Abstract
The pulmonary alveolus, terminal gas-exchange unit of the lung, is composed of alveolar epithelial and endothelial cells separated by a thin basement membrane and interstitial space. These cells participate in the maintenance of a delicate system regulated not only by biological factors but also by the mechanical environment of the lung, which undergoes dynamic deformation during breathing. Clinical and animal studies as well as cell culture studies point toward a strong influence of mechanical forces on lung cells and tissues including effects on growth and repair, surfactant release, injury, and inflammation. However, despite substantial advances in our understanding of lung mechanics over the last half century, there are still many unanswered questions regarding the micromechanics of the alveolus and how it deforms during lung inflation. Therefore, the aims of this review are to draw a multidisciplinary account of the mechanics of the alveolus on the basis of its structure, biology, and chemistry and to compare estimates of alveolar deformation from previous studies.
Keywords: lung injury, mechanotransduction, distension, stretch
the alveolus, the location of gas exchange in the lung, has a complex three-dimensional structure that is composed of an epithelial cell-lined air space and endothelial cell-lined capillaries separated by a thin basement membrane and interstitial space. The basement membrane, composed of extracellular matrix (ECM) proteins including collagen and elastin (54, 70, 80), provides support for the alveolar epithelial cells, which include both type I (AEI) and type II (AEII) cells.
During normal tidal breathing, the alveolus undergoes volume fluctuations that have been estimated to cause 4% linear distension of the basement membrane (28, 29, 58). The alveolar distension, experienced by the alveolar basement membrane and alveolar epithelial cells, is described as a “mechanical stretch load.” In an injured lung, changes in structure and mechanical properties can lead to changes in the magnitude of stretch experienced by the alveolus compared with a healthy lung. For example, in the case of acute lung injury, fluid leakage into the interstitium and into the alveoli causes changes in the properties of the tissue, loss or dilution of surfactant, and changes in the inflation of non-fluid-filled regions. This causes changes in the overall compliance of the lung that will affect not only gas exchange locally, but also the distension of the tissue in both injured and noninjured parts of the lung (91). It has become increasingly apparent over the last two decades that the levels of mechanical stress influence the biological function and signaling of alveolar epithelial cells (82, 85, 99). For example, mechanical stretch of cultured type II cells was shown to stimulate changes in surfactant secretion (25, 72, 99), cell injury or death (3, 36, 84), permeability (12, 14), and cell migration (22).
From the clinical perspective, a study by the ARDSNet demonstrated a significant reduction in mortality when acute respiratory distress syndrome (ARDS) patients were ventilated with lower tidal volumes (1, 50, 74, 88). Since the lower tidal volume is thought to correlate with lower distension of the lung, the outcome of the ARDSNet trial provides strong evidence that alterations in lung mechanics can significantly impact both the basic biology of the lung as well as clinical outcomes. However, because of the complexities of lung mechanics and limitations in imaging capabilities, our knowledge of the micromechanics of the individual alveolus is limited. One aim of this article is to summarize the previous studies of alveolar mechanics and to extrapolate quantitative information about the alveolar strain field.
Mechanical Determinants of the Alveolus
When examining the mechanical environment of the alveolus, we often consider factors either at the level of the whole organ (macroscale) or at the level of the alveolus (microscale), but these considerations across scale are interrelated (see Fig. 1). We can think of the lung as a prestressed network structure whose overall response to a mechanical stimulus depends not only on the properties of the network elements (alveolar structures) but also on how those elements are connected to one another. The mechanical properties, composition, and geometry of underlying constituents, the mechanical loads, and the edge displacement conditions (boundary conditions) are all factors that are known to contribute directly or indirectly to the mechanical response of the alveolus. Therefore, it is convenient to depict two length scales for discussion of the important factors that regulate lung mechanics, with the understanding that there is a strong communication between these two scales.
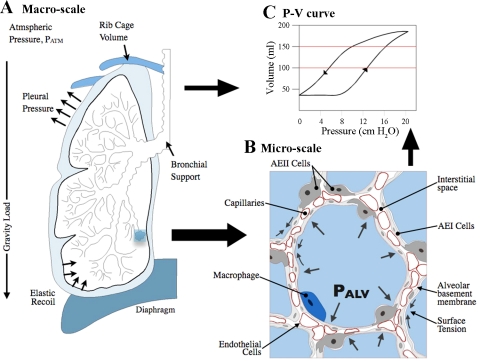
Fig. 1.
A: mechanical determinants of the lung include loads, tissue properties, and boundary conditions at 2 length scales. B: close-up, 2D cross-section of a group of alveoli. Alveoli are separated by the alveolar septa, which are composed of alveolar basement membrane, capillaries, and alveolar epithelial cells. This illustration is representative of an inflated, but not an overdistended alveolus. The outline of the alveoli was adapted by tracing one of the microscopic images of rat lungs found in Ref. 64 corresponding to 5 cmH2O inflation pressure. PALV, alveolar pressure; AEI and AEII, type I and II alveolar epithelial cells, respectively. The schematic is not to scale for illustration purposes. C: pressure-volume (P-V) curve.
The major determinants of lung mechanics during normal tidal breathing are depicted at two length scales in Fig. 1. The loads at the organ level include alveolar pressure (PALV), pleural pressure (PPL), elastic recoil, and gravity. PPL is regulated by changes in the volume of the thoracic cage primarily controlled by its elastic recoil and contraction of the diaphragm. The difference between the pressure levels in the alveolus (PALV) and in the pleural space (PPL), the transpulmonary pressure (PT), determines the volume of the lung, whereas the difference between atmospheric pressure and PALV drives air flow into and out of the lungs. An increase in PT causes the lungs to expand, and air flows into the lungs when alveolar pressure is less than the pressure at the mouth. At the alveolar level, the surface tension of the air-fluid interface, the elastic recoil of the tissue, interdependence, and the PALV are the predominant loads. As described by Mead et al. (53), PALV will be the same in all alveoli, and there will be no pressure differences across the walls except at the pleural surface. Thus the forces that distend air spaces are derived from tissue attachments that transmit stresses due to the PT. One boundary condition of the lung tissue is the volumetric limitation provided by the relatively more rigid tissue of the rib cage. Intercostal muscles (not shown in the illustration) help in the expansion of the rib cage during forced inspiration; thus the boundary conditions of the lung tissue are not static. Moreover, since the lung is not attached to the rib cage or the diaphragm, it slides against these surfaces during breathing with low frictional resistance due to the presence of a thin layer of pleural fluid (18, 90).
At the microscale level, the key structure of the lung parenchyma is the alveolus, which has a three-dimensional structure resembling an octahedron with an approximate diameter on the order of 100 μm at physiological pressure levels (6, 35, 42, 55, 58, 83). A cross-sectional illustration of a group of alveoli is depicted in Fig. 1B in a partially inflated configuration. Because alveoli share structural walls with neighboring alveoli, there is an interdependence in which neighboring units resist the collapse of one another and also allow for uniform expansion (53). The space that separates an alveolus from its adjacent neighbor is referred to as the alveolar septum, which is composed of a layer of epithelial cells on the surfaces in contact with air, endothelial cells that line the capillaries that allow blood flow through the septal tissue, and matrix materials that comprise the basement membrane and interstitial space. The alveolar epithelial cells include AEI and AEII cells, of which the AEI cells cover ∼93% of the alveolar surface area (17), whereas the AEII cells tend to reside near the cornerlike areas of the alveolus. Alveolar epithelial cells form a relatively impermeable barrier that is dependent on the formation and maintenance of tight junctions. Typically, macrophages reside in the alveolar air space, but other immune cells can also be recruited into the interstitial space or into the air space. Although epithelial cells, endothelial cells, and fibroblasts are abundant within the lung parenchyma, their direct contribution to the mechanical strength of the lung parenchyma is generally considered to be small (5, 11); we will consider this topic further below. However, epithelial cells and fibroblasts do contribute to the mechanical properties of the alveolus by synthesizing and secreting ECM proteins that form the basement membrane (16). For example, dysregulated repair of alveolar epithelial cells following injury has been suggested to induce epithelial-mesenchymal transition (EMT), resulting in excessive matrix production and fibrosis (69, 96, 97). It has also been suggested that changes in the mechanical environment might promote EMT or that fibrosis itself alters the mechanical environment (37).
