Abstract
Asthma is a chronic lung disease characterized by local inflammation that can result in structural alterations termed airway remodeling. One component of airway remodeling involves fibroblast accumulation and activation, resulting in deposition of collagen I around small bronchi. Prostaglandin E2 (PGE2) is the main eicosanoid lipid mediator produced by lung fibroblasts, and it exerts diverse anti-fibrotic actions. Dysregulation of the PGE2 synthesis/response axis has been identified in human pulmonary fibrotic diseases and implicated in the pathogenesis of animal models of lung parenchymal fibrosis. Here we investigated the relationship between the fibroblast PGE2 axis and airway fibrosis in an animal model of chronic allergic asthma. Airway fibrosis increased progressively as the number of airway challenges with antigen increased from 3 to 7 to 12. Compared with cells from control lungs, fibroblasts grown from the lungs of asthmatic animals, regardless of challenge number, exhibited no defect in the ability of PGE2 or its analogs to inhibit cellular proliferation and collagen I expression. This correlated with intact expression of the EP2 receptor, which is pivotal for PGE2 responsiveness. However, cytokine-induced upregulation of PGE2 biosynthesis as well as expression of cyclooxygenase-2 (COX-2) and microsomal PGE synthase-1 declined with increasing numbers of antigen challenges. In addition, treatment with the COX-2-selective inhibitor nimesulide potentiated the degree of airway fibrosis following repeated allergen challenge. Because endogenous COX-2-derived PGE2 acts as a brake on airway fibrosis, the inability of fibroblasts to upregulate PGE2 generation in the inflammatory milieu presented by repeated allergen exposure could contribute to the airway remodeling and fibrosis observed in chronic asthma.
Keywords: prostaglandin E2, airway, prostanoid, inflammation
asthma is a chronic inflammatory disease of the airways that in 2005 was estimated to affect over 300 million people around the world. Allergens play an important role in driving asthma, and animal models of allergic asthma have contributed greatly to the understanding of its pathogenesis. The inflammatory process in allergic asthma is typically characterized by increased numbers of Th2 lymphocytes, eosinophils, and activated mast cells. A variety of structural changes in and around the airways, collectively termed airway remodeling, are also observed (4, 7, 10, 39, 43). These include mucus metaplasia of goblet cells, hyperplasia and hypertrophy of airway smooth muscle cells, excessive angiogenesis, and airway fibrosis (2). Airway fibrosis reflects the accumulation, activation, and differentiation of fibroblasts which elaborate type I collagen and other extracellular matrix proteins in the subepithelial region of the small airways (4, 5, 43).
The fibrogenic functions of parenchymal lung fibroblasts have long been studied in the context of pulmonary fibrosis. Indeed, a number of functional differences in cells from fibrotic lung tissue that would be expected to contribute to fibrosis have been identified (45); moreover, many of these differences have been found to persist through numerous passages, implying a stable phenotypic alteration. The apparent stability of some of these phenotypic alterations can now be explained on the basis of epigenetic mechanisms (11, 26). Many of these same stable differences in fibroblast phenotype have likewise been observed in both human (30) and mouse models (47) of asthma. Although activation by mediators such as transforming growth factor-β (TGF-β) and interleukin (IL)-13 of both parenchymal and airway fibroblasts has been studied extensively, the importance of endogenous anti-fibrotic mediators has received far less attention. Prostaglandin E2 (PGE2) is the major arachidonic acid metabolite of lung fibroblasts (34, 36, 46), and it has been shown to inhibit virtually all fibroblast functions, including migration (32, 50), proliferation (3, 12), survival (29), myofibroblast differentiation (33), and collagen accumulation (13, 17, 35).
However, fibroblasts isolated from the lungs of patients with idiopathic pulmonary fibrosis (IPF) exhibit diminished synthesis of PGE2 and diminished expression of the inducible enzyme responsible for its biosynthesis, cyclooxygenase-2 (COX-2), in response to various stimuli (51). PGE2 can ligate four different G protein-coupled receptors (EP1 through EP4), which are coupled to different G proteins and thereby mediate distinct signaling responses. Virtually all of the inhibitory effects of PGE2 on lung fibroblasts are mediated predominantly by its ligation of EP2 (25, 33, 50) and subsequent ability to increase intracellular levels of cAMP. Furthermore, however, we have shown that fibroblasts from some patients with IPF also manifest resistance to the suppressive actions of PGE2 (28), in part because of downregulation of EP2 (28), and this defect was recapitulated in lung fibroblasts from mice following bleomycin fibrosis (40).
Although they affect different compartments of the lung, both asthma and IPF are characterized by the deposition of scar tissue that alters normal architecture and physiological function. In addition, both IPF (23) and allergic asthma are characterized by Th2 skewing of immune responses. Because parenchymal lung fibrosis is characterized by defects in fibroblast synthesis of and responsiveness to PGE2, and because airway fibrosis is an important component of airway remodeling in asthma, in this study we utilized a model of repeated allergen challenge to the airways to determine if airway fibrosis is accompanied by similar defects in the fibroblast PGE2 axis.
MATERIALS AND METHODS
Reagents.
Grade V ovalbumin (OVA) and BSA were purchased from Sigma-Aldrich (St. Louis, MO). Aluminum hydroxide (ImJect Alum Adjuvant) was obtained from Thermo Scientific (Waltham, MA). Dulbecco's modified Eagle's medium (DMEM) and penicillin/streptomycin were obtained from Invitrogen (Carlsbad, CA). FBS was purchased from HyClone (Logan, UT). PGE2 and PGE2 enzyme immunoassay (EIA) kits were obtained from Cayman Chemical (Ann Arbor, MI). Mouse IL-1β was obtained from Thermo Scientific, TGF-β was purchased from R&D Systems (Minneapolis, MN), and forskolin was from Cayman Chemical. [3H]thymidine and enhanced chemiluminescence (ECL) reagent were obtained from GE Healthcare (Piscataway, NJ). Antibodies used for immunoblotting and their sources were as follows: mouse collagen I, Cedarlane Laboratories (Burlington, ON, Canada); COX-2 and microsomal PGE synthase-1 (mPGEs1), Cayman Chemical; α-tubulin, Sigma-Aldrich; and GAPDH, Santa Cruz Biotechnology (Santa Cruz, CA). The collagen-I antibody used for immunohistochemistry was purchased from Abcam (Cambridge, UK). Nimesulide was obtained from Sigma-Aldrich.
Mice.
Female BALB/c mice 7–8 wk old were obtained from Jackson Laboratory (Bar Harbor, ME) and kept under specific pathogen-free conditions at the University of Michigan. All experiments were approved by The University of Michigan Committee on the Use and Care of Animals.
Induction of allergic asthma.
