Abstract
There is no effective intervention to prevent or treat bronchopulmonary dysplasia (BPD). Curcumin has potent antioxidant and anti-inflammatory properties, and it modulates signaling of peroxisome proliferator-activated receptor-γ (PPARγ), an important molecule in the pathobiology of BPD. However, its role in the prevention of BPD is not known. We determined 1) if curcumin enhances neonatal lung maturation, 2) if curcumin protects against hyperoxia-induced neonatal lung injury, and 3) if this protection is mediated by blocking TGF-β. Embryonic day 19 fetal rat lung fibroblasts were exposed to 21% or 95% O2 for 24 h following 1 h of treatment with curcumin. Curcumin dose dependently accelerated e19 fibroblast differentiation [increased parathyroid hormone-related protein (PTHrP) receptor, PPARγ, and adipocyte differentiation-related protein (ADRP) levels and triolein uptake] and proliferation (increased thymidine incorporation). Pretreatment with curcumin blocked the hyperoxia-induced decrease (PPARγ and ADRP) and increase (α-smooth muscle actin and fibronectin) in markers of lung injury/repair, as well as the activation of TGF-β signaling. In a separate set of experiments, neonatal Sprague-Dawley rat pups were exposed to 21% or 95% O2 for 7 days with or without intraperitoneal administration of curcumin. Analysis for markers of lung injury/repair [PTHrP receptor, PPARγ, ADRP, fibronectin, TGF-β receptor (activin receptor-like kinase 5), and Smad3] and lung morphology (radial alveolar count) demonstrated that curcumin effectively blocks TGF-β activation and hyperoxia-induced lung injury. Therefore, curcumin accelerates lung maturation by stimulating key alveolar epithelial-mesenchymal interactions and prevents hyperoxia-induced neonatal lung injury, possibly by blocking TGF-β activation, suggesting that it is a potential intervention against BPD.
Keywords: bronchopulmonary dysplasia, hyperoxia, transforming growth factor-β
approximately 30% of premature infants with birth weights <1,000 g develop bronchopulmonary dysplasia (BPD) (15). The “new” BPD is characterized by abnormal lung cytoarchitecture, characterized by deranged alveolarization, with minimal large and small airway changes, and relatively mild inflammation and fibrosis (9). Infants with BPD are at significant risk of increased morbidity and mortality. They are unable to oxygenate efficiently and often require O2 supplementation well into childhood. Although the pathogenesis of BPD is not completely understood, hyperoxia, inflammation, and/or ventilator exposure of the premature lung are the principal causative factors (33). More importantly, there is no effective intervention to prevent or treat BPD (3).
Mesenchymal interstitial fibroblasts play an important role in lung development and injury/repair (16, 34, 36, 38). During lung development and injury/repair, alveolar interstitial fibroblasts (AIFs) differentiate into lipid-laden AIFs (lipofibroblasts), which promote alveolar epithelial cell proliferation and differentiation (36). However, if AIFs lose their lipogenic phenotype and transdifferentiate to a myogenic phenotype, i.e., myofibroblasts, they are not conducive to lung epithelial cell growth and differentiation (36). In fact, abnormally located myofibroblasts are the “hallmark” of chronic lung diseases, including BPD (20, 37). Peroxisome proliferator-activated receptor-γ (PPARγ), a member of the retinoid X-receptor heterodimer family of the retinoid/steroid/thyroid hormone superfamily of ligand-activated nuclear receptors, is the “key molecular switch” that determines adipocyte differentiation (2, 11, 17, 21, 24). PPARγ is sufficient to trigger the adipocyte differentiation cascade and confer a lipid-storing phenotype on mesenchymal cells in culture, characterized by the expression of other lipid-related proteins, e.g., adipocyte differentiation-related protein (ADRP). In the developing rat lung, PPARγ expression peaks just prior to delivery on embryonic day 21 (e21) to e22 (e0 = day of mating) and is increased by paracrine mediators that enhance fetal lung maturation (31). In contrast, loss of PPARγ expression leads to transdifferentiation of lipofibroblasts to myofibroblasts.
In a neonatal rat model, we previously showed that exposure to short-term (24 h) and long-term (7 days) hyperoxia (95% O2) disrupts the alveolar epithelial-mesenchymal paracrine homeostatic signaling pathway, characterized by downregulation of the lipofibroblastic phenotype (PPARγ and ADRP) and upregulation of the myogenic phenotype [α-smooth muscle actin (SMA), fibronectin, and calponin] (5, 23). This is accompanied by pulmonary morphological changes, such as larger alveoli, decreased alveolar number, and increased interstitial thickness, all changes consistent with development of the new BPD.
Transforming growth factor (TGF)-β signaling is critical for normal lung development and the lung injury/repair response. This signaling is initiated by ligand-induced serine/threonine receptor kinases and phosphorylation of the cytoplasmic signaling molecules Smad2 and Smad3. Smad6 and Smad7 are part of the negative-feedback loop, which is upregulated by TGF-β activation. We previously demonstrated dose-dependent increases in intracellular reactive oxygen species production on exposure to hyperoxia under in vitro conditions (31). More recently, in a rat model, we demonstrated TGF-β activation under in vivo conditions, as evidenced by increased activin receptor-like kinase (ALK)-5, phosphorylated Smad3, and total Smad7 expression, accompanying myofibroblast proliferation on exposure to hyperoxia (5).