The alveolar basement membrane supports the epithelial cells; is composed of collagen, elastin, proteoglycans, and other ECM proteins (54, 92, 93); and is regulated by matrix metalloproteinases (60, 61). The relatively higher strength collagen is generally considered to be the load-bearing protein, because it resists loads induced by large distension, whereas elastin allows the lung parenchyma to return to its original shape following deformation (80). A recent study by Cavalcante et al. (11) examined lung slices to demonstrate that proteoglycans contribute significantly to the mechanical structure of lung tissue, since they act as connectors between various load-bearing proteins. The overall elastic recoil of the lungs is also greatly dependent on the ratio of these underlying constituents found elsewhere in the lung, such as the airways, blood vessels, and parenchymal tissue (26, 54, 70, 79, 80). Both compliance and elastic recoil can be affected by lung disease that alters the underlying composition of the alveolar constituents. For example, the destruction of alveolar units and loss of elastic tissue in emphysema results in increased compliance and decreased elastic recoil (15, 52), and bleomycin-induced fibrosis increased the local tissue elasticity by sixfold measured with atomic force microscopy (AFM) in indentation mode (47).
In addition to the role of material properties in regulating alveolar mechanics, alveolar deformation is also determined by interactions between levels of prestress, the stiffness of individual wall components, and resistance to changes in alveolar geometry. Because PT balances the elastic recoil of the lungs and keeps the air spaces open at the end of exhalation, tension is maintained in the alveolar walls, and there is resistance to changes in the geometry of the structures due to the interdependence between neighboring units. Stiffness is a term that describes the mechanical response of a material to deforming stress inclusive of the geometric factors. Thus the overall mechanical response to changes in distending pressure is complex and involves multiple factors.
Are epithelial and endothelial cells mechanical determinants of the alveolar strain field?
In examining the mechanics of the alveolus, the composition of the alveolar basement membrane and the interfacial forces at the air-liquid interface are considered to be major determinants of the mechanical response to normal breathing loads (26, 79). However, the potential contribution of epithelial and endothelial cells at the micromechanics scale has not been clearly defined. There are two potential reasons why epithelial cells in particular may be contributors of the overall mechanical response of the lung: 1) the mechanical properties of alveolar epithelial cells have been measured to be of the same order of magnitude as the measured mechanical response of lung tissue slices, and 2) the epithelial cells are loaded parallel to their surface, which implies loading of the cell-to-cell connections in tension that are known to form strong connections between cells. Using a continuum mechanics framework, an indirect estimate of the strength of cell-to-cell junctions could be computed using information regarding cell mechanical properties, morphology of tight junctions, and a measured strain level at which confluent monolayers begin to form intercellular gaps. However, at this time we lack direct quantitative mechanical measurements of the tensile strength of the cell-to-cell connections of epithelial cells.
Despite this limitation in our capability for measuring the strength of connections between cells similar to the in vivo loading of alveolar epithelial cells, there are a number of studies using techniques that measure a variety of mechanical responses of cells (4, 27, 45, 89). In general, studies utilizing beads (micrometers in diameter), translated or rotated with either magnetic or optical forces, result in measurements that yield an elastic modulus on the order of 1 kPa or less (27, 45). In comparison, the elastic moduli of cultured alveolar epithelial cells and A549 cells (an adenocarcinoma-derived human lung epithelial cell line) were measured to be 2.5 ± 1.0 kPa (4) and 0.91 ± 0.47 kPa (71), respectively, using an AFM in indentation mode. Although these two measurement approaches resulted in comparable elastic moduli, each technique measures different aspects of the mechanical response of cells. The microbead tests apply a load to move a bead that is tethered to the cytoskeleton, resulting mainly in a tensile load on the cytoskeletal proteins. If the bead is not fully immersed, the measurement is more of an indication of the cellular response near the plasma membrane. With AFM indentation, the contact between the AFM cantilever tip and the cell can generate an indentation of up to a few micrometers, reaching a level that is a significant proportion of the cell height. Therefore, AFM indentation is capable of measuring the contribution of the underlying cytoplasmic constituents, including the nucleus, which has been shown to have a significantly higher elastic modulus (9, 51).
Although these techniques are informative, none of them are capable of providing information regarding the tensile strength of an epithelial sheet that experiences a stretch loading in a direction parallel to the substrate. In other words, it is important to note that none of the current microscale techniques have been adapted to apply a tensile load in a way that can measure the strength of cell-to-cell contacts. Moreover, most of our measurements of cell mechanical responses are conducted on substrates that are relatively more rigid than lung tissue (such as plastic), and evidence in the literature suggests that the mechanical response of cells is dependent on the stiffness of the substrate (41, 77). In addition, cell stiffness has been shown to respond to mechanical stretch (82), and this may also impact how cells contribute to the overall mechanical properties of the lung. Because of these limitations, we do not have an accurate assessment of the contribution of cells to overall lung mechanics.
Nevertheless, the measurements of alveolar epithelial cell mechanical response indicate that isolated cells have an elastic modulus that is less than but of the same order of magnitude as the lung tissue strips. The elastic modulus of the alveolar wall estimated from the extension of lung tissue strips was ∼5 kPa (11). In this study by Cavalcante et al. (11), the contractile cells of the lung parenchyma were assumed to be inactive at the time of experiments. Interestingly, three recent studies demonstrated that the scaffold material of decellularized rat or mouse lungs had significantly lower compliance than native lungs (59, 67, 68), suggesting that the presence of the cells made the lungs less stiff. This could potentially be due to the lack of surfactant in the decellularized lungs, since lungs with repopulated cells capable of producing surfactant had increased compliance (albeit lower than native lungs). However, the decellularization process may have affected the structure and content of the matrix, and the potential presence of fluid or foam in the air space may have affected the measurements. More experimental studies will be required to examine the possibility of epithelial and endothelial cells contributing to the overall mechanical properties of the lung. A combination of experimental approaches, imaging studies, and computational models may be necessary to determine whether the epithelial or endothelial cells contribute to the overall lung mechanical response.
Surface tension.
The alveolar epithelial cells, basement membrane, and endothelial cells form a barrier that allows the efficient transport of gas molecules and prevents the leakage of fluids into the air space. At the interface between the air space and the epithelial cells, a thin layer of fluid covers the epithelial cells. The surface tension of this air-fluid interface results in significant forces that tend to collapse the alveolus. Although the magnitude of the surface tension varies with the inflation of the alveolus (and thus the pressure), a maximum surface tension value was measured to be 30 mN/m in rabbit lungs (7, 29). The magnitude of the surface tension is reduced by surfactant produced and released by AEII cells, which prevents the collapse of the alveolar walls at low pressures (5, 8, 76). Inadequate surfactant production in premature infants (38) or surfactant depletion in acute lung injury (95) and other diseases can result in increased surface tension and difficulty in inflation of alveoli. Although surface tension plays an important role in the balance of forces involved in individual alveolar inflation, the considerable size of the alveolar surface area dictates that surface tension impacts overall lung mechanics, for which pressure-volume (P-V) curves similar to the one in Fig. 1C serve as an indication or a measurement.
