Abstract
Microscopic lymphatics produce nitric oxide (NO) during contraction as flow shear activates the endothelial cells. The valve leaflets and bulbous valve housing contain a large amount of endothelial nitric oxide synthase (eNOS) due both to many endothelial cells and increased expression of eNOS. Direct NO measurements indicate the valve area has a 30–50% higher NO concentration ([NO]) than tubular regions although both regions generate equivalent relative increases in [NO] with each contraction. We hypothesize that 1) the greater eNOS and [NO] of the bulb region would have greater effects to lower pumping activity of the overall lymphatic than occurs in tubular regions and 2), the elevated [NO] in the bulb region may be because of high NO production in the valve leaflets that diffuses to the wall of the bulb. Measurement of [NO] with a micropipette inside the lymphatic bulb revealed the valve leaflets generate ∼50% larger [NO] than the bulb wall in the in vivo rat mesenteric lymphatics. The valves add NO to the lymph that quickly diffuses to the bulb wall. Bradykinin locally released iontophoretically from a micropipette on both bulbs and tubes increased the [NO] in a dose-dependent manner up to ∼50%, demonstrating agonist activation of the NO pathway. However, pumping output determined by contraction frequency and stroke volume decreased much more for the bulb than tubular areas in response to the bradykinin. In effect, NO generation by the bulb area and its valves limits the pumped flow of the total lymphatic by lowering frequency and stroke volume of individual contractions.
Keywords: lymphatic, valve, nitric oxide, lymph flow
during contractions of microscopic lymphatics, our measurements of nitric oxide (NO) with microelectrodes have revealed a transient increase in NO concentration ([NO]) within 1–3 s after the contraction first begins (7). The assumption is that the increase in [NO] is caused by increased fluid shear interacting with the lymphatic endothelium. The elevation of [NO] then presumably contributes to the subsequent relaxation of the lymph vessel. These assumptions are based on multiple studies involving both pharmacological and increased fluid shear activation of endothelial nitric oxide synthase (eNOS) (14, 16, 18, 23, 26, 33, 36) that demonstrated that NO suppressed lymphatic contractions. However, the NO process is not as simple as first was assumed. As the frequency of lymphatic contractions was increased by systemic administration of saline to increase mesenteric lymph flow (2), the basal [NO] in lymphics also increased with the frequency of contraction (7). In effect, elevated pumping by lymphatics increased their production of NO presumably because of increased flow shear forces. Therefore, NO appears to limit individual contractions as well as provide a generalized inhibition of pumping during periods of high lymph flow. In both cases, NO supports the diastolic relaxation processes of the lymphatic.
The role of NO in lymphatic regulation is essential because relaxation after a contraction, or diastole in the context defined by Granger (19), McHale and Meharg (24), and Zawieja et al. (39), is severely compromised if eNOS is pharmacologically blocked (16, 18, 33). Blocking relaxation during diastole impairs refilling of the lymph pump and diminishes the subsequent stroke volume. The blockade of NO increased the frequency of lymphatic contractions at any given stretch and impaired the fall in contraction frequency with elevated lymph flow (16, 18, 33, 34). Additionally, exogenous sources of NO slowed the lymphatic contraction frequency (35). In effect, NO has negative chronotropic effects on contraction frequency in addition to its negative effects on tonic contractility. The end result is the lymphatic has time to fill and sufficient relaxation to be filled because of the dual effects of NO.
The chronotropic and inotropic effects of NO occur in a condition where there are very different [NO] along the lymph vessel. In measuring [NO] with microelectrodes, we found that the highest [NO] occurred in the valve-bulb region with much lower concentrations (by about one-third to one-half) in the tubular portions of the rat mesenteric lymphatics (7). Immunohistological analysis of eNOS in the bulb and tubular regions indicated a higher content of total eNOS in the bulb area because of greater numbers of endothelial cells near the valve leaflets and a greater eNOS expression in the cells of the bulb and valve than the tubular section. However, both regions are capable of increased basal [NO] with increased lymph flow and have comparable relative increases in [NO] with each lymphatic contraction (7).
Even though all areas of the rat mesenteric lymphatics appear quite capable of generating NO to flow shear events during contractions (7), this does not necessarily signify that the role of NO is uniformly important for all sections of the lymph vessel during the contraction and relaxation process. The passage of the contraction cycle from upstream tubular lymphatics through the bulb region and on to the downstream tubular section has been shown to combine mechanical and conducted electrophysiological events, as the Zawieja (39) and von der Weid (10, 34) groups have documented. The relaxation phase of the cycle has a component of locally generated NO in response to transiently elevated shear forces. With the higher generation of NO in the bulb-valve than tubular areas demonstrated by direct NO measurements (8), this NO could both have effects to initiate a wave of relaxation by cell-to-cell conduction and use the NO in the lymph to influence distal portions of the lymph vessel. For example, we have considered that NO generated in the lymphatic endothelium will diffuse into the flowing lymph within the valve area and might survive to downstream tubular sites and perhaps contribute to tubular relaxation. This possibility is supported to some extent by our recent finding that NO survives at vasodilatory concentrations within blood plasma of in vivo microvessels after being produced by upstream small arteries or larger arterioles (8). In our past studies of lymphatic NO, we were technically unable to reliably measure the [NO] within the lumen of microscopic lymphatics. However, this technical problem was resolved and allowed the evaluation of several hypotheses. First, we hypothesize that the lymphatic valve leaflets generate a very high [NO] relative to the rest of the lymphatic luminal wall, even the walls of the lymphatic bulb region. This premise is based on the expected high shear forces over the valve leaflets during bulb contraction (12) and the mass of eNOS represented by the endothelial cells covering all sides of the two valve leaflets (7). Assuming this hypothesis is correct, the second hypothesis to be tested is that NO formed by the leaflet endothelial cells might survive to downstream sites within the tubular portions of the lymph vessel. In measuring in vivo lymphatic oxygen tension, we consistently find that the partial pressure is below 20 mmHg even when near arterioles (unpublished observations). Consequently, NO might survive many seconds in such a low-oxygen and -protein environment as occurs in lymph (32, 38). The third hypothesis is the greater NO generation by bulb areas of the lymphatic has a greater impact on the frequency and magnitude of lymphatic contractions than occurs in tubular regions. This type of analysis has never been performed because selectively increasing and then measuring NO in a very localized area requires integrating multiple difficult microelectrode technologies with intravital microscopy. We used local release of bradykinin to activate NO production and test this hypothesis.
METHODS
Animal and tissue set up.