Curcumin, a lipophilic polyphenol compound that is an active ingredient of the Indian spice turmeric, is known to have potent antioxidant, anti-inflammatory, and antimicrobial properties. It is known to inhibit the inflammatory response by modulation of cyclooxygenase-2 and lipoxygenases; production of inflammatory cytokines such as TNF-α, IL-1, -2, -6, -8, and -12, monocyte chemoattractant protein, and migration inhibitory protein; and activation of mitogen-activated protein kinases and NFκB (1, 6, 8, 30). Furthermore, it has been shown to inhibit bleomycin-induced pulmonary fibrosis by modulating TGF-β signaling (4, 28). As outlined above, BPD pathogenesis is closely linked to oxidant damage and the inflammatory response following exposure of the premature lung to stimuli that predispose to BPD. However, it is not known if curcumin has an effect on the development of BPD. We hypothesize that curcumin, by upregulating homeostatic AIF PPARγ signaling, enhances neonatal lung maturation and prevents hyperoxia-induced neonatal lung injury and that this protective effect is mediated by blocking hyperoxia-induced activation of TGF-β signaling.
METHODS
In vitro studies.
Fetal rat lung fibroblasts were isolated from e19 Sprague-Dawley rats and cultured at 37°C in chamber slides, six-well plates, and 100-mm dishes, as described by us previously (21, 36, 38). At near confluence, cells were exposed to 21% or 95% O2 for 24 h with or without 1 h of pretreatment with curcumin (1, 5, 10, or 20 μM). The rate of cell proliferation was determined on the basis of [3H]thymidine incorporation into DNA (25). Cell differentiation was determined on the basis of Western analysis for fibroblast differentiation markers, i.e., parathyroid hormone-related protein (PTHrP) receptor (1:100 dilution; catalog no. sc-12722), PPARγ (1:250 dilution; catalog no. sc-7196), ADRP (1:250 dilution; catalog no. sc-32888), and fibronectin (1:1,000 dilution; catalog no. sc-9068), which were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and α-SMA (1:100,000 dilution; catalog no. A2547), which was obtained from Sigma (St. Louis, MO). TGF-β signaling was assessed by Western analysis for Smad3 (1:200 dilution; catalog no. sc-101154) and TGF-β receptor 1 (ALK-5, 1:350 dilution; catalog no. sc-398), which were obtained from Santa Cruz Biotechnology, and phosphorylated Smad3 (1:500 dilution; catalog no. 9514), which was obtained from Cell Signaling (Danvers, MA). Cell apoptosis was assessed by Western analysis for Bcl-2 (1:500 dilution; catalog no. sc-492) and Bax (1:1,000 dilution; catalog no. sc-493), which were obtained from Santa Cruz Biotechnology.
Triolein uptake.
Triolein uptake was determined as described by us previously (32). Briefly, culture medium was replaced with DMEM containing 20% adult rat serum mixed with [3H]triolein (1 μCi/ml). The cells were incubated at 37°C in 5% CO2-balance air for 4 h. At the termination of the incubation, the medium was decanted, and the cells were rinsed twice with 1 ml of ice-cold PBS and then thoroughly homogenized. An aliquot of the cell homogenate was taken for protein assay, and the remaining suspension was extracted for neutral lipid content.
Oxidative stress.
Oxidative stress was assessed by determination of the NADP-to-NADPH ratio following the manufacturer's protocol (catalog no. ab65349, Abcam, Cambridge, MA).
In vivo hyperoxia exposure system and animal protocol.
First-time-pregnant Sprague-Dawley rat dams were housed in humidity- and temperature-controlled rooms on a 12:12-h light-dark cycle and allowed food and water ad libitum. On day 22 of pregnancy, the dams delivered naturally. The pups were pooled, randomized, and returned to the nursing dams. One set of pups was maintained in 95% (vol/vol) O2, while the other set was maintained in room air-21% (vol/vol) O2. Nursing dams were rotated between hyperoxia- and room air-exposed litters every 24 h to prevent O2 toxicity in the dams. Continuous 95% O2 exposure was achieved in a Plexiglas chamber (77 × 64 × 37 cm) by a flow-through system. The O2 level inside the Plexiglas chamber was monitored continuously with an O2 analyzer (MAXO2, Ceramatec). Experimental pups were grouped as controls (21% O2 for 7 days + placebo intraperitoneal saline administration), 21% O2 + curcumin, hyperoxia only (95% O2 for 7 days + placebo), and hyperoxia with curcumin (95% O2 for 7 days + curcumin). Curcumin (Sigma) was first dissolved in DMSO (16 μg/μl) and then, according to the body weight of each animal (5 mg/kg), further diluted with sterile saline to 50 μl volume and administered intraperitoneally with a microsyringe once a day. After the 7-day experimental period, the pups were killed using 0.1 ml of Euthasol (390 mg/ml pentobarbital sodium + 50 mg/ml phenytoin; Virbac Animal Health) per pup, and lungs were collected and processed for Western analysis and immunofluorescence staining (see below). All animal procedures were performed following National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Los Angeles Biomedical Research Institute Animal Care and Use Committee.