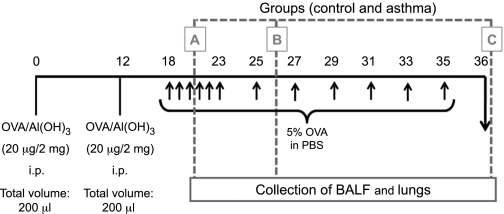
Mice were sensitized on days 0 and 12 by an intraperitoneal injection of a mixture containing 20 μg of OVA and 2 mg of Al(OH)3 in PBS (a total volume of 0.1 ml). Sensitized mice were then challenged by multiple exposures to an aerosol of OVA at 5% in PBS generated by an ultrasonic nebulizer (ICEL US-800), delivering particles of 0.5–10 μm diameter at ∼0.75 ml/min for 20 min. An initial dose-finding experiment compared three different numbers of cumulative airway challenges to determine the most appropriate protocol for inducing histologic airway remodeling as well as the kinetics of this response. The experimental groups were as follows: group A received the sensitizations and 3 challenges (days 18, 19, and 20); group B received the sensitizations and 7 challenges (days 18–23 and 25); and group C received the sensitizations and 12 challenges (days 18–23, 25, 27, 29, 31, 33, and 35), as depicted in Fig. 1. Animals were killed for evaluation following the last allergen challenge on the days indicated in Fig. 1. The control groups consisted of animals sensitized with OVA as described above but challenged the same number of times with PBS solution. Because no differences were detected among the three control groups, we used the control of the group C (2 sensitizations and 12 challenges with PBS) mice as standard controls for all experiments. Four independent experiments were performed, with five animals per group in each of them. For the dose-finding experiment, we examined five animals per group.
Fig. 1.
Dose-finding experimental protocol. Animals were sensitized ip 2 times with ovalbumin (OVA) and alum and then subjected to varying numbers of aerosol antigen challenges to the airways with 5% OVA (wt/vol) in PBS or PBS alone (indicated by arrows). Groups received 3, 7, and 12 challenges (groups A, B, and C, respectively). One day after the last challenge in each of the groups, lungs and bronchoalveolar lavage fluid (BALF) were collected for further analysis.
BAL.
Mice were killed via CO2 asphyxiation, the trachea was cannulated with polyethylene tubing (PE-50, Intramedic; Clay Adams, Parsippany, NJ) attached to a 25-gauge needle on a tuberculin syringe, and the lungs were lavaged two times with 0.75 ml cold PBS for a total lavage volume of 1.5 ml. In >95% of the mice, the recovery volume was 1.3–1.4 ml. The bronchoalveolar lavage (BAL) fluid was centrifuged at 1,500 rpm, and the supernatant was removed. The pelleted cells were collected and enumerated by counting on a hemocytometer in the presence of trypan blue. Cytospins were prepared from suspended BAL cells.
Differential staining.
Cytospins of the BAL were made by centrifuging 50,000–100,000 cells on microscope slides using a Shandon Cytospin 3 (Shandon, Astmoore, UK). The slides were allowed to air-dry and were then stained using a modified Wright-Giemsa (WG) stain. For WG staining, the slides were fixed/prestained for 2 min using a one-step methanol-based WG stain (Harleco; EM Diagnostics, Gibbstown, NJ) followed by steps 2 and 3 of the Diff-Quick whole blood stain (Diff-Quick; Baxter Scientific, Miami, FL). A total of 300 cells were counted from randomly chosen high-power microscope fields for each sample. The differential percentage was multiplied by the total leukocyte number to derive the number of monocyte/macrophages, neutrophils, and eosinophils per sample.
Light microscopy processing.
Lung samples were fixed for 24 h in 10% neutral buffered formalin, dehydrated in ethanol, and embedded in Paraplast (Sigma-Aldrich) at 60°C. Five-micrometer sections were adhered on glass slides precoated with 0.1% poly-l-lysine (Sigma-Aldrich) and then dried at 37°C.
Histology.
Animals were killed and perfused via the left ventricle with 3 ml normal saline. Lungs were inflated with 1 ml 10% neutral buffered formalin before being dehydrated in 70% ethanol. Lungs were then processed using standard procedures and embedded in paraffin. Sections of 5 μm were cut, mounted on slides, and stained with hematoxylin and eosin, picrosirius-hematoxylin, and antibody against type I collagen. The material was analyzed under a Nikon Eclipse E600 microscope, and images were captured using a Nikon DXM1200C digital camera. Photographs were analyzed, and morphometric analysis was performed using the NIS Elements AR 2.30 Imaging Software. Specifically, we quantified the stained area in the maximum number of small airways (0.4–0.7 mm in diameter) in each slide (one slide/animal). The areas of staining of each animal were averaged, and this number was considered representative of that individual animal. Results are presented as the mean of the stained area in square micrometers.
Immunohistochemistry.
Lung sections were deparaffinized and hydrated, and antigenic retrieval was performed by incubating the slides in 10 mM sodium citrate buffer, pH 6.0 with 0.05% vol/vol Tween 20, at 90°C for 20 min. Each of the succeeding steps was followed by a thorough rinse in PBS. All steps were performed in a humidified chamber. Slides were then treated with 3% H2O2 in PBS for 30 min to block endogenous peroxidase activity. Nonspecific staining was blocked by incubating the sections for 30 min in PBS containing 10% BSA. Rabbit polyclonal anti-collagen I antibody was diluted 1:200 in PBS containing 0.3% Tween 20 and incubated overnight at 4°C. The sections were incubated with biotin-conjugated goat anti-rabbit immunoglobulin G (Vector Laboratories, Burlingame, CA), diluted 1:1,000 in PBS, for 1 h at room temperature. After washes in PBS, sections were incubated in streptavidin-peroxidase ABC complex (Vector Laboratories) for 1 h at room temperature. Peroxidase was visualized using 0.03% 3,3′-diaminobenzidine in PBS with 0.03% H2O2. The sections were counterstained with Mayer's hematoxylin. For each immunohistochemical reaction, controls were obtained by omitting the primary antibody. Microscopy, image capture, morphometric analysis, and data presentation were as described above for histochemical staining.
Fibroblast purification.
A previous report (47) identified phenotypic differences between fibroblasts from asthmatic and control lungs but noted no differences between cells isolated from the trachea and the lung parenchyma. Because our focus was on the process of remodeling of small bronchi, we used parenchymal lung tissue as a source of fibroblasts in these studies. Mouse lungs were perfused via the right ventricle with 5 ml cold PBS and removed under aseptic conditions. The left lung was minced with scissors in DMEM containing 10% FBS and placed in 10 ml of medium in 10-cm-diameter tissue culture plates. Fibroblasts were allowed to grow out of the minced tissue, and, when cells reached 70% confluence, they were passaged following trypsinization. Fibroblasts were grown for 10–15 days (2–3 passages) before being used.
