Abstract
Cyclooxygenase metabolites stimulate or sensitize group III and IV muscle afferents, which comprise the sensory arm of the exercise pressor reflex. The thromboxane (TP) receptor binds several of these metabolites, whose concentrations in the muscle interstitium are increased by exercise under freely perfused conditions and even more so under ischemic conditions, which occur in peripheral artery disease. We showed that the exercise pressor reflex is greater in rats with simulated peripheral artery disease than in rats with freely perfused limbs. These findings prompted us to test the hypothesis that the TP receptor contributes to the exaggerated exercise pressor reflex occurring in a rat model of peripheral artery disease. We compared the cardiovascular responses to static contraction and stretch before and after femoral arterial injections of daltroban (80 μg), a TP receptor antagonist. We performed these experiments in decerebrate rats whose femoral arteries were ligated 72 h before the experiment (a model of simulated peripheral artery disease) and in control rats whose hindlimbs were freely perfused. Daltroban reduced the pressor response to static contraction in both freely perfused (n = 6; before: Δ12 ± 2 mmHg, after: Δ6 ± 2 mmHg, P = 0.024) and 72-h-ligated rats (n = 10; before: Δ25 ± 3 mmHg, after: Δ7 ± 4 mmHg, P = 0.001). Likewise, daltroban reduced the pressor response to stretch in the freely perfused group (n = 9; before: Δ30 ± 3 mmHg, after: Δ17 ± 3 mmHg, P < 0.0001) and in the ligated group (n = 11; before: Δ37 ± 5 mmHg, after: Δ23 ± 3 mmHg, P = 0.016). Intravenous injections of daltroban had no effect on the pressor response to contraction. We conclude that the TP receptor contributes to the pressor responses evoked by contraction and stretch in both freely perfused rats and rats with simulated peripheral artery disease.
Keywords: static contraction, tendon stretch, neural control of the circulation, thin fiber muscle afferents
during exercise, mean arterial pressure (MAP), heart rate (HR), myocardial contractility, and ventilation increase. These circulatory and ventilatory increases ensure that the working muscle is provided with adequate blood flow and oxygen. The exercise pressor reflex (30), first evidenced by Alam and Smirk (1), is one mechanism thought to be responsible for these cardiovascular and ventilatory effects. The afferent arm of the reflex is comprised of thinly myelinated group III afferents and unmyelinated group IV afferents (25). The majority of group III afferents are mechanically sensitive and are activated by distortion of their receptive fields, whereas most group IV afferents are chemically sensitive and are activated by the metabolic by-products of working muscle (17–19, 27, 28, 36). While both afferent components of the exercise pressor reflex function to increase blood pressure and HR during exercise, the metabolically sensitive one is thought to signal a mismatch in contracting muscle between blood supply and metabolic demand (39). While much research has been done to identify the muscle by-products and their associated receptors that mediate metaboreflex activation, to date, the role played by the thromboxane (TP) receptor is unknown.
When cyclooxygenase metabolites of arachidonic acid are hydrolyzed by thromboxane A synthase, thromboxane A2 (TxA2) is formed (31, 32). TxA2, when bound to the TP receptor, facilitates platelet aggregation and constricts vascular smooth muscle (3, 5). Moreover, the TP receptor has several other agonists, including 8-isoprostane, PGH2, PGF2α, PGE2, and PGD2 (9). In addition, human and animal studies have shown that cyclooxygenase metabolites of arachidonic acid sensitize and/or stimulate group III and IV afferent fibers and, in turn, contribute to the magnitude of the exercise pressor reflex (4, 6, 11, 20, 29, 35, 38, 41). The extent to which this effect on the exercise pressor reflex is caused by stimulation of the TP receptor is not known.
Peripheral artery disease, which can be caused by smoking, diabetes, hypertension, and aging, often leads to chronic or acute ischemia (21). A common symptom of peripheral artery disease that results in insufficient blood flow to the legs during exercise is intermittent claudication, an effect that is relieved by rest (21). Recently, experiments performed in a rat model of peripheral artery disease (34, 48) showed that the exercise pressor reflex in rats whose femoral arteries were ligated for 72 h before the start of the experiment was significantly greater than that in rats whose hindlimbs were freely perfused (44). The receptors responsible for the exaggerated exercise pressor reflex in the rat model of peripheral artery disease have not been identified. Because substantial evidence exists showing that hindlimb ischemia increases arachidonic acid levels as well as its cyclooxygenase metabolites in the muscle interstitium during contraction or exercise (37, 42), we were prompted to test the hypothesis that the TP receptor contributed to the exaggerated exercise pressor reflex occurring in the rat model of peripheral artery disease.
METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University, Hershey Medical Center. The following experimental techniques were performed in adult, male Sprague-Dawley rats (n = 101). All rats were housed in a temperature-controlled room (24 ± 1°C) with a 12:12-h light-dark cycle and fed a standard diet and tap water ad libitum.