The protocols in these studies were approved by both the Indiana University Medical School and Texas A&M Institutional Animal Care and Use Committees. All studies were done at the Indiana University Medical School in Indianapolis. Sprague Dawley male rats (Harlan Industry, Indianapolis, IN) that weighed 300–400 g were studied. The animals were allowed water but not food for 12–18 h. Sodium thiopental (200 mg/kg; Abbott, Chicago, IL) diluted to 50 mg/ml of saline was used for general anesthesia and given subcutaneously at four sites over the lower back and thighs. This approach is essential to avoid anesthetic interaction with the fragile lymphatic vessels as would occur with intraperitoneal injection. A warmed water heating mat (35–37°C) was used to maintain body temperature at ∼37.5–38°C. Core temperature was measured in the stomach using a flexible probe advanced from the mouth (Yellow Springs Instruments).
After tracheal cannulation, the animal was mechanically ventilated at 70 breaths/min, a typical conscious ventilation frequency for the weight of rats used, and at a tidal volume predicted by the Harvard Apparatus (Harvard Apparatus, Holliston, MA) nomogram for weight, ventilation frequency, and tidal volume interaction. The tidal volume was increased to compensate for dead space in the ventilation tubing. An ear oximeter (Nonin Pulse Oximeter model 8600V, Plymouth, MN) measured the percent saturation of hemoglobin with oxygen. Percent saturations of 93–97% were achieved with ventilation, and saturation >97% was avoided to prevent hypocapnia. We chose these ventilation parameters to prevent hypocapnia based on measuring end-tidal percent carbon dioxide using a Pyron SC-210 CO2 Monitor (Pyron, Menomonee Falls, WI) in past studies (28) (1).
The cannulated right femoral artery was used to measure the mean arterial pressure and provide an infusion route for saline. The basal lymphatic activity in unfed rats is quite low and was stimulated by giving a bolus of saline (0.5 ml/100 g of body wt) followed by a continuous arterial saline infusion (0.5 ml·100 g of body wt−1·h−1). This approach provided a very stable lymphatic activity profile after ∼1 h, as several of our studies have shown (2, 7).
A loop of distal jejunum or early ileum was exposed through a midline incision (1.5–2 cm) and placed in a tissue support system. The “arterial” side of the mesentery, which is consistently the left side of the mesentery, was used for all studies because the lymph vessels typically have less adipose tissue covering them. This allows easier access to the lymphatic with microelectrodes and facilitates diameter measurements. The tissue support is a hollow stainless steel shell used routinely by this laboratory (5, 37). To secure the tissue, 4-O silk sutures were nontraumatically tied to the intestinal wall along the antimesenteric border. The sutures are held by a metal and plastic housing over the tissue that was used to direct bicarbonate-buffered complete physiological solution over the intestine and mesentery (7). The buffer flow was 5 ml/min over the tissue through a chamber volume of ∼7 ml and was immediately removed by suction. The buffer was bubbled with 5% oxygen, 5% carbon dioxide, and balance nitrogen to generate gas tensions comparable to venular gas tensions, which are quite similar to fluid aspirated from the abdominal cavity (4). Concentrated calcium chloride solution (10% in distilled water) was added after the buffer was bubbled with carbon dioxide, which reduced the pH to ∼7.4 to avoid calcium bicarbonate crystallization. The chamber system is heated and the chamber fluid also preheated with a fluid heater (heated distilled water, 1.5–1.7 l/min). The bath temperature was held at 37.5 ± 0.2°C.
Lymph vessels close to the main trunk of mesenteric arteries in route to the intestine were studied because they are not as extensively arborized as those near the bowel wall. Lymphatics in contact with an artery, vein, or large arteriole were avoided because the arteries and arterioles can often operate at higher [NO] than lymphatics, and potential interactions of blood and lymph vessel [NO] complicate the NO interpretations.
NO measurements.
NO-sensitive microelectrodes based on those developed by Buerk et al. (9) and Friedemann et al. (15) were modified to achieve the best characteristics of both designs used in earlier experiences from this laboratory (6). The open glass enclosed tip of the electrode surrounded a 7- to 8-μm-diameter carbon fiber, and the total outer width of the beveled tip was 11–14 μm. Nafion (Sigma Chemical, St. Louis, MO) was electrically deposited on the surface of the carbon fiber at +0.7 V for ∼15–20 min. Nafion forms a barrier that limits false sensing of negatively charged organic molecules (30) and nitrite/nitrate at physiological concentrations (15). For NO measurements, the microelectrodes were polarized at either +0.7 or +0.9 volts, depending on the most stable “0” NO baseline and largest current response to 600 and 1,200 nM NO gas calibrations for that microelectrode. Highly stable microelectrodes were essential because of the long periods of measurement. To improve stability, the electrodes were polarized for 12–24 h before evaluation. What small electronic drift with time did occur was compensated by determining a virtual baseline for 0 NO over time. To establish the 0 NO over living tissue, the tip of the electrode was placed 200 μm above the tissue in the flowing bathing media. The microelectrode can be moved up or down vertically ∼50 μm from this location with no change in basal current. As the microelectrode approaches within ∼100 μm of the tissue surface, a signal current begins to occur and gradually increases as the microelectrode advances toward the tissue. The actual [NO] was known from the difference of the baseline 0 current and tissue current values in concert with the [NO]/current relationship generated by the microelectrode. Currents in the 3- to 100-pA range were measured by a Keithley model 6517A Electrometer (Cleveland, OH). The electrometer was monitored with a PowerLab analog-to-digital chart recorder system (AD Instruments, Colorado Springs, CO) along with blood pressure. Data were displayed at 1-s intervals.
Lymphatic vessels are difficult to penetrate with NO microelectrodes because the tip diameters must necessarily be ∼10–12 μm to house the internal 8-μm-diameter carbon fiber. However, by using specially sharpened microelectrodes for a single use, this problem is improved substantially. To make sure that the glass of the micropipette barrel surrounded the carbon fiber, which had been cut by hand to extend ∼5 mm from the glass tip, the remaining extending carbon fiber was electrolytically etched at 3–8 volts (microelectrode positively charged) until a perceptible void was seen in the tip of the electrode at ×100. The microelectrodes were sharpened on 5-Å aluminum carbide particles deposited to a 1- to 3-mm depth in distilled water over a rotating (1 rpm) glass disc (12 cm diameter). The microelectrode tip-to-particle layer interaction was viewed with a dissecting microscope as a very shallow furrow was formed when the tip touched the surface of the water-covered alumina powder. The electrode was held at a 30° angle to the surface of the rotating glass plate. Ten minutes of contact were usually sufficient to sharpen the tip. The microelectrode was then cleaned in a stream of distilled water and soaked in absolute ethanol for 5–10 min, and then a thin layer of Nafion was electroplated as described above. With these procedures, a given electrode could routinely penetrate the wall of lymph vessels two to three times before being too dull to easily pass through the vessel wall. Fortunately, electrodes can be resharpened along the same original axis of the bevel by realigning the sharpened tip relative to the sharpening stand. However, the microelectrode requires new Nafion coverage and calibration.