Cell proliferation.
Proliferation was measured by [3H]thymidine incorporation in disintegrations per minute measured by β-scintillation counting as described previously (25). Cells were incubated in medium alone or in 3% FBS in the presence or absence of PGE2 (1 μM) or the direct adenyl cyclase activator forskolin (200 μM) with the simultaneous addition of [3H]thymidine for 18 h. Proliferation in the presence of PGE2 and other cAMP agonists was expressed as a percentage of that determined in the absence of PGE2/cAMP agonists. All proliferation experiments were run in six replicates for each treatment, and results are presented as a percentage of untreated nonasthmatic animals.
Determination of PGE2 synthesis.
PGE2 was measured in both BAL fluid and cell culture supernatants using an EIA kit according to the manufacturer's instructions. Mouse lung fibroblasts were adhered and then cultured for 18 h with or without 2.5 ng/ml IL-1β. Supernatants were collected and kept at −80°C before immunoassay. BAL results are given as picograms of PGE2 per milliliter of BAL fluid, whereas PGE2 in the cell culture supernatant results were normalized to the total amount of protein in the cell lysates.
Immunoblot analysis.
For collagen I analysis, the cells were plated, serum-starved overnight, and then treated for 18 h with TGF-β (5 ng/ml) alone or in combination with forskolin (200 μM) or PGE2 (1 μM). Cells were washed in PBS and disrupted in lysis buffer (PBS containing 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM orthovanadate, and Roche protease cocktail inhibitor). Equal amounts of lysate protein were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked in 7% nonfat milk for collagen I and 5% BSA in Tris-buffered solution with 0.1% Tween for the other proteins of interest. They were then incubated overnight at 4°C with respective primary antibodies against collagen I (1:500 in 7% milk), COX-2 (1:2,000 in 2% PBS/BSA), and mPGEs-1 (1:2,000 in 2% PBS/BSA), followed by peroxidase-conjugated secondary antibodies to rabbit or mouse IgG (1:5,000). Proteins of interest were detected using the ECL method. Densitometry of the bands was calculated with Scion Image software (NIH) and normalized for α-tubulin or GAPDH, which were used as loading controls. Results are expressed as a percentage of the nontreated cells.
Semiquantitative real-time RT-PCR.
Semiquantitative real-time RT-PCR was performed on an ABI Prism 7000 Thermocycler (Applied Biosystems) attached to a Dell Latitude laptop computer. Gene-specific primers and probes were designed using Primer Express software (Perkin Elmer/Applied Biosystems). The sequences were as follows: EP2 forward, 5-TGCGCTCAGTCCTCTGTTGT-3; EP2 reverse, 5-TGGCACTGGACTGGGTAGAAC-3; EP2 probe, 5–6FAM-CACTGAGAACACAAGAAGCTCAGCAAACAT-TAMRA-3; β-actin (housekeeping gene against which EP2 expression was normalized) forward, 5-CTGCCTGACGGCCAAGTC-3; β-actin reverse, 5-CAAGAAGGAAGGCTGGAAAAGAG-3; and β-actin probe, 5–6FAMAACGAGAGGTTCCGATGCCCTG-TAMRA-3. Briefly, the reaction mixture contained 250 ng of RNA, 12.5 μl of TaqMan Universal PCR Master Mix, 0.625 μl of ×40 MultiScribe and RNase Inhibitor Mix (Applied Biosystems and Roche), 250 nM probe, and forward and reverse primers at 300 nM in a final volume of 25 μl. For each time point, samples from each individual mouse (5 animals/group) were run in triplicate. The average cycle threshold (Ct) was determined for each group of animals from a given experiment at each time point. Relative gene expression was calculated using the comparative Ct method, which assesses the difference in gene expression between the gene of interest and β-actin for each sample to generate the ΔΔCt. Relative gene expression was then determined by the formula 2−ΔΔCt.
Nimesulide treatment.
Mice received an intraperitoneal dose of the selective COX-2 inhibitor nimesulide (5 mg/kg) in sterile saline solution daily from days 27 to 35 of the group C asthma protocol to assess the role of COX-2-derived prostanoids during the fibrotic phase of chronic asthma. All other animals in these experimental groups received the same volume of vehicle alone intraperitoneally on the same days.
Data analysis.
Data are presented as mean values ± SE. Statistical significance was analyzed using GraphPad Prism 5 (version 5.01; GraphPad Software). Significance was assessed by ANOVA and a post hoc Bonferroni test for three or more groups. P < 0.05 was considered significant.
RESULTS
Inflammatory cell recruitment to the airways.
Total and differential BAL cell counts were determined as a means to evaluate airway inflammation. Control mice exhibited a small number of leukocytes, most of which were mononuclear cells (Table 1). An increase in the cumulative number of allergen challenges resulted in a modest gradual increase in the accumulation of mononuclear cells as well as a modest increase in neutrophils that peaked in group C. Numbers of recovered eosinophils increased substantially in all groups, peaking in group B. This analysis confirmed that the protocol employed resulted in robust eosinophil-predominant allergic inflammation.
Table 1.
Cell counts in bronchoalveolar lavage fluid
| Group | Total | Mononuclear | Eosinophils | Neutrophils | |
|---|---|---|---|---|---|
| Control | A | 5 ± 0.54 | 5 ± 0.54 | 0 ± 0 | 0 ± 0 |
| B | 10.4 ± 1.8 | 9.49 ± 1.57 | 0 ± 0 | 0.19 ± 0.13 | |
| C | 12 ± 1.22 | 11.98 ± 1.23 | 0 ± 0 | 0.02 ± 0.02 | |
| Asthma | A | 52 ± 10.81 | 24.75 ± 3.12 | 23.64 ± 7.86 | 3.72 ± 1.04 |
| B | 316.8 ± 78.33* | 35.88 ± 9.02 | 276.7 ± 70.51# | 4.72 ± 1.66 | |
| C | 198.8 ± 22.78** | 44.64 ± 8.34*** | 125.1 ± 24.83## | 28.56 ± 3.39### |
Cell nos. are expressed as 104 cells/ml and represent means ± SE from 4 independent experiments. Cells were pelleted and resuspended in 1 ml total volume. Total cells were counted on a hemocytometer. Cytospins were prepared and stained with a modified Wright-Giemsa stain for the differential cell counts. Nos. of each cell type were compared among the experimental groups.
P < 0.05 compared with total cells from controls and asthma from groups A and B.
P < 0.05 compared with total cells from controls and asthma from groups A and C.
P < 0.05 compared with mononuclear cell counts of controls and asthma from groups A and B.
