Abstract
Systolic and diastolic dysfunction of the left ventricle (LV) is a hallmark of most cardiac diseases. In vivo assessment of heart function in animal models, particularly mice, is essential to refining our understanding of cardiovascular disease processes. Ultrasound echocardiography has emerged as a powerful, noninvasive tool to serially monitor cardiac performance and map the progression of heart dysfunction in murine injury models. This review covers current applications of small animal echocardiography, as well as emerging technologies that improve evaluation of LV function. In particular, we describe speckle-tracking imaging-based regional LV analysis, a recent advancement in murine echocardiography with proven clinical utility. This sensitive measure enables an early detection of subtle myocardial defects before global dysfunction in genetically engineered and rodent surgical injury models. Novel visualization technologies that allow in-depth phenotypic assessment of small animal models, including perfusion imaging and fetal echocardiography, are also discussed. As imaging capabilities continue to improve, murine echocardiography will remain a critical component of the investigator's armamentarium in translating animal data to enhanced clinical treatment of cardiovascular diseases.
Keywords: murine echocardiography, systolic and diastolic function, speckle-tracking imaging, strain analysis, heart failure
this article is part of a collection on Assessing Cardiovascular Function in Mice: New Developments and Methods. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Rodents are invaluable models for cardiovascular research, in part because of the extensive knowledge of their genome, homogeneity of study population, reproducible pathological phenotypes, and relative ease of creating genetically modified models. Surgical techniques that induce myocardial overload, infarction, and dysfunction in mice and rats have enabled a reliable identification and assessment of key physiological, molecular, and biochemical mechanisms of cardiovascular diseases (43). With the use of noninvasive imaging tools such as ultrasound echocardiography, cardiovascular evaluation of rodents has further led to the translational development of new diagnostic techniques and therapeutic strategies to predict and prevent cardiovascular disease complications in humans (82).
Echocardiography remains a gold standard for a reliable assessment of cardiovascular structure and function in humans (19). The technology's allure lies primarily in its portability, relative affordability, widespread availability, noninvasive nature, and rapid real-time imaging capabilities. With advancements in clinical echocardiography, the technicalities and conceptual framework of the methodology and equipment have been extended from humans to small animals. Ultrasound imaging greatly facilitates the evaluation of cardiac function in transgenic animals, as well as surgically induced mouse models of cardiovascular disease (11, 30). Furthermore, it permits a serial acquisition and an assessment of cardiac anatomy and function without inducing significant changes to the animal or its physiology. Importantly, with these tools, longitudinal studies within the same animal are feasible for a continuous assessment of pathological remodeling and treatment strategies (14, 34).
While echocardiographic image and data acquisition are relatively standard in humans, murine myocardial characteristics require high spatial and temporal resolution. Over the last decade, challenges such as small animal size (mouse, ∼18 g), orientation of the heart, and high heart rates (HRs, 500–650 beats/min) have been overcome via high-frequency transducers (up to 70 MHz), improved signal processing, and superior imaging frame rates (700 frames/s), providing superior resolution (∼30 μm) and image uniformity throughout the field with novel postacquisition analysis (48). Along with these technological improvements, high-quality examination and careful data interpretation by an experienced, blinded operator to obtain meaningful and reproducible cardiac functional data are essential.
Small animal echocardiography has become a relatively routine procedure for the interpretation and analysis of factors associated with the development and progression of left ventricular (LV) myocardial remodeling in cardiovascular disease. However, several key factors warrant consideration. First, while intra- and interobserver variability is typically low (< 20%) for most ventricular measurements, measurements of parameters such as wall thickness can be quite variable (76). Second, the cardiovascular traits of inbred mouse strains can have naturally different cardiovascular performance indexes (31). For example, the C57BL/6J mouse has reduced LV function with eccentric LV hypertrophy compared with the A/J mouse. Third, while image acquisition performed under mild anesthesia is recommended (59), complications of anesthesia (54), such as reduced HR, and hypothermia can impact cardiac function and ventricular repolarization (3). Although conscious human transthoracic echocardiography is standard, echocardiography in a conscious mouse, while feasible (76), may not be ideal for all applications due to the need for training regimens, difficulty in positioning and placement of transducer, and alterations in sympathetic and parasympathetic tone that inevitably result upon restraining the animal. Fourth, a standardization of technique (39) is essential to maintain consistency between animals. High-quality image acquisition necessitates a correct positioning of the animal, as well as the ultrasound probe. Adjustable animal handling platforms and imaging stations on an adjustable rail system enable reproducible customization of animal handling, probe positioning and image optimization, modulation of body temperature, and, importantly, integration of respiration, HR, and electrocardiogram with cardiac function (48).
With these thoughts as a backdrop, the goal of this review is to provide an update of techniques, imaging modalities, and practical applications of rodent echocardiography. We describe echocardiographic approaches with proven clinical utility that are now amenable to rodent studies, enabling profound insights into the assessment of murine heart function. Examples of specific research applications are included for conventional imaging formats, as well as for more specialized imaging techniques, to illustrate the manifold utility of small animal echocardiography.
Transthoracic Imaging and Assessment of Rodent Hearts
High-frequency sound beams penetrating the thoracic cavity are reflected back to the ultrasound transducer when reaching an interface between tissues of different acoustic impedance such as myocardium, valves, and blood. This reverberated signal when processed by the software produces a real-time image of the heart. The signals are displayed as various points on the image and the brightness dependent on the amplitude of the returning signal. In the adult rodent heart, the myocardium reflects more ultrasound (appears white, hyperechoic) than the blood (appears black, hypoechoic). Echocardiographic probes operating at ultrahigh frequencies use linear array technologies with improved near-field imaging for superior spatial resolution (48), defined as a function of both depth and width (Fig. 1). Comprehensive transthoracic echocardiography in rodents uses four principal imaging formats to assess cardiac function: brightness mode (B-mode), motion mode (M-mode), Doppler imaging, and three-dimensional (3-D) imaging.
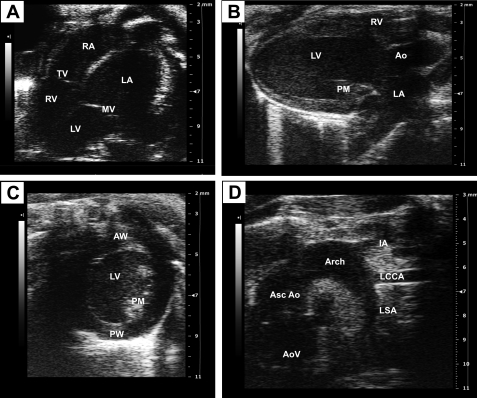
Fig. 1.
B-mode image views of a mouse heart. A: apical four-chamber view of the mouse heart. B: parasternal long-axis view at the level of the papillary muscle. C: short-axis view of the ventricle. D: suprasternal view of the aorta and its branches. Images were acquired using the Vevo 2100 ultrasound system (Visualsonics). AoV, aortic valve; Ao, aorta; Asc Ao, ascending aorta; AW, anterior wall; IA, innominate artery; LA, left atrium; LCCA, left common carotid artery; LSA, left subclavian artery; LV, left ventricle; PM, papillary muscle; PW, posterior wall; MV, mitral valve; RA, right atrium; RV, right ventricle; TV, tricuspid valve.
B-mode imaging.
B-mode is the most basic of the echo modes and simply produces a real-time black and white image of the targeted site. B-mode images display two-dimensional (2-D) views of the heart and other vasculatures such as the aortic arch, pulmonary artery, and carotid artery (9). This mode allows a nonquantitative assessment of cardiac phenotype, chamber dimensions, and heart function and the visualization of fine cardiac structures such as the chordae, papillary muscle, and valvular structures. Short- and long-axis cineloops may be traced in diastole and systole to assess cardiac function. Figure 1 illustrates B-mode images of a normal heart from a wild-type mouse showing an apical four-chamber view of both the mitral and tricuspid valves (Fig. 1A), parasternal long-axis view (Fig. 1B), short-axis views at the level of the papillary muscle (Fig. 1C), and a right parasternal view of the aorta and its branches (Fig. 1D). In general, the right ventricle (RV) may not be clearly visualized in short-axis views, in part because of the interference of the sternum, preventing a complete analysis of the RV (60). By allowing a regular distribution of lateral resolution over the entire field, B-mode can serve as a guidance platform to the operator for the correct positioning of structures that require further evaluation using other imaging formats such as M-mode and color Doppler imaging.
M-mode imaging.
