Abstract
Transverse (t) tubules are surface membrane invaginations that are present in all mammalian cardiac ventricular cells. The apposition of L-type Ca2+ channels on t tubules with the sarcoplasmic reticulum (SR) constitutes a “calcium release unit” and allows close coupling of excitation to the rise in systolic Ca2+. T tubules are virtually absent in the atria of small mammals, and therefore Ca2+ release from the SR occurs initially at the periphery of the cell and then propagates into the interior. Recent work has, however, shown the occurrence of t tubules in atrial myocytes from sheep. As in the ventricle, Ca2+ release in these cells occurs simultaneously in central and peripheral regions. T tubules in both the atria and the ventricle are lost in disease, contributing to cellular dysfunction. The aim of this study was to determine if the occurrence of t tubules in the atrium is restricted to sheep or is a more general property of larger mammals including humans. In atrial tissue sections from human, horse, cow, and sheep, membranes were labeled using wheat germ agglutinin. As previously shown in sheep, extensive t-tubule networks were present in horse, cow, and human atrial myocytes. Analysis shows half the volume of the cell lies within 0.64 ± 0.03, 0.77 ± 0.03, 0.84 ± 0.03, and 1.56 ± 0.19 μm of t-tubule membrane in horse, cow, sheep, and human atrial myocytes, respectively. The presence of t tubules in the human atria may play an important role in determining the spatio-temporal properties of the systolic Ca2+ transient and how this is perturbed in disease.
Keywords: atria, t-tubule heart
t tubules are invaginations of the surface membrane that penetrate deep within the cell. They occur at the z-line and are present in ventricular myocytes of all mammalian species studied to date. Many of the proteins involved in excitation contraction coupling are located on, or in close proximity to, the t-tubule membrane (16). In cardiac muscle, excitation contraction coupling is initiated by opening of L-type Ca2+ channels and subsequent Ca2+ entry (ICa,L) triggering release of Ca2+ from the intracellular Ca2+ store, the sarcoplasmic reticulum (SR). T tubules allow close coupling of ICa,L to ryanodine receptors (RyRs) on the SR membrane resulting in rapid triggered Ca2+ release in the cell interior upon electrical excitation. Thus, in ventricular cells, which have a regular t-tubule network penetrating the entire cell, the rise in intracellular Ca2+ responsible for contraction is both rapid and synchronous throughout the entire cell. Chemically induced t-tubule removal with formamide results in this initial rise in intracellular Ca2+ concentration being localized to the periphery and then propagating to the cell center (46).
To date, t tubules have not been reported in amphibian (6), reptilian (6, 17), or avian (7) cardiac tissue. Within the mammals, t tubules are absent in ventricular myocytes of neonates and increase in number throughout development (21, 39). In adult ventricular myocytes, t tubules form a regular network associated with the z-line. Conversely, t tubules are generally accepted to be absent in the atria (9) with the exception of a few rudimentary structures in a subset of rat atrial myocytes, e.g., Ref. 25. The lack of t tubules in these species generally results in action potential-induced Ca2+ release being isolated to the cell periphery upon stimulation (26, 33). Work from this laboratory (11) and Lenaerts et al. (28) has shown the existence of an extensive t-tubule network in the atria of the sheep. T tubules are also present in dog atrial myocytes (8, 15, 43). Furthermore, immunohistochemistry suggests the presence of t tubules in the cow atria (34). In sheep, atrial cell-triggered Ca2+ release occurs simultaneously at the cell center and periphery (11), and in the dog atria Ca2+ signals are similar in subsarcolemmal and central regions (43). In the rat atrium, the rudimentary t-tubule system is more apparent in larger diameter myocytes (25, 40) consistent with the hypothesis that atrial myocytes with a comparatively large width require t tubules to allow rapid propagation of the Ca2+ transient to the cell interior.
T tubules are labile structures; in ventricular muscle, their numbers decrease in cell culture (29, 35) and there is a loss and/or disorganization in disease with important consequences for function (4, 19, 30). T-tubule loss in heart failure and type 2 diabetes can be partially reversed by exercise training (24, 42). In the sheep, atrial t tubules virtually disappear following rapid ventricular pacing (11) and are reduced in atrial fibrillation (AF; Ref. 28). In addition, t tubules in dog atrial myocytes have recently been shown to be depleted following atrial tachycardia (43). Since human atrial tissue is regularly obtained from diseased patients, the labile nature of t tubules makes their identification difficult (for example Ref. 14).
However, despite the functional importance of t tubules, it remains unknown if t tubules are present in the atria only in specific mammals or, alternatively, are a common feature in the atria of large mammals, including humans. Given the above considerations, the aim of the present work was to 1) determine if healthy adult large mammalian species, including humans, possess atrial t tubules; and 2) establish the extent of atrial t-tubule networks and if this correlates with cell width. In this study, we have shown the existence of atrial t-tubule networks in other large mammals (cow, horse, and human) and that the extent of these networks is strongly correlated with cell width. The presence of t tubules in the human atria is significant since it has important implications for our understanding of how excitation contraction coupling occurs in the healthy atria as well its deterioration in disease.
METHODS
All procedures accord to the United Kingdom Animals (Scientific Procedures) Act of 1986 and the University of Manchester's ethical review process. Human samples were obtained from patients undergoing coronary artery bypass surgery following ethical approval from the North West 9 Research Ethics Committee (Manchester, UK) or from the ethical review committee of the Medical Faculty Mannheim (2011-216N-MA, Heidelberg University, Germany).
Tissue localization and preparation.
Human atrial samples were obtained from the right atrial appendage (or auricle) at the time of surgery. While the atrial appendages are highly trabeculated, they do not contain the cardiac pacemaker, the sinoatrial node, or any specialized conduction system. Thus in this investigation cells studied were deemed to be cardiac muscle cells. Tissue samples were embedded in optimal cutting temperature embedding matrix. Sections were cut such that each extended through the entire wall, i.e., from subepicardial to subendocardial surface.