P < 0.05 compared with eosinophils of controls and asthma from groups A and C.
P < 0.05 compared with eosinophils of controls and asthma from groups A and B.
P < 0.05 compared with neutrophil counts of controls and asthma from groups A and B.
Allergen-induced histopathologic alterations in lung structure.
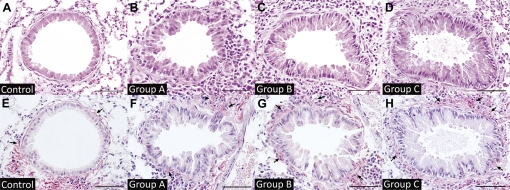
Control lungs exhibited no inflammation or structural abnormalities (Fig. 2A) and, regardless of the cumulative number of PBS challenges, were identical to untreated controls in their lack of inflammation (data not shown). Lungs from group A mice exhibited inflammatory cell infiltrates around small bronchi and blood vessels but almost no collagen deposition (Fig. 2F). A modest amount of collagen deposition around small airways was observed in the group B animals, and this was more extensive in those from group C (Fig. 2, E–H).
Fig. 2.
Morphologic analysis of airways with increasing allergen challenges. A–D: hematoxylin and eosin (H&E) staining of small bronchi from control and group A, B, and C animals, respectively. E–H: picrosirius-hematoxylin staining of lungs from control and group A, B, and C mice. Bars = 50 μm. Arrows indicate areas of Periodic acid-Schiff (PAS) staining.
In vitro properties of fibroblasts during the evolution of airway fibrosis.
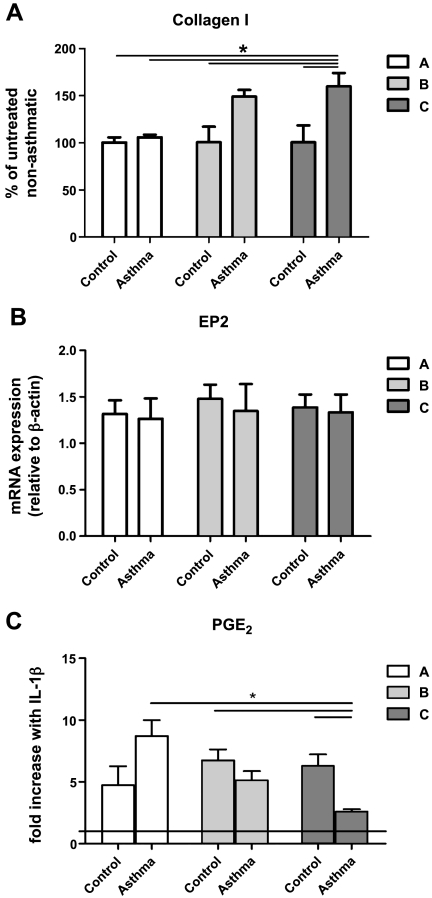
Having defined the kinetics for the evolution of airway fibrosis as described above, we next performed parallel in vitro experiments with lung fibroblasts isolated from mice following 3 (group A), 7 (group B), or 12 (group C) challenges with OVA or PBS. Functional properties assessed included baseline collagen I expression, baseline EP2 expression, and PGE2 production in the presence and absence of IL-1β stimulation. At baseline, control fibroblasts from groups A, B, and C expressed similar amounts of type I collagen. While baseline collagen I expression in asthmatic cells did not differ from that of control cells from group A mice, asthmatic cells from group B mice showed a trend toward greater baseline collagen I than observed in controls, and this difference reached significance in those from group C (Fig. 3A). Because immunodetection of EP proteins by available commercial antibodies is of limited success in mice, real-time RT-PCR was used to determine receptor expression. EP2 expression was similar among the experimental groups and not altered by the severity of airway inflammation or fibrosis (Fig. 3B). As shown in Fig. 3C, control cells from all three groups were able to upregulate PGE2 synthesis approximately five- to sevenfold in response to IL-1β. Asthmatic fibroblasts from group A mice exhibited a capacity to upregulate the synthesis of PGE2 to an even greater extent than corresponding control cells. However, this capacity was progressively lost with increased numbers of allergen challenges, and in group C this upregulation was clearly impaired; in fact, asthmatic cells from group C mice exhibited only one-third the capacity for upregulated PGE2 synthesis as did asthmatic cells from group A mice. These results identify functional alterations in fibroblasts that accompany and parallel the development of airway fibrosis by histopathologic assessment. Because airway fibrosis and alterations in fibroblast function were both maximal in group C, we focused on this group to carefully evaluate the PGE2 axis in a subsequent series of four independent experiments.
Fig. 3.
Properties of mouse lung fibroblasts as a function of numbers of allergen challenges. A: baseline type I collagen expression in mouse lung fibroblasts from control and asthmatic animals as determined by Western blot. *P < 0.05 compared with group A and control from groups B and C. B: EP2 expression as determined by real-time RT-PCR in nonstimulated mouse lung fibroblasts from control and asthmatic animals using the comparative cycle threshold method, with data expressed relative to β-actin. C: prostaglandin E2 (PGE2) synthesis as measured by enzyme-linked immunosorbent assay (ELISA) and expressed as pg/μg of total protein in the cell lysate sample; the horizontal line indicates the no-IL-1β value. *P < 0.05 compared with groups A, B, and C. Data represent means ± SE from 5 animals in each group.
Fibroblast responsiveness to PGE2.