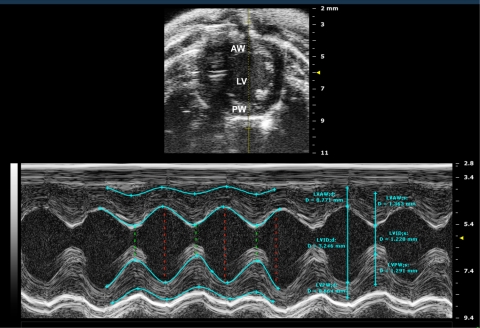
M-mode images are obtained by a rapid sequence of B-mode scans along a single line or axis that are displayed over time. A pencil beam of sound reflected from the moving endocardial and epicardial walls is converted to continuous waves depicting motion of the myocardium as it moves between systole and diastole (depth on the y-axis and time on the x-axis). This mode provides a very high temporal resolution (1,000 frames/s) of tissue motion along a narrow ultrasound beam, enabling precise measurements. Images accurately reflect the wall motion and allow an assessment of the LV contractile pattern. A tracing of the wall along the epicardial and endocardial borders allows an assessment of global LV functional parameters including ejection fraction (EF), fractional shortening (FS), cardiac output, and stroke volume, as well as wall and interventricular diameters, abnormal segmental wall contraction, and LV mass (12, 37, 78). M-mode images may be taken from the long- or short-axis and are typically at the level of the papillary muscle. An ice-pick M-mode view of the ventricle obtained from a short-axis view is illustrated in Fig. 2.
Fig. 2.
M-mode imaging. M-mode image of the LV displays dimensions of the ventricular walls, LV cavity, and cardiac function measurements. y-axis represents the distance (in mm) from the transducer; time (in ms) is on the x-axis. The M-mode images show the LV AW, LV chamber, and LV PW throughout diastole (d) and systole (s). Echogenic peaks visible along the PW during systole represent the papillary muscle entering the field of view. Images were acquired using the Vevo 2100 ultrasound system (Visualsonics). LVID, s, left ventricular internal diameter (systole); LVID, d, left ventricular internal diameter (diastole).
Doppler imaging.
Doppler imaging uses the Doppler shift principle reflected by the moving target (i.e., blood cells) to determine blood flow velocity and direction. An increase in the Doppler shift correlates with increasing velocity of blood flow. The Doppler shift is affected by the alignment of the ultrasound beam and the flow of blood: the more parallel the beam, the less attenuation of the Doppler shift signal.
In pulsed-wave Doppler, the transducer transmits and receives the sound waves, allowing for a determination of blood flow velocity profile from a precise location using 2-D image guidance. Transvalvular flow velocity waveforms obtained from pulsed-wave Doppler can be used to calculate peak velocities, ejection time (ET), and velocity time intervals. Particularly, transmitral flow velocity profiles have been useful in the assessments of diastolic function, including isovolumic contraction and relaxation times, ratio of early (E)-to-late (atrial, A) ventricular filling velocities (E/A), and deceleration of E wave (Fig. 3A). Rapid physiological HR of the mouse often results in partial or complete fusion of E and A waves (21). Notably, a fusion of the waves may also be an indicator of diastolic dysfunction in genetically engineered mice at comparable HRs to wild-type control littermates (80).
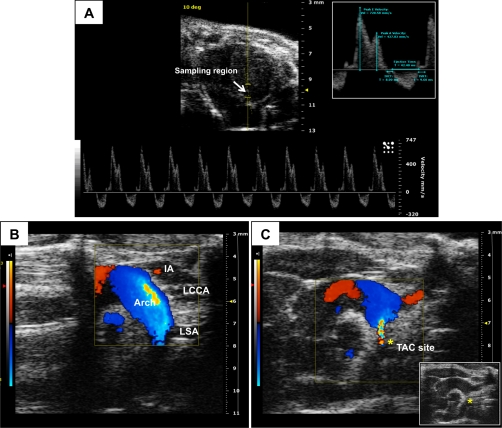
Fig. 3.
Pulsed-wave and color Doppler imaging. A: mitral inflow velocity obtained using pulsed-wave Doppler imaging. The flow velocity profile is obtained within the sampling region shown from the apical four-chamber view. The velocity (in mm/s, y-axis) is shown over time (in ms, x-axis). Inset: early (E) velocity peak during LV relaxation, and the atrial (A) velocity peak during atrial contraction along with other relevant measurements. B: color Doppler image of the aorta from a normal mouse. Flow toward the transducer (red) depicts blood flow through the ascending aorta and innominate artery. Flow away from the transducer (blue) displays blood flow through the aortic arch. C: color Doppler imaging of the aorta after constriction of the arch. The turbulent blood flow is shown in multiple color hues around the suture. Inset: right parasternal B-mode image of the aorta depicting constriction of the aortic arch (*). Images were acquired using the Vevo 2100 ultrasound system (Visualsonics). IVCT, isovolumic contraction time; IVRT, isovolumic relaxation time; TAC, transverse aortic constriction.
In color Doppler imaging, a color-encoded map of flow velocity and direction is superimposed on the 2-D image. Blood flowing toward the ultrasound transducer has an increase in echo frequency and is identified by the color red; flow away from the transducer has a decrease in echo frequency and is identified by the color blue. Blood flowing horizontally is not detected; an alignment of the ultrasound beam as close as possible to the direction of the flow of blood is, therefore, critical to enable detection directly toward or away from the transducer. Figure 3B illustrates color Doppler patterns through the aortic arch from a normal mouse. The colors red and blue show the direction of flow through the arch, and the various hues represent differing velocities. Doppler evaluation of blood flow and velocity can be obtained in nearly any vascular bed; it is particularly useful to determine the severity of transverse aortic constriction (TAC) surgery to induce LV hypertrophy (Fig. 3C). In the presence of turbulent flow, a mosaic of colors results at the site of stenosis. Pressure/flow gradients attained through pulsed-wave Doppler allow for an assessment of the severity and reproducibility of the stenosis.
Other Doppler-based measurements such as Doppler tissue imaging (DTI) and myocardial performance index (MPI, discussed in the following section) are used alternatively or in combination to assess global and regional cardiac function as well as vascular properties in rodents. DTI obtained from the mitral annulus or LV posterior myocardium measures tissue motion velocity. MPI to assess systolic and diastolic function is obtained from pulse or tissue Doppler. Importantly, these values are less influenced by preload and afterload unlike other routinely measured functional parameters such as EF and FS.
3-D imaging.
As described above, B-mode and M-mode are a grayscale display of amplitude in two and one dimensions, respectively. A challenge is the restriction of the dynamic range from weak scatter reflections being lost at conversion without logarithmic conversion of signal amplitude. Acquisition of 3-D echocardiography based on reconstruction from multiple 2-D images (from sequential cardiac cycle) is a superior and feasible approach for visualization of cardiac structures from any spatial point of view. Through the use of electrocardiogram and respiration-gated 2-D image acquisition (14, 83), true end-systolic and true end-diastolic frames with excellent temporal resolution are captured and reconstructed into a 3-D image that is near real time, spatially precise, and without the burden of any motion artifacts. Semiautomated 3-D acquisition imaging obtained from varying transducer positions at the same points in cardiac cycle provides high throughput analysis of LV volume and mass (Fig. 4). These images provide absolute LV chamber volume and function in normal and chronically infarcted mice without the need for geometric assumptions (14, 58), a limitation in 2-D and M-mode imaging. Using this approach, Dawson and colleagues (14) constructed 3-D reconstruction images from 500-μm consecutive short-axis slices to determine LV volumes. A quantitative analysis strongly correlated with values obtained from MRI imaging, and infarct size measurements were accurate. While this modality is feasible with 2-D transducers, it is time consuming, requires high-quality 2-D images, and necessitates stable HRs. Furthermore, with the progression of myocardial infarction (MI), an accurate LV mass calculation may be skewed with scar thinning and fusion of epicardial and endocardial walls over time. Real-time 3-D echocardiography is an exciting future possibility for accurate and reproducible detection and quantification of LV dyssynchrony.
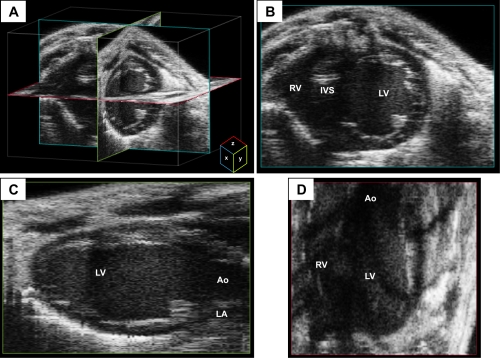
Fig. 4.
Three-dimensional (3-D) reconstruction imaging from a mouse heart. A: multiple two-dimensional (2-D) images obtained during a sweep of the transducer beam are used to create a dataset of voxels to reconstruct a 3-D image of the heart. Representative x-, y-, and z-planes of the heart are depicted in teal, green, and red, respectively. B–D: representative views from each of the planes are depicted. The x-plane image has good axial and lateral resolution of the 2-D image as a function of the transducer beam. The y- and z-planes have lower resolution than the x-plane because the resolution is a function of the size of the voxel in addition to that of the transducer beam. Images were acquired using the Vevo 2100 ultrasound system (Visualsonics). IVS, interventricular septum.