To test that suppression of eNOS resulted in a decline in the measured [NO] and confirm that actions of bradykinin predominately occurred because of increased formation of NO, NO responses of lymph vessels generated by bradykinin were compared before and after exposure to nitro-l-arginine methyl ester (l-NAME) for 20–30 min. l-NAME (1 mM) in normal saline was applied from a large-tip-diameter micropipette (∼50 μm) at 50 μl/min to approximately a 500-μm length of the outer lymph vessel wall by microinjection. Lissamine green dye (1 mM) in the l-NAME solution gave a visual indication of the “cloud” of l-NAME solution over the vessel of interest. The combination of a bath suffusion flow was 5 ml/min, and the low flow of l-NAME solution (50 nl/min) would very quickly dilute l-NAME to an innocuous concentration (∼1 nM) and avoid changing overall lymph production by the small intestine. To ensure that l-NAME was effective, the [NO] was measured as the drug was released, and drug exposure continued until the [NO] reached a stable low concentration. This procedure cannot fully depress the local [NO] to near 0 nM because there is some NO in the lymph entering the small area of eNOS-blocked lymph vessel from intact upstream lymph vessels. However, the technique did completely eliminate responses to supramaximal bradykinin as will be explained in results.
Lymphatic motion measurements.
A major goal of the study was to equate changes in [NO] with frequency of contractions and changes in diameter between resting and contracted states as they interact to influence the total pumped flow/minute of the vessel. The diameter measurements were made at the same site as the NO measurement in all cases to ensure temporal correlation of mechanical and NO events. The contraction of lymph vessels is typically described in cardiac nomenclature (19, 24). The maximum diameter between contractions was termed end-diastolic diameter (EDD), the diameter at the peak contraction was end-systolic diameter (ESD), and stroke volume was calculated assuming a round structure of the lymph vessel. To follow rapid contractile and relaxation events, the vessel was time lapse recorded using a video camera and Metamorph Imaging software (Molecular Devices, Sunnyvale, CA). Images were taken and stored at 1-s intervals for 3 min. The vessel diameters were measured by the operator using previously determined magnification parameters for the images based on a stage micrometer marked in 10- and 100-μm units. Images were not recorded until it was obvious that the vessel was at steady-state behavior either at rest or when being stimulated by a given amount of bradykinin. Correlation of NO and diameter events was assured by simultaneously starting the time lapse video and adding a timing mark in the PowerLab recording program.
Bradykinin activation.
To highly localize the actions of bradykinin to a specific area of a lymph vessel, iontophoretic release of bradykinin from a micropipette was used. These microelectrodes had a tip diameter of 3–5 μm after intentionally breaking the tip under precise micromanipulator control. Because the very thin mesenteric membrane was in direct contact with the lymphatic outer wall, there was no need for the bradykinin micropipette to pierce mesenteric tissue but simply rest against the joint mesenteric-lymphatic walls. The bradykinin concentration in distilled water was 1 mM, and currents of 50, 100, 200, and 400 nA were used. To make sure the NO microelectrodes did not react to bradykinin, the tips of the bradykinin and NO microelectrodes were placed almost touching each other's tips in bath media. Maximum electrical current and consequently release of bradykinin did not influence the current being generated by the NO microelectrode. The release current for the iontophoretic electrode was controlled by a World Precision Instruments model 260-B Iontophoresis Programmer with a retain current of 10 nA to prevent leakage of bradykinin during control periods. During experiments, the bradykinin was released for up to 10 min, and data were taken for 3 min once the vessel response was stable, which usually required ∼2–3 min of measurements. The NO microelectrode was placed immediately beside the tip of the bradykinin microelectrode. In these experiments, the enhanced sharpening technique of the NO microelectrodes was avoided so that the microelectrode tip would not enter the vessel wall, but the tip was beveled. The beveled tip could pass through the mesenteric membrane and be literally pressed against the lymphatic wall with a very slight bend in the microelectrode shaft near the tip. This allowed the electrode to move with the lymphatic wall during contractions. After stoppage of the bradykinin release current, the lymphatic vessels resumed normal contractions within ∼3 min.
To evaluate the effects of bradykinin before and after suppression of eNOS with l-NAME, the [NO], EDD, ESD, and the frequency of contractions were compared for resting conditions and during exposure to a maximum iontophoretic dosage of bradykinin (400 nA) during control conditions and after exposure to l-NAME for 20–30 min. The measurements were chosen because they are the primary response changes to bradykinin. Measurements were analyzed during the 2nd and 3rd min of a 3-min release of bradykinin on a second-by-second basis.
Statistics.
Statistical differences were evaluated with one-way ANOVA if comparing regionally different values or two-way ANOVA for repeated measures when control and response conditions were measured and compared between regions. Each ANOVA was followed by the Tukey's Least-Significant Difference test to determine specific significant events. The analysis was performed with Statistica Software (Statsoft, Tulsa, OK). Data are reported as means and SE.
RESULTS
Lymphatic wall and lumen [NO].
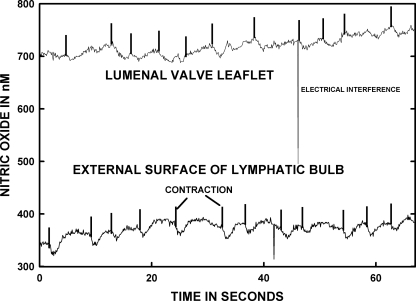
Figure 1 presents representative real-time measurements of lymphatic wall and valve leaflet [NO] from two different areas on the same lymphatic bulb. For the sake of consistency for all measurements in Figs. 1 and 2, the external bulb recordings were all taken on the bulb surface very near the tip ending areas of lymphatic valve leaflets. Data presented in Figs. 2 and 3 illustrate that the bulb region has a higher lymphatic wall [NO] than tubular regions, and the location near the valve tips has the highest [NO] along the entire lymphatic bulb.
Fig. 1.