To allow comparison with the human, transverse sections of the right atrial appendage were dissected from the sheep and horse. Tissue samples from sheep were obtained as previously described (12). Briefly, sheep were killed with an overdose of pentobarbitone (200 mg/kg iv). Right atrial appendage and ventricular samples were removed and washed in a Ca2+ free solution containing the following (in mmol/l): 134 NaCl, 11 glucose, 10 HEPES, 10 2,3-butanedione monoxime, 4 KCl, 1.2 MgSO4, 1.2 NaH2PO4, and 0.5 mg/ml BSA pH 7.34 with NaOH. Cow and horse hearts were purchased from an abattoir, and tissue was dissected within 1 h of death, washed (as above), and transported to the laboratory on ice. Samples were dissected, embedded in optimal cutting temperature, and frozen in isopentane precooled in liquid nitrogen. We were unable to obtain right atrial appendage samples in the cow, and therefore the left atrial appendage was sectioned in the same way. Samples of sheep left ventricular free wall were used as a positive control. The tissue was sectioned at 40 μm (or 20 μm for staining of blood vessels) in a cryostat at −20°C, collected on polylysine-coated slides, and stored at −80°C until use.
Immunostaining, confocal, and epi-fluorescence microscopy.
Tissue sections were fixed in freshly prepared 4% paraformaldehyde (PFA) for 30 min before incubation with wheat germ agglutinin (WGA), Alexa Fluor 488 conjugate prepared in PBS (Invitrogen, Glasgow, UK) at 20 μg/ml for 30 min to visualize t tubules.
A subset of human sections (20-μm thickness) was fixed and permeabilized with 1% Triton X-100 (Sigma), and nonspecific binding sites were blocked using 10% goat serum and 1% BSA in PBS. Sections were stained with mouse anti-CD31 (5 μg/ml; Dako) for 1 h at room temperature followed by goat anti-mouse secondary antibodies conjugated to Texas red (1:200; Invitrogen) to allow visualization of the endothelial layer of any blood vessels. Sections were washed before application of FITC-conjugated WGA (20 μg/ml) for 30 min.
Coverslips were mounted using Vectashield with 1.5 μg/ml DAPI (Vector Labs) to allow visualization of the nuclei. Slides stained with CD31 were visualized using a Leica CTR5000 microscope (Leica, Germany) using phase contrast and fluorescent modes with appropriate filter blocks for each fluorophore. All imaging for t tubules was performed on a Leica SP2 confocal microscope. The point spread function of the imaging system (63 × 1.2 numeric aperture water immersion lens on a Leica SP2 confocal) was measured using polystyrene beads (100-nm diameter; Molecular Probes) imaged at an x-y resolution of 100 nm and vertical z-stacks of 162-nm separation as described previously (41, 44). Fluorescence was excited at 488 nm, and emitted light was collected >515 nm. Tissue sections were imaged in the same way as the beads to identify t tubules and excited at 351 to 364 nm with emitted light collected at >400 nm to visualize nuclear regions stained with DAPI.
Image analysis.
Images were deconvolved with the measured point spread function using Huygens Essential (Scientific Volume Imaging) to correct for out of focus fluorescence (11, 44). The distance of every voxel (3-dimensional pixel) within the cell to the nearest membrane was calculated using routines written in IDL as described previously (11). Briefly, the confocal stack for a single cell was digitally isolated from the tissue section using Image J (National Institutes of Health), and a series of sections from the cell center was used for analysis taking care to avoid the top and bottom surfaces where it becomes difficult to clearly separate t-tubular and surface membranes. Samples were costained using DAPI to visualize nuclear regions. The area occupied by nuclei was excluded from subsequent analysis. Image stacks were thresholded and resampled to make the z spacing the same as the x-y. The distance of the nearest membrane from any voxel within the cell was then calculated considering the xy as well as the z-direction using an IDL routine (11). To calculate the distance to the nearest surface membrane, t tubules were manually removed in Image J. Similarly, to calculate the distance to the nearest t-tubule membrane, the surface membrane was manually removed from the image stack.
Statistics.
All data are presented as means ± SE. Data were compared using a one-way ANOVA, an ANOVA on ranks for nonnormally distributed data, or a Mann-Whitney rank sum test where appropriate. Data were considered significant when P < 0.05.
RESULTS
Are t tubules present in horse and cow atrial myocytes?
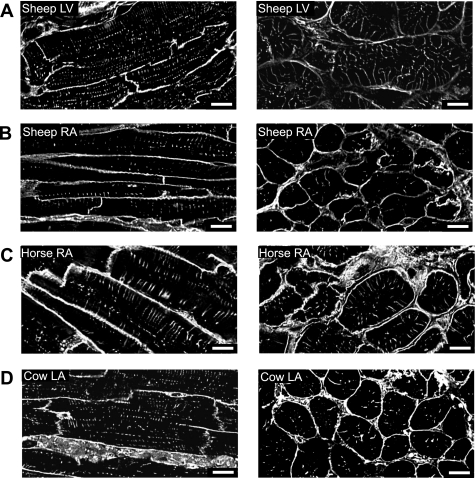
WGA-stained tissue sections from the atria of cow, horse, and sheep reveal substantial t-tubule networks (Fig. 1). A stack of confocal z-sections was taken to capture the full depth (where possible) of the cell of interest. Images in Fig. 1 show typical single central sections from such a series in both longitudinal (left) and transverse orientations (right). Sheep ventricle tissue sections were used as a positive control. T tubules were observed in all atrial cells from horse, cow, and sheep. In sheep ventricular myocytes, as expected, t tubules were regularly spaced at the z-line (Fig. 1A), although gaps were present in some areas consistent with previous data for the pig (20).
Fig. 1.
Surface and t-tubular membrane staining of ventricular and atrial tissue sections. Example deconvolved images of cell membranes in tissue sections labeled with wheat germ agglutinin (WGA). A–D, left: longitudinal view of sheep left ventricle (LV), sheep right atrium (RA), horse RA, and cow left atrium (LA), A–D, right: transverse sections of tissue in the same species. All scale bars are 10 μm and are shown at bottom right.
What is the role of t tubules in determining the distance of the cell interior to membrane?
