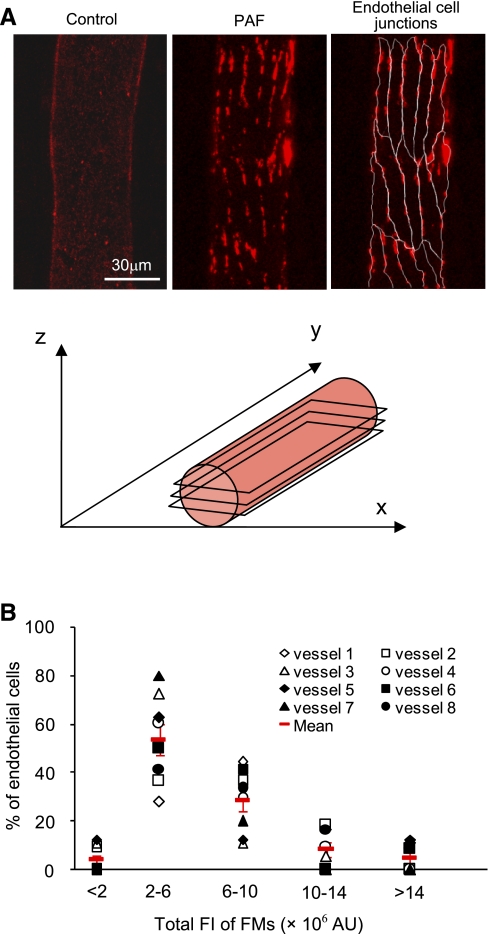
Platelet-activating factor (PAF)-induced endothelial gap formation. A, top: confocal images of fluorescent microspheres (FMs) from two representative experiments that were perfused with albumin-Ringer solution (left) and after an exposure to PAF (10 nM; middle and right), respectively. The right image shows the outline of endothelial junctions based on the profile of the accumulated FMs. Bottom, diagram showing the z-stack of confocal images in relation to vessel orientation. B: histograms showing the magnitude distribution of the accumulated FMs around each endothelial cell (EC) in 103 cells of 8 vessels. Red symbols show means ± SE of eight vessels. FI, fluorescence intensity; AU, arbitrary units.
First, we evaluated the variations of PAF-induced endothelial gap formation (data shown in Fig. 1). The magnitude of the endothelial gaps was evaluated by quantification of accumulated FMs (100 nm in diameter) at endothelial clefts. A helium-neon laser (543 nm, 1.2 mV) was used for excitation, and the emission band was 570–630 nm. Image stacks were collected using a 1,024 × 1,024 scan format with 0.5-μm vertical steps (z-axis). Control images were acquired after each vessel was perfused with albumin-Ringer solution containing FMs for 10 min and followed by albumin-Ringer perfusion alone for 10 min to remove the free FMs from the vessel lumen. Images of PAF-induced gap formation were collected after perfusing each vessel with a solution containing PAF and FMs for 10 min followed by washing away the free FMs from the vessel lumen. Details of the experimental procedures and method evaluations have been previously described (17).
To quantify the relationship of either endothelial [Ca2+]i or NO with the formed endothelial gaps, [Ca2+]i and NO were measured in the confocal system where FM accumulation was determined. To fit our laser line spectrum, fluo-4 was selected for confocal Ca2+ imaging and DAF-2 DA was used for confocal NO imaging. An argon laser (488 nm) at 50% power was used for excitation, and the emission band was 510–530 nm. To minimize photobleaching, both fluo-4 and DAF-2 images were collected using a 512 × 512 scan format at a gain of 500 V and a z-step of 2 μm. A xyzt scan mode was applied for real-time image stacks. No vasomotion was generated during PAF perfusion under our experimental conditions, which enabled a relatively constant focal plane during the image acquisition period.
For endothelial [Ca2+]i and gap experiments, each vessel was first loaded with fluo-4 AM (5 μM) for 20 min followed by albumin-Ringer perfusion to wash out the lumen fluo-4 AM before control images were collected. Confocal NO images were collected with the continuous perfusion of DAF-2 DA (5 μM) after 40 min of dye loading (41). After control images were collected, the fluo-4- or DAF-2-loaded vessel was perfused with a solution containing both PAF and FMs. Stacks of images were collected from the same group of endothelial cells of the vessel wall for 20 min, with 2-min intervals for fluo-4 and 10-min intervals for DAF-2. Identical instrument settings were applied to all of the experiments. Endothelial [Ca2+]i and NO at the cellular level were quantified by calculating the mean FI of each stack of ROIs after the subtraction of background autofluorescence. Each ROI covered the area of one endothelial cell, and the drawing was based on the endothelial junctions outlined by the accumulated FMs after PAF stimulation. Endothelial [Ca2+]i was converted from the mean FI of each cell based on the in vitro fluo-4 calibration curve. NO production was calculated using the same method described for conventional DAF-2 fluorescence imaging (41), with the exception that FIDAF was the mean of a whole stack of ROIs and the FI was expressed as confocal arbitrary units (AUconfocal).