Nitric oxide concentration ([NO]) of the external bulb surface and internal valve leaflet are shown for the same lymphatic. The data were not obtained simultaneously but within 10 min of each other. When the nitric oxide (NO) micropipette is very near and essentially touching the valve leaflet surface, the recorded [NO] was consistently much higher than on the outer surface of the bulb wall. The vertical marks on each record indicate the onset of mechanical contraction by the lymphatic bulb followed by a transient decrease in [NO] as the valve closed and then an increase in [NO] presumably activated by the transient increase in lymph flow before the valve closed. The same mechanical correlation to changes in [NO] was more evident for the bulb external surface than in the lumen near the lymphatic valves.
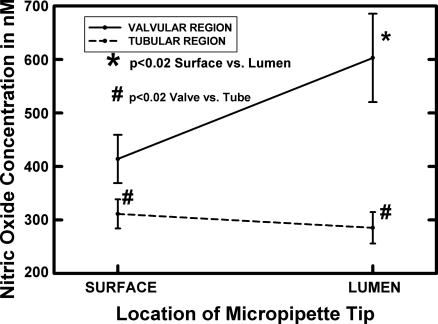
Fig. 2.
The luminal and surface [NO] of lymphatic bulb and tubular regions are shown as averages for 11 bulb and 12 tube regions in 9 animals with dual bulb and downstream tubular measurements in all but one animal. The tubular region was ∼500 μm downstream from the terminal part of the lymphatic bulb. The valve lumen [NO] was consistently much higher than that of the bulb surface near the terminal portion of the valve leaflets. The tubular surface and luminal [NO] were very similar and within the resolution of the NO microelectrode technology in most cases. In all animals, the tubular wall [NO] was lower than that of the bulb surface. *Significant difference from the bulb origin.
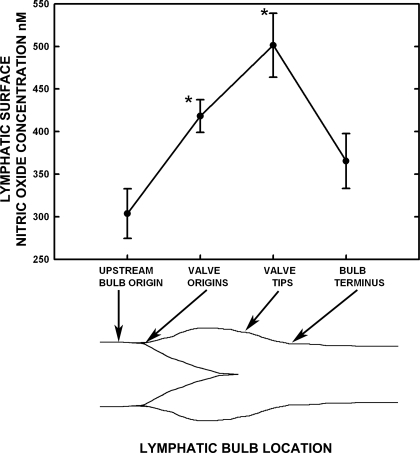
Fig. 3.
The [NO] along the lymphatic bulb region is highly influenced by the proximity of valvular tissue. Four locations from the origin of the bulb, the origin of the valve leaflets on the bulb surface, the bulb surface at the valve tips, and bulb terminus were easy to consistently identify and compare [NO] between 12 bulbs in 9 animals. The inset tracing of a lymphatic bulb image identifies these regions. The tips of the valves were associated with the highest bulb surface [NO], and even the origin of the valves raised the [NO] relative to the bulb origin and terminus. The [NO] from valve tip regions to the terminus of bulbs decreased dramatically, and the terminal [NO] were only marginally higher than the origin surface [NO]. *Significant difference from the bulb origin.
The vertical lines in the graphs for Fig. 1 mark the lymphatic contraction. In general, as the bulb contracts and the valve leaflets close, where a mark occurs in the record, the [NO] decreases shortly thereafter because flow through the valve has ceased momentarily. Next, the relaxation phase occurs, the valve reopens, and, with the resumption of flow, the [NO] increased. Flow of lymph was judged by the motion of lymphocytes in the vicinity. For the majority of measurements, there was a delay of ∼1.5–2.5 s after contraction began before the peak increase in lymphatic wall and lumen [NO] occurred for both bulb wall and valve leaflets. This delay matches the delay we have recorded previously between the onset of the contraction to the peak velocity (∼1.75 s) (12). This timing delay of contraction and the following increase in [NO] also applied to tubular regions of lymph vessels, as shown in our prior publication using identical methodology (8).
The averaged data for measurements of [NO] inside and outside of the lymphatic wall in the valve bulb region, as well as the tubular region in 9 animals, are shown in Fig. 2. In these measurements, the tubular area was ∼500 μm downstream from the terminus of the valve/bulb. The luminal tube [NO] was essentially identical to that of its outer wall. The comparable [NO] on the surface and lumen of the tubular regions indicated the lymph was equilibrated with the vessel wall [NO]. By contrast, the averaged [NO] very near the valve leaflet was 150–300 nM higher than at the outer surface of the nearby lymphatic bulb wall. Also, the valve area surface [NO] was consistently higher by 100–150 nM than the downstream tube wall [NO].
The [NO] along the outside of the lymphatic bulb region from its origin to termination were measured and are shown in Fig. 3. These data are the time average during repeated systole and diastoles for several minutes. For practical purposes, the [NO] at the origin of the bulb just as it begins to enlarge is the same as just upstream for the tubular regions. This is demonstrated by the tubular exterior [NO] in Fig. 2 of 311 ± 27 nM compared with that of 330 ± 29 nM at the bulb origin in Fig. 3. Similar comparisons can be made for data in Fig. 4, which used different animals. In the in vivo images, the valve leaflet interface with the wall of the bulb was marked clearly by a dense curved line representing the valve leaflet connection to the bulb exterior wall. At the origin of the bulb/valve leaflet region, there was a distinct increase in [NO] of ∼100 nM compared with the very beginning of the bulb, as shown in Fig. 3. There was an additional increase in [NO] of 100 nM on the exterior of the bulb at the tips of the lymphatic leaflets relative to the valve origin. In total, [NO] in the region of the bulb exterior at the valve tips was ∼200 nM higher than at the bulb origin. At the terminus of the bulb where the lymphatic diameter first reaches the tubular diameter, [NO] had decreased by 100–200 nM to a value only 50–100 nM above that at the bulb origin. In a prior study (7), measurements of [NO] along tubular regions of lymph vessels were found to be quite consistent for a given vessel, and these measurements were not repeated.
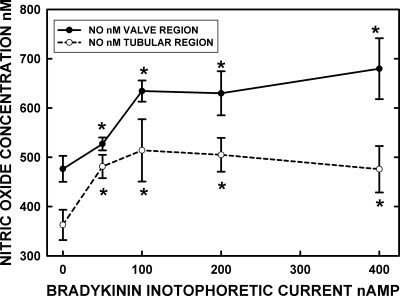
Fig. 4.