In summary, because of spatially and temporally nonuniform loading and edge conditions, the lung is a complex organ in which changes in mechanical strains have been shown to be critical determinants of biological function. As described in the next section, the alveolar strain field, the microscale representation of the bulk and alveolar lung mechanical environment, is challenging to describe because of the complex structure and limited imaging modalities that can measure alveolar deformation as a function of volume or pressure.
Alveolar Strain Field: How Do Alveoli Expand?
Tissue deformation is often expressed in terms of mechanical strain, which is described as the change in a linear dimension over its initial or reference value. For most bodies undergoing deformations, the linear change in length can be linked to a change in area or volume. The strain levels in soft tissue are usually distributed nonuniformly; thus such a tissue is considered to experience a nonuniform strain field. In the case of lung tissue, because the deformation often involves positive changes in dimension, the term stretch or distension is often used in place of strain. Compression is also used to describe negative changes in dimension, for example, during constriction of airways. There have been several studies focusing on the magnitude and distribution of strain within the alveolus, and these are summarized in the next section.
Morphometric assessments of alveolar inflation.
Early attempts to determine lung distension were based on the assumption of isotropic expansion of the lung upon inflation, and estimates of linear mechanical strain of the alveoli were made on the basis of changes in lung volume (6, 28, 42, 55, 58, 78, 87). Many studies have used morphometric measurements of isolated lung tissue fixed at varying pressure levels or fractions of the total lung capacity (TLC) to determine surface area, volume, surface tension, and pressure relationships. [It is important to recognize that TLC in humans is defined as the volume at maximal inspiratory effort, whereas in studies with whole animals or isolated lungs TLC is often based on specific criteria (pressure) that might vary from study to study.] Perfusion fixation was a common method in these studies, but freeze-drying techniques were also utilized in some studies (42). In these studies, the lungs were excised and the P-V curves were determined to identify the volumes at TLC. Lungs were then fixed during either inflation or deflation portions of the P-V curves. This is important to note because the overall mechanical response of the lung exhibits a strong hysteresis due to the presence of the surface tension (as discussed above). In these studies, animal models also varied between cat, rabbit, rat, and guinea pig.
Examination of lung microstructure using isolated fixed tissue was popular in obtaining morphological information during the earlier stages of lung biomechanics. In 1929, Macklin (49) suggested that the alveoli were merely static terminal structures and that the airways experienced the volume changes. Later, many challenged this view with studies involving alveolar duct and alveolar measurements, questioning whether the alveoli experienced major alterations in shape during inflation or whether their shape remained nearly constant with an increase in surface area (24, 28, 78). Storey and Staub (78) provided evidence toward the latter by showing that the mean alveolar diameter increased with inflation pressure in cats. In 1967, Dunnill provided data in dogs indicating that the relationship between alveolar surface area (SA) and volume (V) was approximated by SA∝V2/3, which would suggest that the alveolar shape remains constant and that there was expansion rather than recruitment of previously collapsed alveoli. In contrast, Forrest (28) found that total alveolar surface area increased linearly as a function of the total lung volume, suggesting recruitment. Macklem (48) later suggested that both relationships fit the data well, probably because of the limited sensitivity of the morphometric techniques. The reader is referred to this discussion by Macklem for a more extensive analysis of this relationship (48). However, it is worth noting that the question of whether the alveolar surface area increases as SA ∝ V or SA ∝ V2/3 still remains unresolved because of challenges in obtaining dynamic in vivo measurements. Particularly important for this review is the fact that direct measurement of alveolar strain was not possible in these earlier studies because a single alveolus could not be imaged at varying pressures.
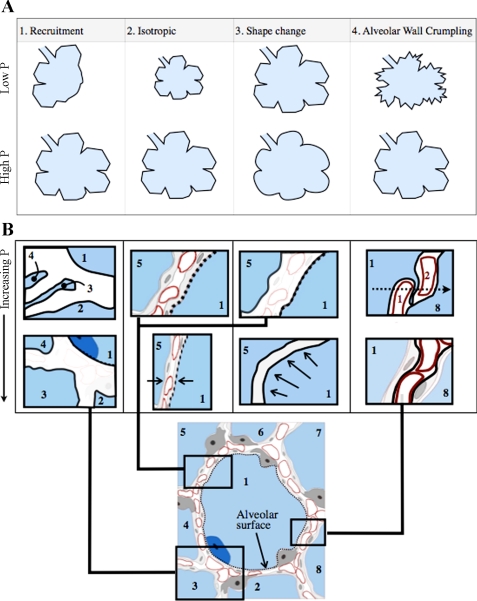
On the basis of these and other early morphological studies using fixed tissue, discussion arose regarding the mechanism of lung volume expansion: were alveoli recruited and derecruited by opening and closing of units, did they expand by an accordion-like change in shape, or did they expand from a prestressed state like a balloon (23) to account for the volumetric increase of the lungs? Klingele and Staub (42) concluded that the alveolar walls became unfolded with inflation on the basis of their experimental observations of fixed tissue. Later, Gil et al. (35) outlined four mechanisms by which the lung could inflate and deflate (illustrated in Fig. 2): 1) recruitment and derecruitment of alveoli SA ∝ V; 2) balloonlike increases and decreases in volume with SA ∝ V2/3; 3) equal alveolar volumetric change accompanied by a shape change, from a truncated octahedron to a sphere; and 4) accordion-like “crumpling” of the alveolar walls.
Fig. 2.
Illustration of how alveolar structure changes with different inflation pressures (P). A: 4 mechanisms by which the lung expands according to Gil et al. (5). Alveoli close or collapse at low volumes in recruitment (mechanism 1). In the case of mechanisms 2 and 3, the alveoli grow in an isotropic manner, but rounding of the alveolar wall is a characteristic of the third mechanism only. In the case of crumpling (mechanism 4) the walls fold in an accordion-like fashion, maintaining the total number of alveoli the same. B: an alveolus with a moderate inflation pressure (∼5 cmH2O) at the bottom and depictions of the most significant effects of changes in pressure in the alveolar walls for each mechanism. Isotropic expansion thins the walls, whereas recruitment results from opening of previously collapsed alveolar walls. The alveolar surface area would be estimated from the dashed line indicated in B. The alveolar wall crumpling illustration shows a septal wall in an alveolus at low inflation pressure (∼0 cmH2O), indicating septal wall folding. In this case the epithelial basement membrane surface area would be estimated by the solid dark lines.
The same authors proposed that these different mechanisms may regulate the alveolar geometry with varying significance as a function of inflation volume (35). They suggested that the alveoli experience volume changes due to folding and unfolding of the alveolar septa (termed pleating) at low pressures as shown in Fig. 2B. Figure 2 is a schematic indicating how alveolar structures may change at different inflation pressures. The size of the cells is somewhat exaggerated to distinguish the changes in basement membrane. Figure 2B shows an alveolus at a moderate level of inflation (∼5 cmH2O). The dashed line indicates the alveolar surface area. Figure 2B also shows a diagram of a septal wall at low inflation pressure (∼0 cmH2O), indicating septal wall folding. However, many investigators questioned whether the images of septal wall folding from the earlier studies were artifacts of the fixation process (57, 58, 64). In 1991, Oldmixon and Hoppin (58) systematically addressed this possibility by examining fixed lungs using light and electron microscopy. They defined septal folding according to the appearance of apposition of epithelial surfaces on electron microscopy, or by capillary bunching within septal tissue that separated two air spaces (as indicated in Fig. 2B), or rounding of air space profiles observed by light microscopy. They suggested that folding occurred only at lower PT values in excised lungs and was unlikely to occur in vivo. Although they could not absolutely rule out folding and unfolding, they concluded that there was a “reasonable possibility” that this proposed mechanism was an artifact of the ex vivo approach. In 1999, Tschumperlin and Margulies (84) examined the question of whether or not there were changes in epithelial basement membrane (and thus deformation of epithelial cells) at different levels of inflation in isolated rat lungs. Because Oldmixon and Hoppin had shown that alveolar folding was highly dependent on the volume history of the lungs prior to fixation, Tschumperlin and Margulies fixed the lungs during deflation following three cycles of inflation at high lung volume (10–25 cmH2O). On the basis of measurements of epithelial basement membrane from electron microscopy, they concluded that the alveolar expansion in isolated rat lungs at low volumes was by septal folding and unfolding but that stretching of septal tissue occurred at higher lung volumes.