Our previous study (17) demonstrated that maximal gap formation occurred at 10 min after the initiation of PAF perfusion and that entrapped FMs at the open endothelial clefts locked the gap size and could not be washed away. Therefore, two channel images of FMs with either DAF-2 or fluo-4 were collected after a time series of fluo-4 or DAF-2 image stacks were obtained and after the free FMs in the vessel lumen had been washed away. A 1,024 × 1,024 scan format and 0.5-μm step at the z-axis were applied. Fluo-4 or DAF-2 images that were taken simultaneously with FMs were for spatial registration and not used for FI quantifications. The endothelial cells selected for FM images were the same as those in fluo-4 or DAF-2 images in each of the vessels. The ROIs selected for FM quantification were based on the outline of endothelial junctions observed in the merged two-channel confocal images. The magnitude of endothelial gap formation was determined by FM accumulation at the endothelial clefts, which was calculated as the total FI of the stack of ROIs around each endothelial cell. The FM FI at the endothelial cleft applied to both adjacent cells during data analysis. The total FI of the accumulated FMs around each endothelial cell was then correlated with the peak [Ca2+]i or the ΔFIDAF measured in that cell.
Calibration of fluo-4.
In vitro calibration of fluo-4 was conducted on the same confocal microscope used for the in vivo experiments. Calibration solutions were prepared with a Ca2+ calibration buffer kit containing 5 μM fluo-4 pentapotassium salt with a pH of 7.3. The confocal settings were identical to those used for Ca2+ imaging in intact vessels. The in vitro calibration curve was obtained at 37°C, and each depicted value is the mean of three measurements. The curve well fit the following relation: [Ca2+] = Kd × (F − Fmin)/(Fmax − F) (32) with r2 = 0.9894 (P < 0.001), where Kd is the dissociation constant of fluo-4, F is the measured FI, Fmin is the FI at zero free [Ca2+], and Fmax is the FI with saturated free [Ca2+]. The calculated values in this study were Kd = 230 nM, Fmin = 0.04 AUconfocal, and Fmax = 68.01 AUconfocal.
Solutions and reagents.
Mammalian Ringer solution was used for the experiments (14). The composition of mammalian Ringer solution was (in mM) 132 NaCl, 4.6 KCl, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, 5.0 NaHCO3, 20 HEPES, and Na-HEPES. All perfusates contained 10 mg/ml BSA. Fura-2 AM, DAF-2 DA, fluo-4 AM, fluo-4 pentapotassium salt, and the Ca2+ calibration buffer kit were all purchased from Invitrogen. All fluorescent dyes except for fluo-4 pentapotassium salt were prepared in DMSO for a stock solution, and a dilution of at least 1:1,000 was made for the final solution. FMs were purchased from Duke Scientific (Palo Alto, CA). The final perfusate concentration of FMs was 3× 1011 FMs/ml. PAF (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine, Sigma, St. Louis, MO) was initially dissolved in 95% ethyl alcohol and then diluted to a final concentration of 10 nM. All perfusates containing the test agents were freshly prepared before each cannulation.
Data analysis and statistics.
All values are presented as means ± SE; n represents the number of vessels. One-way ANOVA was used to compare data between groups. A paired t-test was used for paired data analysis. P values of <0.05 were considered statistically significant. Correlation was conducted by a linear regression analysis. The degree of heterogeneity was quantified as the coefficient of variance (CV), which is the SD normalized by the mean value.
RESULTS
Spatial quantification of PAF-induced endothelial gap formation in venular microvessels.
FMs were used to perfuse individual microvessels under control conditions or during PAF application to evaluate endothelial gap formation. Control experiments were conducted in three vessels. After each vessel was perfused for 10 min with albumin-Ringer solution containing FMs followed by a 10-min washout to remove free FMs from the vessel lumen, confocal images demonstrated a thin and uniform distribution of FMs on the surface of the vascular wall (Fig. 1A, left). These results are consistent with our previous observations (17). PAF-induced endothelial gap formation at the individual cell level was studied in eight vessels (8–18 endothelial cells/vessel, 103 cells in total). In contrast to the uniform distribution of FMs under control conditions, a 10-min perfusion of FMs with PAF followed by a 10-min washout with PAF alone resulted in the preferential accumulation of FMs at endothelial junctions (Fig. 1A). The maximum accumulation occurred at 10 min after the initiation of PAF perfusion, which was correlated with the PAF-induced Lp peak (17). No FMs extravasated across the vessel wall in either control or PAF-stimulated vessels. PAF-induced FM accumulation was observed at the junctions of all endothelial cells. However, the magnitude of FM accumulation varied among endothelial cells in each vessel. Approximately 83% of the endothelial cells of the eight vessels formed middle-sized gaps (FIs from 2 to 10 × 106 AU), and 4% and 13% of the cells formed small (FI < 2 × 106 AU) and large gaps (FI > 10 × 106 AU), respectively (Fig. 1B). The mean CV of the accumulated FM FI among endothelial cells in each vessel was 34 ± 5% (n = 8).