Lung morphology.
Lung morphometry was assessed by determination of radial alveolar count by an investigator unaware of the treatment groups, as described by us previously (5).
Western analysis.
The isolated lungs were flash-frozen in liquid nitrogen and then homogenized and sonicated in ice-cold RIPA lysis buffer (500 μl/25 mg of lung tissue) containing 50 mM Tris·HCl (pH 7.4), 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS (catalog no. BP-115, Boston Bioproducts, Ashland, MA) with 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 2 μg/ml pepstatin A. After centrifugation at 13,200 g for 15 min at 4°C, the supernatant was used for Western blot analysis for PPARγ, α-SMA, fibronectin, phosphorylated and total Smad3, Smad7, ALK-5, Bcl-2, Bax, and 3-nitrotyrosine. The protein concentration of the supernatant was measured by the Bradford method, with bovine serum albumin as the standard. Aliquots of the supernatant, each containing 25 μg of protein, were separated by SDS-PAGE and electrically transferred to nitrocellulose membranes. Nonspecific binding sites were blocked with Tris-buffered saline (0.1% TBS) containing 5% nonfat dry powdered milk (wt/vol) for 1 h at room temperature. After a brief rinse with TBS containing 0.1% Tween 20 (TBST), the protein blots were incubated in primary antibody [PPARγ (1:500 dilution; catalog no. sc-7196), ADRP (1:250 dilution; catalog no. sc-32888), fibronectin (1:600 dilution; catalog no. sc-9068), Smad3 (1:200 dilution; catalog no. sc-101154), Smad6/7 (1:350 dilution; catalog no. sc-7004), ALK-5 (1:200 dilution; catalog no. sc-398), Bcl-2 (1:350 dilution; catalog no. sc-492), and Bax (1:600 dilution; catalog no. sc-493), all from Santa Cruz Biotechnology; phosphorylated Smad3 (1:250 dilution; catalog no. 9514, Cell Signaling); α-SMA (1:10,000 dilution; catalog no. A2547) and 3-nitrotyrosine (1:1,000 dilution; catalog no. N5663, both from Sigma); and GAPDH (1:10,000 dilution; MAb 374, Chemicon, Temecula, CA)] overnight at 4°C and then with the appropriate secondary antibody for 1 h at room temperature. After three washes in TBST, the blots were exposed to X-ray film using SuperSignal West Pico chemiluminescent substrate (Pierce Biotechnology, Rockford, IL) and developed. The relative densities of the protein bands were determined with UN-SCAN-IT software (Silk Scientific, Orem, UT) and normalized to that of GAPDH.
Immunofluorescence staining.
At e19, AIFs were subjected to hyperoxia (95% O2) for 6 h with or without 1 h of curcumin pretreatment (10 μM). Control cells were kept in 21% O2. Confocal immunofluorescent analysis for Smad3 (green; catalog no. C67H9, Cell Signaling) and nuclei (blue; TO-Pro-3 Iodide, Invitrogen) was performed as described previously (22). For tissue immunofluorescence staining for the relevant proteins, rat lungs were inflated in situ with 4% paraformaldehyde in phosphate buffer at a standard inflation pressure of 5 cmH2O for 4 h at 4°C. The lungs were subsequently transferred to PBS containing 30% sucrose (wt/vol) until equilibrated in the cold (4°C). After fixation, 5-μm paraffin sections were treated three times with Histo-Clear for 5 min and then rehydrated by a sequential ethanol wash. Sections were then washed twice for 10 min with PBS and blocked for 1 h in PBS-5% normal goat serum-0.2% Triton X-100. Sections were incubated with primary antibodies for 1 h at room temperature and then with the appropriate secondary antibody for 1 h, also at room temperature. Antibodies included α-SMA [primary antibody, 1:100 dilution; catalog no. A2547 Sigma; secondary antibody, 1:100 dilution; Alexa Fluor, goat anti-mouse 488 (green)]; PPARγ [primary antibody, 1:100 dilution, catalog no. sc-7196, Santa Cruz Biotechnology; secondary antibody, 1:100 dilution, Alexa Fluor, anti-rabbit 568 (red)]; platelet endothelial cell adhesion molecule [primary antibody, 1:10 dilution, catalog no. 550300, BD Biosciences; secondary antibody, 1:100 dilution, Alexa Fluor, anti-rabbit 568 (red)]; Bcl-2 [primary antibody, 1:100 dilution, catalog no. sc-492, Santa Cruz Biotechnology; secondary antibody, 1:100 dilution, Alexa Fluor, anti-rabbit 488 (green)]; and Bax [primary antibody, 1:100 dilution, catalog no. sc-493, Santa Cruz Biotechnology; secondary antibody, 1:100 dilution, Alexa Fluor, anti-rabbit 568 (red)]. Sections were washed with PBS and then mounted with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen) for visualization under a fluorescence microscope.