Imaging approaches.
Because of the limitations of morphometric measurements from fixed tissue, several investigators have developed imaging approaches for the measurement of alveolar shape change as a function of volume and pressure in situ or ex vivo. In 1983, Smaldone et al. (75) measured the deposition of aerosol without flow on excised dog lungs and suggested that alveolar diameters remained unchanged for most of the deflation from TLC. Later, Carney et al. (10) used intravital microscopy to examine the subpleural alveoli of porcine lungs and concluded that lung volumetric changes occurred by recruitment-derecruitment (RD) of the alveoli since they did not observe alveolar shape change up to 80% TLC upon inflation from a (nonphysiological) degassed state. Other studies by this group supported RD of the alveoli and suggested that the folding and unfolding of alveolar septa was the mechanism in which unstable alveoli change volume (62) and that the alveolar recruitment was dependent on the magnitude of the recruiting pressure and its duration (2).
Volumetric change of the lungs by RD has been suggested by studies involving ARDS patients, which may provide clues related to how alveoli expand in one diseased condition (13, 31–33, 63). Using computed tomography (CT), this group showed the heterogeneous nature of injured lungs suggesting the existence of fluid and air filled regions of the lung that are unrecruitable and recruitable. A discussion provided by Hubmayr (40) raises concerns regarding three underlying assumptions of the CT studies of injured lungs: 1) the strong dependence of lung volumetric change on the weight of the tissue, 2) the nature of the relationship between the alveolar volume change and CT scan grayscale, and 3) the dependence of the change in the P-V curves in ARDS patients predominantly on the alveolar recruitment. A partial dependence of lung mechanics on the gravitational forces within the lung and the existence of many mechanical contributors of the P-V curves raise concerns about the use of CT scan data to deduce lung stress and strain levels. Although CT scan analyses are useful in the clinical setting for assessing regional heterogeneity and recruitment of air spaces, it is important to understand their limitations in measuring stress and strain levels in the lung. It is unlikely that alveolar level information can be obtained from gross CT scan grayscale measurements of injured lungs.
Recent developments in real-time fluorescence microscopy and optical coherence tomography (OCT) of the lung have provided an opportunity to monitor the volumetric evolution of a given alveolus as a function of pressure (44, 56). Perlman and Bhattacharya (64) combined real-time laser scanning confocal fluorescence microscopy with optical sectioning to measure length changes of the alveolar perimeter in subpleural alveoli following changes in alveolar pressure in isolated lungs. They concluded that alveolar strain was heterogeneous both within a particular alveolus and throughout the lung. They observed that the AEI cells experienced greater strains than AEII cells. In images with alveolar pressures ranging from 5 to 20 cmH2O, they reported no evidence of recruitment and derecruitment. In another study, Perlman et al. (65) utilized the same technique to image adjacent air filled and fluid-filled alveoli. The liquid-filled alveoli tended to shrink, causing the adjacent air-filled alveoli to expand. Their observation of increased distension with pressure and the lack of collapse and reopening agreed with the findings of Mertens et al. (56), who used OCT to visualize alveolar dynamics in normal and acid-injured mouse lungs without immobilizing the pleural surface. They concluded that alveolar overdistension could occur in injured lungs owing to redistribution of volume from small alveolar clusters with reduced compliance to larger alveoli. These observations are important because they may indicate one source of overdistension injury that is commonly accompanied by lung edema.
Although how alveoli change volume still remains a controversial topic, the cumulative weight of evidence suggests that alveoli expand in vivo in a manner best represented by a combination of mechanisms involving isotropic expansion and shape change as summarized above. Because physiological levels of pressure do not normally allow deflation below the residual volume, accordion-like crumpling seems unlikely during tidal breathing. However, it is likely that alveoli in injured lungs will undergo heterogeneous levels of expansion in different regions of the lungs. CT imaging suggests that regions of the lung also become recruited and derecruited due to regional variations in compliance and changes in fluid accumulation and distribution, but direct visualization remains elusive. It is also plausible that alveolar wall crumpling could occur in an injured lung due to nonuniform compliance and fluid distribution, but this has not been directly visualized either.
Interpretation of subpleural alveolar imaging.
Although recent studies involving images of alveoli in situ or ex vivo have reported new techniques capable of tracking subpleural alveoli during volumetric evolution, there are limitations to these approaches. The use of suction pressure to fix the microscope objective to the surface of the lung may affect the deformation of the tissue and result in a significant deviation from in vivo conditions. However, even if the in vivo conditions were fully achieved, from the mechanics perspective, the alveoli near the pleura that are constrained by a glass coverslip with or without suction pressure are likely to have a mechanical environment that is significantly different from the lung interior.
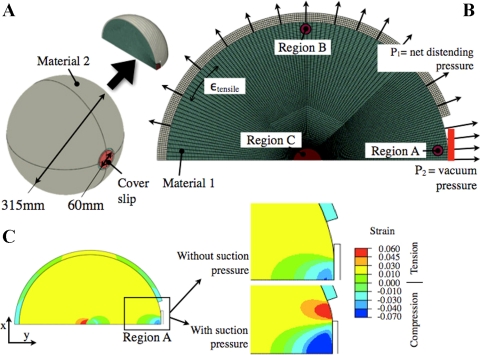
To expand this point, a simple finite element (FE) model of a spherical structure with an encapsulation was generated (see Fig. 3) using ABAQUS (Simulia, Providence, RI). In this model, the internal sphere represents the lung, whereas the outer structure represents the collective response of the rib cage and tissue level boundary conditions (see Fig. 3A). A two-dimensional model (Fig. 3B) of the spherical assembly was generated to accurately implement the axisymmetric boundary conditions and reduce the computational cost. As an initial approximation, the sphere (material 1) and the cover (material 2) were assigned elastic moduli of E1 = 5 kPa (11) and E2 = 10 kPa, respectively. A circular opening with 60-mm diameter was created such that material 1 is exposed on the right-hand side of the sphere. A rigid structure, resembling a glass coverslip was modeled to fit this opening. All interfaces were allowed to slide against each other in a frictionless manner.
Fig. 3.
A simple finite element (FE) model showing the effect of additional suction pressure near a glass slide placed on the surface of a spherical lung. A: full geometry of the composite spherical assembly. B: FE model of the simplified geometry with pressure loads and the coverslip. All structures are allowed to slide freely against each other. C: contour plot of the strains in the y-direction showing a close-up of the compressive strains near the subpleural region closest to the coverslip (with and without suction pressure). A total of 12,646 2D elements were utilized to discretize this assembly.
Two analyses were conducted using this model with and without the suction pressure applied near the glass slide. During the first analysis, a 250-Pa (2.55 cmH2O) uniform pressure load (P1) was applied on the outer surface of the sphere as a net distending pressure. The vacuum pressure load (P2) was selectively raised to 500 Pa (5.1 cmH2O) near the outer surface of the inner sphere that was in contact with the rigid glass slide. The magnitude of the vacuum pressure load is right at the lower end of suction pressure that was applied in Carney et al. (10) near the glass slide. Material 1 falling within region C was prohibited from moving in the y-direction to stabilize the FE model in a way that is similar to the lung; i.e., the connection between the large airways and parenchyma stabilizes the lung.