PAF-induced changes in endothelial [Ca2+]i at the individual endothelial cell level in intact venules.
PAF-induced changes in endothelial [Ca2+]i at the cellular level were measured in five vessels (13–23 endothelial cells/vessel) by fluorescence imaging with fura-2. The mean baseline endothelial [Ca2+]i was 54 ± 1 nM (n = 5), with small variations among endothelial cells (CV: 9 ± 0.9%). When PAF was applied to each vessel, [Ca2+]i in all endothelial cells reached a peak at 0.7 ± 0.09 min. The mean peak [Ca2+]i was 708 ± 21 nM (n = 5), which varied from 339 to 1,101 nM among cells and declined to 239 ± 31 nM after 15 min. The mean CV was 28 ± 1.5%, which was significantly higher than that of baseline endothelial [Ca2+]i (P < 0.001). The variations of individual endothelial [Ca2+]i responses to PAF of the five vessels are shown in Table 1. Figure 2, A and B, show the fura-2 ratio images and the individual endothelial [Ca2+]i responses after PAF stimulation from one vessel. The distribution of PAF-induced peak endothelial [Ca2+]i from all endothelial cells of five vessels is shown in Fig. 2C.
Table 1.
PAF-induced changes in endothelial [Ca2+]i at the cellular level
| Baseline [Ca2+]i |
PAF-Induced Peak [Ca2+]i |
||||||
|---|---|---|---|---|---|---|---|
| Experiment | Number of Cells | Mean ± SE, nM | Range, nM | CV, % | Mean ± SE, nM | Range, nM | CV, % |
| PAF1 | 18 | 53 ± 1 | 39–61 | 11 | 757 ± 47 | 459–1,100 | 26 |
| PAF2 | 23 | 53 ± 1 | 43–67 | 9 | 659 ± 34 | 393–1,101 | 24 |
| PAF3 | 15 | 54 ± 1 | 49–61 | 6 | 659 ± 41 | 362–1,024 | 28 |
| PAF4 | 19 | 57 ± 1 | 47–68 | 11 | 716 ± 56 | 339–1,041 | 33 |
| PAF5 | 13 | 53 ± 1 | 43–62 | 10 | 750 ± 59 | 414–1,048 | 27 |
Mean endothelial intracellular Ca2+ concentration ([Ca2+]i) responses to 10 nM platelet-activating factor (PAF) in all endothelial cells from five individual rat mesenteric venules are shown. Variability is presented as the coefficient of variance (CV).
Relationship between PAF-induced peak endothelial [Ca2+]i and the magnitude of endothelial gap formation at the individual endothelial cell level in intact venules.
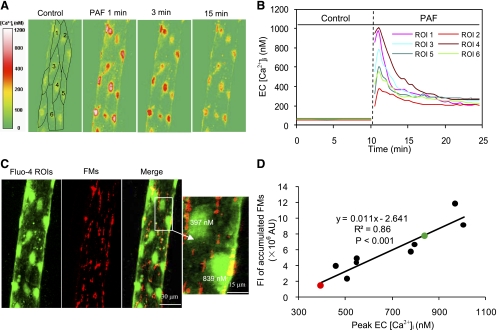
The relationship between PAF-induced peak endothelial [Ca2+]i and the magnitude of gap formation was examined by the simultaneous quantification of endothelial [Ca2+]i and FM accumulation in four vessels (8–15 cells/vessel) using confocal imaging. PAF-induced changes in endothelial [Ca2+]i measured with fluo-4 by confocal microscopy was similar to that measured with fura-2 using conventional fluorescence imaging. The mean baseline endothelial [Ca2+]i was 55 ± 2 nM, with low variations among cells (CV: 10 ± 2.1%). No FM accumulation was observed at endothelial junctions under basal conditions. Endothelial [Ca2+]i increased to a mean peak value of 690 ± 20 nM (n = 4) within 0.7 ± 0.04 min of PAF stimulation and varied from 320 to 1,176 nM at the cellular level (Fig. 3, A and B). A larger magnitude of FM accumulation was found around endothelial cells with a higher peak value of [Ca2+]i (Fig. 3C). Figure 3D shows peak [Ca2+]i in each endothelial cell as a function of its surrounding FM accumulation, which delineated a linear correlation between the magnitude of peak endothelial [Ca2+]i and the degree of endothelial gap formation (n = 4, r2 = 0.8 ± 0.1, P < 0.01).
Fig. 3.