The dose/NO response to microiontophoretic application of bradykinin to the wall of valve and tubular regions indicated the peak response of NO occurred at ∼100-nA release currents. However, at both 200- and 400-nA currents, the NO response extended upstream and downstream from the release site by at least 100 μm but was highly localized for the lower-release currents. Both diffusion of bradykinin and cell-to-cell conduction events are presumed to explain the distance-NO response issues. The data are based on 9 sets of bulb and tubular regions in 5 rats. *Significant (P < 0.05) increase in [NO] from 0 current (retain current) for the bradykinin microelectrode.
Bradykinin effects on lymphatic wall [NO] and contraction events.
The first tests we did with iontophoretic release of bradykinin on the walls of mesenteric lymphatic vessels were to establish the dose-response curve of [NO] vs. ejection current. As shown in Fig. 4, [NO] increased in a dose-dependent fashion up to 100–200 nA for both the valve and tubular regions of the lymph vessel. However, these data did not reflect that, as the eject current was increased, the section of the lymph vessel that responded to bradykinin with an increase in [NO] occurred over a substantially longer distance. While the stimulated NO release was highly localized at low release currents for bradykinin, the region of increased NO extended ∼200 μm in the up- and downstream directions at 200- and 400-nA currents. This presumably reflects diffusion of bradykinin to some extent and perhaps some form of cell-to-cell conduction along the lymphatic that induced greater NO formation. Once again, we found the [NO] at the valve region was substantially higher than [NO] at the tubular region, and likewise the magnitude of the increase in [NO] after bradykinin application at the valve region was higher than the change in [NO] at the tubular region.
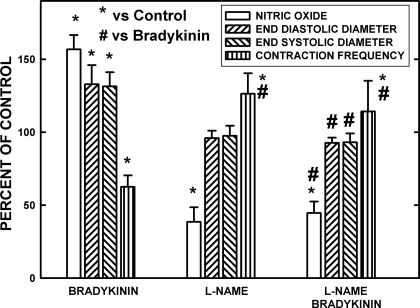
Figure 5 presents the effects of supramaximal dosage of bradykinin (400 nA) under control conditions before and after suppression of eNOS with l-NAME. All measurements were made on tubular regions of seven lymph vessels in five rats. Bradykinin under control conditions increased the [NO], EDD, and ESD but caused a significant decrease in lymphatic contraction frequency. l-NAME alone lowered the resting [NO] by ∼60% and increased the frequency of lymphatic contractions ∼30%. Very localized application of l-NAME as used in this study had no effect on the resting EDD and ESD; however, exposure of over 1 mm of lymph vessel caused localized diastolic and ESD constriction, as previously reported (7). After l-NAME pretreatment, bradykinin had no effect on any of the parameters measured. Therefore, under these conditions, actions of bradykinin through nonnitronergic mechanisms appeared to be very limited.
Fig. 5.
The effects of bradykinin at a maximal dosage (400 nA) and nitro-l-arginine methyl ester (l-NAME) suppression of endothelial nitric oxide synthase (eNOS) on [NO], end-diastolic diameter (EDD), end-systolic diameter (ESD), and frequency of contractions is shown for control and bradykinin-stimulated conditions. Seven vessels in 5 rats were used to obtain the data. Under control conditions, bradykinin raised the [NO] as the vessels dilated in diastole and systole, and the frequency of contraction declined. Very localized application of l-NAME decreased the resting [NO] to 38.5% of control, and the frequency of contraction increased. After l-NAME, bradykinin had no effects on the [NO], diameters, or frequency of contractions. Therefore, bradykinin predominately influenced rat mesenteric lymphatics through increasing NO production. *Significant change from control. #Significant change from bradykinin-induced responses.
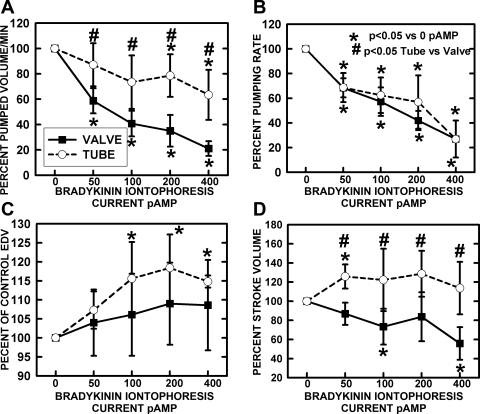
Once the dose-response characteristics of bradykinin and its dependence on NO formation were established, the pumped volume per minute (stroke volume × frequency), pumping rate or frequency, end diastolic volume (3.14 × radius2), end systolic volume, and stroke volume were measured over the range of bradykinin current dosages. These data are shown in Fig. 6. Generally, the change in these responses reached maximum at a bradykinin dosage of 200 nA. The pumping frequency of both valve and tubular areas decreased quite similarly as the release current of bradykinin increased (Fig. 6B). The pumped volume per minute (Fig. 6A), which took into mathematical account the effects on stroke volume (Fig. 6D) and pumping rate, decreased much more in the valve than tubular regions. Bradykinin at all dosages did increase the EDD of both tubular and valvular areas (Fig. 6C), but particularly for the tubular areas. However, from the larger EDD, the stroke volume was increased for the tubular region but decreased for the valve region. The interaction of stroke volume and contraction frequency lowered pumped volume per minute to some degree for both sections, but it was particularly evident for the valve region.
Fig. 6.
Chronotropic and mechanical responses of tubular and bulb regions to bradykinin iontophoretic application. A: pumped volume/min is analogous to cardiac output calculated from frequency and stroke volume. B: the frequency of pumping decreased essentially identically for tubular and valvular regions. C: end-diastolic volume (EDV) increased significantly for the tubular regions and, as shown in D, stroke volume significantly increased for the tubular regions but significantly decreased for the valve regions as bradykinin release was increased. The combination of data as pumped volume/min indicated a substantial drop in valve region flow due to the combination of decreased frequency and stroke volume. A much smaller decrease in flow occurred in the tube region due to increased stroke volume to offset the decline in contraction frequency. *Significant change from control. #Significant difference between valve and tube regions.