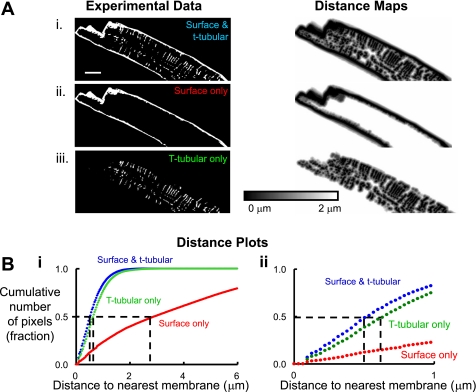
Since t tubules allow close coupling of L-type Ca2+ channels to RyRs, we have calculated the distance of every voxel (in a 3-dimensional reconstructed section of the cell) to the nearest membrane (11). Longitudinal images as shown in Fig. 1, left, were analyzed as detailed in Fig. 2 using the horse. First, the image stack was deconvolved and the background was removed (using the polygon tool in Image J to draw around the background and delete it) leaving only the z-series of the cell of interest, e.g., Fig. 2Ai, left. Where necessary, nonspecific staining of the nuclear envelope was also removed. To assess the importance of t tubules and surface membrane in the close coupling of L-type Ca2+ channels to RyRs, we generated three sets of experimental data for each cell as shown in Fig. 2A, left. This consists of 1) both surface membrane and t tubules together (Fig. 2Ai); 2) surface membrane only following deletion of t tubules in Image J (Fig. 2Aii); and 3) t tubules only, following deletion of the surface membrane in Image J (Fig. 2Aiii). Each of these image stacks was entered into the analysis software, and example distance maps generated are shown in the Fig. 2A, right. Here pixel intensity represents distance to the nearest membrane component where the darker the pixel the closer this pixel is to membrane in either the x, y, or z direction. Distance maps in Fig. 2 are scaled from 0 to 2 μm for clarity, but for analysis in this study the scale was extended to 8 μm. Figure 2B shows a distance plot generated from the distance maps. Here, the distance to membrane was calculated for each pixel intensity and plotted against the cumulative number of pixels in each distance map resulting in a data series for each of the three data types input, i.e., for t tubules and surface membrane together (blue), t tubules only (green), and surface membrane only (red). In this example, it is evident that all the pixels lie within ∼2 μm of any membrane (both surface membrane and t tubules together, blue). Furthermore, the distance plot shows that t tubules are more important than the surface membrane in ensuring the center of the horse atrial myocyte is close to membrane. The half distance, or distance at which 50% of pixels are from a given membrane, is illustrated by the dashed lines in Fig. 2, Bi and ii. Thus the half distance of the surface and t-tubule membranes together is 0.52 μm and those of the t-tubule membrane only and surface membrane only are 0.64 and 2.91 μm, respectively, in this example (see magnified example in Fig. 2Bii).
Fig. 2.
Measurement of the distance of voxels within a horse atrial myocyte to t-tubular and surface membrane. A: deconvolved cellular images (left) and resultant distance maps (right). Ai: image of a typical cell (left) and a distance map (right) to show the distance of voxels to the nearest membrane (t tubule or surface). Aii: cell image of surface membrane only (left) following digital removal of t tubules from Ai and resultant distance map (right) showing the distance of voxels to the surface membrane. Aiii: cell image of t tubules only (left) following digital removal of the surface membrane from Ai and resultant distance map (right) showing the distance of voxels to the t-tubular membrane. Scale bar for left is shown in Ai and is 10 μm. Bi: distance plot calculated from distance maps in Ai-iii; blue line shows the distance of voxels to the nearest t-tubular and surface membrane together, green line to the nearest t-tubular membrane, and red line to the nearest surface membrane. Dashed lines show 50% of the voxels in the cell are closer to membrane than the indicated distance on the x-axis (termed half distance). Bii: distance plot from Bi on an expanded x-axis.
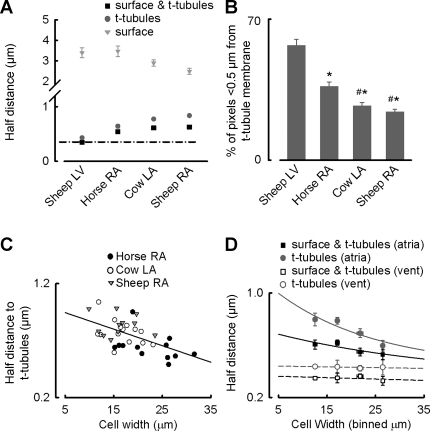
Similar distance plots were generated for cow and sheep atria as well as sheep ventricle. Half distance values for surface membrane, t-tubule membrane or t-tubule and surface membrane together for each species are summarized in Fig. 3A. Considering the sheep ventricle first, it is evident that the proximity of pixels to membrane largely depends on t tubules rather than surface membrane with half-distance values of 0.43 ± 0.02 vs. 3.39 ± 0.24 μm, respectively (n = 13 cells from 4 animals). Similarly, in sheep, cow, and horse atrial cells there is a strong reliance on t tubules over surface membrane in ensuring the cell interior is close to membrane. Consequently, 50% of voxels are between 2.48 ± 0.15 and 3.46 ± 0.26 μm from surface membrane in horse, cow, and sheep atrial cells but only between 0.84 ± 0.03 and 0.64 ± 0.03 μm of t-tubule membrane (n = 11 to 14 cells from 4 to 5 animals). This is not, however, as strong a reliance on t tubules as ventricular cells since t tubules are not present on every sarcomere in the atria. Figure 3A shows that the half distance for atrial t tubules is smallest in the horse and greatest in the sheep (0.64 ± 0.03 vs. 0.84 ± 0.03 μm; n = 11 to 12 cells from 4 to 5 animals; P < 0.05). In addition, the percentage of pixels that are <0.5 μm from the t tubule (dark grey) are shown in Fig. 3B. In the sheep ventricle, 57 ± 3.1% of pixels are closer than 0.5 μm to t tubules compared with only 9.8 ± 0.6% of pixels being <0.5 μm from the surface membrane (surface membrane data not shown; n = 13 cells from 4 animals). The percent of pixels less than 0.5 μm from t tubules was greater in the horse atria compared with the cow and sheep atria (37 ± 2.2 vs. 27 ± 1.6 and 24 ± 1.2%; n = 11 to 14 cells from 4 to 5 animals; P < 0.05), while values for the surface membrane were similar throughout (n = 11 to 14 cells from 4 to 5 animals; P = 0.09).