Correlation of PAF-induced peak endothelial [Ca2+]i with the magnitude of endothelial gap formation at the cellular level. A: fluo-4 confocal images from one representative experiment before and after the start of PAF perfusion at 1, 3, and 15 min. Each confocal image is the projection of 5 consecutive sections from the bottom half of the vessel. B: time course of PAF-induced changes in endothelial [Ca2+]i in 6 of 10 ROIs from the image in A. C: confocal images of fluo-4 (green) and FMs (red) projected from the bottom half of one vessel. The magnified image shows two ECs. The cell with a higher peak endothelial [Ca2+]i (839 nM) was surrounded by more entrapped FMs (7.7 × 106 AU), and less FM accumulation (1.4 × 106 AU) occurred around the cell with a lower peak endothelial [Ca2+]i (397 nM). D: linear correlation between peak endothelial [Ca2+]i and the magnitude of FM accumulation around each EC from the image in A. The green and red circles represent the two ECs in C.
Relationship between PAF-induced NO and peak increase in endothelial [Ca2+]i at the individual endothelial cell level in intact venules.
PAF-induced changes in endothelial [Ca2+]i and NO production were simultaneously measured in four vessels (7–14 endothelial cells/vessel) using DAF-2 and fura-2. The mean baseline endothelial [Ca2+]i and NO production rate was 53 ± 1 nM and 0.12 ± 0.001 AU/min, respectively. Both of the variations under control conditions were small among individual cells (CV: 9 ± 1.1% and 6 ± 0.3%, respectively). When each vessel was perfused with PAF, [Ca2+]i in all endothelial cells reached a peak at 0.7 ± 0.06 min. Mean peak [Ca2+]i was 749 ± 19 nM and varied from 425 to 1,251 nM among cells. These endothelial Ca2+ responses were similar to that measured by fura-2 alone, suggesting that DAF-2 loading in the same vessel does not affect the fura-2 imaging. PAF-induced NO production was also observed in all endothelial cells (Fig. 4A). The mean peak NO production rate was 1.4 ± 0.09 AU/min, which occurred at 1.3 ± 0.07 min after the initiation of PAF perfusion and returned to the control level at 21 ± 0.8 min (n = 4). When the peak NO production rate was compared with the timing of peak [Ca2+]i, there was a 0.6 ± 0.01-min delay in the NO response at the individual endothelial cell level. The time course of PAF-induced sequential changes in endothelial [Ca2+]i and NO production pooled from all endothelial cells of one vessel is shown in Fig. 4D. The magnitude of the net increase in FIDAF varied from 8.1 to 15.8 AU among cells (Fig. 4E). The mean CV of the change in FIDAF was 26 ± 1.9% (n = 4), which was significantly higher than that under basal conditions (P < 0.01) but was not significantly different from the variations in PAF-induced peak endothelial [Ca2+]i (CV: 28 ± 0.5%, P > 0.05). Cells with a higher magnitude of FIDAF increase also had a higher peak endothelial [Ca2+]i (Fig. 4, A and B). The results shown in Fig. 4F demonstrate the linear relationship between the magnitude of NO production and the peak endothelial [Ca2+]i in each individual endothelial cell (n = 4, r2 = 0.9 ± 0.02, P < 0.001).
Fig. 4.
Correlation between PAF-induced nitric oxide (NO) production and peak endothelial [Ca2+]i at the cellular level. A: fura-2 and 4,5-diaminofluorescein (DAF-2) fluorescence images from one representative experiment before and after PAF stimulation. The two ECs indicated by arrows have different responses. The cell with a higher NO response [net increase in DAF-2 FI (ΔFIDAF) = 15.5 AU] has a higher peak endothelial [Ca2+]i (951 nM), and the cell with a lower NO response (ΔFIDAF = 8 AU) has a lower peak endothelial [Ca2+]i (581 nM). B and C: time course of PAF-induced changes in endothelial [Ca2+]i and NO production in 6 of 14 ROIs. Time 0 in C indicates the time when the DAF-2 loading reached steady state. The vertical dotted line indicates the time when the PAF-induced NO production rate declined to baseline levels. D: temporal relationship between PAF-induced changes in endothelial [Ca2+]i and NO production grouped from all ECs of the vessel in A. E: histogram showing the magnitude distribution of PAF-induced NO production in 44 endothelial cells of 4 vessels. F: linear correlation between PAF-induced peak endothelial [Ca2+]i and the amount of NO production in 14 ECs from the image in A. The green and red circles represent the two ECs that are in A.
Relationship of PAF-induced NO with the magnitude of endothelial gap formation at the individual endothelial cell level in intact venules.