In addition to the above described imaging formats and modes, echocardiography offers several other sophisticated formats for diagnostic imaging, such as contrast echocardiography, ultrasound biomicroscopy, and fetal imaging. These techniques, successfully used for imaging mice and rats, are briefly described in the latter part of this review.
Evaluation of Cardiac Function in Murine Hearts
Myocardial performance is affected by three main factors: geometry of the ventricles (ellipsoid LV and crescent-shaped RV), orientation of myocardial fibers (longitudinal in the subendocardium, radial subepicardium, and circumferential midwall), and wall elasticity (for synchronous interaction of the myocardial segments). The complex interplay between these heart muscle sections produces longitudinal and circumferential shortening, plus radial thickening in cardiac systole. In response to cardiac injury and conditions of increased load, e.g., post-MI, myocardial ischemia-reperfusion (I/R), or TAC, cardiac remodeling manifests as alterations in shape, size, and function. Serial transthoracic imaging used to evaluate the progression of myocardial dysfunction (6, 44) and characterize phenotypes of genetically altered mice (1) aid our understanding of clinical cardiac pathology. Echocardiographic measures to evaluate cardiac function, such as the assessment of ventricular volume, EF, FS, isovolumic relaxation and ventricular filling, myocardial thickening, and regional wall contractility are described in the following sections.
Systolic function.
The most common functional surrogate of cardiac function is EF (in %) and FS (in %). Ventricular dimensions (such as thickness of the anterior wall, posterior wall, and the interventricular septum) measured at end systole and end diastole can be used to reproducibly calculate indexes of LV function, LV mass, stroke volume, and cardiac output (based on assumptions of a “normal” LV geometry). The average LV function measurements from 36 normal mice, under light inhaled isoflurane anesthesia, are shown in Table 1. Echocardiographic analysis under injectable or inhaled anesthesia is typically reported at HRs ranging from 300 to 550 (36, 54, 76) compared with 658 ± 9 beats/min in a conscious animal (76). The impact of anesthetic agents on the hemodynamic state, HR, and cardiac LV function is well recognized. Particularly, low HR associated with deep anesthesia (ketamine-xylazine, pentobarbital sodium, etc.) is accompanied with cardiac depression, LV dilatation, and reduced LV functional parameters (54, 70). HR positively correlated with FS (75); high HR (>550 beats/min) results in stable LV contractility and echocardiographic measures, allowing for a minimal force-frequency dependence in these conditions (22). The LV internal diameter at end diastole negatively correlated, i.e., low HR results in slowed diastolic filling (75), maintaining a stable HR is particularly crucial in evaluating cardiac function under conditions of LV hypertrophy and remodeling. Accordingly, an echocardiographic assessment under carefully modulated low grade-inhaled volatile anesthesia allows for near-physiological HR (i.e., >500 beats/min) and cardiac function for echocardiographic imaging (Table 1) with minimal anesthesia-related cardiac depression.
Table 1.
Baseline conventional two-dimensional echocardiographic measurements
| Echocardiographic Measures | Wild-Type Mouse |
|---|---|
| n | 36 |
| Heart rate, beats/min | 538 ± 6 |
| Systolic internal diameter, mm | 1.6 ± 0.04 |
| Diastolic internal diameter, mm | 3.1 ± 0.03 |
| Ejection fraction, % | 81 ± 1 |
| Fractional shortening, % | 49 ± 1 |
| Stroke volume, ml | 31.3 ± 0.7 |
| Cardiac output, ml/min | 16.8 ± 0.5 |
| Systolic anterior wall diameter, mm | 1.3 ± 0.02 |
| Diastolic anterior wall diameter, mm | 0.8 ± 0.1 |
| Systolic posterior wall diameter, mm | 1.2 ± 0.02 |
| Diastolic posterior wall diameter, mm | 0.7 ± 0.01 |
| LV mass, mg | 68 ± 2 |
Values are means ± SE; n, number of mice (8–10 wk old). Echocardiography measures were obtained from a short-axis view at the level of the papillary muscle. LV, left ventricular.
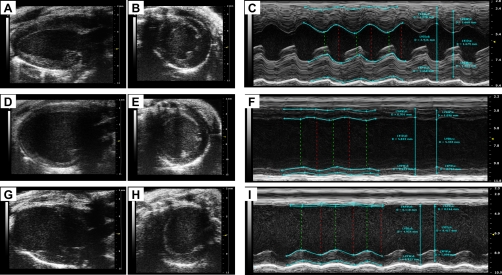
Myocardial remodeling (after MI and pressure and volume overload) promotes geometric maladaptation of the LV over time. Myocardial stress due to pressure overload initially results in reduced LV volume and increased wall thickness with no change in cardiac size. Conditions of volume overload then cause cavity dilatation, thinning of the ventricular wall, and increase in LV mass from LV enlargement. Reduced contractile function and progression of LV remodeling ultimately leads to the development of heart failure (HF) (53). Figure 5 illustrates B- and M-mode images of LV hypertrophy (Fig. 5, A–C), LV dilatation (Fig. 5, D–F), and MI (Fig. 5, G–I), demonstrating the structural and functional characteristics of LV remodeling. LV hypertrophy is associated with wall thickening and prominent papillary muscle. Ventricular chamber dilatation, decreased EF and FS, and changes in thickness of myocardial wall reflect impaired ventricular filling and myocardial contractility on echocardiography after myocardial injury. Unlike remodeling from pressure and volume overload, M-mode-based echocardiographic assessment of infarct size poorly correlates with the functional parameters after MI and histologically derived infarct size values (20, 34, 69). Thus regional LV function measurements from a single M-mode measurement may not always be extrapolated to estimate global LV function. To account for these geometric changes within the ventricle, multiple short-axis views roughly identified by visualizing anatomic landmarks may need to be obtained. In a recent study (81), an endocardial length-based approach using four equally spaced (1 mm apart) short-axis views strongly correlated with histologically assessed infarct size. While this approach may only be feasible when assessing transmural MI, it may not reflect an accurate determination of infarct size following I/R injury.
Fig. 5.
Transthoracic views of the heart from mouse with LV remodeling. B-mode and M-mode images depicting visualization of LV structure and function from a mouse with cardiac hypertrophy (A–C), cardiac dilatation (D–F), and myocardial infarction (G–I). Parasternal long-axis views (A, D, and G), short-axis views (B, E, and H), and M-mode images (C, F, and I) are shown. Images were acquired using the Vevo 2100 ultrasound system (Visualsonics).
An estimation of LV volume and mass is obtained using the area-length method (29) from a parasternal long-axis view or using the Simpsons method (61) from the short axis. The former method assumes a uniform geometric shape of the LV, whereas the latter represents the LV cavity as a stack of disks. Thus LV volume is calculated as a summation volume of all disks. LV mass is calculated (12) by subtracting the volume of LV cavity at end systole from end diastole and multiplying by the myocardial density (1.055). Echocardiographic estimates of LV mass have been shown to correlate well with heart weight as assessed by necropsy following TAC surgery (12). The above-described method of LV mass calculation is typically applied to adult mouse models. The assessment of LV mass becomes mandatory in neonate and adolescent transgenic models. Accordingly, Ghanem et al. (24) have demonstrated an accurate estimation of systolic LV mass measurements in hearts weighing <50 mg. Future advancement and refinement of geometric methods and 3-D volumetric techniques may be useful for a more accurate determination of LV mass measurements.
Diastolic function.
LV filling with the opening of the mitral valve at low left atrial pressures defines the diastole of the cardiac cycle. Ventricular filling velocity, described by the magnitude of the A wave and E/A ratio (passive/active filling estimates LV relaxation, using pulsed Doppler across the mitral valve, Fig. 3) is a functional parameter of diastolic function in small animal models (17, 33, 56). Impaired relaxation, pseudonormalization, and restriction depict abnormal ventricular filling patterns (21) and have been used to characterize the abnormal phenotype of numerous genetic and surgical mouse models, including the phospholamban knockout and mutant mice (62). While these diastolic indexes have been correlated with varying E/A ratios in humans, a similar categorization has not been well defined for murine pathophysiology. Mitral annual diastolic motion (E′ and A′ waves) analysis correlates with increased LV filling pressures in mouse models of cardiomyopathy (42) and in aged mice with mutation of cardiac troponin I (17). A decrease in the E/A ratio is a strong indicator of diastolic dysfunction and may be due to a stiff or fibrotic LV. The ventricle is unable to fill as well during the early phase of diastole and has an increased reliance on active filling (A wave) to passive filling (E wave). The acceleration time of the transmitral Doppler E wave is prolonged in the initial phase of LV filling and further in the assessment of the pseudonormalization and restrictive phase in murine models (80). Other parameters derived from transmitral Doppler waveform such as ventricular ET, diastolic intervals of isovolumic relaxation time (IVRT), and deceleration times of the E wave have been strongly correlated with invasive measurements in the spontaneous hypertensive rat model (65). Impaired LV relaxation is further associated with prolonged IVRT and prolonged E-wave deceleration times (11).