A contour plot of the averaged strain in a plane that is normal to the glass coverslip, is shown in Fig. 3C. Although the majority of the strain levels in the sphere were positive or tensile, indicating stretch, the strain levels near the coverslip (region A) were negative, indicating compressive strain in the alveoli beneath the coverslip. In this model, the compressive-to-tensile transition of the strains occurred at 34.3 mm away from the glass slide, which is 11.4% of the diameter of the inner sphere. Moreover, the tensile strain parallel to the perimeter of the sphere, as indicated in Fig. 3B, is significantly lower for region A relative to region B. In summary, this simplified FE analysis indicates an increased compression and a reduced tension (opening) near the subpleural alveoli adjacent to the glass coverslip. This indicates that both the glass slide and a suction pressure can affect the localized mechanical response of the lung and result in a unique response in the region of interest. Certainly, the presence of the suction pressure amplifies the magnitude of the compressive stress.
This FE model of the lung at the macroscale is simplistic because it does not include all of the mechanical determinants of the lung as shown in Fig. 1. However, it does indicate some of the potential artifacts that can result from this approach for imaging alveoli. The presence of compressive strain near the coverslip may encourage movement of alveoli toward the surface. Moreover, the reduced tensile strain parallel to the surface of the coverslip may indicate a lack of change in surface area with a given pressure relative to an unconstrained area. These are both consistent with findings indicating lack of alveolar volume increase of subpleural alveoli and the increase in the number of alveoli beneath the coverslip (2, 10). This FE model shows how subpleural alveoli are constrained by the presence of a glass coverslip and how these alveoli experience a mechanical environment that is significantly different from other subpleural regions and internal or parenchymal environment. This underlines the importance of further consideration when extending subpleural findings to the remainder of the lung parenchyma when the actual measurements are conducted near a region in which the mechanical environment is greatly modified.
Estimates of change in tensile strain in the alveolus.
In addition to addressing fundamental questions regarding the mechanisms of lung volumetric increase, previous studies have provided data that can be utilized to calculate the change in tensile strains experienced in the lung during tidal or TLC inflation/deflation. A summary of the change in strain levels estimated from the literature is presented in Table 1. These articles were selected on the basis of providing specific numerical information about surface area or diameter of the alveoli at either a specific pressure level or at a fraction of total lung capacity (TLC). The majority of the studies reported changes in surface area (ΔSA), and we either listed the reported surface area strain (or distension) or calculated the strain by ΔSA = (SAf − SAo)/SAo where ΔSA is the area strain for a particular change in lung volume from an initial (o) and final (f) pressure. To compare the area strain with the linear strain
where
λ is the stretch ratio, and Lf and Li represent the initial and final lengths (or alveolar diameters).
Table 1.
Alveolar strain at physiological pressure levels calculated from previously published morphometric data
| Reference No. | Species | Fixation Method/Imaging | Surface Area Distension ΔSA, % | Linear Distension ε, % | Initial/Final, %TLC |
|---|---|---|---|---|---|
| 6 | Rabbit | Vascular perfusion, EM | 20.6(I) −15.2(D) | 9.8(I)–7.9(D) | 80⇔100 |
| 79.2(I)–19.8(D) | 33.9(I)–10.4(D) | 40⇔80 | |||
| 28 | Guinea pig | Rapid freezing and vascular perfusion, LM | 64.4(I) | 28.2(I) | 40⇒75.7 |
| 11.3(I) | 5.5(I) | 28.5⇒40 | |||
| 35 | Rabbit | Vascular perfusion, EM | 20.2(I) | 9.6(I) | 40⇒80 |
| 48.1(I) | 21.7(I) | 80⇒100 | |||
| 42 | Cat | Rapid freezing and freeze drying, LM | n/a | 4.7(D)* | 75⇐100 |
| n/a | 11.2(I)–9.9(D)* | 50⇔75 | |||
| n/a | 4.2(D)* | 37⇐50 | |||
| 55 | Rat | Vascular perfusion, EM | 5.1(I)–8.0(D) | 2.5(I)–4.1(D) | 95.8⇔100 |
| 30.8(I) | 14.4(I) | 73.2⇒95.8 | |||
| 142.6(I)–51.0(D) | 55.8(I)–30.0(D) | 24.8⇔95.8 | |||
| 83–85 | Rat | Vascular perfusion, EM | 19.5(D) | 10.3(D) | 82⇐100 |
| 7.0(D) | 3.5(D) | 42⇐82 | |||
| 4.4(D) | 2.2(D) | 24⇐40 | |||
| 13.8(D) 12(D)† | 7.2(D) | 24⇐60 | |||
| 11.1(D) 25(D)† | 5.7(D) | 24⇐82 | |||
| 28.5(D) 37(D)† | 15.4(D) | 24⇐100 | |||
| In situ | |||||
| 66 | Rat | Laser scanning fluorescence microscopy | n/a | 14 | 5⇒20 cmH2O (82%TLC) |
Our values are computed with (SAf − SAo)/SAo. %TLCf>%TLCo for inflation and %TLCf<%TLCo for deflation. SA, alveolar surface area; TLC, total lung capacity; subscript o, initial value; subscript f, final value; LM, light microscopy; EM, electron microscopy; (I), inflation; (D), deflation.
Linear strain (ε) is computed from change in diameter.
ΔSA values of 12, 25, and 37% were obtained from the synthesis of a series of studies in which an empirical equation was derived from experimental data. The reported data is shown in the first column under ΔSA, and estimates from the empirical equation are shown in the second column. We report these values (12, 25, and 37%) since they have been used extensively in other studies.
We selected only limited measurements from each study for comparison. For example, 40% TLC is the initial and 80% TLC is the final configuration for many of the measurements recorded during inflation because of the physiological relevance of this particular range. It is important to recognize that strain values can vary greatly depending on the selection of the initial starting point. Ideally, we would estimate changes in strain based on an initial unstressed state, but that is difficult to define in a lung in vivo. Therefore, we referred to strain levels as % distension to account for the possibility of different reference lengths within the studies. Furthermore, as described above, the deformation of the lung differs during inflation and deflation, and inflation causes positive changes in dimensions (stretch) whereas deflation causes negative changes (compression or relaxation). In this table, those measurements corresponding to lungs that were fixed during the deflationary stage have a final condition (f) that was at a lower %TLC value than the initial one, i.e., lower surface area. Instead of denoting the change in surface area for these measurements with only a negative sign, we marked these deflationary distension and ΔSA values with (D), and we indicated inflationary ones with (I). We also reported the initial and final configurations corresponding to the computed distension values with arrows indicating the direction of the pressure change. It is also important to recognize that the type of distension that may be of interest will depend on the perspective of the reader: if one is concerned with cellular mechanotransduction, then levels of linear distension are important, whereas those concerned with gas exchange and organ level physiology may be more interested in changes in surface area.
Among the studies presented in the table, Tschumperlin and Margulies (84) and Bachofen et al. (6) measured the epithelial basement membrane surface area (EBMSA), which measures area based on the basement membrane including the alveolar folds, whereas other studies reported alveolar surface area. The difference between the EBMSA and alveolar surface area is indicated in Fig. 2, where the EBMSA was calculated by using the perimeter of the alveolus from two-dimensional images including basement membrane folds, as seen in the close-up image of the alveolar wall in this figure. The alveolar basement membrane folds were excluded when the alveolar surface area was calculated. Therefore, the EBMSA are inherently larger than the alveolar surface area and may be less sensitive to changes in pressure, because the unfolding of these features will precede an increase in surface area. As discussed above, it is unclear whether these folds exist in an in vivo lung in the same way as an excised lung (58). Therefore, although Bachofen et al. measured both EBMSA and total surface area, we calculated the change in distension utilizing only the total surface area.