Statistical analysis.
Experiments were done at least three times independently. Differences between the groups were evaluated by one-way ANOVA followed by Newman-Keuls post hoc test and unpaired Student's t-test as needed. P < 0.05 was considered to be statistically significant. Values are means ± SE.
RESULTS
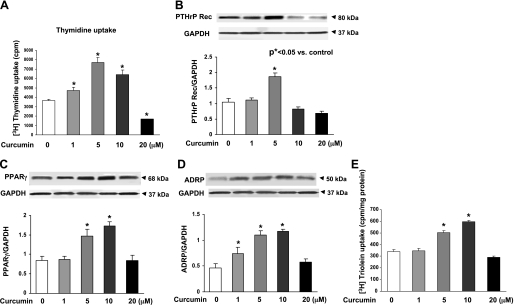
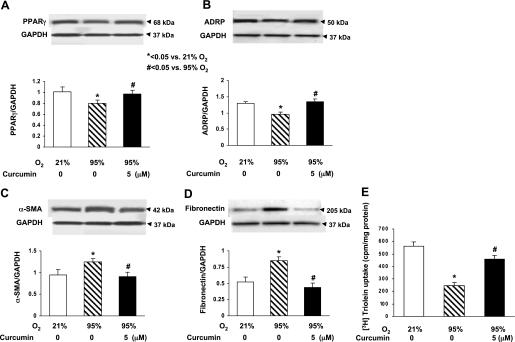
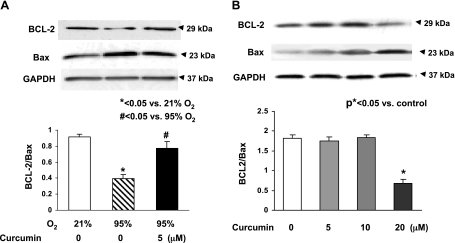
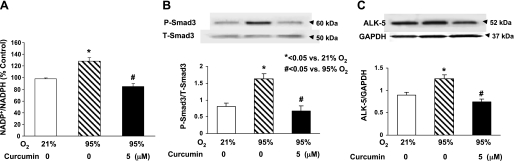
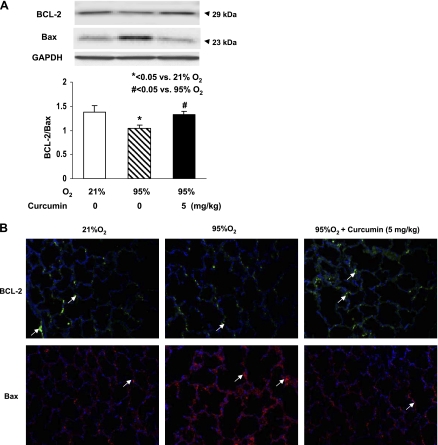
Since our working hypothesis is that curcumin stimulates lung maturation, protecting the lung against oxidant injury, we first examined the effect of curcumin on lung fibroblast differentiation, the determinant of alveolar maturation, in cell culture. Curcumin stimulated e19 fetal rat lung fibroblast proliferation and maturation, as indicated by dosimetric increases in thymidine incorporation (28 ± 4.9%, 110 ± 11.2%, and 75 ± 7.3% vs. control at 1, 5, and 10 μM curcumin, respectively; Fig. 1A) and PTHrP receptor (79 ± 6.6% at 5 μM curcumin; Fig. 1B), PPARγ (74 ± 3.2% and 104 ± 5.9% at 5 and 10 μM curcumin; Fig. 1C), and ADRP (59 ± 3.8%, 136 ± 7.5%, and 145 ± 6.9% at 0.1, 5, and 10 μM curcumin; Fig. 1D) protein levels. Curcumin also stimulated triolein uptake by AIFs (48 ± 1.8% and 75 ± 5.9% at 5 and 10 μM curcumin; Fig. 1E), indicating stimulation of their lipofibroblastic phenotype. In cultured e19 fetal rat lung fibroblasts, 95% O2 exposure for 24 h inhibited PPARγ (20 ± 3.9%) and ADRP (27 ± 2.1%) protein levels, which were blocked by 5 μM curcumin (Fig. 2, A and B), and hyperoxia increased α-SMA (33 ± 3.1%; Fig. 2C) and fibronectin (55 ± 7.3%; Fig. 2D) protein levels; both effects were blocked by 5 μM curcumin. Again, as an overall measure of lipofibroblast function, hyperoxia exposure decreased triolein uptake by 56 ± 3.8% (Fig. 2E), an effect that was markedly attenuated (27 ± 3.2%) by curcumin treatment. The protective effects of curcumin on hyperoxia-induced changes in e19 AIF proliferation and differentiation were also accompanied by blockage of the hyperoxia-induced increase in fibroblast apoptosis, as demonstrated by blockage of the hyperoxia-induced decrease in the Bcl-2-to-Bax ratio (Fig. 3A). However, at 20 μM, curcumin by itself increased fibroblast apoptosis (Fig. 3B). The mechanism for the cytoprotective action of curcumin is shown in Fig. 4: hyperoxia increased the NADP-to-NADPH ratio, a marker of oxidative stress (Fig. 4A), as well as the phosphorylated-to-total Smad3 ratio (67 ± 3.3%) and the expression of ALK-5 (36 ± 4.2%), both indicating TGF-β activation (Fig. 4, B and C); all these effects were blocked by curcumin at 5 μM. Consistent with these molecular cytoprotective effects, hyperoxia increased the immunostaining for Smad3 and α-SMA (Fig. 5), effects that were blocked by 10 μM curcumin.