The MPI, Tei, is a noninvasive global indicator of systolic and diastolic function (71) and is obtained from mitral valve pulsed-wave Doppler measurements: MPI = (IVRT + IVCT)/ET. Myocardial relaxation is dependent on ventricular load conditions. MPI can be obtained within the same cardiac cycle and is therefore independent of HR and LV shape (57) and is a reliable predictor of diastolic dysfunction (increased MPI). Changes in Tei value are particularly useful indicators of cardiac function following MI in rats (57) and nutrient restriction in mice (38). Furthermore, Tei values strongly correlate with invasively derived contractility indexes and LV pressure over a range of hemodynamic conditions in mice (5).
Pulmonary artery Doppler imaging, like aortic Doppler imaging, is relatively straightforward in rodents. Pulmonary venous Doppler technology, while routinely used in patients for the assessment of diastolic function, has not been noticeably used in the small animals. A primary distinction in the mouse is the presence of a single large pulmonary vein, which imposes technical challenges to visualize on apical four-chamber views. Furthermore, a large pulmonary vein in relation to the left atrium of the mouse results in lower pressure and less robust flow profile. The Doppler flow waveforms in the mice suggest an increased forward flow volume during ventricular diastole in the mice, and therefore a useful indicator of diastolic dysfunction (80). Additional studies imaging the pulmonary vein at higher resolutions are warranted.
Regional Ventricular Function
Global LV function is an index of murine myocardial performance. A limitation of commonly measured LV function indexes (EF and FS) is its dependence on preload and/or afterload (49). Furthermore, regional specificity is particularly important when measuring dyssynchronous contraction and function of reversibly injured yet viable myocardium following MI or I/R and in detecting subtle myocardial dysfunction not determinable by conventional echocardiography. Load-independent measures are thus ideal for a complete insight into LV mechanics. The relationship between LV wall stress and rate-corrected velocity of fiber shortening (10), LV pressure-volume assessment, and LV systolic rotation using MRI (25) provides valuable, load-independent analysis of LV contractility. However, the ease of availability and superior temporal resolution of echocardiography makes it attractive for a load-independent assessment of ventricular function (56, 77) with clinical utility. In addition to MPI (described above), using one dimensional (1-D)-based DTI and 2-D-based speckle-tracking techniques, myocardial motion, and deformation for specified regions of the heart, particularly of the ventricle, can be assessed as described below.
Doppler tissue imaging.
DTI measures in humans is a sensitive measure to predict early cardiac dysfunction in the absence of histological abnormalities and normal LV systolic function (79). Indexes of DTI in rats and large animals have been validated as sensitive measures of ischemia (16), pressure overload (15), and cardiomyopathy (7). Tissue Doppler velocity is based on the same principle as color and pulsed-Doppler technology used for blood flow assessment. High- and low-pass filters differentiate signals originating from the blood flow and moving tissue. Myocardial velocities and DTI parameters [strain and strain rate (SR)] in mice are derived through a parasternal short-axis view reflecting radial motion (18). The three basic velocity waveforms include two in the early and late diastole (Ea and Aa, respectively) and one in systole (Sa). Normal peak endocardial velocities in the mice average 3.4 ± 0.1 cm/s, and epicardial averages are 2.3 ± 0.2 cm/s (56). A decrease in the Ea-to-Aa ratio indicates diastolic dysfunction. Peak LV systolic and diastolic endocardial velocities detect subtle changes in LV function, for example with doxorubicin treatment before conventional echocardiography measures (41). Diastolic velocities are also altered in genetic heart failure models, such as the phospholamban knockout mice (62), fatty-acid induced cardiac injury (8), and after TAC (56).
Tissue Doppler measures are derived from 1-D velocity measurement in the direction of a single scan line and detects the component of motion that is parallel to the ultrasound beam. Thus myocardial segment velocity may be influenced from the tethering of adjacent myocardial segments with each other (18). Myocardial deformation calculations, such as strain and SR, are therefore attractive parameters, since passive myocardial motions do not affect these parameters. Radial SR is derived from the velocity gradient between endocardium and epicardium over wall thickness, and longitudinal SR is derived over a segment with a fixed distance. Peak radial strain averages in mice are 14.2 ± 0.7 s−1 (56) and correlates with invasive measures of systolic function (41). Reductions in SR predict transmural infarct in early ischemia (72) and can detect subtle cardiac injury after doxorubicin treatment (41). Particularly, strain measures from subendocardium are a marker of myocardial dysfunction in the setting of myocardial hypoperfusion and mechanical stress (28).
While Doppler technology has a high temporal resolution, the spatial resolution is limited and DTI indexes are sensitive to transducer orientation and Doppler angle dependency. Myocardial torsion can move the sample out of the sampling field and is an important consideration when assessing nonuniform myocardial function. It may also be emphasized that SR is also extremely sensitive to signal noise since it is dependent on the quality of the velocity data.
Speckle-tracking imaging.
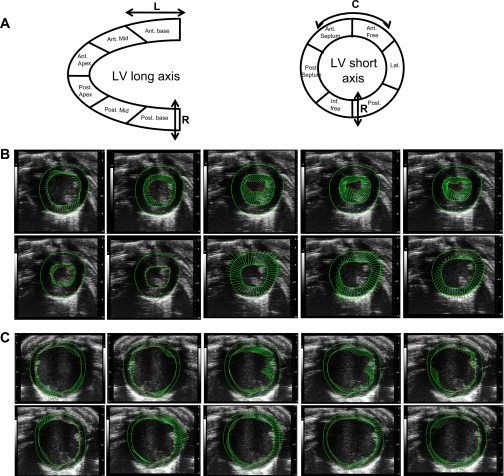
Recent echocardiographic imaging techniques based on tissue deformation provide an improved accuracy of myocardial contractility and quantification of regional myocardial function. Speckle-tracking imaging (STI) is a novel, non-Doppler-based technique used to detect myocardial wall motion (velocity, displacement) and myocardial deformation (strain and SR). A summary of the terms used in speckle tracking-based measurement is listed in Table 2. The basic principle of speckle tracking is that the interference of the reflected ultrasound gives rise to an irregular speckle pattern (68). Endocardial and epicardial borders of the LV myocardium may be semiautomatically traced, and subsequent speckle patterns are identified along the path of the trace. Randomness ensures that each region of the myocardium has its own unique speckle pattern that can enable differentiation of a single region from any other region. Each unique group of speckles, called kernels, remains unchanged as they follow the myocardial wall; these kernels can be tracked from frame to frame through the use of a speckle-tracking algorithm (e.g., VevoStrain, VisualSonics). The resulting geometric shift can be used to calculate regional velocity, displacement, strain, and SR along the radial, circumferential, and longitudinal planes of the heart (Fig. 6A) (46). This is superior in contrast to DTI that is Doppler dependent, and measurements can only be obtained from the anterior and posterior ventricular wall. Speckle-tracking assessment of myocardial performance may be obtained from long- and short-axis B-mode cineloops. Quantitative data may be presented as curves, tables, segmental color wheels, or as color display (parametric 2-D/3-D imaging) to allow a quick visual assessment of LV functional data over a larger area.
Table 2.
Definition of terms used in speckle tracking-based measurement
| Term | Definition | Unit/B-Mode Views |
|---|---|---|
| Displacement | Distance that speckle or cardiac structure moved between two consecutive frames | cm |
| Velocity | Displacement per unit time | cm/s |
| Strain | Fractional change in length of myocardial segment | % |
| Strain rate | Rate of change of strain | 1/s |
| Time to peak | Time at which the curve reaches its maximum absolute value | ms |
| Longitudinal strain | Myocardial deformation from the base to the apex | Long axis |
| Radial strain | Myocardial deformation toward the center of the LV cavity, indicates LV wall thickening and thinning motion during cardiac cycle | Long axis, short axis |
| Circumferential strain | LV myocardial fiber shortening along the circular perimeter | Short axis |
Fig. 6.
Strain-based vector mapping of cardiac function. A: normal ventricular function involves deformation in longitudinal (L), circumferential (C), and radial (R) planes. In both long- and short-axis, the LV segment is divided into six regional segments. B and C: serial vectors (arrows) reflecting tracking of segmental myocardial deformation during LV contraction from systole to diastole from a normal mouse (B, top) and after myocardial infarction (MI; C, bottom). Tracing of endocardial and epicardial borders are also depicted. Note that the vectors depict rotational direction as well as velocity and displacement. Images were acquired using the Vevo 2100 ultrasound system (Visualsonics). Ant, anterior; Inf, inferior; Lat, lateral; Post, posterior.