A number of the studies shown in Table 1 reported morphometric information comparing deformation from ∼40 to 80% TLC during inflation. Estimates of linear distension values range from 9.6 to 33.9% during inflation from 40 to 80% TLC. The change in linear distension was substantially lower during deflation in this range with estimates ranging from 3.5 to 10.4%. The lower estimate may reflect differences in measurements of EBMSA vs. total surface area since Bachofen et al. (6) showed differences in strain dependent on the use of the EBMSA or alveolar surface area in the computation of strain.
To examine changes in alveolar dimensions directly, Perlman and Bhattacharya (64) utilized optical sectioning methods (OSM) with confocal fluorescence microscopy to image isolated lungs, and focused on a single alveolus to measure its volumetric evolution in situ. Their results reflect the strain of a single segment in the alveolar perimeter, which was shown to vary between 5% and 25% with an average of 14% at 82% TLC or Δ15 cmH2O. This is the first study to directly measure the alveolar wall deformation in an intact lung. It provides direct evidence that the alveolar walls experience a nonuniform strain throughout.
Establishing a consensus for the magnitude of strain in the alveolus at different levels of inflation based on the morphometric measurements is challenging. To determine the actual (or physiological) distension level, stress (normalized force) and strain relationships in alveolar walls must be described. It is not currently possible to measure the alveolar wall stress in situ. As discussed above, it is also difficult to establish a reference or equilibrium condition for in vivo lungs by which to describe subsequent changes. Some studies have determined the stress-free volume in ex vivo lungs (86). Although informative, such ex vivo studies are limited in capturing this reference condition in vivo. Once the lungs are removed from the thoracic cavity and fixed, a number of critical mechanical determinants, such as the rib cage volumetric limitation and the PPL, are eliminated from the overall mechanical system. Therefore, the excised lung, without any compensating increase in PT, is more inclined to exhibit a configuration in which the alveolar walls may fold or collapse, potentially indicating a stress- and strain-free condition. As discussed in detail in Oldmixon and Hoppin (58), the inflation-deflation cycling of the excised lungs may result in atelectasis when pressures cycle below 3 cmH2O. It should also be pointed out that many of the studies summarized in the table utilized different standards for volume cycling and fixation, and this makes it more difficult to compare results directly (39). In addition, TLC is often defined by using an arbitrary pressure that may vary in different species or models. The majority of the studies shown in Table 1 inflated the lungs to 25 to 30 cmH2O and referenced percent inflation relative to this.
Nevertheless, the morphometric measurements have provided invaluable estimates of the alveolar strain field. However, given the many caveats discussed above, we must be careful in our interpretation of these estimates. On the basis of the various studies to date, we estimate that normal tidal breathing results in linear distension in the range of 0 to 5%, whereas inflation from functional residual capacity to TLC likely causes more variation in strain ranging from ∼15 to ∼40% (and potentially higher). We expect that there will be much greater variation in injured or diseased lungs, but little information is available. A final note of caution is that none of the morphometric or imaging studies have provided direct estimates of alveolar strain in human lungs.
Computational models.
Computational models have been used extensively in an attempt to describe the mechanics of lung parenchyma and alveoli. Initial models were developed in an attempt to interpret pressure-volume loops measured in vivo (19, 20, 43, 98). In a number of these studies, a mathematical analog of the alveolus was generated with the use of a truncated octahedron (19, 30). Utilizing this three-dimensional analog as a repeat unit, Denny and Schroter (20) generated a model of the lung alveolar duct and concluded that the alveolar duct must be included in the mechanical analysis of the lung parenchyma to accurately describe the overall mechanical behavior of the lung. Denny and Schroter (21) later used their repeat unit truncated octahedron model to conclude that the large deformation and anisotropic behavior of the lung parenchyma were not negligible when the aim was to capture the nonuniformity of the distortions in the lung.
A number of targeted computational models have been generated to predict the contribution of the alveolar interaction to lung mechanics (73) and the mechanics of lungs with emphysema (34, 79). Gefen et al. (34) utilized scanning electron microscopic images in the generation of the “alveolar sac” geometry and concluded that emphysemic lungs experienced higher strains than normal lungs at a given pressure. However, it should be recognized that their results were based on a two-dimensional FE model without the surface tension loading condition. Although mechanical models such as these are useful for examining the complexities of lung mechanics, they have not yet provided a detailed description of the alveolar strain field.
One aspect of recent multiscale, multidisciplinary computational models is the incorporation of patient-specific data into the models, which is coupled with one type of global imaging modality. Werner et al. (94) concluded that in patients with lung tumors the overall lung deformation can be estimated using FE models of four-dimensional patient CT scans. Their study was aimed at improving the image-guided radiation therapy outcomes by controlling the dose distribution. In another study, Sundaresan et al. (81) utilized a mechanical model of the lung to capture the “recruitment status of lung units” to determine the optimal positive end-expiratory pressure. This type of progress in patient-specific modeling in the lungs is in line with the overall progress made in this field, which is still in its early stages and necessitates substantial clinical validation and usability tests (56a).
In general, computational mechanics approaches to determine function in a healthy or diseased lung have proven to be useful in explaining or measuring observations that are not captured by imaging modalities. However, for these models to fully explain complex physiological mechanical events, appropriate mechanical properties, boundary conditions, and mechanical loads must be identified. Moreover, validation of such computational models, which is an essential component of any computational mechanics approach, remains to be a challenge in the analysis of soft tissue mechanics.
Concluding Remarks
The extent of mechanical deformation in the alveolus affects the functionality of the alveolus in a number of critical ways, including by controlling surfactant release, permeability, inflammation, and cell injury and repair. Lung injury and disease can result in substantial alterations of the alveolar mechanical environment, which is then transmitted to the cells through changes in the strain field. Despite substantial progress, there are still many unanswered questions about this mechanical environment. This limitation is based mostly on the complexity of the lung tissue, which is composed of a relatively soft ECM and cells that are continuously undergoing large deformations. The complexity of experimental and theoretical analysis of the soft tissue mechanics coupled with the lack of imaging techniques to fully describe the lung shape at each inflation pressure contributes greatly to our lack of understanding. In this review, we attempted to unify some of the key findings over many years of mechanics studies in the literature and to summarize some of the key measurements and studies. Sophisticated experimental, computational, and theoretical approaches combining biology, chemistry, mechanics, and imaging will be necessary to fully describe the alveolar mechanical environment and its effects on alveolar cells.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-094366.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Kaushik Parthasarathi and Dr. Scott Sinclair for helpful discussions regarding this manuscript.