To simulate peripheral artery disease in rats, we ligated the left femoral artery 72 h before the experiment. Briefly, rats were anesthetized with isoflurane gas (2–3%) in pure oxygen. Under sterile procedure, the femoral artery was surgically exposed and ligated with suture (5–0, silk) just distal to the inguinal ligament. This technique has been shown to reduce blood flow capacity to ∼10–20% of normal while having little effect on resting blood flow (34, 48).
Surgical Techniques
On the day of the experiment, rats were anesthetized with isoflurane gas (2–3%) in pure oxygen, after which the trachea was cannulated and their lungs mechanically ventilated with the gaseous anesthetic until the decerebration procedure was completed. Both carotid arteries and a jugular vein were cannulated (PE-50) to measure arterial blood pressure and to administer fluids, respectively. Arterial blood gases and pH were measured throughout the experiment using an automated blood gas analyzer (ABL 700 Series; Radiometer). Dexamethasone (0.2 mg) was given intravenously to minimize edema caused by decerebration (43). Body temperature was maintained between 36.5 and 38.0°C by an isothermal heating pad and lamp.
To administer all agonists and antagonists, the circulation of the left hindlimb was isolated. A catheter (PE-10, polyethylene tubing) was inserted in a retrograde manner into the right femoral artery and its tip advanced to the abdominal aorta. A reversible balloon occluder was tied around the abdominal aorta and the inferior vena cava, which when inflated helped to maintain the injectate within the circulation of the left hindlimb.
A laminectomy was performed to expose the spinal cord and the lower lumbar roots (L2-L6). Next, the L4 and L5 ventral roots, which innervate the muscles of the hindlimb, were identified and sectioned. The cut peripheral ends of L4 and L5 ventral roots were placed on a bipolar shielded-stimulating electrode, and all exposed neural tissue was immersed in warm mineral oil. The rats were then secured in a customized spinal frame, and the calcaneal bone was sectioned and its tendon connected to a force transducer (FT10; Grass Instruments) that in turn was attached to a rack-and-pinion.
Anesthetized rats were placed in a stereotaxic head unit (Kopf Instruments), and a precollicular decerebration was performed using the method described previously by Tsuchimochi et al. (44). Briefly, a dull blade was passed through the brain stem ∼0.5 mm rostral to the superior colliculi, and the remaining neural tissue rostral to the section was aspirated. To minimize bleeding, small pieces of oxidized regenerated cellulose (Ethicon; Johnson & Johnson) were placed on the internal skull surface, and the cranial cavity was packed with gauze. Immediately after precollicular transection, anesthesia was discontinued. Each rat was allowed to stabilize for at least 1 h.
Experimental Protocols
Exercise pressor and muscle mechanoreceptor reflexes.
The exercise pressor reflex was evoked by electrical stimulation of the cut peripheral ends of the left L4 and L5 ventral roots (1–3 times motor threshold, 0.1-ms pulse duration, 40 Hz) for 30 s. TP receptors in the left hindlimb were blocked by injecting daltroban (80 μg), a TP receptor antagonist, into the arterial supply of the left hindlimb (8, 47). Before injection, the balloon occluder on the abdominal aorta and inferior vena cava was inflated to trap the injectate within the circulation of the left hindlimb. After the drug had been trapped for 5 min, the balloon was deflated, and the muscles were freely perfused for 10 min before we again initiated contraction.
The muscle mechanoreceptor reflex (40) was activated by manually stretching the left triceps surae muscles for 30 s by turning the rack-and-pinion attached to the calcaneal tendon. Baseline tension was set between 50 and 100 g for both static contraction and tendon stretch. Passive hindlimb stretch was performed before and after injection of the TP receptor antagonist daltroban (80 μg) in the arterial supply of the left hindlimb. As in the contraction protocol, the injectate was trapped in the hindlimb vasculature for 5 min after which the muscles were freely perfused for 10 min, and hindlimb stretch was repeated. In the rats tested with tendon stretch, laminectomies were not performed in 7 of the 9 having patent femoral arteries; likewise, laminectomies were not performed in 8 of the 11 having ligated femoral arteries. Consequently, there was less blood loss in rats not undergoing laminectomies, which in turn may have led to higher baseline arterial pressures than in rats in which laminectomies were performed.
Muscle microdialysis to measure thromboxane B2.
Microdialysis probes were manufactured by gluing both ends of a 2-cm length of capillary microdialysis membrane (0.20 mm in diameter with a 13-kDa molecular cutoff) into nylon tubing. The nylon tubing was attached to a Luer tip adapter stub that connects the probe and the perfusate-filled syringe. Each rat had four microdialysis probes placed in the gastrocnemius and soleus muscles, with the fibers being spaced ∼0.25 cm apart. The probes were inserted in the muscles via a 20-gauge cannula and were positioned parallel to the muscle fiber orientation. The insertion and exit points were ∼3 cm apart. The microdialysis probes were threaded through the lumen of the cannula, and the cannula was withdrawn once the probes were inserted in the muscle. Each rat underwent a 2-h stabilization period after probe insertion before triceps surae contraction.