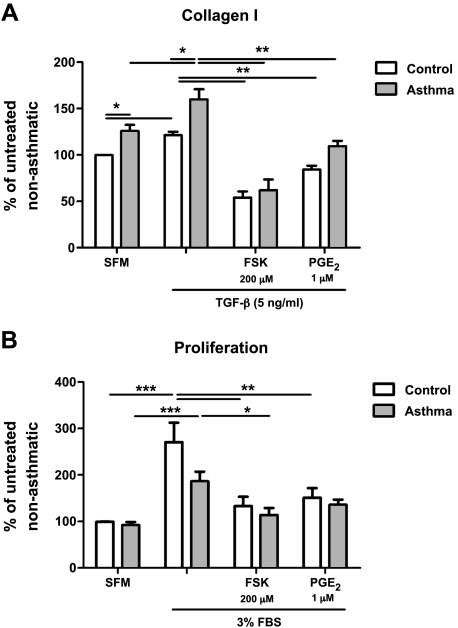
To more thoroughly assess if the histopathologic alterations of chronic asthma reflected by group C animals are accompanied by a defect in the responsiveness of lung fibroblasts to PGE2, we first analyzed the gene expression of EP2, the G protein-coupled receptor through which PGE2 exerts most of its inhibitory effects on these cells. Real-time RT-PCR results showed no difference in EP2 expression in lung fibroblasts from group C control vs. asthmatic mice (data not shown), consistent with the results (Fig. 3B) of initial dose-finding studies. In addition to the phenomenon of PGE2 resistance attributable to EP2 downregulation, we have also observed that fibroblasts from some IPF patients can exhibit resistance to the inhibitory actions of PGE2 despite intact EP2 expression (28). Because of this possibility of a postreceptor defect in cell signaling, we investigated asthmatic cell responses to PGE2 or its analogs even though EP2 expression was preserved. As endpoints for these experiments, we examined type I collagen expression and proliferation. As suggested in the dose-finding experiment, fibroblasts isolated from group C mice expressed higher amounts of collagen I at baseline than did their control counterparts (Fig. 4A). To determine their responsiveness to a profibrotic stimulus, fibroblasts from group C (control and asthmatic) mice were incubated with TGF-β (5 ng/ml) either alone or together with PGE2 (1 μM) or the direct adenyl cyclase activator forskolin (200 μM), both of which increase intracellular levels of cAMP. As also shown in Fig. 4A, TGF-β significantly potentiated collagen I accumulation in lung fibroblasts from both control and asthmatic animals, and TGF-β-stimulated collagen I accumulation was abrogated by PGE2 and forskolin to the same extent in control and asthmatic cells. In contrast to collagen I levels, baseline proliferation was comparable between control and asthmatic group C cells (Fig. 4B). Proliferation was stimulated by 3% (vol/vol) serum in both control and asthmatic cells, although stimulation was slightly less in asthmatic cells. Serum-stimulated proliferation was likewise diminished by PGE2 and forskolin to a comparable degree in lung fibroblasts from both control and asthmatic animals.
Fig. 4.
Responsiveness to PGE2 and forskolin in group C fibroblasts. A: effect of PGE2 and forskolin (FSK) on collagen I expression in mouse lung fibroblasts from control and asthmatic group C animals. B: effect of PGE2 and forskolin on proliferation, measured as [3H]thymidine incorporation, in mouse lung fibroblasts from control and asthmatic group C animals. Data represent means ± SE from 5 animals in each of 4 independent experiments. SFM, serum-free medium; TGF, transforming growth factor. *P < 0.05 compared with forskolin-treated asthma group. **P < 0.05 compared with control cells treated with forskolin and PGE2. ***P < 0.05 compared with untreated controls.
PGE2 synthesis by lung fibroblasts.
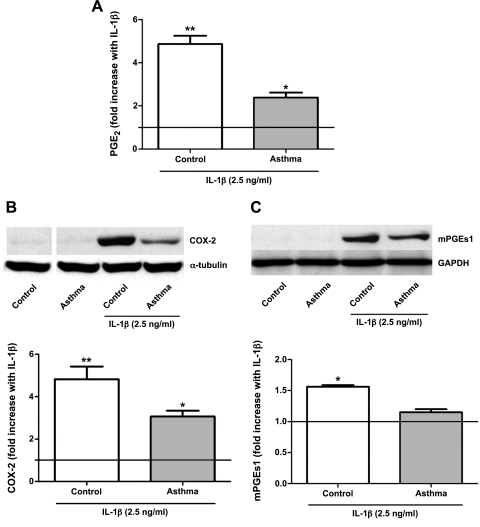
Next we examined the capacity of lung fibroblasts for stimulated PGE2 synthesis. Cells were plated and incubated overnight with serum-free medium or treated with IL-1β (2.5 ng/ml). Because IL-1β is well-known to induce COX-2 expression and PGE2 synthesis in lung fibroblasts (51), it was employed as a tool to evaluate the ability of lung fibroblasts from asthmatic mice to upregulate the biosynthesis of this prostanoid. However, it is also a relevant constituent of the inflammatory milieu to which lung fibroblasts are exposed in vivo, as IL-1β has been reported to be elevated in the BAL of asthma patients (38) and to play a role in the development of airway hyperresponsiveness (42). Results from the initial dose-finding experiment were corroborated here, since we saw an impaired ability to upregulate PGE2 synthesis by asthmatic fibroblasts in group C (Fig. 5A). PGE2 synthesis from arachidonic acid depends on the sequential actions of COX and PGE enzymes. We therefore compared the IL-1β-induced upregulation of the inducible synthetic enzymes, COX-2 and mPGEs-1, in control and asthmatic fibroblasts from group C animals. Compared with control cells, lung fibroblasts from asthmatic animals exhibited a diminished capacity to upregulate COX-2 (Fig. 5B) and, to a lesser extent, mPGEs-1 (Fig. 5C), in response to IL-1β.
Fig. 5.
Inducible PGE2 synthesis and biosynthetic enzyme expression in group C mouse lung fibroblasts. A: fibroblasts from control and asthmatic mouse lungs of group C were incubated without or with interleukin (IL)-1β for 18 h, and PGE2 levels in medium were quantitated by immunoassay. Data are expressed as the fold increase with IL-1β above unstimulated conditions (which are indicated by the horizontal line) and represent the means ± SE from 5 animals in each of 4 independent experiments. B and C: cyclooxygenase (COX)-2 (B) and microsomal PGE synthase-1 (mPGEs1, C) expression, determined by Western blot, in fibroblasts from group C animals. Data are expressed as the fold increase with IL-1β above the unstimulated level, depicted as a horizontal line, and represent the means ± SE from 5 animals in each of 4 independent experiments. A and B: *P < 0.05 compared with baseline. **P < 0.05 compared with baselne and IL-1β-treated asthma group. C: *P < 0.05 compared with baselne and IL-1β-treated asthma group.
PGE2 in BAL fluid.
PGE2 was measured in the BAL fluid of group C asthmatic and control animals. PGE2 was not different between the experimental groups. In BAL from control animals, PGE2 levels were 185.1 ± 22.82 pg/ml, whereas levels in the asthmatic animals were 232.8 ± 15.41 pg/ml.
Effect of nimesulide treatment on histopathology.
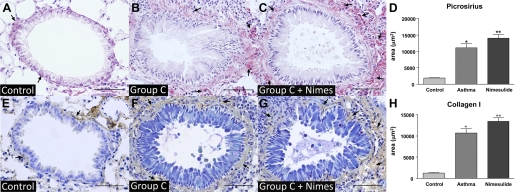
To determine if endogenous COX-2-derived prostanoids generated during the fibrotic phase of airway remodeling influence the eventual extent of fibrosis, we treated animals with a daily intraperitoneal dose (5 mg/kg) of nimesulide, a highly selective COX-2 inhibitor (1, 14), from days 27 to 35 of the group C protocol. Lung sections were stained with picrosirius-hematoxylin to visualize total collagen and also immunostained for type I collagen and submitted to morphometric analysis as described in materials and methods. By both methods, animals treated with nimesulide exhibited a greater degree of airway fibrosis than their vehicle-treated asthmatic counterparts (Fig. 6, A–C and E–G). This conclusion was supported by morphometric quantitation of the mean total area (μm2) of collagen deposition around all small bronchi (0.4–0.7 mm in diameter) (Fig. 6, D and H).