REFERENCES
- 1. Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Albert S, DiRocco J, Allen G, Bates J, Lafollette R, Kubiak B, Fischer J, Maroney S, Nieman G. The role of time and pressure on alveolar recruitment. J Appl Physiol 106: 757–765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arold S, Bartolák-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol 296: L574–L581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azeloglu E, Bhattacharya J, Costa K. Atomic force microscope elastography reveals phenotypic differences in alveolar cell stiffness. J Appl Physiol 105: 652–661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bachofen H, Schürch S. Alveolar surface forces and lung architecture. Comp Biochem Physiol A Mol Integr Physiol 129: 183–193, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Bachofen H, Schürch S, Urbinelli M, Weibel E. Relations among alveolar surface tension, surface area, volume, and recoil pressure. J Appl Physiol 62: 1878–1887, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Bachofen H, Schurch S, Urbinelli M, Weibel ER. Relations among alveolar surface tension, surface area, volume, and recoil pressure. J Appl Physiol 62: 1878–1887, 1987 [DOI] [PubMed] [Google Scholar]
- 8. Butler J, Brown R, Stamenović D, Morris J, Topulos G. Effect of surface tension on alveolar surface area. J Appl Physiol 93: 1015–1022, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Caille N, Thoumine O, Tardy Y, Meister J. Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech 35: 177–187, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Carney D, Bredenberg C, Schiller H, Picone A, McCann U, Gatto L, Bailey G, Fillinger M, Nieman G. The mechanism of lung volume change during mechanical ventilation. Am J Respir Crit Care Med 160: 1697–1702, 1999 [PubMed] [Google Scholar]
- 11. Cavalcante F, Ito S, Brewer K, Sakai H, Alencar A, Almeida M, Andrade JJ, Majumdar A, Ingenito E, Suki B. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol 98: 672–679, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Cavanaugh KJ, Oswari J, Margulies S. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol 25: 584–591, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, Tallarini F, Cozzi P, Cressoni M, Colombo A, Marini J, Gattinoni L. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 178: 346–355, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Cohen T, Cavanaugh K, Margulies S. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur Respir J 32: 854–861, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Colebatch H, Finucane K, Smith M. Pulmonary conductance and elastic recoil relationships in asthma and emphysema. J Appl Physiol 34: 143–153, 1973 [DOI] [PubMed] [Google Scholar]
- 16. Corvol H, Flamein F, Epaud R, Clement A, Guillot L. Lung alveolar epithelium and interstitial lung disease. Int J Biochem Cell Biol 41: 1643–1651, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 126: 332–337, 1982 [DOI] [PubMed] [Google Scholar]
- 18. D'Angelo E, Loring S, Gioia M, Pecchiari M, Moscheni C. Friction and lubrication of pleural tissues. Respir Physiol Neurobiol 142: 55–68, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Dale P, Matthews F, Schroter R. Finite element analysis of lung alveolus. J Biomech 13: 865–873, 1980 [DOI] [PubMed] [Google Scholar]
- 20. Denny E, Schroter R. The mechanical behavior of a mammalian lung alveolar duct model. J Biomech Eng 117: 254–261, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Denny E, Schroter R. A model of non-uniform lung parenchyma distortion. J Biomech 39: 652–663, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Desai L, Chapman K, Waters C. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell Mol Physiol 295: L958–L965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dunnill M. Effect of lung inflation on alveolar surface area in the dog. Nature 214: 1013–1014, 1967 [DOI] [PubMed] [Google Scholar]
- 24. Dunnill MS. Effect of lung inflation on alveolar surface area in the dog. Nature 214: 1013–1014, 1967 [DOI] [PubMed] [Google Scholar]
- 25. Edwards Y. Stretch stimulation: its effects on alveolar type II cell function in the lung. Comp Biochem Physiol A Mol Integr Physiol 129: 245–260, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Faffe D, Zin W. Lung parenchymal mechanics in health and disease. Physiol Rev 89: 759–775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Féréol S, Fodil R, Pelle G, Louis B, Isabey D. Cell mechanics of alveolar epithelial cells (AECs) and macrophages (AMs). Respir Physiol Neurobiol 163: 3–16, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Forrest J. The effect of changes in lung volume on the size and shape of alveoli. J Physiol 210: 533–547, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fredberg J, Kamm R. Stress transmission in the lung: pathways from organ to molecule. Annu Rev Physiol 68: 507–541, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Fung Y. A model of the lung structure and its validation. J Appl Physiol 64: 2132–2141, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Gattinoni L, Chiumello D, Cressoni M, Valenza F. Pulmonary computed tomography and adult respiratory distress syndrome. Swiss Med Wkly 135: 169–174, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Gattinoni L, D'Andrea L, Pelosi P, Vitale G, Pesenti A, Fumagalli R. Regional effects and mechanism of positive end-expiratory pressure in early adult respiratory distress syndrome. JAMA 269: 2122–2127, 1993 [PubMed] [Google Scholar]
- 33. Gattinoni L, Pesenti A, Baglioni S, Vitale G, Rivolta M, Pelosi P. Inflammatory pulmonary edema and positive end-expiratory pressure: correlations between imaging and physiologic studies. J Thorac Imaging 3: 59–64, 1988 [DOI] [PubMed] [Google Scholar]
- 34. Gefen A, Halpern P, Shiner R, Schroter R, Elad D. Analysis of mechanical stresses within the alveolar septa leading to pulmonary edema. Technol Health Care 9: 257–267, 2001 [PubMed] [Google Scholar]
- 35. Gil J, Bachofen H, Gehr P, Weibel E. Alveolar volume-surface area relation in air- and saline-filled lungs fixed by vascular perfusion. J Appl Physiol 47: 990–1001, 1979 [DOI] [PubMed] [Google Scholar]
- 36. Hammerschmidt S, Kuhn H, Gessner C, Seyfarth H, Wirtz H. Stretch-induced alveolar type II cell apoptosis: role of endogenous bradykinin and PI3K-Akt signaling. Am J Respir Cell Mol Biol 37: 699–705, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Heise R, Stober V, Hollingsworth JW, Garantziotis S. Mechanical stretch induces epithelial to mesenchymal transition of alveolar type II epithelium through hyaluronan expression and innate immune activation. Am J Respir Crit Care Med 181: 2010 [Google Scholar]
- 38. Hermansen C, Lorah K. Respiratory distress in the newborn. Am Fam Physician 76: 987–994, 2007 [PubMed] [Google Scholar]
- 39. Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181: 394–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hubmayr R. Perspective on lung injury and recruitment: a skeptical look at the opening and collapse story. Am J Respir Crit Care Med 165: 1647–1653, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Janmey PA, Winer JP, Murray ME, Wen Q. The hard life of soft cells. Cell Motil Cytoskeleton 66: 597–605, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klingele T, Staub N. Alveolar shape changes with volume in isolated, air-filled lobes of cat lung. J Appl Physiol 28: 411–414, 1970 [DOI] [PubMed] [Google Scholar]
- 43. Kowe R, Schroter R, Matthews F, Hitchings D. Analysis of elastic and surface tension effects in the lung alveolus using finite element methods. J Biomech 19: 541–549, 1986 [DOI] [PubMed] [Google Scholar]
- 44. Kuebler W, Parthasarathi K, Lindert J, Bhattacharya J. Real-time lung microscopy. J Appl Physiol 102: 1255–1264, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Laurent V, Hénon S, Planus E, Fodil R, Balland M, Isabey D, Gallet F. Assessment of mechanical properties of adherent living cells by bead micromanipulation: comparison of magnetic twisting cytometry vs optical tweezers. J Biomech Eng 124: 408–421, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190: 693–706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Macklem PT. Respiratory mechanics. Annu Rev Physiol 40: 157–184, 1978 [DOI] [PubMed] [Google Scholar]