We used microdialysis to obtain interstitial fluid from the triceps surae muscles. Saline was administered through the probes via a perfusion pump (model 402; CMA) at 20 μl/min. At the end of the 2-h stabilization period, dialysate samples were collected during 60 s of static contraction. We needed to contract the muscles for 60 s to obtain enough dialysate fluid to perform the enzyme-linked immunosorbent assay (ELISA) that measured thromboxane B2 (TxB2) concentrations, a stable metabolite of TxA2. Contractions were initiated by electrical stimulation of the tibial nerves (40 Hz; 0.025 ms). The current intensity was adjusted so that the peak tension developed approximated that generated when the exercise pressor reflex was evoked by stimulation of the ventral roots. The dialysate was sealed in ice-cold microcentrifuge tubes and stored at −80°C until the quantitative determination of TxB2 by commercially available ELISA methods (Cayman Chemical). TxB2 concentrations are expressed in picograms per milliliter.
Data Analysis
In all experiments, baseline and reflex changes in MAP, HR, and developed tension were recorded continuously with a Spike 2 data acquisition system (CED) and stored on a computer hard drive (Dell). The initial 30 s before stimuli were taken as baseline to compare peak responses with experimental maneuvers. MAP is expressed in millimeters mercury (mmHg), and HR is in beats per minute. The tension-time index (TTI) (33) was calculated by integrating the area between the tension trace and the baseline level and is expressed in kilograms per second.
All values are expressed as means ± SE. Statistical comparisons were performed with a one between-one within ANOVA. If the overall F value was significant, post hoc tests were performed with the Tukey test between individual means. The criterion for statistical significance was set as P < 0.05.
RESULTS
Intra-Arterial Injection of a TxA2 Mimetic and Its Blockade by Daltroban
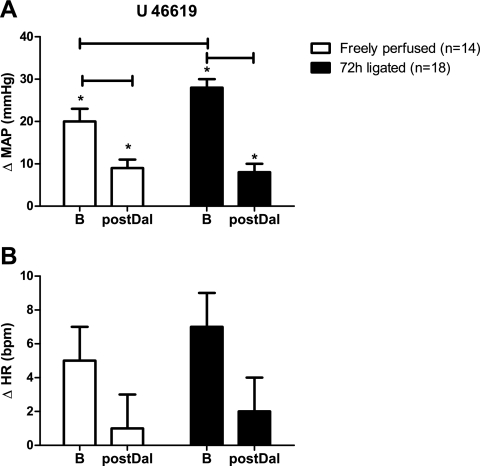
To establish the efficacy of the TP receptor blockade, we determined the pressor response to hindlimb intra-arterial injections of the TxA2 mimetic U-46619 (0.4 μg), before and after injecting the TP receptor blocker daltroban (80 μg) in the left hindlimb arterial supply. Daltroban significantly reduced the cardiovascular responses to U-46619 injections in both rats whose femoral arteries were ligated (n = 18) and in rats whose hindlimbs were freely perfused (n = 14) (P < 0.05) (Fig. 1). The pressor responses to U-46619 injection in the rats with ligated femoral arteries were significantly greater than were the pressor responses to injection of this TXA2 mimetic in the rats with patent femoral arteries (P = 0.02; Fig. 1). Daltroban injection had no effect on baseline arterial blood pressure or HR (Table 1).
Fig. 1.
Effect of hindlimb intra-arterial injection of the thromboxane (TP) receptor agonist U-46619 (0.4 μg) on reflex increases in mean arterial pressure (MAP; in mmHg) and heart rate (HR; in beats/min) before (B) and after (postDal) hindlimb intra-arterial injection of the TP receptor antagonist daltroban (80 μg). Values are means ± SE. The horizontal brackets signify that the responses were significantly different from each other (P < 0.05). *Values significantly increased from baseline (P < 0.05).
Table 1.
Baseline MAP and HR immediately before static contraction and tendon stretch both before and after the intra-arterial administration of daltroban
| Condition | MAP, mmHg | HR, beats/min | |
|---|---|---|---|
| Freely perfused | |||
| U-46619 | Before | 97 ± 5 | 454 ± 14 |
| After | 103 ± 13 | 496 ± 35 | |
| Static contraction | Before | 74 ± 5 | 436 ± 5 |
| After | 75 ± 3 | 524 ± 55 | |
| Tendon stretch | Before | 111 ± 9 | 445 ± 13 |
| After | 103 ± 12 | 484 ± 18 | |
| 72-h Ligated | |||
| U-46619 | Before | 102 ± 8 | 455 ± 13 |
| After | 107 ± 11 | 481 ± 20 | |
| Static contraction | Before | 84 ± 6 | 427 ± 7 |
| After | 96 ± 14§ | 394 ± 29§ | |
| Tendon stretch | Before | 139 ± 14 | 467 ± 9 |
| After | 129 ± 16 | 469 ± 16 |
Values are means ± SE. MAP, mean arterial pressure; HR, heart rate. There were no significant differences between baselines before and after daltroban (80 μg).