Confocal imaging was used to examine the spatial relationship between the magnitude of NO production and endothelial gap formation. Experiments were conducted in five vessels (7–11cells/vessel). The basal NO production rate was 0.2 ± 0.01 AUconfocal/min, and the CV was 9.0 ± 0.8% (n = 5). After PAF stimulation, increases in FIDAF occurred in all endothelial cells (Fig. 5, A and B), and the mean ΔFIDAF was 14.6 ± 1.6 AUconfocal, ranging from 11.0 to 28.7 AUconfocal. The CV was 24 ± 2.3%, which was significantly higher than that under control conditions (P < 0.01). A larger magnitude of FM accumulation was found around cells that showed higher increases in FIDAF (Fig. 5C). The correlation between the amount of NO production and the magnitude of gap formation at the cellular level is shown in Fig. 5D (n = 5, r2 = 0.9 ± 0.02).
DISCUSSION
By combining fluorescence imaging of endothelial [Ca2+]i and NO production with the simultaneous quantification of endothelial gaps, our study demonstrates the temporal and spatial relationship between inflammatory mediator-induced changes in cell signaling and endothelial gap formation at the individual endothelial cell level in intact venules. Our new findings are that 1) PAF-induced increases in endothelial [Ca2+]i, NO production, and gap formation occurred in all endothelial cells rather than a small percentage of cells along the microvessel wall; 2) the magnitude of PAF-induced peak endothelial [Ca2+]i, NO production, and gap formation varied among endothelial cells in each vessel; and 3) PAF-induced peak endothelial [Ca2+]i preceded the peak increase in NO production and the magnitude of the peak increase in endothelial [Ca2+]i was linearly correlated with the magnitude of NO production and gap formation at the individual endothelial cell level. These results demonstrate that inflammatory mediator-induced increases in microvessel permeability are associated with generalized gap formation around all endothelial cells, but that the sizes of the formed gaps vary. The fact that the magnitude of the peak endothelial [Ca2+]i level determines the amount of downstream NO production and the degree of gap formation suggests that the heterogeneity in permeability increases along the vascular wall is mainly initiated by variations in the Ca2+ response from individual endothelial cells of each vessel.
Generalized endothelial gap formation versus local leaky sites along the microvessel wall.
Endothelial gap formation has been recognized as the main transport pathway responsible for the increased permeability to fluid and macromolecules during inflammation (1, 3, 15, 18, 21, 36). Previously, the identification of endothelial gaps mainly relied on ultrastructural experiments by electron microscopy (15, 18, 36). The limited region of each electron micrograph made it impossible to illustrate the changes in endothelial junctions over an entire vessel segment. In this study, we characterized the spatial variations of PAF-induced endothelial gap formation from entire vessel segments by the quantification of entrapped FMs at open endothelial clefts. Our results revealed that at the peak of the Lp response to PAF, endothelial gaps occurred in all endothelial cells of the microvessel wall, suggesting that the increased microvessel permeability did not only occur at local regions. Histamine-induced albumin leakage has also been reported to be along the entire perfused vessel wall (8), which suggests that the involvement of all endothelial cells is unlikely to be PAF specific.
However, when inflammatory mediators were applied topically in whole vascular bed studies (15, 19, 20, 30), leakages of fluorescently labeled macromolecules were usually reported at local regions. The sensitivity of different experimental approaches may explain this difference in leakage patterns. Small leakages have been reported to be confined to the vessel wall and are difficult to visualize if fluorescently labeled macromolecules were continuously present in the vasculature (2). By the systemic injection of fluorescently labeled macromolecules, the observed local leaky sites may only represent leakages through large endothelial gaps. In PAF-stimulated vessels, our results showed that ∼13% of endothelial cells formed large gaps. This can be converted to 2.1 large gaps/100-μm vessel length in a 40-μm diameter vessel based on the endothelial cell dimensions of rat tracheal venules (21). This calculation is close to the number of bradykinin-induced leaky spots (1.8 leaky sites/100-μm vessel length) observed in hamster cheek pouch venular microvessels (15).
Temporal and spatial relationship of PAF-induced increases inendothelial [Ca2+]i, NO production, and endothelial gap formation at the individual endothelial cell level.
Although all endothelial cells responded to PAF and formed gaps, we observed a marked variability in the magnitude of gap formation among endothelial cells in the same vessel. The single vessel perfusion technique with well-controlled perfusion pressure enabled the testing agents to be uniformly distributed to the vessel wall at the distance we studied, which avoided the uncertainties of hemodynamic effects on agonist distribution. Therefore, the variability we observed is the result of individual endothelial cells responding differently to PAF. Variations in endothelial gaps have also been demonstrated in rat tracheal postcapillary venules in response to different inflammatory stimuli by silver nitrate staining (21).