DISCUSSION
Our first concern in this study was if the lymphatic valve leaflets could generate a very high [NO] relative to the walls of the lymphatic bulb region due to high shear rates of fluid during the bulb contraction cycle (12) and the large mass of eNOS represented by the valvular endothelial cells (7). Our past study (7) using NO-sensitive microelectrodes with in vivo lymphatics indicated a much higher [NO] at the bulb surface than the tubular lymphatic surface, and we have confirmed these observations in Fig. 2. From our past study on the quantitative immunocytochemistry of eNOS in rat mesenteric lymphatics (7), we assumed the greater eNOS content found for the combined valve leaflet plus bulb cells compared with that in just the wall cells in the tubular regions partially explained the regional differences in [NO]. The data in Figs. 1 and 2 confirm that the [NO] very near the leaflet surface was indeed on average ∼50% greater than at the surface of the bulb wall. By comparison, the lumen and wall [NO] for tubular regions are equivalent almost within the measurement error of ± 10–20 nM (Fig. 2). The consequence of the large source of NO made by the valve leaflets was higher [NO] on the outer surface of bulb regions at the valve origins and tips of the valve leaflet areas, as shown in Fig. 3. As a frame of reference, the [NO] of the bulb region near the valve leaflet origin and terminus was quite similar to the perivascular [NO] of the largest arterioles in the wall of the small intestine (6, 8, 20). This indicates that lymphatics of the mesentery, particularly in the bulb regions, are very competent generators of NO.
Our working hypothesis based on the new data and past experience (7) is that the flow of lymph during lymphatic diastole and even greater flow during parts of systole activate the valve leaflet endothelial cells to generate a substantial [NO]. Image analysis techniques using high-speed image acquisition (11, 12) and visual observation have demonstrated lymphocytes moving through the valve region during the diastolic phase, which indicated ongoing lymph flow due to upstream tubular contractions. The contraction of the bulb in the vicinity of the valves accelerates lymph flow through the bulb before the valves close (11, 12) and presumably caused the transient increases in [NO] seen ∼1.5–2 s after each contraction began (Fig. 1). However, with each contraction of the lymph bulb, there was first a drop in [NO] as lymph velocity momentarily stopped during valve closure followed by a 50- to 200-nM increase in [NO]. Presumably the rush of lymph through the bulb during the contraction cycle activated eNOS, and a slight delay occurred before additional NO was made and diffused to the surface of the valve leaflet. To improve the reliability of NO measurements on the valve leaflets, the tip of the microelectrode must lightly press against the leaflet so that contact is maintained even during valve closure. This approach did occasionally artifactually activate the endothelial cells with a large but transient increase in [NO] for up to 5–10 min before normal behavior resumed. However, with appropriate conditions, as the peak of the contraction of the bulb passed by the terminus of the leaflet, shown by the upward marks in Fig. 1, the valves closed, and a fall in [NO] was noted. The closure of the valves would lessen the shear forces on the valve leaflets and, as the data show, quickly allow the [NO] to begin declining. These data demonstrate that NO generation by the valve is both remarkably fast and highly dependent on the mechanical activity of the bulb. These observations have shown the value of real-time measurement of [NO] and lymphatic motion to reveal the physical interactions of the bulb and valves with NO generated by the valves as a feedback system to the bulb wall to limit contraction. Valves in other parts of the cardiovascular system, such as the heart valves and venous valves, may use somewhat similar mechanisms linked to shear forces and receptor activation to locally generate NO. For example, Odashiro et al. (29) and Eguchi and Katusic (13) have shown endothelial cells of venous valves release vasoactive molecules. In the heart valves where high flow shear is routine, release of NO has been documented in in vitro studies both pharmacologically by Ku et al. (22) and by measurements of NO by Siney and Lewis (31) and Moesgaard et al. (27).
It is very important to note in Fig. 3 that the upstream bulb origin and downstream terminus had similar [NO]. These data indicate that the valves interacting with flowing lymph cause a localized large increase in [NO] that quickly dissipated downstream from the valve leaflets as lymph exited the bulb region. Therefore, our second hypothesis that NO formed by the leaflet endothelial cells might survive to downstream sites is supported, but the consequences are small because of the rapid dissipation of NO in the vessel wall and probably interaction of NO with oxygen to form nitrate. However, substantial lateral diffusion of NO from the valve leaflets through the lymph to the wall of the bulb was confirmed because the highest [NO] of the bulb occurred just as the valve leaflets terminated (Fig. 3). The comparable lumen and wall [NO] for tubular regions indicated that the lymph in the lumen was equilibrated with the [NO] of the tubular wall, as shown in Fig. 2. The increase in tubular [NO] during very localized bradykinin release presented in Fig. 4 indicated that tubular sections are quite capable of increased NO generation through receptor-mediated mechanisms, just as occurred for bulbs. In this context, local stimulation of NO production in bulb and tubular regions could be quite autonomous to shear forces and local chemical mediators, in keeping with our earlier finding that both regions have similar relative increases in [NO] with each contraction cycle and during equivalent increases in contraction frequency (7). Therefore, the role of NO to suppress an ongoing contraction cycle is a predominantly localized event that is coordinated along the lymphatic vessel primarily by the flow of lymph to regionally increase shear forces and any vasoactive NO-dependent mediators in the lymph or tissue.
With the results indicating autonomy of NO production in lymphatic bulb and tubular regions during lymphatic contraction, our next concern was whether NO generation by bulb or tubular areas of the lymphatic had a greater impact on the frequency and magnitude of lymphatic contractions. In part, this concern stems from the large contribution of valve leaflet NO to the bulb wall [NO] shown in Figs. 2 and 3. In effect, the lymphatic valves in flowing lymph stream are ideally situated to sense lymph flow and use NO to ensure relaxation of the bulb wall occurred. As such, NO generation in the bulb region could be the limiting factor to pumping lymph downstream by influencing the frequency and stroke volume of the bulb contractions. To increase NO generation, bradykinin was released onto the wall of lymphatic vessels using a microiontophoretic technique to have a point source of the endothelial-dependent dilator. As mentioned in methods, the increase in NO was highly localized at low ejection currents for bradykinin but occurred over a longer length of vessel at high-release currents, presumably because of some combination of bradykinin diffusion and cell-to-cell conduction of a signal to increase NO production. As shown in Fig. 4, the bulb and tubular region [NO] increased both in dose-response fashion to increased release of bradykinin, and the actual increases by 150–175 nM in [NO] were quite similar for the two regions of the lymph vessel. As shown in Fig. 5, the inotropic and chronotropic actions of bradykinin are predominately through increased formation of NO. The evidence for this conclusion is that, after suppression of eNOS with l-NAME, supramaximal stimulation with bradykinin had no effects on the vessel wall [NO], the diastolic and systolic diameters, or the frequency of contractions. These variables were the primarily effected entities during bradykinin release at natural conditions, as shown by the first set of data Fig. 5, left. The decline in [NO] at rest after l-NAME exposure also indicated that the NO-sensitive microelectrode followed the decline in local [NO] that would be expected with suppression of eNOS. The local [NO] was not reduced to near 0 nM [NO] by very localized application of l-NAME because there was [NO] in lymph entering from intact regions of the upstream lymphatic. However, the absence of any increase in [NO] during bradykinin release did indicate localized suppression on the NO generation mechanism.