Fig. 1.
Effect of curcumin on alveolar maturation by examination of embryonic day 19 (e19) fetal rat lung alveolar interstitial fibroblast (AIF) proliferation and differentiation. Treatment of cultured e19 AIFs with curcumin for 24 h significantly increased thymidine incorporation (A), which was accompanied by increased parathyroid hormone-related protein (PTHrP) receptor (PTHrP Rec; B), peroxisome proliferator-activated receptor (PPAR)-γ (C), and adipocyte differentiation-related protein (ADRP; D) protein levels and triolein uptake (E). Values are means ± SE; n = 6 (A and E) and 3 (B–D). All biomarker responses were curcumin-dose-dependent; increases were significant at 5 μM curcumin.
Fig. 2.
Hyperoxia-induced pulmonary damage markers and cytoprotective effect of curcumin against hyperoxia in cultured e19 AIFs. When exposed to 95% O2 for 24 h, PPARγ (A) and ADRP (B) protein levels decreased significantly, α-smooth muscle actin (α-SMA, C) and fibronectin (D) protein levels increased significantly, and triolein uptake decreased significantly (E). These hyperoxia-induced effects were completely blocked by 1 h of pretreatment with 5 μM curcumin. Values are means ± SE; n = 3 (A–D) and 6 (E).
Fig. 3.
Effect of curcumin on hyperoxia-induced apoptosis in e19 AIFs. Exposure of cultured e19 AIFs to 95% O2 for 24 h resulted in decreased Bcl-2-to-Bax ratio (A), an effect that was blocked by 1 h of pretreatment with 5 μM curcumin. Under normoxic conditions, 24 h of treatment with 20 μM curcumin increased fibroblast apoptosis (decreased Bcl-2-to-Bax ratio), while 5 and 10 μM curcumin had no effect (B). Values are means ± SE.
Fig. 4.
Hyperoxia-induced increase in oxidative stress and activation of the TGF-β pathway and their blockage by curcumin pretreatment. In cultured e19 AIFs exposed to 95% O2 for 24 h (to determine oxidative stress) and 30 min (to determine TGF-β activation), NADP-to-NADPH ratio and phosphorylated-to-total Smad3 ratio (P-Smad3/T-Smad3) were significantly increased. Similarly, 95% O2 exposure of e19 AIFs for 24 h caused a significant increase in activin-like receptor kinase (ALK)-5 expression. All these effects were completely blocked by 5 μM curcumin pretreatment. Values are means ± SE; n = 3.
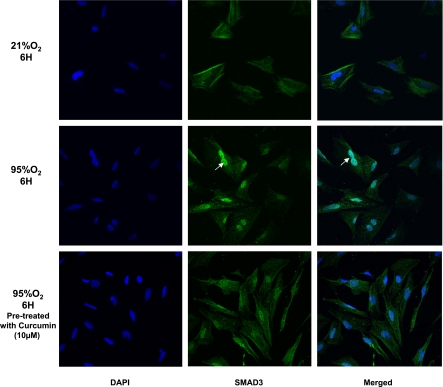
Fig. 5.
Hyperoxia-induced increase in Smad3 and its nuclear translocation and their blockage by curcumin pretreatment. In cultured e19 AIFs exposed to 95% O2 for 6 h, Smad3 translocated to the nucleus (white arrows), an effect that was at least partially blocked by 1 h of pretreatment with 10 μM curcumin. Original magnification ×20.