It has been shown that endothelial gap formation results from cell retraction and/or the reorganization of adherens junctions (1, 9). A previous study (37) on pulmonary artery endothelial cells demonstrated that endothelial cell retraction involves Ca2+/calmodulin-dependent myosin light chain phosphorylation and that Ca2+ signaling plays essential roles in endothelial gap formation and barrier dysfunction. Furthermore, studies (11, 12, 14, 40) on intact microvessels showed that the magnitude of the inflammatory mediator-induced peak increase in endothelial [Ca2+]i determines the degree of increase in microvessel permeability. An augmented Ca2+ influx by either membrane hyperpolarization or increasing extracellular Ca2+ concentration potentiated ionomycin- and PAF-induced increases in microvessel permeability (12, 40). Depolarization of the cell membrane or the depletion of extracellular Ca2+ concentration attenuated ionomycin-induced increases in endothelial [Ca2+]i and microvessel permeability (11). At the individual endothelial level, the present study demonstrates that endothelial cells with larger gap formation have a higher magnitude of peak endothelial [Ca2+]i in PAF-perfused venules (Fig. 3, C and D). These results provide further in vivo evidence that Ca2+ plays an essential role in the regulation of endothelial gap formation.
This study showed that PAF induced a spatial heterogeneity in endothelial [Ca2+]i responses among endothelial cells in each vessel. These observations provide a basis for the future exploration of the mechanisms of these heterogeneous responses. Although differences in the number or affinity of PAF receptors (16, 33) might play a role, factors that regulate Ca2+ influx could also be involved. Nonreceptor-mediated stimuli, such as ionomycin, also produce variations in endothelial [Ca2+]i responses in intact microvessels (23, 26). Agonist-induced Ca2+ influx in endothelial cells is regulated by the electrochemical driving force through passive conductance pathways (7, 11, 12, 14). Since the concentration gradient of Ca2+ across the endothelial membrane is constant for all the cells, any variations of agonist-induced membrane hyperpolarization among endothelial cells may result in a different magnitude of Ca2+ influx (12).
In addition to Ca2+ signaling, NO has also been implicated in mediating PAF-induced and other inflammatory mediator-induced increases in microvessel permeability (4, 5, 10, 25, 27, 35, 40, 43, 44). Although Ca2+-independent eNOS activation has been reported in some studies, eNOS activation associated with increased microvessel permeability has been suggested to be Ca2+ dependent (38, 40, 43). The depletion of extracellular Ca2+ attenuated both PAF- and bradykinin-induced NO production in perfused and isolated vessels (38, 43). An increase of extracellular Ca2+ concentration that potentiated PAF-induced Ca2+ influx augmented NO production (40). In addition, inhibition of NO production by N-monomethyl-l-arginine or the caveolin-1 scaffolding domain attenuated PAF-induced increases in microvessel permeability without affecting the initial increase in endothelial [Ca2+]i, suggesting that NO regulates microvessel permeability downstream from Ca2+ (40, 43, 44). This study also demonstrated the temporal relationship between PAF-induced Ca2+ and NO at the cellular level (Fig. 4D). The 0.6-min delay of the PAF-induced NO peak from the peak increase in endothelial [Ca2+]i suggests a causal link between these two sequential events. This causal relationship is further supported by our findings showing that the magnitude of peak endothelial [Ca2+]i is linearly correlated with the level of increased NO production at the individual endothelial cell level. However, at the recovery phase, PAF-induced NO production declined to basal levels, whereas [Ca2+]i remained significantly elevated (Fig. 4D). This suggests that the inactivation of eNOS may be regulated by mechanisms other than a decline of endothelial [Ca2+]i levels. The mechanisms responsible for the recovery phase, i.e., the closure of endothelial gaps and the return of increased permeability toward the baseline level in the presence of agonist, still need to be identified.
Our previous studies (40, 43) have demonstrated a direct correlation between the magnitude of PAF-induced NO production and the degree of increases in microvessel permeability at the single vessel level. As an extension of our previous studies, this study revealed that variations of NO production and gap formation at the cellular level are well correlated with variations of peak endothelial [Ca2+]i. This suggests that initial levels of endothelial [Ca2+]i induced by the inflammatory mediator determine the downstream NO production and endothelial gap formation. It appears that heterogeneous downstream signaling and permeability responses are mainly initiated at the Ca2+ level. Other mechanisms that regulate eNOS activity and endothelial gap formation may play less important roles than Ca2+ in the spatial heterogeneity of NO production and gap formation among endothelial cells. Future studies that demonstrate a relatively uniform eNOS expression in all endothelial cells of intact microvessels will further support this hypothesis.
Currently, the detailed downstream signaling pathways linking NO with endothelial gap formation remain obscure. The soluble guanylate cyclase-derived cGMP pathway has been suggested to play important roles in NO-mediated vascular barrier dysfunction (13, 34, 39). However, exactly how the activation of the NO-cGMP pathway results in endothelial gap formation is not yet understood. Although increased endothelial [Ca2+]i plays an essential role in the regulation of inflammatory mediator-induced endothelial gap formation (28, 31, 37), blocking PAF-induced NO production by NOS inhibition nearly abolished the permeability increase without affecting the initial increase in endothelial [Ca2+]i in intact venules (40, 43, 44). These results suggest that increases in endothelial [Ca2+]i are necessary but not sufficient to increase permeability. Whether increased NO alone via a Ca2+-independent pathway is sufficient to increase microvessel permeability remains to be determined. The present experimental evidence strongly suggests that an interdependent relationship between endothelial Ca2+ and NO is essential for endothelial gap formation.