During the release of bradykinin under control conditions, the mechanical consequences of increased NO generation in the valvular and tubular regions were substantially different. The percent changes in pumped flow per minute for sequential bulb and tube regions are shown in Fig. 6A. Stimulation of NO production in the valve area decreased pumped flow much more than occurred for the tubular region. This difference was not the result of variations in the frequency of contraction with increased bradykinin release, as shown in Fig. 6B. The tubular and bulb regions had essentially identical reductions in contraction frequency, with increasing NO caused by bradykinin release. The difference in pumped volume per minute was the result of a reduced stroke volume in the valve area compared with a marginal increase in stroke volume in the tubular area, as shown in Fig. 6D. The enlarged end diastolic volume of tubular regions (Fig. 6C) indicated relaxation during the increased formation of NO caused by bradykinin. It is possible that this distention of the lymphatic during diastole would contribute to increased stroke volume of subsequent contractions, as shown in Fig. 6, C and D, and minimize the decline in minute pumped volume as the frequency of contractions decreased, as shown in Fig. 6, A and B. Based on earlier studies, we know the enhanced filling as lymph accumulated induced a stronger phasic contraction to increase the stroke volume (3, 17). Additionally, we have previously seen in isolated lymphatics that phasic lymphatic contraction frequency and lymphatic tone appear more sensitive to be reduced by NO generation than does the phasic contraction strength, which is positively influenced by stretch (16, 18). Thus, an increase in NO production that is the result of pump flow/shear in isolated lymphatics, as well as bradykinin applied to in vivo lymphatics in the current study (Figs. 4 and 6), reduces pump contraction frequency and diastolic tone compared with what was seen with NO blockade but not stroke volume directly (Figs. 6B and 7). In the isolated lymphatic, these events combine to then increase diastolic filling, which enhanced the subsequent stroke volume, presumably via increased preload effects. If we further increased NO generation by imposing a pressure gradient across the isolated lymphatics to increase flow/shear, we observed further reductions in phasic lymphatic contraction frequency and lymphatic tone and a direct decrease in phasic stroke volume, as shown diagrammatically in Fig. 7B (16, 18). Thus we propose that the difference in basal [NO] we observed between the bulb and tubular sections sets the stage for the different contractile effects on pumped flow, end-diastolic volume, and stroke volume we observed upon further [NO] increase by bradykinin (Figs. 2 and 4). As shown in Fig. 4, the increase in [NO] at the bulb caused by bradykinin while well developed only increased end-diastolic volume ∼10% while substantially lowering contraction frequency. In effect, the resting [NO] in the bulb is high enough that this segment of the lymphatic is chronically dilated, but NO has effects through mildly increased lusitropy and a diminished contraction frequency. The basal [NO] in the tubular section is 30–50% lower than that found in the bulb (Fig. 4), and, as a result, it is relatively less dilated than the bulb and can increase EDV (Fig. 4C) by ∼20% as the [NO] is increased by bradykinin. The net effect is that, in the tubular region, with a greater ability to increase lusitropy and thus enhance stroke volume, pumped volume per minute (Fig. 5A) increased despite an inhibited pacemaker frequency seen in the valve region (Fig. 5B). When we increase the NO generation at the bulb, we get modest further decreases in tone/increased diameter, but these now lead to a fall in stroke volume as we have presumably reached the declining phase of the stretch-stroke volume relationship. Increasing NO generation in the tubular section produces further decreases in tone/increased diameter, which lead to an enhanced stroke volume as we are presumably still on the inclining phase of the stretch-stroke volume relationship. For both sections of the lymphatic, the increase in [NO] decreases the pacemaker similarly, which is expected, since we have previously shown that a dominant pacemaker will drive long multilymphangion segments of the lymphatic (39). This reduction in contraction frequency with increasing [NO] is the predominant factor reducing lymph pump flow from the bulb in the tubular sections of the lymphatic. However, the effects of this reduction in contraction frequency on pump flow in the tubular section are mitigated by a slightly increased stroke volume, whereas, in the bulb, it is exacerbated by a decreased stroke volume (Fig. 6) and shown diagrammatically in Fig. 7. If the data are taken in the context that the downstream tubular region only receives lymph based on the contractile function of the upstream valve and bulb, the greater suppression of stroke volume by NO in the valve than tubular region predicts NO effects are most important for valve regions. Thus, under basal conditions, the bulbous region of the lymphatic is operating at a relatively optimum state of [NO] and accompanying pump characteristics, whereas the tubular section at a lower [NO] still has some lusitropic reserves. When conditions change to increase [NO] either via increased flow/shear or by agonist-induced NO release, the vessel as a whole becomes a dilated, weaker pump with the bulb exhibiting the greatest influence in pump status. In situations where lymph flow and pressure generated by the upstream organ is high, the contractility and frequency of contraction of lymph vessels does increase, as many studies have shown (2: 7, 17, 21, 25, 26, 39). However, simultaneous increases in shear elevate [NO] and modulate the contractile response to ensure proper diastolic relaxation and, based on the current data, have inhibitory chronotropic effects. Because valves appear to generate most of the NO in the bulb region, which in turn relaxes the contractile components of the bulb, the valves not only control the physical movement of lymph along the lymphatic but also have a regulatory role in influencing the pumping rate and force of contraction of the overall lymphatic structure through the negative chronotropic and ionotropic effects of NO.
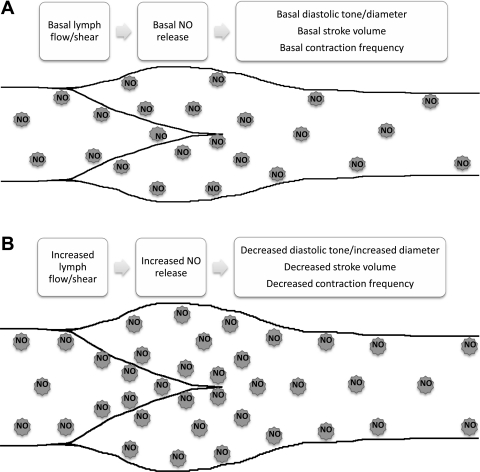
Fig. 7.
Summary of the effects of the differential production of NO in the valve/sinus vs. tubular sections of the lymphatic on [NO] and the lymphatic contractile characteristics. Schematic of a lymphatic vessel depicting the wall, valve leaflets, and sinus and tubular regions of the lymphatic where [NO] is highest near the valve/sinus region. A: effects under normal basal lymph flow. B: changes observed during increases in lymph flow or other mechanisms that elevate NO production.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01 HL-70308.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We express sincere appreciation to Randall Bills for excellent technical service in preparing animals for in vivo microscopy.