Fig. 3.
T-tubule density and correlation with cell width. A: half distance of voxels within the cell to membrane for the species indicated; surface membrane is grey triangles, t-tubular membrane is dark grey circles, and both membranes together are black squares throughout. Black dashed line indicates distance of voxels to surface and t-tubular membranes together in sheep ventricular cells. B: histogram to show the percentage of voxels that are <0.5 μm from the t-tubular membrane. *Significant difference vs. sheep LV; #significant difference vs. horse RA. C: individual cell data displaying the half distance to t-tubular membrane for horse atria (closed circles), cow atria (open circles), and sheep atria (grey triangles). Data are fit with a linear regression where the gradient is −0.015 ± 0.004. Individual cells were binned according to their width, and data for half distance are displayed in D for both atrial (closed symbols) and ventricular (vent) cells (open symbols) for t tubules (dark grey circles) and both t-tubular and surface membrane (black squares). Ventricular data are fitted with linear regressions to guide the eye, and atrial data are fitted by single exponentials where half distance = mean ventricular half distance + a * exp(−b*x); thus for the atria a minimum value is reached at the mean ventricular half distance. Values for the constant of the exponent (b) are 0.049 for atrial t tubules only (solid grey line) and 0.028 for atrial surface and t tubules together (solid black line).
Does the prevalence of atrial t tubules increase with cell width?
We have tested the hypothesis that atrial cells from horse, cow, and sheep possess t tubules due to their large cell width and that in wider cells the prevalence of t tubules is increased. The width of horse atrial myocytes is larger than that of cow or sheep atrial myocytes (22.7 ± 1.5 vs. 16.8 ± 0.93 and 16.0 ± 1.16 μm, respectively; n = 11 to 14 cells from 4 to 5 animals; P < 0.01, data points distributed normally) and is not different from that of sheep ventricular myocytes in this study (20.7 ± 1.36 μm; n = 13 cells from 4 animals; P = 0.3, data points distributed normally). Figure 3C therefore examines the relationship between t-tubule density (or the half distance to t tubules) and cell width. Data points are fitted with a linear regression resulting in a correlation coefficient of 0.32 and a slope significantly different from zero (P < 0.001) showing that the wider the cell the smaller the half distance to t-tubule membranes. Data in Fig. 3C and similar data for ventricular myocytes were binned according to cell width in 5-μm groups and are displayed in Fig. 3D for t tubules (grey circles) and surface and t-tubule membranes together (black squares). In Fig. 3D, sheep ventricular data (open symbols) are fitted with linear regressions that show the half distance of t tubules is not correlated with the width of the cell; t tubules lie on every z-line in the ventricle regardless of cell width. In Fig. 3D, it would be inappropriate to fit atrial data with a linear regression, since this would suggest half-distance values lower than those of the ventricle could exist in very wide cells and also that a point could be reached (at the x-axis intercept) where the half distance would be zero and the cell entirely made up of t tubules. Half-distance values lower than those of the ventricle are unlikely to occur, since a minimum half-distance value would be reached when a t tubule was present on every z-line as in the ventricle. Thus atrial cell data are fitted with a single exponential where a minimum level was defined at the mean half-distance value for the ventricular data. However, it should be noted that t tubules do possess longitudinal or axial extensions that, in the rat ventricle, form dyadic junctions containing up to 19% of the RyRs of the cell (3). Since these extensions are not restricted to z-line localization, it would be possible for a cell to increase t-tubule numbers beyond those of sheep ventricular cells in this study but it would not be possible for the half distance to decrease to zero. It is evident from Fig. 3D that the half distance to atrial t tubules (grey filled circles) shows an association with cell width where the wider the cell the smaller the half distance to the t-tubule membrane. Similar data considering both surface and t-tubule membrane together are also shown (black filled symbols). This relationship is less sensitive to cell width compared with that for t tubules alone highlighting the importance of t tubules in wide cells.
Are t tubules present in the human atria?
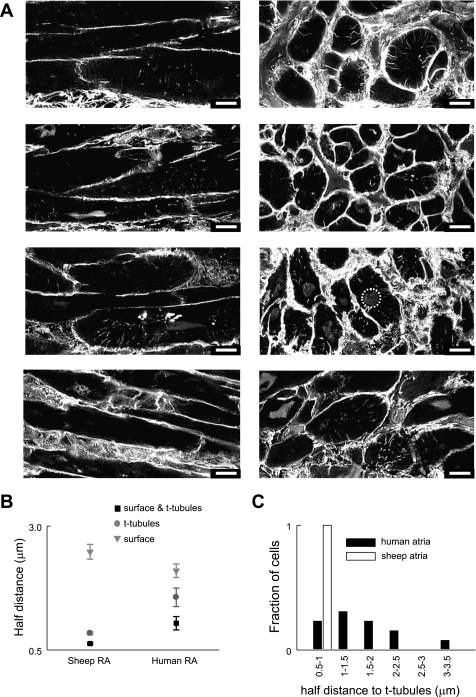
It is of great importance to determine if t tubules are present in the human atria given their demonstrated functional role in large mammals and the fact that this is altered by disease (11, 28, 43). Since atrial t-tubule expression is dramatically reduced in models of disease (11, 28, 43), we have taken atrial samples from nine patients who were in sinus rhythm. The study was blinded to other patient information. Figure 4A shows eight 2-μm-thick human right atrial tissue sections from four patients in both the longtitudinal (left) and transverse (right) orientations stained with WGA. Clear t tubules are present as shown either by punctuate staining (where the t tubule extends perpendicular to the plane of view) or staining along the t-tubule length from the surface membrane towards the cell interior. Atrial cells analyzed from each human showed a heterologous population of cells where t tubules were either present or absent. T tubules were observed in some cells from every patient and in a total of 59 from 86 cells (49 tissue sections), i.e., 69% of human atrial cells showed at least some t-tubule structures.
Fig. 4.