Conclusions.
In summary, this study provides direct in vivo evidence showing that PAF-induced increases in microvessel permeability are associated with the generalized formation of endothelial gaps along the microvessel wall but that the magnitude varies among individual cells. The temporal and spatial relationship of PAF-induced peak endothelial [Ca2+]i, NO production, and endothelial gap formation suggests that the heterogeneities in endothelial NO and gap formation among endothelial cells are initiated at the endothelial [Ca2+]i level. The spatial differences in the molecular signaling at the cellular level contribute to the heterogeneity in permeability increases along the microvessel wall during inflammation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-56237 and HL-084338 (to P. He) and by an American Heart Association predoctoral fellowship (to X. P. Zhou).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Adamson RH, Zeng M, Adamson GN, Lenz JF, Curry FE. PAF- and bradykinin-induced hyperpermeability of rat venules is independent of actin-myosin contraction. Am J Physiol Heart Circ Physiol 285: H406–H417, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Baldwin AL. Modified hemoglobins produce venular interendothelial gaps and albumin leakage in the rat mesentery. Am J Physiol Heart Circ Physiol 277: H650–H659, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Braverman IM, Keh-Yen A. Three-dimensional reconstruction of endothelial cell gaps in psoriatic vessels and their morphologic identity with gaps produced by the intradermal injection of histamine. J Invest Dermatol 86: 577–581, 1986 [DOI] [PubMed] [Google Scholar]
- 4. Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 6: 1362–1367, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Bucci M, Roviezzo F, Posadas I, Yu J, Parente L, Sessa WC, Ignarro LJ, Cirino G. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc Natl Acad Sci USA 102: 904–908, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clough G, Michel CC, Phillips ME. Inflammatory changes in permeability and ultrastructure of single vessels in the frog mesenteric microcirculation. J Physiol 395: 99–114, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curry FE. Modulation of venular microvessel permeability by calcium influx into endothelial cells. FASEB J 6: 2456–2466, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Curry FE, Joyner WL. Modulation of Capillary Permeability: Methods and Measurements in Individually Perfused Mammanlian and Frog Microvessels. Boca Raton, FL: CRC, 1988 [Google Scholar]
- 9. Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Hatakeyama T, Pappas PJ, Hobson RW, 2nd, Boric MP, Sessa WC, Duran WN. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol 574: 275–281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He P, Curry FE. Depolarization modulates endothelial cell calcium influx and microvessel permeability. Am J Physiol Heart Circ Physiol 261: H1246–H1254, 1991 [DOI] [PubMed] [Google Scholar]
- 12. He P, Curry FE. Endothelial cell hyperpolarization increases [Ca2+]i and venular microvessel permeability. J Appl Physiol 76: 2288–2297, 1994 [DOI] [PubMed] [Google Scholar]
- 13. He P, Zeng M, Curry FE. cGMP modulates basal and activated microvessel permeability independently of [Ca2+]i. Am J Physiol Heart Circ Physiol 274: H1865–H1874, 1998 [DOI] [PubMed] [Google Scholar]
- 14. He P, Zhang X, Curry FE. Ca2+ entry through conductive pathway modulates receptor-mediated increase in microvessel permeability. Am J Physiol Heart Circ Physiol 271: H2377–H2387, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Hulstrom D, Svensjo E. Intravital and electron microscopic study of bradykinin-induced vascular permeability changes using FITC-dextran as a tracer. J Pathol 129: 125–133, 1979 [DOI] [PubMed] [Google Scholar]
- 16. Hwang SB, Lee CS, Cheah MJ, Shen TY. Specific receptor sites for 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine (platelet activating factor) on rabbit platelet and guinea pig smooth muscle membranes. Biochemistry 22: 4756–4763, 1983 [DOI] [PubMed] [Google Scholar]
- 17. Jiang Y, Wen K, Zhou X, Schwegler-Berry D, Castranova V, He P. Three-dimensional localization and quantification of PAF-induced gap formation in intact venular microvessels. Am J Physiol Heart Circ Physiol 295: H898–H906, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Majno G, Palade GE. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol 11: 571–605, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mayhan WG. Role of nitric oxide in modulating permeability of hamster cheek pouch in response to adenosine 5′-diphosphate and bradykinin. Inflammation 16: 295–305, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Mayhan WG, Joyner WL. The effect of altering the external calcium concentration and a calcium channel blocker, verapamil, on microvascular leaky sites and dextran clearance in the hamster cheek pouch. Microvasc Res 28: 159–179, 1984 [DOI] [PubMed] [Google Scholar]