- 49. Macklin C. The musculature of the bronchi and lungs: a retrospect. Can Med Assoc J 20: 404, 1929 [PMC free article] [PubMed] [Google Scholar]
- 50. Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med 357: 1113–1120, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maniotis A, Chen C, Ingber D. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA 94: 849–854, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mead J, Lindgren I, Gaensler E. The mechanical properties of the lungs in emphysema. J Clin Invest 34: 1005–1016, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 28: 596–608, 1970 [DOI] [PubMed] [Google Scholar]
- 54. Mercer R, Crapo J. Spatial distribution of collagen and elastin fibers in the lungs. J Appl Physiol 69: 756–765, 1990 [DOI] [PubMed] [Google Scholar]
- 55. Mercer R, Laco J, Crapo J. Three-dimensional reconstruction of alveoli in the rat lung for pressure-volume relationships. J Appl Physiol 62: 1480–1487, 1987 [DOI] [PubMed] [Google Scholar]
- 56. Mertens M, Tabuchi A, Meissner S, Krueger A, Schirrmann K, Kertzscher U, Pries AR, Slutsky AS, Koch E, Kuebler WM. Alveolar dynamics in acute lung injury: heterogeneous distension rather than cyclic opening and collapse. Crit Care Med 37: 2604–2611, 2009 [DOI] [PubMed] [Google Scholar]
- 56a. Neal ML, Kerckhoffs R. Current progress in patient-specific modeling. Brief Bioinform 2: 111–126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oeckler R, Hubmayr R. Alveolar microstrain and the dark side of the lung. Crit Care 11: 177, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oldmixon E, Hoppin FJ. Alveolar septal folding and lung inflation history. J Appl Physiol 71: 2369–2379, 1991 [DOI] [PubMed] [Google Scholar]
- 59. Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16: 927–933, 2010 [DOI] [PubMed] [Google Scholar]
- 60. Parks W. Matrix metalloproteinases in lung repair. Eur Respir J Suppl 44: 36s–38s, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Parks W, Shapiro S. Matrix metalloproteinases in lung biology. Respir Res 2: 10–19, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pavone L, Albert S, DiRocco J, Gatto L, Nieman G. Alveolar instability caused by mechanical ventilation initially damages the nondependent normal lung. Crit Care 11: R104, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pelosi P, Crotti S, Brazzi L, Gattinoni L. Computed tomography in adult respiratory distress syndrome: what has it taught us? Eur Respir J 9: 1055–1062, 1996 [DOI] [PubMed] [Google Scholar]
- 64. Perlman C, Bhattacharya J. Alveolar expansion imaged by optical sectioning microscopy. J Appl Physiol 103: 1037–1044, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Perlman C, Lederer D, Bhattacharya J. The micromechanics of alveolar edema. Am J Respir Cell Mol Biol 44: 34–39, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Perlman CE, Bhattacharya J. Alveolar expansion imaged by optical sectioning microscopy. J Appl Physiol 103: 1037–1044, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Petersen T, Calle E, Zhao L, Lee E, Gui L, Raredon M, Gavrilov K, Yi T, Zhuang Z, Breuer C, Herzog E, Niklason L. Tissue-engineered lungs for in vivo implantation. Science 329: 538–541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A 16: 2581–2591, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Radisky D, Kenny P, Bissell M. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem 101: 830–839, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Raghu G, Striker L, Hudson L, Striker G. Extracellular matrix in normal and fibrotic human lungs. Am Rev Respir Dis 131: 281–289, 1985 [DOI] [PubMed] [Google Scholar]
- 71. Rico F, Roca-Cusachs P, Gavara N, Farré R, Rotger M, Navajas D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys Rev E Stat Nonlin Soft Matter Phys 72: 021914, 2005 [DOI] [PubMed] [Google Scholar]
- 72. Sanchez-Esteban J, Cicchiello L, Wang Y, Tsai S, Williams L, Torday J, Rubin L. Mechanical stretch promotes alveolar epithelial type II cell differentiation. J Appl Physiol 91: 589–595, 2001 [DOI] [PubMed] [Google Scholar]
- 73. Schirrmann K, Mertens M, Kertzscher U, Kuebler W, Affeld K. Theoretical modeling of the interaction between alveoli during inflation and deflation in normal and diseased lungs. J Biomech 43: 1202–1207, 2010 [DOI] [PubMed] [Google Scholar]
- 74. Slutsky A, Ranieri V. Mechanical ventilation: lessons from the ARDSNet trial. Respir Res 1: 73–77, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Smaldone G, Mitzner W, Itoh H. Role of alveolar recruitment in lung inflation: influence on pressure-volume hysteresis. J Appl Physiol 55: 1321–1332, 1983 [DOI] [PubMed] [Google Scholar]
- 76. Smith J, Stamenovic D. Surface forces in lungs. I. Alveolar surface tension-lung volume relationships. J Appl Physiol 60: 1341–1350, 1986 [DOI] [PubMed] [Google Scholar]
- 77. Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J 93: 4453–4461, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Storey W, Staub N. Ventilation of terminal air units. J Appl Physiol 17: 391–397, 1962 [DOI] [PubMed] [Google Scholar]
- 79. Suki B, Bates J. Extracellular matrix mechanics in lung parenchymal diseases. Respir Physiol Neurobiol 163: 33–43, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Suki B, Ito S, Stamenovic D, Lutchen K, Ingenito E. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol 98: 1892–1899, 2005 [DOI] [PubMed] [Google Scholar]
- 81. Sundaresan A, Yuta T, Hann C, Chase J, Shaw G. A minimal model of lung mechanics and model-based markers for optimizing ventilator treatment in ARDS patients. Comput Methods Programs Biomed 95: 166–180, 2009 [DOI] [PubMed] [Google Scholar]
- 82. Trepat X, Deng L, An S, Navajas D, Tschumperlin D, Gerthoffer W, Butler J, Fredberg J. Universal physical responses to stretch in the living cell. Nature 447: 592–595, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tschumperlin D, Margulies S. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol 86: 2026–2033, 1999 [DOI] [PubMed] [Google Scholar]
- 84. Tschumperlin D, Margulies S. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 275: L1173–L1183, 1998 [DOI] [PubMed] [Google Scholar]
- 85. Tschumperlin D, Oswari J, Margulies A. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 162: 357–362, 2000 [DOI] [PubMed] [Google Scholar]
- 86. Vawter D. Stress-free equilibrium volume of the lung. J Appl Physiol 43: 3–7, 1977 [DOI] [PubMed] [Google Scholar]
- 87. Vawter D, Matthews F, West J. Effect of shape and size of lung and chest wall on stresses in the lung. J Appl Physiol 39: 9–17, 1975 [DOI] [PubMed] [Google Scholar]
- 88. Villar J, Kacmarek R, Pérez-Méndez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 34: 1311–1318, 2006 [DOI] [PubMed] [Google Scholar]
- 89. Wagh A, Roan E, Chapman K, Desai L, Rendon D, Eckstein E, Waters C. Localized elasticity measured in epithelial cells migrating at a wound edge using atomic force microscopy. Am J Physiol Lung Cell Mol Physiol 295: L54–L60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang N. Anatomy of the pleura. Clin Chest Med 19: 229–240, 1998 [DOI] [PubMed] [Google Scholar]
- 91. Ware L, Matthay M. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 92. Weber M. Basement membrane proteins. Kidney Int 41: 620–628, 1992 [DOI] [PubMed] [Google Scholar]
- 93. Welling L, Zupka M, Welling D. Mechanical properties of basement membrane. Physiology 10: 30–35, 1995 [Google Scholar]
- 94. Werner R, Ehrhardt J, Schmidt R, Handels H. Patient-specific finite element modeling of respiratory lung motion using 4D CT image data. Med Phys 36: 1500–1511, 2009 [DOI] [PubMed] [Google Scholar]
- 95. Westaby S. Mechanisms of membrane damage and surfactant depletion in acute lung injury. Intensive Care Med 12: 2–5, 1986 [DOI] [PubMed] [Google Scholar]
- 96. Willis B, Borok Z. TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 293: L525–L534, 2007 [DOI] [PubMed] [Google Scholar]
- 97. Willis B, Liebler J, Luby-Phelps K, Nicholson A, Crandall E, du Bois R, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 166: 1321–1332, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wilson T. Nonuniform lung deformations. J Appl Physiol 54: 1443–1450, 1983 [DOI] [PubMed] [Google Scholar]
- 99. Wirtz H, Dobbs L. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250: 1266–1269, 1990 [DOI] [PubMed] [Google Scholar]