Significantly different from freely perfused baseline (P < 0.05).
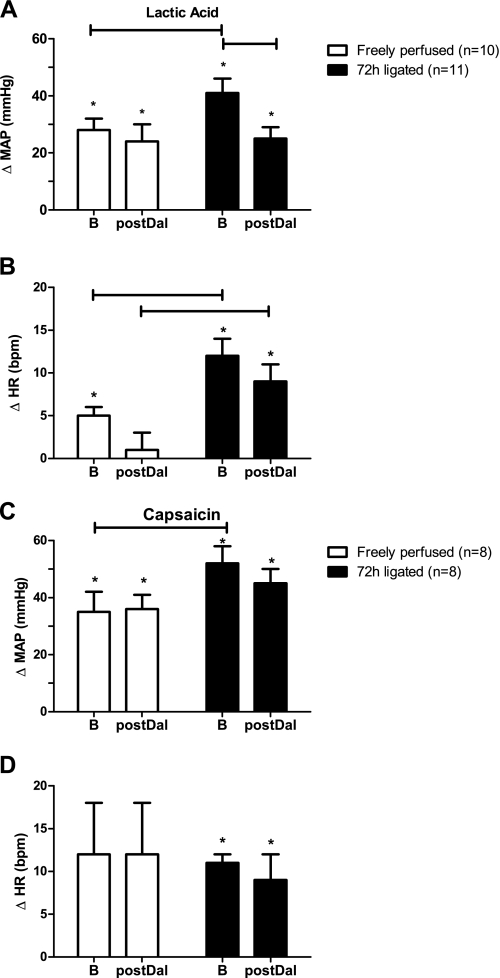
In 22 rats that were not used in the above experiments, we measured the pressor responses to intra-arterial injection of lactic acid (24 mM; 0.4 ml) and capsaicin (0.2 μg in 0.l ml) both before and after intra-arterial injection of daltroban (80 μg). We found that TXA2 receptor blockade with daltroban had no effect on the pressor and cardioaccelerator responses to either lactic acid or capsaicin in the rats with patent femoral arteries (Fig. 2). Likewise, daltroban had no effect on the pressor and cardioaccelerator responses to capsaicin in the rats with ligated femoral arteries. Daltroban, however, attenuated the pressor, but not the cardioaccelerator, response to lactic acid injection in the rats with ligated femoral arteries (Fig. 2).
Fig. 2.
Effects of hindlimb intra-arterial injection of lactic acid (24 mM; 0.4 ml) and capsaicin (0.2 μg in 0.1 ml) on the reflex increases in MAP and HR before and after hindlimb intra-arterial injection of the TP receptor antagonist daltroban (80 μg). Values are means ± SE. The horizontal brackets signify that the responses were significantly different from each other (P < 0.05). *Values significantly increased from baseline (P < 0.05).
Exercise Pressor Reflex and Daltroban
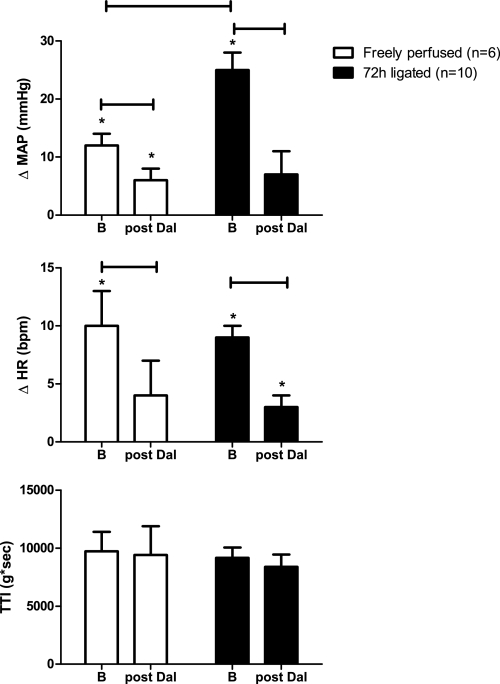
In both the “72-h-ligated” (n = 10) and the “freely perfused” rats (n = 6), we statically contracted the left hindlimb muscles (i.e., evoked the exercise pressor reflex) before and after intra-arterial injection of the TP receptor antagonist daltroban (80 μg). We confirmed our previous finding (44) that the pressor responses to static contraction in rats who had undergone femoral artery ligation 72 h prior were significantly greater than those observed in control rats whose hindlimbs were freely perfused (Fig. 3).
Fig. 3.
Effect of static contraction on the peak reflex increases in MAP and HR before and after intra-arterial injection of daltroban (80 μg), a TP receptor antagonist. Tension-time index (TTI) values for all hindlimb contractions were not different across groups. Values are means ± SE. The horizontal brackets signify that the responses were significantly different from each other (P < 0.05). *Values significantly increased from baseline (P < 0.05).