- 21. McDonald DM. Endothelial gaps and permeability of venules in rat tracheas exposed to inflammatory stimuli. Am J Physiol Lung Cell Mol Physiol 266: L61–L83, 1994 [DOI] [PubMed] [Google Scholar]
- 22. McDonald DM, Thurston G, Baluk P. Endothelial gaps as sites for plasma leakage in inflammation. Microcirculation 6: 7–22, 1999 [PubMed] [Google Scholar]
- 23. McKnight TR, Curry FE. Mechanisms of heterogeneous endothelial cytoplasmic calcium increases in venular microvessels. Microcirculation 9: 537–550, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Michel CC, Curry FE. Microvascular permeability. Physiol Rev 79: 703–761, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Nguyen LS, Villablanca AC, Rutledge JC. Substance P increases microvascular permeability via nitric oxide-mediated convective pathways. Am J Physiol Regul Integr Comp Physiol 268: R1060–R1068, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Pagakis SN, Curry FE. Imaging of Ca2+ transients in endothelial cells of single perfused capillaries: correlation of peak [Ca2+]i with sites of macromolecule leakage. Microcirculation 1: 213–230, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Ramirez MM, Quardt SM, Kim D, Oshiro H, Minnicozzi M, Duran WN. Platelet activating factor modulates microvascular permeability through nitric oxide synthesis. Microvasc Res 50: 223–234, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Sandoval R, Malik AB, Naqvi T, Mehta D, Tiruppathi C. Requirement for Ca2+ signaling in the mechanism of thrombin-induced increase in endothelial permeability. Am J Physiol Lung Cell Mol Physiol 280: L239–L247, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Simionescu N, Simionescu M, Palade GE. Open junctions in the endothelium of the postcapillary venules of the diaphragm. J Cell Biol 79: 27–44, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Svensjo E, Arfors KE, Raymond RM, Grega GJ. Morphological and physiological correlation of bradykinin-induced macromolecular efflux. Am J Physiol Heart Circ Physiol 236: H600–H606, 1979 [DOI] [PubMed] [Google Scholar]
- 31. Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol 39: 173–185, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Tsien RY. Fluorescent indicators of ion concentrations. Methods Cell Biol 30: 127–156, 1989 [DOI] [PubMed] [Google Scholar]
- 33. Valone FH, Goetzl EJ. Specific binding by human polymorphonuclear leucocytes of the immunological mediator 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycero-3-phosphorylcholine. Immunology 48: 141–149, 1983 [PMC free article] [PubMed] [Google Scholar]
- 34. Varma S, Breslin JW, Lal BK, Pappas PJ, Hobson RW, 2nd, Duran WN. p42/44MAPK regulates baseline permeability and cGMP-induced hyperpermeability in endothelial cells. Microvasc Res 63: 172–178, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Wu HM, Huang Q, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. Am J Physiol Heart Circ Physiol 271: H2735–H2739, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Wu NZ, Baldwin AL. Transient venular permeability increase and endothelial gap formation induced by histamine. Am J Physiol Heart Circ Physiol 262: H1238–H1247, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Wysolmerski RB, Lagunoff D. Involvement of myosin light-chain kinase in endothelial cell retraction. Proc Natl Acad Sci USA 87: 16–20, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yi FX, Zhang AY, Campbell WB, Zou AP, Van Breemen C, Li PL. Simultaneous in situ monitoring of intracellular Ca2+ and NO in endothelium of coronary arteries. Am J Physiol Heart Circ Physiol 283: H2725–H2732, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Yuan Y, Granger HJ, Zawieja DC, DeFily DV, Chilian WM. Histamine increases venular permeability via a phospholipase C-NO synthase-guanylate cyclase cascade. Am J Physiol Heart Circ Physiol 264: H1734–H1739, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Zhou X, He P. Endothelial [Ca2+]i and caveolin-1 antagonistically regulate eNOS activity and microvessel permeability in rat venules. Cardiovasc Res 87: 340–347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou X, He P. Improved measurements of intracellular nitric oxide in intact microvessels using 4,5-diaminofluorescein diacetate. Am J Physiol Heart Circ Physiol 301: H108–H114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou X, Wen K, Yuan D, Ai L, He P. Calcium influx-dependent differential actions of superoxide and hydrogen peroxide on microvessel permeability. Am J Physiol Heart Circ Physiol 296: H1096–H1107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu L, He P. Platelet-activating factor increases endothelial [Ca2+]i and NO production in individually perfused intact microvessels. Am J Physiol Heart Circ Physiol 288: H2869–H2877, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Zhu L, Schwegler-Berry D, Castranova V, He P. Internalization of caveolin-1 scaffolding domain facilitated by Antennapedia homeodomain attenuates PAF-induced increase in microvessel permeability. Am J Physiol Heart Circ Physiol 286: H195–H201, 2004 [DOI] [PubMed] [Google Scholar]