Displacement and velocity represented as vectors can be used for characterization of global and regional myocardial mechanics. An example of velocity/displacement vector imaging over a full cardiac cycle comparing a normal and MI heart is illustrated in Fig. 6, B and C. Displacement (in cm) along the radial and longitudinal axes, measured as the distance traveled by the kernels from peak diastole to full systole, is presented as a positive bell-shaped curve. Radial and longitudinal velocities (in cm/s) are positive during systole (myocardium shortens and progresses inward) and negative during diastole (myocardium lengthens and progresses outward). Circumferential displacement and velocity are measured as degrees and degrees per second, respectively, unlike the units for radial and longitudinal axes. A positive and negative value for displacement represents a counterclockwise and clockwise rotational change of the myocardium, respectively. A positive and negative value for circumferential velocity represents a counterclockwise and clockwise rate of rotational change of the myocardium, respectively.
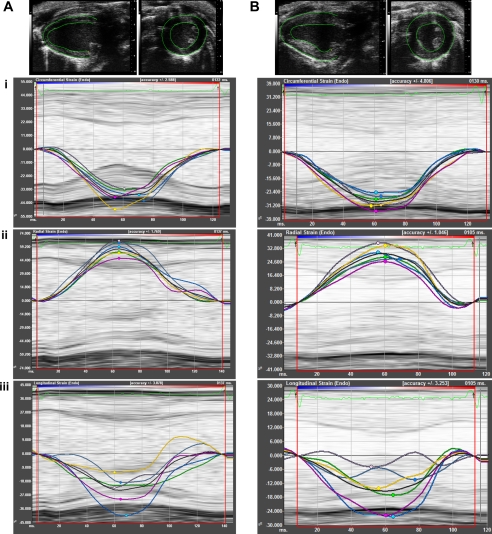
Strain and SR are useful in the detection of regional myocardial function. Radial strain, defined as the percent change in myocardial wall thickness, can be measured in both the short- and long-axis views. Consequently, radial strain is a positive curve reflecting increasing myocardial thickness during systole and diminishing wall thickness during diastole. Circumferential strain, representing the percent change in myocardial circumference, is measured from a short-axis view. Longitudinal strain detects the percent change in length of the ventricle, typically measured from the endocardial wall in the long-axis view. During systole, the myocardial fibers shorten from base to the apex of the heart, with a decrease in LV circumference, reflected as a reduction of distance between kernels. Consequently, circumferential and longitudinal strain decreases to a negative value. With an increase in LV circumference and length during diastole, strain moves in the positive direction. Figure 7 illustrates speckle tracking-based strain measures and curvilinear data of normal ventricular function and after cardiac hypertrophy following TAC surgery. For a review on the fundamentals and concepts of myocardial strain and STI, the readers are kindly referred to Geyer et al. (23).
Fig. 7.
Speckle tracking-based strain analysis. Strain imaging is shown for a normal mouse at baseline (A, left) and after TAC (B, right). Representative B-mode tracing of epicardial and endocardial borders in short- and long-axis are shown (A and B, top). Curvilinear strain data with M-mode tracing are depicted for circumferential (i), radial (ii), and longitudinal (iii) planes. Each color corresponds to individual segment of the left ventricle; strain for each segment on the left ventricle is plotted during a single cardiac cycle. Note the decrease in %circumferential, radial, and longitudinal strain due to LV hypertrophy following TAC surgery compared with baseline. Images were acquired using the Vevo 2100 ultrasound system (Visualsonics).
Time to peak analysis for strain and SR is a useful tool in evaluating the synchrony of segments through the cardiac cycle. The LV myocardium is divided into six distinct segments for regional calculations (Fig. 6A). During normal LV function, all myocardial LV segments have similar velocity and therefore peak at relatively similar times (synchronized). Conversely, during abnormal LV function, certain myocardial segments moving at different velocity may result in delayed peak, resulting in dyssynchrony. Global strain value is the summation of peak strain and SR measurements across all six segments; regional strain values may be obtained at specific segments of the LV. Figure 8 illustrates the curves and time to peak for radial strain from a normal mouse and dyssynchrony after MI, along with vector and parametric visualization.
Fig. 8.
Time to peak analysis of strain. Vector, time to peak, and parametric imaging from normal mouse are shown at baseline (A–C) and after MI (D–F). B-mode long-axis views depict myocardial deformation in systole (A and D). Radial myocardial strain curves and time to peak (inset) are represented in B and E. Parametric display of myocardial deformation (C and F) are shown in three dimensions (time in x-axis, location of measurement in y-axis, and strain value in z-axis). Regions a, b, and c of the left ventricle (from B-mode) correspond to the strain peaks in parametric curves. Myocardial dyssynchrony is observed in all three imaging views after MI compared with control. Images were acquired using the Vevo 2100 ultrasound system (Visualsonics).
Strain and SR derived from STI reflect an improved assessment of myocardial contractility and quantification of regional myocardial deformation in humans (2, 40). Particularly in the clinical setting, cardiovascular risk factors contributing to altered STI measures occur before the development of global LV dysfunction (13). Technical advances have now permitted strain imaging measures in small animal models and are found to correlate well with conventional measures of LV function. STI can accurately predict pathophysiology during the evolution of MI (4, 50) and TAC-induced heart failure in mice (45). The average values for speckle-tracking measurements from normal mice and after 2 and 4 wk following TAC surgery are presented in Table 3. STI-derived radial strain and SR (from both long and short axis) and circumferential strain significantly decreased during early stages of TAC-mediated LV remodeling at a time when there was no appreciable reduction in global LV function. These findings are in accordance with a previous publication showing sensitivity of circumferential and radial strain during TAC-mediated LV dysfunction (45). Strain measures across all three planes, radial, circumferential, and longitudinal, are useful. The former two axes reflect the activity of the midmyocardium. Furthermore, longitudinal strain measures along with circumferential SR are an independent predictor of mortality in high-risk patients with MI (32). Accordingly, Dr. Liao's group recently found in a mouse model of MI (4) that longitudinal strain from global and regional (at infarct and remote MI) LV segments were highly sensitive in identifying myocardial dysfunction early in the course of MI and subsequently in the follow-up period, where detrimental effects of MI can be clearly seen. Thus a serial application of STI in this study was useful in the identification of beneficial effects following rescue therapy on myocardial function before changes in global LV function (4).
Table 3.
Speckle tracking-based strain measurements in mice at baseline and 2 and 4 wk following TAC surgery
| Measured Parameter | Baseline | TAC (2 wk) | TAC (4 wk) |
|---|---|---|---|
| n | 29 | 29 | 29 |
| M-mode-derived measures | |||
| Ejection fraction, % | 81.4 ± 0.8 | 76.9 ± 1.9 | 76.9 ± 2.5 |
| Fractional shortening, % | 49.7 ± 0.9 | 45.5 ± 1.6 | 46.2 ± 2.1 |
| LV anterior wall, diastole, mm | 0.81 ± 0.02 | 0.99 ± 0.02*** | 1.10 ± 0.02***,# |
| LV anterior wall, systole, mm | 1.26 ± 0.02 | 1.44 ± 0.02*** | 1.50 ± 0.02*** |
| LV posterior wall, diastole, mm | 0.71 ± 0.01 | 0.91 ± 0.02*** | 0.94 ± 0.02*** |
| LV posterior wall, systole, mm | 1.20 ± 0.02 | 1.33 ± 0.02** | 1.37 ± 0.03*** |
| LV mass, mg | 52.4 ± 1.4 | 83.3 ± 3.1*** | 94.5 ± 4.0***,† |
| Speckle-tracking measures | |||
| Displacement, cm | |||
| Circumferential | 6.08 ± 0.54 | 3.82 ± 0.39** | 4.21 ± 0.53* |
| Radial (short axis) | 0.63 ± 0.02 | 0.59 ± 0.02 | 0.59 ± 0.02 |
| Longitudinal | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.21 ± 0.02 |
| Radial (long axis) | 0.54 ± 0.01 | 0.57 ± 0.02 | 0.53 ± 0.02 |
| Velocity, cm/s | |||
| Circumferential | 409.27 ± 21.38 | 341.16 ± 26.42 | 389.40 ± 36.84 |
| Radial (short axis) | 2.02 ± 0.07 | 1.58 ± 0.05*** | 1.67 ± 0.07*** |
| Longitudinal | 1.19 ± 0.06 | 1.06 ± 0.05 | 1.14 ± 0.05 |
| Radial (long axis) | 1.62 ± 0.05 | 1.47 ± 0.06 | 1.47 ± 0.07 |
| Strain measures | |||
| Short axis, % | |||
| Circumferential strain | −35.62 ± 1.08 | −31.61 ± 1.19* | −32.34 ± 1.45 |
| Circumferential SR | −18.02 ± 0.79 | −13.87 ± 0.90* | −15.82 ± 1.79 |
| Radial strain | 50.05 ± 1.44 | 37.83 ± 1.47*** | 37.07 ± 1.84*** |
| Radial SR | 12.91 ± 0.37 | 8.96 ± 0.33*** | 9.29 ± 0.36*** |
| Long axis, % | |||
| Longitudinal strain | −19.25 ± 1.01 | −18.58 ± 1.03 | −17.20 ± 0.81 |
| Longitudinal SR | −10.20 ± 0.65 | −9.10 ± 0.53 | −9.32 ± 0.59 |
| Radial strain | 43.81 ± 1.17 | 35.54 ± 1.32*** | 33.47 ± 1.49*** |
| Radial SR | 10.43 ± 0.29 | 7.95 ± 0.28*** | 7.79 ± 0.35*** |
Values are means ± SE; n, number of mice. Statistical analysis was computed using repeated-measures one-way ANOVA.