In both the freely perfused and the 72-h-ligated rats, the pressor component of the exercise pressor reflex was reduced significantly by TP receptor blockade with daltroban injected intra-arterially (Fig. 3). In both groups of rats, the cardioaccelerator component was also reduced significantly (P < 0.05). Peak tension development and TTIs during static contraction were similar between groups both before and after TP receptor blockade (Fig. 3).
In contrast to the attenuating effect on the exercise pressor reflex of intra-arterial injection of daltroban, in three rats, intravenous injection of this TP receptor antagonist (80 μg) had no effect on the reflex. In two, the hindlimb was freely perfused, and the pressor responses to contraction were 40 and 12 mmHg before intravenous daltroban and 38 and 13 mmHg afterward. Likewise, in the one remaining rat, the femoral artery was ligated 72 h before the start of the experiment, and the pressor response before intravenous daltroban was 33 and 29 mmHg afterward.
Muscle Mechanoreceptor Reflex and Daltroban
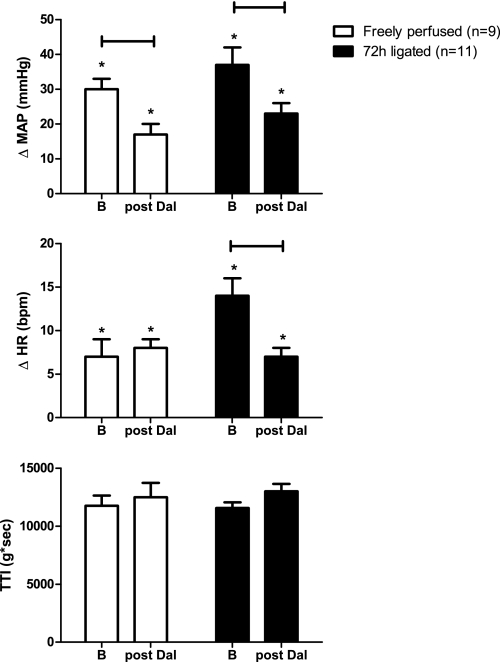
In both the 72-h-ligated (n = 11) and the freely perfused rats (n = 9), we examined the effect of daltroban (80 μg), injected intra-arterially on the reflex cardiovascular response to stretch of the calcaneal tendon, a maneuver that evokes the muscle mechanoreceptor reflex (40). The pressor responses to tendon stretch in rats whose femoral arteries were ligated 72 h before the start of the experiment were not significantly greater than those in rats whose hindlimbs were freely perfused (Fig. 4). Daltroban significantly reduced the pressor responses to tendon stretch in both groups (Fig. 4). Also, daltroban attenuated the cardioaccelerator responses to tendon stretch in the 72-h-ligated rats but did not do so in the freely perfused rats. Triceps surae tension development and TTIs during tendon stretch were not altered by daltroban (Fig. 3). Daltroban had no effect on baseline MAP in either treatment group (Table 1).
Fig. 4.
Effect of stretch on reflex increases in MAP and HR before and after intra-arterial injection of daltroban (80 μg). All TTI values were similar across groups. Values are means ± SE. The horizontal brackets signify that the responses were significantly different from each other (P < 0.05). *Values significantly increased from baseline (P < 0.05).
Effect of Static Contraction on Interstitial Levels of TxB2
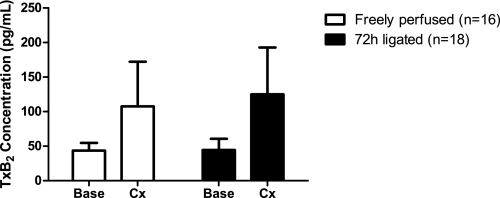
Although there was a tendency for an increase, static contraction for 60 s did not significantly alter TxB2 concentrations in the microdialysis fluid collected from either group of rats. Moreover, the results were highly variable (Fig. 5). For example, in the freely perfused group, TxB2 levels increased in 12 and decreased in 4 rats during contraction. In the 72-h-ligated group, TxB2 levels increased in eight, decreased in six, and remained the same in four rats. Increases in MAP, HR, and TTI were not significantly different between groups.
Fig. 5.
Effects of 1 min of static contraction on interstitial thromboxane B2 (TxB2) concentrations (pg/ml) in “freely perfused” and “72-h-ligated” rats. The TxB2 concentration data show a trend for increasing during static hindlimb contraction in both groups of rats. However, the results were highly variable. Values are means ± SE. Base, baseline; Cx, contraction.
DISCUSSION
We have shown that daltroban, injected in the arterial supply of the hindlimb, attenuated both the exercise pressor reflex and the muscle mechanoreceptor reflex in decerebrated unanesthetized rats. Daltroban, a TP receptor antagonist, attenuated both reflexes in rats whose hindlimbs were freely perfused and in rats whose femoral arteries were ligated 72 h before the start of the experiment. Although we found that injecting U-46619, a TxA2 mimetic, in the arterial supply of the hindlimb evoked pressor responses that could be blocked with daltroban in both groups of rats, the TXA2 mimetic evoked a greater pressor response in the rats whose femoral arteries were ligated than it did in the rats with patent femoral arteries.