T tubules are present in human atrial cells. A: example of deconvolved 2-μm-thick images of human atrial cell membranes from 4 patients in tissue sections labeled with WGA. T tubules are clearly present in some cells and absent in others in the plane shown. Images at left are longitudinal in orientation while those at right are transverse. None specific staining of the nucleus was observed in some cells an example of which is circled at right. All scale bars are 10 μm and are located at bottom right. B: half distance of voxels within the cell to membrane for sheep and human right atrial cells; surface membrane is grey triangles, t-tubular membrane is dark grey circles, and both membranes together are black squares. C: frequency histogram displaying binned values for the half distance to t tubules for human atrial cells (filled bars) and sheep atrial cells (open bars).
Image analysis for human tissue was performed on transverse (Fig. 4A, right) rather than longitudinal sections (Fig. 4A, left) due to improved image clarity. In some sections, nonspecific staining of the nucleus was observed as shown by in Fig. 4A, right (dotted circle). This was removed before analysis was performed. Half-distance values were calculated (as described in Fig. 2) for the surface and t-tubular membranes together, t tubules alone, and surface membrane alone from transverse sections (1.04 ± 0.14, 1.56 ± 0.19, and 2.10 ± 0.14 μm respectively; n = 13 to 14 cells from 4 patients) and are displayed in Fig. 4B with similar data from the sheep atria for comparison. Compared with other species studied human atrial cells rely less on t tubules in bringing the cell interior close to membrane. In the human, 50% of voxels lie within 1.56 ± 0.19 μm of t-tubular membrane compared with 0.84 ± 0.03 μm in the sheep atria (n = 11 to 14 cells from 4 patients or 5 animals; P < 0.001). However, from the subset of human atrial cells analyzed, t tubules were more important than the surface membrane in defining the proximity of the cell interior to membrane (50% of voxels were 1.56 ± 0.19 μm from t-tubule membrane vs. 2.1 ± 0.14 μm from surface membrane; P < 0.05; n = 13 to 14 cells from 4 patients). Figure 4C highlights the variability in human atrial myocytes compared with sheep atrial myocytes by binning the half-distance value to t tubules for each cell. While the half distance (or the distance of 50% of pixels) to t tubules for all the sheep atrial cells fell between 0.5 and 1 μm, values for human cells reached >3 μm and some cells had no t-tubule structures.
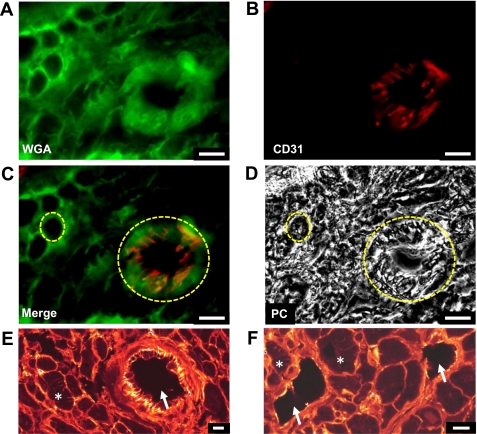
Performing analysis on transverse rather than longitudinal human sections would not be expected to affect half-distance values. This was confirmed by analyzing a subset of sheep ventricular cells in the transverse orientation and comparing values to those from a longitudinal orientation (data not shown). Here 50% of the voxels were 3.39 ± 0.24 vs. 3.29 ± 0.27 from the surface membrane and 0.43 ± 0.02 vs. 0.47 ± 0.02 from the t-tubular membrane calculated from longitudinal vs. transverse images (n = 5 to 13 cells; P = 0.83 and 0.35). Analysis of transverse sections such as those in Fig. 4, right, may inadvertently result in the inclusion of blood vessels mistaken for cardiac myocytes with no t tubules. This would act to decrease assessment of t-tubule contribution to the human atrium in subsequent analysis.
One concern with the above measurements is the possibility that a small blood vessel might appear similar to a cardiac myocyte without t tubules. The contribution of vessels to human transverse tissue sections was investigated as shown in Fig. 5. Tissue sections were costained with WGA to highlight cell membrane (Fig. 5A), CD31 was used as a marker of blood vessels (Fig. 5B), and images were taken on a Leica epi-fluorescence microscope. Figure 5B highlights a transverse section through a blood vessel ∼25 μm in diameter. Images from Fig. 5, A and B, are overlaid in Fig. 5C, and the corresponding phase contrast image is shown in Fig. 5D. These data show that the transverse vessel (Fig. 5C, large yellow circle) is distinguished by CD31 binding as well as a dark region, void of light diffraction, in the phase contrast image (Fig. 5D, large yellow circle). The small yellow circle highlights WGA staining of a single myocyte in Fig. 5C with no CD31 staining and the same myocyte in Fig. 5D under phase contrast where the interior of the cell appears filled in contrast to the vessel. The transverse vessel could potentially be mistaken for a myocyte devoid of t tubules. We have therefore calculated the contribution of such vessels to tissue sections by counting the number of myocytes and vessels in 50 sections from 5 patients. We identified 561 vessels in sections totaling 3,014 cells (where cells were over ∼5 μm in diameter), i.e., 18.6% of structures were blood vessels. The majority of these blood vessels were, however, very small with 69% being <5 μm in diameter. Thus vessels >5 μm in diameter only contributed 5.3% to the total number of structures (cells and vessels together). All human atrial cells analyzed in this study were >10 μm in diameter, and vessels of this size contributed only 1.5% of the total structures. Therefore, while some overlap in diameter exists between vessels and myocytes, the presence of vessels was deemed to have minimal impact on the study. It is important to note that any vessels mistaken for atrial cells devoid of t tubules will decrease the importance of human t tubules in this study. Importantly, WGA-stained confocally imaged blood vessels shown by arrows in Fig. 5, E and F, do not contain structures that could be mistaken for t tubules.
Fig. 5.
Contribution of blood vessels to human atrial sections. A: WGA-stained section revealing circular structures in the transversely cut myocardium. B: anti-CD31-staining labeling vascular endothelial cells. C: merged image showing microvasculature present in the myocardium. D: same image shown under phase contrast. In C and D, the large yellow circle highlights a vessel and the smaller one a single atrial myocyte. Blood vessels could be identified using phase contrast where the lumen was void of light diffraction as opposed to myocytes where diffraction was present. E and F: WGA-stained myocardium imaged using confocal microscopy confirming the lack of t-tubule-like structures in the lumen of blood vessels (arrows). T tubules are clearly present in some myocytes; examples are highlighted by asterisks. All scale bars are 10 μm and are shown at bottom right.