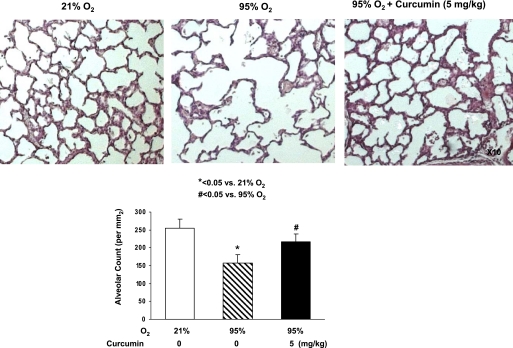
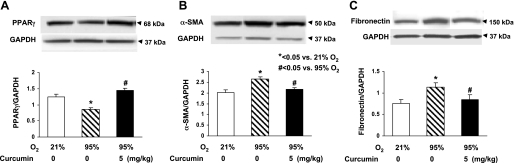
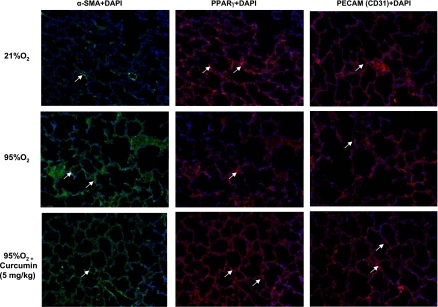
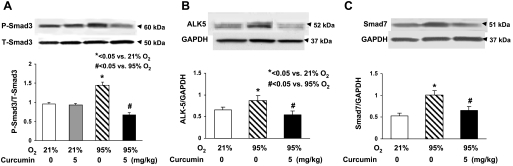
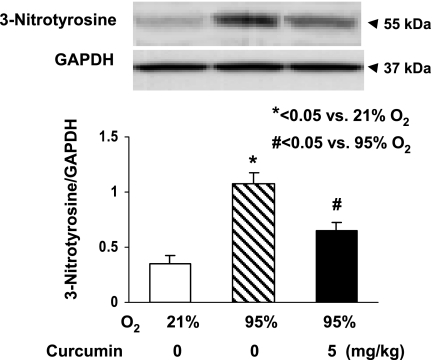
The in vivo cytoprotective effects of curcumin are shown in Fig. 6: hyperoxia decreased the alveolar count by 39 ± 4.0%, an effect that was significantly prevented by once-daily intraperitoneal treatment with curcumin (5 mg/kg). The cellular mechanism by which the hyperoxia exposure inhibited alveolarization is shown in Fig. 7: it decreased PPARγ levels by 16 ± 3.0%, while it increased α-SMA (25 ± 4.7%) and fibronectin (50 ± 2.8%) levels, all of which were blocked by curcumin treatment. Blockage of the hyperoxia-induced increase in α-SMA and the decreases in PPARγ and platelet endothelial cell adhesion molecule (CD31) by curcumin treatment were corroborated by immunostaining (Fig. 8). Examination of TGF-β signaling (Fig. 9) revealed that hyperoxia increased the phosphorylated-to-total Smad3 ratio (67 ± 2.9%), an effect that was significantly inhibited by curcumin. Treatment with curcumin under normoxic conditions did not affect the basal phosphorylated Smad3 level (Fig. 9A). Moreover, the hyperoxia-induced increase in ALK-5 (31 ± 2.0%; Fig. 9B) and Smad7 (92 ± 8.5%; Fig. 9C) protein levels was also blocked by curcumin treatment. Since, under in vitro conditions, we observed that curcumin blocked oxidative stress (Fig. 4A), we next examined the effect of curcumin on hyperoxia-induced changes in 3-nitrotyrosine protein levels, a marker of nitrosative damage as a consequence of oxidative stress under in vivo conditions. Not surprisingly, the hyperoxia-induced increase in lung 3-nitrotyrosine protein level was blocked by curcumin (Fig. 10). Finally, consistent with our in vitro data (Fig. 3), the in vivo hyperoxia-induced increase in cellular apoptosis was also blocked by curcumin, as demonstrated by Western blotting (Fig. 11A) and immunostaining (Fig. 11B) for Bcl-2 and Bax proteins.
Fig. 6.
In vivo protective effects of curcumin against hyperoxia. Exposure to 95% O2 for 7 days caused a significant decrease in the alveolar count of experimental rats, an effect that was effectively blocked by concurrent treatment with curcumin (5 mg/kg). Values are means ± SE; n = 3.
Fig. 7.
Molecular mechanism by which hyperoxia exposure inhibits alveolarization and how curcumin blocks this effect. Exposure of newborn rat pups to 95% O2 for 7 days significantly reduced PPARγ protein level (A) and increased α-SMA (B) and fibronectin (C) protein levels. All these effects were blocked by concurrent treatment with 5 mg/kg curcumin. Values are means ± SE; n = 3.
Fig. 8.
Molecular mechanism by which hyperoxia exposure inhibits alveolarization and how curcumin blocks this effect. Increase in α-SMA and decreases in PPARγ and platelet endothelial cell adhesion molecule (PECAM, CD31) protein levels induced by 7 days of hyperoxia (95% O2) in vivo were corroborated by immunostaining. All these effects were blocked by concurrent treatment with 5 mg/kg curcumin. Representative images are shown, and locations of the specific proteins are indicated by white arrows; nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Images are representative of results from 3 experiments.
Fig. 9.
In vivo hyperoxia-induced activation of TGF-β pathway and blockage by curcumin treatment. A and B: phosphorylated-to-total Smad3 ratio and expression of ALK-5 were significantly increased in newborn rats exposed to 95% O2 for 7 days; these effects were completely blocked by concurrent treatment with 5 mg/kg curcumin. Under normoxic conditions, treatment with curcumin did not affect basal phosphorylated Smad3 levels. C: Smad7 was significantly induced by hyperoxia exposure and blocked by concurrent treatment with 5 mg/kg curcumin. Values are means ± SE; n = 3.