P < 0.0001,
P < 0.01, and
P < 0.05 vs. baseline. #P < 0.0001 and
P < 0.001 vs. transverse aortic constriction (TAC) (2 wk). Note that the speckle-tracking measures, particularly of strain and strain rate (SR), are highly significant within 2 and 4 wk of TAC compared with baseline. These parameters enable detection of subtle changes in LV performance before changes in global LV function (ejection fraction and fractional shortening).
While STI is a highly attractive technology, it is relatively new and evolving. Strain measurement using STI is a semiautomated process dependent on the quality of image and hardware and therefore is easily reproducible with minimal operator variability. An accurate delineation of the endocardial and epicardial borders is dependent on high-resolution echocardiographic imaging. An optimal frame rate of ∼233–347 frames/s gives high temporal resolution and spatial definition to obtain quality speckle-tracking information. The utility of STI can be further improved with higher frame rates to accommodate for faster HRs in mice, while still preserving temporal and spatial resolution. Lastly, it is important to determine diastolic SRs as additional surrogates of regional wall abnormalities.
Myocardial Perfusion Assessment
LV remodeling and coronary angiogenesis are closely interlinked with altered myocardial perfusion in human cardiac pathology. An assessment of the coronary vasculature using echocardiography permits an estimation of coronary flow velocity reserve. Color Doppler and myocardial contrast echocardiography techniques have been developed to measure myocardial perfusion in rodent models.
Color Doppler ultrasound has been shown to permit the detection and measurement of the left coronary artery flow velocity in both the proximal and the distal segments (74, 83). Measures of coronary velocity reserve under conditions of hypoxia (55), hypertrophy after aortic banding (TAC) (26), and vasodilatation (isoflurane inhalation) (27) have enabled the assessment of coronary microdysfunction during early and late pathological changes in the development of atherosclerosis and heart failure. In myocardial contrast echocardiography, contrast microbubbles agents filled with inert gas reflect sound waves returning to the transducer with increased signal-to-noise ratio. In its simplest application, contrast microbubbles enable an excellent visualization of ventricular cavity and myocardial walls in mice (Fig. 9) and thus the measurement of perfusion defects following ischemia and infarction (59). A continuous injection of microbubbles permits an evaluation of coronary perfusion following MI via coronary artery ligation (51) and coronary dobutamine stress in mice (52).
Fig. 9.
Myocardial contrast imaging. A: long-axis view of mouse ventricle before contrast injection. Image was captured in nonlinear contrast mode that eliminates tissue signal to identify contrast signal after a bolus injection. Contrast agent was administered via the tail vein. B: long-axis view of mouse ventricle after contrast injection (50-μl bolus injection). Contrast agent is seen in the cavity as well as in the AW and PW, which have been outlined in the image. C: intensity changes in myocardium after a bolus injection. Two regions of interest (PW and AW) are outlined, and the increase in intensity due to contrast uptake is plotted. Various types of perfusion kinetics may be extrapolated from these types of graphs. Images were acquired using the Vevo 2100 ultrasound system (Visualsonics). AU, arbitrary units.
Contrast agents react to sound waves; microbubbles can be ruptured by high-energy sound waves and thus be used for a targeted delivery of compounds for functional imaging. With the use of this property, microbubbles coated with recombinant adenovirus for β-galactosidase and constitutive promoter increased myocardial transfection and β-galactosidase activity compared with conventional viral construct injection or nondisrupted microbubbles (64). Very recently, pioneering work in using conjugated microbubbles targeted to markers such as VCAM-1 (35) and ICAM-1 (73) allows for monitoring of inflammatory changes during atherosclerosis and acute transplant rejection. In addition to these technologies, ultrasound biomicroscopy application is emerging in adult rats and mice to visualize myocardial tissue at microscopic resolution. With this, lumen-narrowing plaques may be identified and measured in pathological mouse models of atherosclerosis (74).
Murine Fetal Echocardiography
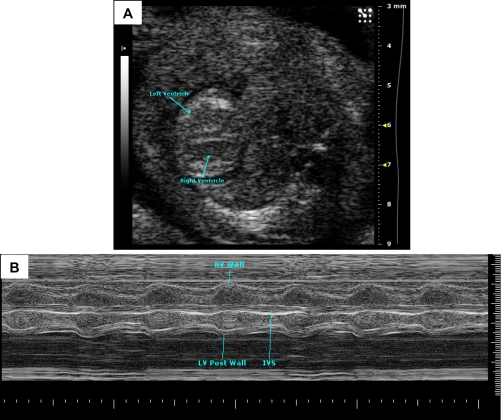
Fetal and perinatal death is not uncommon when studying genetic alterations affecting the cardiovascular system. Ultrasound imaging of the live fetus in utero is valuable for early recognition of abnormalities and longitudinal follow-up of disease progression to study the role of genes in the early development of cardiac function (Fig. 10). An application of high-frequency probes with conventional 2-D and pulsed-Doppler imaging have been shown to provide measurements of cardiac contraction and HRs at embryonic day (E)14.5 (66). FS measured in normal C57BL6 fetuses was found to be ∼50% at E16.5 and E18.5. Color Doppler has been particularly useful for in utero screening of valvular regurgitation and structural cardiovascular abnormalities in the fetal mice (63). Ultrasound biomicroscopy imaging of the fetus has improved 2-D resolution and can provide excellent information on the early development of cardiac structures [from E8.5, (67)] and can be used as a tool for interventional procedures such as injections. This system also allows imaging of blood flow velocities for excellent hemodynamic assessment of the fetal circulatory system. Nuclear factor of activated T cell-deficient embryos has been shown to have a reversal of flow in dorsal aorta and a reduced ventricular compliance with the rapid progression to heart failure (47). Fetal imaging technology, developed over a decade ago, although not widely used in routine procedures, holds a promising future for high throughput fetal cardiovascular screening.
Fig. 10.
Embryonic day (E)15.5 mouse embryonic heart. A: in embryonic stages, blood produces a very echogenic (bright) signal with high-frequency ultrasound (contrast to hypoechoic in the adult). The ventricles can be seen in this image. B: M-mode image of E16 mouse embryo showing myocardial walls, interventricular septum, and ventricles. y-axis shows depth (in mm), and x-axis shows time (in s). Images were acquired using the Vevo 2100 ultrasound system (Visualsonics).
Conclusions
Small animals are powerful model systems for a mechanistic understanding of both normal cardiovascular function and the pathological basis of cardiovascular diseases. Recent advances in imaging technology provide improved spatial and temporal resolution of rodent myocardium, allowing an extraction of increasingly precise physiological information. The evaluation of cardiac function, beyond the use of conventional modalities, is an emerging field in small animal models. Importantly, speckle tracking-based myocardial deformation can predict adverse remodeling of regional ventricular function before changes in global cardiac function. Such exciting advances lend credence to the notion that an improved assessment of cardiac structure and function in rodent models will effectuate diagnostic and therapeutic benefit in humans.
GRANTS
This work was supported by American Heart Association Postdoctoral Fellowship 10POST4150039 (to R. Ram) and by National Institutes of Health Grants R01-HL-89885, 3R01-HL-089885-02S1, R01-HL-091475, and 1S10-RR-027946 (to B. C. Blaxall).
DISCLOSURES
C. Theodoropoulos is an employee of Visualsonics, Inc.
ACKNOWLEDGMENTS
We thank Drs. Stephen L. Belmonte and Zhao-Yang Hu (University of Rochester) for valuable insights in this review.