Several lines of evidence suggest that ligation of a femoral artery for 24–72 h markedly increased the responsiveness of thin fiber afferents to a variety of stimuli. For example, lactic acid, an acid-sensing ion channel agonist, injected in the arterial supply of the hindlimb evoked a larger reflex pressor response in rats whose femoral arteries were ligated than it did in rats whose hindlimbs were freely perfused (23). Likewise, capsaicin, a transient receptor potential vanilloid (TRPV) 1 receptor agonist, injected in the same manner as lactic acid also evoked a larger pressor response in rats whose femoral arteries were ligated than it did in rats whose hindlimbs were freely perfused (45). These findings can also be extended to our present results in which U-46619, a TP receptor agonist, evoked a larger pressor response in rats with ligated arteries than it did in rats with patent arteries. In addition, static contraction of the hindlimb muscles in rats whose femoral arteries were ligated evoked a larger pressor reflex than did static contraction of the hindlimb muscles in rats whose hindlimbs were freely perfused (44). We speculate that, in part, these exaggerated pressor effects evoked in the 72-h-ligated rats were caused by an increase in nerve growth factor (NGF) whose levels in dorsal root ganglion cells were increased by femoral artery ligation (46). Support for our speculation comes from the finding that infusion of NGF by osmotic minipump was shown to increase the responsiveness of dorsal root ganglion cells to capsaicin (46).
One might be surprised by our finding that daltroban, a TP receptor antagonist, attenuated the exercise pressor reflex even though we were not able to show in either the freely perfused or in the 72-h-ligated groups that static contraction significantly increased interstitial levels of TxB2, a stable by-product of TxA2. In our experiments, the variability across rats was great, some showing large contraction-induced increases in TxB2 levels and others showing no increase or even a decrease. Consequently, we cannot claim with any certainty that daltroban prevented TxA2 from binding to the TP receptor to attenuate the exercise pressor reflex in our experiments. The inability to find a significant contraction-induced increase in interstitial levels of TxB2 has also been reported in human studies using muscle microdialysis (14, 15). The TP receptor has several other agonists in addition to TxA2. These include 8-isoprostane, PGH2, PGF2α, PGE2, and PGD2 (9). Static contraction of hindlimb muscles has been shown to increase the interstitial concentrations of several of these prostanoids (13, 26, 42) and acting in combination with each other and at times with TxA2 may have stimulated the TP receptor in our experiments. In our experiments, the TP receptor may have contributed to the augmented exercise pressor reflex in rats with a ligated femoral artery by either being upregulated or by being stimulated by increased concentrations of 8-isoprostane, PGH2, PGF2α, PGE2, and PGD2. A limitation of our experiments is that they cannot distinguish between these two possibilities.
We found that daltroban significantly decreased the exercise pressor reflex and the muscle mechanoreceptor reflex in both the freely perfused and the 72-h-ligated groups. These findings prompted us to test the specificity of the blockade by this supposed TP receptor antagonist. Consequently, we showed that daltroban had no effect on the pressor response to either lactic acid or capsaicin injection in rats with patent femoral arteries. Similarly, daltroban had no effect on the reflex pressor responses to injection of capsaicin, a TRPV1 receptor agonist, in rats with ligated femoral arteries, but it did attenuate the pressor responses to lactic acid injection in these rats. The attenuation of the pressor response to lactic acid by daltroban might be explained by the fact that lactic acid injection is likely to produce cyclooxygenase metabolites of arachidonic acid, which in turn can stimulate the TP receptor (12, 24). Why this happened in rats with ligated femoral arteries and did not happen in rats with patent femoral arteries is unclear, but it may be related to a heightened TP receptor sensitivity or number in the former state.
The muscle mechanoreceptor reflex, which is evoked by tendon stretch (40), is used by some investigators as an index of the mechanical component of the exercise pressor reflex (25). Although the afferent arm of both reflexes is comprised of group III afferents with mechanosensitive endings in muscle, about one-half of the ones that respond to stretch do not respond to static contraction (10). As a result, any attempt to use the muscle mechanoreceptor reflex as an index of the mechanical component of the exercise pressor reflex is fraught with difficulty. Nevertheless, the muscle mechanoreceptor reflex, by itself, has utility because it allows one to assess whether or not the TP receptor plays a role in determining the mechanical sensitivity of thin fiber muscle afferents regulating cardiovascular function. Our findings showing that daltroban attenuated the pressor reflex evoked by tendon stretch provide further evidence that the mechanical responsiveness of group III afferents can be influenced by receptors that are sensitized by their surrounding chemical environment (38). Because direct pressure and shear stress have been known to increase cyclooxygenase metabolites from human endothelial cells (2), the possibility exists that tendon stretch in our experiments released these metabolites, which in turn binded to the TP receptor. Furthermore, blocking TP receptors might have reduced the overall excitability level of thin fiber muscle afferents, which in turn made the afferents less responsive to stretch.