Fig. 10.
Effect of curcumin on in vivo hyperoxia-induced increase in lung tissue nitrosative stress. After 7 days of exposure to 95% O2, Western blotting on lung tissue from newborn rat pups showed increased 3-nitrotyrosine protein level, an effect that was significantly attenuated by concurrent treatment with 5 mg/kg curcumin. Values are means ± SE; n = 3.
Fig. 11.
Effect of curcumin on in vivo hyperoxia-induced increase in lung cellular apoptosis. Exposure of newborn rat pups to 95% O2 for 7 days significantly increased lung cellular apoptosis, as reflected by a decrease in Bcl-2 and an increase in Bax protein levels by Western blotting (A) and immunostaining (B). Both effects were blocked by concurrent treatment with 5 mg/kg curcumin. Values are means ± SE; n = 3. Original magnification ×20 for representative images in B.
DISCUSSION
BPD was originally identified by Northway et al. (19) and later redefined by Jobe (9) as the new BPD. It is essentially a disease of prematurity that is caused by a wide variety of proinflammatory factors, including barotrauma, oxotrauma, and infection (3, 7, 9, 15, 18). All these agonal insults seem to funnel through the common pathway leading to delayed alveolarization and myofibroblast proliferation (33). Conversely, by stimulating the lipofibroblast phenotype (36), as determined by PPARγ expression (16), we have been able to prevent all these injuries (33). Because of the close relationship between lipofibroblast maturation and these diverse effectors of BPD, we have devised a developmental model for the prevention and treatment of lung injury that leads to a “BPD-like” picture experimentally (33) and integrates all these factors mechanistically.
In the present series of studies, we have investigated the putative effect of curcumin on lung fibroblast differentiation, since these cells provide all the growth factors necessary for normal lung development. Curcumin was found to stimulate the key genes necessary for lipofibroblast differentiation (22), which protects the lung against oxidant injury (35). Furthermore, oxidant injury, which downregulates these genes, was inhibited by curcumin treatment in vitro and in vivo, thereby preserving the molecular and structural integrity of the alveoli. At the cellular-molecular level, curcumin sustained the lipofibroblast phenotype, maintaining its ability to take up triolein, which is cytoprotective against oxidant injury (35) and provides substrate for surfactant phospholipid synthesis by the neighboring alveolar type II cell (26, 32). Mechanistically, it inhibited oxidative stress and hyperoxic activation of TGF-β signaling, which is thought to be an important pathway in the pathogenesis of BPD (5, 12, 14, 28).
Curcumin, an active ingredient of the Indian spice turmeric, is known to have potent antioxidant, anti-inflammatory, and antimicrobial properties, making it an interesting dietary therapy for BPD. However, it is important to point out that a number of studies have stressed the limited bioavailability of oral curcumin, thereby necessitating parenteral administration to achieve its effects (13, 27, 29). Therefore, we opted for the intraperitoneal route over the oral route for its administration. The dose of curcumin used by us was based on its optimal anti-TGF-β effects at 5 mg/kg body wt in our preliminary experiments with 2.5, 5, and 20 mg/kg body wt. This dose is markedly lower than those used in adults by others, either by the oral or parenteral route (10, 29). Our investigation into the mechanism of curcumin's protective effect on neonatal lung injury suggests blockage of hyperoxia-induced oxidative stress and TGF-β activation. Similarly, curcumin has been shown to inhibit bleomycin-induced pulmonary fibrosis by modulating TGF-β signaling (4, 29). In the same vein, curcumin has been shown to counteract TGF-β-mediated downregulation of PPARγ in hepatic stellate cells, which are the liver equivalent of AIFs (39). However, our study is the first to show that parenterally administered curcumin prevents neonatal lung injury by blocking oxidant damage to the developing lung, suggesting its potential role in preventing BPD.