DISCUSSION
This study is the first to investigate t-tubule expression in the atria of humans and other large mammals. The main findings are 1), t tubules are abundant in the atria of horse and cow as well as sheep; 2) t-tubule abundance correlates with cell width in horse, cow and sheep atria where wider cells are infiltrated more fully by the t-tubule network; and 3), t tubules are present in the human atria.
Presence of t tubules in atrial myocytes.
T tubules have recently received much attention primarily focused on their role in the ventricle in terms of normal physiology and pathological conditions (reviewed by Refs. 10, 31). Following extensive research on small mammals such as the rat and cat (5, 9, 22), it was assumed that atrial cells were generally devoid of these important structures, although a rudimentary transverse axial tubule system has been observed in a subset of rodent atrial myocytes (25, 40, 45). Work from our laboratory and others has however, shown extensive t-tubule networks in the atria of the sheep (11, 28) and identified their presence in the dog (15, 43). In much the same way as the ventricle, the presence of t tubules in the sheep atria allows Ca2+ release to be triggered in the cell interior, increasing the rate of rise of the systolic Ca2+ transient (11). Thus the presence of t-tubule structures in the atria has fundamentally altered our view of the functional properties of the atrial systolic Ca2+ transient in these species. This study has shown that atrial t tubules are present in the horse and cow to a similar extent as we have previously observed in the sheep, suggesting, that in large mammals generally, t tubules play an integral role in excitation contraction coupling in the atria. Previously, immunohistochemical staining of bovine atrial and ventricular muscle fibers for the membrane-cytoskeletal protein vinculin showed consistency with t-tubule structures, suggesting the presence of a bovine atrial t-tubule system that was less developed than that in the ventricle (34). That study did not quantify t tubules, and the relatively faint atrial staining makes comparison of t-tubule numbers to the current study difficult; nevertheless, this work also supports the presence of atrial t tubules in the cow.
Importantly, the existence of t-tubule networks in the human atria, described in the current study, suggests they may be more functionally comparable to the sheep than to small mammals. Previously, it has been suggested that the majority of human atrial cells do not have a t-tubular system (27). The study by Legato (27) does not include the anatomical origin of these atrial cells, but their dimensions were reported to be 6 to 8 μm in diameter and 20 to 30 μm in length. These cells are extremely small compared with cell diameters for sheep, horse, cow, and human cells in the present study. Other studies (36, 37) have measured dimensions of human atrial myocytes from the right atrial appendage reporting values of 94.2 or 81 μm length and 17.9 or 17 μm width, which are in good agreement with a diameter of 17.3 ± 1.2 μm measured in the current study. The small cells in the study by Legato (27), obtained from an unspecified atrial region, may form part of the specialized sinoatrial node, and since we have shown a correlation between cell width and the presence of t tubules, perhaps in these small cells t tubules may not be needed. Immunohistochemical staining of cytoskeletal proteins in the human left atrium has highlighted irregular spoke-like extensions penetrating towards the cell interior, suggesting the presence of at least some t tubules (2).
T-tubule networks are more apparent in wider atrial cells.
T-tubule abundance governs temporal and spatial properties of the systolic Ca2+ transient and influences the amplitude of the systolic Ca2+ transient and thus contraction of the atria. This concept has been clearly demonstrated in rat ventricular myocytes where detubulation results in Ca2+ rising initially at the cell periphery and then propagating to the cell center giving rise to a Ca2+ transient that is reduced in amplitude (9, 23).
In this study, we have shown that the abundance of t tubules in the atria of horse, cow, and sheep correlates with the width of the cell consistent with the notion that wider cells require t tubules to bring the Ca2+ signal more rapidly to the cell interior. Given that rat atrial cells are ∼10 μm in width (44), their lack of t tubules would be consistent with this hypothesis. Horse atrial myocytes are wider than those of sheep and cow, and t tubules become more important as shown by the smaller distance to the nearest t tubule. The increased presence of t tubules in wide cells more than compensates for the large cell width in ensuring the cell interior is close to membrane. This suggests that the rate of rise of Ca2+ during systole and the amplitude of the Ca2+ transient and thus contraction may be enhanced in wide vs. narrow atrial cells. As expected, in ventricular cells there is no correlation between cell width and half distance to t-tubule membrane, since t tubules occur regularly on each sarcomere regardless of cell width.
Why atrial cell width and thus t-tubule abundance differ between mammalian species while ventricular cells from a mouse, rabbit, and horse are similar in size (32) remains unknown. Interestingly, atrial cell width in this study inversely correlates with resting heart rate whereby the horse with the lowest resting heart rate has the widest cells and most abundant atrial t-tubule system.
Role of atrial t tubules in disease.
A large body of evidence now suggests that the loss or disorganization of t tubules contributes to impaired Ca2+ homeostasis in heart failure in the ventricle (for review see Ref. 31). Similarly, t tubule loss from the atria may also play a role in dysfunction; during disease t tubules have been shown to be lost from the sheep atria in heart failure and the canine atria in a model of atrial tachycardia remodeling, and this appears to be to a greater extent than that reported for the ventricle in a similar study in the dog (11, 18, 43). AF is the most common cardiac arrhythmia and is becoming an increasing problem in the aging Western world. Changes in intracellular Ca2+ handling, most notably decreased ICa,L have been shown to occur in AF (for review see Ref. 13). Work by Lenaerts et al. (28) demonstrates t-tubule loss in a sheep model of AF, indicating that the reduction in ICa,L, commonly associated with AF correlates with a loss of atrial t tubules. Thus the identification of t tubules in the human atria may represent an important mechanism by which their loss could contribute to the pathology of AF. While human atrial samples in this study were from patients in sinus rhythm, other underlying health problems could contribute to the heterogeneity of t-tubule distribution in the human atrium compared with that of sheep, cow, and horse. Interestingly, in ventricular cells t-tubule loss following severe pathological remodeling has been shown to be partially restored following exercise training (24).