Fig. 6.
Effect of nimesulide on airway remodeling in asthma. The COX-2 inhibitor nimesulide was administered ip to animals in the group C asthma protocol daily from days 27 to 35, and small bronchi were analyzed for staining by picrosirius-hematoxylin (top) and collagen I antibody (bottom). A: control. B: asthma. C: asthma + nimesulide. Bars = 50 μm. D: morphometric analysis of the stained area in μm2. Data represent means ± SE from 5 mice in each of 3 independent experiments. *P < 0.05 compared with the control group. **P < 0.05 compared with the asthma group. Arrows indicate areas of positive staining to PAS and collagen I.
DISCUSSION
A variety of structural changes in the airways, collectively termed “airway remodeling,” are thought to contribute to airway hyperresponsiveness and obstruction in chronic asthma (10, 22). Fibrosis is one component of this remodeling response. Common pathogenic elements driving fibrogenesis of both pulmonary airways and parenchyma include a Th2 immune response dominated by cytokines such as IL-5 and IL-13, epithelial injury, and elaboration of growth factors such as TGF-β. These events ultimately result in the accumulation and activation of mesenchymal cells, including fibroblasts, which synthesize and secrete matrix proteins such as collagen that comprise scars. Fibrosis in asthma has been associated with physiologic airflow limitation and loss of airway distensibility (49). No effective therapeutic options for fibrotic processes are currently available, underscoring the need to gain a better understanding of fibrogenesis that might lead to novel therapeutic strategies. Importantly, existing data in both humans (9a) and in animal models (19, 20) suggest that corticosteroids, well known to exert anti-inflammatory effects in asthma, fail to ameliorate airway remodeling.
As noted in the Introduction, a substantial body of literature now supports the conclusion that the PGE2 axis is an important endogenous brake on fibrogenesis that is dysregulated in parenchymal lung fibrosis. PGE2 can be synthesized by virtually all cell types in the lung, but epithelial cells, mesenchymal cells, and macrophages are likely its predominant sources. This prostanoid possesses ideal properties of an anti-fibrotic molecule, since it inhibits virtually all profibrotic functions of activated fibroblasts while at the same time promoting the survival, proliferation, and migration of epithelial cells (24). Its suppressive actions in fibroblasts are mediated by ligation of EP2 and to a lesser extent, EP4, receptors that signal via increases in intracellular cAMP. Downstream mechanisms that have been identified for these actions of PGE2 and cAMP include activation of protein kinase A, guanine nucleotide exchange protein activated by cAMP, and phosphatase and tensin homolog activated on chromosome 10, with subsequent inhibition of protein kinase C, phosphatidylinositol 3-kinase, and the anti-apoptotic protein survivin (27, 29, 50).
Given that defects in both PGE2 synthesis (21, 31, 44, 51) and responsiveness (28, 40) have been reported in fibroblasts isolated from lungs of patients and mouse models exhibiting parenchymal fibrosis, we wished to evaluate the fibroblast PGE2 axis in an experimental model of airway fibrosis, since this has not previously been examined. We initially utilized a model consisting of two sensitizations with OVA/alum followed by either 3 (group A), 7 (group B), or 12 (group C) aerosol OVA challenges. Eosinophil numbers in BAL fluid verified allergic inflammation in all three groups. However, preliminary histologic analyses revealed that airway fibrosis was modest in group B animals but extensive in group C animals. In the absence of in vitro stimulation, expression of collagen I in fibroblasts from group A asthmatic animals was no different from that in cells from control animals. However, baseline collagen I expression was noted to increase with additional allergen challenges, reaching statistical significance in group C. This finding is consistent with a previous report that identified upregulation of a variety of profibrotic properties in lung fibroblasts isolated from asthmatic mice compared with cells from control animals, although collagen expression was not examined therein (47). This initial dose-finding study led us to focus our investigation of the PGE2 axis on cells from group C, in which more robust collagen I expression at baseline and an impaired ability to upregulate PGE2 synthesis paralleled the robust degree of airway remodeling seen histopathologically.
We first compared the response arm of the PGE2 axis between fibroblasts isolated from group C and control animals. As expected, both TGF-β1-driven synthesis of collagen I and 3% serum-stimulated cellular proliferation were inhibited by PGE2 in fibroblasts from control animals. Inhibition by PGE2 remained intact in asthmatic fibroblasts, in contrast to what has been observed in fibroblasts from mice with bleomycin-induced parenchymal fibrosis (40) and from some, but not all, patients with IPF (28). As expected, responses to forskolin mirrored those to PGE2, emphasizing the cAMP dependence of fibroblast suppression. Likewise, because PGE2 responsiveness was preserved, it was not surprising that gene expression of the EP2 receptor, which mediates most of the inhibitory effects of PGE2 in lung fibroblasts, was also intact in the asthmatic fibroblasts. EP2 downregulation and corresponding PGE2 resistance in lung fibroblasts from mice and humans with parenchymal pulmonary fibrosis have been attributed to silencing of the EP2 gene promoter by methylation (26). It remains to be determined why this defect was not observed in fibroblasts from our group C model of airway fibrosis.
In contrast to their intact PGE2 responsiveness, a loss of PGE2 synthesis in response to IL-1β stimulation was noted in asthmatic fibroblasts isolated from mice subject to increasing numbers of allergen challenges. This was accompanied by diminished capacity of the fibroblasts from group C asthmatic animals to upregulate both COX-2, the biosynthetic enzyme that converts arachidonic acid into PGH2, and mPGES-1, the inducible enzyme that converts PGH2 to the bioactive terminal product PGE2. Of note, impaired COX-2 induction and inducible PGE2 synthesis have previously been reported for bronchial fibroblasts from aspirin-sensitive asthmatics (44) and for airway smooth muscle cells from asthmatics (9). However, to our knowledge, this is the first demonstration of an impairment in inducible PGE2 synthesis in mesenchymal cells that is specifically associated with fibrosis of the airways, as opposed to the parenchyma of the lung. Although the mechanism(s) accounting for impaired expression of PGE2 biosynthetic enzymes in our model remain to be determined, possibilities include inhibition by IL-13 or other Th2 cytokines (48) and epigenetic silencing of COX-2 by histone deacetylation (11). PGE2 levels in the BAL fluid were not different between control and asthmatic animals. However, sampling of the airway surface via lavage would not be expected to adequately reflect concentrations in the subepithelial region of the airway. Moreover, since allergic inflammation and the infiltration of inflammatory cells was extensive in the lungs of asthmatic animals, we speculate that the contribution of inflammatory cells in vivo could compensate for the diminished production of PGE2 by lung fibroblasts. Nevertheless, a deficiency of PGE2 production in the subepithelial compartment could allow unchecked activation of lung fibroblasts and promote airway fibrosis.