REFERENCES
- 1. Bauser-Heaton HD, Bohlen HG. Cerebral microvascular dilation during hypotension and decreased oxygen tension: a role for nNOS 2. Am J Physiol Heart Circ Physiol 293: H2193–H2201, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress 7. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989 [DOI] [PubMed] [Google Scholar]
- 4. Bohlen HG. Intestinal mucosal oxygenation influences absorptive hyperemia. Am J Physiol Heart Circ Physiol 239: H489–H493, 1980 [DOI] [PubMed] [Google Scholar]
- 5. Bohlen HG. Integration of intestinal structure, function, and microvascular regulation. Microcirculation 5: 27–37, 1998 [PubMed] [Google Scholar]
- 6. Bohlen HG. Mechanism of increased vessel wall nitric oxide concentrations during intestinal absorption. Am J Physiol Heart Circ Physiol 275: H542–H550, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol 297: H1319–H1328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bohlen HG, Zhou X, Unthank JL, Miller SJ, Bills R. Transfer of nitric oxide by blood from upstream to downstream resistance vessels causes microvascular dilation. Am J Physiol Heart Circ Physiol 297: H1337–H1346, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buerk DG, Riva CE, Cranstoun SD. Nitric oxide has a vasodilatory role in cat optic nerve head during flicker stimuli. Microvas Res 52: 13–26, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Crowe MJ, von der Weid PY, Brock JA, van Helden DF. Co-ordination of contractile activity in guinea-pig mesenteric lymphatics. J Physiol 500: 235–244, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dixon JB, Gashev AA, Zawieja DC, Moore JE, Jr, Cote GL. Image correlation algorithm for measuring lymphocyte velocity and diameter changes in contracting microlymphatics. Ann Biomed Eng 35: 387–396, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13: 597–610, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Eguchi D, Katusic ZS. Inhibitory effect of valves on endothelium-dependent relaxations to calcium ionophore in canine saphenous vein. Am J Physiol Heart Circ Physiol 280: H892–H898, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Ferguson MK, DeFilippi VJ. Nitric oxide and endothelium-dependent relaxation in tracheobronchial lymph vessels. Microvasc Res 47: 308–317, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Friedemann MN, Robinson SW, Gerhardt GA. o-Phenylenediamine-modified carbon fiber electrodes for the detection of nitric oxide. Anal Chem 68: 2621–2628, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gashev AA, Wang W, Laine GA, Stewart RH, Zawieja DC. Characteristics of the active lymph pump in bovine prenodal mesenteric lymphatics. Lymphat Res Biol 5: 71–79, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575: 821–832, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Granger HJ. Role of the interstitial matrix and lymphatic pump in regulation of transcapillary fluid balance. Microvasc Res 18: 209–216, 1979 [DOI] [PubMed] [Google Scholar]
- 20. Kempson S, Thompson N, Pezzuto L, Glenn BH. Nitric oxide production by mouse renal tubules can be increased by a sodium-dependent mechanism. Nitric Oxide 17: 33–43, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koller A, Mizuno R, Kaley G. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: role of endothelial prostanoids. Am J Physiol Regul Integr Comp Physiol 277: R1683–R1689, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Ku DD, Nelson JM, Caulfield JB, Winn MJ. Release of endothelium-derived relaxing factors from canine cardiac valves. J Cardiovasc Pharmacol 16: 212–218, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Leak LV, Cadet JL, Griffin CP, Richardson K. Nitric oxide production by lymphatic endothelial cells in vitro. Biochem Biophys Res Commun 217: 96–105, 1995 [DOI] [PubMed] [Google Scholar]
- 24. McHale NG, Meharg MK. Co-ordination of pumping in isolated bovine lymphatic vessels. J Physiol 450: 503–512, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mizuno R, Dornyei G, Koller A, Kaley G. Myogenic responses of isolated lymphatics: modulation by endothelium. Microcirculation 4: 413–420, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins 2. Am J Physiol Regul Integr Comp Physiol 274: R790–R796, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Moesgaard SG, Olsen LH, Aasted B, Viuff BM, Pedersen LG, Pedersen HD, Harrison AP. Direct measurements of nitric oxide release in relation to expression of endothelial nitric oxide synthase in isolated porcine mitral valves. J Vet Med A Physiol Pathol Clin Med 54: 156–160, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Nase GP, Tuttle J, Bohlen HG. Reduced perivascular Po2 increases nitric oxide release from endothelial cells. Am J Physiol Heart Circ Physiol 285: H507–H515, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Odashiro T, Komori K, Ishii T, Okadome K, Sugimachi K. Comparison of endothelial function between in situ and reversed vein graft: differences in endothelium-dependent responses. Surgery 117: 179–188, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Pezzuto L, Bohlen HG. Extracellular arginine rapidly dilates in vivo intestinal arteries and arterioles through a nitric oxide mechanism. Microcirculation 15: 123–135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siney L, Lewis MJ. Nitric oxide release from porcine mitral valves. Cardiovasc Res 27: 1657–1661, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci USA 98: 355–360, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsunemoto H, Ikomi F, Ohhashi T. Flow-mediated release of nitric oxide from lymphatic endothelial cells of pressurized canine thoracic duct. Jpn J Physiol 53: 157–163, 2003 [DOI] [PubMed] [Google Scholar]
- 34. von der Weid PY, Crowe MJ, van Helden DF. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J Physiol 493: 563–575, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. von der Weid PY, Zhao J, van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol 280: H2707–H2716, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Yokoyama S, Ohhashi T. Effects of acetylcholine on spontaneous contractions in isolated bovine mesenteric lymphatics 6. Am J Physiol Heart Circ Physiol 264: H1460–H1464, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Zani BG, Bohlen HG. Sodium channels are required during in vivo sodium chloride hyperosmolarity to stimulate an increase in intestinal endothelial nitric oxide production. Am J Physiol Heart Circ Physiol 288: H89–H95, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Zawieja DC, Barber BJ. Lymph protein concentration in initial and collecting lymphatics of the rat. Am J Physiol Gastrointest Liver Physiol 252: G602–G606, 1987 [DOI] [PubMed] [Google Scholar]
- 39. Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ. Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol Heart Circ Physiol 264: H1283–H1291, 1993 [DOI] [PubMed] [Google Scholar]