REFERENCES
- 1. Aguilar F, Belmonte SL, Ram R, Noujaim SF, Dunaevsky O, Protack TL, Jalife J, Todd Massey H, Gertler FB, Blaxall BC. Mammalian enabled (Mena) is a critical regulator of cardiac function. Am J Physiol Heart Circ Physiol 300: H1841–H1852, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Stoylen A, Ihlen H, Lima JA, Smiseth OA, Slordahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 47: 789–793, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Appleton GO, Li Y, Taffet GE, Hartley CJ, Michael LH, Entman ML, Roberts R, Khoury DS. Determinants of cardiac electrophysiological properties in mice. J Interv Card Electrophysiol 11: 5–14, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, Trujillo A, Lin FC, Liao R. Echocardiographic speckle-tracking-based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res 108: 908–916, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broberg CS, Pantely GA, Barber BJ, Mack GK, Lee K, Thigpen T, Davis LE, Sahn D, Hohimer AR. Validation of the myocardial performance index by echocardiography in mice: a noninvasive measure of left ventricular function. J Am Soc Echocardiogr 16: 814–823, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, Vorobiof G, Dunaevsky O, Matavel A, Lopes CM, Smrcka AV, Blaxall BC. Small molecule disruption of G beta gamma signaling inhibits the progression of heart failure. Circ Res 107: 532–539, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chetboul V, Escriou C, Tessier D, Richard V, Pouchelon JL, Thibault H, Lallemand F, Thuillez C, Blot S, Derumeaux G. Tissue Doppler imaging detects early asymptomatic myocardial abnormalities in a dog model of Duchenne's cardiomyopathy. Eur Heart J 25: 1934–1939, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res 96: 225–233, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Coatney RW. Ultrasound imaging: principles and applications in rodent research. ILAR J 42: 233–247, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Colan SD, Borow KM, Neumann A. Use of the calibrated carotid pulse tracing for calculation of left ventricular pressure and wall stress throughout ejection. Am Heart J 109: 1306–1310, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics 13: 227–239, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Collins KA, Korcarz CE, Shroff SG, Bednarz JE, Fentzke RC, Lin H, Leiden JM, Lang RM. Accuracy of echocardiographic estimates of left ventricular mass in mice. Am J Physiol Heart Circ Physiol 280: H1954–H1962, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Cottrell C, Kirkpatrick JN. Echocardiographic strain imaging and its use in the clinical setting. Expert Rev Cardiovasc Ther 8: 93–102, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Dawson D, Lygate CA, Saunders J, Schneider JE, Ye X, Hulbert K, Noble JA, Neubauer S. Quantitative 3-dimensional echocardiography for accurate and rapid cardiac phenotype characterization in mice. Circulation 110: 1632–1637, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Derumeaux G, Mulder P, Richard V, Chagraoui A, Nafeh C, Bauer F, Henry JP, Thuillez C. Tissue Doppler imaging differentiates physiological from pathological pressure-overload left ventricular hypertrophy in rats. Circulation 105: 1602–1608, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Derumeaux G, Ovize M, Loufoua J, Andre-Fouet X, Minaire Y, Cribier A, Letac B. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation 97: 1970–1977, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Du J, Liu J, Feng HZ, Hossain MM, Gobara N, Zhang C, Li Y, Jean-Charles PY, Jin JP, Huang XP. Impaired relaxation is the main manifestation in transgenic mice expressing a restrictive cardiomyopathy mutation, R193H, in cardiac TnI. Am J Physiol Heart Circ Physiol 294: H2604–H2613, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fayssoil A. Tissue Doppler characterization of cardiac phenotype in mouse. Eur J Radiol 72: 82–84, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Feigenbaum H. Echocardiography. St. Louis, MO: William & Wilkins, 1994 [Google Scholar]
- 20. Force T, Kemper A, Perkins L, Gilfoil M, Cohen C, Parisi AF. Overestimation of infarct size by quantitative two-dimensional echocardiography: the role of tethering and of analytic procedures. Circulation 73: 1360–1368, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol 19: 1550–1558, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Georgakopoulos D, Kass D. Minimal force-frequency modulation of inotropy and relaxation of in situ murine heart. J Physiol 534: 535–545, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 23: 351–369, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Ghanem A, Roll W, Hashemi T, Dewald O, Djoufack PC, Fink KB, Schrickel J, Lewalter T, Luderitz B, Tiemann K. Echocardiographic assessment of left ventricular mass in neonatal and adult mice: accuracy of different echocardiographic methods. Echocardiography 23: 900–907, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Gotte MJ, Germans T, Russel IK, Zwanenburg JJ, Marcus JT, van Rossum AC, van Veldhuisen DJ. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: studies in normal and impaired left ventricular function. J Am Coll Cardiol 48: 2002–2011, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Hartley CJ, Reddy AK, Madala S, Michael LH, Entman ML, Taffet GE. Doppler estimation of reduced coronary flow reserve in mice with pressure overload cardiac hypertrophy. Ultrasound Med Biol 34: 892–901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hartley CJ, Reddy AK, Madala S, Michael LH, Entman ML, Taffet GE. Effects of isoflurane on coronary blood flow velocity in young, old and ApoE−/− mice measured by Doppler ultrasound. Ultrasound Med Biol 33: 512–521, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hashimoto I, Li X, Hejmadi Bhat A, Jones M, Zetts AD, Sahn DJ. Myocardial strain rate is a superior method for evaluation of left ventricular subendocardial function compared with tissue Doppler imaging. J Am Coll Cardiol 42: 1574–1583, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, Tambouret R, Jassal DS, Raher MJ, Furutani E, Ichinose F, Gladwin MT, Rosenzweig A, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 291: H379–H384, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Hoit BD. New approaches to phenotypic analysis in adult mice. J Mol Cell Cardiol 33: 27–35, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Hoit BD, Kiatchoosakun S, Restivo J, Kirkpatrick D, Olszens K, Shao H, Pao YH, Nadeau JH. Naturally occurring variation in cardiovascular traits among inbred mouse strains. Genomics 79: 679–685, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Hung CL, Verma A, Uno H, Shin SH, Bourgoun M, Hassanein AH, McMurray JJ, Velazquez EJ, Kober L, Pfeffer MA, Solomon SD. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol 56: 1812–1822, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Jegger D, Jeanrenaud X, Nasratullah M, Chassot PG, Mallik A, Tevaearai H, von Segesser LK, Segers P, Stergiopulos N. Noninvasive Doppler-derived myocardial performance index in rats with myocardial infarction: validation and correlation by conductance catheter. Am J Physiol Heart Circ Physiol 290: H1540–H1548, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Kanno S, Lerner DL, Schuessler RB, Betsuyaku T, Yamada KA, Saffitz JE, Kovacs A. Echocardiographic evaluation of ventricular remodeling in a mouse model of myocardial infarction. J Am Soc Echocardiogr 15: 601–609, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, Lindner JR. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation 116: 276–284, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Kawahara Y, Tanonaka K, Daicho T, Nawa M, Oikawa R, Nasa Y, Takeo S. Preferable anesthetic conditions for echocardiographic determination of murine cardiac function. J Pharm Sci 99: 95–104, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Kiatchoosakun S, Restivo J, Kirkpatrick D, Hoit BD. Assessment of left ventricular mass in mice: comparison between two-dimensional and m-mode echocardiography. Echocardiography 19: 199–205, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Knight BS, Sunn N, Pennell CE, Adamson SL, Lye SJ. Developmental regulation of cardiovascular function is dependent on both genotype and environment. Am J Physiol Heart Circ Physiol 297: H2234–H2241, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Kutschka I, Sheikh AY, Sista R, Hendry SL, Chun HJ, Hoyt G, Kutschka W, Pelletier MP, Quertermous T, Wu JC, Robbins RC. A novel platform device for rodent echocardiography. ILAR J 49: E1–E7, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 17: 1021–1029, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Neilan TG, Jassal DS, Perez-Sanz TM, Raher MJ, Pradhan AD, Buys ES, Ichinose F, Bayne DB, Halpern EF, Weyman AE, Derumeaux G, Bloch KD, Picard MH, Scherrer-Crosbie M. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. Eur Heart J 27: 1868–1875, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Parlakian A, Charvet C, Escoubet B, Mericskay M, Molkentin JD, Gary-Bobo G, De Windt LJ, Ludosky MA, Paulin D, Daegelen D, Tuil D, Li Z. Temporally controlled onset of dilated cardiomyopathy through disruption of the SRF gene in adult heart. Circulation 112: 2930–2939, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail 2: 138–144, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Pawlinski R, Tencati M, Hampton CR, Shishido T, Bullard TA, Casey LM, Andrade-Gordon P, Kotzsch M, Spring D, Luther T, Abe J, Pohlman TH, Verrier ED, Blaxall BC, Mackman N. Protease-activated receptor-1 contributes to cardiac remodeling and hypertrophy. Circulation 116: 2298–2306, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peng Y, Popovic ZB, Sopko N, Drinko J, Zhang Z, Thomas JD, Penn MS. Speckle tracking echocardiography in the assessment of mouse models of cardiac dysfunction. Am J Physiol Heart Circ Physiol 297: H811–H820, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Perk G, Tunick PA, Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography—from technical considerations to clinical applications. J Am Soc Echocardiogr 20: 234–243, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Phoon CK, Ji RP, Aristizabal O, Worrad DM, Zhou B, Baldwin HS, Turnbull DH. Embryonic heart failure in NFATc1−/− mice: novel mechanistic insights from in utero ultrasound biomicroscopy. Circ Res 95: 92–99, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Pistner A, Belmonte S, Coulthard T, Blaxall B. Murine echocardiography and ultrasound imaging. J Vis Exp 2010. August 8;(42). pii: 2100 doi:10.3791/2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pollick C, Hale SL, Kloner RA. Echocardiographic and cardiac Doppler assessment of mice. J Am Soc Echocardiogr 8: 602–610, 1995 [DOI] [PubMed] [Google Scholar]