A number of studies have demonstrated that pharmacologic (18) or genetic (8) COX inhibition as well as mPGEs-1 deficiency (37) can worsen the allergic inflammation in mouse models of allergic asthma (15). Moreover, one report has demonstrated that allergen challenge of mPGEs-1-deficient mice led to enhanced remodeling of the pulmonary vasculature (37). It has previously been shown that COX-2 knockout mice develop worse allergic lung inflammation (15) and worse pulmonary fibrosis (21) than their wild-type counterparts. However, the phenotype of mice with a constitutive deletion of COX-2, PGE synthase, or EP2 receptor might in fact be somewhat unpredictable. This is because, in some contexts, these genotypes have been shown to exhibit diminished acute inflammation, and this could counterbalance the exaggerated fibrosis resulting from loss of the PGE2 brake. To assess the possible role of endogenous COX-2-derived prostanoids, including PGE2, in limiting airway fibrosis in vivo, we administered nimesulide, a highly selective COX-2 inhibitor, beginning at day 27 (corresponding to group B) after airway remodeling had already begun and harvested mice at the group C endpoint, day 35. The time window for nimesulide administration was selected so that it would interfere with prostanoid synthesis exclusively during the peak fibrogenic phase rather than during the peak inflammatory phase of the response. The histopathology and morphometric analyses indicated that nimesulide treatment of asthmatic animals worsened airway fibrosis compared with vehicle-treated group C asthmatic animals. Its ability to do so may in part reflect the fact that fibroblast responsiveness to PGE2 remained intact in group C asthmatic mice. This result is similar to our previous finding that bleomycin-induced parenchymal fibrosis was worsened by treatment with the COX-1/COX-2 inhibitor indomethacin administered exclusively during the postinflammatory fibrotic phase of this response (41). These in vivo data provide a basis for envisioning that the impaired ex vivo COX-2 function and PGE2 synthesis that characterizes fibroblasts during the evolution of airway fibrosis may likewise promote fibrogenesis. Our data also prompt the speculation that the well-established ability of corticosteroids to inhibit COX-2 induction (16) may help to explain their inability to ameliorate or prevent airway fibrosis in asthma and provide a basis for the possibility that corticosteroids may even promote fibrosis.
In summary, we have shown that, in a mouse model of chronic allergic inflammation, the development of airway fibrosis with an increasing number of allergen challenges is associated with an acquired defect in the ability of lung fibroblasts to upregulate PGE2 generation in response to a cytokine stimulus. Given that endogenous PGE2 acts as a brake on fibrogenesis, our results suggest the possibility that this defect may contribute to the evolution of airway fibrosis (27).
GRANTS
This work was supported by National Institutes of Health Grant HL-094311 and the Brazilian agencies Fundação de Amparo a Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The technical assistance of Teresa Murphy and Casey Lewis is acknowledged. We thank Drs. Steven Huang, Carlos Henrique Serezani, and David M. Aronoff for insightful discussions.
REFERENCES
- 1. Bennett A, Villa G. Nimesulide: an NSAID that preferentially inhibits COX-2, and has various unique pharmacological activities. Expert Opin Pharmacother 1: 277–286, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bentley JK, Hershenson MB. Airway smooth muscle growth in asthma: proliferation, hypertrophy, and migration. Proc Am Thorac Soc 5: 89–96, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bitterman PB, Wewers MD, Rennard SI, Adelberg S, Crystal RG. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest 77: 700–708, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boulet LP, Sterk PJ. Airway remodelling: the future. Eur Respir J 30: 831–834, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol 121: 560–570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Busse W, Elias J, Sheppard D, Banks-Schlegel S. Airway remodeling and repair. Am J Respir Crit Care Med 160: 1035–1042, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Card JW, Carey MA, Bradbury JA, Graves JP, Lih FB, Moorman MP, Morgan DL, DeGraff LM, Zhao Y, Foley JF, Zeldin DC. Cyclooxygenase-1 overexpression decreases Basal airway responsiveness but not allergic inflammation. J Immunol 177: 4785–4793, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chambers LS, Black JL, Ge Q, Carlin SM, Au WW, Poniris M, Thompson J, Johnson PR, Burgess JK. PAR-2 activation, PGE2, and COX-2 in human asthmatic and nonasthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 285: L619–L627, 2003 [DOI] [PubMed] [Google Scholar]
- 9a. Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med 343: 1054–1063, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol 22: 789–815, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Coward WR, Watts K, Feghali-Bostwick CA, Knox A, Pang L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol Cell Biol 29: 4325–4339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elias JA, Rossman MD, Zurier RB, Daniele RP. Human alveolar macrophage inhibition of lung fibroblast growth. A prostaglandin-dependent process. Am Rev Respir Dis 131: 94–99, 1985 [DOI] [PubMed] [Google Scholar]
- 13. Fine A, Poliks CF, Donahue LP, Smith BD, Goldstein RH. The differential effect of prostaglandin E2 on transforming growth factor-beta and insulin-induced collagen formation in lung fibroblasts. J Biol Chem 264: 16988–16991, 1989 [PubMed] [Google Scholar]
- 14. Fukutake M, Nakatsugi S, Isoi T, Takahashi M, Ohta T, Mamiya S, Taniguchi Y, Sato H, Fukuda K, Sugimura T, Wakabayashi K. Suppressive effects of nimesulide, a selective inhibitor of cyclooxygenase-2, on azoxymethane-induced colon carcinogenesis in mice. Carcinogenesis 19: 1939–1942, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, Tiano HF, Lee CA, Langenbach R, Roggli VL, Zeldin DC. Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J Clin Invest 104: 721–732, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilroy DW, Tomlinson A, Willoughby DA. Differential effects of inhibition of isoforms of cyclooxygenase (COX-1, COX-2) in chronic inflammation. Inflamm Res 47: 79–85, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Goldstein RH, Polgar P. The effect and interaction of bradykinin and prostaglandins on protein and collagen production by lung fibroblasts. J Biol Chem 257: 8630–8633, 1982 [PubMed] [Google Scholar]