GRANTS
This study was supported by National Institutes of Health Grants HL-75405, HD-51857, HD-058948, HD-67319, and HL-55268 and Tobacco-Related Disease Research Program Grants 15IT-0250 and 17RT-0170.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Bisht K, Wagner KH, Bulmer AC. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology 278: 88–100, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Boros LG, Torday JS, Paul Lee WN, Rehan VK. Oxygen-induced metabolic changes and transdifferentiation in immature fetal rat lung lipofibroblasts. Mol Genet Metab 77: 230–236, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Cerny L, Torday JS, Rehan VK. Prevention and treatment of bronchopulmonary dysplasia: contemporary status and future outlook. Lung 186: 75–89, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Chen B, Zhang DP, Gao W. [Effect of curcumin on the expression of collagen type I protein and transforming growth factor-β1 mRNA in pulmonary fibrosis rats]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 26: 257–261, 2008 [PubMed] [Google Scholar]
- 5. Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rehan VK. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-β and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol 296: L1031–L1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr 103: 1545–1557, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Hayes D, Jr, Feola DJ, Murphy BS, Shook LA, Ballard HO. Pathogenesis of bronchopulmonary dysplasia. Respiration 79: 425–436, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Jacob A, Wu R, Zhou M, Wang P. Mechanism of the anti-inflammatory effect of curcumin: PPAR-γ activation. PPAR Res 2007: 89369, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 46: 641–643, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Kalpana C, Menon VP. Inhibition of nicotine-induced toxicity by curcumin and curcumin analog: a comparative study. J Med Food 7: 467–471, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA 91: 7355–7359, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwong KY, Niang S, Literat A, Zhu NL, Ramanathan R, Jones CA, Minoo P. Expression of transforming growth factor β (TGF-β1) by human preterm lung inflammatory cells. Life Sci 79: 2349–2356, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 6: 10, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lecart C, Cayabyab R, Buckley S, Morrison J, Kwong KY, Warburton D, Ramanathan R, Jones CA, Minoo P. Bioactive transforming growth factor-β in the lungs of extremely low birthweight neonates predicts the need for home oxygen supplementation. Biol Neonate 77: 217–223, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, Fanaroff AA, Stark A, Carlo W, Tyson JE, Donovan EF, Shankaran S, Stevenson DK. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 107: E1, 2001 [DOI] [PubMed] [Google Scholar]
- 16. McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol 59: 43–62, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Mendelson CR. In: Endocrinology of the Lung. Totowa, NJ: Humana, 2000 [Google Scholar]
- 18. Miller NE. Techniques of early respiratory management of very low and extremely low birth weight infants. Neonatal Netw 29: 153–160, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 276: 357–368, 1967 [DOI] [PubMed] [Google Scholar]
- 20. Pache JC, Christakos PG, Gannon DE, Mitchell JJ, Low RB, Leslie KO. Myofibroblasts in diffuse alveolar damage of the lung. Mod Pathol 11: 1064–1070, 1998 [PubMed] [Google Scholar]
- 21. Rehan V, Torday J. Hyperoxia augments pulmonary lipofibroblast-to-myofibroblast transdifferentiation. Cell Biochem Biophys 38: 239–250, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Rehan VK, Sakurai R, Corral J, Krebs M, Ibe B, Ihida-Stansbury K, Torday JS. Antenatally administered PPAR-γ agonist rosiglitazone prevents hyperoxia-induced neonatal rat lung injury. Am J Physiol Lung Cell Mol Physiol 299: L672–L680, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rehan VK, Wang Y, Patel S, Santos J, Torday JS. Rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr Pulmonol 41: 558–569, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science 289: 950–953, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, Torday JS, Rehan VK. 1α,25(OH)2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol 297: L496–L505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schultz CJ, Torres E, Londos C, Torday JS. Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells. Am J Physiol Lung Cell Mol Physiol 283: L288–L296, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer 41: 1955–1968, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Shi W, Xu J, Warburton D. Development, repair and fibrosis: what is common and why it matters. Respirology 14: 656–665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith MR, Gangireddy SR, Narala VR, Hogaboam CM, Standiford TJ, Christensen PJ, Kondapi AK, Reddy RC. Curcumin inhibits fibrosis-related effects in IPF fibroblasts and in mice following bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 298: L616–L625, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun J, Guo W, Ben Y, Jiang J, Tan C, Xu Z, Wang X, Bai C. Preventive effects of curcumin and dexamethasone on lung transplantation-associated lung injury in rats. Crit Care Med 36: 1205–1213, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr Opin Genet Dev 5: 571–576, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Torday J, Hua J, Slavin R. Metabolism and fate of neutral lipids of fetal lung fibroblast origin. Biochim Biophys Acta 1254: 198–206, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Torday JS, Rehan VK. Developmental cell/molecular biologic approach to the etiology and treatment of bronchopulmonary dysplasia. Pediatr Res 62: 2–7, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Torday JS, Rehan VK. Lung evolution as a cipher for physiology. Physiol Genomics 38: 1–6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Torday JS, Torday DP, Gutnick J, Qin J, Rehan V. Biologic role of fetal lung fibroblast triglycerides as antioxidants. Pediatr Res 49: 843–849, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Torday JS, Torres E, Rehan VK. The role of fibroblast transdifferentiation in lung epithelial cell proliferation, differentiation, and repair in vitro. Pediatr Pathol Mol Med 22: 189–207, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Toti P, Buonocore G, Tanganelli P, Catella AM, Palmeri ML, Vatti R, Seemayer TA. Bronchopulmonary dysplasia of the premature baby: an immunohistochemical study. Pediatr Pulmonol 24: 22–28, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Santos J, Sakurai R, Shin E, Cerny L, Torday JS, Rehan VK. Peroxisome proliferator-activated receptor γ agonists enhance lung maturation in a neonatal rat model. Pediatr Res 65: 150–155, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng S, Chen A. Disruption of transforming growth factor-β signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-γ in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 292: G113–G123, 2007 [DOI] [PubMed] [Google Scholar]