- 50. Popovic ZB, Benejam C, Bian J, Mal N, Drinko J, Lee K, Forudi F, Reeg R, Greenberg NL, Thomas JD, Penn MS. Speckle-tracking echocardiography correctly identifies segmental left ventricular dysfunction induced by scarring in a rat model of myocardial infarction. Am J Physiol Heart Circ Physiol 292: H2809–H2816, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Raher MJ, Thibault H, Poh KK, Liu R, Halpern EF, Derumeaux G, Ichinose F, Zapol WM, Bloch KD, Picard MH, Scherrer-Crosbie M. In vivo characterization of murine myocardial perfusion with myocardial contrast echocardiography: validation and application in nitric oxide synthase 3 deficient mice. Circulation 116: 1250–1257, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Raher MJ, Thibault HB, Buys ES, Kuruppu D, Shimizu N, Brownell AL, Blake SL, Rieusset J, Kaneki M, Derumeaux G, Picard MH, Bloch KD, Scherrer-Crosbie M. A short duration of high-fat diet induces insulin resistance and predisposes to adverse left ventricular remodeling after pressure overload. Am J Physiol Heart Circ Physiol 295: H2495–H2502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res 61: 269–280, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J., Jr Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol 282: H2134–H2140, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Saraste A, Kyto V, Saraste M, Vuorinen T, Hartiala J, Saukko P. Coronary flow reserve and heart failure in experimental coxsackievirus myocarditis. A transthoracic Doppler echocardiography study. Am J Physiol Heart Circ Physiol 291: H871–H875, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Schaefer A, Klein G, Brand B, Lippolt P, Drexler H, Meyer GP. Evaluation of left ventricular diastolic function by pulsed Doppler tissue imaging in mice. J Am Soc Echocardiogr 16: 1144–1149, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Schaefer A, Meyer GP, Hilfiker-Kleiner D, Brand B, Drexler H, Klein G. Evaluation of Tissue Doppler Tei index for global left ventricular function in mice after myocardial infarction: comparison with Pulsed Doppler Tei index. Eur J Echocardiogr 6: 367–375, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Scherrer-Crosbie M, Steudel W, Hunziker PR, Liel-Cohen N, Ullrich R, Zapol WM, Picard MH. Three-dimensional echocardiographic assessment of left ventricular wall motion abnormalities in mouse myocardial infarction. J Am Soc Echocardiogr 12: 834–840, 1999 [DOI] [PubMed] [Google Scholar]
- 59. Scherrer-Crosbie M, Steudel W, Ullrich R, Hunziker PR, Liel-Cohen N, Newell J, Zaroff J, Zapol WM, Picard MH. Echocardiographic determination of risk area size in a murine model of myocardial ischemia. Am J Physiol Heart Circ Physiol 277: H986–H992, 1999 [DOI] [PubMed] [Google Scholar]
- 60. Scherrer-Crosbie M, Thibault HB. Echocardiography in translational research: of mice and men. J Am Soc Echocardiogr 21: 1083–1092, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vancon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation 104: 1286–1291, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Schmidt AG, Gerst M, Zhai J, Carr AN, Pater L, Kranias EG, Hoit BD. Evaluation of left ventricular diastolic function from spectral and color M-mode Doppler in genetically altered mice. J Am Soc Echocardiogr 15: 1065–1073, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Shen Y, Leatherbury L, Rosenthal J, Yu Q, Pappas MA, Wessels A, Lucas J, Siegfried B, Chatterjee B, Svenson K, Lo CW. Cardiovascular phenotyping of fetal mice by noninvasive high-frequency ultrasound facilitates recovery of ENU-induced mutations causing congenital cardiac and extracardiac defects. Physiol Genomics 24: 23–36, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, Grayburn PA. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation 101: 2554–2556, 2000 [DOI] [PubMed] [Google Scholar]
- 65. Slama M, Ahn J, Peltier M, Maizel J, Chemla D, Varagic J, Susic D, Tribouilloy C, Frohlich ED. Validation of echocardiographic and Doppler indexes of left ventricular relaxation in adult hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol 289: H1131–H1136, 2005 [DOI] [PubMed] [Google Scholar]
- 66. Spurney CF, Leatherbury L, Lo CW. High-frequency ultrasound database profiling growth, development, and cardiovascular function in C57BL/6J mouse fetuses. J Am Soc Echocardiogr 17: 893–900, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Srinivasan S, Baldwin HS, Aristizabal O, Kwee L, Labow M, Artman M, Turnbull DH. Noninvasive, in utero imaging of mouse embryonic heart development with 40-MHz echocardiography. Circulation 98: 912–918, 1998 [DOI] [PubMed] [Google Scholar]
- 68. Stoylen A. Strain Rate Imaging. Cardiac Deformation Imaging by Ultrasound/Echocardiography Tissue Doppler and Speckle Tracking. Trodheim, Norway: Norwegian University of Science and Technology, 2011 [Google Scholar]
- 69. Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol 102: 2104–2111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tan TP, Gao XM, Krawczyszyn M, Feng X, Kiriazis H, Dart AM, Du XJ. Assessment of cardiac function by echocardiography in conscious and anesthetized mice: importance of the autonomic nervous system and disease state. J Cardiovasc Pharmacol 42: 182–190, 2003 [DOI] [PubMed] [Google Scholar]
- 71. Tei C, Nishimura RA, Seward JB, Tajik AJ. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr 10: 169–178, 1997 [DOI] [PubMed] [Google Scholar]
- 72. Thibault H, Gomez L, Donal E, Pontier G, Scherrer-Crosbie M, Ovize M, Derumeaux G. Acute myocardial infarction in mice: assessment of transmurality by strain rate imaging. Am J Physiol Heart Circ Physiol 293: H496–H502, 2007 [DOI] [PubMed] [Google Scholar]
- 73. Weller GE, Lu E, Csikari MM, Klibanov AL, Fischer D, Wagner WR, Villanueva FS. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation 108: 218–224, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Wikstrom J, Gronros J, Bergstrom G, Gan LM. Functional and morphologic imaging of coronary atherosclerosis in living mice using high-resolution color Doppler echocardiography and ultrasound biomicroscopy. J Am Coll Cardiol 46: 720–727, 2005 [DOI] [PubMed] [Google Scholar]
- 75. Xu Q, Ming Z, Dart AM, Du XJ. Optimizing dosage of ketamine and xylazine in murine echocardiography. Clin Exp Pharmacol Physiol 34: 499–507, 2007 [DOI] [PubMed] [Google Scholar]
- 76. Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol Heart Circ Physiol 277: H1967–H1974, 1999 [DOI] [PubMed] [Google Scholar]
- 77. Yip GW, Zhang Q, Xie JM, Liang YJ, Liu YM, Yan B, Lam YY, Yu CM. Resting global and regional left ventricular contractility in patients with heart failure and normal ejection fraction: insights from speckle-tracking echocardiography. Heart 97: 287–294, 2011 [DOI] [PubMed] [Google Scholar]
- 78. Youn HJ, Rokosh G, Lester SJ, Simpson P, Schiller NB, Foster E. Two-dimensional echocardiography with a 15-MHz transducer is a promising alternative for in vivo measurement of left ventricular mass in mice. J Am Soc Echocardiogr 12: 70–75, 1999 [DOI] [PubMed] [Google Scholar]
- 79. Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol 49: 1903–1914, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Yuan L, Wang T, Liu F, Cohen ED, Patel VV. An evaluation of transmitral and pulmonary venous Doppler indices for assessing murine left ventricular diastolic function. J Am Soc Echocardiogr 23: 887–897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yuan LJ, Wang T, Kahn ML, Ferrari VA. High-resolution echocardiographic assessment of infarct size and cardiac function in mice with myocardial infarction. J Am Soc Echocardiogr 24: 219–226, 2011 [DOI] [PubMed] [Google Scholar]
- 82. Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, Tarin C, Mas S, Ortiz A, Egido J. Animal models of cardiovascular diseases. J Biomed Biotechnol 2011: 497841, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhou YQ, Foster FS, Nieman BJ, Davidson L, Chen XJ, Henkelman RM. Comprehensive transthoracic cardiac imaging in mice using ultrasound biomicroscopy with anatomical confirmation by magnetic resonance imaging. Physiol Genomics 18: 232–244, 2004 [DOI] [PubMed] [Google Scholar]