TxA2 is not stable in the blood and, consequently, has a very short half-life. These properties make it unsuitable for testing its capacity to stimulate thin fiber visceral or somatic afferents. Instead, a stable TxA2 analog, U-44619, has been developed as a surrogate compound. U-44619 has been shown to stimulate almost one-half of the group III and IV muscle afferents tested (20). In addition, this TP analog has been shown to stimulate ischemically sensitive cardiac sympathetic afferents with both Aδ and C fibers (7). Likewise, this analog has been shown to stimulate vagal afferent C fibers accessible from the pulmonary circulation (16). In combination, these findings suggest that TP receptors are found across a wide variety of sensory nerves and that their stimulation may have potent reflex effects on the cardiovascular system (7).
At first glance, one might conclude from perusing our findings that the TP receptor plays an essential role both in generating the exercise pressor reflex in rats with freely perfused hindlimbs as well as in generating its exaggerated counterpart evoked by femoral arterial ligation. The recent finding by Light et al. (22) strongly suggests that this conclusion needs to be qualified. Specifically, Light et al. showed that the “adequate stimulus” for the cell bodies of thin fiber muscle afferents was a combination of metabolic by-products of contraction. A corollary to the finding of Light et al. (22) is that the removal of the input of any one metabolite, such as that done by us with daltroban, could greatly reduce thin fiber muscle afferent discharge and therefore the magnitude of the exercise pressor reflex. If this corollary is correct, then blockade of any receptor or mechanogated channel activated by contraction or stretch would have the same effect as did daltroban in our experiments.
In closing, part of our motivation for performing our experiments was to identify an antagonist that was effective in determining the receptor on group III and IV muscle afferents responsible for the exaggerated exercise pressor reflex in rats whose femoral arteries were ligated. Perhaps the simplest way to determine this was to obtain results in which an antagonist reduced the exaggerated exercise pressor reflex in the ligated rats but had no effect on the reflex in the freely perfused rats. In our experiments, TP receptor blockade with daltroban did not allow us to make this distinction because daltroban attenuated the exercise pressor reflex in both rats with freely perfused hindlimbs as well as in rats whose femoral arteries were ligated.
GRANTS
This work was supported by National Institutes of Health Grants PO1 HL-096570, RO1 AR-059397, and F32 HL-108406-1.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Sarah Simmonds for technical assistance.
REFERENCES
- 1. Alam M, Smirk FH. Observation in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bouaziz A, de Ficquelmont-Loizos MM, Richert A, Caprani A. Direct physical factors and PGI2 and TXA2 secretions by a human endothelial cell line: in vitro investigation of pressure and shear stress applied independently or in synergy. Thromb Res 90: 279–289, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Bunting S, Moncada S, Vane JR. The effects of prostagladin endoperoxides and thromboxane A2 on strips of rabbit coeliac artery and certain other smooth muscle preparations [proceedings]. Br J Pharmacol 57: 462P–463P, 1976 [PMC free article] [PubMed] [Google Scholar]
- 4. Cui J, McQuillan P, Momen A, Blaha C, Moradkhan R, Mascarenhas V, Hogeman C, Krishnan A, Sinoway LI. The role of the cyclooxygenase products in evoking sympathetic activation in exercise. Am J Physiol Heart Circ Physiol 293: H1861–H1868, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ellis EF, Nies AS, Oates JA. Cerebral arterial smooth muscle contraction by thromboxane A2. Stroke 8: 480–483, 1977 [DOI] [PubMed] [Google Scholar]
- 6. Fontana GA, Pantaleo T, Bongianni F, Gresci F, Lavorini F, TostiGuerra C, Panuccio P. Prostaglandin synthesis blockade by ketoprofen attenuates the respiratory and cardiovascular responses to static handgrip. J Appl Physiol 78: 449–530, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Fu LW, Guo ZL, Longhurst JC. Undiscovered role of endogenous thromboxane A2 in activation of cardiac sympathetic afferents during ischaemia. J Physiol 586: 3287–3300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fu LW, Phan A, Longhurst JC. Myocardial ischemia-mediated excitatory reflexes: a new function for thromboxane A2? Am J Physiol Heart Circ Physiol 295: H2530–H2540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol 146: 834–845, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol 290: H2239–H2246, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol 99: 1891–1896, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Ikeda Y, Ueno A, Naraba H, Oh-Ishi S. Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing responses of mice. Life Sci 69: 2911–2919, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Karamouzis I, Christoulas K, Grekas D, Giannoulis K, Vamvakoudis E, Mandroukas K. The response of muscle interstitial F2-isoprostane (8-ISO-PGF2alpha) during dynamic muscle contractions in humans. Prostaglandins Leukot Essent Fatty Acids 71: 87–90, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Karamouzis M, Karamouzis I, Vamvakoudis E, Ampatzidis G, Christoulas K, Angelopoulou N, Mandroukas K. The response of muscle interstitial prostaglandin E(2)[PGE(2)], prostacyclin I(2)[PGI(2)] and thromboxane A(2)[TXA(2)] levels during incremental dynamic exercise in humans determined by in vivo microdialysis. Prostaglandins Leukot Essent Fatty Acids 64: 259–263, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Karamouzis M, Langberg H, Skovgaard D, Bulow J, Kjaer M, Saltin B. In situ microdialysis of intramuscular prostaglandin and thromboxane in contracting skeletal muscle in humans. Acta Physiol Scand 171: 71–76, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Karla W, Shams H, Orr JA, Scheid P. Effects of the thromboxane A2 mimetic, U46,619, on pulmonary vagal afferents in the cat. Respir Physiol 87: 383–396, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res 50: 133–139, 1982 [DOI] [PubMed] [Google Scholar]
- 18. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 19. Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Kenagy J, VanCleave J, Pazdernik L, Orr JA. Stimulation of group III and IV afferent nerves from the hindlimb by thromboxane A2. Brain Res 744: 175–178, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Kollerits B, Heinrich J, Pichler M, Rantner B, Klein-Weigel P, Wolke G, Brasche S, Strube G, Kronenberg F. Intermittent claudication in the Erfurt Male Cohort (ERFORT) Study: its determinants and the impact on mortality. A population-based prospective cohort study with 30 years of follow-up. Atherosclerosis 198: 214–222, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X and TRPV1. J Neurophysiol 100: 1184–1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299: H1357–H1364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Longhurst JC, Tjen-A-Looi SC, Fu LW. Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion. Mechanisms and reflexes. Ann NY Acad Sci 940: 74–95, 2001 [DOI] [PubMed] [Google Scholar]
- 25. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCord JL, Hayes SG, Kaufman MP. PPADS does not block contraction-induced prostaglandin E2 synthesis in cat skeletal muscle. Am J Physiol Heart Circ Physiol 295: H2043–H2045, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mense S, Meyer H. Bradykinin-induced modulation of the response behavour of different types of feline group III and IV muscle receptors. J Physiol 398: 49–63, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mense S, Stahnke M. Responses in muscle afferent fibers of slow conduction velocity to contractions and ischemia in the cat. J Physiol 342: 383–397, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Middlekauff HR, Chiu J. Cyclooxygenase products sensitize muscle mechanoreceptors in healthy humans. Am J Physiol Heart Circ Physiol 287: H1944–H1949, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- 31. Needleman P, Moncada S, Bunting S, Vane JR, Hamberg M, Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature 261: 558–560, 1976 [DOI] [PubMed] [Google Scholar]
- 32. Palmer MA, Piper PJ, Vane JR. The release of rabbit aorta contracting substance (RCS) from chopped lung and its antagonism by anti-inflammatory drugs. Br J Pharmacol 40: 581P–582P, 1970 [PMC free article] [PubMed] [Google Scholar]
- 33. Perez-Gonzalez JF. Factors determining the blood pressure responses to isometric exercise. Circ Res 48: I-76–I-86, 1981 [PubMed] [Google Scholar]
- 34. Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates the responses of group IV muscle afferents to static contraction. Am J Physiol Heart Circ Physiol 259: H745–H750, 1990 [DOI] [PubMed] [Google Scholar]
- 36. Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 37. Rotto DM, Massey KD, Burton KP, Kaufman MP. Static contraction increases arachidonic acid levels in gastrocnemius muscles of cats. J Appl Physiol 66: 2721–2724, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by products of arachidonic acid metabolism. J Appl Physiol 68: 861–867, 1990 [DOI] [PubMed] [Google Scholar]
- 39. Rowell L, O'Leary D. Reflex control of the circulation during exercise: Chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol 65: 1539–1547, 1988 [DOI] [PubMed] [Google Scholar]
- 41. Stebbins CL, Maruoka Y, Longhurst JC. Prostaglandins contribute to cardiovascular reflexes evoked by static muscular contraction. Circ Res 59: 645–654, 1988 [DOI] [PubMed] [Google Scholar]
- 42. Symons JD, Theodossy SJ, Longhurst JC, Stebbins CL. Intramuscular accumulation of prostaglandins during static contraction of the cat triceps surae. J Appl Physiol 71: 1837–1842, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 111: 178–186, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral artery occlusion augments TRPV1-mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol 295: H1262–H1269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol 296: H1380–H1387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yanagisawa A, Smith JA, Brezinski ME, Lefer AM. Mechanism of antagonism of thromboxane receptors in vascular smooth muscle. Eur J Pharmacol 133: 89–96, 1987 [DOI] [PubMed] [Google Scholar]
- 48. Yang HT, Ogilvie RW, Terjung RL. Peripheral adaptations in trained aged rats with femoral artery stenosis. Circ Res 74: 235–243, 1994 [DOI] [PubMed] [Google Scholar]