Study limitations.
WGA is selective for N-acetylglucosamine oligomers and sialic acid residues and has been used to specifically label t tubules in live cardiac myocytes (e.g., Ref. 38). In this study, we have used fixed permeabilized tissue sections where WGA stains the t-tubule system but could also result in nonspecific staining. We observed nonspecific staining of the nucleus, which was digitally removed where necessary.
While tissue from the left atrial free wall or pulmonary vein sleeves would be preferable to the atrial appendage tissue used in this study given their role in atrial contraction and AF, it was not possible to obtain these tissue regions in the human. Accordingly, appendage tissue was selected in other species for comparison to the human. Evidence suggests that, at least in the left atrium, active contraction of the atrial appendage does occur in the cardiac cycle (reviewed by Ref. 1). The left atrial appendage shows dramatic t-tubule loss following rapid ventricular pacing in the sheep, and this could play an important role in atrial dysfunction in disease (11). A similar loss of t tubules in the right atria has been shown to occur in AF (28). This suggests that appendage tissue is functionally relevant in both health and disease settings and that our findings might apply to other atrial regions including the left atrium.
In this study, we have expressed t-tubule abundance as a function of cell width. Two factors may have resulted in inaccurate cell width measurements: 1) difficulty determining if cells were obliquely orientated (overestimate of cell width) or if cells lay on their sides (underestimate of cell width); and 2) tissue sections were fixed with PFA that can result in shrinkage of the tissue and therefore a decrease in cellular dimensions. In this study, the width of sheep atrial myocytes was 16.0 ± 1.2 μm compared with 15.2 ± 1.48 μm in our previous study (11) on live single isolated cells, suggesting PFA did not result in significant shrinkage.
The presence of t tubules in the transverse human atrium sections may be underestimated due to mistaking vessel lumens for myocytes devoid of t tubules. We have used CD31 staining of the endothelial layer as well as phase-contrast microscopy to identify blood vessels. Since vessels >10 μm in diameter only contributed 1.5% of structures in this study they were deemed to have minimal impact on the analysis. While some human cells clearly show an abundant t-tubular system, other cells, sometimes smaller in size, appear to be largely devoid of these structures (Fig. 4). In the case of smaller cells, it was impossible to tell if these cells are truly smaller and therefore t tubules are sparse or if these cells appear smaller as they represent tapered cell ends and have t tubules in their center (not included in the 40-μm tissue section). In light of this, as well as the heterogeneous nature of human atrial cells with respect to t tubules, we have not included relationships to cell width or a detailed cross-species comparison with the human data. Detailed analysis of t tubules in the healthy human atrium as well as a comparison with disease states requires further investigation.
Conclusions.
While it has long been assumed that atrial cells lack t tubules, we have shown they are an important feature of large mammalian atrial cells. The identification of t tubules in human atria is important; it not only revises our view of the control of intracellular Ca2+ but also highlights a potential mechanism for dysfunction in human disease states. Intriguingly, t tubules may represent a useful target for future treatments aimed at increasing contractile performance in conditions such as heart failure and AF.
GRANTS
This work was supported by the British Heart Foundation, including an Intermediate Basic Science Research Fellowship (to K. M. Dibb), a Manchester Biomedical Research Center George Lancashire Award, and a grant from Fondation Leducq (European-North American Atrial Fibrillation Research Alliance, 07CVD03; to D. Dobrev).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the cardiac surgeons from Manchester Healthcare Trust and the University of Heidelberg for providing human atrial tissue.
REFERENCES
- 1. Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart 82: 547–554, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anastasi G, Cutroneo G, Gaeta R, Di MD, Arco A, Consolo A, Santoro G, Trimarchi F, Favaloro A. Dystrophin-glycoprotein complex and vinculin-talin-integrin system in human adult cardiac muscle. Int J Mol Med 23: 149–159, 2009 [PubMed] [Google Scholar]
- 3. Asghari P, Schulson M, Scriven DR, Martens G, Moore ED. Axial tubules of rat ventricular myocytes form multiple junctions with the sarcoplasmic reticulum. Biophys J 96: 4651–4660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res 59: 67–77, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Blatter LA, Kockskamper J, Sheehan KA, Zima AV, Huser J, Lipsius SL. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J Physiol 546: 19–31, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bossen EH, Sommer JR. Comparative stereology of the lizard and frog myocardium. Tissue Cell 16: 173–178, 1984 [DOI] [PubMed] [Google Scholar]
- 7. Bossen EH, Sommer JR, Waugh RA. Comparative stereology of the mouse and finch left ventricle. Tissue Cell 10: 773–779, 1978 [DOI] [PubMed] [Google Scholar]
- 8. Boyden PA, Hoffman BF. The effects of atrial electrophysiology and structure of surgically induced right atrial enlargement in dogs. Circ Res 49: 1319–1331, 1981 [DOI] [PubMed] [Google Scholar]
- 9. Brette F, Komukai K, Orchard CH. Validation of formamide as a detubulation agent in isolated rat cardiac cells. Am J Physiol Heart Circ Physiol 283: H1720–H1728, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Brette F, Orchard C. Resurgence of cardiac t-tubule research. Physiology (Bethesda) 22: 167–173, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Dibb KM, Clarke JD, Horn MA, Richards MA, Graham HK, Eisner DA, Trafford AW. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ Heart Fail 2: 482–489, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Dibb KM, Rueckschloss U, Eisner DA, Isenberg G, Trafford AW. Mechanisms underlying enhanced cardiac excitation contraction coupling observed in the senescent sheep myocardium. J Mol Cell Cardiol 37: 1171–1181, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Dobrev D, Nattel S. Calcium handling abnormalities in atrial fibrillation as a target for innovative therapeutics. J Cardiovasc Pharmacol 52: 293–299, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Dobrev D, Teos LY, Lederer WJ. Unique atrial myocyte Ca2+ signaling. J Mol Cell Cardiol 46: 448–451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dolber PC, Bauman RP, Rembert JC, Greenfield JC., Jr Regional changes in myocyte structure in model of canine right atrial hypertrophy. Am J Physiol Heart Circ Physiol 267: H1279–H1287, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Franzini-Armstrong C, Protasi F, Tijskens P. The assembly of calcium release units in cardiac muscle. Ann NY Acad Sci 1047: 76–85, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Galli GL, Warren DE, Shiels HA. Ca2+ cycling in cardiomyocytes from a high-performance reptile, the varanid lizard (Varanus exanthematicus). Am J Physiol Regul Integr Comp Physiol 297: R1636–R1644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He JQ, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res 49: 298–307, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von WF, Claus P, Dymarkowski S, Maes F, Bogaert J, Rademakers F, D′hooge J, Sipido K. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res 102: 338–346, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Heinzel FR, Bito V, Volders PGA, Antoons G, Mubagwa K, Sipido KR. Spatial and temporal inhomogeneities during Ca2+ release from the sarcoplasmic reticulum in pig ventricular myocytes. Circ Res 91: 1023–1030, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Hirakow R, Krause WJ. Postnatal differentiation of ventricular myocardial cells of the opossum (Didelphis virginiana Kerr) and T-tubule formation. Cell Tissue Res 210: 95–100, 1980 [DOI] [PubMed] [Google Scholar]
- 22. Hüser J, Lipsius SL, Blatter LA. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol 494: 641–651, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawai M, Hussain M, Orchard CH. Excitation-contraction coupling in rat ventricular myocytes after formamide-induced detubulation. Am J Physiol Heart Circ Physiol 277: H603–H609, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Kemi OJ, Hoydal MA, MacQuaide N, Haram PM, Koch LG, Britton SL, Ellingsen O, Smith GL, Wisloff U. The effect of exercise training on transverse tubules in normal, remodeled, and reverse remodeled hearts. J Cell Physiol 226: 2235–2243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirk MM, Izu LT, Chen-Izu Y, McCulle SL, Wier WG, Balke CW, Shorofsky SR. Role of the transverse-axial tubule system in generating calcium sparks and calcium transients in rat atrial myocytes. J Physiol 547: 441–451, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kockskamper J, Sheehan KA, Bare DJ, Lipsius SL, Mignery GA, Blatter LA. Activation and propagation of Ca2+ release during excitation-contraction coupling in atrial myocytes. Biophys J 81: 2590–2605, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Legato MJ. Ultrastructure of the atrial, ventricular, and Purkinje cell, with special reference to the genesis of arrhythmias. Circulation 47: 178–189, 1973 [DOI] [PubMed] [Google Scholar]
- 28. Lenaerts I, Bito V, Heinzel FR, Driesen RB, Holemans P, D'hooge J, Heidbuchel H, Sipido KR, Willems R. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ Res 105: 876–885, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules–a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res 62: 63–73, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, Sejersted OM. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol 574: 519–533, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Louch WE, Sejersted OM, Swift F. There goes the neighborhood: pathological alterations in T-tubule morphology and consequences for cardiomyocyte Ca2+ handling. J Biomed Biotechnol 2010: 503906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loughrey CM, Smith GL, MacEachern KE. Comparison of Ca2+ release and uptake characteristics of the sarcoplasmic reticulum in isolated horse and rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol 287: H1149–H1159, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Mackenzie L, Bootman MD, Berridge MJ, Lipp P. Predetermined recruitment of calcium release sites underlies excitation- contraction coupling in rat atrial myocytes. J Physiol 530: 417–429, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pardo JV, Siliciano JD, Craig SW. Vinculin is a component of an extensive network of myofibril-sarcolemma attachment regions in cardiac muscle fibers. J Cell Biol 97: 1081–1088, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pavlovic D, McLatchie LM, Shattock MJ. The rate of loss of T-tubules in cultured adult ventricular myocytes is species dependent. Exp Physiol 95: 518–527, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Polontchouk L, Haefliger JA, Ebelt B, Schaefer T, Stuhlmann D, Mehlhorn U, Kuhn-Regnier F, De Vivie ER, Dhein S. Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol 38: 883–891, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Porciatti F, Pelzmann B, Cerbai E, Schaffer P, Pino R, Bernhart E, Koidl B, Mugelli A. The pacemaker current I(f) in single human atrial myocytes and the effect of beta-adrenoceptor and A1-adenosine receptor stimulation. Br J Pharmacol 122: 963–969, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Savio-Galimberti E, Frank J, Inoue M, Goldhaber JI, Cannell MB, Bridge JH, Sachse FB. Novel features of the rabbit transverse tubular system revealed by quantitative analysis of three-dimensional reconstructions from confocal images. Biophys J 95: 2053–2062, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A, Tohse N. Fetal and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovasc Res 58: 535–548, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Smyrnias I, Mair W, Harzheim D, Walker SA, Roderick HL, Bootman MD. Comparison of the T-tubule system in adult rat ventricular and atrial myocytes, and its role in excitation-contraction coupling and inotropic stimulation. Cell Calcium 47: 210–223, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res 84: 266–275, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Stolen TO, Hoydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, Larsen T, Rolim N, Condorelli G, Smith GL, Wisloff U. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res 105: 527–536, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Wakili R, Yeh YH, Yan Q, X, Greiser M, Chartier D, Nishida K, Maguy A, Villeneuve LR, Boknik P, Voigt N, Krysiak J, Kaab S, Ravens U, Linke WA, Stienen GJ, Shi Y, Tardif JC, Schotten U, Dobrev D, Nattel S. Multiple potential molecular contributors to atrial hypocontractility caused by atrial tachycardia remodeling in dogs. Circ Arrhythm Electrophysiol 3: 530–541, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Walden AP, Dibb KM, Trafford AW. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol 46: 463–473, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Woo SH, Cleemann L, Morad M. Diversity of atrial local Ca2+ signalling: evidence from 2-D confocal imaging in Ca2+-buffered rat atrial myocytes. J Physiol 567: 905–921, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang Z, Pascarel C, Steele DS, Komukai K, Brette F, Orchard CH. Na+-Ca2+ exchange activity is localized in the t-tubules of rat ventricular myocytes. Circ Res 91: 315–322, 2002 [DOI] [PubMed] [Google Scholar]