- 18. Hashimoto K, Sheller JR, Morrow JD, Collins RD, Goleniewska K, O'Neal J, Zhou W, Ji S, Mitchell DB, Graham BS, Peebles RS., Jr Cyclooxygenase inhibition augments allergic inflammation through CD4-dependent, STAT6-independent mechanisms. J Immunol 174: 525–532, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Henderson WR, Jr, Chiang GK, Tien YT, Chi EY. Reversal of allergen-induced airway remodeling by CysLT1 receptor blockade. Am J Respir Crit Care Med 173: 718–728, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henderson WR, Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med 165: 108–116, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Hodges RJ, Jenkins RG, Wheeler-Jones CP, Copeman DM, Bottoms SE, Bellingan GJ, Nanthakumar CB, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E(2) production. Am J Pathol 165: 1663–1676, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holgate ST. Pathogenesis of asthma. Clin Exp Allergy 38: 872–897, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Honda T, Imaizumi K, Yokoi T, Hashimoto N, Hashimoto I, Kawabe T, Matsuo M, Iwano S, Shimokata K, Hasegawa Y. Differential Th1/Th2 chemokine expression in interstitial pneumonia. Am J Med Sci 339: 41–48, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Horowitz JC, Peters-Golden M. Prostaglandin E2's new trick: “decider” of differential alveolar cell life and death. Am J Respir Crit Care Med 182: 2–3 [DOI] [PubMed] [Google Scholar]
- 25. Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling Am J Physiol Lung Cell Mol Physiol 292: L405–L413, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Huang SK, Fisher AS, Scruggs AM, White ES, Hogaboam CM, Richardson BC, Peters-Golden M. Hypermethylation of PTGER2 confers prostaglandin E2 resistance in fibrotic fibroblasts from humans and mice. Am J Pathol 177: 2245–2255, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol 39: 482–489, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang SK, Wettlaufer SH, Hogaboam CM, Flaherty KR, Martinez FJ, Myers JL, Colby TV, Travis WD, Toews GB, Peters-Golden M. Variable prostaglandin E2 resistance in fibroblasts from patients with usual interstitial pneumonia. Am J Respir Crit Care Med 177: 66–74, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang SK, White ES, Wettlaufer SH, Grifka H, Hogaboam CM, Thannickal VJ, Horowitz JC, Peters-Golden M. Prostaglandin E(2) induces fibroblast apoptosis by modulating multiple survival pathways. FASEB J 23: 4317–4326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ingram JL, Huggins MJ, Church TD, Li Y, Francisco DC, Degan S, Firszt R, Beaver DM, Lugogo NL, Wang Y, Sunday ME, Noble PW, Kraft M. Airway fibroblasts in asthma manifest an invasive phenotype. Am J Respir Crit Care Med 183: 1625–1632, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keerthisingam CB, Jenkins RG, Harrison NK, Hernandez-Rodriguez NA, Booth H, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 158: 1411–1422, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kohyama T, Ertl RF, Valenti V, Spurzem J, Kawamoto M, Nakamura Y, Veys T, Allegra L, Romberger D, Rennard SI. Prostaglandin E2 inhibits fibroblast chemotaxis. Am J Physiol Lung Cell Mol Physiol 281: L1257–L1263, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol 29: 537–544, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Korn JH. Fibroblast prostaglandin E2 synthesis. Persistence of an abnormal phenotype after short-term exposure to mononuclear cell products. J Clin Invest 71: 1240–1246, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Korn JH, Halushka PV, LeRoy EC. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest 65: 543–554, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levine L, Alam I. Arachidonic acid metabolism by cells in culture: analyses of culture fluids for cyclooxygenase products by radioimmunoassay before and after separation by high pressure liquid chromatography. Prostaglandins Med 3: 295–304, 1979 [DOI] [PubMed] [Google Scholar]
- 37. Lundequist A, Nallamshetty SN, Xing W, Feng C, Laidlaw TM, Uematsu S, Akira S, Boyce JA. Prostaglandin E(2) exerts homeostatic regulation of pulmonary vascular remodeling in allergic airway inflammation. J Immunol 184: 433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mahajan B, Vijayan VK, Agarwal MK, Bansal SK. Serum interleukin-1beta as a marker for differentiation of asthma and chronic obstructive pulmonary disease. Biomarkers 13: 713–727, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Mauad T, Bel EH, Sterk PJ. Asthma therapy and airway remodeling. J Allergy Clin Immunol 120: 997–1009, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Moore BB, Ballinger MN, White ES, Green ME, Herrygers AB, Wilke CA, Toews GB, Peters-Golden M. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol 174: 5644–5649, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Moore BB, Coffey MJ, Christensen P, Sitterding S, Ngan R, Wilke CA, McDonald R, Phare SM, Peters-Golden M, Paine R, 3rd, Toews GB. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J Immunol 165: 4032–4039, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Nakae S, Komiyama Y, Yokoyama H, Nambu A, Umeda M, Iwase M, Homma I, Sudo K, Horai R, Asano M, Iwakura Y. IL-1 is required for allergen-specific Th2 cell activation and the development of airway hypersensitivity response. Int Immunol 15: 483–490, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol 116: 477–487, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Pierzchalska M, Szabo Z, Sanak M, Soja J, Szczeklik A. Deficient prostaglandin E2 production by bronchial fibroblasts of asthmatic patients, with special reference to aspirin-induced asthma. J Allergy Clin Immunol 111: 1041–1048, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest 132: 1311–1321, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Shindo N, Saito T, Murayama K. Rapid quantification of 11 prostanoids by combined capillary column gas chromatography and negative ion chemical ionization mass spectrometry: application to prostanoids released from normal human embryonic lung fibroblasts WI38 in a culture medium. Biomed Environ Mass Spectrom 15: 25–32, 1988 [DOI] [PubMed] [Google Scholar]
- 47. Sugiura H, Liu X, Duan F, Kawasaki S, Togo S, Kamio K, Wang XQ, Mao L, Ahn Y, Ertl RF, Bargar TW, Berro A, Casale TB, Rennard SI. Cultured lung fibroblasts from ovalbumin-challenged “asthmatic” mice differ functionally from normal. Am J Respir Cell Mol Biol 37: 424–430, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trudeau J, Hu H, Chibana K, Chu HW, Westcott JY, Wenzel SE. Selective downregulation of prostaglandin E2-related pathways by the Th2 cytokine IL-13. J Allergy Clin Immunol 117: 1446–1454, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Ward C, Johns DP, Bish R, Pais M, Reid DW, Ingram C, Feltis B, Walters EH. Reduced airway distensibility, fixed airflow limitation, and airway wall remodeling in asthma. Am J Respir Crit Care Med 164: 1718–1721, 2001 [DOI] [PubMed] [Google Scholar]
- 50. White ES, Atrasz RG, Dickie EG, Aronoff DM, Stambolic V, Mak TW, Moore BB, Peters-Golden M. Prostaglandin E(2) inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol 32: 135–141, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest 95: 1861–1868, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]